Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.758
Peer-review started: February 28, 2023
First decision: March 14, 2023
Revised: March 25, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: June 15, 2023
Processing time: 106 Days and 22.6 Hours
The global burden of diabetic foot ulcers (DFUs) is a significant public health concern, affecting millions of people worldwide. These wounds cause considerable suffering and have a high economic cost. Therefore, there is a need for effective strategies to prevent and treat DFUs. One promising therapeutic approach is the use of adiponectin, a hormone primarily produced and secreted by adipose tissue. Adiponectin has demonstrated anti-inflammatory and anti-atherogenic properties, and researchers have suggested its potential therapeutic applications in the treatment of DFUs. Studies have indicated that adiponectin can inhibit the production of pro-inflammatory cytokines, increase the production of vascular endothelial growth factor, a key mediator of angiogenesis, and inhibit the activation of the intrinsic apoptotic pathway. Additionally, adiponectin has been found to possess antioxidant properties and impact glucose metabolism, the immune system, extracellular matrix remodeling, and nerve function. The objective of this review is to summarize the current state of research on the potential role of adiponectin in the treatment of DFUs and to identify areas where further research is needed in order to fully understand the effects of adiponectin on DFUs and to establish its safety and efficacy as a treatment for DFUs in the clinical setting. This will provide a deeper understanding of the underlying mechanisms of DFUs that can aid in the development of new and more effective treatment strategies.
Core Tip: The global burden of diabetic foot ulcers (DFUs) is significant, both in terms of human suffering and healthcare costs. Therefore, effective strategies to prevent and treat DFUs are urgently needed. Adiponectin, a hormone produced by adipose tissue, shows promise as a therapeutic option for DFUs due to its anti-inflammatory, antioxidant, and pro-angiogenic effects. While adiponectin has potential therapeutic applications, further research is necessary to establish its safety and efficacy in clinical settings. This review aims to summarize current research on adiponectin’s potential role in treating DFUs and identify areas requiring further investigation.
- Citation: Abdalla MMI, Mohanraj J, Somanath SD. Adiponectin as a therapeutic target for diabetic foot ulcer. World J Diabetes 2023; 14(6): 758-782
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/758.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.758
Diabetic foot ulcers (DFUs), a serious complication of diabetes mellitus, pose a significant economic burden on patients and healthcare systems worldwide. The development of these ulcers is often due to poor foot care, inadequate glycaemic control, underlying neuropathy, and peripheral vascular disease. Left untreated, these ulcers can result in amputations. The global prevalence of DFUs ranges from 3% in Oceania to 13% in North America, with a global average of 6.4%[1].
Healing time for these ulcers can take up to 12 mo, with a recurrence rate estimated to be 65% within 5 years[2]. Studies have shown that the impact of DFUs on individuals is profound, with loss of ambulatory function, financial strain, and emotional suffering being common outcomes[3]. The economic impact on patients and their families due to medical bills, loss of income, and emotional distress can be significant. Participants in a recent study reported experiencing depression, isolation, and hurtful comments from others[3].
DFUs continue to pose a significant public health challenge, and they are a major cause of morbidity and mortality worldwide[4].
Adiponectin, a fat-derived hormone, has been shown to protect against insulin resistance, type 2 diabetes (T2DM), and atherosclerosis. Reduced circulating levels of adiponectin are thought to play a role in the development of T2DM. In cases of obesity, the production of endogenous adiponectin is impaired. It is, therefore, suggested that pharmacological or dietary interventions be considered to restore the capacity of adipose tissue to secrete adiponectin[5].
Diabetes mellitus (DM), is a main cause of death and poor quality of life worldwide, affecting 463 million individuals in 2019 and is estimated to reach 700 million by 2045[2]. People with diabetes often have foot problems that impose an economic burden on the individual, and about half of all foot amputations are observed to be among diabetics. The lifetime chance of a diabetic having a foot ulcer is as high as 25%[3], and it is estimated that every 30 s a lower limb is lost due to diabetes somewhere in the globe[4]. DFUs can be prevented by ensuring that diabetics get regular foot exams and treating any neuropathy that may be present[5]. The International Diabetes Foundation has called for greater awareness of diabetes foot concerns due to the psychological, social, medical, and economic effects of what should be one of the most preventable long-term complications of diabetes[6,7]. In most Western nations, the yearly incidence of DFUs is roughly 2%, however, greater rates have been observed in select populations, including Medicare recipients (6%) and United States veterans (5%)[8]. The projected annual cost to the NHS in the United Kingdom is around 580 million, with 307 million spent on ulceration in primary care[9].
Diabetic foot syndrome is defined, according to the World Health Organization, as “ulceration of the foot (distally from the ankle and including the ankle) associated with neuropathy and different grades of ischemia and infection”[10]. It is a significant, long-term consequence of diabetes that can result in amputations, disability, and diminished quality of life.
Since peripheral neuropathy and vascular disease are present in more than 10% of individuals at the time of diagnosis of T2DM[11] and because the first year following the diagnosis of diabetes is a risky time for foot ulcers and amputations, the burden of diabetic foot disease is expected to rise in the future[12]. Furthermore, emerging nations in Africa, Asia, and South America, where foot ulcers are more likely to have neuropathic origins[13] and are thus very avoidable, are anticipated to have the biggest growth in the prevalence of T2DM[6]. Deploying screening, education, and treatment programmes most effectively around the globe is still the dilemma facing the worldwide diabetes community[14].
The simplest definition of diabetic foot infection is “any infra-malleolar infection in a diabetic patient”. These include paronychia, cellulitis, myositis, abscesses, necrotizing fasciitis, septic arthritis, tendonitis, and osteomyelitis. DFUs are complex and rarely caused by a single condition. Several risk factors cause DFUs[15,16]. Understanding pathobiology helps diagnose and treat DFUs, which is one of the leading indicators for amputations.
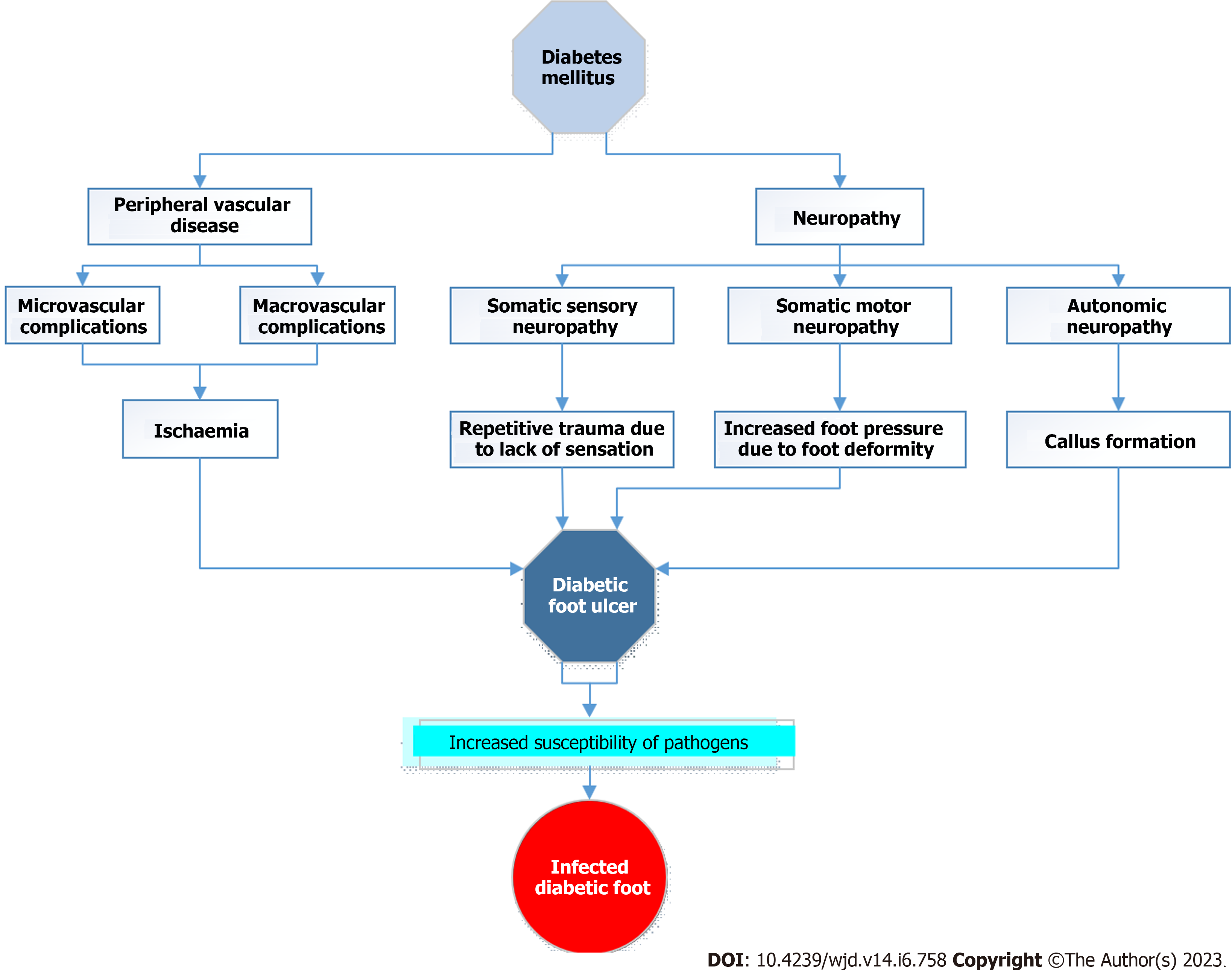
Neuropathy is the primary contributing factor leading to ulceration, in diabetics. Diabetic peripheral neuropathy (DPN) is a disruption of normal nerve function that can change autonomic, motor, and sensory functioning throughout the body[17]. Due to the absence of protective sensation in patients with sensory neuropathy, the foot is more likely to sustain untreated minor injuries as a result of excessive pressure as well as mechanical or thermal damage. Individuals with diabetes who also have sensory neuropathy were found to be at the highest risk for developing ulcers, as revealed by a significant prospective multicentred investigation[18].
There are various types of neuropathies, and some of them may cause foot ulcers. Motor neuropathy may lead to foot deformities, decreased joint mobility, and abnormal foot loading. These modifications may cause a shift in the distribution of loads that are experienced when walking, with a subsequent reactive thickening of the skin known as callus at unusual load areas. Ischaemic necrosis of the tissues underneath the callus also contributes to the development of a neuropathic ulcer. Autonomic neuropathy often results in changes to the skin’s texture and turgor, such as dryness and fissuring, which makes the skin more susceptible to infection since it provides an entry site to the bacteria[19].
Another condition that contributes to the development of foot ulcers is peripheral vascular disease, which affects both small and major blood vessels. It is possible for both macrovascular and micro
The ulcerated diabetic foot is the result of a complex interaction between several factors, including neuropathy, peripheral vascular disease, trauma, and infections. Neuropathy and ischaemia, also called neuro ischaemia, are the initial mechanisms, while the infection is typically a result of this condition. Studies have indicated that diabetics acquire peripheral vascular disease at a younger age more frequently than others in the same age group[22]. In 35% of cases, proximal arterial disease-related peripheral ischaemia was cited as an important cause of ulceration among diabetics in a two-center study of causal pathways[22]. In another study that compared diabetic patients with peripheral artery disease to non-diabetic patients with the same condition, it was found that diabetic patients had more distal disease and a worse prognosis in terms of amputation and mortality[23].
Hence the pathogenesis of DFUs, a complication of longstanding uncontrolled diabetes, involves multifactorial influences such as neuropathy, peripheral vascular disease, foot deformity, trauma, infection, and inadequate glycaemic control. The loss of sensation brought on by neuropathy can result in repeated damage to the foot, while the peripheral vascular disease can reduce blood flow and slow healing. Injuries and infections can exacerbate already-existing ulcers, while foot abnormalities can create pressure points and raise the risk of skin deterioration. Moreover, poor glycaemic management might hinder wound healing and raise the danger of infection. Thus, for the prevention and management of DFUs, a multidisciplinary strategy that takes these aspects into account is essential. Figure 1 summarizes the factors contributing to the development of DFUs in diabetic patients.
Multiple variables contribute to the emergence of DFUs. Peripheral neuropathy and ischaemia that result from the peripheral vascular disease that reduces the protective components of the tissues are the primary underlying causes. In addition, the skin can be subjected to stress, such as pressure, shear, or trauma, which also contributes to the condition. Antonio et.al. in their study identified general and local factors predisposing to the development of DFUs[24]. The general factors include duration and severity of diabetes, and associated comorbidities such as hypertension, dyslipidaemia, chronic renal disease, peripheral vascular disease and age while the local factors included foot deformity, trauma, callus presence, previous amputation, impaired joint mobility and shoe defects[25].
Individuals who have diabetes are required to have their neurological, vascular, dermatological, and musculoskeletal conditions evaluated on a yearly basis, at the very least. The American Diabetes Association (ADA) developed a comprehensive foot examination and risk assessment tool that is fast and requires very little specialised medical equipment[26]. Patients who come in exhibiting tissue loss are placed in a higher risk category than those who do not. In situations like these, an evaluation of the overall level of limb threat should be performed.
Many measures exist to assess the severity of a diabetic ulcer by analysing the ulcer’s features, ischaemia, and infection. Wagner, University of Texas, and PEDIS are the most widely used and globally recognised scales[27,28]. These scales have demonstrated their utility in correlating the degree of severity of the ulcers with the risk of amputation[29]. The wound scales are a valuable tool for classifying the severity of DFUs, but they should not be used to determine the need for amputation. The microbiology of wounds should be examined in each region to further determine the appropriate empiric therapy in the management of DFU.
DFUs are the major cause of hospitalisation and amputation in diabetes patients[5,25]. Foot ulcer complications include excruciating pain, infection, gangrene, osteomyelitis, amputation, and death[30]. Coexisting diabetes-related problems, such as diminished peripheral sensations and absence of pain along with this sustained ambulation further incite additional damage[31].
Studies demonstrated a higher death rate in diabetic patients with DFUs, with a death rate almost double that of diabetic patients without foot ulcers[22,32]. DFUs have been also reported to be associated with a greater frequency of major cardiovascular risk factors, subclinical signs of past and new-onset cardiovascular and cerebrovascular events [33].
Current treatment emphasises patient education, regular foot self-examinations, and annual diabetic foot evaluations. These annual examinations comprise patient history, peripheral vascular exam, and sensory nerve function evaluation to detect DPN early. Pressure analysis studies on lowering foot pressure or changing gait offer promising technology for the early detection and prevention of DFU[34,35]. Depending on DFU categorization, DFU patients need unloading, infection or ischaemia treatment, wound debridement, and wound dressings[36]. Tissue volume and type are often used to classify DFUs[37]. Granulation tissue is red/pink and symbolises healing tissue, whereas slough tissue is more yellow and represents infected tissue and necrotic tissue is dark/black and shows tissue death. Many studies show that DFU diagnosis and treatment can greatly reduce or prevent serious consequences[37,38]. Despite national and international guidelines, DFU administration varies. Under this ambit, patients suffering from DFUs need reliable and quick therapy, which can only be facilitated with deeper understanding of the metabolic marker of DFU such as advanced glycated end-products (AGE’s), inflammatory markers, lipid profile, while newer markers such as adiponectin as a prospective diagnostic tool needs to be further explored. Emerging technologies such as bioprinting and electrospinning[39], stem and somatic cell monotherapy[40] and grafting techniques[41] offer promising alternatives by overcoming the limitation in conventional approaches.
Adipose tissue produces adipokines, which are peptides that communicate with other tissues such as the brain, liver, pancreas, immune system, vasculature, and muscle about their functional state. Thus, adipose tissue dysfunction is often related with alterations in the secretion of adipokines such as leptin, adiponectin, fibroblast growth factor 21 (FGF21), retinol-binding protein 4, dipeptidyl peptidase 4, bone morphogenetic protein (BMP)-4, BMP-7, vaspin, apelin, and progranulin. Although the complete repertoire of human adipokines has not yet been described, it has been established that adipose tissue is a reservoir for more than 600 secretory proteins[42].
Adipokines control many physiological processes, including appetite and fullness, fat distribution, insulin secretion and sensitivity, energy expenditure, endothelial function, inflammation, blood pressure, and blood clotting[43,44]. As the mRNA transcript for adipokines was most robustly expressed in adipocytes, adiponectin was first discovered in mice shortly after leptin’s discovery in 1995[45]. Two different adiponectin receptors, ADIPOR1 and ADIPOR2, are responsible for relaying signals from the 30-kilodalton, 244-amino-acid protein, adiponectin, to its target cells[45].
Adiponectin undergoes post-translational modifications that lead to the secretion of oligomers of 90-kDa trimers, which are subsequently detected in the bloodstream as 180-kDa hexamers (low molecular weight)[45,46]. Adiponectin structure consists of trimers, hexamers, and higher order complexes that can be formed in the collagen domain of adiponectin before secretion[47,48].
Many different receptors, including adiponectin receptors 1 and 2, play roles in mediating adiponectin’s effects[49]. These receptors are functionally dissimilar from G-protein-coupled receptors, primarily due to the fact that their polarity is in the opposite direction. It is projected that they include seven transmembrane sections. large level of functional redundancy appears to exist between the adiponectin receptors, as suggested by both single- and double-knockout mice for the receptors[50]. Although the relative ratios of ADIPOR1 and ADIPOR2 expression in different tissues may differ, in general, both are expressed in a very high proportion of tissues. T-cadherin is the name given to a newly discovered molecule that may be found on the cell surface and possesses a considerable affinity for the protein adiponectin[51]. It is not technically a signalling receptor since it does not have an intracellular signalling domain, even though it is capable of binding adiponectin. T-cadherin is necessary, however, in order for adiponectin to reach its full potential in terms of its cardioprotective effects[52].
Adiponectin is a secretory protein that is only produced by adipocytes. Constitutively synthesized, it accounts for 0.01%-0.05% of plasma protein, which places it in the range of 2-20 g/mL and makes it a component of plasma that is reasonably abundant. Adiponectin is a protein that is fairly stable in circulation, despite the fact that its plasma half-life is only 45-75 min[53]. Other cell types, such as beta cells in the pancreas and certain cell types in the heart and kidneys, also have a strong affinity for adiponectin and can bind to it. Adiponectin is primarily removed from the bloodstream in the liver, making it an important organ in this process[53]. In spite of the fact that adiponectin is secreted by adipose tissue, circulating levels mysteriously decrease when there is an increase in the amount of central adiposity[54]. Despite this, greater degrees of adiposity in the lower extremities and the truncal region are associated with greater concentrations of adiponectin.
Adiponectin’s insulin-sensitizing, anti-inflammatory, and antiapoptotic effects have generated considerable interest[45,55]. In addition, numerous cohort studies in various groups have shown that adiponectin levels are inversely related to either the presence of glucose intolerance or the risk of developing T2DM[56].
The beta cells of pancreatic islets express both ADIPOR1 and ADIPOR2, the two receptors for adiponectin[57,58]. Recombinant adiponectin given to adiponectin-deficient mice shows that it targets beta cells. Adiponectin may enhance glucose-mediated insulin production and promote insulin and related gene transcription[59], however, the effect of adiponectin on insulin release in individuals with normal insulin sensitivity is not well-established[57,60].
The strong and long-standing correlation between adiponectin levels and the development of cardiovascular disease has been well-documented. Pischon et al[61] found in a large cohort that men with high plasma adiponectin levels had a lower risk of myocardial infarction. In preclinical ischaemia/reperfusion trials, the Walsh group showed that recombinant adiponectin strongly improves cardiomyocyte survival[62]. However, why end-stage cardiovascular disease has a significant positive correlation between mortality and high adiponectin levels, unlike early stages, is unknown[63].
A similar scenario exists in the kidney, where low adiponectin levels correlate with albuminuria in both animals and humans[64]. In animal models with adiponectin gene knockout, the lack of adiponectin has been linked to increased podocyte damage and albuminuria, and adiponectin therapy has demonstrated the ability to reverse certain renal dysfunction[65]. In patients with chronic kidney disease, adiponectin levels are positively correlated with proteinuria[31]. This upregulation is similar to that seen in cardiovascular disease, particularly end-stage cardiovascular disease. The mechanisms are unknown. This is especially challenging given that adiponectin is not cleared through the kidney except in cases of severe proteinuria. This is especially challenging given that adiponectin is not cleared through the kidney except in cases of severe proteinuria[66], making it difficult to determine which mechanisms are responsible.
Skeletal muscle is an important factor in insulin sensitivity because it is the primary source of glucose for the body as a whole. It should not come as a surprise, consequently, that a substantial amount of attention has been paid to the potential metabolic effects that adiponectin has on this tissue. High-molecular-weight adiponectin correlated better with systemic insulin sensitivity than low-molecular weight in rodents and humans[45,46]. Skeletal muscle has a high concentration of ADIPOR1, through which adiponectin regulates energy metabolism[67]. Most investigations into the effects of adiponectin have focused on its binding to globular adiponectin, which exhibits greater binding strength and biological activity in skeletal muscle compared to most other tissues[68,69]. Adiponectin binding leads to increased glucose uptake and nonoxidative glycolysis, while simultaneously reducing intramyocellular triacylglycerol content and enhancing fatty acid oxidation[68,69]. Additionally, adiponectin influences the number of mitochondria and the types of oxidative fibers present in skeletal muscle[70]. However, in diseased states, the effects of adiponectin on skeletal muscle are attenuated.
The liver is affected in a number of different ways by adiponectin. One of the most notable effects is hepatic glucose production inhibition, which lowers body glucose levels. Hepatocytes are insulin-sensitive at physiological adiponectin levels. As a result, glucose production is significantly inhibited in response to any given dose of insulin[50]. Adiponectin inhibits both the expression[68,71] and activity of important regulators in the process of gluconeogenesis[71,72]. Studies using murine euglycemic clamps have shown that the rates of glucose disposal, glycolysis, and glycogen synthesis are not affected by the presence of intravenous adiponectin infusion[72]. This suggests that the primary mechanism by which adiponectin lowers blood sugar levels is through the suppression of hepatic glucose output, rather than through enhancing glucose disposal.
The adiponectin receptors (ADIPOR1 > R2) are also reported to be expressed by adipocytes. This data further implies that adiponectin may alter adipose tissue function locally, either with modifying autocrine or paracrine function.
Adiponectin’s impact on inflammation is not limited to adipose tissue, and its anti-inflammatory effects have been observed in other contexts. This is significant because systemic inflammation is thought to play a role in the development of insulin resistance[73]. These researchers have shown that adiponectin can inhibit the development and proliferation of bone marrow-derived granulocyte and macrophage progenitors, but it does not have this effect on other haematopoietic cell lines. In addition, it is also reported that inflammatory processes in macrophages can be disrupted, by suppressing the phagocytic activity in human macrophages that have been treated with adiponectin [73], as is the production of pro-inflammatory cytokines[73]. In the setting of the development of atherosclerosis, adiponectin is shown to limit the transition of macrophages into lipid-laden foam cells[74].
Adiponectin works in the brain to increase the amount of energy that is expended, which might lead to weight reduction[46]. In clinical research, circulating adiponectin has been shown to have an independent and unfavourable relationship with components of metabolic syndrome. These components include insulin resistance, body weight, blood pressure, and serum lipids[43,55].
Adiponectin’s molecular functions imply that the molecule or agonists of its receptors might cure obesity and related comorbidities[45]. Studies showed that adiponectin improved insulin sensitivity, glucose metabolism, insulin secretion, and body weight in rodent models[75]. Recently, it was shown that a synthetic small molecule adiponectin receptor agonist, known as “AdipoRon”, greatly increased insulin sensitivity and decreased glucose intolerance in rats[76]. AdipoRon treatment prolonged the lives of high-fat-fed db/db mice, adding support to the idea that higher blood adiponectin levels are associated with a later average age of mortality in obese people[76]. Levels of adiponectin have been shown to have a negative correlation with obesity, visceral fat, T2DM, and other complications that are associated with obesity[45,55]. Not only adiponectin has a promising and readily detectable stable marker for a variety of illnesses, but it also has the potential to play a big role in the future of clinical importance as a therapeutic agent. This is because adiponectin has the ability to regulate fat storage[44].
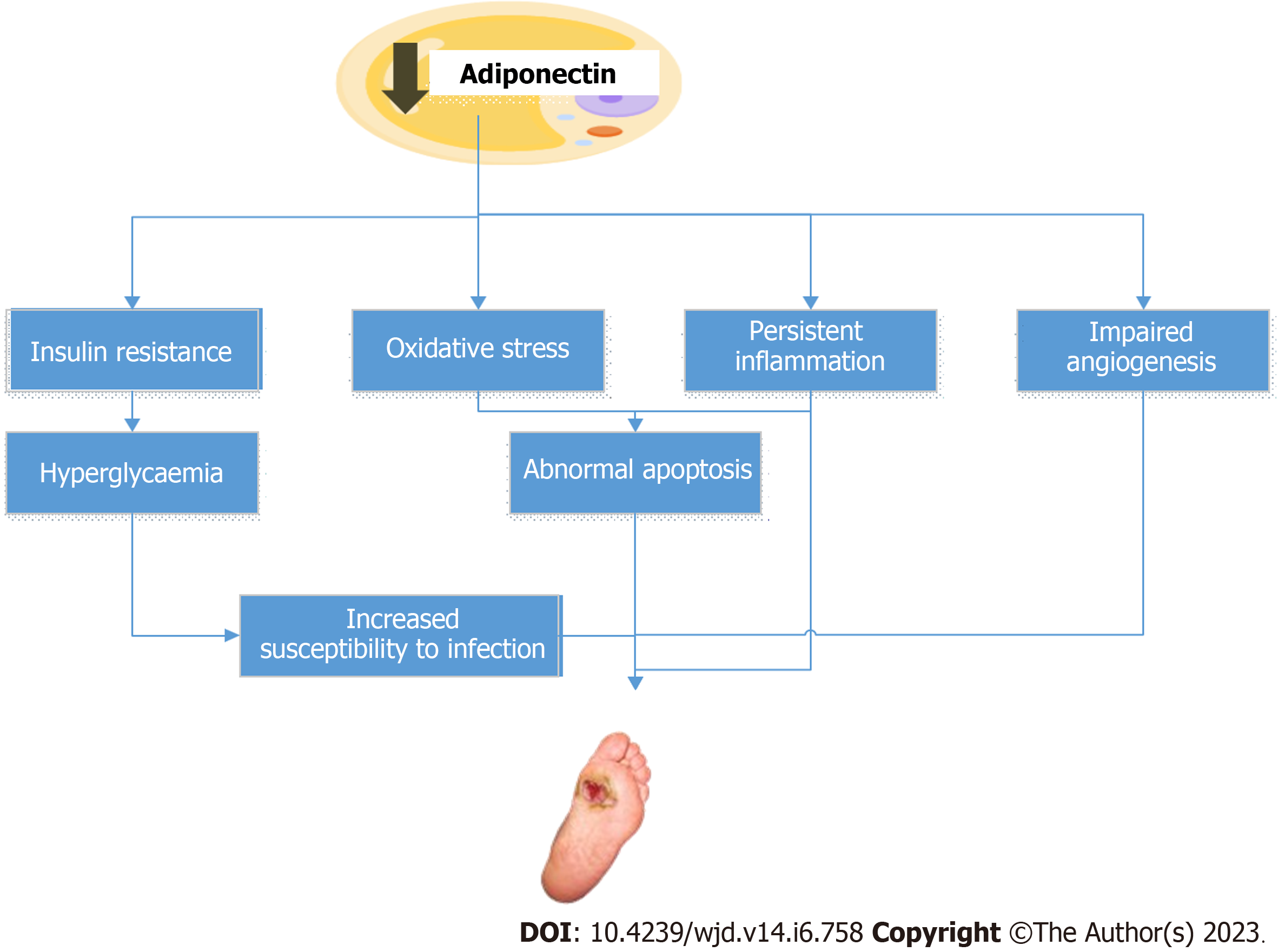
Many studies have shown that diabetic patients have lower adiponectin levels than healthy controls[77-79]. Adiponectin deficiency has been correlated with increased susceptibility to diabetes and its associated complications, such as DFUs[80,81]. Some investigations have also indicated that low adiponectin levels may be a potential biomarker of poor wound healing and increased amputation risk in diabetic foot ulcer patients[78,81,82]. Figure 2 depicts the probable mechanisms via which reduced adiponectin levels may contribute to the development of DFUs. Nonetheless, more research is needed to completely understand the role of adiponectin in the pathophysiology and therapy of DFUs.
Diabetic patients often experience impaired wound healing due to continuous hyperglycaemia, leading to alterations in various stages of the healing process such as haemostasis, inflammation, proliferation, and remodelling. Factors that contribute to this include hypercoagulability, impairment of skin function[83], imbalanced inflammatory and growth factors[84], reduced neutrophil function[85], and insufficient wound re-epithelialization[86]. Adipose tissue has recently been recognized as a key endocrine organ with a role in wound healing. This role is due to the secretion of bioactive substances known as adipokines, which regulate paracrine signaling[87]. There is evidence from numerous research that adipose tissue contributes to the healing of wounds[88-90]. Despite the current understanding, the exact role of adipocytes in the wound healing process remains unknown. However, it has been demonstrated that applying adipose tissue extracts over skin wounds can improve wound repair[91]. The healing process is believed to take place via paracrine signaling, highlighting the significance of the adipokines released by adipose tissue in wound healing[92].
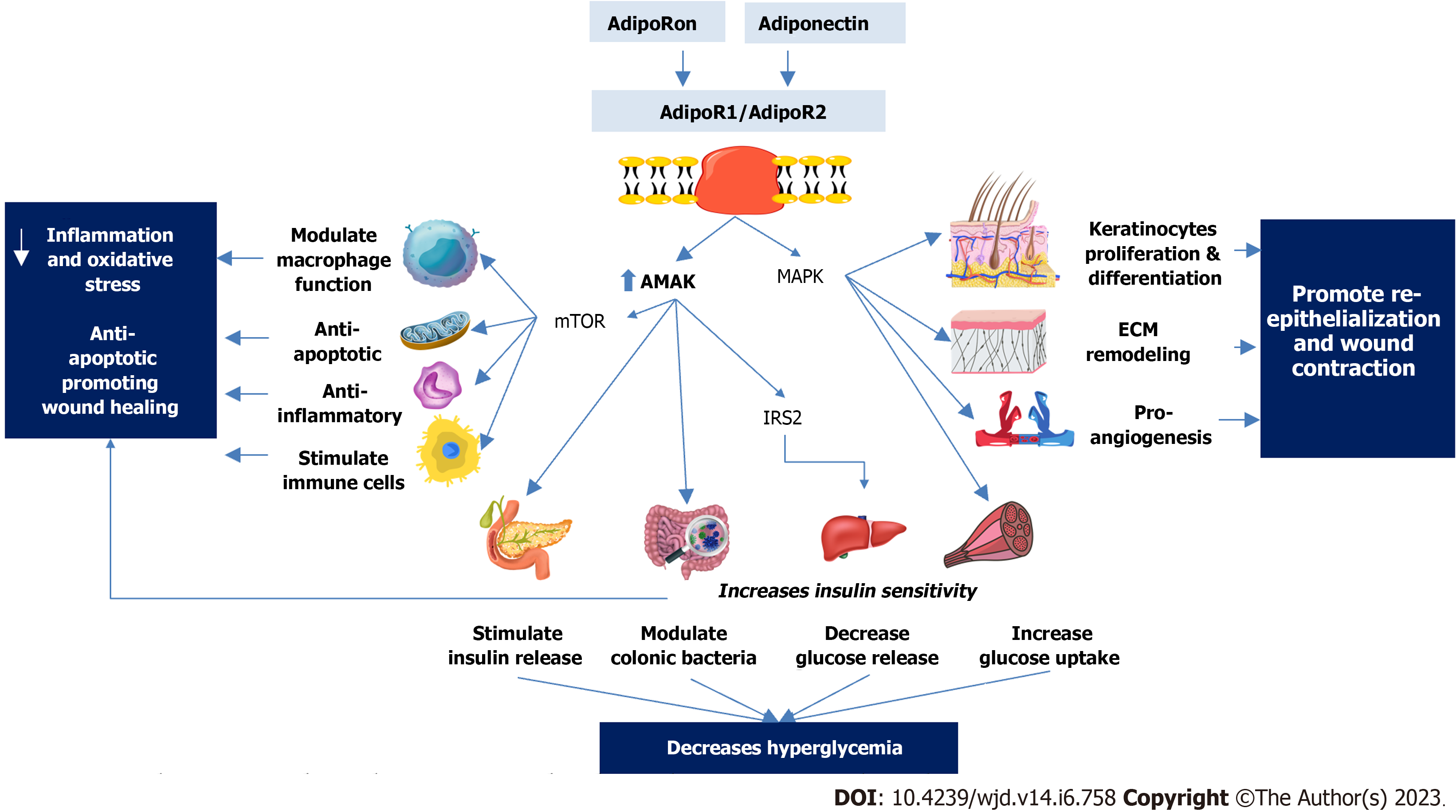
Adiponectin, that is secreted from adipocytes has been found to aid in wound healing through its effects on keratinocytes, the most abundant cellular component of the epidermis[93]. Adiponectin promotes keratinocyte proliferation and migration, which is crucial for proper re-epithelialization and wound closure. This is mediated via the AdipR1/AdipR2 and ERK signaling pathways[94]. Adiponectin also elevates the intracellular and reconstructed epidermal lipid content of keratinocytes, and regulates the expression of lipid biosynthesis enzymes and nuclear hormone receptors, which helps maintain skin barrier integrity, an action that is mediated through SIRT1 signaling molecule (SIRT1)[95].
Furthermore, adiponectin possesses reactive oxygen species (ROS)-scavenging abilities and can mitigate oxidative stress-induced DNA damage while regulating antimicrobial peptide production in senescent keratinocytes[96-98]. Studies have shown that adiponectin can reverse premature cellular aging in keratinocytes and restore normal antimicrobial peptide levels by activating AMP-activated protein kinase (AMPK), increasing SIRT1 deacetylation, recovering FoxO1 and FoxO3 transcription activity, and suppressing NF-κB p65, thereby preventing abnormal expression of human β-defensin 2 induced by hydrogen peroxide[99]. Additionally, it restores filaggrin expression and normalizes keratinocyte activity, which is crucial for maintaining skin integrity as an immune barrier[100,101]. Therefore, one way in which adiponectin may promote DFU healing is through its impact on skin integrity, keratinocyte proliferation, and migration. However, further research is necessary to fully understand the potential mechanisms of adiponectin in DFU healing.
The extracellular matrix (ECM) that is produced, assembled, and remodeled by fibroblasts is crucial for maintaining skin integrity, but when it is damaged, as in skin ulcers, it undergoes repair and remodeling. Matrix metalloproteinases (MMPs), a family of proteins that includes collagenases and gelatinases, are ECM enzymes that break down damaged fibrils during ECM remodeling[102]. Normal ECM remodeling includes a delicate balance of ECM breakdown, generation, and maturation. In poor wound healing, such as DFU, the process of ECM remodeling tends to yield more degraded, non-soluble fibrils, resulting in a disorderly ECM network and callus formation[103,104].
Abnormal expression of MMPs and differential expression of ECM contribute to poor healing in DFUs[105-107]. Elevated MMP activity and imbalanced tissue inhibitors of metalloproteinases (TIMPs) have been reported in the skin of diabetic ulcer patients. A study reported that enhanced expression of MMP-1 is necessary for wound healing in DFU, while enhanced MMP-8 and MMP-9 contribute to delayed wound healing. Furthermore, a higher MMP-1/TIMP-1 ratio may indicate a proteolytic environment in the wound[106,107]. Adiponectin has been found to suppress fibroblast proliferation, migration, and ECM formation[108], as well as increase the expression of fibroblasts and type 1 collagen components of the ECM[109,110]. The endogenous expression of adiponectin and its malfunctioning may play a fundamental role in skin fibrosis and exert a substantial negative regulatory impact on fibrosis[111].
In summary, adiponectin has been shown to influence ECM composition by regulating the activity of ECM-associated molecules, such as collagen, elastin, and glycosaminoglycans, implicating that as a potential mechanism through which adiponectin may help promote DFU healing.
Another contributing factor to poor wound healing in diabetic patients is the presence of excessive inflammatory reaction[112,113]. DFUs which are characterized by chronic inflammation and infection can lead to tissue necrosis and amputation[114-116]. In individuals with diabetes, the wound healing process is often hindered as the wounded tissues remain in the late inflammatory phase. In such cases, macrophages are unable to transition into the repair phenotype and release the necessary factors that promote tissue repair. As a result, the wound fails to progress from the inflammatory to the proliferative phase of healing, leading to persistent inflammation[117]. The persistent inflammation in DFUs is contributed by the activation of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway[112,113,118]. In addition, the downregulation of neuropeptides that are essential for wound healing and biofilm development also contributes to the persistent inflammatory state in DFUs. Biofilms, which disrupt wound healing by creating a prolonged inflammatory response, limit macrophage phagocytosis and keratinocyte growth migration, and transmit antimicrobial resistance genes[119-121].
Adiponectin has an anti-inflammatory effect and is a potential therapeutic option for preventing and treating DFUs. It has been demonstrated that adiponectin reduces the expression of pro-inflammatory markers and inhibits the activation of the NF-κB signaling pathway in human aortic endothelial cells and monocytes[122-124]. Activation of AdipoR1 and AdipoR2 receptors increases the activity of AMPK and inhibit the NF-κB signaling in various cells including macrophages, liver, and skeletal muscle. Both contribute to adiponectin’s anti-inflammatory properties[50,125]. The crucial role of AMPK signaling activity in wound healing is highlighted by the successful improvement of DFU healing achieved through the reactivation of AMPK signaling[126]. Adiponectin may also inhibit the activation of the inflammasome, a complex of proteins which plays a key role in the inflammatory response[127,128].
Adiponectin stimulates the nuclear receptor peroxisome proliferator-activated receptor-gamma (PPAR-γ), which affects glucose and lipid metabolism[129,130]. When PPAR- is activated, pro-inflammatory cytokines are suppressed, and anti-inflammatory genes are activated. Adiponectin also suppresses the creation of reactive oxygen species and the activation of NADPH oxidase[131], which contribute to inflammation and oxidative stress. Adiponectin may also limit the migration and proliferation of vascular smooth muscle cells, as well as the development of new blood vessels[132-134], while promoting the regression of existing blood vessels, which can also contribute to its anti-inflammatory effects[135-137]. Hence, adiponectin’s anti-inflammatory properties may aid wound healing by minimising prolonged inflammation and accelerating the wound’s transition into the proliferative phase of recovery.
A meta-analysis by Macdonald et al[138] found that DFUs are caused by several genera of bacteria, mainly gram-positive. Another study by Smith et al[139] revealed populations of gram-positive bacteria and both aerobes and anaerobes. These bacteria can form biofilms, making it more difficult for antimicrobials to access and thus slowing down the healing process[140].
Diabetics are also susceptible to periodontitis, which is associated with dysbiotic plaque biofilms and eventually leads to the destruction of the tooth-supporting structures. DFUs are similar in that they comprise bacteria that form biofilms and eventually lead to destruction of the underlying bone structures. A study by Wang et al[140] suggested that the level of adiponectin has an inverse association with periodontitis. Treatment with adiponectin in animal experiments better improved tissue destruction and suppressed inflammation, which improved bone regeneration[141]. There is little literature on the use of adiponectin as an antibacterial for DFUs. However, a study by Wang et al[140] suggests that adiponectin may inhibit inflammation stimulated by obesity or by periodontal pathogens and somehow influence antibacterial outcomes.
Given these findings, further research is needed to explore the antibacterial effects of adiponectin in DFUs and its use as a candidate for the treatment of this chronic condition.
One contributing factor to the delayed healing and susceptibility to bacterial infection in DFUs is the low immune response. Research has shown that adiponectin has the ability to modulate immune cell activity by inhibiting the activation and differentiation of T-helper 1 (Th1) cells, which leads to the emergence of inflammatory and autoimmune diseases, while promoting the activation and differentiation of Th2 cells, which regulate immune responses[142]. Adiponectin achieves its anti-inflammatory effects by regulating multiple signaling pathways and modulating cellular processes involved in inflammation, making it a promising therapeutic target for various inflammatory and metabolic disorders. Additionally, studies suggest that adiponectin can modulate bacterial infection by regulating the activity of molecules responsible for bacterial uptake and killing[143,144].
Fibroblast growth factors (FGFs) are proteins that are expressed in various tissues and play a crucial role in wound repair[145]. There are two types of FGFs: Paracrine and endocrine. Endocrine FGF regulates various metabolic processes and cell survival, while paracrine FGFs regulate neural development, angiogenesis, and wound healing. Studies have shown that specific types of FGFs for instance aFGF, bFGF, and the FGF 15/19 subfamily may have a positive effect on diabetic wound healing. aFGF aids in diabetic ulcer healing by stimulating capillaries, fibroblasts, and proliferative proteins in ulcer tissue[146]. Regulating the release of bFGF has also been shown to enhance skin wound healing and epithelium development in diabetic mice, while also minimizing scar formation by promoting fibroblast and myofibroblast apoptosis[147]. Additionally, FGF-19 and FGF-21 have been found to be excessively expressed in the serum of diabetes patients[148,149]. FGFs are more potent angiogenesis factors than platelet-derived growth factor and vascular endothelial growth factor (VEGF). FGFs enhance the development of granulation tissue by increasing fibroblast proliferation and angiogenesis[150].
Studies have shown that impaired angiogenesis contributes to the poor healing of DFUs[151,152]. This is due to various factors such as the failure of macrophages to change into a repair phenotype[151], elevated levels of plasma pigment epithelium-derived factor (PEDF), and dysregulation of angiopoietin-1 (Ang 1) and Ang 2. Macrophages are a key source of VEGF and other pro-angiogenic substances in wounds. On the other hand, PEDF has been found to delay wound healing and decrease angiogenesis in diabetic wounds[152].
Adiponectin has both pro-angiogenic and anti-angiogenic effects, depending on the signaling pathways involved. Adiponectin can promote the formation of new blood vessels through various mechanisms. For example, it increases the production of pro-angiogenic factors like VEGF and FGF-2, and stimulates the migration, proliferation, and differentiation of endothelial cells. This is thought to happen because adiponectin activates signaling pathways like Akt and AMPK[153,154]. However, adiponectin can also inhibit angiogenesis in some contexts. It decreases the production of pro-angiogenic factors and inhibits the expression of angiogenic factors like FGF-2. Additionally, it can inhibit the migration and invasion of certain cancer cells through the modulation of signaling pathways like Akt and AMPK[155].
Hence, the effects of adiponectin on angiogenesis could help promote wound healing. However, those effects are context-dependent and complex. Further research is needed to understand the molecular mechanisms behind these effects and to determine its potential therapeutic applications in different contexts.
Impaired apoptosis is another factor that contributes to the poor healing of DFUs[156]. During the wound healing process, different cell groups go through various stages of clearance, culminating in apoptosis. DFU trauma causes mitochondrial damage, which increases the expression of pro-apoptotic proteins while decreasing the expression of anti-apoptotic proteins such as B-cell lymphoma-2 (Bcl-2). This results in apoptosis in cells such as fibroblasts and vascular smooth muscle cells. Low expression of FGF-2, a factor related to fibroblast mitosis and cell survival, has been observed in diabetic wound cells. Reduced expression of other factors related to fibroblast regeneration, such as adiponectin, also contributes to this process[157].
Furthermore, delayed apoptosis has been reported during the inflammatory phase of wound healing in diabetic mice, which may contribute to the persistent inflammatory state in DFUs[158]. Excessive cell death due to hyperglycaemia can lead to poor structural recombination and difficulty in generating granulation tissue, making the wound more susceptible to infection[156]. In addition, chronic hyperglycaemia associated with altered lipid and glucose metabolism promotes a condition of oxidative stress, which results in long-term chronic inflammation of wounds across all stages of wound healing.
Adiponectin has been shown to have anti-apoptotic effects, which may prevent cell death in the wound area and promote wound healing. Studies have shown that adiponectin can inhibit the activation of the intrinsic apoptotic pathway, leading to the prevention of cell death and promotion of wound healing. For example, a study showed that treatment with recombinant human adiponectin promoted wound healing in diabetic mice by inhibiting the activation of the intrinsic apoptotic pathway. However, further research is needed to understand the molecular mechanisms behind adiponectin’s effects on apoptosis and its potential therapeutic applications in different contexts[159].
Adiponectin has been identified as a mediator of wound contraction, a process that involves the reduction of wound size through the convergence of wound edges. This action is considered to occur via a variety of molecular mechanisms, including collagen synthesis stimulation, MMP inhibition, and myofibroblast migration and proliferation boosting.
Adiponectin has been found to increase the production of collagen; a critical component of the ECM necessary for wound healing. This is achieved through the activation of the Transforming Growth Factor-β (TGF-β) signalling pathway, which stimulates collagen synthesis via the phosphorylation of Smad2 and Smad3. Adiponectin also enhances the activity of procollagen type I and III mRNA, which are necessary for collagen synthesis[160].
Additionally, adiponectin suppresses MMPs, enzymes that degrade the ECM and hinder wound healing. By decreasing the expression of MMP-2 and MMP-9 and increasing the expression of TIMP-1, an inhibitor of MMPs, adiponectin promotes the maintenance of the ECM[161,162].
Furthermore, myofibroblasts play a crucial role in wound healing and contraction. However, excessive myofibroblast proliferation during the late stage of wound healing can lead to the formation of pathological scars that greatly reduce the quality of wound healing[163]. Studies have shown that adiponectin may prevent the formation of pathological scars by inhibiting myofibroblast synthesis, proliferation, and migration[164,165].
Therefore, adiponectin may increase wound contraction by increasing collagen synthesis, inhibiting MMPs, and modulating myofibroblast migration as well as proliferation. More research is needed to understand the molecular mechanisms of these effects and to assess the therapeutic potential of adiponectin in wound healing.
Excessive oxidative stress is a hallmark of diabetic wounds, where high levels of ROS are present. The balance between ROS creation and elimination is crucial for proper wound healing. In diabetes, high glucose levels lead to an increase in energy metabolism substrates, which, in turn, result in elevated levels of superoxide and oxidative stress. This increased oxidative stress enhances the production of advanced glycation end products (AGEs)[166,167]. Moreover, nitric oxide synthase decoupling in diabetes leads to decreased nitric oxide production[168], further complicating the healing process. These findings highlight the crucial role that oxidative stress plays in diabetic wound healing and the need to address this issue to improve therapeutic outcomes.
Adiponectin has demonstrated wound healing benefits through its antioxidant properties. Specifically, adiponectin has been shown to increase insulin release[75], enhance insulin sensitivity[169], promote glucose uptake[68,170], and scavenge ROS[171]. These antioxidant properties of adiponectin provide new avenues for the development of effective therapeutic strategies for diabetic wound healing.
According to a review conducted by Woodward et al[172], adiponectin has additional anti-inflammatory, anti-apoptotic, and antioxidative effects that can reduce cardiovascular oxidative stress. Matsuda and Shimomura[173] also suggested that adiponectin may protect against oxidative-stress-induced damage in the cardiovascular system, and that circulating adiponectin levels and increased oxidative stress may contribute to the pathogenesis of obesity-associated metabolic diseases. Nguyen[174] proposed that adiponectin could be explored as a focus of new treatment strategies in various metabolic diseases due to its antioxidative, anti-inflammatory, and anti-fibrotic effects, which help regulate glucose levels, lipid metabolism, and insulin sensitivity. However, further research is needed to investigate the antibacterial effects of adiponectin in DFUs and its potential use as a treatment strategy.
The development and poor healing of DFUs are influenced by peripheral neuropathy, a complex and multi-factorial condition. Among the identified contributors to DPN are oxidative stress, hypoxia, AGEs, activation of T lymphocytes, and insufficiency of nerve growth factors. Reduced expression of neuropeptides is a hallmark of neuropathy in both autonomic and sensory nerve fibers that arise from diabetes mellitus. These neuropeptides, which act as neuromodulators, play a crucial part in the process of diabetic wound healing[175]. Adiponectin has been suggested to promote wound healing in diabetics through its neuroprotective role, although further research is required to fully understand the underlying mechanisms involved[176].
Persistent hyperglycaemia in diabetic patients is a main factor contributing to delayed wound healing and progression of DFUs[177]. Several studies reported the beneficial effect of adiponectin on insulin sensitivity through its metabolic effects on various tissues, including skeletal muscle, liver, and adipose tissue. Skeletal muscle, which is a key factor in insulin sensitivity and glucose metabolism, has a high concentration of adiponectin receptors and has been shown to have increased glucose uptake and decreased intramyocellular triacylglycerol content in response to adiponectin binding[178,179]. The liver, on the other hand, experiences decreased glucose production and increased insulin sensitivity when physiological levels of adiponectin are present[180]. Adiponectin has also been shown to have anti-inflammatory effects in various tissues, including adipose tissue and liver[181-184]. In addition, adiponectin has been linked to weight reduction and improved insulin sensitivity, glucose metabolism, and insulin secretion in rodents as evidenced by a recent study[185]. In addition, adiponectin improves insulin sensitivity through modulating the gut microbiome[186].
Thus, adiponectin has been proposed to have potential therapeutic and preventive applications in DFUs through various mechanisms, as outlined in Figure 3.
To review the available evidence on the measurement of adiponectin levels in DFUs in patients with T2DM, a comprehensive search of relevant databases such as PubMed and Google Scholar was conducted to identify relevant studies. The findings of seven selected studies are presented chronologically in Table 1. The results of these studies revealed a consistent pattern, with lower plasma levels of adiponectin found in patients with DFUs compared to those without DFUs. A negative correlation between the duration of diabetes and the development of DFUs was also observed. The findings further indicated a positive association between low plasma levels of adiponectin and DFUs, and that low adiponectin levels could serve as a predictor for DFUs. The results of these investigations imply that reduced levels of adiponectin in the blood of individuals with T2DM and DFUs may play a role in the emergence of foot ulcers by means of microvascular and inflammatory processes.
| No. | Ref. | Country | Study objective | Study design and sample size | Results | Adiponectin levels (ng/ mL) | Conclusion | |||
| Non-Diabetic | Diabetic without FUs | DFU | P value | |||||||
| 1 | Tuttolomondo et al[203], 2010 | Italy | To investigate the plasma levels of adiponectin, resistin and IL-6 in subjects with diabetic foot in comparison with subjects without foot complication | Case-control; sample size: 34 patients with type 2 DM with FU and 37 patients with type 2 DM without FUs | The patients with DFUs exhibited higher CRP, HbA1c, lipid profile, IL-6, resistin and lower levels of adiponectin; DFU patients have lower median; plasma levels of adiponectin; patients with foot ulcers had a longer duration of DM, higher percentage was associated with nephropathy, peripheral artery diseases, ischemic heart diseases, transient ischemic attacks or stroke | NA | 8.48 × 103 (5.15 × 103-12.87 × | 7.145 × 103 (4.470 × 103-12.170 × | 0.022 | Adiponectin levels are negatively correlated with the duration of diabetes and the development of DFUs |
| 2 | Zubair et al[81], 2012 | India | To investigate the association between inflammation and acute foot syndrome | Case-control; sample size: 162 diabetics with FUs & 162 diabetics without FUs | Adiponectin levels were lower in DFU patients than in subjects without DFU; multiple linear regression analysis showed a significant negative correlation between adiponectin levels and DFU (R2 = -0.0189) | NA | 13.4 (12.1-14.2)1 | 8.4 (7.1-9.2)1 | < 0.0001 | Diabetic subjects with various grades of diabetic foot ulcer showed a higher IL-6, hsCRP, TNF-α, and lower adiponectin plasma levels in comparison with diabetes without foot ulcer, independent of the concomitant infections |
| 3 | Ahmad et al[82], 2012 | India | To evaluate plasma levels of Cathepsin D, adiponectin, TNF-α, IL-6, and hsCRP in subjects with diabetic foot in comparison with subjects without foot complications | Prospective cohort multicentric hospital-based study; sample size: 211 diabetics with FUs, 208 diabetics without FUs | The median levels of adiponectin were lower in patients with DFUs; adiponectin plasma levels were found to be negatively correlated with various cardiovascular risk factors, including hypertension, dyslipidemia, and microvascular complications such as neuropathy, retinopathy, nephropathy, and PAD; this was found through both multiple linear regression analysis and forward stepwise regression analysis | NA | 13.3 (12.1-14.2)1 | 8.5 (7.1-9.5)1 | < 0.0001 | Low plasma adiponectin is a predictor for DFUs; the study suggests that low levels of adiponectin in diabetic patients with foot ulcers could be linked to the development of foot ulcers through microvascular and inflammatory mechanisms. The findings also indicate that adiponectin may play a role in inhibiting the expression of adhesion molecules on endothelial cells, which are involved in the inflammatory vascular response |
| 4 | Dhamodharan et al[204], 2015 | India | To investigate the genetic association of IL-6, TNF-α, and SDF-1 polymorphisms with serum cytokine, adiponectin, leptin and hsCRP levels in diabetic foot ulcers | Case-control; sample size: A total of 515 subjects were divided into four study groups: Group-I (NGT)/control; n = 106), group-II known T2DM without DFU (T2DM; n = 139); group-III T2DM with neuropathic DFU (DFU-DN; n = 191); group-IV T2DM with PVD (DFU-PVD; n = 79) | The levels of adiponectin were significantly lower in the diabetic groups (T2DM, DFU-DN, and DFU-PVD) compared to the NGT group | 536.0 (0.1-1787.0)2 | 528.6 (6.2-1255.0)2 | 524.0 (63.3-1641.0)2 in DFU+ DN; 453.5 (164.9-1078.0)2 in DFU + PVD | < 0.05 | Low adiponectin levels can be a biomarker of DFUs; SNPs in cytokine/chemokine genes are useful biomarkers for DFU and can help predict the risk of developing DFU |
| 5 | Viswanathan et al[205], 2018 | India | To examine the involvement of IL-6, TNF-α, and SDF-1) polymorphisms in determining the susceptibility to foot microbial infection, grade of the ulcer) and treatment-outcome; (Debridement vs amputation) in DFU subjects and further, the effect of these SNPs on serum cytokine levels and biomarkers such as leptin, adiponectin, CRP and HOMA-IR | Cross-sectional; sample size: 270 DFU subjects | Data on adiponectin levels are not reported | NA | NA | NA | NA | Screening for SNPs in TNF-α, SDF-1, and IL-6; among DFU subjects would help in identifying high risk individuals and might aid in better patient care |
| 6 | Anguiano-Hernandez et al[206], 2019 | México | To assess the modification in adiponectin, HIF-1α, NF-κB, IGFBP-3, VEGF and adiponectin in diabetic foot ulcers treated with hyperbaric oxygen | Study design: Not specified; sample size: 17 ambulatory patients and one hospitalized; patient with DFUs; 15 were males & 3 females; 17 T2DM and 1 T1DM; grade 3 and 4 on Wagner scale | Adiponectin levels increase after therapy | NA | NA | -14943 ± 79152 (before therapy); -17281 ± 79622 (after therapy) | 0.035 | The study found that while treatment increased adiponectin levels, the increase was not significant; however, all patients showed an increase in angiogenesis and fibrosis and a decrease in ulcer size and infection signs after undergoing HBO2 therapy. The results suggest that HBO2 stimulates the expression of IGFBP-3, NF-κB, and HIF-1α and modulates the inflammatory response related to hypoxia |
| 7 | Vangaveti et al[207], 2022 | Australia | To determine vildagliptin’s effect on inflammatory markers and wound healing in patients with type 2 diabetic foot ulcer | Prospective, randomized, double-blind, placebo controlled, single-centre study; sample size: 50 participants; 25 were assigned to the placebo and 25 to the treatment group | Vildagliptin treatment led to significant improvements in key health markers, including reduced HbA1c, hematocrit, total cholesterol, LDL cholesterol, and total/HDL cholesterol ratio compared to the placebo group. Additionally, vildagliptin demonstrated a protective effect on DFU wound healing | NA | NA | 11822 ± 2584.03; Placebo; following; treatment 13138 ± 26712 | 1.0 | The vildagliptin treatment in DFU patients improve wound healing with an associated reduction in some inflammatory biomarkers and a non-significant increase in adiponectin |
Adiponectin has been found to play a crucial role in wound healing, both in vivo and in vitro. Kumada et al[187] found that the incubation of human monocyte-derived macrophages with human recombinant adiponectin increased tissue inhibitor of metalloproteinases; TIMP-1 mRNA levels in a dose-dependent manner without affecting MMPs mRNA levels. Adiponectin selectively increased TIMP-1 expression in human monocyte-derived macrophages through the induction of IL-10[187].
Kawai et al[188] investigated the effect of human recombinant adiponectin on an immortalized human keratinocyte cell line (HaCaT) and found that adiponectin suppressed the gene expression of involucrin, a marker of keratinocyte differentiation, in a dose-dependent manner. Adiponectin also upregulated the expression of TGFb1 in HaCaT cells and promoted apoptosis in keratinocytes, which could inhibit hyperkeratosis during wound healing in diabetic patients[188,189].
Shibata et al[94] found that adiponectin was a powerful mediator in the regulation of cutaneous wound healing. Adiponectin receptors were found in normal human keratinocytes, and adiponectin increased keratinocyte proliferation and migration via AdipoR1/AdipoR2 and the ERK signaling pathway. Wound closure was significantly delayed in adiponectin-deficient mice compared to wild-type mice, and both systemic and topical adiponectin treatment improved wound healing in adiponectin-deficient and diabetic mice[94].
In 2013, an orally active adiponectin receptor agonist, AdipoRon, was developed and was found to have similar effects to adiponectin[76,190], improving insulin sensitivity and glucose tolerance, lipid metabolism[190], and vascular dysfunction in type 2 diabetic mice[192].
Salathia et al[193] found that the injection of adiponectin into the skin edges of a wound accelerated healing and enhanced epithelialization at the wound margin. Jin et al[194] found that adiponectin promoted the growth and migration of preadipocytes in an adipose tissue wound healing study. FGF21 has also been shown to stimulate the production of adiponectin, which could contribute to the expansion of subcutaneous fat and improvement of systemic insulin sensitivity[195].
Kim et al[196] conducted a study on the effects of AdipoRon, a synthetic adiponectin receptor agonist, on diabetic nephropathy in T2DM patients. The study found that AdipoRon treatment reversed kidney abnormalities caused by diabetes in mice. The renoprotective benefits of AdipoRon were achieved through the activation of AdipoR1 and AdipoR2 receptors in the kidneys, which improved pathways related to lipid accumulation and endothelial impairment. AdipoRon also increased intracellular Ca2+ levels and activated a CaMKK/phosphorylated Ser431LKB1/phosphorylated Thr172AMPK/PPAR pathway, reducing oxidative stress and apoptosis, and improving endothelial dysfunction. The study also found that AdipoRon had cardioprotective benefits through the same mechanism as shown in the kidney[196].
Hong et al[95] conducted a study examining the impact of recombinant human full-length adiponectin on human epidermal keratinocyte cell culture. The results showed that adiponectin improved the differentiation and lipid content of keratinocytes through modulation of the expression of multiple enzymes involved in lipid synthesis and regulation within these cells[95].
Adiponectin replacement therapy has the potential to treat various human diseases, but due to the challenges in using the intact protein, efforts have focused on developing peptide and small molecule agonists of the adiponectin receptor. One such example is ADP355, a peptide that has low nanomolar cellular activity and efficacy in treating fibrotic and inflammation-related diseases. On the other hand, small-molecule therapies like AdipoRon can be taken orally and target multiple metabolic conditions. However, the difficulty in comparing the efficacy of different drug classes due to the use of various in vivo models and the limitations of in vitro measures makes it challenging to determine their effectiveness. Adiponectin receptor antagonists can still be useful in target validation studies, but direct receptor agonists have been shown to be more effective in controlling direct signalling than therapies that aim to increase adiponectin production[197].
Studies have shown the potential benefits of AdipoRon, a small-molecule therapy for multiple metabolic conditions, in improving various aspects of health. A 2020 study by Choi et al[192] found that chronic oral intake of AdipoRon improved vascular function in type 2 diabetic mice through an endothelium-independent mechanism. Lindfors et al[198] discovered that AdipoRon reduced proinflammatory cytokine expression and improved glomerular inflammation and injury in diet-induced obese mice and cultured podocytes. Sun et al[199] showed that AdipoRon reduced inflammation markers and apoptosis, improved mitochondrial function, and accelerated wound healing in aged skin. Zatorski et al[200] found that AdipoRon had a gastroprotective effect and reduced inflammation in stomach ulcers. Tarkhnishvili et al[201] found that AdipoRon changed myocardial substrate preference towards higher glucose consumption in type 2 diabetic mice, although it was insufficient to enhance cardiac output and efficiency. Li et al[202] reported that topical adiponectin was effective in improving clinical signs and reducing inflammation in a mouse model of dry eye or alkali burn, while Baradaran-Rafii et al[137] showed that topical adiponectin decreased recent corneal neovascularization in rabbits.
Hence, adiponectin plays a vital role in wound healing, tissue regeneration, and metabolic regulation. It is typically administered through injections or orally via an adiponectin receptor agonist, such as AdipoRon. Studies have shown that adiponectin enhances wound healing, keratinocyte differentiation, and improves insulin sensitivity, glucose tolerance, lipid metabolism, and vascular dysfunction in diabetic patients. AdipoRon, a small-molecule therapy, has demonstrated similar effects to adiponectin and can be taken orally, targeting multiple metabolic conditions. Although challenges exist in comparing the effectiveness of various drug classes due to differing in vivo models and in vitro limitations, direct receptor agonists have shown promise in controlling signaling better than therapies aiming to increase adiponectin production. Overall, adiponectin-based therapies appear to be safe and hold potential for treating a range of human diseases.
Further research is needed to comprehensively understand the effects of adiponectin on DFUs. Although the existing literature has shown favourable outcomes, there is a need for a more detailed exploration into the mechanisms underlying adiponectin-mediated promotion of wound healing and tissue regeneration. Research studies should be carried out to establish the safety and efficacy of adiponectin as a therapeutic intervention for DFUs in the clinical setting. Despite positive preclinical outcomes, clinical trials are essential to determine the appropriate dose and treatment schedule, as well as any potential adverse effects. Furthermore, additional research is required to identify subgroups of patients that may benefit the most from adiponectin therapy. This could include examining whether specific genetic or demographic factors influence the effectiveness of adiponectin treatment. Studies should be conducted to identify the optimal delivery method for adiponectin therapy, considering that while the topical application has been successful in some studies, other delivery methods such as injection or implantation may be more efficacious in specific cases. Furthermore, research should be conducted to determine whether adiponectin can be used in combination with other treatments for DFUs, such as antibiotics or growth factors, to enhance wound healing and tissue regeneration. Further investigation is also required into the long-term effects of adiponectin therapy, including its impact on wound recurrence rates and overall wound healing outcomes.
In conclusion, the available evidence suggests that adiponectin and its receptors agonist may hold promise as therapeutic targets for the management of DFUs. However, to fully comprehend the role of adiponectin in DFU pathogenesis and treatment, additional research is necessary. Future studies should focus on conducting longitudinal investigations to establish a causal relationship between adiponectin levels and DFU incidence. Moreover, exploring treatment strategies aimed at elevating adiponectin levels in patients with DFUs could provide insights into the potential benefits of adiponectin as a therapeutic target. Investigating the specific cellular and molecular mechanisms underlying the relationship between adiponectin and DFUs is also necessary for a comprehensive understanding of the role of adiponectin in the condition. Additionally, clinical trials that evaluate the efficacy and safety of interventions targeting adiponectin levels in DFU prevention and management are needed. Such research could help to identify novel therapeutic targets for DFUs, ultimately leading to more effective management of the condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leung PC, China; Siddalingam R, Malaysia S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 970] [Article Influence: 121.3] [Reference Citation Analysis (4)] |
| 2. | Bhuyan K. Identification of socioeconomic variables responsible for diabetic heart disease among Bangladeshi adults. ARC J Diabetes Endocrinol. 2019;5:1-8. [DOI] [Full Text] |
| 3. | Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 453] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 4. | Federation I. Time to act: diabetes and foot care. Brussels: International Diabetes Federation. 2005. [cited 20 February 2023]. Available from: https://www.worlddiabetesfoundation.org/files/diabetes-and-foot-care-time-act. |
| 5. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1536] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 6. | Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47:1343-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Jeffcoate W, Bakker K. World Diabetes Day: footing the bill. Lancet. 2005;365:1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Lipsky BA; International consensus group on diagnosing and treating the infected diabetic foot. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev. 2004;20 Suppl 1:S68-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med. 2014;31:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Jeffcoate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Diabet Med. 1993;10:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The lancet. 1998;352 ):837-853. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12653] [Article Influence: 468.6] [Reference Citation Analysis (0)] |
| 12. | New JP, McDowell D, Burns E, Young RJ. Problem of amputations in patients with newly diagnosed diabetes mellitus. Diabet Med. 1998;15:760-764. [PubMed] [DOI] [Full Text] |
| 13. | Morbach S, Lutale JK, Viswanathan V, Möllenberg J, Ochs HR, Rajashekar S, Ramachandran A, Abbas ZG. Regional differences in risk factors and clinical presentation of diabetic foot lesions. Diabet Med. 2004;21:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bandyk DF. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018;31:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 15. | Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 316] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Frykberg RG. Diabetic foot ulcers: current concepts. J Foot Ankle Surg. 1998;37:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 231] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Walters DP, Gatling W, Mullee MA, Hill RD. The distribution and severity of diabetic foot disease: a community study with comparison to a non-diabetic group. Diabet Med. 1992;9:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Syafril S. Pathophysiology diabetic foot ulcer. Proceedings of the IOP Conference Series. IOP Conf Ser Earth Environ Sci. 2018;125:012161. [DOI] [Full Text] |
| 20. | Noor S, Zubair M, Ahmad J. Diabetic foot ulcer--A review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 21. | Williams DT, Hilton JR, Harding KG. Diagnosing foot infection in diabetes. Clin Infect Dis. 2004;39 Suppl 2:S83-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 659] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 23. | Abbott RD, Brand FN, Kannel WB. Epidemiology of some peripheral arterial findings in diabetic men and women: experiences from the Framingham Study. Am J Med. 1990;88:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome as a possible cardiovascular marker in diabetic patients. J Diabetes Res. 2015;2015:268390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Schaper NC, Andros G, Apelqvist J, Bakker K, Lammer J, Lepantalo M, Mills JL, Reekers J, Shearman CP, Zierler RE, Hinchliffe RJ. Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2012;28 Suppl 1:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL Sr, Mueller MJ, Sheehan P, Wukich DK; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 562] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 27. | Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT, Li W. A "traffic control" role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093-1105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Sun JH, Tsai JS, Huang CH, Lin CH, Yang HM, Chan YS, Hsieh SH, Hsu BR, Huang YY. Risk factors for lower extremity amputation in diabetic foot disease categorized by Wagner classification. Diabetes Res Clin Pract. 2012;95:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1152] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 30. | Kapłon-Cieślicka A, Postuła M, Rosiak M, Peller M, Kondracka A, Serafin A, Trzepla E, Opolski G, Filipiak KJ. Association of adipokines and inflammatory markers with lipid control in type 2 diabetes. Pol Arch Med Wewn. 2015;125:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Aziz Z, Lin WK, Nather A, Huak CY. Predictive factors for lower extremity amputations in diabetic foot infections. Diabet Foot Ankle. 2011;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 290] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Pinto A, Tuttolomondo A, Di Raimondo D, La Placa S, Di Sciacca R, Fernandez P, Di Gati M, Raffa A, Licata G. Ischemic stroke in patients with diabetic foot. Int Angiol. 2007;26:266-269. [PubMed] |
| 34. | Fernando M, Crowther R, Lazzarini P, Sangla K, Cunningham M, Buttner P, Golledge J. Biomechanical characteristics of peripheral diabetic neuropathy: A systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clin Biomech (Bristol, Avon). 2013;28:831-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 35. | Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, Armstrong DG, Agrawal CM. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 36. | Coppini D. New NICE guidelines on diabetic foot disease prevention and management. Practical Diabetes. 2015;32:286-286. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Santema TB, Lenselink EA, Balm R, Ubbink DT. Comparing the Meggitt-Wagner and the University of Texas wound classification systems for diabetic foot ulcers: inter-observer analyses. Int Wound J. 2016;13:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2000;16 Suppl 1:S84-S92. [PubMed] [DOI] [Full Text] |
| 39. | Glover K, Stratakos AC, Varadi A, Lamprou DA. 3D scaffolds in the treatment of diabetic foot ulcers: New trends vs conventional approaches. Int J Pharm. 2021;599:120423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Krasilnikova OA, Baranovskii DS, Lyundup AV, Shegay PV, Kaprin AD, Klabukov ID. Stem and Somatic Cell Monotherapy for the Treatment of Diabetic Foot Ulcers: Review of Clinical Studies and Mechanisms of Action. Stem Cell Rev Rep. 2022;18:1974-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Smith OJ, Leigh R, Kanapathy M, Macneal P, Jell G, Hachach-Haram N, Mann H, Mosahebi A. Fat grafting and platelet-rich plasma for the treatment of diabetic foot ulcers: A feasibility-randomised controlled trial. Int Wound J. 2020;17:1578-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 43. | Blüher M. Importance of adipokines in glucose homeostasis. Diabetes Manage. 2013;3:389. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Blüher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 45. | Anderson AS, Good DJ. Increased body weight affects academic performance in university students. Prev Med Rep. 2017;5:220-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 467] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 47. | Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 692] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 48. | Sathyapalan T, Mellor D, Atkin SL. Obesity and gestational diabetes. Semin Fetal Neonatal Med. 2010;15:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452-5455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 50. | Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 601] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 51. | Gartland D, Riggs E, Muyeen S, Giallo R, Afifi TO, MacMillan H, Herrman H, Bulford E, Brown SJ. What factors are associated with resilient outcomes in children exposed to social adversity? BMJ Open. 2019;9:e024870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 52. | Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342-4352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 53. | Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Ihm JJ, An SY, Seo DG. Do Dental Students' Personality Types and Group Dynamics Affect Their Performance in Problem-Based Learning? J Dent Educ. 2017;81:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 56. | Boström EA, d'Elia HF, Dahlgren U, Simark-Mattsson C, Hasséus B, Carlsten H, Tarkowski A, Bokarewa M. Salivary resistin reflects local inflammation in Sjögren's syndrome. J Rheumatol. 2008;35:2005-2011. [PubMed] |
| 57. | Staiger K, Stefan N, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Machicao F, Kellerer M, Stumvoll M, Fritsche A, Häring HU. Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J Clin Endocrinol Metab. 2005;90:6707-6713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Huypens P, Moens K, Heimberg H, Ling Z, Pipeleers D, Van de Casteele M. Adiponectin-mediated stimulation of AMP-activated protein kinase (AMPK) in pancreatic beta cells. Life Sci. 2005;77:1273-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285:33623-33631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 60. | Winzell MS, Nogueiras R, Dieguez C, Ahrén B. Dual action of adiponectin on insulin secretion in insulin-resistant mice. Biochem Biophys Res Commun. 2004;321:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1297] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 62. | Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 812] [Cited by in RCA: 791] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 63. | Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 64. | Zoccali C, Mallamaci F. Adiponectin and leptin in chronic kidney disease: causal factors or mere risk markers? J Ren Nutr. 2011;21:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 66. | von Eynatten M, Liu D, Hock C, Oikonomou D, Baumann M, Allolio B, Korosoglou G, Morcos M, Campean V, Amann K, Lutz J, Heemann U, Nawroth PP, Bierhaus A, Humpert PM. Urinary adiponectin excretion: a novel marker for vascular damage in type 2 diabetes. Diabetes. 2009;58:2093-2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Debard C, Laville M, Berbe V, Loizon E, Guillet C, Morio-Liondore B, Boirie Y, Vidal H. Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia. 2004;47:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3047] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 69. | Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1088] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 70. | Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 790] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 71. | Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest. 2011;121:2518-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 72. | Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1868] [Cited by in RCA: 1832] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 73. | Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723-1732. [RCA] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 858] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 74. | Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202:152-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 75. | Okamoto M, Ohara-Imaizumi M, Kubota N, Hashimoto S, Eto K, Kanno T, Kubota T, Wakui M, Nagai R, Noda M, Nagamatsu S, Kadowaki T. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Kadowaki T, Yamauchi T, Okada-Iwabu M, Iwabu M. Adiponectin and its receptors: implications for obesity-associated diseases and longevity. Lancet Diabetes Endocrinol. 2014;2:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Wang Y, Meng RW, Kunutsor SK, Chowdhury R, Yuan JM, Koh WP, Pan A. Plasma adiponectin levels and type 2 diabetes risk: a nested case-control study in a Chinese population and an updated meta-analysis. Sci Rep. 2018;8:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 78. | Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm J. 2015;23:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res Care. 2016;4:e000194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop. 2015;6:62-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 81. | Zubair M, Malik A, Ahmad J. Plasma adiponectin, IL-6, hsCRP, and TNF-α levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian J Endocrinol Metab. 2012;16:769-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Ahmad J, Zubair M, Malik A, Siddiqui MA, Wangnoo SK. Cathepsin-D, adiponectin, TNF-α, IL-6 and hsCRP plasma levels in subjects with diabetic foot and possible correlation with clinical variables: a multicentric study. Foot (Edinb). 2012;22:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Erem C, Hacihasanoğlu A, Celik S, Ovali E, Ersöz HO, Ukinç K, Deger O, Telatar M. Coagulation and fibrinolysis parameters in type 2 diabetic patients with and without diabetic vascular complications. Med Princ Pract. 2005;14:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Xiao J, Li J, Cai L, Chakrabarti S, Li X. Cytokines and diabetes research. J Diabetes Res. 2014;2014:920613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 86. | Lan CC, Liu IH, Fang AH, Wen CH, Wu CS. Hyperglycaemic conditions decrease cultured keratinocyte mobility: implications for impaired wound healing in patients with diabetes. Br J Dermatol. 2008;159:1103-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 649] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 88. | Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 628] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 89. | Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, André M, Cousin B, Gourmelon P, Benderitter M, Casteilla L, Tamarat R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 90. | Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 91. | Fu X, Fang L, Li H, Li X, Cheng B, Sheng Z. Adipose tissue extract enhances skin wound healing. Wound Repair Regen. 2007;15:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | López JF, Sarkanen JR, Huttala O, Kaartinen IS, Kuokkanen HO, Ylikomi T. Adipose tissue extract shows potential for wound healing: in vitro proliferation and migration of cell types contributing to wound healing in the presence of adipose tissue preparation and platelet rich plasma. Cytotechnology. 2018;70:1193-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int. 2008;17:105-112. [PubMed] |
| 94. | Shibata S, Tada Y, Asano Y, Hau CS, Kato T, Saeki H, Yamauchi T, Kubota N, Kadowaki T, Sato S. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012;189:3231-3241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Hong SP, Seo HS, Shin KO, Park K, Park BC, Kim MH, Park M, Kim CD, Seo SJ. Adiponectin Enhances Human Keratinocyte Lipid Synthesis via SIRT1 and Nuclear Hormone Receptor Signaling. J Invest Dermatol. 2019;139:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Ryu YS, Kang KA, Piao MJ, Ahn MJ, Yi JM, Bossis G, Hyun YM, Park CO, Hyun JW. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp Mol Med. 2019;51:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 97. | Lewis DA, Yi Q, Travers JB, Spandau DF. UVB-induced senescence in human keratinocytes requires a functional insulin-like growth factor-1 receptor and p53. Mol Biol Cell. 2008;19:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Kim M, Park KY, Lee MK, Jin T, Seo SJ. Adiponectin Suppresses UVB-Induced Premature Senescence and hBD2 Overexpression in Human Keratinocytes. PLoS One. 2016;11:e0161247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Jin T, Kim MJ, Heo WI, Park KY, Choi SY, Lee MK, Hong SP, Kim SJ, Im M, Moon NJ, Seo SJ. Adiponectin corrects premature cellular senescence and normalizes antimicrobial peptide levels in senescent keratinocytes. Biochem Biophys Res Commun. 2016;477:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Jin T, Park KY, Seo SJ. Adiponectin Upregulates Filaggrin Expression via SIRT1-Mediated Signaling in Human Normal Keratinocytes. Ann Dermatol. 2017;29:407-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Choi SY, Kim MJ, Ahn GR, Park KY, Lee MK, Seo SJ. The Effect of Adiponectin on the Regulation of Filaggrin Expression in Normal Human Epidermal Keratinocytes. Ann Dermatol. 2018;30:645-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Apte SS, Parks WC. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015;44-46:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 103. | Karamanos NK, Theocharis AD, Neill T, Iozzo RV. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75-76:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 104. | Maione AG, Smith A, Kashpur O, Yanez V, Knight E, Mooney DJ, Veves A, Tomic-Canic M, Garlick JA. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 2016;24:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 105. | Burrage PS, Brinckerhoff CE. Molecular targets in osteoarthritis: metalloproteinases and their inhibitors. Curr Drug Targets. 2007;8:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 107. | Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 409] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 108. | Luo L, Li J, Liu H, Jian X, Zou Q, Zhao Q, Le Q, Chen H, Gao X, He C. Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 109. | Yang J, Lin SC, Chen G, He L, Hu Z, Chan L, Trial J, Entman ML, Wang Y. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol. 2013;24:1644-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 110. | Luo L, Li J, Wu Y, Qiao J, Fang H. Adiponectin, but Not TGF-β1, CTGF, IL-6 or TNF-α, May Be a Potential Anti-Inflammation and Anti-Fibrosis Factor in Keloid. J Inflamm Res. 2021;14:907-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Marangoni RG, Masui Y, Fang F, Korman B, Lord G, Lee J, Lakota K, Wei J, Scherer PE, Otvos L, Yamauchi T, Kubota N, Kadowaki T, Asano Y, Sato S, Tourtellotte WG, Varga J. Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci Rep. 2017;7:4397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 112. | Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol. 2017;199:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 113. | Theocharidis G, Baltzis D, Roustit M, Tellechea A, Dangwal S, Khetani RS, Shu B, Zhao W, Fu J, Bhasin S, Kafanas A, Hui D, Sui SH, Patsopoulos NA, Bhasin M, Veves A. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes. 2020;69:2157-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 114. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2301] [Article Influence: 287.6] [Reference Citation Analysis (2)] |
| 115. | Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 116. | Malhotra R, Chan CS, Nather A. Osteomyelitis in the diabetic foot. Diabet Foot Ankle. 2014;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Boniakowski AE, Kimball AS, Joshi A, Schaller M, Davis FM, denDekker A, Obi AT, Moore BB, Kunkel SL, Gallagher KA. Murine macrophage chemokine receptor CCR2 plays a crucial role in macrophage recruitment and regulated inflammation in wound healing. Eur J Immunol. 2018;48:1445-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 118. | Liu D, Yang P, Gao M, Yu T, Shi Y, Zhang M, Yao M, Liu Y, Zhang X. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci (Lond). 2019;133:565-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 119. | Yang S, Hu L, Han R, Yang Y. Neuropeptides, Inflammation, Biofilms, and diabetic Foot Ulcers. Exp Clin Endocrinol Diabetes. 2022;130:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Ramirez HA, Pastar I, Jozic I, Stojadinovic O, Stone RC, Ojeh N, Gil J, Davis SC, Kirsner RS, Tomic-Canic M. Staphylococcus aureus Triggers Induction of miR-15B-5P to Diminish DNA Repair and Deregulate Inflammatory Response in Diabetic Foot Ulcers. J Invest Dermatol. 2018;138:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 121. | Stone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, Tomic-Canic M. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 122. | Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, Funahashi T, Takata M, Temaru R, Sato A, Yamazaki K, Nakamura N, Kobayashi M. Adiponectin inhibits endothelial synthesis of interleukin-8. Circ Res. 2005;97:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 123. | Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1540] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 124. | Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1243] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 125. | Lee KH, Jeong J, Woo J, Lee CH, Yoo CG. Globular Adiponectin Exerts a Pro-Inflammatory Effect via IκB/NF-κB Pathway Activation and Anti-Inflammatory Effect by IRAK-1 Downregulation. Mol Cells. 2018;41:762-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 126. | Huang H, Wang L, Qian F, Chen X, Zhu H, Yang M, Zhang C, Chu M, Wang X, Huang X. Liraglutide via Activation of AMP-Activated Protein Kinase-Hypoxia Inducible Factor-1α-Heme Oxygenase-1 Signaling Promotes Wound Healing by Preventing Endothelial Dysfunction in Diabetic Mice. Front Physiol. 2021;12:660263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 127. | Dong Z, Zhuang Q, Ye X, Ning M, Wu S, Lu L, Wan X. Adiponectin Inhibits NLRP3 Inflammasome Activation in Nonalcoholic Steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS Signaling Pathways. Front Med (Lausanne). 2020;7:546445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 128. | Wang F, Liu Y, Yang W, Yuan J, Mo Z. Adiponectin inhibits NLRP3 inflammasome by modulating the AMPK-ROS pathway. Int J Clin Exp Pathol. 2018;11:3338. [DOI] [Full Text] |
| 129. | Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 324] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 130. | Massaro M, Scoditti E, Pellegrino M, Carluccio MA, Calabriso N, Wabitsch M, Storelli C, Wright M, De Caterina R. Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazar in attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol Res. 2016;107:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 131. | Li X, Guo H, Zhao L, Wang B, Liu H, Yue L, Bai H, Jiang H, Gao L, Feng D, Qu Y. Adiponectin attenuates NADPH oxidase-mediated oxidative stress and neuronal damage induced by cerebral ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3265-3276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 132. | Zhang W, Shu C, Li Q, Li M, Li X. Adiponectin affects vascular smooth muscle cell proliferation and apoptosis through modulation of the mitofusin-2-mediated Ras-Raf-Erk1/2 signaling pathway. Mol Med Rep. 2015;12:4703-4707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Motobayashi Y, Izawa-Ishizawa Y, Ishizawa K, Orino S, Yamaguchi K, Kawazoe K, Hamano S, Tsuchiya K, Tomita S, Tamaki T. Adiponectin inhibits insulin-like growth factor-1-induced cell migration by the suppression of extracellular signal-regulated kinase 1/2 activation, but not Akt in vascular smooth muscle cells. Hypertens Res. 2009;32:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 134. | Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 135. | Peng J, Chen Q, Wu C. The role of adiponectin in cardiovascular disease. Cardiovasc Pathol. 2023;64:107514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 136. | Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 137. | Baradaran-Rafii A, Ashnagar A, Heidari Keshel S, Jabbehdari S, Baradaran-Rafii G. Regression of corneal neovascularization: Adiponectin vs bevacizumab eye drops. Eur J Ophthalmol. 2021;31:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 138. | Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21:770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 139. | Smith K, Collier A, Townsend EM, O'Donnell LE, Bal AM, Butcher J, Mackay WG, Ramage G, Williams C. One step closer to understanding the role of bacteria in diabetic foot ulcers: characterising the microbiome of ulcers. BMC Microbiol. 2016;16:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 140. | Wang Z, Chen Z, Fang F, Qiu W. The role of adiponectin in periodontitis: Current state and future prospects. Bio Pharm. 2021;137:111358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 141. | Malone M, Johani K, Jensen SO, Gosbell IB, Dickson HG, Hu H, Vickery K. Next Generation DNA Sequencing of Tissues from Infected Diabetic Foot Ulcers. EBioMedicine. 2017;21:142-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 142. | Xiao Y, Deng T, Shang Z, Wang D. Adiponectin inhibits oxidization-induced differentiation of T helper cells through inhibiting costimulatory CD40 and CD80. Braz J Med Biol Res. 2017;50:e6227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 143. | Liu H, Lu XJ, Chen J. Full-length and a smaller globular fragment of adiponectin have opposite roles in regulating monocyte/macrophage functions in ayu, Plecoglossus altivelis. Fish Shellfish Immunol. 2018;82:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Rossi A, Lord J. Adiponectin inhibits neutrophil phagocytosis of Escherichia coli by inhibition of PKB and ERK 1/2 MAPK signalling and Mac-1 activation. PLoS One. 2013;8:e69108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 145. | Shi GJ, Shi GR, Zhou JY, Zhang WJ, Gao CY, Jiang YP, Zi ZG, Zhao HH, Yang Y, Yu JQ. Involvement of growth factors in diabetes mellitus and its complications: A general review. Biomed Pharmacother. 2018;101:510-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 146. | Xie L, Zhang M, Dong B, Guan M, Lu M, Huang Z, Gao H, Li X. Improved refractory wound healing with administration of acidic fibroblast growth factor in diabetic rats. Diabetes Res Clin Pract. 2011;93:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Huang C, Orbay H, Tobita M, Miyamoto M, Tabata Y, Hyakusoku H, Mizuno H. Proapoptotic effect of control-released basic fibroblast growth factor on skin wound healing in a diabetic mouse model. Wound Repair Regen. 2016;24:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 148. | Cheng X, Zhu B, Jiang F, Fan H. Serum FGF-21 Levels in type 2 diabetic patients. Endocr Res. 2011;36:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 149. | Rysz J, Gluba-Brzózka A, Mikhailidis DP, Banach M. Fibroblast growth factor 19-targeted therapies for the treatment of metabolic disease. Expert Opin Investig Drugs. 2015;24:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 150. | Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 151. | Nolfi AL, Behun MN, Yates CC, Brown BN, Kulkarni M. Beyond Growth Factors: Macrophage-Centric Strategies for Angiogenesis. Curr Path Reports. 2020;8: 111-120. |
| 152. | Isidori AM, Venneri MA, Fiore D. Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J Endocrinol Invest. 2016;39:1235-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 153. | Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 610] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 154. | Herold J, Kalucka J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front Physiol. 2021;11. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 155. | Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res. 2008;78:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 156. | Shaikh-Kader A, Houreld NN, Rajendran NK, Abrahamse H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem Funct. 2019;37:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 157. | Januszyk M, Chen K, Henn D, Foster DS, Borrelli MR, Bonham CA, Sivaraj D, Wagh D, Longaker MT, Wan DC, Gurtner GC. Characterization of Diabetic and Non-Diabetic Foot Ulcers Using Single-Cell RNA-Sequencing. Micromachines (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 158. | Bhan S, Mitra R, Arya AK, Pandey HP, Tripathi K. A study on evaluation of apoptosis and expression of bcl-2-related marker in wound healing of streptozotocin-induced diabetic rats. ISRN Dermatol. 2013;2013:739054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 159. | Liu Z, Gan L, Wu T, Feng F, Luo D, Gu H, Liu S, Sun C. Adiponectin reduces ER stress-induced apoptosis through PPARα transcriptional regulation of ATF2 in mouse adipose. Cell Death Dis. 2016;7:e2487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | Haugen S, He J, Sundaresan A, Stunes AK, Aasarød KM, Tiainen H, Syversen U, Skallerud B, Reseland JE. Adiponectin Reduces Bone Stiffness: Verified in a Three-Dimensional Artificial Human Bone Model In Vitro. Front Endocrinol (Lausanne). 2018;9:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 161. | Berg G, Barchuk M, Miksztowicz V. Behavior of Metalloproteinases in Adipose Tissue, Liver and Arterial Wall: An Update of Extracellular Matrix Remodeling. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 162. | Cheng M, Hashmi S, Mao X, Zeng QT. Relationships of adiponectin and matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio with coronary plaque morphology in patients with acute coronary syndrome. Can J Cardiol. 2008;24:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Teng YY, Zou ML, Zhou XJ, Wu JJ, Liu SY, Yuan ZD, Jia Y, Zhang KW, Li X, Ye JX, Yuan FL. Novel prospects for scarless wound healing: The roles of myofibroblasts and adipocytes. J Cell Mol Med. 2022;26:5113-5121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 164. | Kattan WM, Alarfaj SF, Alnooh BM, Alsaif HF, Alabdul Karim HS, Al-Qattan NM, Al-Qattan MM, El-Sayed AA. Myofibroblast-Mediated Contraction. J Coll Physicians Surg Pak. 2017;27:38-43. [PubMed] |
| 165. | Vallée A, Lecarpentier Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019;9:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 166. | Wu H, Cai L, de Haan JB, Giacconi R. Targeting Oxidative Stress in Diabetic Complications: New Insights. J Diabetes Res. 2018;2018:1909675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 167. | Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 168. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3608] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 169. | Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46:1369-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 477] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 170. | Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 171. | Ryu J, Loza CA, Xu H, Zhou M, Hadley JT, Wu J, You H, Wang H, Yang J, Bai J, Liu F, Bialowas C, Dong LQ. Potential Roles of Adiponectin Isoforms in Human Obesity with Delayed Wound Healing. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Woodward L, Akoumianakis I, Antoniades C. Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol. 2017;174:4007-4020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 173. | Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 174. | Nguyen TMD. Adiponectin: Role in Physiology and Pathophysiology. Int J Prev Med. 2020;11:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 175. | Theocharidis G, Veves A. Autonomic nerve dysfunction and impaired diabetic wound healing: The role of neuropeptides. Auton Neurosci. 2020;223:102610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 176. | Ma OK, Ronsisvalle S, Basile L, Xiang AW, Tomasella C, Sipala F, Pappalardo M, Chan KH, Milardi D, Ng RC, Guccione S. Identification of a novel adiponectin receptor and opioid receptor dual acting agonist as a potential treatment for diabetic neuropathy. Biomed Pharmacother. 2023;158:114141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 177. | Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr Diab Rep. 2018;18:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 178. | Karbowska J, Kochan Z. Role of adiponectin in the regulation of carbohydrate and lipid metabolism. J Physiol Pharmacol. 2006;57 Suppl 6:103-113. [PubMed] |
| 179. | Lee B, Shao J. Adiponectin and lipid metabolism in skeletal muscle. Acta Pharmaceutica Sinica B. 2012;2:335-340. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 180. | Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic β cells and adipocytes. Best Pract Res Clin Endocrinol Metab. 2014;28:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 181. | Park PH, Sanz-Garcia C, Nagy LE. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Curr Pathobiol Rep. 2015;3:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 182. | Wolf AM, Wolf D, Avila MA, Moschen AR, Berasain C, Enrich B, Rumpold H, Tilg H. Up-regulation of the anti-inflammatory adipokine adiponectin in acute liver failure in mice. J Hepatol. 2006;44:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 183. | Febriza A, Ridwan R, As’ ad S, Kasim VN, Idrus HH. Adiponectin and its role in inflammatory process of obesity. Mol Cell Bio Sci. 2019;3:60-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 184. | Esmaili S, Hemmati M, Karamian M. Physiological role of adiponectin in different tissues: a review. Arch Physiol Biochem. 2020;126:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 185. | Wang Y, Liu H, Zhang R, Xiang Y, Lu J, Xia B, Peng L, Wu J. AdipoRon exerts opposing effects on insulin sensitivity via fibroblast growth factor 21-mediated time-dependent mechanisms. J Biol Chem. 2022;298:101641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 186. | Kim SW, Park KY, Kim B, Kim E, Hyun CK. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun. 2013;431:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 187. | Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 188. | Kawai K, Kageyama A, Tsumano T, Nishimoto S, Fukuda K, Yokoyama S, Oguma T, Fujita K, Yoshimoto S, Yanai A, Kakibuchi M. Effects of adiponectin on growth and differentiation of human keratinocytes--implication of impaired wound healing in diabetes. Biochem Biophys Res Commun. 2008;374:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 189. | Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 4175] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 190. | Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 569] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 191. | Holland WL, Scherer PE. Cell Biology. Ronning after the adiponectin receptors. Science. 2013;342:1460-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 192. | Choi SK, Kwon Y, Byeon S, Haam CE, Lee YH. AdipoRon, adiponectin receptor agonist, improves vascular function in the mesenteric arteries of type 2 diabetic mice. PLoS One. 2020;15:e0230227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 193. | Salathia NS, Shi J, Zhang J, Glynne RJ. An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. J Invest Dermatol. 2013;133:812-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 194. | Jin CE, Xiao L, Ge ZH, Zhan XB, Zhou HX. Role of adiponectin in adipose tissue wound healing. Genet Mol Res. 2015;14:8883-8891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 195. | Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B, Wu L, Bao Y, Li P, Xu A, Jia W. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 196. | Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, Choi BS, Kim YS, Chang YS, Park CW. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J Am Soc Nephrol. 2018;29:1108-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 197. | Otvos L Jr. Potential Adiponectin Receptor Response Modifier Therapeutics. Front Endocrinol (Lausanne). 2019;10:539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 198. | Lindfors S, Polianskyte-Prause Z, Bouslama R, Lehtonen E, Mannerla M, Nisen H, Tienari J, Salmenkari H, Forsgård R, Mirtti T, Lehto M, Groop PH, Lehtonen S. Adiponectin receptor agonist AdipoRon ameliorates renal inflammation in diet-induced obese mice and endotoxin-treated human glomeruli ex vivo. Diabetologia. 2021;64:1866-1879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 199. | Sun J, Liu X, Shen C, Zhang W, Niu Y. Adiponectin receptor agonist AdipoRon blocks skin inflamm-ageing by regulating mitochondrial dynamics. Cell Prolif. 2021;54:e13155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 200. | Zatorski H, Salaga M, Zielińska M, Majchrzak K, Binienda A, Kordek R, Małecka-Panas E, Fichna J. AdipoRon, an Orally Active, Synthetic Agonist of AdipoR1 and AdipoR2 Receptors Has Gastroprotective Effect in Experimentally Induced Gastric Ulcers in Mice. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 201. | Tarkhnishvili A, Koentges C, Pfeil K, Gollmer J, Byrne NJ, Vosko I, Lueg J, Vogelbacher L, Birkle S, Tang S, Bon-Nawul Mwinyella T, Hoffmann MM, Odening KE, Michel NA, Wolf D, Stachon P, Hilgendorf I, Wallner M, Ljubojevic-Holzer S, von Lewinski D, Rainer P, Sedej S, Sourij H, Bode C, Zirlik A, Bugger H. Effects of Short Term Adiponectin Receptor Agonism on Cardiac Function and Energetics in Diabetic db/db Mice. J Lipid Atheroscler. 2022;11:161-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 202. | Li Y, Jin R, Li L, Hsu HH, You IC, Yoon HJ, Yoon KC. Therapeutic Effect of Topical Adiponectin-Derived Short Peptides Compared with Globular Adiponectin in Experimental Dry Eye and Alkali Burn. J Ocul Pharmacol Ther. 2020;36:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 203. | Tuttolomondo A, La Placa S, Di Raimondo D, Bellia C, Caruso A, Lo Sasso B, Guercio G, Diana G, Ciaccio M, Licata G, Pinto A. Adiponectin, resistin and IL-6 plasma levels in subjects with diabetic foot and possible correlations with clinical variables and cardiovascular co-morbidity. Cardiovasc Diabetol. 2010;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 204. | Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-α and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene. 2015;565:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 205. | Viswanathan V, Dhamodharan U, Srinivasan V, Rajaram R, Aravindhan V. Single nucleotide polymorphisms in cytokine/chemokine genes are associated with severe infection, ulcer grade and amputation in diabetic foot ulcer. Int J Biol Macromol. 2018;118:1995-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 206. | Anguiano-Hernandez YM, Contreras-Mendez L, de Los Angeles Hernandez-Cueto M, Muand Oz-Medina JE, Santillan-Verde MA, Barbosa-Cabrera RE, Delgado-Quintana CA, Trejo-Rosas S, Santacruz-Tinoco CE, Gonzalez-Ibarra J, Gonzalez-Bonilla CR. Modification of HIF-1α, NF-aκB, IGFBP-3, VEGF and adiponectin in diabetic foot ulcers treated with hyperbaric oxygen. Undersea Hyperb Med. 2019;46:35-44. [PubMed] [DOI] [Full Text] |
| 207. | Vangaveti VN, Jhamb S, Hayes O, Goodall J, Bulbrook J, Robertson K, Biros E, Sangla KS, Malabu UH. Effects of vildagliptin on wound healing and markers of inflammation in patients with type 2 diabetic foot ulcer: a prospective, randomized, double-blind, placebo-controlled, single-center study. Diabetol Metab Syndr. 2022;14:183. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |