Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1091
Peer-review started: January 10, 2023
First decision: January 31, 2023
Revised: February 20, 2023
Accepted: May 17, 2023
Article in press: May 17, 2023
Published online: July 15, 2023
Processing time: 184 Days and 1 Hours
Cardiovascular disease (CVD) is the leading cause of death globally, and diabetes mellitus (DM) is a well-established risk factor. Among the risk factors for CVD, DM is a major modifiable factor. In the fatal CVD outcomes, acute myocardial infarction (AMI) is the most common cause of death.
To develop a long-term quality-of-care score for predicting the occurrence of AMI among patients with type 2 DM on the basis of the hypothesis that good quality of care can reduce the risk of AMI in patients with DM.
Using Taiwan’s Longitudinal Cohort of Diabetes Patient Database and the medical charts of a medical center, we identified incident patients diagnosed with type 2 DM from 1999 to 2003 and followed them until 2011. We constructed a summary quality-of-care score (with values ranging from 0 to 8) with process indicators (frequencies of HbA1c and lipid profile testing and urine, foot and retinal examinations), intermediate outcome indicators (low-density lipoprotein, blood pressure and HbA1c), and co-morbidity of hypertension. The associations between the score and the incidence of AMI were evaluated using Cox regression models.
A total of 7351 patients who had sufficient information to calculate the score were enrolled. In comparison with participants who had scores ≤ 1, those with scores between 2 and 4 had a lower risk of developing AMI [adjusted hazard ratio (AHR) = 0.71; 95% confidence interval (95%CI): 0.55-0.90], and those with scores ≥ 5 had an even lower risk (AHR = 0.37; 95%CI: 0.21-0.66).
Good quality of care can reduce the risk of AMI in patients with type 2 DM. The quality-of-care score developed in this study had a significant association with the risk of AMI and thus can be applied to guiding the care for these patients.
Core Tip: Cardiovascular disease is the leading cause of death globally, and diabetes mellitus (DM) is a major modifiable factor. Hypothesizing that good quality of care can reduce the risk of acute myocardial infarction (AMI) in patients with DM, we developed a long-term quality-of-care score for predicting the occurrence of AMI in patients with type 2 DM. In 7351 patients, we observed a good association between the score and the risk of AMI. Therefore, good quality of care can reduce the risk of AMI in patients with DM, and the score can be applied to guiding the care for these patients.
- Citation: Li PI, Guo HR. Long-term quality-of-care score for predicting the occurrence of acute myocardial infarction in patients with type 2 diabetes mellitus. World J Diabetes 2023; 14(7): 1091-1102
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1091.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1091
Diabetes mellitus (DM) is prevalent worldwide, and it was approximated that there were 422 million individuals suffering from it in 2014[1]. It was projected that this number will reach 592 million by 2035[2]. In Taiwan, it was estimated that around 1.6 million people (7% of the total population) had DM in 2012, and 90% of them had type 2 DM. For over 30 years, this has been one of the most frequent causes of mortality, resulting approximately 11.5% of overall health care costs in recent times[3]. In addition, DM is associated with a two- to three-fold increased risk of heart attacks and strokes[4], and cardiovascular disease (CVD) is the leading cause of death and disability for those with type 2 DM[5,6].
Results from randomized controlled trials have demonstrated conclusively that strict glycemic control reduces microvascular complications (retinopathy, nephropathy and neuropathy) in patients with type 1[7,8] and type 2 DM[9-11]. However, there is a lack of firm evidence of the beneficial effects of intensive glycemic control on great vessel disease, especially CVD, from large, long-term randomized controlled trials[12,13]. According to the United Kingdom Prospective Diabetes Study, intensive control (median HbA1c < 7.0%) could reduce the overall microvascular complication rate by 25%, but had only a slight benefit for the prevention of CVD (16% decrease; P = 0.052)[11,13,14].
The argument that strict control of blood sugar control has no benefit in terms of reducing mortality is largely driven by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, which did not observe a positive effect[15,16]. However, other studies such as the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive)[17], have suggested that controlling blood sugar can lead to improvements. Interventions that simultaneously control common comorbidities of DM, such as hypertension and hyperlipidemia, have been shown to be more effective in reducing deaths related to CVD than solely focusing on regulating blood sugar levels[18]. Adherence to frequent blood testing for blood sugar and lipid profile has been linked to fewer hospital visits for people with DM, including those for vascular and renal complications[19]. Multifactorial risk factor reduction (controlling blood sugar levels, stopping smoking, keeping blood pressure (BP) in check, treating cholesterol issues, and daily use of aspirin for secondary prevention) appears to be the most effective preventive approach for the macrovascular complications of type 2 DM. Nonetheless, studies found that screening tests, including those for HbA1c and lipid, as well as urine and retinal examinations, generally fell well below the frequencies recommended by the American Diabetes Association[19].
Many initiatives have been focused on the evaluation and enhancement of healthcare for people suffering from DM[20-24]. The Diabetes Quality Improvement Program (DQIP), one of the most important such initiatives, has proposed a uniform set of process and intermediate outcome indicators for quality of care, selected under the hypothesis that as a whole they can predict macrovascular complications of type 2 DM[25]. Only a small number of studies have combined process (e.g., the frequency of HbA1c testing) and intermediate outcome (e.g., HbA1c < 8.0%) indicators to predict the occurrence of specific complications of DM, and the combination of DQIP process indicators and intermediate outcome indicators was found to be associated with CVD events and mortality[26,27]. While DQIP chose HbA1c as an intermediate outcome indicator of blood sugar control and applied 9.5% (80 mmol/mol) as the cut-off[25], some recent studies used 8% (64 mmol/mol) based on American Diabetes Association recommendations under the hypothesis that stricter blood sugar control leads to a lower risk of macrovascular complications[25-28]. Nonetheless, the choice of process indicators remained a problem[26,27]. According to the American Diabetes Association, blood sugar should be tested at least twice yearly as an indicator of effective healthcare management. However, studies conducted on an Italian insurance database suggest that less frequent testing may result in better diabetic control[26,27]. Despite the studies having a 28-mo[26,27] average follow-up period, it may not be enough time to get an accurate assessment of the long-term effects like macrovascular complications.
We took into account past research while combining process indicators, intermediate outcome indicators, and the presence of hypertension to construct a score that allowed us to analyze its relationship with AMI. In this research, we obtained information from both hospital medical charts and national health insurance claims. We followed the American Diabetes Association’s advice concerning the frequency of testing to measure the quality of healthcare and kept tabs on the progress over an extended period. Among intermediate outcome indicators, we adopted the American Diabetes Association recommended cut-off of 100 mg/dL for low-density lipoprotein (LDL) instead of 130 mg/dL, which was adopted by DQIP and some other previous studies[28].
Patients who had type 2 DM and were covered by the National Health Insurance system in Taiwan were enrolled from a medical facility located in the southern of Taiwan. The insurance program was launched in March 1995 and had reached a coverage rate of 99% in 2014. For research purpose, the National Health Research Institute of Taiwan constructed and maintains a Longitudinal Cohort of Diabetes Patient Database (LHDB), which contains claim data on 120000 individuals who are randomly selected annually since 1999 from incident patients of DM, identified using the International Classification of Diseases, Clinical Modification (ICD-9-CM) codes 250, A181, and 648.0. The inclusion criteria are having at least: (1) One hospitalization for DM or receiving a prescription for DM medication during hospitalization; (2) Two outpatient visits for DM within one year; or (3) One outpatient visit for DM and receiving at least one prescription for DM medication within one year. The incident year was defined as the year when the first claim for DM was filed, and all the patients included were traced back to January 1, 1997 for their claim records.
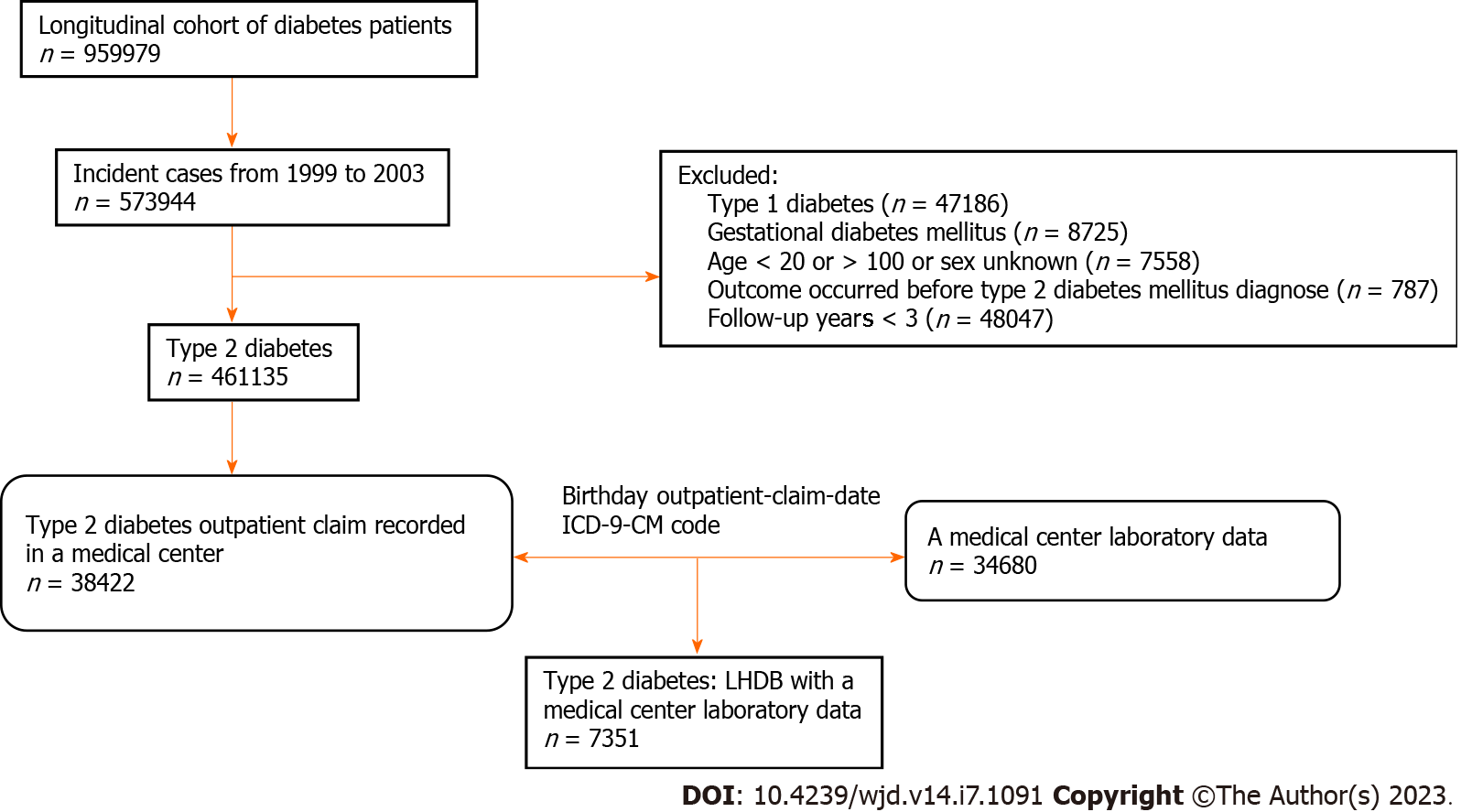
In the current study, participants were identified from the LHDB in 2013. We identified incident patients of DM who were diagnosed between January 1, 1999 and December 31, 2003, with a two-year washout period from January 1, 1997 to December 31, 1998, and followed them till December 31, 2011. In 2011, the Taiwanese health authority initiated a quality control campaign of diabetes care, in which the care indicators of each hospital are compared with the whole country. Because the frequency of care indicators is an important component of the quality of care in our study, this campaign will interfere the study results. Therefore, we used the data before 2011 for this study. Candidates who were diagnosed with Type 1 DM or gestational diabetes were excluded. We also excluded those who had myocardial infarction events before the diagnosis of DM, who were under 20 years of age, who had no information on sex, and who were followed up for less than 3 years (Figure 1).
The LHDB does not have information about lab tests, so we figured out which patients got care at the medical center by pairing their outpatient visit times, ICD-9-CM codes, and date of birth in the LHDB, and then gathering the information from the patient’s medical charts. We extracted information from the medical charts of each participant until the end of follow-up (Figure 1). The medical facility eliminated any identifying details from the medical charts prior to making them public, in order to protect the confidentiality of the information. The study protocol was reviewed and approved by the Ethics Committees of the Chi Mei Medical Center.
On the basis of the scoring systems used in previous studies[26,27], we constructed a quality-of-care score (Table 1). The score includes items of process indicators (frequencies of tests), intermediate outcome indicators (values of test results), and co-morbidity of hypertension for which clear associations with CVD complications have been documented and effective preventive measures are available. The intermediate outcome indicators included LDL < 100 mg/dL, BP < 130/80 mmHg, and HbA1c < 8.0%. The process indicators encompassed how often HbA1c and lipid profiles were examined, along with the regularity of urine, foot, and retinal examinations. Data on the process indicators and co-morbidity of hypertension were extracted from the LHDB, and data on intermediate outcome indicators were extracted from the medical charts retrieved from the medical center.
| Item | Score |
| HbA1c | |
| HbA1c measurement < 2/yr & HbA1c ≥ 8% (64 mmol/mol) | 0 |
| HbA1c measurement < 2/yr & HbA1c < 8% (64 mmol/mol) | 1 |
| HbA1c measurement ≥ 2/yr & HbA1c ≥ 8% (64 mmol/mol) | 1 |
| HbA1c measurement ≥ 2/yr & HbA1c < 8% (64 mmol/mol) | 2 |
| Blood pressure | |
| Co-morbidity of hypertension, never used anti- hypertension agents | 0 |
| SBP ≥ 130 mmHg or DBP ≥ 80 mmHg, never used anti-hypertension agents | 0 |
| No blood pressure data, ever used anti-hypertension agents | 0 |
| SBP ≥ 130 mmHg or DBP ≥ 80 mmHg, ever used anti-hypertension agents | 0 |
| No blood pressure data, no co-morbidity of hypertension, and never used anti-hypertension agents | 1 |
| SBP < 130 mmHg and DBP < 80 mmHg | 1 |
| Lipid profile | |
| Lipid profile measurement < 1/yr & LDL cholesterol ≥ 100 | 0 |
| Lipid profile measurement < 1/yr & LDL cholesterol < 100 | 1 |
| Lipid profile measurement ≥ 1/yr & LDL cholesterol ≥ 100 | 1 |
| Lipid profile measurement ≥ 1/yr & LDL cholesterol < 100 | 2 |
| Eye exam | |
| Eye measurement < 1/yr | 0 |
| Eye measurement ≥ 1/yr | 1 |
| Foot exam | |
| Foot exam < 1/yr | 0 |
| Foot exam ≥ 1/yr | 1 |
| Urine exam | |
| Urine exam < 1/yr | 0 |
| Urine exam ≥ 1/yr | 1 |
We modified cut-offs values of the intermediate outcome indicators according to the most recent American Diabetes Association guidelines, and so they were not exactly the same as those used in the previous studies: 130 mg/dL instead of 100 mg/dL for LDL, 130 mmHg instead of 140 mmHg for systolic BP, and 80 mmHg instead of 90 mmHg for diastolic BP. Similarly, cut-offs for the process indicators were also modified: ≥ 2/year instead of < 1/year for tests of HbA1c, ≥ 1/year instead of < 1/year for tests of lipid profile, and ≥ 1/year instead of < 1/year for urine examination. In addition, we included frequencies of foot examination and retinal examinations (both with 1/year as the cut-off) as process indicators.
We assigned the scores according to the data during the 3-year period before the censor date. In scoring the control of lipid and blood sugar, we assigned the value 2 when both the process and the intermediate outcome indicators met the targets, the value 1 when only one of the indicators met the target, and 0 when none of the indicators met the target. For the frequency of examinations of urine, foot and retinal, the value 1 was assigned when the target was met, and 0 otherwise. For BP, the values were assigned to the status during the study period: 1 to cases with good BP control and cases with no co-morbidity of hypertension. When the information on a specific indicator was missing, a value of 0 was assigned. Consequently, the quality-of-care score has a range between 0 and 8, and a higher score indicates better quality of care.
We identified AMI events using ICD-9-CM diagnostic codes[29]. On the basis of prior research[30,31], the event date was determined to be the day when an applicable ICD-9-CM diagnostic code appeared on claims for outpatient visits for a second time or on claims for inpatient care for its initial time. For those who have survived till end of the study period without any AMI events, a censoring date of December 31, 2011 was assigned.
To evaluate the differences in continuous variables among groups, we used one-way ANOVA. For categorical variables, we used χ2 tests to evaluate differences among groups. We used the Kaplan-Meier method to calculate the probability of AMI in each group defined by the score and the Breslow test to evaluate differences in the AMI disease event-free probabilities among groups. To evaluate the association between the score and AMI, we used Cox proportional hazards regressions. We looked into age, sex, types of medication, compliance with treatment, the Diabetes Complications Severity Index (DCSI)[32], and BP or lipid disorder history in Cox proportional hazards analyses to account for and adjust for possible distorting effects. The DCSI was constructed in a previous study using automated diagnostic, pharmacy, and laboratory data, and a score from 0 to 13 can be assigned accordingly.
Taking into account the stability of estimates, we defined “high” quality of care as having a score higher than half of the maximum value (≥ 5). Accordingly, we divided the participants into three groups: With scores ≤ 1 (the reference group), with scores between 2 and 4, and with scores ≥ 5 (the high-quality group).
Because a portion of the participants did not have information on all the variables evaluated, we conducted a sensitivity analysis by including participants with complete data only. There are two possible reasons why the information is missing. One is that the test/examination was not ordered or administered on the patient. The other is that the patient received the test/examination at other facilities, not the medical center, which rarely happens. Besides, due to the potential for large fluctuations in BP and the lack of routine foot examinations in Taiwan, these two items were excluded from the quality-of-care score in the sensitivity analysis. In other words, participants included in the sensitivity analysis were those who had a complete set of data, except for data on BP or foot examinations. All the statistical analyses were performed using SAS software, Version 9.2 (SAS, Cary, NC).
A total of 7351 participants with type 2 DM were enrolled in this study, including 3963 (53.9%) men and 3388 (46.1%) women (Table 2). The mean age at diagnosis was 56.0 years old, and 66.5% of them were between 40 and 65 years old. Most of the participants (64.3%) took oral antidiabetic drugs (OAD) only, followed by those who received insulin injections only, and then those who received both OAD and insulin treatment. Using records of pharmacy refill, we defined a ratio between 90% and 110% as good adherence[33], which was found in 23.1% of the participants. According to the DCSI, we divided the participants into six groups, from 0 to ≥ 5, as in a previous study[32] and found 66.7% of them were categorized in the first group while only 0.4% were categorized in the last group. During the one-year period before diagnosis, 25.3% of the participants had hypertension, 3.6% had dyslipidemia, and 6.0% had both.
| n (%) | P value | ||||
| Total, n = 7351 | Score ≤ 1, n = 3858 | 1 < score < 5, n = 2819 | Score ≥ 5, n = 674 | ||
| Age (mean ± SD) | 55.96 ± 11.94 | 57.08 ± 12.70 | 54.99 ± 10.94 | 53.62 ± 10.70 | < 0.0001 |
| ≤ 40 | 643 (8.8) | 330 (8.6) | 245 (8.7) | 68 (10.1) | < 0.0001 |
| 40 < age ≤ 65 | 4886 (66.5) | 2369 (61.4) | 2022 (71.7) | 495 (73.4) | |
| > 65 | 1822 (24.8) | 1159 (30.0) | 552 (19.6) | 111 (16.5) | |
| Sex | |||||
| Male | 3963 (53.9) | 2146 (55.6) | 1477 (52.4) | 340 (50.5) | < 0.01 |
| Female | 3388 (46.1) | 1712 (44.4) | 1342 (47.6) | 334 (49.6) | |
| Duration of diabetes mellitus (mean ± SD) | 9.95 ± 1.94 | 9.76 ± 2.06 | 10.14 ± 1.81 | 10.32 ± 1.60 | < 0.0001 |
| ≤ 5 yr | 216 (2.9) | 161 (4.2) | 52 (1.8) | 3 (0.5) | < 0.0001 |
| > 5 yr | 7135 (97.1) | 3697 (95.8) | 2767 (98.2) | 671 (99.6) | |
| Anti-diabetic drugs | |||||
| Oral only | 4729 (64.3) | 2483 (64.4) | 1818 (64.5) | 428 (63.5) | < 0.0001 |
| Insulin only | 1607 (21.9) | 919 (23.8) | 608 (21.6) | 80 (11.9) | |
| Oral + insulin | 1015 (13.8) | 456 (11.8) | 393 (13.9) | 166 (24.6) | |
| Adherence to medication (%) | |||||
| < 90 | 5340 (72.6) | 3087 (80.0) | 1872 (66.4) | 381 (56.5) | < 0.0001 |
| 90 ≤ adherence < 110 | 1698 (23.1) | 637 (16.5) | 786 (27.9) | 275 (40.8) | |
| ≥ 110 | 313 (4.3) | 134 (3.5) | 161 (5.7) | 18 (2.7) | |
| Comorbidity (DCSI) | |||||
| 0 | 4904 (66.7) | 2444 (63.4) | 1951 (69.2) | 509 (75.5) | < 0.0001 |
| 1 | 1160 (15.8) | 634 (16.4) | 436 (15.5) | 90 (13.4) | |
| 2 | 911 (12.4) | 546 (14.2) | 313 (11.1) | 52 (7.7) | |
| 3 | 227 (3.1) | 131 (3.4) | 82 (2.9) | 14 (2.1) | |
| 4 | 122 (1.7) | 80 (2.1) | 34 (1.2) | 8 (1.2) | |
| ≥ 5 | 27 (0.4) | 23 (0.6) | 3 (0.1) | 1 (0.2) | |
| Hypertension/dyslipidemia | |||||
| None | 4782 (65.1) | 2458 (63.7) | 1845 (65.5) | 479 (71.1) | < 0.0001 |
| Hypertension only | 1861 (25.3) | 1054 (27.3) | 669 (23.7) | 138 (20.5) | |
| Dyslipidemia only | 265 (3.6) | 124 (3.2) | 118 (4.2) | 23 (3.4) | |
| Both | 443 (6.0) | 222 (5.8) | 187 (6.6) | 34 (5.0) | |
| Acute myocardial infarction event | |||||
| No | 7043 (95.8) | 3666 (95.0) | 2716 (96.4) | 661 (98.1) | < 0.001 |
| Yes | 308 (4.2) | 192 (5.0) | 103 (3.7) | 13 (1.9) | |
| Incidence rate (per 1000 person-year) | 4.21 | 5.1 | 3.6 | 1.87 | |
While 52% of the participants had a quality-of-care score of ≤ 1, only 9% had high quality of care (score ≥ 5). In comparison with those in the other two groups, participants in the lowest score group were older, predominantly male, and more likely to be prescribed with insulin only. This group also had the worst adherence to treatment and the shortest history of DM (Table 2).
We followed up the participants for a mean period of 9.95 years, and more than 97% of them were followed for more than 5 years. During the follow-up period, 308 (4.2%) participants had AMI, and the incidence rate correlated with the quality-of-care score: 5.1 per 1000 person-years in those having a score of ≤ 1, 3.6 per 1000 person-years in those having a score between 2 and 4, and 1.87 per 1000 person-years in those having a score of ≥ 5. Kaplan-Meier curves also show that a score of ≥ 5 was associated with a lower likelihood of developing AMI.
After adjusting for age, sex, type of DM medicine, adherence to medication, DCSI, and past history of hypertension or dyslipidemia, we found that participants with a score of ≥ 5 had a lower risk of developing AMI [adjusted hazard ratio (AHR) = 0.37; 95% confidence interval (95%CI): 0.21-0.66] in comparison with those with a score of ≤ 1 (Table 3). Female participants had a lower risk of developing AMI (AHR = 0.53; 95%CI: 0.42-0.67) in comparison with male participants (Table 3). Other independent predictors identified in this study included age 40 years to 65 years (AHR = 1.90; 95%CI: 1.10-3.28 in comparison with those ≤ 40 years old), age older than 65 years (AHR = 2.48; 95%CI: 1.39-4.40 in comparison with those ≤ 40 years old), and a history of both hypertension and dyslipidemia (AHR = 1.82; 95%CI: 1.20-2.75 in comparison with those who had no history of hypertension nor dyslipidemia).
| Item | Crude HR (95%CI) | Adjusted HR (95%CI) |
| Quality-of-care score | ||
| Score ≤ 1 | 1 | 1 |
| 1 < Score < 5 | 0.69 (0.55-0.88) | 0.71 (0.55-0.90) |
| Score ≥ 5 | 0.36 (0.20-0.63) | 0.37 (0.21-0.66) |
| Age | ||
| ≤ 40 | 1 | 1 |
| 40 < Age ≤ 65 | 1.93 (1.12-3.32) | 1.90 (1.10-3.28) |
| > 65 | 2.64 (1.51-4.62) | 2.48 (1.39-4.40) |
| Sex | ||
| Male | 1 | 1 |
| Female | 0.57 (0.45-0.73) | 0.53 (0.42-0.67) |
| Anti-diabetic drugs | ||
| Oral only | 1 | 1 |
| Insulin only | 0.80 (0.60-1.07) | 0.78 (0.59-1.05) |
| Oral + insulin | 0.70 (0.49-1.01) | 0.77 (0.54-1.11) |
| Adherence to medication (%) | ||
| < 90 | 0.89 (0.69-1.15) | 0.79 (0.61-1.02) |
| 90 ≤ adherence < 110 | 1 | 1 |
| ≥ 110 | 0.71 (0.37-1.37) | 0.66 (0.34-1.28) |
| Comorbidity (DCSI) | ||
| 0 | 1 | 1 |
| 1 | 1.13 (0.83-1.54) | 0.95 (0.69-1.31) |
| 2 | 1.31 (0.94-1.81) | 1.02 (0.72-1.43) |
| 3 | 1.27 (0.69-2.33) | 0.96 (0.52-1.80) |
| 4 | 1.56 (0.73-3.31) | 1.07 (0.49-2.31) |
| ≥ 5 | 1.04 (0.15-7.43) | 0.66 (0.09-4.77) |
| Hypertension/dyslipidemia | ||
| None | 1 | 1 |
| Hypertension only | 1.38 (1.07-1.78) | 1.30 (0.99-1.71) |
| Dyslipidemia only | 1.19 (0.64-2.18) | 1.17 (0.64-2.17) |
| Both | 1.89 (1.27-2.79) | 1.82 (1.20-2.75) |
To compare the scoring system developed in this study with a well-established system[26,27], we used 5-point increments to assign the scores. When applying that scoring system to the data in this study, we did not observe an association between the score and the risk of developing AMI (Table 4). There was a U-shaped relationship between the score and the risk of AMI. Initially, the risk went down as the score increased, reaching its lowest at 25. After that, the risk increased again, peaking when the score was between 35 and 40. When evaluating the system developed in this research, we observed similar risks for scores ranging from 0 to 10. Afterwards, there was a reduction in the risk as scores decreased, up until scores between 35 and 40. However, the number of individuals included in this group was small (only 103 people), so the risk assessment might not be accurate.
| Score | Present study (n = 7351) | Score | Previous study1 (n = 7351) | Sensitivity analysis2 (n = 3433) | ||||
| n (%) | Crude HR | Adjusted HR | n (%) | Adjusted HR | n (%) | Adjusted HR | ||
| 0 | 2107 (28.7) | 1 | 1 | 0 | 6 (0.1) | 1 | 969 (28.2) | 1 |
| 1 | 1751 (23.8) | 1.21 (0.91-1.61) | 1.22 (0.92-1.63) | 5 | 720 (9.8) | 0.19 (0.03-1.46) | 828 (24.1) | 1.17 (0.77-1.76) |
| 2 | 1355 (18.4) | 0.97 (0.70-1.33) | 0.98 (0.71-1.35) | 10 | 1333 (18.1) | 0.23 (0.03-1.69) | 654 (19.1) | 1.00 (0.63-1.58) |
| 3 | 925(12.6) | 0.56 (0.36-0.86) | 0.57 (0.37-0.89) | 15 | 1148 (15.6) | 0.24 (0.03-1.78) | 420 (12.2) | 0.49 (0.25-0.98) |
| 4 | 539 (7.3) | 0.60 (0.36-1.02) | 0.63 (0.37-1.07) | 20 | 2932 (39.9) | 0.27 (0.04-1.98) | 249 (7.3) | 0.62 (0.28-1.38) |
| 5 | 343 (4.7) | 0.59 (0.31-1.14) | 0.63 (0.33-1.26) | 25 | 857 (11.7) | 0.18 (0.02-1.35) | 173 (5.0) | 0.49 (0.17-1.36) |
| 6 | 228 (3.1) | 0.27 (0.08-0.84) | 0.26 (0.08-0.83) | 30 | 344 (4.7) | 0.20 (0.03-1.54) | 97 (2.8) | 0.00 (0.00-NA) |
| 7-8 | 103 (1.4) | 0.00 (0.00-4.23E266) | 0.00 (0.00-1.57E265) | 35-40 | 11 (0.2) | 0.77 (0.05-12.47) | 43 (1.3) | 0.00 (0.00-NA) |
The sensitivity analysis, based on data from people who had all the indicators present, yielded a similar dose-response relationship as seen in the main investigation; however, the risk decreased (> 50%) even at a score of 3 (Table 4).
It is well known that DM has a close association with major CVD[34], including ischemic heart disease, heart failure, stroke, and peripheral artery disease, which may affect as many as 50% of the patients[35]. Despite the advances in our understanding of the pathophysiology underlying its relationship with CVD, the effects of DM still remain not fully understood. DM, in particularly type 2, is often fraught with additional risk factors contributing to the risk for developing CVD[36]. The additional risk factors include, but are not limited to, dyslipidemia, hypertension, poor blood sugar control, hypercoagulability, smoking, obesity, and lack of physical activity[37].
The relative risk of myocardial infarction is 50% greater in diabetic males and 150% greater in diabetic females[38], and the prevalence of AMI is 3 to 5 times higher in patients with DM in population studies in the United States[39,40]. Women with DM had a lower risk for myocardial infarction than men with DM to experience whichever myocardial infarction events[41]. In our study, the risk of developing AMI was 47% lower in female patients (AHR = 0.53; 95%CI: 0.42-0.67) in comparison with male patients, which is compatible with findings in the United States.
Diabetic patients are at increased risk of developing coronary artery disease (CAD)[42] and experience worse clinical outcomes following AMI[43]. Due to the high prevalence of AMI in diabetic patients, the quality-adjusted life years associated with diabetes lost was 32.8 years[44]. DM is an independent risk factor for the development of CAD[34] and clinical outcomes following the various manifestations of CAD. Despite a clear improvement in the treatment and survival rate of myocardial infarction, the mortality and morbidity of myocardial infarction remain high in diabetic patients[45,46].
DM is a complex chronic progressive metabolic disorder which requires continuous medical care as well as multifactorial risk-reduction strategies extending beyond blood sugar control. Research has proven that managing hypertension and cholesterol levels properly can lead to remarkable declines in CVD[47-49]. For this reason, it is important for those with diabetes to control these factors in combination for reducing the chance of CVD[50,51]. All six components of our quality care score, which are HbA1c, BP, LDL, urine examination, foot examination, and retinal examination, are also included in the conditions established by the Taiwanese government’s pay for performance (P4P) program for diabetes[52,53]. The program incentivizes healthcare providers to register patients who have diabetes, with the intention of increasing the quality of care. Those who join the P4P program are more likely to obtain tests associated with diabetes, and an extended investigation assessing the sustained impacts of the program found it to be economical[52]. Our study confirmed the finding and supports that good quality of care can greatly reduce the risk of developing AMI, and even other CVD, in patients with DM. Therefore, the quality-of-care score developed in this study can be used for prediction and surveillance.
DM poses huge financial burdens to many countries, but data on the clinical care for DM have varied substantially across countries[54]. In Italy, the Quality of Care and Outcomes in Type 2 Diabetes Study combined HbA1c, BP, LDL, and microalbuminuria to construct a quality-of-care score for DM ranging from 0 to 40 and found a close relationship between the score and long-term CVD outcomes[27]. The Quality Assessment Score and Cardiovascular Outcomes in Italian Diabetes Patients study confirmed the finding[26]. However, a large variation in the quality-of-care score among participating centers was observed[26]. Our investigation collected all the information from the same healthcare facility and used factual details to calculate the scores directly. Our scoring system follows the guidelines laid out by the American Diabetes Association in order to properly care for those with diabetes. In addition to using the scoring system developed in this study, we adopted the scoring system used by previous studies[26,27] and found that the other scoring system had a poor correlation with the risk of AMI. Results of this comparison showed that the same scoring system may not work well in prediction of CVD in different countries. It seems that the quality of care may differ from one nation to another, and the indicator used to measure it could have different effects in different health care systems. In Italy, frequent testing may be regarded as a sign of poor care quality[26,27], while in Taiwan it signifies good quality of care, which is in agreement with the American Diabetes Association’s guidelines.
While our study has the strength coming with a large study population and a long follow-up period, it still suffers from some limitations. First of all, lifestyle characteristics such as diet, smoking, and exercise are also predictors for AMI, but was not included in our scoring system because the LHDB does not have the information. Nonetheless, these predictors were not included in the scoring system used by previous studies. Furthermore, although we could not adjust for the effect of smoking, due to the low prevalence of smoking in female Taiwanese (e.g., 2.3% in adults above 18 years of age in 2017[55]), it has been roughly adjusted indirectly when we adjusted for the effects of sex. Secondly, some of the data required for the calculation of the quality-of-care score were missing on a portion of the participants. Nonetheless, in the sensitivity analysis that included only patients with complete data, we observed findings similar to those in the main analysis. It should also be noted that our study was conducted in Taiwan, where there is a health insurance program with an almost complete coverage rate and a high density of medical care facilities. Subsequently, research must be conducted to determine if the observed results are also true in regions where healthcare is limited or costly.
The new quality-of-care score developed in this study had a good correlation with the risk of AMI. Thus, the score can be utilized to recognize those receiving substandard treatment, as well as the components of care that should be advanced. In fact, the scoring systems have been demonstrated as having good correlations with other long-term complications. A previous study revealed that the likelihood of developing chronic kidney illness dropped as the score rose, so strategies focusing on each indicator should be adjusted to reduce the development of diabetes-induced nephropathy[56]. Another study showed that a reduction in macrovascular complication events was associated with a score of 5 or higher[57], similar to findings in this study. Therefore, in order to reduce the risk of AMI in patients with DM, multifactorial interventions should be taken. Checking laboratory tests and combining treatments directed at high blood sugar, high BP, and unhealthy cholesterol levels are among the steps that can be taken. The score we developed is easy to calculate. It can also be applied to comparison of performance across health care facilities and evaluation of the efficacy of quality improvement programs. Nonetheless, it should be kept in mind that various healthcare systems may modify the scoring system to make it more useful.
Cardiovascular disease (CVD) is the leading cause of death globally and diabetes mellitus (DM) is a well-established risk factor. Of the fatal outcomes of CVD, acute myocardial infarction (AMI) is the most common.
DM is a major modifiable factor for CVD, and good quality of care can reduce the risk of AMI in patients with DM. Therefore, a long-term quality-of-care score for DM may predict the occurrence of AMI among patients with type 2 DM and thus guide the care.
To develop a long-term quality-of-care score for predicting the occurrence of AMI among patients with type 2 DM.
Using Taiwan’s Longitudinal Cohort of Diabetes Patients Database and the medical charts of a medical center, we identified incident patients diagnosed with type 2 DM. We constructed a summary quality-of-care score consists of process indicators, intermediate outcome indicators, and a hallmark co-morbidity. The associations between the score and the incidence of AMI were evaluated using Cox regression models.
A total of 7351 patients were enrolled. In comparison with participants who had scores ≤ 1, those with scores between 2 and 4 had a lower risk of developing AMI [adjusted hazard ratio (AHR) = 0.71; 95% confidence interval (95%CI): 0.55-0.90], and those with scores ≥ 5 had an even lower risk (AHR = 0.37; 95%CI: 0.21-0.66). The performance of this score in predicting the risk of AMI is better than that of a widely used scoring system.
Good quality of care can reduce the risk of AMI in patients with type 2 DM. The quality-of-care score developed in this study had a significant association with the risk of AMI and thus can be applied to guiding the care for these patients.
The quality-of-care score developed in this study can be applied to guiding the care for these patients, but different healthcare systems may make modifications to the scoring system for better application.
This study is based in part on the data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SY, China; Tang P, China S-Editor: Chen YL L-Editor: A P-Editor: Ma YJ
| 1. | Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The Growing Epidemic of Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 2. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2956] [Article Influence: 268.7] [Reference Citation Analysis (1)] |
| 3. | Tsai MK, Wang HM, Shiang JC, Chen IH, Wang CC, Shiao YF, Liu WS, Lin TJ, Chen TM, Chen YH. Sequence variants of ADIPOQ and association with type 2 diabetes mellitus in Taiwan Chinese Han population. ScientificWorldJournal. 2014;2014:650393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3857] [Cited by in RCA: 3456] [Article Influence: 230.4] [Reference Citation Analysis (0)] |
| 5. | Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 7. | Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 750] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 8. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16273] [Article Influence: 508.5] [Reference Citation Analysis (3)] |
| 9. | Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2132] [Cited by in RCA: 1931] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 10. | Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5771] [Cited by in RCA: 5268] [Article Influence: 195.1] [Reference Citation Analysis (0)] |
| 11. | Sun S, Hisland L, Grenet G, Gueyffier F, Cornu C, Jaafari N, Boussageon R. Reappraisal of the efficacy of intensive glycaemic control on microvascular complications in patients with type 2 diabetes: A meta-analysis of randomised control-trials. Therapie. 2022;77:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36:2288-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Woo JT, Park KS, Byun DW, Ko KS, Chung YS, Kim DM, Park TS, Cha BS, Lee IK, Park JY, Son HS, Lee MK, Kim KW, Son HY. Regulation of glucose control in people with type 2 diabetes: a review and consensus. Korean Diabetes J. 2010;34:16-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] |
| 15. | Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 16. | Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5608] [Article Influence: 329.9] [Reference Citation Analysis (0)] |
| 17. | Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3109] [Cited by in RCA: 2958] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 18. | Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 2861] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 19. | Sloan FA, Bethel MA, Lee PP, Brown DS, Feinglos MN. Adherence to guidelines and its effects on hospitalizations with complications of type 2 diabetes. Rev Diabet Stud. 2004;1:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Amini M, Timori A, Aminorroaya A. Quality of care for first-degree relatives of type 2 diabetes patients diagnosed with diabetes at a screening program one year after diagnosis. Rev Diabet Stud. 2008;5:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Peters AL, Legorreta AP, Ossorio RC, Davidson MB. Quality of outpatient care provided to diabetic patients. A health maintenance organization experience. Diabetes Care. 1996;19:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Martin TL, Selby JV, Zhang D. Physician and patient prevention practices in NIDDM in a large urban managed-care organization. Diabetes Care. 1995;18:1124-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | TRIAD Study Group. The Translating Research Into Action for Diabetes (TRIAD) study: a multicenter study of diabetes in managed care. Diabetes Care. 2002;25:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Ilag LL, Martin CL, Tabaei BP, Isaman DJ, Burke R, Greene DA, Herman WH. Improving diabetes processes of care in managed care. Diabetes Care. 2003;26:2722-2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Rossi MC, Lucisano G, Comaschi M, Coscelli C, Cucinotta D, Di Blasi P, Bader G, Pellegrini F, Valentini U, Vespasiani G, Nicolucci A; AMD-QUASAR Study Group. Quality of diabetes care predicts the development of cardiovascular events: results of the AMD-QUASAR study. Diabetes Care. 2011;34:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | De Berardis G, Pellegrini F, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH, Rossi MC, Sacco M, Tognoni G, Valentini M, Nicolucci A; QuED (Quality of Care and Outcomes in Type 2 Diabetes) Study Group. Quality of diabetes care predicts the development of cardiovascular events: results of the QuED study. Nutr Metab Cardiovasc Dis. 2008;18:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Standards of medical care in diabetes--2015: summary of revisions. Diabetes Care. 2015;38 Suppl:S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 29. | Chang CH, Chang YC, Wu LC, Lin JW, Chuang LM, Lai MS. Different angiotensin receptor blockers and incidence of diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol. 2014;13:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Kuo HW, Tsai SS, Tiao MM, Yang CY. Epidemiological features of CKD in Taiwan. Am J Kidney Dis. 2007;49:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Laliberté F, Bookhart BK, Vekeman F, Corral M, Duh MS, Bailey RA, Piech CT, Lefebvre P. Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: a managed care perspective. J Manag Care Pharm. 2009;15:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)-Update and ICD-10 translation. J Diabetes Complications. 2017;31:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1299] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 35. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1336] [Article Influence: 190.9] [Reference Citation Analysis (2)] |
| 36. | Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 472] [Cited by in RCA: 556] [Article Influence: 50.5] [Reference Citation Analysis (6)] |
| 37. | Triplitt C, Alvarez CA. Best Practices for Lowering the Risk of Cardiovascular Disease in Diabetes. Diabetes Spectr. 2008;21:177-189. [DOI] [Full Text] |
| 38. | Gomez-Arbelaez D, Sánchez-Vallejo G, Perez M, Garcia RG, Arguello JF, Peñaherrera E, Duarte YC, Casanova ME, Accini JL, Sotomayor A, Camacho PA, Lopez-Jaramillo P. [Hyperglycaemia is associated with worse outcomes in Latin-American individuals with acute myocardial infarction]. Clin Investig Arterioscler. 2016;28:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 484] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 40. | Behl T, Sehgal A, Grover M, Singh S, Sharma N, Bhatia S, Al-Harrasi A, Aleya L, Bungau S. Uncurtaining the pivotal role of ABC transporters in diabetes mellitus. Environ Sci Pollut Res Int. 2021;28:41533-41551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Ballotari P, Venturelli F, Greci M, Giorgi Rossi P, Manicardi V. Sex Differences in the Effect of Type 2 Diabetes on Major Cardiovascular Diseases: Results from a Population-Based Study in Italy. Int J Endocrinol. 2017;2017:6039356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Maffei E, Seitun S, Nieman K, Martini C, Guaricci AI, Tedeschi C, Weustink AC, Mollet NR, Berti E, Grilli R, Messalli G, Cademartiri F. Assessment of coronary artery disease and calcified coronary plaque burden by computed tomography in patients with and without diabetes mellitus. Eur Radiol. 2011;21:944-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Zhao T, Gong HP, Dong ZQ, Du YM, Lu QH, Chen HQ. Predictive value of fasting blood glucose for serious coronary atherosclerosis in non-diabetic patients. J Int Med Res. 2019;47:152-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 977] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 45. | Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord. 2017;17:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 46. | Schmitt VH, Hobohm L, Münzel T, Wenzel P, Gori T, Keller K. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab. 2021;47:101211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 2614] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 48. | Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1796] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 49. | Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, Woodward M, MacMahon S; Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 50. | Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2459] [Cited by in RCA: 2351] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 51. | Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 506] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 52. | Cheng SH, Lee TT, Chen CC. A longitudinal examination of a pay-for-performance program for diabetes care: evidence from a natural experiment. Med Care. 2012;50:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Lu JR, Chen YI, Eggleston K, Chen CH, Chen B. Assessing Taiwan's pay-for-performance program for diabetes care: a cost-benefit net value approach. Eur J Health Econ. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Si D, Bailie R, Wang Z, Weeramanthri T. Comparison of diabetes management in five countries for general and indigenous populations: an internet-based review. BMC Health Serv Res. 2010;10:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Huang HW, Hsueh KC, Li WW, Huang CL. Characteristics of Hardcore Male Smokers in Taiwan: A Qualitative Study. Asian Pac Isl Nurs J. 2020;5:55-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Li PI, Wang JN, Guo HR. Long-term quality-of-care score predicts incident chronic kidney disease in patients with type 2 diabetes. Nephrol Dial Transplant. 2018;33:2012-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Li PI, Wang JN, Guo HR. A long-term quality-of-care score for predicting the occurrence of macrovascular diseases in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;139:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |