INTRODUCTION
According to current data from the International Diabetes Federation (IDF), more than 1 in 10 ten persons has diabetes. Diabetes prevalence among adults (20 to 79 year of age) has more than quadrupled from 151 million (4.6% of the global population) in 2000 to 537 million (10.5% of the global population) in 2021. More shockingly, it is estimated that without changes to intervention strategy, 643 million people (11.3% of the world population) will develop diabetes by 2030[1].
Diabetes complications are primarily attributed to vascular and metabolic factors associated with the disease. Among the major complications are cardiovascular disease, stroke, peripheral artery disease, nephropathy, retinopathy, neuropathy, dental disease, and immunocompromise. These are also expected to become increasingly pervasive, affecting both the local and global burden of illness[2]. Diabetes is a systemic disease, as it can affect nearly every body system. For instance, diabetes can disrupt proper cardiovascular, gastrointestinal, immune, or nervous function. The functional impairment of the peripheral nervous system can lead to diabetic foot and, in the worst cases, amputation and associated physical disability. Diabetic retinopathy can lead to loss of vision and blindness. A wide range of cognitive dysfunction can also occur as a consequence of diabetes, and can manifest as mild cognitive impairment (MCI) to dementia[3].
There is an increased risk of cognitive decline and dementia in patients with diabetes[4]. This has major implications for patient care, particularly in older adults with dementia or pre-dementia with cognitive impairment, which are the most typical manifestations in communities worldwide[4]. The stages of diabetes-associated cognitive decline depend on the type of diabetes and also the patient’s age. For type 1 diabetes (T1DM), the impairment of cognitive function progresses with age. However, for type 2 diabetes (T2DM), there are three stages of cognitive function loss, including diabetes-associated cognitive decline, MCI, and in the final stage, dementia[2].
Several investigations have revealed that patients with T1DM may suffer from severe impairments in information processing, psychomotor efficiency, attention, visuoconstruction, and mental flexibility[5]. However, T2DM has been associated more with problems in executive function, psychomotor speed, and memory. As a result, older diabetic patients often have slower walking pace, poorer coordination, a higher chance of falling, and more fractures, all of which can affect quality of life. In addition, executive dysfunction has been linked to the incapacity to carry out daily tasks.
The effects of diabetes on brain function and cognitive decline have received little attention in academia. However, a study using brain magnetic resonance spectroscopy discovered various metabolic criteria for dementia in diabetic patients and established new links between dementia and diabetes. This study also found extremely low levels of N-acetyl aspartate (which affects neuronal integrity), high levels of myoinositol, high levels of excitatory neurotransmitters (e.g., glutamate and glycine), and low levels of inhibitory neurotransmitters [e.g., gamma-aminobutyric acid (GABA)], which has been linked to pain perception problems in diabetic patients[6]. Diabetes also causes brain atrophy, myelin degradation, and vacuole dispersion throughout the white matter of the brain in rats[7]. Diabetic patients are also thought to have irregularities in the metabolism of neurotransmitters in the brain, which leads to neuronal dysfunction and destruction, eventually contributing to the development of dementia.
Research regarding the actions of insulin has mainly focused on peripheral diseases rather than brain function[8]. However, insulin has been shown to play a role in cognition and neuroprotection in brain. Insulin also has an indirect effect on brain function by acting on peripheral tissue. Many circulating mediators that fluctuate due to obesity and diabetes can pass across the blood-brain barrier (BBB) and contribute to dysfunction in neurons, astrocytes, and microglia[7]. Nonetheless, the mechanism of diabetes-induced cognitive decline is still uncertain. Interestingly, this condition shares many cellular and molecular pathways with Alzheimer’s disease (AD), the most common form of dementia[9]. Here, we describe the putative pathophysiology of diabetes that may contribute to cognitive decline and review diabetic animal models used to study this condition. Finally, we discuss the obstacles and future directions for elucidating the diabetes-related mechanisms associated with cognitive decline.
FACTORS AND PATHOPHYSIOLOGY OF DIABETES INDUCES A COGNITIVE DECLINE
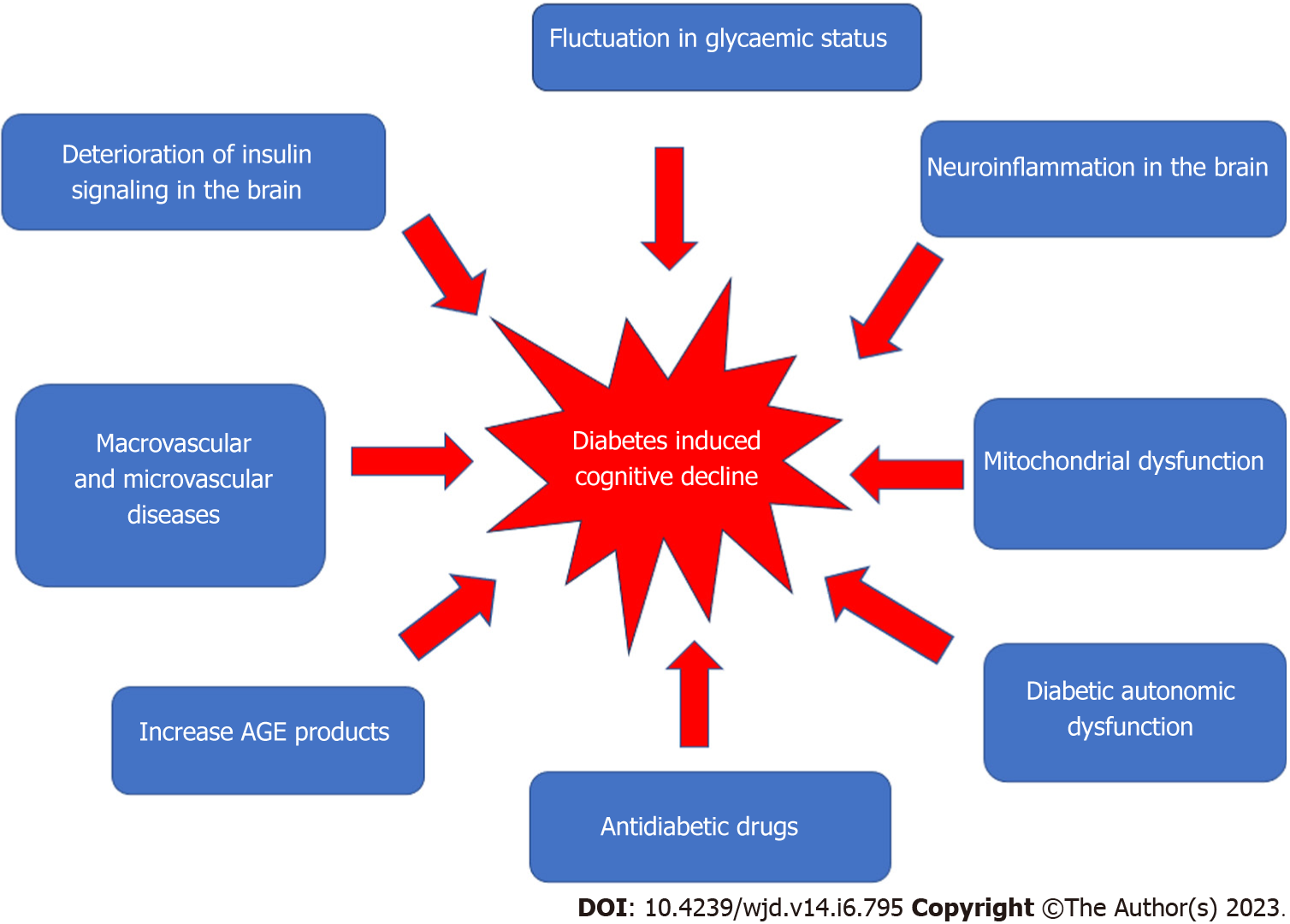
Figure 1 shows the factors that may contribute to the development of diabetes-induced cognitive decline.
Figure 1 Multifactorial pathophysiology of diabetes-induced cognitive decline.
Macrovascular and microvascular diseases
Many vascular, metabolic, and psychosocial factors have been linked to diabetic-induced cognitive decline. Vascular disease, including hypertension and dyslipidemia, has been linked to an increased risk of stroke; in diabetic patients, this risk is estimated to be 115% higher for every 1% increase in glycated hemoglobin (HbA1c)[10]. Furthermore, cardio- or cerebrovascular abnormalities in the brain can lead to cognitive decline and dementia. Patients with T1DM who frequently have cognitive difficulties may have both subclinical and overt cerebrovascular disease[3]. T2DM patients with elevated plasma triglycerides and higher cholesterol levels have been demonstrated to have poorer cognitive function[11]. Studies have also revealed a relationship between cognitive dysfunction and hypertension in T2DM[6,7]. However, due to inconsistent findings from observational studies in the general population[8,9], the roles of dyslipidemia and hypertension in the development of cognitive decline in diabetics is still uncertain and needs further investigation.
Microvascular dysfunction also has been associated with cognitive decline[3,12]. Chronic hyperglycemia increases the risk of microvascular dysfunction, which can affect many organs, including the eye (retinopathy), kidney (nephropathy), and nerves (neuropathy). There is also a positive correlation between the development of cognitive decline and the presence of nephropathy and/or retinopathy[13]. Retinopathy has been linked to cognitive decline in adult diabetic patients as it is thought to affect intelligence, attention/concentration, and information processing[14].
Hyperglycemia
Hyperglycemia has been linked to cognitive decline[15], and can affect cognitive function in the long and short term time. Hyperglycemia has been shown to correlate with impaired working memory, attention, and depression. Acute variations in blood glucose have a negative effect on cognitive performance, and glycemic control improvement is advantageous for regulating cognitive function. It does not cause microvascular structural changes, but has been linked to regional cerebral blood flow or osmotic shifts across the neuronal membrane[16]. In contrast, chronic hyperglycemia affects cognitive function through the production of advanced glycosylation end-products (AGEs), formation of senile plaques and neurofibrillary tangles, and cerebral microvascular disease[17]. A reduction in white matter volume has also been connected to diminished executive function and a reduction in the processing of information[18].
The negative impact of hyperglycemia on cognitive impairment was validated in a zebrafish study in which T1DM was induced with injection of streptozotocin (STZ)[19]. It was discovered that exposing zebrafish to water-diluted glucose for 14 d caused sustained memory impairment accompanied by an increase in acetylcholinesterase activity. On the other hand, galantamine therapy reversed the memory-damaging effects of hyperglycemia. These findings revealed a link between acetylcholinesterase activity and cognitive impairment in T1DM patients[19].
However, cross-sectional studies investigating the association of chronic hyperglycemia (as evidenced by HbA1c) and cognitive decline in people with T2DM have yielded inconclusive results[20,21]. However, this association is apparent in older patients, as the improvement in glycemic control also improves cognitive function[22]. One study demonstrated that treatment of T2DM with either rosiglitazone or glibenclamide (glyburide) improved working memory over 24 wk[16]. Metformin has also been shown to reduce the risk of cognitive impairment in diabetic patients[23], but other evidence suggests that it may increase the risk[24] or have no effect[25]. Treatment with metformin may reduce tau phosphorylation as well as interleukin-1ß-mediated activation of the phosphokinases Akt and mitogen-activated protein kinase (MAPK). Furthermore, it can inhibit the mitochondrial respiratory chain, increasing cyclic adenosine monophosphate (AMP) and activating protein kinase A and AMP-activated protein kinase (AMPK)[26]. AMPK activation has been shown to improve memory and learning in female animal models[27]. However, when the evidence from observational studies and randomized controlled trials is combined, it seems that hyperglycemia and glucose excursions are both weakly associated with poorer cognitive function in T2DM patients[28]. As a result, further research is needed regarding hyperglycemia as a potentially modifiable risk factor for cognitive decline in diabetes.
Hypoglycemia
The presence of hypoglycemic episodes in diabetic patients has also been linked to cognitive decline and an increased risk of dementia[17,18]. The human brain, which accounts for 20% of the body’s metabolic consumption, has a greater need for glucose as a fuel source than other parts of the body. As a result, if the brain is temporarily depleted of glucose, cognitive and emotional functions are impaired. If left untreated, neuroglycopenia can lead to coma, seizure, or brain damage. There is evidence that repeated severe hypoglycemia in patients with early-onset diabetes can contribute to slower mental development and lower intellectual quotient (IQ)[29]. While the cerebral effects of severe hypoglycemia in adults are still not fully clear, insulin-dependent diabetic adults with repeated episodes of severe hypoglycemia performed worse on neuropsychological tests than diabetic patients who had never experienced severe hypoglycemia[30,31]. Another study found a weak link between the reported frequency of severe hypoglycemia and IQ decrement, lower levels of current IQ, and slowed variable reaction times[32]. However, this study found no cognitive differences between diabetic patients receiving intensive insulin therapy with severe hypoglycemia and those receiving conventional therapy[33]. The impact of several episodes of severe hypoglycemia between the ages of 5 year and 15 year is considered mild among young adults dependent on insulin. Strict glycemic control is thought to have a significant benefit in reducing target organ damage and slowing the progression of nephropathy, retinopathy, and neuropathy; however, it increases the risk of severe hypoglycemia. Further research on hypoglycemia and cognitive decline is needed to assist diabetic patients and their physicians in making the best treatment decisions.
Hyperinsulinemia
Hyperinsulinemia caused by endogenous insulin hypersecretion is common in the early stages of T2DM as a result of insulin resistance (IR). Hyperinsulinemia in adults without diabetes is associated with poorer cognitive function[26-28] and an increased risk of AD[34]. When compared to normal patients, patients with moderate to severe AD had higher levels of insulin in plasma but lower levels in cerebrospinal fluid[35]. Insulin therapy, both intravenous and intranasal, has been shown to improve cognitive function in AD patients[36]. Insulin injection into the cerebral ventricles of rats has also been shown to improve memory in a study that demonstrated an insulin signaling defect similar to that found in peripheral tissues could also occur in the hippocampus, resulting in functional insulin deficiency and cognitive decline[37]. Rosiglitazone can prevent disruption in memory tasks and reduce β-amyloid protein in the brain in transgenic mice that overexpress human amyloid precursor protein and develop AD pathology[38,39]. However, more research is needed to determine the link between hyperinsulinemia and cognitive decline.
Peripheral and cerebral insulin resistance
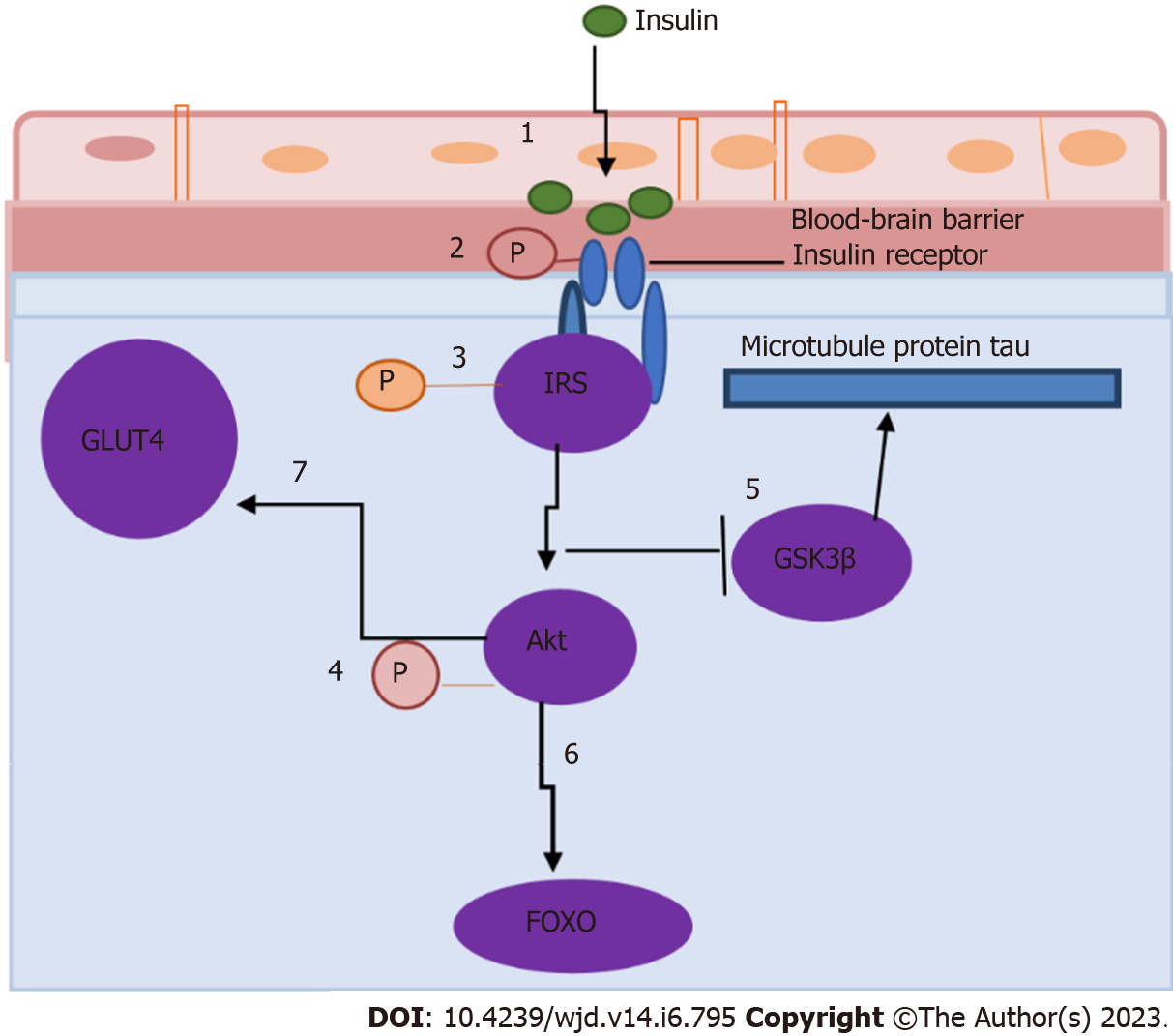
Insulin is released into the circulation by the pancreas and can pass the BBB via a carrier-facilitated process. The BBB comprises ependymal and endothelial cells, and the blood-cerebral spinal fluid barrier has insulin-binding sites that allow insulin to pass through[7]. Insulin receptors are found in the hypothalamus, prefrontal cortex, and hippocampus, among other central nervous system (CNS) sites[9]. The activation of hippocampal insulin receptors is thought to mediate insulin-induced cognitive improvement in healthy mammalian brains by facilitating long-term hippocampal potential (LTP), which is linked to learning and memory, as well as by increasing the expression of N-methyl-D-aspartate (NMDA) receptor[10] (Figure 2). Insulin also regulates the production of other neurotransmitters involved in learning and memory, including acetylcholine, norepinephrine, and adrenaline[3], and stimulates the accumulation of GABA-A receptors on the postsynaptic membrane[11]. A transient surge in peripheral insulin is thought to cause an increase in CNS insulin, which reaches the brain.
Figure 2 Insulin receptor signaling in the hippocampus (adapted from Biessels and Reagan[40], 2015).
Cerebral insulin resistance causes downregulation of insulin transporters at the blood-brain barrier, limiting the amount of insulin that can enter the brain (step 1), decreasing the expression and/or activity of insulin receptors (step 2), modulating the phosphorylation state of insulin receptor substrates (step 3), phosphorylation of Akt (step 4), affecting several downstream components in the insulin signaling cascade (including glycogen synthase kinase 3β) (step 5), regulating the phosphorylation state of the microtubule protein tau and forkhead box O family of transcription factors (step 6), and impairing the trafficking of GLUT4 to the plasma membrane of the brain (step 7). GSK3β: Glycogen synthase kinase 3β; FOXO: Forkhead box O.
In healthy individuals, insulin binds to insulin receptor α-subunits and stimulates the tyrosine kinase domain of βsubunits, resulting in autophosphorylation. This autophosphorylation has the potential to activate the phosphoinositide 3-kinase (PI3K)-Akt (also known as PKB) signaling pathway. Akt molecules (Akt1, Akt2, and Akt3) are serine/threonine kinases that are activated by PI3K in response to growth factors and other cellular stimuli. In the brain, Akt mediates the translocation of glucose transporter type 4 (GLUT 4; also known as SLC2A4) to the plasma membrane. Akt also phosphorylates and inactivates the forkhead box O (FOXO) transcription factor family and glycogen synthase kinase 3β (GSK3β), reducing GSK3β’s ability to phosphorylate the microtubule-associated protein tau.
Chronic peripheral hyperinsulinemia induced by diabetes, obesity, or hyperlipidemia produces peripheral IR associated with the brain’s functional and structural changes. It also contributes to the dysregulation of insulin signaling in the brain and the development of cerebral IR. Cerebral IR causes the downregulation of insulin transporters at the BBB, limiting the amount of insulin that can enter the brain, decreasing the expression and/or activity of insulin receptors, and modulating the phosphorylation state of insulin receptor substrates such as Akt[7]. T2DM patients have lower Akt activation in their adipocytes and skeletal muscle, leading to many damaging effects on neuronal and glial cells[40]. Lower Akt activation affects several downstream components in the insulin signaling cascade, including GSK3β, which regulates the phosphorylation state of the microtubule protein tau and FOXO family of transcription factors. As a result, trafficking of GLUT4 to the plasma membrane is impaired. In addition, memory problems, diminished neuroprotective effects, and impaired synaptic transmission may result from cerebral IR, all of which may also contribute to the development of neurodegenerative illness[20]. However, the mechanisms underlying the relationships between systemic metabolic and vascular consequences of peripheral IR and cerebral IR are still largely undefined. Observational studies in humans are unlikely to fully elucidate the complex interplay between local and systemic factors of IR in the brain and periphery with respect to the mechanism of diabetes-induced cognitive decline[41,42].
Mitochondrial dysfunction
Mitochondria are involved in oxidative respiration, energy metabolism, free radical production, and apoptosis among other physiological processes[43]. The brain has a high energy requirement, and as such it is particularly sensitive to mitochondrial dysfunction. Mitochondria play an important role in anti-aging and neurodegenerative disease prevention[44]. The pathogenesis of diabetes and many neurodegenerative diseases includes mitochondrial dysfunction, attributed to the production of reactive oxygen species (ROS) that can damage proteins, carbohydrates, and lipids. Dysfunctional mitochondria are less effective in generating ATP but rather produce more ROS, leading to the oxidative imbalance seen in cognitive decline[45].
One study has reported that hyperglycemia in diabetes enhances mitochondrial oxidative stress and ROS generation, which can lead to calcium homeostasis disruption, apoptosis, and memory impairment[45]. Diabetic rats also had higher levels of superoxide, protein oxidation, and thiobarbituric acid reactive substances[46] as well as reduced activities of catalase, superoxide dismutase, and glutathione peroxidase in the brain[47]. Excessive oxidative stress causes release of cytochrome C, which starts the apoptotic cascade and leads to mitochondrial dysfunction[44]. These findings suggest that diabetes may worsen mitochondrial dysfunction and oxidative stress in memory and cognition-related brain regions, and may be the fundamental cause of diabetes-related cognitive decline.
Neuroinflammation in the Brain
Diabetes raises the levels of pro-inflammatory cytokines in the brain, which can lead to neuronal damage[48]. Additionally, vascular endothelial dysfunction also elevates inflammatory mediators and compromises the BBB. When BBB function is impaired, neurotoxic blood proteins such as thrombin, fibrin, plasmin, and hemoglobin can potentially enter the brain parenchyma, causing abnormal neuronal activity[49]. The pro-inflammatory nuclear factor-kappa B (NF-κB) has been implicated in diabetic cognitive decline, and a pharmacological inhibitor that inhibits NF-κB activation has been shown to reduce levels of interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) and improve cognitive decline[50].
A post-mortem examination of a diabetic patient’s hippocampus revealed microglia activity similar to that seen in AD patients[17]. TNF-α levels and microglial activation in the brain were found to be higher in mice fed a high-fat diet. It has also been suggested that diabetes and obesity cause decreased spatial recognition memory in db/db mice, which is linked to increased levels of pro-inflammatory cytokines, establishing a relationship between inflammation and cognitive loss[18]. In addition, there is a relationship between neuroinflammation and ROS in cognitive impairment. The creation of ROS in the diabetic brain has been shown to stimulate several cellular pathways, including the advanced glycation end products and its receptor (AGE/RAGE), polyol, and protein kinase C pathways, leading to increased brain inflammation and neurodegeneration[49].
Increase in AGEs
Hyperglycemia in diabetes damages tissues and increases intracellular glucose. This condition triggers mitochondrial overproduction of reactive oxygen and nitrogen species (RONS) such as superoxide anion radical, peroxynitrite, and hydrogen peroxide[51]. RONS, in turn, cause DNA damage and overstimulate peroxisome proliferator-activated receptor, a repair enzyme that increases NAD consumption while decreasing the activity of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which is already compromised by RONS[52]. As a result, endothelial dysregulation occurs, as does the initiation of pro-apoptotic signals, such as the production of AGEs. When AGEs interact with specific receptors (RAGEs), a complex pro-inflammatory cascade involving IL-1, IL-6, TNF-α, transforming growth factor-β (TGF-β), and vascular cell adhesion molecule-1 (VCAM-1) is activated, increasing oxidative stress[51-53]. AGE formation alters the structural and functional properties of proteins in both the extracellular matrix and the intracellular region.
Effects of drugs used in the treatment of diabetes
When compared to untreated patients, treated diabetic patients have less improperly aggregated protein and less vascular damage[54,55]. Metformin, an insulin sensitizer, can reduce the risk of dementia and the rate of cognitive decline in diabetics[54]. Biguanides and sulfonylureas, among other diabetes medications, can alter the relationship between tau pathology and diabetes, slowing the onset of cognitive decline[55].
It is postulated that the best way to delay the onset of dementia is to improve early prevention strategies. Both elderly and middle-aged people with diabetes have poorer cognitive functioning and faster cognitive deterioration[56]. A retrospective study found that the association between DM and AD is stronger in middle-aged people than in the elderly, implying that age is a significant factor in the relationship between DM and AD.
Diabetic autonomic dysfunction and cognitive impairment
Diabetes autonomic dysfunction (DAD) is a complication of diabetes with unexplained and undiscovered pathogenesis. DAD is related to poor blood pressure regulation and a higher risk of stroke, both of which are risk factors for cognitive impairment[57]. Cognitive decline and autonomic dysfunction have comparable fundamental pathologic mechanisms. Autonomic function is compromised in patients with MCI, AD, frontotemporal dementia, dementia with Lewy bodies, and Parkinson’s disease with dementia. In comparison to age-matched controls with normal cognition, there is evidence of sympathetic cardiac autonomic dysfunction in patients with MCI[30]. The correlation between blood pressure dysregulation, silent cerebral infarcts, and cognitive decline reveals that intermittent chronic declines in cerebral blood flow caused by high blood pressure can lead to cognitive decline. This process, however, is still not fully clear and requires additional investigation.
ANIMAL MODELS OF DIABETES-INDUCED COGNITIVE DECLINE
Zucker diabetic fatty rat
Zucker diabetic fatty (ZDF) rats are a genetically derived Zucker fatty strain model of pathological alterations associated with T2DM. Obese ZDF rats have T2DM symptoms such as increased insulin levels, obesity, and increased triglyceride levels. Genetically, the ZDF rat model has also been reported to have abnormally low brain insulin content[58]. Studies have found that this model has altered memory tests and hippocampal-dependent learning due to hyperinsulinemia[59,60]. It is believed that leptin receptor deficiency in the hippocampus of ZDF rats impairs LTP in the hippocampal CA1 region and affects spatial memory[61]. Another study found that the brains of ZDF rats produced more ROS and nitric oxide, as well as suffered more complications of redox homeostasis, mitochondrial function, and ATP synthesis[62]. Astrogliosis was discovered in the hippocampus and frontal and parietal cortices, and there was an increase in the number of glial fibrillary acidic protein (GFAP) immunoreactive astrocytes in ZDF rats[63]. ZDF rats also exhibit reduced hypothalamic corticotropin releasing factor tone due to dysregulation of the hypothalamic-pituitary-adrenal axis[64].
The db/db mouse
The db/db mice are now being used to generate diabetes in rodents to better understand the underlying mechanism and etiology of T2DM. This model includes a leptin receptor gene mutation that causes hepatic IR, hyperglycemia, hyperinsulinemia, hyperlipidemia, and obesity[24]. The Morris water-maze (MWM) test reveals impaired spatial memory in these mice due to decreases in their leptin receptors in the hippocampus[61]. The interaction between cytokines and central processes involving the hippocampus contributes to cognitive behavioral alteration in this db/db mice[65]. Reportedly, changes in hippocampal plasticity and function in db/db mice can be reversed when normal physiological levels of corticosterone are maintained, indicating that cognitive impairment in this model may be caused by glucocorticoid-mediated deficits in neurogenesis and synaptic plasticity[66].
The released cytokines, such as IL-1, due to obesity and diabetes in this model can also mediate the neuroinflammation process and impair hippocampal synaptic plasticity[66]. The debilitation of memory and learning process in db/db mice due to metabolic changes has the ability to reduced membrane metabolism and tricarboxylic cycle and also restrain the cycle of Gln-Glu/GABA, impartially triggering a rise in anaerobic glycolysis[67]. Similarly, Yermakov et al[68], who observed a study on the db/db model in the MWM test’s reversal phase, confirmed that it would impact cognitive flexibility. Another study using this model was executed to examine the importance of neutrophils in the db/db mice model after exposure to hypoxic/ischemic (H/I) insults, which might generate higher morbidity and acute ischemic stroke[69].
The ob/ob mouse
Ob/ob mice are a naturally occurring genetic model in which a mutation in the leptin gene causes leptin insufficiency. As a result, they have large appetites, develop obesity, and are considered an appropriate model for T2DM. A study has been done in ob/ob mice to identify the effect of T2DM disease on tau phosphorylation, which concluded that tau hyperphosphorylation affected thermoregulation resulting in hypothermia in ob/ob mice[68]. The ob/ob mice with leptin-deficiency showed an increase in LTP in the amygdala, indicating that diabetes can have an impact on emotional state[70]. A previous study on ob/ob mice showed acute behavioral dysfunction and disability of spatial memory with higher pro-inflammatory cytokine levels and NF-κB activation compared to the control[71]. Another study looked at the lifespan of the ob/ob mouse and found a link between this and the dysregulation of microglia and astrocytes. Higher levels of GFAP and decreased levels of microglial markers followed this finding[72].
Goto-Kakizaki rat
The Goto-Kakizaki (GK) rat was developed from a polygenic non-obese Wistar substrain as a non-obese diabetic animal model for spontaneous T2DM. A study of brain energy metabolism in diabetic GK rats using 13C magnetic resonance spectroscopy found that the glutamate-glutamine cycle between astrocytes and neurons was impaired due to astrocytes having a greater TCA cycle rate than neurons[73]. Soares et al[74] (2019) also demonstrated that diminished brain glycogen metabolism could interfere with memory and learning capability in the GK rat model. The present findings resulted in the successful induction of aging as one of the characteristics of AD in advanced-age GK rats by increasing phosphorylation of tau. Furthermore, there was an increase in amyloid-β levels along with a reduction in the levels of synaptic proteins in GK rats[75].
High-fat diet rats and streptozotocin injection
In comparison to the regular diet of rats, a high-fat diet (HFD) represents a diet with a high-fat content mixed with fructose or glucose. One study showed that C57BI/6 mice fed a high-fat lard diet increased their body weight and had impaired cognitive function due to increased brain inflammation and decreased BDNF levels[76]. Rats fed high-calorie diets such as HFD, high glucose, and high fructose diets demonstrated changes in energy and lipid metabolism similar to those seen in clinical diabetes, including elevated blood glucose, cholesterol, and triglycerides. This high-calorie diet also decreased spatial learning ability, hippocampal dendritic spine density, and LTP at Schaffer collateral-CA1 synapses. These changes occurred in tandem with a decrease in BDNF levels in the hippocampus[77-79]. This effect has also been proposed due to increased corticosterone and peripheral IR, which may contribute to cerebral IR and increase oxidative stress reaction in the brain[80,81].
Many studies have found that rats fed HFD paired with low-dose STZ also developed obesity and cerebral IR, two key hallmarks of T2DM[82,83]. The T2DM rat model closely resembled the natural history of disease events to induce IR, impair β cell malfunction and metabolic characteristics of T2DM. STZ is an anti-neoplastic and antibiotic drug isolated initially from Streptomyces achromogenes in 1960 and consists of a nitrosourea moiety that is interposed between a methyl group and glucosamine. Due to its severe toxicity to mammalian pancreatic β cells, this drug is commonly used in research to generate experimental animal models of T1DM and AD. Its diabetogenic effects are manifested as hypoinsulinemia, hyperglycemia, polydipsia, and polyurea in animals, all of which are characteristic features of diabetes in humans. Although high-dose STZ causes severe impairment in insulin secretion comparable to that seen in T1DM, low-dose STZ has been shown to cause a modest impairment in insulin secretion, which is similar to T2DM in its later stages[83]. This model is easily available, cheaper, and valuable for future research.
CHALLENGES AND FUTURE DIRECTION
The role of insulin in the brain, particularly the hippocampal region, has been demonstrated to be critical for functional and structural changes in the brain for cognitive processes. Insulin plays a trophic role in the brain and serves as a metabolic homeostasis regulator, promoting neuroplasticity and high energy regulation. Understanding the molecular mechanisms of insulin on brain plasticity is critical for identifying the mechanisms that regulate neural plasticity in health and metabolic disease, such as diabetes-induced cognitive decline, as well as in neurodegenerative disease, particularly AD[80].
To date, research has confirmed the hypothesis that boosting hippocampal insulin receptor signaling could reverse or ameliorate IR-induced neuroplasticity deficits in animal models of T2DM[40]. Previous research has also shown that pharmacological and lifestyle interventions can effectively restore hippocampal neuroplasticity in a T2DM animal model[81]. Several studies study also looking into the efficacy of intranasal insulin administration as an innovative therapeutic strategy to alleviate cognitive decline in T2DM, as it allows insulin to be delivered directly to the CNS and avoids systemic hormone effects[36,84]. Nonetheless, the findings of these studies raise important questions about the localization and effects of intervention strategies, whether they are mediated peripherally or centrally.
The diabetic animal model, which has been used to replicate human cognitive decline, has some limitations and is unreliable in determining the exact human brain condition in diabetes. In addition, diabetes-related cognitive decline has a convoluted etiology with several variables, such as IR and insulin insufficiency, as well as pancreatic cell malfunction, all of which can lead to multiorgan deficits. Thus, additional new characteristics of animal models, along with clinical evidence, should be empowered.
As in T1DM, the induction of STZ is involved in pharmacologic toxicity by destroying pancreatic β cells, which is carcinogenic[85]. The challenge of the STZ-induced animal model involves higher mortality of rats due to toxicity is a stumbling block in research. As the toxicity of STZ can impact multiple organs, it can resemble a contribution to death instead of diabetic complications[86].
Regarding the development of T2DM animal models, potential systemic consequences of disrupted leptin signaling in ob/ob mice to exhibit diabetic peripheral neuropathy should be contemplated[87]. Ob/ob and db/mice are assigned as an appropriate model for neuropathy diabetes, exhibiting early onset and approximate nature of neuropathy. However, numerous studies have shown that these models can result in infertility. Furthermore, they could not perpetuate hyperglycemia levels that are inconsistent with the reduction in fasting blood glucose started at the age of 4 wk[88].
HFD rat models can be developed for future investigations that imitate other human conditions. Nevertheless, the diet composition may not work well with interstudy data. To better understand disease pathogenesis and therapeutic approaches by employing animal models, standardization of induction methods and extensive phenotyping should be prioritized.
Furthermore, the HFD and STZ injection models are more expensive and require a long time to develop. However, animal experimental models that carry significant heterogeneity of diabetes pathology across a broad spectrum of phenotypes seen in patients with cognitive decline must be developed and improved in order to make progress in investigating the causative mechanisms of cognitive decline in diabetes, particularly in T2DM.