Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1137
Peer-review started: December 17, 2022
First decision: February 20, 2023
Revised: February 27, 2023
Accepted: June 5, 2023
Article in press: June 5, 2023
Published online: July 15, 2023
Processing time: 204 Days and 17.4 Hours
Maturity-onset diabetes of the young (MODY) is a monogenic genetic disease often clinically misdiagnosed as type 1 or type 2 diabetes. MODY type 9 (MODY9) is a rare subtype caused by mutations in the PAX4 gene. Currently, there are limited reports on PAX4-MODY, and its clinical characteristics and treatments are still unclear. In this report, we described a Chinese patient with high autoimmune antibodies, hyperglycemia and a site mutation in the PAX4 gene.
A 42-year-old obese woman suffered diabetes ketoacidosis after consuming substantial amounts of beverages. She had never had diabetes before, and no one in her family had it. However, her autoantibody tested positive, and she managed her blood glucose within the normal range for 6 mo through lifestyle inter-ventions. Later, her blood glucose gradually increased. Next-generation sequencing and Sanger sequencing were performed on her family. The results revealed that she and her mother had a heterozygous mutation in the PAX4 gene (c.314G>A, p.R105H), but her daughter did not. The patient is currently taking liraglutide (1.8 mg/d), and her blood glucose levels are under control. Previous cases were retrieved from PubMed to investigate the relationship between PAX4 gene mutations and diabetes.
We reported the first case of a PAX4 gene heterozygous mutation site (c.314G>A, p.R105H), which does not appear pathogenic to MODY9 but may facilitate the progression of latent autoimmune diabetes in adults.
Core Tip: Maturity-onset diabetes of the young type 9 (MODY9), as a subtype of MODY caused by mutations in the PAX4 gene, has been poorly reported, and its clinical features and treatments remain unclear. We reported a heterozygous mutation in the PAX4 gene (c.314G>A, p.R105H) in a patient with latent autoimmune diabetes in adults (LADA). Based on the analysis of the cases indexed in PubMed, it is the first reported case of PAX4 with LADA. The PAX4 heterozygous mutation reported in the present case may not be considered for MODY9 and may be facilitated for the onset and progress of LADA.
- Citation: Zhou GH, Tao M, Wang Q, Chen XY, Liu J, Zhang LL. Maturity-onset diabetes of the young type 9 or latent autoimmune diabetes in adults: A case report and review of literature. World J Diabetes 2023; 14(7): 1137-1145
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1137.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1137
Maturity-onset diabetes of the young (MODY) is a monogenic genetic disease inherited predominantly and is often associated with impaired pancreatic β cell function[1,2]. The prevalence in adults is estimated to be 1 in 10000 and in children to be 1 in 23000, accounting for 1%-3% of diabetes cases[3,4]. A definitive diagnosis of MODY relies on genetic testing. According to the Standard of Care for Diabetes proposed in 2022[5], children diagnosed with diabetes within 6 mo or children or young adults who do not have typical characteristics of type 1 or type 2 diabetes but have a family history of diabetes for several generations should have genetic testing for MODY. MODY is often misdiagnosed as type 1 or type 2 diabetes[6,7].
MODY is classified into subtypes based on genetic mutations; 14 gene mutations have been proven to cause MODY. The most common types are HNF4A, GCK and HNF1A[8]. MODY9 is a subtype caused by mutations in the PAX4 gene. PAX4 belongs to the paired cassette homology domain family primarily expressed in pancreatic islets and is a key factor in the normal differentiation of β cells and δ cells[9]. Inactivation of PAX4 causes a lack of mature β and δ cells in the pancreas, resulting in the body’s inability to produce sufficient insulin and growth inhibitory hormone[10]. Numerous studies have shown that PAX4 can promote the differentiation of stem cells to β cells[11,12], promote β cell survival and proliferation[13,14], induce the conversion of mature α cells to β cells[15,16], regulate cell cycle proteins[17] and maintain endoplasmic reticulum integrity[18] and other pathways that play a crucial role in diabetes. Reports on the diagnosis of PAX4 mutations are still controversial, and the clinical features and treatment of PAX4-related hyperglycemia have not been identified. Here, we reported a patient with high autoimmune antibodies and hyperglycemia with a novel site mutation in the PAX4 gene.
A 42-year-old woman presented with xerostomia, polydipsia, polyuria and blurred vision for 4 d.
The patient experienced xerostomia, polydipsia and polyuria after consuming substantial amounts of beverages and fruits 4 d before admission to the local hospital. She also had blurred vision and fatigue. She went to the local hospital, where her lab results revealed that her fasting blood glucose (FBG) was 18.15 mmol/L, and her glycated hemoglobin (HbA1c) was 10.3%. She was then prescribed metformin and another oral drug (details unknown) to control her blood glucose. However, her symptoms were not relieved, and her FBG remained at 14.54 mmol/L at the time of admission.
The patient had a history of cesarean section 18 years prior to admission and had uterine fibroids for 12 years.
The patient reported no knowledge of diabetes in her family.
The patient was sane, conscious and had dry lips. Her body mass index was 31.85 kg/m2, and her blood pressure was 133/96 mmHg. She was generally in good condition, and no other obvious abnormality was detected at admission.
At admission, the patient arterial pH was 7.29, PO2 was 93 mmHg, bicarbonate was 14.6 mmol/L, FBG was 14.54 mmol/L, islet cell antibody was 45 times higher than normal, glutamic acid decarboxylase (GAD) was 200 times higher than normal, and insulin autoantibody was two times higher than normal. Her urine ketone was significantly positive. Her liver function was slightly abnormal, but her blood lipids, albumin/creatinine ratio and thyroid function were normal (Table 1).
| Parameter | Values |
| Age at onset (yr) | 42 |
| Weight (kg) | 79.5 |
| Height (cm) | 158 |
| BMI (kg/m2) | 31.85 |
| FBG (mmol/L) | 14.54 |
| HbA1c (%) | 10.3 |
| pH | 7.29 |
| HCO3- (mmol/L) | 14.6 |
| ABE | 16.6 |
| SBE | 16.7 |
| ICA (COI) | 45.20 |
| GAD (IU/mL) | > 2000.00 |
| IAA (COI) | 2.10 |
| KET (mmol/L) | +- |
| UA (µmol/L) | 484.7 |
| TG (mmol/L) | 1.23 |
| TC (mmol/L) | 4.22 |
| HDL (mmol/L) | 1.01 |
| LDL (mmol/L) | 2.89 |
| ALT (U/L) | 44.8 |
| AST (U/L) | 40.4 |
| ALP (U/L) | 66.6 |
| GGT (U/L) | 34.0 |
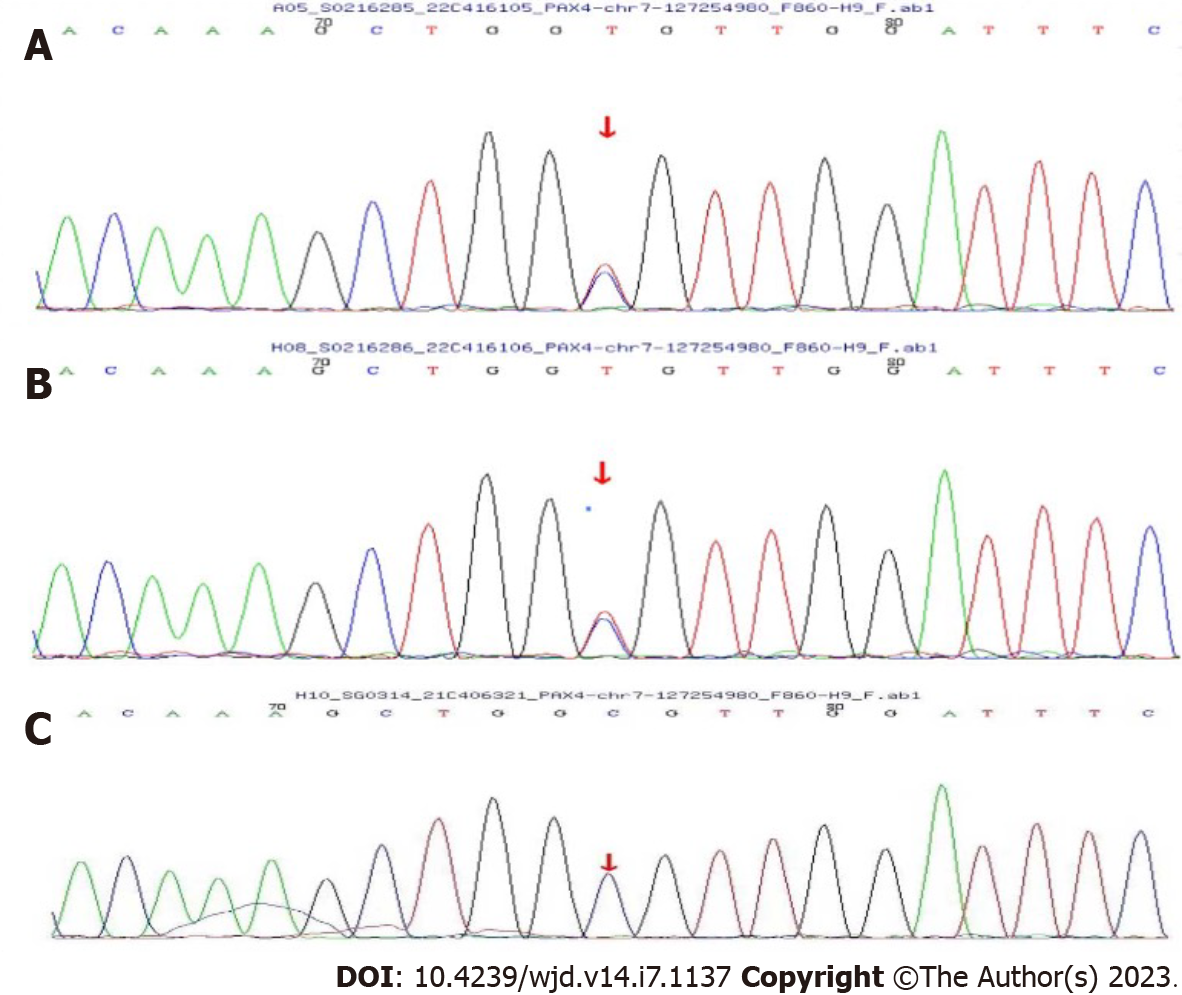
The patient was tested with next-generation sequencing (DNBSEQ-T7) to detect 130 genes related to diabetes, which include 14 pathogenic genes associated with MODY (HNF4A, GCK, HNF1A, PDX1, HNF1B, NEUROD1, KLF11, CEL, PAX4, INS, BLK, ABCC8, KCNJ11, APPL1). The patient’s mother and daughter also underwent Sanger validation. The findings revealed that she had a heterozygous mutation in the PAX4 gene (c.314G>A, p.R105H), and subsequent Sanger validation revealed that her mother also suffered the same mutation. Her daughter was normal (Figure 1).
Diabetic ketoacidosis and type 1 diabetes mellitus (T1DM).
The patient was given a fluid replacement and insulin treatment at admission until her arterial pH and urine ketone levels returned to normal. She was then administered a hypodermic injection of mixed protamine zinc recombinant human insulin injection (70/30), 8 IU before breakfast and 8 IU before dinner, and her FBG level was 6-7 mmol/L at discharge. She maintained lifestyle interventions (balanced diet and regular exercise 30 min/d). One month after discharge, the patient discontinued insulin therapy, and her blood glucose appeared to be normal with lifestyle interventions.
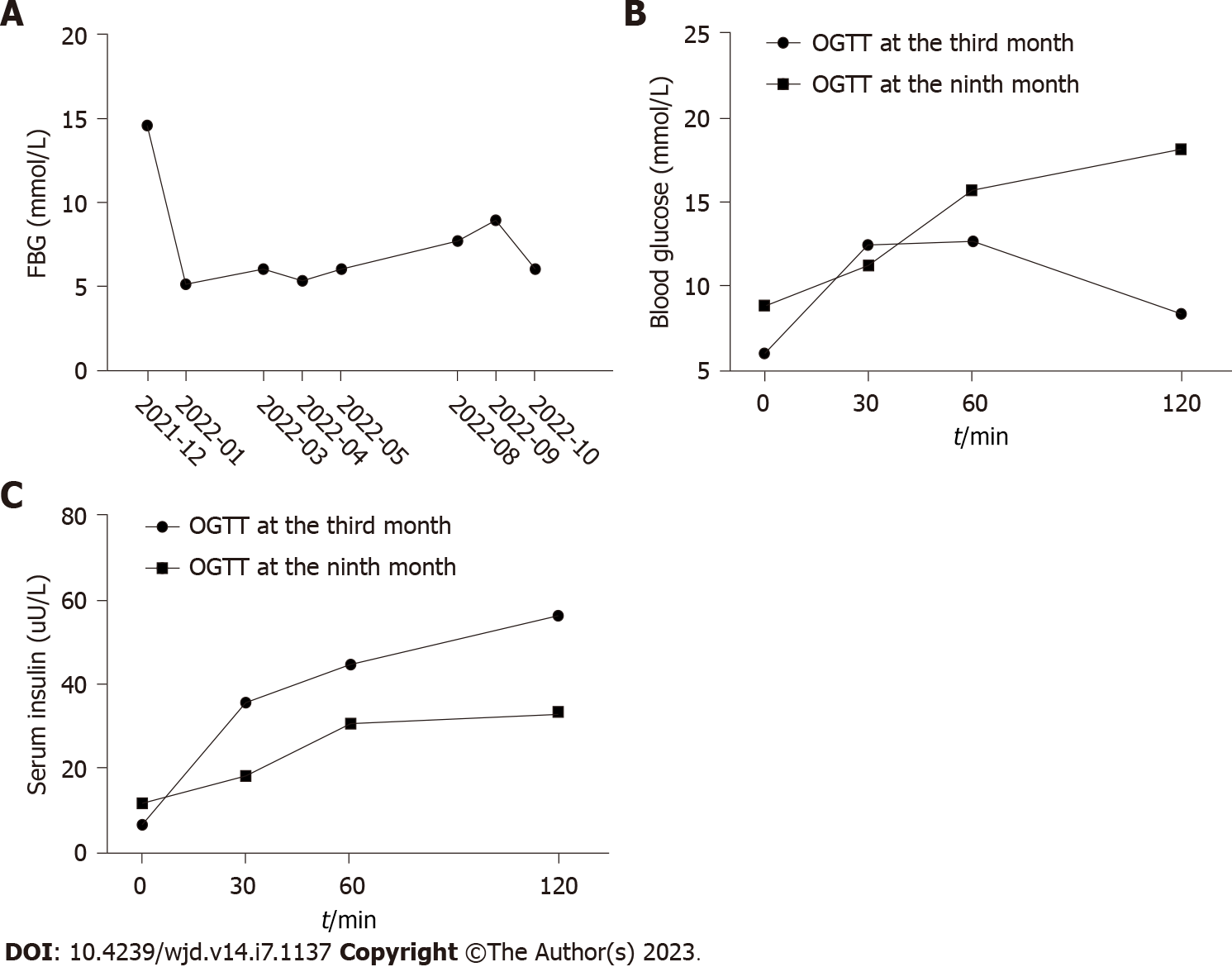
The patient visited our outpatient clinic regularly for check-ups. She also regularly tested capsular blood glucose at home, and the data showed her blood glucose was well controlled. About 3 mo after discharge, we administered an oral glucose tolerance test (OGTT) to evaluate her cell function. Her HbA1c was 6.2%, OGTT (fasting, 30 min, 1 h and 2 h) was 5.96 mmol/L, 12.44 mmol/L, 12.64 mmol/L and 8.33 mmol/L, respectively, oral glucose-insulin release test (fasting, 30 min, 1 h and 2 h) was 6.82 µU/mL, 35.97 µU/mL, 44.81 µU/mL and 56.74 µU/mL, respectively, and the autoantibodies of GAD were still higher than the upper limit. At the 9-mo follow-up, she informed us that her capsular blood glucose was always around 7 mmol/L or slightly higher; hence, we further scheduled an HbA1c and an OGTT test. Her HbA1c was 7.3%, OGTT (fasting, 30 min, 1 h and 2 h) was 8.88 mmol/L, 11.26 mmol/L, 15.72 mmol/L and 18.17 mmol/L, respectively, and oral glucose-insulin release test (fasting, 30 min, 1 h and 2 h) was 11.93 µU/mL, 18.26 µU/mL, 30.93 µU/mL and 33.13 µU/mL. Furthermore, her GAD was still higher than the upper limit (GAD ≥ 10.0 IU/mL). Considering her gradually increasing blood glucose and relatively remaining cell function, she was administered liraglutide 1.8 mg once a day. Her fasting blood glucose was 5-6 mmol/L, and her postprandial blood glucose was 6-8 mmol/L (Figure 2).
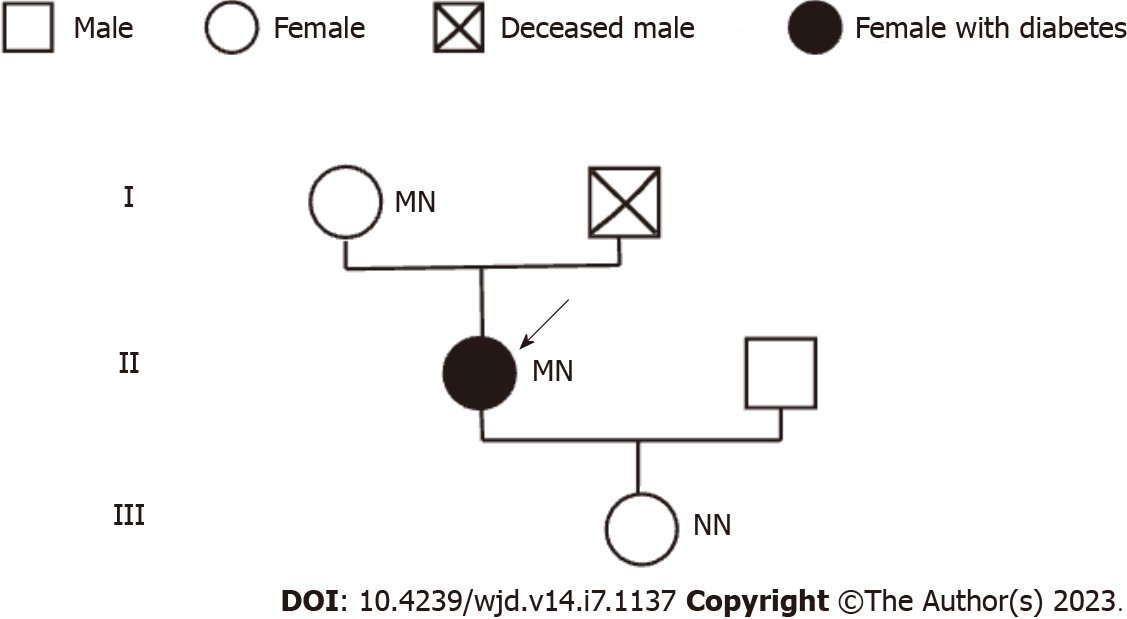
Here, we reported a rare case of diabetes with a heterozygous mutation in the PAX4 gene (c.314G>A, p.R105H). The patient, a middle-aged obese woman, had no obvious diabetic syndrome until she consumed substantial amounts of beverages and fruits. Her HbA1c was 10.3%, indicating that her blood glucose was increased for at least 3 mo. Her high body mass index and insidious onset diabetes are characteristics of type 2 diabetes. However, the repeated high level of autoantibodies (GAD, islet cell antibody and insulin autoantibody) suggested the diagnosis of latent autoimmune diabetes in adults (LADA). Furthermore, this was further supported by her short remission time after lifestyle interventions (about 3-6 mo) and progressive declining cell function and increased blood glucose. We performed genetic testing to exclude other reasons for hyperglycemia. We found that the patient and her mother had a heterozygous mutation in the PAX4 gene (c.314G>A, p.R105H), while her daughter did not. We then drew her family pedigree (Figure 3), which confirmed that the mutation was indeed heterozygous, and the mother carried the mutation but with normal blood glucose. Therefore, we concluded that the mutation might not be the primary cause of her hyperglycemia. So, we did not diagnose her with MODY. To the best of our knowledge, this is the first case of LADA combined with a heterozygous mutation in the PAX4 gene.
MODY9 is the result of a PAX4 mutation. However, few studies have reported MODY9 in detail. Here, we conducted a literature review of case reports of PAX4 mutation. We searched the PubMed database with the terms “maturity-onset diabetes of the young or MODY” and “paired cassette homology domain or PAX4” and selected the case reports, pedigree analyses, and cross-sectional studies. If the article was not related to MODY9 or PAX4 gene mutations, or if the specifics of the patient were not described, it was excluded. Finally, nine articles with 17 cases were included[19-27] (Table 2).
| Ref. | Diagnosis | PAX4 variant | Ethnicity | Family history | Diagnostic age (yr) | Sex | BMI (kg/m2) | HbA1c, % | Insulin antibody, +/- | Treatment | HbA1c % at remission |
| Sujjitjoon et al[22] | MODY9 | Heterozygous IVS7-1G>A | Thailand | Yes | 44 | Female | NA | NA | - | NA | NA |
| Chapla et al[25] | MODY | Heterozygous c.92G>T | Asian-Indian | Yes | 14 | Male | 23 | NA | - | Glimepiride and insulin | NA |
| Jo et al[19] | MODY | Heterozygous c.374-412 del 39 | Japanese | Yes | 15 | Male | 18.2 | 14.5 | - | Insulin | 7.4 |
| Cho et al[20] | MODY | Homozygous c.575G>a | Korean | No | 22 | Male | 25.3 | 13.8 | NA | NA | NA |
| Abreu et al[24] | MODY | Heterozygous c.491G>A | Brazilian | Yes | 32 | Female | 21.6 | NA | - | Insulin | NA |
| Brazilian | Yes | 56 | Female | 29.48 | 11.3 | - | Metformin and gliclazide | NA | |||
| Brazilian | Yes | 49 | Female | 23.61 | 6 | - | Metformin | NA | |||
| Schmidt et al[21] | Ketosis-prone diabetes | Heterozygous c.109C>T | African | No | 38 | Male | 28.4 | > 14 | - | Insulin | 7.0 |
| Mauvais-Jarvis et al[26] | Ketosis-prone diabetes | Homozygous R133W | West African | Yes | 47 | Male | 29.1 | 13.8 | - | Drugs | 6.6 |
| West African | Yes | 22 | Male | 18.5 | 12.2 | - | Drugs | 5.1 | |||
| West African | Yes | 38 | Male | 28.3 | 14.1 | - | Insulin | 6.2 | |||
| West African | Yes | 20 | Male | 26.5 | 12.5 | - | Insulin | 7.3 | |||
| Heterozygous R37W | West African | Yes | 39 | Male | 30.4 | 11.6 | - | Insulin | 8.2 | ||
| Kanatsuka et al[27] | Late-onset diabetic | Homozygous R121W | Japanese | Yes | 37 | Male | 21.5 | 7.6 | - | Insulin | NA |
| Japanese | No | 71 | Male | 22.8 | 7.1 | - | Insulin | NA | |||
| Japanese | Yes | 71 | Female | 20.3 | 6.2 | + | Drugs | NA | |||
| Shimajiri et al[23] | T2DM | Homozygous R121W | Japanese | No | 29 | Female | 22.2 | 12.6 | - | Insulin | 7.3 |
| Present case | T1DM | Heterozygous c.314G>A | Chinese | No | 42 | Female | 31.85 | 10.3 | + | Lifestyle control | 7.3 |
Of these cases, 6 cases[19,22,24,25] with heterozygous PAX4 mutation and 1 case[20] with homozygous PAX4 mutation were diagnosed with MODY, indicating that both homozygous and heterozygous mutations were pathogenic. However, in our case, the patient’s mother had normal blood glucose, possibly because the present site mutation had little pathogenic function, or the mother may progress to diabetes in the future and have longer follow-up needs. The above 6 cases with heterozygous mutations had a family history, while the patient with the homozygous mutation had no family history. Moreover, our case also had no family history. Therefore, it is difficult to determine whether diabetic family history is a characteristic of PAX4 mutation.
Six cases[21,26] were diagnosed with ketosis-prone diabetes, two-thirds of them were homozygous mutation, all were male, and most of them had a family history. One Japanese case of homozygous mutation[23] was diagnosed with type 2 diabetes mellitus (T2DM), and three Japanese cases of homozygous mutation[27] were diagnosed with late-onset diabetes. All of these patients were lean and had no obvious sex and family history differences. Of the 17 cases, only 1 female case with the homozygous mutation had a slightly high level of positive insulin antibody but with a relatively low HbA1c. She was treated with an oral drug and no detailed follow-ups; that case was diagnosed with late-onset diabetes.
Although the c.314G>A mutation has been reported in the dbSNP database, there is no article reporting the specific clinical features of the patients with this mutation nor has it been reported that this mutation is related to LADA. Therefore, our case is significant since it is the first to be reported in China with a mutation site and a high level of autoimmune antibodies. It had a 1-year follow-up to assess the changes in cell function and the progression of the disease.
The literature on the diagnosis of PAX4 mutation with hyperglycemia was controversial. Of the above 17 cases, only 1 case was diagnosed with MODY9, 6 cases were diagnosed only as MODY, and the other cases were diagnosed with ketosis-prone diabetes, late-onset diabetes and T2DM. No case was diagnosed as LADA. While cross-sectional studies found PAX4 gene mutations to be associated with T2DM or ketosis-prone diabetes[21,23,26], population-based studies from China[28], Finland, Hungary[29] and the United Kingdom[30] found no significant association between the PAX4 gene and the risk of developing T1DM. After Biason-Lauber et al[31] proposed that the PAX4 variant 1168C>A was associated with T1DM, Geng et al[32] rejected this point the same year. Mechanically, PAX4 plays a crucial role in the normal differentiation of β cells[9], including promoting the differentiation of stem cells to β cells[11,12], converting mature α cells to β cells[15,16] and maintaining β cell survival and proliferation[13,14]. Therefore, in our case, we considered that the heterozygous mutation in the PAX4 gene might facilitate cell function decline, which coupled with autoimmune antibody destruction accelerates the progression of diabetes. However, this hypothesis also depends on the outcome of her mother’s follow-up.
According to the treatment, in cases with mutations in the PAX4 gene, 9 patients were treated with insulin (52.9%) and 6 patients with oral medication (35.3%). Liraglutide, an incretin hormone that can increase glucose-stimulated insulin secretion, has also been demonstrated to promote β cell proliferation, reduce apoptosis[33,34] and improve β cell function in high-lipid environments by activating the PI3K/Akt pathway[35]. For obese T1DM patients, clinical trials have demonstrated that liraglutide can improve blood glucose, stimulate lipid oxidation and increase thermogenesis while maintaining lean body mass[36]. In T1DM patients with residual islet function, adjuvant therapy with liraglutide has also been proven to reduce HbA1c levels, reduce insulin requirements and increase C-peptide levels[37-39]. We finally added liraglutide to control blood glucose levels and was effectively controlling the patient’s glucose levels at the last follow-up.
In this report, we discovered a heterozygous mutation in PAX4 (c.314G>A, p.R105H) that can coexist with LADA and does not appear pathogenic to MODY9 but may facilitate the progression of LADA. Further functional experiments are needed to confirm this in future.
The authors gratefully acknowledge the patient and family members that agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yahaya TO, Nigeria; Yalcintepe S, Turkey S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Yorifuji T, Kurokawa K, Mamada M, Imai T, Kawai M, Nishi Y, Shishido S, Hasegawa Y, Nakahata T. Neonatal diabetes mellitus and neonatal polycystic, dysplastic kidneys: Phenotypically discordant recurrence of a mutation in the hepatocyte nuclear factor-1beta gene due to germline mosaicism. J Clin Endocrinol Metab. 2004;89:2905-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Stanik J, Dusatkova P, Cinek O, Valentinova L, Huckova M, Skopkova M, Dusatkova L, Stanikova D, Pura M, Klimes I, Lebl J, Gasperikova D, Pruhova S. De novo mutations of GCK, HNF1A and HNF4A may be more frequent in MODY than previously assumed. Diabetologia. 2014;57:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Pihoker C, Gilliam LK, Ellard S, Dabelea D, Davis C, Dolan LM, Greenbaum CJ, Imperatore G, Lawrence JM, Marcovina SM, Mayer-Davis E, Rodriguez BL, Steck AK, Williams DE, Hattersley AT; SEARCH for Diabetes in Youth Study Group. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98:4055-4062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K, Oram RA, Knight B, Hyde C, Cox J, Mallam K, Moudiotis C, Smith R, Fraser B, Robertson S, Greene S, Ellard S, Pearson ER, Hattersley AT; UNITED Team. Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes. Diabetes Care. 2016;39:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 5. | American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45 (Suppl 1):S1-S2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 220] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 6. | Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 7. | Petruzelkova L, Dusatkova P, Cinek O, Sumnik Z, Pruhova S, Hradsky O, Vcelakova J, Lebl J, Kolouskova S. Substantial proportion of MODY among multiplex families participating in a Type 1 diabetes prediction programme. Diabet Med. 2016;33:1712-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Urbanova J, Brunerova L, Broz J. Hypoglycemia and antihyperglycemic treatment in adult MODY patients - A systematic review of literature. Diabetes Res Clin Pract. 2019;158:107914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lenoir O, Flosseau K, Ma FX, Blondeau B, Mai A, Bassel-Duby R, Ravassard P, Olson EN, Haumaitre C, Scharfmann R. Specific control of pancreatic endocrine β- and δ-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9. Diabetes. 2011;60:2861-2871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 579] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Açiksari A, Duruksu G, Karaöz E. Improved insulin-secreting properties of pancreatic islet mesenchymal stem cells by constitutive expression of Pax4 and MafA. Turk J Biol. 2017;41:979-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ, Moore HD, Cosgrove KE, Andrews PW. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS One. 2008;3:e1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Parajuli KR, Zhang Y, Cao AM, Wang H, Fonseca VA, Wu H. Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation. Cell Transplant. 2020;29:963689720958655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol. 2004;167:1123-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 431] [Article Influence: 26.9] [Reference Citation Analysis (1)] |
| 16. | Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, Lacas-Gervais S, Ambrosetti D, Benizri E, Hecksher-Sorensen J, Gounon P, Ferrer J, Gradwohl G, Heimberg H, Mansouri A, Collombat P. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26:86-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Lee G, Jang H, Kim YY, Choe SS, Kong J, Hwang I, Park J, Im SS, Kim JB. SREBP1c-PAX4 Axis Mediates Pancreatic β-Cell Compensatory Responses Upon Metabolic Stress. Diabetes. 2019;68:81-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Mellado-Gil JM, Jiménez-Moreno CM, Martin-Montalvo A, Alvarez-Mercado AI, Fuente-Martin E, Cobo-Vuilleumier N, Lorenzo PI, Bru-Tari E, Herrera-Gómez Ide G, López-Noriega L, Pérez-Florido J, Santoyo-López J, Spyrantis A, Meda P, Boehm BO, Quesada I, Gauthier BR. PAX4 preserves endoplasmic reticulum integrity preventing beta cell degeneration in a mouse model of type 1 diabetes mellitus. Diabetologia. 2016;59:755-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Jo W, Endo M, Ishizu K, Nakamura A, Tajima T. A novel PAX4 mutation in a Japanese patient with maturity-onset diabetes of the young. Tohoku J Exp Med. 2011;223:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Cho YK, Lee JM, Song G, Choi HS, Cho EH, Kim SW. The ominous trio of PCSK1, CHD7 and PAX4: Normosmic hypogonadotropic hypogonadism with maturity-onset diabetes in a young man. Clin Endocrinol (Oxf). 2020;92:554-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Schmidt W, Lankers H. Co-inheritance of PAX4 and BLK Mutations (MODY 7 and 9) in an 38 Year old African Patient with Ketosis-Prone Diabetes. Gemeinschaftspraxis für Humangenetik & Genetische Labore 2016. [DOI] [Full Text] |
| 22. | Sujjitjoon J, Kooptiwut S, Chongjaroen N, Tangjittipokin W, Plengvidhya N, Yenchitsomanus PT. Aberrant mRNA splicing of paired box 4 (PAX4) IVS7-1G>A mutation causing maturity-onset diabetes of the young, type 9. Acta Diabetol. 2016;53:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Shimajiri Y, Sanke T, Furuta H, Hanabusa T, Nakagawa T, Fujitani Y, Kajimoto Y, Takasu N, Nanjo K. A missense mutation of Pax4 gene (R121W) is associated with type 2 diabetes in Japanese. Diabetes. 2001;50:2864-2869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Abreu GM, Soares CAPD, Tarantino RM, da Fonseca ACP, de Souza RB, Pereira MFC, Cabello PH, Rodacki M, Zajdenverg L, Zembrzuski VM, Campos Junior M. Identification of the First PAX4-MODY Family Reported in Brazil. Diabetes Metab Syndr Obes. 2020;13:2623-2631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Chapla A, Mruthyunjaya MD, Asha HS, Varghese D, Varshney M, Vasan SK, Venkatesan P, Nair V, Mathai S, Paul TV, Thomas N. Maturity onset diabetes of the young in India - a distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf). 2015;82:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Mauvais-Jarvis F, Smith SB, Le May C, Leal SM, Gautier JF, Molokhia M, Riveline JP, Rajan AS, Kevorkian JP, Zhang S, Vexiau P, German MS, Vaisse C. PAX4 gene variations predispose to ketosis-prone diabetes. Hum Mol Genet. 2004;13:3151-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kanatsuka A, Tokuyama Y, Nozaki O, Matsui K, Egashira T. Beta-cell dysfunction in late-onset diabetic subjects carrying homozygous mutation in transcription factors NeuroD1 and Pax4. Metabolism. 2002;51:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Xiao X, Liu Y, Zhu X, Wenhui L, Li N, Yuan T, Wang H. The association of the PAX4 gene with type 1 diabetes in Han Chinese. Diabetes Res Clin Pract. 2008;81:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Hermann R, Mantere J, Lipponen K, Veijola R, Soltesz G, Otonkoski T, Simell O, Knip M, Ilonen J. Lack of association of PAX4 gene with type 1 diabetes in the Finnish and Hungarian populations. Diabetes. 2005;54:2816-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Martin RJ, Savage DA, Carson DJ, Maxwell AP, Patterson CC. The PAX4 gene variant A1168C is not associated with early onset Type 1 diabetes in a UK population. Diabet Med. 2006;23:927-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Biason-Lauber A, Boehm B, Lang-Muritano M, Gauthier BR, Brun T, Wollheim CB, Schoenle EJ. Association of childhood type 1 diabetes mellitus with a variant of PAX4: possible link to beta cell regenerative capacity. Diabetologia. 2005;48:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Geng DG, Liu SY, Steck A, Eisenbarth G, Rewers M, She JX. Comment on: Biason-Lauber A, Boehm B, Lang-Muritano M, et al (2005) Association of childhood type 1 diabetes mellitus with a variant of PAX4: possible link to beta cell regenerative capacity. Diabetologia 48:900-905. Diabetologia. 2006;49:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Tamura K, Minami K, Kudo M, Iemoto K, Takahashi H, Seino S. Liraglutide improves pancreatic Beta cell mass and function in alloxan-induced diabetic mice. PLoS One. 2015;10:e0126003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Ding M, Fang QH, Cui YT, Shen QL, Liu Q, Wang PH, Yu DM, Li CJ. Liraglutide prevents β-cell apoptosis via inactivation of NOX2 and its related signaling pathway. J Diabetes Complications. 2019;33:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Shao S, Nie M, Chen C, Chen X, Zhang M, Yuan G, Yu X, Yang Y. Protective action of liraglutide in beta cells under lipotoxic stress via PI3K/Akt/FoxO1 pathway. J Cell Biochem. 2014;115:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Ghanim H, Batra M, Green K, Abuaysheh S, Hejna J, Makdissi A, Borowski R, Kuhadiya ND, Chaudhuri A, Dandona P. Liraglutide treatment in overweight and obese patients with type 1 diabetes: A 26-week randomized controlled trial; mechanisms of weight loss. Diabetes Obes Metab. 2020;22:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Kuhadiya ND, Prohaska B, Ghanim H, Dandona P. Addition of glucagon-like peptide-1 receptor agonist therapy to insulin in C-peptide-positive patients with type 1 diabetes. Diabetes Obes Metab. 2019;21:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, Jensen T, Jensen AK, Holst JJ, Tarnow L, Knop FK, Madsbad S, Andersen HU. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 39. | Dejgaard TF, Schmidt S, Frandsen CS, Vistisen D, Madsbad S, Andersen HU, Nørgaard K. Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: The Lira Pump trial-a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab. 2020;22:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |