Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.632
Peer-review started: December 17, 2022
First decision: March 24, 2023
Revised: April 1, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: June 15, 2023
Processing time: 179 Days and 23.3 Hours
Obesity and type-2 diabetes mellitus (T2DM) are metabolic disorders. Obesity increases the risk of T2DM, and as obesity is becoming increasingly common, more individuals suffer from T2DM, which poses a considerable burden on health systems. Traditionally, pharmaceutical therapy together with lifestyle changes is used to treat obesity and T2DM to decrease the incidence of comorbidities and all-cause mortality and to increase life expectancy. Bariatric surgery is increasingly replacing other forms of treatment of morbid obesity, especially in patients with refractory obesity, owing to its many benefits including good long-term outcomes and almost no weight regain. The bariatric surgery options have markedly changed recently, and laparoscopic sleeve gastrectomy (LSG) is gradually gaining popularity. LSG has become an effective and safe treatment for type-2 diabetes and morbid obesity, with a high cost-benefit ratio. Here, we review the me-chanism associated with LSG treatment of T2DM, and we discuss clinical studies and animal experiments with regard to gastrointestinal hormones, gut microbiota, bile acids, and adipokines to clarify current treatment modalities for patients with obesity and T2DM.
Core Tip: Obesity and type-2 diabetes mellitus (T2DM) incidence are currently increasing, and these afflictions have become important global health issues. Bariatric surgery is safe and effective for treating obesity and T2DM. The precise processes associated with this treatment, however, are somewhat unclear. Here, we review associated findings with respect to gastrointestinal hormones, intestinal microbiota, bile acids, and adipokines involved in laparoscopic sleeve gastrectomy (the most popular bariatric surgery) of T2DM patients.
- Citation: Liu FS, Wang S, Guo XS, Ye ZX, Zhang HY, Li Z. State of art on the mechanisms of laparoscopic sleeve gastrectomy in treating type 2 diabetes mellitus. World J Diabetes 2023; 14(6): 632-655
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/632.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.632
Obesity, a complicated chronic metabolic illness induced by excessive lipid accumulation, has replaced smoking as the leading cause of early mortality linked to lifestyle[1,2]. More than one-third of all nations have experienced a two-fold increase in the frequency of obesity during the 1980s, and most countries still report an increasing trend[3]. In 2015, more than 700 million adults and children were globally reported to be obese[4]. Numerous disorders, including type-2 diabetes mellitus (T2DM), afflictions of the cardiovascular system, hyperlipidemia, chronic renal disease, sleep apnea syndrome, non-alcoholic fatty liver disease (NAFLD), osteoarthritis, and metabolic syndrome, are closely associated with obesity[5].
T2DM is a prevalent metabolic condition that can damage various physiological systems and is defined by glucose metabolism problems elicited by poor insulin production and decreased insulin sensitivity[6]. The pronounced global increase in obesity, which is a major driver of T2DM, has markedly increased T2DM prevalence[7]. In 2017, more than 460 million individuals worldwide, i.e., 6.28% of the global population, suffered from T2DM[8]. Obesity and T2DM have developed into important public health problems that constitute a heavy burden for the affected patients.
In addition to regular lifestyle behavior adjustments and medication, laparoscopic sleeve gastrectomy (LSG) has been acknowledged by worldwide diabetic organizations as a potent treatment of obesity and T2DM[9]. Even though the advantages of LSG for treating obesity and T2DM are commonly known, the processes by which LSG influences T2DM via several mechanisms, in addition to weight reduction, are still not comprehensively understood. Treatments can be optimized when the mechanisms underlying these metabolic processes and their effects on T2DM are elucidated. In this review, we focus on changes in terms of gastrointestinal hormones (GHs), adipokines, gut microbiota (GM), and bile acids (BAs) after LSG treatment of T2DM.
Since the first bariatric surgery (BS) was performed in 1952, advances have been achieved throughout the past 70 years[10]. BS was intended to help patients lose weight and thereafter maintain normal weight; however, its importance in treating obesity-related comorbidities, particularly T2DM, has increasingly become prominent in clinical practice[11]. To improve surgery results and reduce complication rates, bariatric surgeons continually upgrade and enhance their techniques, and current bariatric operations include vertical-banded gastroplasty, duodenal switch, jejunoileal bypass, biliopancreatic diversion, adjustable gastric banding, Roux-en-Y gastric bypass (RYGB), and sleeve gastrectomy (SG)[12]. Additionally, BS is mostly carried out through laparoscopy due to the advances of lumpectomy surgery.
The most frequently performed BS techniques are RYGB and SG[13]. The first variant of SG was described by Marceau et al[14] in 1993; it is a more physiologic variation of gastroplasty, which is normally a restrictive treatment using a longer, less curved vertical gastric tube to reduce stomach capacity. Despite their anatomical distinctions, both treatments have been proven safe and effective for treating obesity and T2DM[15]. BS can markedly decrease all-cause mortality and enhance life expectancy in obese adult patients, compared to standard obesity therapy, as evidenced by long-term follow-up of a large sample population. In addition, individuals who are overweight and suffer from T2DM benefit more from this treatment than those who suffer from obesity only[16]. A long-term follow-up study of 146 patients approaching 10 years showed complete remission of T2DM after LSG in 72.2%, significant improvement in 25.1%, and no change in only 2.7%[17]. The treatment effect of LSG on T2DM in morbidly obese patients was the same compared to laparoscopic RYGB (LRYGB), as demonstrated by a meta-analysis containing 9 studies, in which the remission rates of T2DM were 82.3% and 80.7% for LRYGB and LSG, respectively[18]. In addition, a meta-analysis containing 33 studies with 4109 patients showed that patients receiving LSG experienced more significant improvement or remission of diabetes than those receiving laparoscopic adjustable gastric banding (LAGB)[19]. A meta-analysis designed for 1108 adult subjects showed that the probability of T2DM mitigation after LSG was 61.4%, significantly higher than in the medication group (2.5%). Based on the above findings, the remission rate of T2DM after LSG was not significantly different from LRYGB but significantly higher than drug treatment and LAGB[20].
Surgeons performing BS and patients tend to choose LSG over other BS because of its lower risk of complications, compared to other surgical procedures; further, it is less invasive, preserves the body's original natural channels, and has better clinical outcomes. Currently, LSG is globally the most common BS[21]. Between 2010 and 2018, the proportion of LSG among BS techniques increased from 2% to 61%, whereas that of RYGB decreased from 55% to 17%[22]. According to the International Federation for Surgery of Obesity Global Registry, 833678 weight-reduction procedures were recorded globally in 2019; however, only 1% of individuals qualified for surgical reasons received surgical treatment[23,24]. Thus, there is considerable room for expansion of bariatric metabolic surgery. Considering the advances in BS options, we focus on the mechanisms of LSG relieving T2DM. The remission rate of T2DM after SG is approximately 65%[25], and this process involves, for example, GHs, GM, BAs, adipokines, the nervous system, and other potential mechanisms that are addressed here.
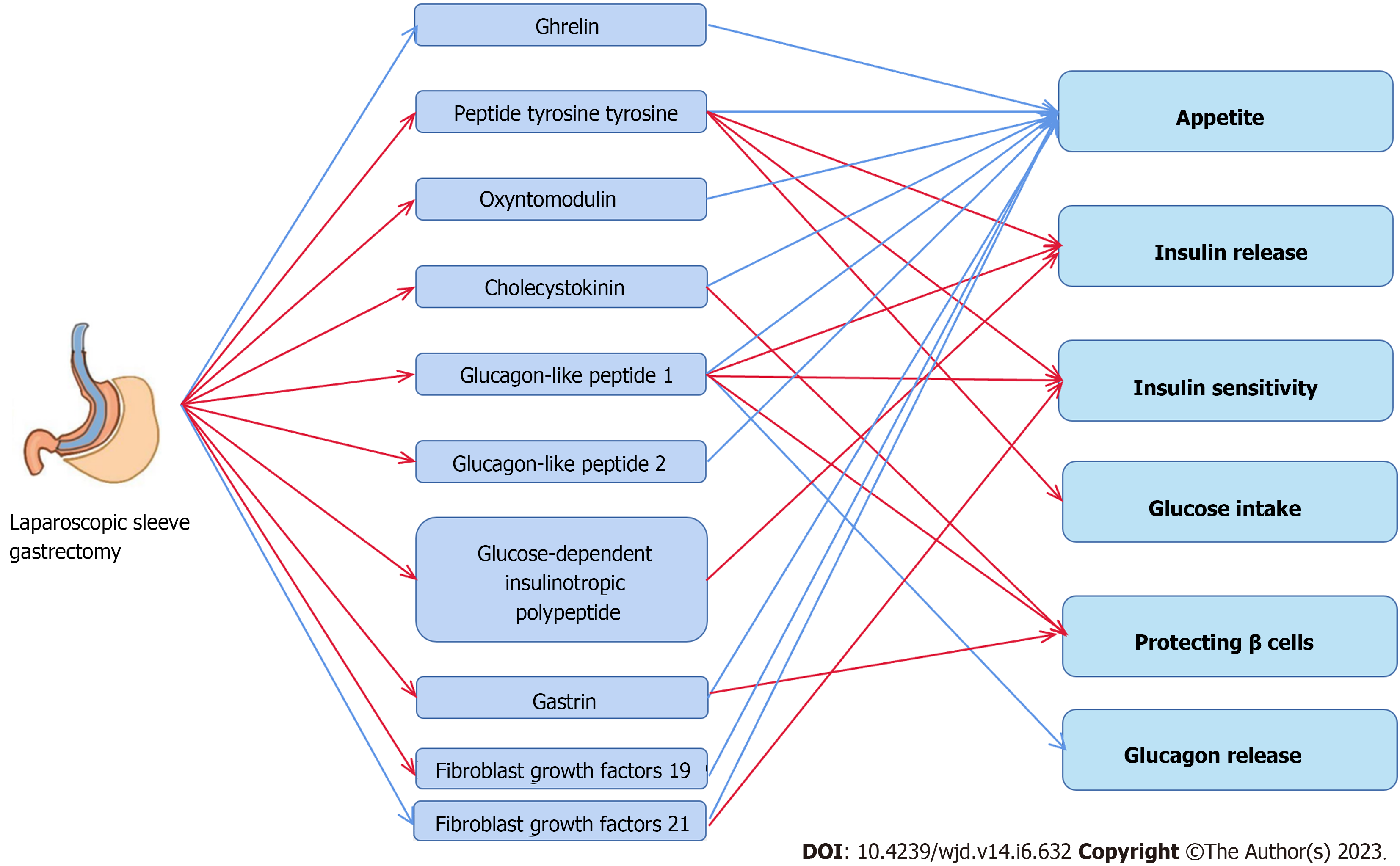
Ghrelin, also referred to as the "hunger hormone", is a peptide of 28 amino acids predominantly generated by gastric fundus X/A cells. During fasting, ghrelin expression increases, and it is reduced after eating[26]. Ghrelin regulates the energy balance, increases the sensation of hunger, stimulates growth hormone release from the hypothalamus and anterior pituitary, and stimulates food intake to facilitate the buildup of adipose tissue[27,28]. Additionally, ghrelin increases muscle insulin resistance (IR) and controls peripheral glucose homeostasis by lowering glucose-stimulated insulin release[29,30]. In extremely obese individuals, ghrelin prevents the appropriate inhibitory response to food intake and does not return to normal after losing weight without surgery[31,32]. Kalinowski et al[33] found that glucose metabolism improved in obese patients with BS, with reduced ghrelin levels after LSG and increased levels after RYGB. The same outcomes were obtained in other long-term follow-up trials, with patients reporting a significant decrease in ghrelin levels after LSG[34]. Stoica et al[35] confirmed this finding in a study on Wistar rats showing that LSG markedly decreased the levels of circulating acylated ghrelin. The primary location of ghrelin production is removed through LSG, which may be the primary cause of reduced ghrelin levels post-surgery. This ghrelin decrease after LSG likely explains the subsequent glycemic improvement as ghrelin is associated with higher circulating insulin and glucagon levels[36]. However, in a study on ghrelin-deficient and wild-type mice, the responses to LSG resembled those after glycemic control, which implies that ghrelin may not be required to improve the glucose metabolism[37]. The studies cited above concluded that LSG substantially affects ghrelin production but that this effect was not the single causative factor of postoperative T2DM remission.
As a member of the pancreatic polypeptide-fold family, peptide tyrosine tyrosine (PYY) is a digestive hormone released after eating by the L-cells among intestinal endocrine cells of the distal ileum and colonic mucosa, and in rodents, it is considered a satiety signal[38]. PYY may also affect insulin sensitivity and glucose absorption by acting on Y2 receptors, and it may modulate insulin secretion by acting on islets[39]. Reduced PYY levels occur in obese people during fasting and after eating, possibly because PYY synthesis, release, or clearance is impeded[39]. Exogenous PYY has recently attracted attention as an anti-obesity agent that can reduce food intake, delay stomach emptying, and lower the glycemic index[40,41]. Potential LSG-induced alterations of PYY levels are currently controversial. One prominent question is whether PYY levels change after LSG surgery. Most studies concluded that PYY is elevated due to LSG[42-44], whereas one study suggested that PYY secretion, although numerically increased, is not statistically different from baseline[45]; however, considering the small number of patients included in this study (only six cases), this may not be a general pattern. The other question is whether increased PYY is restored to its baseline levels within a certain period after LSG.
Arakawa et al[41] observed an increase in PYY 26 wk after surgery but not after 52 wk. Similar results were obtained in a different study, showing higher PYY levels immediately after surgery, which then decreased to baseline levels within one year[44]; PYY secretion did, however, continue to increase postoperatively and remained above baseline levels at 18 mo, according to Alamuddin et al[42]. In an animal study, non-obese diabetic Goto-Kakizaki (GK) rats that were subjected to LSG showed substantial improvements in glycemic control, a significant decrease in glycated hemoglobin, and an increase in diet-induced PYY[46]. Moreover, in diet- and streptozocin (STZ)-induced diabetic obese mice, LSG can increase PYY levels. Animals subjected to surgery also show higher glucose tolerance and fasting insulin improvement, and their insulin secretion increases and peaks faster following glucose infusion[47]. Boza et al[47] additionally performed ileal transposition with LSG, and compound surgery resulted in a considerable reduction in food intake, increased PYY levels, and improved glucose tolerance in obese diabetic mice. Current research suggests that PYY levels are increased in mice and humans subjected to LSG, which is directly related to lower food consumption. Further fundamental research is required to determine whether a direct connection exists between higher PYY and better insulin release and glucose tolerance.
Oxyntomodulin (OXM), like PYY, is produced by intestinal L cells. It participates in the control of satiety, influences the production of hydrochloric acid by gastric secretion glands, and exerts a biological activity similar to that of glucagon[48,49]. OXM has not yet been linked to a particular receptor, but intriguingly, it affects glucagon-like peptide (GLP)-1 receptors in the hypothalamic arcuate nucleus[50]. Furthermore, it exhibits entero-insulinotropic effects and β cell-protecting qualities[51]. According to previous studies, OXM may boost energy expenditure and control blood glucose levels in obese people while suppressing appetite and reducing food intake[52,53]. In obese individuals with T2DM, OXM combined with GLP-1 and PYY has been demonstrated to improve glycemia and body weight[54]. Few studies examined how BS affects OXM, particularly when the surgical strategy is restricted to LSG; thus, little is known about changes in OXM following LSG. Nielsen et al[55] reported that post-LSG patients exhibited increased OXM production, which was correlated with body weight and postoperative dietary preferences. After RYGB, weight reduction may be predicted by early postprandial OXM, according to a different study[56]. Laferrère et al[57] conducted oral glucose tolerance trials and found that peak OXM levels were considerably higher in the surgery group compared to the control diet group and corresponded with an increase in PYY. Further, OXM levels following RYGB surgery did not change while fasting. In mice, exogenous OXM increases glucose-induced insulin secretion, energy expenditure, and weight loss[58]. This effect of OXM may be due to its impact on the GLP-1 receptor (GLP-1R) as it does not stimulate insulin secretion in GLP-1R-/- mice[59]. The effect of exogenous OXM on T2DM has been partly established, however, further research is needed to understand how it is affected by LSG and other types of BS. Intriguingly, two studies have revealed that OXM might be a predictor of weight reduction after BS. We hypothesize that this impact may be associated with changes in dietary practice and satiety.
Cholecystokinin (CCK) was first described in 1982[60], and as suggested by its designation, it is a peptide hormone which can cause gallbladder contraction linked to the gastrointestinal system. According to recent studies, CKK receptors are expressed in the pancreas, central nervous system, gallbladder smooth muscle, and stomach mucosa[61]. CCK interacts with CCK-1 receptors in distinct areas of the hindbrain to signal satiety and decrease food intake[62]. CCK has also been linked to neurophysiological processes, including anxiety, sadness, pain, learning, and memory[63,64]. It controls stomach acid production, reduces BA release, and impacts gastrointestinal motility in the gut[65,66]. In aged mice, CCK expression in β cells increases the area of the pancreas and shields the cells from STZ-induced diabetes and apoptosis, demonstrating a protective impact on β cells[62]. Frequent ravenous hunger of obese patients may be explained by the fact that insensitivity of vagal afferent neurons to CCK is decreased which reduces the drug's impact on satiety[60]. CCK and associated peptide hormones can successfully be used as adjuvant therapy for treating T2DM and obesity[67]. In high-fat diet (HFD) mice, CCK analogs can lower caloric intake, reduce body weight, and increase insulin sensitivity[68]. Numerous studies have shown that LSG significantly affects the levels of circulating CCK, thus improving glucose homeostasis and improving homeostasis model assessment of IR (HOMA-IR)[69,43]. Additionally, elevated CCK appears to inhibit sympathetic action and subsequently inhibits the intrarenal renin-angiotensin system, producing a hypotensive effect[70]. LSG has a stronger CCK-increasing effect than RYGB; however, it seems to be associated with lower remission rates in T2DM patients[71]. According to current research, CCK has a favorable function in preserving glucose homeostasis in T2DM, and one potential explanation may be its protective effects on pancreatic β cells. In cases with obesity, the weight-reduction effect of CCK may be mediated by a response of the central nervous system that re-establishes normal satiety signaling and reduces food ingestion. However, as there is no clear correlation between the increase in CCK and frequency of remission of T2DM after BS, it is not entirely conclusive to explain T2DM by changes in it alone.
GLP-1 is considered the most "successful" peptide hormone currently available. It is predominantly produced by intestinal L cells, and is a fundamental compound of several T2DM and obesity medications and of novel medications currently under research[72]. Under physiological circumstances, ingested food (including carbohydrates, glucose, proteins, and BAs) stimulates L cells scattered throughout the epithelium to release GLP-1 into the blood at a rate corresponding to food absorption[73]. This hormone is important in coordinating postprandial glucose homeostasis. GLP-1 stimulates the release of postprandial insulin, and activation of GLP-1R in pancreatic β cells stimulates the release of insulin, which depends on plasma glucose levels[74]. When β cells perceive elevated plasma glucose levels and GLP-1 signals from the intestine, it enhances insulin release after glucose intake, which is also known as the intestinal proinsulin effect[75]. Meanwhile, GLP-1 prevents pancreatic α cells from releasing glucagon[76], and it regulates gastric emptying, thus influencing appetite and contributing to a sensation of satiety. GLP-1 contributes to the ileal brake, allowing nutrients to enter the duodenum at the same rate as absorbed in the small intestine[77]. By targeting GLP-1R in the brainstem or hypothalamus, GLP-1 decreases hunger and increases satiety, which is complementary to the effects of PYY; however, both originate from L cells[78,79]. In T2DM, GLP-1 secretion is reduced, and the effect of entero-insulin is diminished[80]. However, this may be a consequence of T2DM rather than an etiology because non-T2DM patients with elevated blood glucose show a marked decrease in GLP-1 Levels[81]. The study of Shehata et al[82] showed that in obese adolescents with T2DM, LSG significantly increased GLP-1 Levels in the early postoperative period (until six months after surgery). However, it did not produce the same effect during the late postoperative period (12 mo after surgery). Furthermore, the size of the antrum was not linked to higher GLP-1, better glucose control, or less IR, but to higher T2DM remission rates. Min et al[83] came to similar conclusions, as GLP-1 Levels were increased in the early stage after surgery, but this effect was not persistent. Significant reductions in glycosylated hemoglobin (HbA1c) and IR predict improvement of T2DM. Vigneshwaran et al[84] also found that LSG led to increased GLP-1 Levels six months after surgery in T2DM patients who were not morbidly obese, but they did not record GLP-1 Levels thereafter. Further, obese people without T2DM also showed low insulin sensitivity and high insulin levels in the blood, compared to healthy controls. After LSG intervention. patients showed higher insulin sensitivity and markedly higher GLP-1 Levels[85].
In contrast, Rigamonti et al[86] compared GLP-1 Levels before and after surgery and examined how food ingestion rates affected GLP-1 secretion. They found no significant difference in GLP-1 Levels, but they proposed that LSG would make patients less resistant to insulin. However, who underwent RYGB showed higher GLP-1 Levels, better β cell function, and a higher chance of remission from T2DM[87]. In an animal study, Garibay et al[88] showed that SG helps better control glucose levels by improving β cell GLP-1R signaling and increasing glucose-stimulated insulin secretion. Li et al[89] suggested that improved glucose metabolism in GK rats with SG was caused by increased GLP-1 secretion, which was achieved by increasing the amount of GLP-1 in the plasma through increasing GLP-1 production in the jejunal and ileal mucosa.
Nevertheless, other studies suggest a different perspective. Wilson-Pérez et al[90] used GLP-1R-deficient mice which after SG did not differ significantly from wild-type controls in terms of weight and body fat reduction, improved glucose tolerance, food intake, and food preference. The authors concluded that GLP-1R activity was not required for SG to improve glucose metabolism and reduce body weight. Evidence from recent studies supports the notion that GLP-1 is crucial for maintaining glucose homeostasis, and the prospect of developing effective treatments is encouraging. As a hormone with an intestinal proinsulin effect, production of GLP-1 may be decreased during T2DM. The effect of LSG on GLP-1 currently prefers the ability of LSG to increase GLP-1 Levels in the early postoperative period. It may alter glucose homeostasis and help cure T2DM by boosting intestinal L-cell GLP-1 production and promoting GLP-1 signaling in pancreatic β cells. However, it remains controversial why SG produces the same surgical effect in mice, even without GLP-1R. Therefore, further studies are required to determine how GLP-1 influences glucose metabolism in T2DM after LSG.
GLP-2 consists of 33 amino acids and is encoded at the carboxyl terminus of the GLP-1 sequence in the glucagon gene. Like GLP-1, it is predominantly produced by enteroendocrine L cells in the ileum and large intestine[91]. It is produced in response to food stimulation in the gut, and GLP-2 is primarily responsible for inhibiting gastrointestinal motility and intestinal nutrition (enhancement of intestinal growth, digestion, absorption, barrier function, and blood flow)[92]. Due to its distinct intestinal nutrition effects, the use of GLP-2 analogs for the treatment of intestinal failure can markedly reduce the frequency of required parenteral nourishment[93]. GLP-2 contributes to preserving the energy balance, and in particular, it promotes nutritional absorption in the gastrointestinal system; this is achieved not only by enterotropic action but also by decelerating gastrointestinal motility, which extend the duration of nutrient digestion and absorption. Intriguingly, GLP-2 is a peptide hormone that has been associated with anorexia[94]. Its receptor, GLP-2R, is expressed in the brainstem, hippocampus, and hypo
Furthermore, mice with a specific GLP-2R deficiency in proopiomelanocortin neurons show increased plasma insulin and hepatic glucose production as well as glucose intolerance[97]. Moreover, endo
Following food ingestion, endocrine K cells in the crypt-villi axis produce glucose-dependent insulinotropic polypeptide (GIP), a protein comprising 42 amino acids. This hormone was originally designated gastric inhibitory polypeptide because of its capacity to reduce stomach secretion and motility[102]. However, GIP was then identified as an incretin hormone capable of enhancing glucose-dependent insulin secretion from pancreatic β cells and thus received its current designation[103]. GIP exerts two functions. As a sister hormone of GLP-1, GIP exerts the same proinsulin action, and the loss of effects of entero-functional insulin is the primary cause of poor postprandial glycemic control in T2DM[104]. GIP agonists have been developed for the treatment of T2DM and obesity[105]; however, it is crucial to note that GIP agonists do not effectively reduce blood sugar levels in T2DM; nevertheless, when coupled with GLP-1 and GIP agonists, their benefits are significantly larger than those of GLP-1 alone[106]. GIP, by contrast, may influence the distribution of fat in adipose and non-adipose tissues, causing ectopic fat deposition and stimulating the accumulation of visceral and hepatic fat[107]. The major source of circulating non-esterified fatty acids is visceral fat, and a persistent increase in these acids is linked to the development of IR and T2DM[108]. Additionally, inflammation of pro-inflammatory adipokines and adipose tissue may be exacerbated by GIP[109]. Excessive GIP production contribute to the deve
Gastrin is produced in the G cells of the gastric sinus and duodenum, and it is released in response to stimulation by the vagus nerve and gastrin-releasing peptide[114]. This hormone family comprises numerous peptides, with varying levels of biological activity and lengths[115]. The primary roles of gastrin include inducing gastric acid production in the stomach via a Ca-dependent release mechanism, acting on intestinal chromophobic cells in the fundus to trigger histamine release, stimulating the development and motility of the gastric mucosa, and suppressing hunger[116]. Recent studies focused on the relationship between gastrin and the onset and progression of gastrointestinal cancers, particularly neuroendocrine tumors[117]. IR and abdominal obesity are correlated with low gastrin levels[118]. Gastrin and GLP-1 dual agonists exert immunomodulatory effects that enhance insulin levels and β-cell mass in non-obese diabetic mice, eventually improving glycemic control. Furthermore, in individuals with T2DM, the addition of proton pump inhibitors (PPI) to glucose-lowering medications markedly raised gastrin levels, enhanced β cell activity, and reduced HbA1c levels[119-121]. A trend towards increased gastrin secretion after SG was observed in female patients who had undergone BS compared to patients receiving a protein-rich meal mix. However, no statistically significant difference was observed, while gastrin was significantly lower after RYGB. Notably, a negative correlation occurred between gastrin secretion and glucose levels after SG[118]. Grong et al[122] found that SG had superior effects in inducing hypergastrinemia, lowering HbA1c, and improving glycemic control in a GK rat model. In a subsequent study, the authors assessed the -cell mass in GK rats using three-dimensional optical projection tomography, showing that -cell mass was maximally preserved after SG, which may be related to high gastrin levels and long-term improvement in glycemic parameters following surgery[123]. Grong et al[124] also suggested the presence of circulating high gastrin in GK rats after SG. However, this was similar to the result after PPI intervention, with no difference in glycemic control between the two groups, and SG did not improve β cell mass. Few human studies on gastrin changes after SG are available, and current evidence suggests the presence of high gastrin levels after SG, which may have a positive effect on glycemic control in T2DM; however, the precise mechanisms involved are unclear. In general, the results are inconsistent as to whether high gastrin improves β cell quality.
At least 22 protein family members of fibroblast growth factors (FGFs) are associated with angiogenesis, wound healing, metabolic control, and cell growth, development, and migration differentiation[125]. The majority of these work as paracrine or autocrine factors. FGF19, FGF21, and FGF23 are hormone-like members of the FGF family and have certain structural characteristics that facilitate endocrine effects[126]. FGF19 is produced in the brain, gallbladder, and distal small intestine. It inhibits hunger and regulates BA and nutrition metabolism, glucose and lipid metabolism, energy expenditure, and obesity[127]. FGF21 controls lipid and carbohydrate metabolism, elicits white adipose tissue (WAT) thermogenesis and browning, indirectly increases insulin synthesis in the pancreas, improves insulin sensitivity, and decreases food intake[128]. FGF23 is a hormone produced by osteoblasts and osteoclasts in the skeleton and is primarily involved in mineral metabolism to control phosphate levels[129]. According to several studies, there is a significant increase in FGF19 following SG, and this increase is linked to better glycemic control and reduced systemic inflammation[130-132]. Yang et al[133] observed an increase in FGF19 in VSG but no changes in RYGB. A meta-analysis revealed an increase in FGF19 and a negative correlation between FGF19 and BMI after SG[134]. Huang et al[135] noted that higher FGF19 Levels and reduced BA levels after SG may play a role in T2DM remission and NAFLD improvement; they also hypothesized that low preoperative FGF 19 Levels may predict improvement of NAFLD.
With respect to FGF21, Khan et al[136] found a link between elevated FGF21 and weight loss after SG, indicating that FGF21 may play a part in the postoperative energy balance. By contrast, Nielsen et al[137] did not detect changes in FGF21 after SG, and FGF21 Levels were not related with food choice. FGF19 Levels were decreased and FGF21 Levels were increased in obese patients, and FGF21 Levels further increased when obese patients showed T2DM. SG increased FGF19 Levels while decreasing the unnaturally increased FGF21 Levels. The authors concluded that FGF19 Levels were mostly related to physical obesity, particularly visceral obesity, whereas those of FGF21 were primarily linked to glucose homeostasis[138]. Yen et al[139] confirmed this and further observed a substantial decrease in FGF21 Levels after SG and a strong positive association between FGF21 and C-peptide, insulin, and the homeostasis model evaluation of the postoperative IR index.
In conclusion, the available studies are in line with our findings that FGF19 is typically elevated in the postoperative period and that it may control the release of BAs to produce its effects. The elevation of FGF19 after SG is not specifically correlated with T2DM but is linked to a decrease in the body weight index. Contrarily, FGF21, which is frequently increased in obese patients with T2DM, has an independent function in obesity and is linked to metabolic syndrome, hyperinsulinemia, onset of diabetes, aberrant glucose metabolism, and IR[140]. Due to its potential to ameliorate the FGF21 increase induced by obesity or T2DM, SG may play a significant part in preserving glucose homeostasis. FGF21 should be further studied, and it may be a more important metabolic marker of illness in T2DM than FGF19.
Overall, the control of different components of the gut-brain axis, the gut-adipose tissue axis, the gut-liver axis, the gut-pancreatic axis, and the gut-muscle axis all play a role in the overall complexity of the gastrointestinal hormonal alterations after LSG. The surgical method used in RYGB (partial removal of the small intestine and stomach) may explain endocrine differences between LSG and RYGB; this also suggests that the two treatments affect T2DM differently because of such discrepancies. Although the benefits and drawbacks of the two approaches are not entirely clear, one may infer from the few available data that the potential of LSG ability to relieve T2DM is connected to GHs, which may result from systemic rather than specific hormonal alterations.
Adipose tissue is divided into WAT and brown adipose tissue (BAT), classically considered a long-term storage organ that releases free fatty acids to meet the body's energy requirements during fasting or thermoregulation and has a mechanical protective impact on internal organs[141,142]. According to current studies, adipose tissue is one of the major endocrine organs in the body and plays a significant role in systemic homeostasis[143]. Adipocytes are metabolically active, and they are effective secretory cells that can release large quantities of adipokines. Adipokines may influence several biological processes, including appetite regulation, inflammatory and immune functions, glucose and lipid metabolism, cardiovascular homeostasis and reproduction, and other essential physiological processes[144]. This review focuses on T2DM and obesity; hence, other physiological functions will not be described in any great detail. Leptin, adiponectin, resistin, and vaspin are adipokines associated with glucose metabolism. Insulin sensitivity is linked to leptin, adiponectin, chemerin, and omentin, whereas IR is associated with apeline and nesfatin-1. By contrast, leptin and vaspin are also important in controlling appetite[145,146]. As a result, T2DM and adipokine changes are tightly associated in obese people. Below, we provide more details on how LSG affects specific adipokine metabolism processes and its potential impact on T2DM and also summarize the approximate mechanism in Figure 1.
Leptin has a tertiary structure of a globular protein, comprising 167 amino acids. It is predominantly synthesized in white adipose tissue, and primarily acts on trans-modal receptors to exert its effects[147]. Food consumption, systemic adiposity, and hormones affect the amount of leptin that is secreted, with insulin playing a significant regulatory role[148]. Prolonged hyperinsulinemia leads to an increase in circulating leptin concentration[149]. Considering the IR status of obese patients, high leptin levels are likewise a characteristic of obesity. Leptin thus controls hunger, satiety, food intake, and energy use[150].
Meanwhile, it may play an insulin-sensitizing role and is an important regulator of β cell mass and survival. Recombinant leptin has been established for obesity treatment based on its various important physiological roles. However, little progress has been made, which may be due to long-term leptin-resistance during obesity[151]. When such resistance is reduced, recombinant leptin treatment produces effective weight reduction and glycemic control[152]. Thus, studying the alterations in leptin that occur after LSG and how they affect T2DM and obesity is crucial. Numerous studies have produced similar findings, and the impact of LSG on leptin is generally beneficial, with a discernible decrease in leptin levels after surgery that remained throughout long-term follow-up[33,34,153]. Mazahreh et al[154] concluded that LSG increased the expression level of leptin receptors, which alleviated leptin resistance. Leptin levels and IR were correlated in patients, and pre-LSG leptin levels were predictive of IR, according to Hany et al[155]. Additionally, Arble et al[156] also reported that SG improves ventilatory drive in patients with sleep apnea through a leptin-dependent mechanism. Stoica et al[35] showed that SG decreased leptin expression in mice. Similarly, Du et al[157] discovered that SG lowered leptin expression in HFD-fed mice, which caused translocation of glucose transporter protein 2; resulting in inhibition of intestinal glucose absorption. In leptin receptor-knockout mice, long-term weight reduction following SG was shown to require the action of leptin; however, the improvement in blood glucose does not seem to depend on leptin. The authors concluded that a significant improvement in blood glucose caused by SG through enhanced insulin sensitivity, independent of reduced feeding and weight loss[158]. LSG has a well-documented impact on lowering circulating leptin levels and enhancing leptin resistance, and these beneficial effects have been linked to several healthful physiological processes. However, it remains controversial whether changes in leptin levels have beneficial effects on glucose metabolism in T2DM, which may be involved partly by reducing glucose uptake and improving IR, among other effects. The role of leptin in this process is not all or nothing, but good or better.
WAT secretes adiponectin, one of the most prevalent adipokines in the bloodstream of humans[159]. As a secreted protein, it functions by interacting with the cell membrane receptors adiponectin receptor (AdipoR) 1 and AdipoR2. AdipoR1 is primarily expressed in liver and skeletal muscle tissue, and AdipoR2 is predominantly expressed in the liver[160]. Adiponectin increases skeletal muscle glucose absorption and fatty acid oxidation, thus inhibiting gluconeogenesis in the liver[161,162]. Additionally, adiponectin has anti-diabetic properties and activates the AMP-activated protein kinase (AMPK) pathway, which interacts with the AdipoR1 receptor to elicit insulin sensitization[163]. Furthermore, lipocalin exerts anti-inflammatory effects, it is linked to the onset of atherosclerosis, and it effectively inhibits the activation of the nuclear transcription factor-kappa B (NF-kB) pathway and production of the NF-kB nuclear protein p65[164]. Obese patients with T2DM exhibit reduced adiponectin levels which are associated with increased expression of pro-inflammatory cytokines; this may also be associated with low-grade chronic inflammation[165]. According to previous studies, increasing the amount of lipocalin in the blood would be a viable therapeutic approach to treat disorders caused by obesity. Thiazolidinediones, which act as peroxisome proliferator-activated receptor γ (PPAR-γ) agonists, may raise adiponectin levels and successfully regulate blood sugar. However, their applicability is more constrained owing to lower safety (with adverse side effect including hepatotoxicity, heart failure, edema, and reduced bone density)[166]. Lopez-Nava et al[167] reported increased adiponectin levels after LSG, no equivalent changes were seen after endoscopic SG, and patients exhibited increased weight loss following LSG. Rafey et al[168] obtained similar results with increased circulating adiponectin after LSG, and the authors suggested that the leptin-to-adiponectin ratio was correlated with improved insulin sensitivity and weight loss, and that this ratio decreased significantly after surgery. Šebunova et al[169] took an identical perspective: Adiponectin levels increased after BS, however, the authors did not distinguish between various surgical techniques. In GK rats, SG increased serum adiponectin and adipose tissue PPAR-γ expression, decreased IR, and enhanced adipose tissue health and angiogenesis[170]. Adiponectin may have a role in improving glucolipid metabolism and delaying the development of T2DM in UCD-T2DM mice when SG is performed[100]. In addition, a combination of SG and partial small bowel resection resulted in elevated adiponectin levels, which may contribute to improved glucose homeostasis[171]. Adiponectin exerts a significant role in glucose metabolism, whether in patients with T2DM, obesity, or both. Elevating the circulating adiponectin levels through medication seems to be an effective option; however, this treatment modality should be considered with caution regarding the aspect of safety. The effect of LSG on adiponectin is currently presumed consistent, with a postoperative increase, which may be one of the mechanisms by which LSG can help treat T2DM and obesity. Risks and safety of LSG are manageable for specialist weight loss metabolic surgeons, which is one of its advantages over established pharmacological approaches.
Apelin is a late-discovered adipokine peptide with multiple active isoforms. Its receptor, apelin-angiotensin receptor-like (APJ), is an extensively distributed G protein-coupled receptor[172]. Various tissues and cells in the human body contain apelin/APJ, which perform various physiological tasks, including controlling food intake, cell proliferation, and angiogenesis[173]. Apelin is recognized as a helpful adipokine and, like adiponectin, is thought to be an insulin sensitizer[174]. Exogenous apelin supplementation is still beneficial for IR and for the glucose metabolism, even when endogenous apelin levels are high in obese patients and those with T2DM[175]. Exogenous apelin has been shown to improve insulinotropic activity, adipocyte glucose absorption, and insulin release in obese mice, and it is similarly beneficial in human patients[176,177]. Soriguer et al[175] reported a significant decrease in apelin levels in morbidly obese patients with impaired fasting glucose or T2DM due to BS. Apelin levels were significantly positively correlated with changes in serum glucose and negatively correlated with insulin sensitivity. Arica et al[178] observed that laparoscopic gastric banding reduced elevated apelin levels in obese morbidly obese patients. However, we were unable to identify studies on the effects of LSG on apelin. As a novel therapeutic target and important biomarker for metabolic illnesses, including diabetes and obesity, the apelin/APJ signaling pathway has recently attracted attention. However, few studies on apelin and BS are available, and they suggest that apelin levels decrease postoperatively, which seems to be disadvantageous.
The novel adipokine nesfatin-1 is not only released by adipose tissue, but its synthesis and secretion have also been observed in central nervous tissues including the hypothalamus[179]. So far, the nesfatin-1 receptor remains unknown; however, specific binding sites have been found in the central nervous system, gastrointestinal tract, and pancreas[180]. Nesfatin-1 is considered an efficient anorexigenic peptide with regulatory effects on energy metabolism through reducing food intake[181]. Nesfatin-1 expression is lower in obese people, and its levels are negatively correlated with body mass index, weight, and adiposity[182]. Similar observations were made in T2DM patients, whose nesfatin-1 Levels were lower than those of healthy subjects or T1DM patients[183]. Nesfatin-1 stimulates insulin secretion, increases proinsulinogen mRNA expression, and has antihyperglycemic effects during glucose metabolism[184]. A previous study showed that supplementation with exogenous nesfatin-1 elicited resistance to hyperglycemia in mice, suggesting that nesfatin-1 may be a potential therapeutic target for T2DM[185]. According to several studies, LSG raises postoperative nesfatin-1 Levels in patients. Nesfatin-1 has been linked to a reduction in postoperative appetite, according to Dogan et al[186], whereas Yang et al[187] observed a link between nesfatin-1 and NAFLD. Lee et al[188] demonstrated that nesfatin-1 decreased after SG or RYGB, and they proposed a link between nesfatin-1 and glycemic control.
In contrast, Majorczyk et al[189] came to the exact opposite conclusion, suggesting that LSG decreases nesfatin-1 Levels and that there is no significant correlation between nesfatin-1 and improvement in body weight or glucose metabolism. There is a controversy with regard to LSG's impact on nesfatin-1, with starkly contrasting opinions. The correlation between nesfatin-1 and weight, appetite, and hepatic steatosis after LSG has been demonstrated, however, only one study has shown a correlation between nesfatin-1 and glycemic control after LSG. Thus, nesfatin-1 may play a minor role in the LSG-mediated remission of T2DM.
Resistin is a specific adipokine specifically expressed and secreted by adipose tissue[190]. Its effects involve endocrine, autocrine, and paracrine mechanisms, however, its receptor is unknown[191]. Resistin is considered a connection between obesity and T2DM as it reportedly opposes the action of insulin and interferes with glucose homeostasis in vivo, which results in the progress of T2DM[192]. Resistin is also a pro-inflammatory regulator of macrophages, peripheral blood mononuclear cells, and vascular cells, with pro-inflammatory actions and higher expression during pathological states of inflammation, according to recent studies[193,194]. Resistin levels were positively correlated with IR in T2DM patients with hyperresistinemia and in obese people, according to a meta-analysis of 20 studies. However, no such association was found in patients with normal resistin levels[195]. A study showed that leptin and resistin levels decreased following LSG, and liver histopathology results improved[196]. Similar observations were made in a different study, which concluded that weight reduction after LSG was associated with altered levels of anti-inflammatory adipokines and better glucose metabolism[197]. Šebunova et al[169] observed that resistin was markedly higher after LSG than after RYGB, however, the decrease from the preoperative period was not significant. Farey et al[198] found that postoperative resistin levels exhibited a reducing trend which was not statistically significant, and that resistin levels of obese patients were lower than those of non-obese controls.
Additionally, a meta-analysis revealed that weight reduction surgery had no pronounced impact on resistin levels[199]. Presently available studies seem not to support the hypothesis that LSG regulates resistin levels to facilitate T2DM remission. However, the various limitations of such studies should be considered, particularly with regard to small sample sizes and the fact that resistin is not consistently highly expressed in obese people. Further research is required to determine whether preoperative resistin levels are generally within a normal range to more accurately assess its impact on T2DM.
Chemerin was found to be highly expressed in human WAT in 2007. Chemerin is a novel adipokine that binds to the orphan G protein-coupled receptors chemokine-like receptor 1, chemokine receptor-like 2, and G protein-coupled receptor 1 to exert its potential autocrine and paracrine effects[200,201]. It may have a role in energy balance and metabolism in vivo and is linked to adult obesity, T2DM, and metabolic syndrome, according to recent research[202]. Most respective studies found that people with poor glucose homeostasis had higher serum chemerin levels and that this increase was inversely linked with glycemic control parameters[203]. A meta-analysis suggested a marked decline in chemerin levels after BS, however, various surgical methods were not distinguished[199]. Terra et al[153] reported a significant decrease in chemerin 12 mo after LSG, compared with the baseline levels, in a pattern similar to that after RYGB. Similar findings were reported by Jouan et al[204], who discovered a decrease in chemerin after surgery and suggested that chemerin may be utilized as a predictor of a postoperative inflammation; however, the changes in chemerin after LSG were not uniform. The findings of Cӑtoi et al[205] did not reveal any significant differences in chemerin six months after LSG. Chemerin is a relatively novel adipokine; thus, little information is available, and most conclusions originate from meta-analyses. Fundamental research is thus required to understand the mechanisms of action of chemerin acts, particularly with regard to T2DM. The limited available data do not support a link between chemerin and improved glucose metabolism after LSG.
Omentin-1 is the primary circulating form of omentin, also referred to as intelectin-1, which is mainly expressed in visceral adipose tissue and exerts endocrine effects resembling those of hormones[206]. Omentin-1 increases insulin sensitivity, which is key in maintaining the body's metabolism. In addition, it also has anti-inflammatory properties through the intracellular Akt/AMPK/NF-B and mitogen-activated protein kinase signaling pathways[207]. Glucose/insulin and FGF21 affect how omentin-1 is regulated, with glucose/insulin decreasing its expression and secretion and FGF21 increasing it[208,209]. Omentin-1 expression profiles of obese and T2DM patients showed that its expression and secretion were suppressed in patients suffering from obesity[210], T1DM[211], T2DM[212], and metabolic syndrome. In addition, the chromosomal area of omentin-1 is linked to T2DM in certain groups. Thus, this gene may be associated with T2DM susceptibility[213]. Increased circulating omentin-1 Levels and decreased fecal omentin mRNA after LSG may contribute to surgery-induced metabolic improvement and weight reduction[214]. Sdralis et al[215] proposed that LSG combined with omentotomy reduced the expression of omentin-1, but LSG alone increased it, and a low-calorie diet had no significant effect on omentin-1. The pattern of omentin-1 expression after LSG is intriguing, however, as omentin-1 is influenced by glucose/insulin and FGF21, it is unclear whether the reduction in blood glucose under T2DM remission would prevent the inhibition of omentin-1, causing it to increase, or whether the higher omentin-1 Levels affected T2DM remission. Omentin-1-based medication may be an emerging option for treating obesity and T2DM, considering the link between omentin-1 and IR. However, the mechanisms of action of omentin-1 during surgical operations are unclear.
Visceral fat secretes the adipokine visfatin, which has effects similar to those of insulin[216]. Visfatin interacts with insulin receptors during gluconeogenesis to increase glucose absorption in liver and muscle tissue, thus lowering blood sugar levels[217]. Further, it supports the effects of insulin by causing the phosphorylation of insulin receptors 1 and 2[218]. Additionally, the autocrine activity of visfatin in the liver enhances insulin sensitivity[219], and it also works on the hypothalamus in the center to influence insulin release and reduce IR[220]. According to studies, visfatin contributes to IR and T2DM in a dose-dependent manner, and obese patients with T2DM showed higher intraserum levels of visfatin than obese patients without T2DM[221]. However, only few studies could be identified that examined how LSG affected visfatin, one of which found no evidence of a substantial change in visfatin after LSG[222]. Similar conclusions were drawn in a meta-analysis, which showed that BS had no marked impact on visfatin expression or secretion[195]. Animal experiments produced similar results[223]. Visfatin has a beneficial effect on T2DM or decreased glucose tolerance because of its insulin-like activity. However, uncertainty remains regarding how LSG affects visfatin levels and how visfatin contributes to T2DM remission following LSG.
Retinol binding protein 4 (RBP4) is an adipokine secreted by WAT. The primary function is to transport retinol, the active metabolite of vitamin A, from the liver to target tissues. High levels of RBP4 are associated with developing metabolic diseases such as obesity, IR, metabolic syndrome, and T2DM[224]. In obesity, abnormal levels of RBP4 produce both local and systemic effects (retinol homeostasis and transport in vivo)[225]. It exacerbates the inflammatory state in obesity in vivo by activating Toll-like receptor (TLR) 2 and TLR4/myeloid differentiation protein 2 receptor complexes in macrophages[226]. In T2DM, RBP4 is associated with IR and the progression of several T2DM co-morbidities, such as diabetic nephropathy and diabetic retinopathy[227]. Whether RBP4 is elevated in obesity is controversial, as Yang et al[228] found higher serum RBP4 Levels in obese individuals than in lean individuals. However, similar alterations were not found in the study by Korek et al[229] What is certain is that there is a correlation between elevated blood RBP4 Levels and the incidence of IR, serum lipid levels, and anthropometric parameters[224]. Wang et al[230] reported a significant decrease in RBP4 after LSG and concluded that RBP4 Levels positively correlated with BMI, glucose, fasting C-peptide, and HOMA-IR. In another study, the authors found that RBP4 decreased after LSG in children and adolescents[231]. However, some studies have also shown that LSG did not significantly affect RBP4 Levels[232,233]. In addition, Jüllig et al[234] found that RBP4 decreased more in patients after RYGB than after LSG. Fewer studies have been conducted on the effect of LSG on RBP4, and only sporadic studies have been reported; therefore, it is impossible to determine the changes involved. However, it is worth affirming that RBP4, as a specific adipokine, plays an important role in T2DM, and targeting RBP4 may become a potential therapeutic strategy.
The human gut contains a unique variety of microbes, commonly known as the GM, which comprises approximately 3 million non-redundant microbial genes[235]. The GM may impact host metabolic functions, such as energy generation, steroid hormone synthesis, and bile salt metabolism, and they are intricately related to the development of metabolic diseases[236]. By increasing energy absorption from food, alterations in the GM, in particular, plays a significant role in the onset and progression of obesity and T2DM[237]. In obese people, the GM exhibits particular traits, including altered microbial gene abundance and ecological dysregulation which is linked to inflammation, increased body weight and fat mass, and T2DM[238]. Therefore, modifying the GM may be an option for treating T2DM and obesity. Studies have demonstrated that oral administration of improved GM to rats with metabolic syndrome increased insulin sensitivity[239]. Whether SG causes specific changes in the GM that contribute to improving metabolic disorders remains unclear. Tabasi et al[240] observed changes in the diversity and composition of the GM three months after LSG, and long-term follow-up studies showed that most changes remained for one year after surgery, indicating that SG elicits rapid and sustainable changes[241]. The alterations in GM due to RYGB and SG were varied, with RYGB increasing the relative abundances of the phyla Firmicutes and Actinobacteria but reducing those of Bacteroidetes, whereas SG increased Bacteroidetes abundances. Of note, Roseburia species abundance was increased in all patients who achieved T2DM remission, which was common to SG and RYGB[241]. Changes in GM after LSG occur universally, which has been validated in several studies[242,243]. This contributes to the various concerns regarding the degree to which the GM may impact the outcome of LSG and whether specific changes in the particular flora play a dominant role in improving T2DM or obesity. Surgery based on changed GM or fecal transplantation therapy may open new avenues for treating T2DM and obesity.
BAs are planar amphiphilic molecules with a carboxyl tail that are generated in the liver[244]. Diet regulates the synthesis, secretion, and circulation of BAs. In addition to the typical role of lipid absorption, BAs operate as signaling chemicals through two key receptors, i.e., Farnesoid X receptor (FXR) and Tekeda-G-protein receptor 5 (TGR5)[245]. The hepatic-intestinal cycle occurs when BAs are released into the duodenum after eating, and most of them are reabsorbed and transported back to the liver after they reach the ileum[246]. Current studies showed that BAs play a significant function in controlling lipid, glucose, and energy metabolism and that obesity and T2DM are associated with dysregulated BAs homeostasis in vivo[247]. Most respective studies confirmed that BAs alterations are similar in obese, T2DM, and IR patients, who show higher fasting BA levels than healthy controls[248]. However, this variation is not uniform, and many studies concluded that BA levels are not significantly altered[249]. The effect of LSG on BA levels is also somewhat controversial. Yang et al[133] revealed that BA levels exhibited a transitory decrease following LSG and thereafter a progressive increase. In contrast, following RYGB, BA levels show a consistently increasing trend. While Eiken et al[250] discovered higher BA concentrations after RYGB, increased inflow of BAs into the small intestine and more rapid release, this did not occur after LSG. Cӑtoi et al[251] examined the relationship between IR and BAs after LSG and found no significant changes in BA levels and HOMA-IR in the very early period (1 wk) after surgery. However, one month postoperatively, total BA levels increased, HOMA-IR decreased, and there was a negative correlation between them. In a different study, there was a link between higher BAs levels and better-glycated hemoglobin. Fasting and postprandial levels of total, secondary, and unconjugated BAs were higher after LSG[130]. Wang et al[252] discovered that after SG, total BA levels increased, and the fraction of 12-hydroxylated BAs was reduced in a diabetic rat model. This alteration may be fundamental to improved insulin sensitivity after SG. There are some differences between RYGB and LSG with regard to changes in total BAs after BS. One possible explanation for these differences is that RYBG entails changes in the structure of the gastrointestinal tract that affect the hepatic-intestinal circulation of BAs, whereas LSG does not. LSG and total blood BA levels and BA composition are unarguably linked; however, further research is required to help understand how certain BA species affect postoperative variations in LSG.
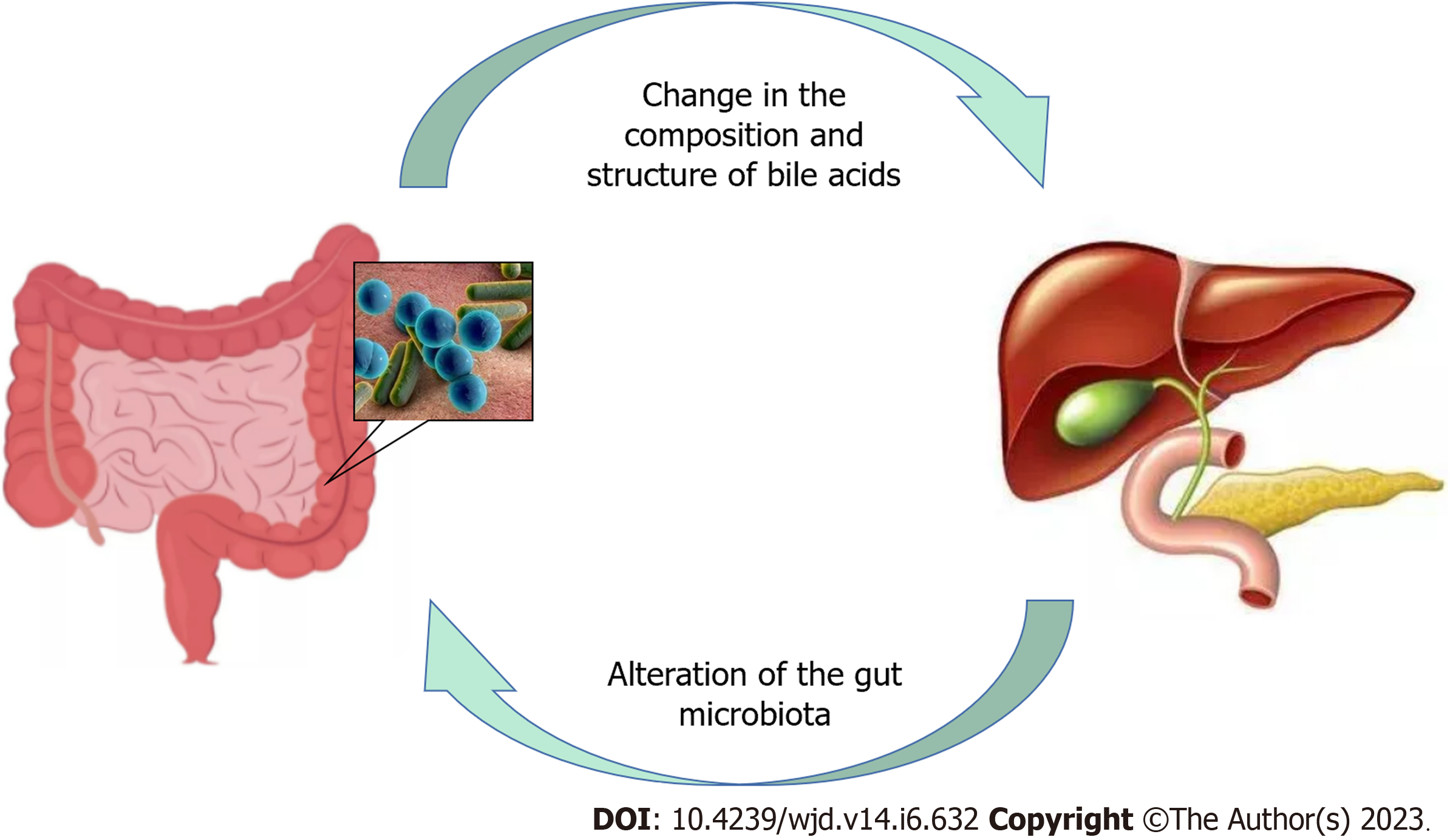
BAs and the GM interact in both directions (Figure 2). In the distal small intestine and colon, where most of the GM occurs, hydroxylation and dihydroxylation occur, through which the GM regulates the composition of BAs and controls the generation of secondary BAs[253]. By modifying the composition structure of BAs, the GM may further regulate FXR and TGR5 functions[254]. Biological agents that affect the GM can alter the BA profile[255], and BAs can affect the GM due to their antimicrobial effects and impact on intestinal mucosal integrity[256]. In conclusion, elucidating the relationship between BAs and the GM may provide a better understanding of the variability in weight reduction and enhanced glucose metabolism between RYGB and LSG. The stronger influence of RYGB on the GM owing to changed physiological channels induces alterations in BAs, whereas this effect is apparently minor after LSG.
LSG is an effective therapy option for the worrying pandemic of obesity and T2DM. LSG entails several therapeutic mechanisms that enhance glucose homeostasis and IR without relying on weight reduction. The gut-brain, gut-adipose tissue, gut-hepatic, gut-pancreatic, and gut-muscle axes are some of these putative entities. These insights may provide novel avenues for T2DM treatment targets focused on the gut. Overall, the understanding of how LSG works to treat T2DM has considerably advanced, however, further research is required. Additionally, while obese and T2DM patients may benefit from LSG, some hazards must be carefully considered, such as higher levels of certain GHs that may cause postprandial hyperinsulinemic hypoglycemia and decreased appetite, leading to malnutrition in non-overweight individuals.
Fa-Shun Liu would like to express his gratitude to his wife Yan-Wen Ran for the support she provided.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beg MMA, Kyrgyzstan; Ghannam WM, Egypt S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 2966] [Article Influence: 494.3] [Reference Citation Analysis (0)] |
| 2. | Lin X, Li H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front Endocrinol (Lausanne). 2021;12:706978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 592] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 3. | Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL, GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5060] [Article Influence: 632.5] [Reference Citation Analysis (2)] |
| 4. | Ataey A, Jafarvand E, Adham D, Moradi-Asl E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J Prev Med Public Health. 2020;53:98-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2895] [Cited by in RCA: 2948] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 6. | Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1385] [Article Influence: 277.0] [Reference Citation Analysis (0)] |
| 7. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1646] [Article Influence: 411.5] [Reference Citation Analysis (2)] |
| 8. | Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1445] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 9. | Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE; Delegates of the 2nd Diabetes Surgery Summit. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Surg Obes Relat Dis. 2016;12:1144-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Pollard S. The current status of bariatric surgery. Frontline Gastroenterol. 2011;2:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Mason EE. History of obesity surgery. Surg Obes Relat Dis. 2005;1:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 14. | Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, Simard S. Biliopancreatic Diversion with a New Type of Gastrectomy. Obes Surg. 1993;3:29-35.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 215] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Sandoval DA, Patti ME. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol. 2023;19:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 16. | Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, Koh ZJ, Chew CA, Loo YE, Tai BC, Kim G, So JB, Kaplan LM, Dixon JB, Shabbir A. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397:1830-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 298] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 17. | Sakran N, Soifer K, Hod K, Sherf-Dagan S, Soued S, Kessler Y, Adelson D, Biton R, Buchwald JN, Goitein D, Raziel A. Long-term Reported Outcomes Following Primary Laparoscopic Sleeve Gastrectomy. Obes Surg. 2023;33:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Guraya SY, Strate T. Surgical outcome of laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass for resolution of type 2 diabetes mellitus: A systematic review and meta-analysis. World J Gastroenterol. 2020;26:865-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 19. | Solé T, Januel L, Denneval A, Williet N, Breton C, Blanc P, Ollier E. Time impact on the antidiabetic effects of key bariatric surgeries: a network meta-analysis of randomized controlled trials with meta-regression. Surg Obes Relat Dis. 2022;18:832-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ding L, Fan Y, Li H, Zhang Y, Qi D, Tang S, Cui J, He Q, Zhuo C, Liu M. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: A network meta-analysis of randomized controlled trials. Obes Rev. 2020;21:e13030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA. 2020;324:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 693] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 22. | Docherty NG, le Roux CW. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat Rev Nephrol. 2020;16:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | English WJ, DeMaria EJ, Hutter MM, Kothari SN, Mattar SG, Brethauer SA, Morton JM. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 24. | Jin ZL, Liu W. Progress in treatment of type 2 diabetes by bariatric surgery. World J Diabetes. 2021;12:1187-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Parikh M, Chung M, Sheth S, McMacken M, Zahra T, Saunders JK, Ude-Welcome A, Dunn V, Ogedegbe G, Schmidt AM, Pachter HL. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg. 2014;260:617-22; discussion 622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Santiago-Fernández C, García-Serrano S, Tome M, Valdes S, Ocaña-Wilhelmi L, Rodríguez-Cañete A, Tinahones FJ, García-Fuentes E, Garrido-Sánchez L. Ghrelin levels could be involved in the improvement of insulin resistance after bariatric surgery. Endocrinol Diabetes Nutr. 2017;64:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D'Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JO, Schmitz O. Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab. 2008;93:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1283] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 31. | Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 269] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Morpurgo PS, Resnik M, Agosti F, Cappiello V, Sartorio A, Spada A. Ghrelin secretion in severely obese subjects before and after a 3-week integrated body mass reduction program. J Endocrinol Invest. 2003;26:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Kalinowski P, Paluszkiewicz R, Wróblewski T, Remiszewski P, Grodzicki M, Bartoszewicz Z, Krawczyk M. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass-results of a randomized clinical trial. Surg Obes Relat Dis. 2017;13:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Salman MA, El-Ghobary M, Soliman A, El Sherbiny M, Abouelregal TE, Albitar A, Abdallah A, Mikhail HMS, Nafea MA, Sultan AAEA, Elshafey HE, Shaaban HE, Azzam A, GabAllah GMK, Salman AA. Long-Term Changes in Leptin, Chemerin, and Ghrelin Levels Following Roux-en-Y Gastric Bypass and Laparoscopic Sleeve Gastrectomy. Obes Surg. 2020;30:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Stoica L, Gadea R, Navolan DB, Lazar F, Duta C, Stoian D, Tarta C, Olaru F, Isaic A, Dobrescu A. Plasma ghrelin, adiponectin and leptin levels in obese rats with type 2 diabetes mellitus after sleeve gastrectomy and gastric plication. Exp Ther Med. 2021;21:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Skuratovskaia D, Vulf M, Chasovskikh N, Komar A, Kirienkova E, Shunkin E, Zatolokin P, Litvinova L. The Links of Ghrelin to Incretins, Insulin, Glucagon, and Leptin After Bariatric Surgery. Front Genet. 2021;12:612501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144:50-52.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Wynne K, Bloom SR. The role of oxyntomodulin and peptide tyrosine-tyrosine (PYY) in appetite control. Nat Clin Pract Endocrinol Metab. 2006;2:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Sam AH, Gunner DJ, King A, Persaud SJ, Brooks L, Hostomska K, Ford HE, Liu B, Ghatei MA, Bloom SR, Bewick GA. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology. 2012;143:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract. 2017;3:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 41. | Arakawa R, Febres G, Cheng B, Krikhely A, Bessler M, Korner J. Prospective study of gut hormone and metabolic changes after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. PLoS One. 2020;15:e0236133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Alamuddin N, Vetter ML, Ahima RS, Hesson L, Ritter S, Minnick A, Faulconbridge LF, Allison KC, Sarwer DB, Chittams J, Williams NN, Hayes MR, Loughead JW, Gur R, Wadden TA. Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-en-Y-Gastric Bypass: an 18-Month Prospective Study. Obes Surg. 2017;27:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 44. | Kowalka AM, Alexiadou K, Cuenco J, Clarke RE, Minnion J, Williams EL, Bech P, Purkayastha S, Ahmed AR, Takats Z, Whitwell HJ, Romero MG, Bloom SR, Camuzeaux S, Lewis MR, Khoo B, Tan TM. The postprandial secretion of peptide YY(1-36) and (3-36) in obesity is differentially increased after gastric bypass versus sleeve gastrectomy. Clin Endocrinol (Oxf). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Eickhoff H, Louro TM, Matafome PN, Vasconcelos F, Seiça RM, Castro E Sousa F. Amelioration of glycemic control by sleeve gastrectomy and gastric bypass in a lean animal model of type 2 diabetes: restoration of gut hormone profile. Obes Surg. 2015;25:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Qiu NC, Li W, Liu ME, Cen XX, Shan CX, Zhang W, Liu Q, Wang Y, Zhu YT, Qiu M. Comparison of Great Curvature Plication with Duodenal-Jejunal Bypass (GCP-DJB) and Sleeve Gastrectomy (SG) on Metabolic Indices and Gut Hormones in Type 2 Diabetes Mellitus Rats. Obes Surg. 2018;28:4014-4021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Boza C, Muñoz R, Yung E, Milone L, Gagner M. Sleeve gastrectomy with ileal transposition (SGIT) induces a significant weight loss and diabetes improvement without exclusion of the proximal intestine. J Gastrointest Surg. 2011;15:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Adamska E, Ostrowska L, Górska M, Krętowski A. The role of gastrointestinal hormones in the pathogenesis of obesity and type 2 diabetes. Prz Gastroenterol. 2014;9:69-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Holst JJ, Albrechtsen NJW, Gabe MBN, Rosenkilde MM. Oxyntomodulin: Actions and role in diabetes. Peptides. 2018;100:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 50. | Fehmann HC, Jiang J, Schweinfurth J, Wheeler MB, Boyd AE 3rd, Göke B. Stable expression of the rat GLP-I receptor in CHO cells: activation and binding characteristics utilizing GLP-I(7-36)-amide, oxyntomodulin, exendin-4, and exendin(9-39). Peptides. 1994;15:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Schjoldager BT, Baldissera FG, Mortensen PE, Holst JJ, Christiansen J. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest. 1988;18:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond). 2006;30:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 53. | Shankar SS, Shankar RR, Mixson LA, Miller DL, Pramanik B, O'Dowd AK, Williams DM, Frederick CB, Beals CR, Stoch SA, Steinberg HO, Kelley DE. Native Oxyntomodulin Has Significant Glucoregulatory Effects Independent of Weight Loss in Obese Humans With and Without Type 2 Diabetes. Diabetes. 2018;67:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Behary P, Tharakan G, Alexiadou K, Johnson N, Wewer Albrechtsen NJ, Kenkre J, Cuenco J, Hope D, Anyiam O, Choudhury S, Alessimii H, Poddar A, Minnion J, Doyle C, Frost G, Le Roux C, Purkayastha S, Moorthy K, Dhillo W, Holst JJ, Ahmed AR, Prevost AT, Bloom SR, Tan TM. Combined GLP-1, Oxyntomodulin, and Peptide YY Improves Body Weight and Glycemia in Obesity and Prediabetes/Type 2 Diabetes: A Randomized, Single-Blinded, Placebo-Controlled Study. Diabetes Care. 2019;42:1446-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Nielsen MS, Ritz C, Wewer Albrechtsen NJ, Holst JJ, le Roux CW, Sjödin A. Oxyntomodulin and Glicentin May Predict the Effect of Bariatric Surgery on Food Preferences and Weight Loss. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Perakakis N, Kokkinos A, Peradze N, Tentolouris N, Ghaly W, Pilitsi E, Upadhyay J, Alexandrou A, Mantzoros CS. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: Evidence from two independent trials. Metabolism. 2019;101:153997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 57. | Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Oliván B, Teixeira J, McGinty J, Rother KI. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4072-4076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, Holland J, Hembree J, Abplanalp W, Grant E, Ruehl J, Wilson H, Kirchner H, Lockie SH, Hofmann S, Woods SC, Nogueiras R, Pfluger PT, Perez-Tilve D, DiMarchi R, Tschöp MH. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 494] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 59. | Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, Liu F, Miller C, Tota LM, Zhou G, Zhang X, Sountis MM, Santoprete A, Capito' E, Chicchi GG, Thornberry N, Bianchi E, Pessi A, Marsh DJ, SinhaRoy R. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258-2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 60. | Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Zeng Q, Ou L, Wang W, Guo DY. Gastrin, Cholecystokinin, Signaling, and Biological Activities in Cellular Processes. Front Endocrinol (Lausanne). 2020;11:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 62. | Kim HT, Desouza AH, Umhoefer H, Han J, Anzia L, Sacotte SJ, Williams RA, Blumer JT, Bartosiak JT, Fontaine DA, Baan M, Kibbe CR, Davis DB. Cholecystokinin attenuates β-cell apoptosis in both mouse and human islets. Transl Res. 2022;243:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Rana T, Behl T, Sehgal A, Singh S, Sharma N, Abdeen A, Ibrahim SF, Mani V, Iqbal MS, Bhatia S, Abdel Daim MM, Bungau S. Exploring the role of neuropeptides in depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2022;114:110478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 64. | Hebb AL, Poulin JF, Roach SP, Zacharko RM, Drolet G. Cholecystokinin and endogenous opioid peptides: interactive influence on pain, cognition, and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1225-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J Nutr. 1994;124:1334S-1339S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Burckhardt B, Delco F, Ensinck JW, Meier R, Bauerfeind P, Aufderhaar U, Ketterer S, Gyr K, Beglinger C. Cholecystokinin is a physiological regulator of gastric acid secretion in man. Eur J Clin Invest. 1994;24:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Pathak V, Flatt PR, Irwin N. Cholecystokinin (CCK) and related adjunct peptide therapies for the treatment of obesity and type 2 diabetes. Peptides. 2018;100:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Irwin N, Hunter K, Montgomery IA, Flatt PR. Comparison of independent and combined metabolic effects of chronic treatment with (pGlu-Gln)-CCK-8 and long-acting GLP-1 and GIP mimetics in high fat-fed mice. Diabetes Obes Metab. 2013;15:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Svane MS, Bojsen-Møller KN, Martinussen C, Dirksen C, Madsen JL, Reitelseder S, Holm L, Rehfeld JF, Kristiansen VB, van Hall G, Holst JJ, Madsbad S. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology. 2019;156:1627-1641.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 70. | Yoshida Y, Gotoh K, Masaki T, Ozeki Y, Tokoro M, Kudo A, Ozaki T, Okamoto M, Chiba S, Watanabe K, Ohta M, Inomata M, Shibata H. Effects of Sleeve Gastrectomy on Blood Pressure and the Renal Renin-Angiotensin System in Rats with Diet-Induced Obesity. Obesity (Silver Spring). 2019;27:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 71. | Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 72. | Gribble FM, Reimann F. Metabolic Messengers: glucagon-like peptide 1. Nat Metab. 2021;3:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 73. | Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 397] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 74. | Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Creutzfeldt W. The [pre-] history of the incretin concept. Regul Pept. 2005;128:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 795] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 77. | Wu T, Rayner CK, Young RL, Horowitz M. Gut motility and enteroendocrine secretion. Curr Opin Pharmacol. 2013;13:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Jepsen SL, Grunddal KV, Wewer Albrechtsen NJ, Engelstoft MS, Gabe MBN, Jensen EP, Ørskov C, Poulsen SS, Rosenkilde MM, Pedersen J, Gribble FM, Reimann F, Deacon CF, Schwartz TW, Christ AD, Martin RE, Holst JJ. Paracrine crosstalk between intestinal L- and D-cells controls secretion of glucagon-like peptide-1 in mice. Am J Physiol Endocrinol Metab. 2019;317:E1081-E1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Svane MS, Jørgensen NB, Bojsen-Møller KN, Dirksen C, Nielsen S, Kristiansen VB, Toräng S, Wewer Albrechtsen NJ, Rehfeld JF, Hartmann B, Madsbad S, Holst JJ. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2016;40:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 80. | Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 924] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 81. | Vollmer K, Gardiwal H, Menge BA, Goetze O, Deacon CF, Schmidt WE, Holst JJ, Meier JJ. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like Peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab. 2009;94:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Shehata MA, Elhaddad A, El-Attar AA, Shehata SM. The Effect of Antrum Size on Weight Loss, Glucagon-Like Peptide-1 (GLP-1) Levels, and Glycemic Control Following Laparoscopic Sleeve Gastrectomy in Adolescents with Obesity and Type 2 Diabetes. Obes Surg. 2021;31:4376-4385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Min T, Prior SL, Churm R, Dunseath G, Barry JD, Stephens JW. Effect of Laparoscopic Sleeve Gastrectomy on Static and Dynamic Measures of Glucose Homeostasis and Incretin Hormone Response 4-Years Post-Operatively. Obes Surg. 2020;30:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Vigneshwaran B, Wahal A, Aggarwal S, Priyadarshini P, Bhattacharjee H, Khadgawat R, Yadav R. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30-35.0 kg/m(2): a Prospective Study. Obes Surg. 2016;26:2817-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 85. | Valderas JP, Irribarra V, Rubio L, Boza C, Escalona M, Liberona Y, Matamala A, Maiz A. Effects of sleeve gastrectomy and medical treatment for obesity on glucagon-like peptide 1 levels and glucose homeostasis in non-diabetic subjects. Obes Surg. 2011;21:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 86. | Rigamonti AE, Bini S, Rocco MC, Giardini V, Massimini D, Crippa MG, Saluzzi A, Casati M, Marazzi N, Perotti M, Cimino V, Grassi G, Sartorio A, Pincelli AI. Post-prandial anorexigenic gut peptide, appetite and glucometabolic responses at different eating rates in obese patients undergoing laparoscopic sleeve gastrectomy. Endocrine. 2017;55:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Fatima F, Hjelmesæth J, Birkeland KI, Gulseth HL, Hertel JK, Svanevik M, Sandbu R, Småstuen MC, Hartmann B, Holst JJ, Hofsø D. Gastrointestinal Hormones and β-Cell Function After Gastric Bypass and Sleeve Gastrectomy: A Randomized Controlled Trial (Oseberg). J Clin Endocrinol Metab. 2022;107:e756-e766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 88. | Garibay D, McGavigan AK, Lee SA, Ficorilli JV, Cox AL, Michael MD, Sloop KW, Cummings BP. β-Cell Glucagon-Like Peptide-1 Receptor Contributes to Improved Glucose Tolerance After Vertical Sleeve Gastrectomy. Endocrinology. 2016;157:3405-3409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | Li L, Wang X, Bai L, Yu H, Huang Z, Huang A, Luo Y, Wang J. The Effects of Sleeve Gastrectomy on Glucose Metabolism and Glucagon-Like Peptide 1 in Goto-Kakizaki Rats. J Diabetes Res. 2018;2018:1082561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62:2380-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 91. | Baggio LL, Drucker DJ. Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2. Best Pract Res Clin Endocrinol Metab. 2004;18:531-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Brubaker PL. Glucagon-like Peptide-2 and the Regulation of Intestinal Growth and Function. Compr Physiol. 2018;8:1185-1210. [PubMed] [DOI] [Full Text] |
| 93. | Sigalet DL. Advances in glucagon like peptide-2 therapy. physiology, current indications and future directions. Semin Pediatr Surg. 2018;27:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Baldassano S, Amato A, Mulè F. Influence of glucagon-like peptide 2 on energy homeostasis. Peptides. 2016;86:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92:442-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 96. | Baldassano S, Bellanca AL, Serio R, Mulè F. Food intake in lean and obese mice after peripheral administration of glucagon-like peptide 2. J Endocrinol. 2012;213:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Shi X, Zhou F, Li X, Chang B, Li D, Wang Y, Tong Q, Xu Y, Fukuda M, Zhao JJ, Burrin DG, Chan L, Guan X. Central GLP-2 enhances hepatic insulin sensitivity via activating PI3K signaling in POMC neurons. Cell Metab. 2013;18:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 98. | Baldassano S, Rappa F, Amato A, Cappello F, Mulè F. GLP-2 as Beneficial Factor in the Glucose Homeostasis in Mice Fed a High Fat Diet. J Cell Physiol. 2015;230:3029-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG, Chouinard ML, Havel PJ. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620-3632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Patel A, Yusta B, Matthews D, Charron MJ, Seeley RJ, Drucker DJ. GLP-2 receptor signaling controls circulating bile acid levels but not glucose homeostasis in Gcgr(-/-) mice and is dispensable for the metabolic benefits ensuing after vertical sleeve gastrectomy. Mol Metab. 2018;16:45-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Buchan AM, Polak JM, Capella C, Solcia E, Pearse AG. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry. 1978;56:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Marks V. The early history of GIP 1969-2000: From enterogastrone to major metabolic hormone. Peptides. 2019;122:170155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Holst JJ, Gasbjerg LS, Rosenkilde MM. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology. 2021;162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 105. | Brown E, Cuthbertson DJ, Wilding JP. Newer GLP-1 receptor agonists and obesity-diabetes. Peptides. 2018;100:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 106. | Thondam SK, Cuthbertson DJ, Wilding JPH. The influence of Glucose-dependent Insulinotropic Polypeptide (GIP) on human adipose tissue and fat metabolism: Implications for obesity, type 2 diabetes and Non-Alcoholic Fatty Liver Disease (NAFLD). Peptides. 2020;125:170208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 107. | Musso G, Gambino R, Pacini G, De Michieli F, Cassader M. Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr. 2009;89:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 109. | Timper K, Grisouard J, Sauter NS, Herzog-Radimerski T, Dembinski K, Peterli R, Frey DM, Zulewski H, Keller U, Müller B, Christ-Crain M. Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am J Physiol Endocrinol Metab. 2013;304:E1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Keyhani-Nejad F, Irmler M, Isken F, Wirth EK, Beckers J, Birkenfeld AL, Pfeiffer AF. Nutritional strategy to prevent fatty liver and insulin resistance independent of obesity by reducing glucose-dependent insulinotropic polypeptide responses in mice. Diabetologia. 2015;58:374-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E1746-E1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 112. | Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 113. | Wang Y, Yan L, Jin Z, Xin X. Effects of sleeve gastrectomy in neonatally streptozotocin-induced diabetic rats. PLoS One. 2011;6:e16383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Xiaoli L, Wu CW, Kim HY, Tian W, Chiang FY, Liu R, Anuwong A, Randolph GW, Dionigi G, Lavazza M. Gastric acid secretion and gastrin release during continuous vagal neuromonitoring in thyroid surgery. Langenbecks Arch Surg. 2017;402:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Majumdar AP, Johnson LR. Gastric mucosal cell proliferation during development in rats and effects of pentagastrin. Am J Physiol. 1982;242:G135-G139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Copps J, Murphy RF, Lovas S. The production and role of gastrin-17 and gastrin-17-gly in gastrointestinal cancers. Protein Pept Lett. 2009;16:1504-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Duan S, Rico K, Merchant JL. Gastrin: From Physiology to Gastrointestinal Malignancies. Function (Oxf). 2022;3:zqab062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 118. | Cowey SL, Quast M, Belalcazar LM, Wei J, Deng X, Given R, Singh P. Abdominal obesity, insulin resistance, and colon carcinogenesis are increased in mutant mice lacking gastrin gene expression. Cancer. 2005;103:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Abulmeaty MMA, Aldisi D, Aljuraiban GS, Almajwal A, El Shorbagy E, Almuhtadi Y, Albaran B, Aldossari Z, Alsager T, Razak S, Berika M, Al Zaben M. Association of Gastric Myoelectrical Activity With Ghrelin, Gastrin, and Irisin in Adults With Metabolically Healthy and Unhealthy Obesity. Front Physiol. 2022;13:815026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57:3281-3288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 121. | Singh PK, Hota D, Dutta P, Sachdeva N, Chakrabarti A, Srinivasan A, Singh I, Bhansali A. Pantoprazole improves glycemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2012;97:E2105-E2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Grong E, Græslie H, Munkvold B, Arbo IB, Kulseng BE, Waldum HL, Mårvik R. Gastrin Secretion After Bariatric Surgery-Response to a Protein-Rich Mixed Meal Following Roux-En-Y Gastric Bypass and Sleeve Gastrectomy: a Pilot Study in Normoglycemic Women. Obes Surg. 2016;26:1448-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Grong E, Kulseng B, Arbo IB, Nord C, Eriksson M, Ahlgren U, Mårvik R. Sleeve gastrectomy, but not duodenojejunostomy, preserves total beta-cell mass in Goto-Kakizaki rats evaluated by three-dimensional optical projection tomography. Surg Endosc. 2016;30:532-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Grong E, Nord C, Arbo IB, Eriksson M, Kulseng BE, Ahlgren U, Mårvik R. The effect of hypergastrinemia following sleeve gastrectomy and pantoprazole on type 2 diabetes mellitus and beta-cell mass in Goto-Kakizaki rats. J Endocrinol Invest. 2018;41:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 2039] [Article Influence: 135.9] [Reference Citation Analysis (1)] |
| 126. | Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 127. | Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 550] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 128. | Patton A, Khan FH, Kohli R. Impact of Fibroblast Growth Factors 19 and 21 in Bariatric Metabolism. Dig Dis. 2017;35:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 130. | Nemati R, Lu J, Dokpuang D, Booth M, Plank LD, Murphy R. Increased Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Correlate with Improvement in Type 2 Diabetes in a Randomized Trial. Obes Surg. 2018;28:2672-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 131. | Chen Y, Lu J, Nemati R, Plank LD, Murphy R. Acute Changes of Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass. Obes Surg. 2019;29:3605-3621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Haluzíková D, Lacinová Z, Kaválková P, Drápalová J, Křížová J, Bártlová M, Mráz M, Petr T, Vítek L, Kasalický M, Haluzík M. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring). 2013;21:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 133. | Yang C, Brecht J, Weiß C, Reissfelder C, Otto M, Buchwald JN, Vassilev G. Serum Glucagon, Bile Acids, and FGF-19: Metabolic Behavior Patterns After Roux-en-Y Gastric Bypass and Vertical Sleeve Gastrectomy. Obes Surg. 2021;31:4939-4946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Ryan PM, Hayward NE, Sless RT, Garwood P, Rahmani J. Effect of bariatric surgery on circulating FGF-19: A systematic review and meta-analysis. Obes Rev. 2020;21:e13038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 135. | Huang HH, Lee WJ, Chen SC, Chen TF, Lee SD, Chen CY. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 136. | Khan FH, Shaw L, Zhang W, Salazar Gonzalez RM, Mowery S, Oehrle M, Zhao X, Jenkins T, Setchell KD, Inge TH, Kohli R. Fibroblast growth factor 21 correlates with weight loss after vertical sleeve gastrectomy in adolescents. Obesity (Silver Spring). 2016;24:2377-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Nielsen MS, Ritz C, Chenchar A, Bredie WLP, Gillum MP, Sjödin A. Does FGF21 Mediate the Potential Decrease in Sweet Food Intake and Preference Following Bariatric Surgery? Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 138. | Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, Valentí V, Salvador J, Giralt M, Villarroya F, Frühbeck G. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr. 2017;36:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 139. | Yen HH, Hsieh ST, Chen CL, Yang WS, Lee PC, Lin MT, Chen CN, Yang PJ. Circulating Diabetic Candidate Neurotrophic Factors, Brain-Derived Neurotrophic Factor and Fibroblast Growth Factor 21, in Sleeve Gastrectomy. Sci Rep. 2020;10:5341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 140. | Keuper M, Häring HU, Staiger H. Circulating FGF21 Levels in Human Health and Metabolic Disease. Exp Clin Endocrinol Diabetes. 2020;128:752-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 141. | Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 142. | Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. 2013;27:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 143. | Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring). 2006;14 Suppl 5:242S-249S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 144. | Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:e12997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 145. | Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 750] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 146. | Blüher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3:230-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 147. | Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 148. | Fried SK, Ricci MR, Russell CD, Laferrère B. Regulation of leptin production in humans. J Nutr. 2000;130:3127S-3131S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 149. | Nogueiras R, Wilson H, Rohner-Jeanrenaud F, Tschöp MH. Central nervous system regulation of adipocyte metabolism. Regul Pept. 2008;149:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 150. | Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567-E584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 151. | Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003-4009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 152. | Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring). 2009;17:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 153. | Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavaldà JM, Hernández M, Sabench F, Porras JA, Llutart J, Martinez S, Aguilar C, Del Castillo D, Richart C. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 154. | Mazahreh TS, Alfaqih M, Saadeh R, Al-Zoubi NA, Hatamleh M, Alqudah A, Aleshawi AJ, Alzoubi A. The Effects of Laparoscopic Sleeve Gastrectomy on the Parameters of Leptin Resistance in Obesity. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 155. | Hany M, Demerdash HM, Agayby ASS, Ibrahim M, Torensma B. Can Leptin/Ghrelin Ratio and Retinol-Binding Protein 4 Predict Improved Insulin Resistance in Patients with Obesity Undergoing Sleeve Gastrectomy? Obes Surg. 2022;32:3942-3950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 156. | Arble DM, Schwartz AR, Polotsky VY, Sandoval DA, Seeley RJ. Vertical sleeve gastrectomy improves ventilatory drive through a leptin-dependent mechanism. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 157. | Du J, Hu C, Bai J, Peng M, Wang Q, Zhao N, Wang Y, Wang G, Tao K, Xia Z. Intestinal Glucose Absorption Was Reduced by Vertical Sleeve Gastrectomy via Decreased Gastric Leptin Secretion. Obes Surg. 2018;28:3851-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 158. | Abu-Gazala S, Horwitz E, Ben-Haroush Schyr R, Bardugo A, Israeli H, Hija A, Schug J, Shin S, Dor Y, Kaestner KH, Ben-Zvi D. Sleeve Gastrectomy Improves Glycemia Independent of Weight Loss by Restoring Hepatic Insulin Sensitivity. Diabetes. 2018;67:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 159. | Choi HM, Doss HM, Kim KS. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 160. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2314] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 161. | Cook WS, Yeldandi AV, Rao MS, Hashimoto T, Reddy JK. Less extrahepatic induction of fatty acid beta-oxidation enzymes by PPAR alpha. Biochem Biophys Res Commun. 2000;278:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 162. | Fang H, Judd RL. Adiponectin Regulation and Function. Compr Physiol. 2018;8:1031-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 163. | Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47-T59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 480] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 164. | Wang X, Chen Q, Pu H, Wei Q, Duan M, Zhang C, Jiang T, Shou X, Zhang J, Yang Y. Adiponectin improves NF-κB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids Health Dis. 2016;15:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 165. | Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1389] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 166. | Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 796] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 167. | Lopez-Nava G, Negi A, Bautista-Castaño I, Rubio MA, Asokkumar R. Gut and Metabolic Hormones Changes After Endoscopic Sleeve Gastroplasty (ESG) Vs. Laparoscopic Sleeve Gastrectomy (LSG). Obes Surg. 2020;30:2642-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 168. | Rafey MF, Fang CEH, Ioana I, Griffin H, Hynes M, O'Brien T, McAnena O, O'Shea P, Collins C, Davenport C, Finucane FM. The leptin to adiponectin ratio (LAR) is reduced by sleeve gastrectomy in adults with severe obesity: a prospective cohort study. Sci Rep. 2020;10:16270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 169. | Šebunova N, Štšepetova J, Kullisaar T, Suija K, Rätsep A, Junkin I, Soeorg H, Lember M, Sillakivi T, Mändar R. Changes in adipokine levels and metabolic profiles following bariatric surgery. BMC Endocr Disord. 2022;22:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 170. | Eickhoff H, Rodrigues T, Neves I, Marques D, Ribeiro D, Costa S, Seiça R, Matafome P. Effect of Sleeve Gastrectomy on Angiogenesis and Adipose Tissue Health in an Obese Animal Model of Type 2 Diabetes. Obes Surg. 2019;29:2942-2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 171. | Kopsombut G, Shoulson R, Milone L, Korner J, Lifante JC, Sebastian M, Inabnet WB 3rd. Partial small bowel resection with sleeve gastrectomy increases adiponectin levels and improves glucose homeostasis in obese rodents with type 2 diabetes. World J Surg. 2012;36:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 172. | Castan-Laurell I, Dray C, Valet P. The therapeutic potentials of apelin in obesity-associated diseases. Mol Cell Endocrinol. 2021;529:111278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 173. | Marsault E, Llorens-Cortes C, Iturrioz X, Chun HJ, Lesur O, Oudit GY, Auger-Messier M. The apelinergic system: a perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann N Y Acad Sci. 2019;1455:12-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 174. | Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 175. | Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, Garcia-Almeida JM, Garcia-Arnes J, Tinahones FJ, Garcia-Fuentes E. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 176. | O'Harte FPM, Parthsarathy V, Hogg C, Flatt PR. Acylated apelin-13 amide analogues exhibit enzyme resistance and prolonged insulin releasing, glucose lowering and anorexic properties. Biochem Pharmacol. 2017;146:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 177. | Gourdy P, Cazals L, Thalamas C, Sommet A, Calvas F, Galitzky M, Vinel C, Dray C, Hanaire H, Castan-Laurell I, Valet P. Apelin administration improves insulin sensitivity in overweight men during hyperinsulinaemic-euglycaemic clamp. Diabetes Obes Metab. 2018;20:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 178. | Arica PC, Aydin S, Zengin U, Kocael A, Orhan A, Zengin K, Gelisgen R, Taskin M, Uzun H. The Effects on Obesity Related Peptides of Laparoscopic Gastric Band Applications in Morbidly Obese Patients. J Invest Surg. 2018;31:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 179. | Abaci A, Catli G, Anik A, Kume T, Bober E. The relation of serum nesfatin-1 level with metabolic and clinical parameters in obese and healthy children. Pediatr Diabetes. 2013;14:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 180. | Luo JJ, Wen FJ, Qiu D, Wang SZ. Nesfatin-1 in lipid metabolism and lipid-related diseases. Clin Chim Acta. 2021;522:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 181. | Tekin T, Cicek B, Konyaligil N. Regulatory Peptide Nesfatin-1 and its Relationship with Metabolic Syndrome. Eurasian J Med. 2019;51:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 182. | Tsuchiya T, Shimizu H, Yamada M, Osaki A, Oh-I S, Ariyama Y, Takahashi H, Okada S, Hashimoto K, Satoh T, Kojima M, Mori M. Fasting concentrations of nesfatin-1 are negatively correlated with body mass index in non-obese males. Clin Endocrinol (Oxf). 2010;73:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 183. | Stengel A. Nesfatin-1 - More than a food intake regulatory peptide. Peptides. 2015;72:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 184. | Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 185. | Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 186. | Dogan U, Bulbuller N, Cakır T, Habibi M, Mayir B, Koc U, Aslaner A, Ellidag HY, Gomceli I. Nesfatin-1 hormone levels in morbidly obese patients after laparoscopic sleeve gastrectomy. Eur Rev Med Pharmacol Sci. 2016;20:1023-1031. [PubMed] |
| 187. | Yang K, Zhang X, Zhou Y, Chen F, Shen M, Wang Y. Changes in Serum Nesfatin-1 After Laparoscopic Sleeve Gastrectomy are Associated with Improvements in Nonalcoholic Fatty Liver Disease. Diabetes Metab Syndr Obes. 2020;13:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 188. | Lee WJ, Chen CY, Ser KH, Chong K, Chen SC, Lee PC, Liao YD, Lee SD. Differential influences of gastric bypass and sleeve gastrectomy on plasma nesfatin-1 and obestatin levels in patients with type 2 diabetes mellitus. Curr Pharm Des. 2013;19:5830-5835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 189. | Majorczyk M, Staszkiewicz M, Szklarczyk J, Major P, Pisarska M, Wysocki M, Stefura T, Kacprzyk A, Droś J, Hołda MK, Pędziwiatr M, Budzyński A, Jaworek J. The influence of bariatric surgery on serum levels of irisin and nesfatin-1. Acta Chir Belg. 2019;119:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 190. | Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 474] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 191. | Acquarone E, Monacelli F, Borghi R, Nencioni A, Odetti P. Resistin: A reappraisal. Mech Ageing Dev. 2019;178:46-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 192. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3211] [Article Influence: 133.8] [Reference Citation Analysis (1)] |
| 193. | Filková M, Haluzík M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 194. | Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 195. | Su KZ, Li YR, Zhang D, Yuan JH, Zhang CS, Liu Y, Song LM, Lin Q, Li MW, Dong J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front Physiol. 2019;10:1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 196. | Salman AA, Sultan AAEA, Abdallah A, Abdelsalam A, Mikhail HMS, Tourky M, Omar MG, Youssef A, Ahmed RA, Elkassar H, Seif El Nasr SM, Shaaban HE, Atallah M, GabAllah GMK, Salman MA. Effect of weight loss induced by laparoscopic sleeve gastrectomy on liver histology and serum adipokine levels. J Gastroenterol Hepatol. 2020;35:1769-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 197. | Salman MA, Salman AA, Nafea MA, Sultan AAEA, Anwar HW, Ibrahim AH, Awad A, Ahmed RA, Seif El Nasr SM, Abouelregal TE, Shaaban HE, Mohamed FAH. Study of changes of obesity-related inflammatory cytokines after laparoscopic sleeve gastrectomy. ANZ J Surg. 2019;89:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 198. | Farey JE, Preda TC, Fisher OM, Levert-Mignon AJ, Stewart RL, Karsten E, Herbert BR, Swarbrick MM, Lord RV. Effect of Laparoscopic Sleeve Gastrectomy on Fasting Gastrointestinal, Pancreatic, and Adipose-Derived Hormones and on Non-Esterified Fatty Acids. Obes Surg. 2017;27:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 199. | Askarpour M, Alizadeh S, Hadi A, Symonds ME, Miraghajani M, Sheikhi A, Ghaedi E. Effect of Bariatric Surgery on the Circulating Level of Adiponectin, Chemerin, Plasminogen Activator Inhibitor-1, Leptin, Resistin, and Visfatin: A Systematic Review and Meta-Analysis. Horm Metab Res. 2020;52:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 200. | Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 703] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 201. | Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 202. | Zhao L, Leung LL, Morser J. Chemerin Forms: Their Generation and Activity. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 203. | Léniz A, González M, Besné I, Carr-Ugarte H, Gómez-García I, Portillo MP. Role of chemerin in the control of glucose homeostasis. Mol Cell Endocrinol. 2022;541:111504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 204. | Jouan Y, Blasco H, Bongrani A, Couet C, Dupont J, Maillot F. Preoperative Chemerin Level Is Predictive of Inflammatory Status 1 Year After Bariatric Surgery. Obes Surg. 2020;30:3852-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 205. | Cӑtoi AF, Pârvu AE, Mironiuc A, Chiorescu Ş, Crӑciun A, Pop ID, Cӑtoi C. Chemerin, Inflammatory, and Nitrooxidative Stress Marker Changes Six Months after Sleeve Gastrectomy. Oxid Med Cell Longev. 2018;2018:1583212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 206. | Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY, Carter RA, Tichansky DS, Madan AK. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes (Lond). 2008;32:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 207. | Watanabe T, Watanabe-Kominato K, Takahashi Y, Kojima M, Watanabe R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr Physiol. 2017;7:765-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 208. | Tan BK, Adya R, Farhatullah S, Lewandowski KC, O'Hare P, Lehnert H, Randeva HS. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 209. | Berti L, Hartwig S, Irmler M, Rädle B, Siegel-Axel D, Beckers J, Lehr S, Al-Hasani H, Häring HU, Hrabě de Angelis M, Staiger H. Impact of fibroblast growth factor 21 on the secretome of human perivascular preadipocytes and adipocytes: a targeted proteomics approach. Arch Physiol Biochem. 2016;122:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 210. | de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, Fried SK, Gong DW, Shuldiner AR, Pollin TI, McLenithan JC. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 534] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 211. | Tan BK, Pua S, Syed F, Lewandowski KC, O'Hare JP, Randeva HS. Decreased plasma omentin-1 levels in Type 1 diabetes mellitus. Diabet Med. 2008;25:1254-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 212. | Greulich S, Chen WJ, Maxhera B, Rijzewijk LJ, van der Meer RW, Jonker JT, Mueller H, de Wiza DH, Floerke RR, Smiris K, Lamb HJ, de Roos A, Bax JJ, Romijn JA, Smit JW, Akhyari P, Lichtenberg A, Eckel J, Diamant M, Ouwens DM. Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studies. PLoS One. 2013;8:e59697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 213. | Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 214. | Urbanová M, Dostálová I, Trachta P, Drápalová J, Kaválková P, Haluzíková D, Matoulek M, Lacinová Z, Mráz M, Kasalický M, Haluzík M. Serum concentrations and subcutaneous adipose tissue mRNA expression of omentin in morbid obesity and type 2 diabetes mellitus: the effect of very-low-calorie diet, physical activity and laparoscopic sleeve gastrectomy. Physiol Res. 2014;63:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 215. | Sdralis E, Argentou M, Mead N, Kehagias I, Alexandridis T, Kalfarentzos F. A prospective randomized study comparing patients with morbid obesity submitted to sleeve gastrectomy with or without omentectomy. Obes Surg. 2013;23:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 216. | Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Retraction. Science. 2007;318:565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 217. | Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15:1851-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 218. | Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 219. | Skop V, Kontrová K, Zídek V, Pravenec M, Kazdová L, Mikulík K, Sajdok J, Zídková J. Autocrine effects of visfatin on hepatocyte sensitivity to insulin action. Physiol Res. 2010;59:615-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 220. | Kim DS, Kang S, Moon NR, Park S. Central visfatin potentiates glucose-stimulated insulin secretion and β-cell mass without increasing serum visfatin levels in diabetic rats. Cytokine. 2014;65:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 221. | Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases. 2022;10:10840-10851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 222. | Beisani M, Pappa S, Moreno P, Martínez E, Tarascó J, Granada ML, Puig R, Cremades M, Puig-Domingo M, Jordà M, Pellitero S, Balibrea JM. Laparoscopic sleeve gastrectomy induces molecular changes in peripheral white blood cells. Clin Nutr. 2020;39:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 223. | Talavera-Urquijo E, Rodríguez-Navarro S, Beisani M, Salcedo-Allende MT, Chakkur A, Arús-Avilés M, Cremades M, Augustin S, Martell M, Balibrea JM. Morphofunctional Changes After Sleeve Gastrectomy and Very Low Calorie Diet in an Animal Model of Non-Alcoholic Fatty Liver Disease. Obes Surg. 2018;28:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 224. | Flores-Cortez YA, Barragán-Bonilla MI, Mendoza-Bello JM, González-Calixto C, Flores-Alfaro E, Espinoza-Rojo M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol Med Rep. 2022;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 225. | Steinhoff JS, Lass A, Schupp M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front Physiol. 2021;12:659977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 226. | Moraes-Vieira PM, Yore MM, Sontheimer-Phelps A, Castoldi A, Norseen J, Aryal P, Simonyté Sjödin K, Kahn BB. Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through Toll-like receptors 2 and 4. Proc Natl Acad Sci U S A. 2020;117:31309-31318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 227. | Zhang L, Cheng YL, Xue S, Xu ZG. The Role of Circulating RBP4 in the Type 2 Diabetes Patients with Kidney Diseases: A Systematic Review and Meta-Analysis. Dis Markers. 2020;2020:8830471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 228. | Yang FC, Xu F, Wang TN, Chen GX. Roles of vitamin A in the regulation of fatty acid synthesis. World J Clin Cases. 2021;9:4506-4519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 229. | Korek E, Gibas-Dorna M, Chęcińska-Maciejewska Z, Krauss H, Łagiedo-Żelazowska M, Kołodziejczak B, Bogdański P. Serum RBP4 positively correlates with triglyceride level but not with BMI, fat mass and insulin resistance in healthy obese and non-obese individuals. Biomarkers. 2018;23:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 230. | Wang X, Huang Y, Gao J, Sun H, Jayachandran M, Qu S. Changes of serum retinol-binding protein 4 associated with improved insulin resistance after laparoscopic sleeve gastrectomy in Chinese obese patients. Diabetol Metab Syndr. 2020;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 231. | Oberbach A, von Bergen M, Blüher S, Lehmann S, Till H. Combined serum proteomic and metabonomic profiling after laparoscopic sleeve gastrectomy in children and adolescents. J Laparoendosc Adv Surg Tech A. 2012;22:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 232. | Faqihi EJ, Alregaiey K, Altuwayjiri MA, Alamri MN, Alshehri BA, Iqbal M. The Effect of Bariatric Surgery on the Relation Between Retinol-Binding Protein 4 (RBP4) and Vitamin D Plasma Levels in Male Obese Population. Cureus. 2022;14:e32733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 233. | Hany M, Demerdash HM, Zidan A, Agayaby ASS, Torensma B. Effect of Weight Regain on Body Composition and Metabolic Biomarkers After Sleeve Gastrectomy: a Cross-Sectional Study from a Hospital Database. Obes Surg. 2023;33:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 234. | Jüllig M, Yip S, Xu A, Smith G, Middleditch M, Booth M, Babor R, Beban G, Murphy R. Lower fetuin-A, retinol binding protein 4 and several metabolites after gastric bypass compared to sleeve gastrectomy in patients with type 2 diabetes. PLoS One. 2014;9:e96489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 235. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7836] [Article Influence: 522.4] [Reference Citation Analysis (4)] |
| 236. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2319] [Article Influence: 257.7] [Reference Citation Analysis (0)] |
| 237. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8752] [Article Influence: 460.6] [Reference Citation Analysis (1)] |
| 238. | Debédat J, Clément K, Aron-Wisnewsky J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr Obes Rep. 2019;8:229-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 239. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2012] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 240. | Tabasi M, Eybpoosh S, Siadat SD, Elyasinia F, Soroush A, Bouzari S. Modulation of the Gut Microbiota and Serum Biomarkers After Laparoscopic Sleeve Gastrectomy: a 1-Year Follow-Up Study. Obes Surg. 2021;31:1949-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 241. | Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. 2017;27:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 242. | Ikeda T, Aida M, Yoshida Y, Matsumoto S, Tanaka M, Nakayama J, Nagao Y, Nakata R, Oki E, Akahoshi T, Okano S, Nomura M, Hashizume M, Maehara Y. Alteration in faecal bile acids, gut microbial composition and diversity after laparoscopic sleeve gastrectomy. Br J Surg. 2020;107:1673-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 243. | Fukuda N, Ojima T, Hayata K, Katsuda M, Kitadani J, Takeuchi A, Goda T, Ueda Y, Iwakura H, Nishi M, Yamaue H. Laparoscopic sleeve gastrectomy for morbid obesity improves gut microbiota balance, increases colonic mucosal-associated invariant T cells and decreases circulating regulatory T cells. Surg Endosc. 2022;36:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 244. | Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 364] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (9)] |
| 245. | Chiang JY, Pathak P, Liu H, Donepudi A, Ferrell J, Boehme S. Intestinal Farnesoid X Receptor and Takeda G Protein Couple Receptor 5 Signaling in Metabolic Regulation. Dig Dis. 2017;35:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 246. | Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1243] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 247. | McGlone ER, Bloom SR. Bile acids and the metabolic syndrome. Ann Clin Biochem. 2019;56:326-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 248. | Cariou B, Chetiveaux M, Zaïr Y, Pouteau E, Disse E, Guyomarc'h-Delasalle B, Laville M, Krempf M. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab (Lond). 2011;8:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 249. | Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond). 2013;37:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 250. | Eiken A, Fuglsang S, Eiken M, Svane MS, Kuhre RE, Wewer Albrechtsen NJ, Hansen SH, Trammell SAJ, Svenningsen JS, Rehfeld JF, Bojsen-Møller KN, Jørgensen NB, Holst JJ, Madsbad S, Madsen JL, Dirksen C. Bilio-enteric flow and plasma concentrations of bile acids after gastric bypass and sleeve gastrectomy. Int J Obes (Lond). 2020;44:1872-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 251. | Cӑtoi AF, Pârvu AE, Mironiuc A, Silaghi H, Pop ID, Andreicuț AD. Ultra-Early and Early Changes in Bile Acids and Insulin After Sleeve Gastrectomy Among Obese Patients. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 252. | Wang M, Wu Q, Xie H, Shao Y, Zhong M, Zhang X, Liu S, He X, Hu S, Zhang G. Effects of Sleeve Gastrectomy on Serum 12α-Hydroxylated Bile Acids in a Diabetic Rat Model. Obes Surg. 2017;27:2912-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 253. | Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 254. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1848] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 255. | Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 553] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 256. | Trauner M, Fickert P, Tilg H. Bile acids as modulators of gut microbiota linking dietary habits and inflammatory bowel disease: a potentially dangerous liaison. Gastroenterology. 2013;144:844-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |