Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.606
Peer-review started: December 23, 2022
First decision: February 20, 2023
Revised: March 2, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: May 15, 2023
Processing time: 143 Days and 10.5 Hours
Uncontrolled type 2 diabetes mellitus (T2DM) may lead to microvascular complications (nephropathy, retinopathy, and neuropathy) and cardiovascular diseases. The beta-glucan content in grains has the potential to improve insulin sensitivity, lowering postprandial glucose response and reducing inflammation degrees. A proper combination of grains not only satisfies human body’s need, but also provides essential and reasonable nutritional contents. However, no trial has been conducted to evaluate the roles of multigrain in T2DM.
To determine the efficacy of multigrain supplementation among T2DM patients.
From October 2020 to June 2021, a total of 50 adults living with T2DM, who were receiving standard diabetes care at Day Care Clinic, were randomized into either a supplementation group or a control group. The supplementation group received twice daily 30 g multigrain supplement (equivalent to 3.4 g beta-glucan) with standard medication for 12 wk, while the control group was prescribed with standard medication. Parameters such as glycemic control (HbA1c, FPG, and HOMO-IR), cardiometabolic profile (lipid profile, renal function test, and liver function test), oxidative stress status, nutritional status, and quality of life (QoL) were assessed at two time points: Baseline and the end of the treatment period (week 12).
The primary outcomes were the mean difference of glycated haemoglobin (%), fasting plasma glucose, and serum insulin as intervention effects. Secondary outcomes included the measurement of cardiometabolic profile, antioxidative and oxidative stress status, nutritional status indices, and QoL. Tertiary outcomes involved the determination of safety and tolerability, and supplementation compliance.
The present clinical trial will reveal the effectiveness of multigrain supplementation among T2DM patients for the improvement of diabetes management.
Core Tip: This is the first human clinical trial aimed to evaluate the effectiveness of multigrain supplementation among type 2 diabetes mellitus patients. The changes of glycemic control, cardiometabolic profile, oxidative stress status, nutritional status, and quality of life were measured. Our study also evaluated the safety, tolerability, and compliance of the supplementation.
- Citation: Mohd Ariffin NA, Mohd Sopian M, Lee LK. Efficacy of multigrain supplementation in type 2 diabetes mellitus: A pilot study protocol for a randomized intervention trial. World J Diabetes 2023; 14(5): 606-616
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/606.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.606
Diabetes mellitus is one of the major global health problems and driving causes of morbidity and mortality around the world. Type 2 diabetes mellitus (T2DM) is a metabolic disease that causes sugar to build up in the bloodstream, characterized by insulin insensitivity as a result of insulin resistance in the muscle and adipose tissue, declining insulin production, and eventual pancreatic beta-cell failure[1]. When the beta-cells in the pancreas malfunction and/or insulin resistance develops in the liver, skeletal muscle, or adipose tissue, hyperglycemia arises, resulting in an excess level of glucose circulating in the blood[2]. T2DM has attained epidemic proportions worldwide with 415 million cases estimated globally in 2015, and the number is expected to increase dramatically in the next decades, reaching 642 million by 2040[3]. T2DM is the foremost common frame of diabetes mellitus, accounting for more than 90% of all cases of adult-onset diabetes mellitus in Malaysia[4]. According to the National Health and Morbidity Survey (2020)[5], one in every five adults in Malaysia has T2DM.
Uncontrolled diabetes mellitus may lead to microvascular complications (nephropathy, retinopathy, and neuropathy) and macrovascular complications, later leading to severe peripheral vascular disease, premature coronary artery disease, and increased risk of cerebrovascular diseases[6]. The main aim of diabetes management is targeted at reducing the acute and chronic diabetes complications, via the effective control of plasma glucose, blood pressure, lipid profile, and body weight concurrently[7]. The distinction between effective treatment and cure is obscured within the case of diabetes, but few individuals can reverse it through diet changes and be able to reach and maintain normal blood sugar levels without or with minimum medication. In particular, nutrition or dietary therapy is one of the trending complementary medicines, with the ultimate goal to control, prevent (occurrence), and reverse (by averting resulting complications after its onset) the disease[8].
Wholegrain is defined as consisting of the entire grain (bran, endosperm, and germ), and most fiber ingredient from the wholegrain is of insoluble origin, including the cellulose, hemicellulose, and lignin, with the exception of barley and oat (relevant sources of soluble fiber such as beta-glucan, pentoses, and arabinoxylan)[9]. Wholegrain is a good source of dietary fiber, resistant starch, antioxidants, and other important micronutrients, such as folic acid and other vitamins[10]. Fiber from the wholegrain has been shown to reduce the risk of T2DM by improving insulin sensitivity, lowering postprandial glucose response, and lowering inflammation[11]. In addition, laboratory and epidemiological investigations have reported that wholegrain, especially barley and oat, contain a high amount of beta-glucan, which has been proven to lower blood glucose levels, improve glucose tolerance, ameliorate hyperlipidemia, improve immunity, and decrease infections[12]. In parallel, the demand of the multigrain source in the commercial market is increasing tremendously due to an increased awareness of managing chronic diseases by ingesting health promoting functional foods[13]. Multigrain, a proper combination of few types of grains, could satisfy human body’s need with essential nutritional benefits[14].
Several published clinical trials were looking into the effect of single grain supplementation on T2DM. Li et al[15] have conducted a clinical trial among overweight T2DM patients, and the results revealed that using oat as a therapeutic dietary regimen for 48 wk improved the body weight and glycemic control. The similar results have been inferred[16], where rice bran as a single treatment diet improved glycemic control and lipid profile in T2DM patients after 12 wk ingestion. In fact, multigrain consumption is more reflective towards human daily consumption. To date, study investigating the role of multigrain supplementation in T2DM patients is scarce.
Hence, the aim of this randomized clinical trial was to evaluate the effect of 12-wk of high beta-glucan multigrain supplementation on glycemic control in patients with T2DM. Secondary outcomes aim to evaluate the roles of the supplementation regimen for the amelioration of cardiometabolic health, antioxidative and oxidative stress, nutritional indices, and quality of life (QoL) among the T2DM patients. Tertiary outcomes involve the determination of safety and tolerability, and supplementation compliance.
This was an open-label, randomized controlled trial, with an allocation ratio for the supplement (S) vs control (C) group at 1:1. All patients were registered T2DM patients. Study recruitment and enrollment began on October 14, 2020, and the completion date for enrollment was June 2021. The study site was the Day Care Clinic in Universiti Sains Malaysia Bertam Medical Center. The medical center serves as the referred medical facility in the northern region of Peninsular Malaysia.
The study population included 50 T2DM patients who were receiving standard diabetes care at Day Care Clinic. Patients aged at least 18 years of age, male or female, clinically diagnosed with T2DM for at least 6 mo duration without clinically manifest complications (retinopathy, nephropathy, neuropathy, vascular diseases, and food ulcer), and currently receiving pharmacological treatment with metformin or insulin, or a combination of metformin and glibenclamide were included in the trial (Table 1). Patients with gluten intolerance were excluded as the supplement contains gluten. Participants who have involved in another supplementary program were also excluded to avoid dilution effects.
| Inclusion criteria | Exclusion criteria |
| Chronological age 18 years and above | Liver disease, kidney disease, or haematological disorders |
| T2DM ≥ 6 mo, stable regimen ≥ 6 mo without clinically manifest complications | Active gastric or duodenal ulcer |
| Male or female | Psychiatric disease or mental retardation |
| Pharmacological treatment with metformin or insulin, or a combination of metformin and glibenclamide | Cancer and other endocrine disorders |
| Free from antioxidant supplements | Alcohol or drug abuse |
| Free from anti-inflammatory supplements | Pregnancy or lactation |
| Hormone replacement therapy | |
| Herbal remedies | |
| Gluten intolerance | |
| Currently under another supplementary program |
The study randomized all trial subjects into either group S or group C. Group S (n = 25) was supplemented with daily 60 g (2 sachets, 30 g each) of high beta-glucan (equivalent to 3.4 g) multigrain supplement for up to 12 wk. This multigrain supplement (Oat King®) was sponsored by TG Ocean Health Food Industries Sdn Bhd, Malaysia. It does not contain any food additives including food preservatives, coloring, flavoring, and sweetener. The main ingredients are oat, barley, brown rice, paddy, rice flour, corn flour, red kidney bean, black bean with kernel, and soybean (Table 2). Group S was required to consume the supplement two times per day (day and night). Patients were attending to the study site to receive and replenish the supplement at baseline, week 4, and week 8. All patients continued their standard medication as prescribed before the trial participation.
| Active ingredient | Scientific name | Percentage (%) |
| Oat | Avena sativa | 11.80 |
| Brown rice | Oryza sativa | 9.55 |
| Paddy | Oryza sativa | 9.38 |
| Rice | Oryza sativa | 6.04 |
| Corn | Zea mays | 6.04 |
| Red kidney bean | Phaseolus vulgaris | 6.04 |
| Black bean | Phaseolus vulgaris | 6.04 |
| Soy bean | Glycine max | 5.17 |
| Barley | Hordeum vulgare | 4.98 |
| Wheat | Triticum | 4.98 |
| Wheat germ | Triticum vulgare | 4.98 |
| Wheat bran | Triticum aestivum L | 4.98 |
| Coix seed | Coix-lacryma-jobi | 4.22 |
| Millet | Pennisetum glaucum | 3.50 |
| Red rice | Oryza longistaminata | 2.46 |
| Black rice | Zizania aqatica | 2.46 |
| Black sesame seed | Sesamum indicum | 2.46 |
| Navy bean | Phaseolus vulgaris | 2.46 |
| Mung bean | Vigna radiata | 2.46 |
Group C (n = 25) continued the standard medication as prescribed prior to the trial. They were reminded not to alter their habitual dietary intake and physical activity level throughout the clinical trial period.
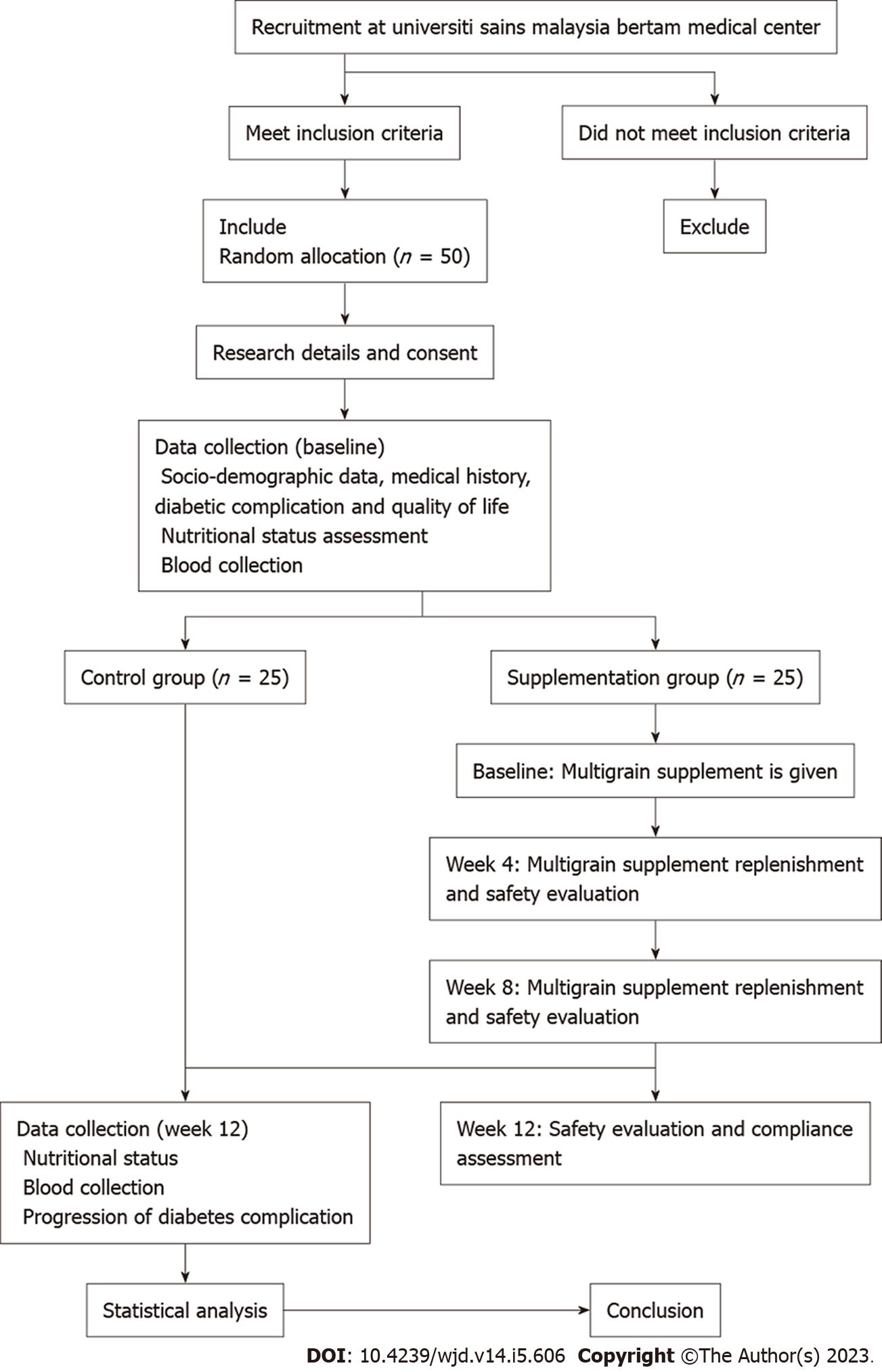
Five categories of study visits have been adopted in this trial: Recruitment, screening, and inform consent form signing, randomization and blinding, enrolment visit, follow-up visits, and post week-12 visit. Figure 1 illustrates the trial flowchart.
All T2DM patients were invited face to face during their routine medical follow-up in the Day Care Clinic. Patient recruitment also occurred through electronic medical record review to identify potential participants. Patients were then invited for a screening session. The research team evaluated the eligibility criteria (both inclusion and exclusion criteria) and explained the research information in detail, followed by obtaining written inform consent. The research team did not coerce or unduly influence a patient to participate in the trial. Eligible patients underwent the randomization procedure.
To generate a random allocation sequence, a computer-generated list of random numbers was used. Simple randomization at a 1:1 allocation ratio (1 group S: 1 group C) has been applied. The allocation sequence was concealed from the investigator enrolling and assessing participants on sequentially numbered, opaque, sealed, and stapled envelopes. To prevent subversion of the allocation sequence, the name and date of birth of the participant were written on the envelope. To randomize the participants, variables such as demographic data (age, gender, and ethnicity), clinical data (years of disease, glycemic status, and the presence of diabetic-related complications), physical activity, and medication (current prescribed medications) were taken into the consideration. To determine whether the patient would be randomized into the multigrain group S or C, randomization was made by reference to a statistical series based on the random sampling number drawn up by the statistician. The details of the series were unknown to any of the investigator or the coordinator. In order to implement blinding, participants were notified individually of the assigned group S or C. However, only data collectors, the coordinator, and the medical officer in charge of the trial were aware of the allocated arm. Investigators, data analyst, and outcome adjudicator are kept blinded to the allocation.
The enrolment visit was consisted of a semi-quantitative questionnaire, physical examination, fasting blood sampling, and laboratory tests. The semi-quantitative questionnaire gathered information with regard to the socio-demographic background and medical history (including medical prescription). Lifestyle health behaviors included alcohol use, cigarettes smoking, and routine exercise practices. Physical examination involved the measurement of systolic and diastolic blood pressure, handgrip strength, and nutritional status assessments (anthropometry and body composition measurements).
A total of 20 mL of fasting venous blood was drawn from each participant for the subsequent clinical laboratory testing. Routine laboratory testing comprised of albumin, total protein and total bilirubin, urea, minerals, uric acid, and creatinine. Fasting plasma glucose, glycated haemoglobin, serum insulin, lipid profile, and liver and kidney function tests were performed. Upon centrifugation, serum and plasma samples were collected, and the antioxidative and oxidative stress statuses were assessed via the measurements of total antioxidant capacity, superoxide dismutase, glutathione, glutathione peroxidase (GPx), malondialdehyde, protein carbonyl, and 8-deoxyguanosine concentrations.
Modified diabetes QoL-17 questionnaire[17] has been used to evaluate the changes of QoL, as assessed using 7 domains (physical functioning, role limitations due to physical health, role limitations due to emotional, energy fatigue, emotional well-being, social functioning, and general health).
The supplement group received the first month supply of multigrain supplement in the form of sachet. Detail use of the supplement was elaborated, and patients returned the used sachets packaging during the follow-up visits.
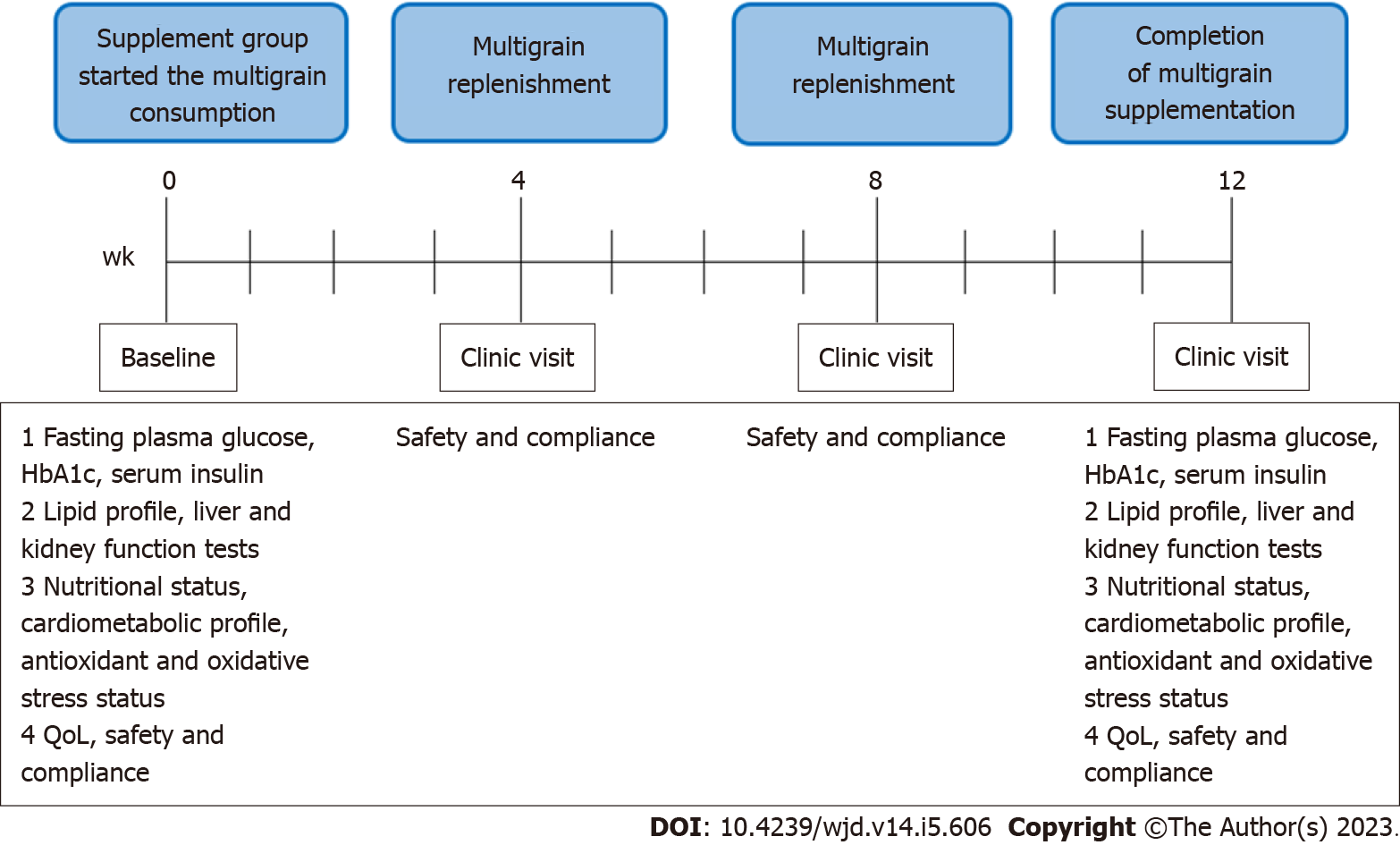
Patients were evaluated at 3 study visits (Figure 2) during the week-4, week-8, and post week-12 follow-ups at Day Care Clinic. At each follow-up, the evaluation of the safety, tolerability, and compliance to the multigrain supplementation was conducted. Adverse effects concomitant to the supplementation regimen, particularly the signs and symptoms of gastrointestinal discomforts, were recorded. Compliance to the supplementation was indicated as the recorded number of consumed sachets. Replenishment of multigrain supplement was implemented during week-4 and week-8 follow-ups, respectively. Disease progression in group C was evaluated following standard medication regimen. Both the supplement and control groups were reminded not to alter their routine dietary intake and physical activity level.
After week 12, study questionnaire, physical examination, blood profile, and QoL assessments were performed in both the supplement and control groups. In-depth interviews have been conducted by the research team members among the patients in group S. The attitudes, positive and negative perceptions towards the supplementation, and perceived general health were interviewed. All study data was recorded into the case report form.
The results from a previous study[18] among T2DM patients were used to determine the trial sample size. The following formula is used to calculate the trial sample size:
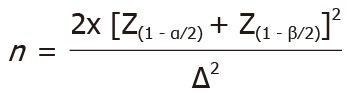
Where n = sample size, Z = 0.8416 (for each arm, a setting of 80% power and 95%CI was used), Z = 1.96, and  = mean difference or standard deviation. Thus, for this study, n = 18 subjects for each arm. With the consideration that the dropout rate was 20%, the needed sample size was 22 patients for each arm.
= mean difference or standard deviation. Thus, for this study, n = 18 subjects for each arm. With the consideration that the dropout rate was 20%, the needed sample size was 22 patients for each arm.
Data analysis in the form of intention to treat will be performed at the end of the study. All statistical analyses will be implemented using the Statistical Package for Social Science (SPSS Inc., Chicago, IL, United States) software. The following statistical methods will be applied:
Assumptions will be checked for normality tests, and transformation will be applied as corrective procedures.
For descriptive statistics, categorical and continuous data, results will be presented as percentages, means with standard deviations, median and range.
For inferential tests, P < 0.05 will be used to indicate statistical significance (type I error) (two-tailed).
Analysis of the primary, secondary, and tertiary outcomes will be measured using Pearson’s correlation, multivariate regression, repeated measures mixed models, logistic regression, and generalized linear models.
The present study is conducted in accordance to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects have been approved by the Human Research Ethics Committee of Universiti Sains Malaysia (No: USM/JEPeM/20030183). Written consent is obtained from all patients, and the study has been registered in the clinical trial registry (ClinicalTrials.gov), with the registration ID: NCT04597229.
The patients’ outcome measure has been assessed at two time points: Enrolment (baseline), and at the end of the treatment period (post week-12).
The primary outcomes were the changes in fasting plasma glucose, HbA1c, and serum insulin from enrolment to post week-12, and the differences in these changes between the two study arms.
Secondary outcomes include the measurement of lipid profile, liver function test and kidney function test comparing between the study groups. The change of nutritional status, antioxidative status, and oxidative stress biomarkers were assessed too.
Tertiary outcomes were the change in QoL, and the difference in this change between the study groups. In term of safety evaluation, a list of gastrointestinal discomfort symptoms has been assessed among the participants in the supplementation group. The intensity of the gastrointestinal symptoms is defined as none, mild, moderate, severe, and very severe according to the symptoms (bloating, abdominal rumbling, flatulence, abdominal pain, nausea, vomiting, heart burn, loss of appetite, diarrhea, and constipation). Patients who showed symptoms have been referred to the physician in charge. Compliance to the supplementation regimen was assessed by counting the number of the consumed sachets during every follow-up visit (week-4, week-8, and week-12). Patients were asked to provide the reason for missed sachet consumption.
No result is provided as this is a pilot study protocol for a human clinical trial.
The current randomized control trial is aimed to evaluate the effects of multigrain supplementation as a complementary regimen vs a control (without supplementation) among patients with T2DM over a period of 12 wk. For the past decades, the underlying mechanisms for an association between grains and T2DM are not entirely clear, but grains may lower the risk of T2DM by improving insulin sensitivity[19]. Particularly, the potency of medium glycemic index multigrain flour to reduce glycemia in T2DM has been highlighted for the implementation of a better dietary plan for diabetes control[20]. Our study is designed to determine if multigrain supplementation, instead of single grain diet, is effective to ameliorate T2DM. Multigrain consumption is relatively a ‘pure’ dietary routine for human being.
Beta-glucan, pentose, and arabinoxylan are found in wholegrain fiber, especially in barley and oats, and other insoluble fibers, including cellulose, hemicellulose, and lignin[21]. These components play a vital role in a collective way, by improving the glycemic metabolism and reducing T2DM risk factor. Soluble fiber from oats and barley (with 3 g of beta-glucan intake per day) has been found to be effective in lowering total cholesterol and low-density lipoprotein (about 5% to 10% reduction, respectively)[15]. The latest finding also outlined the possible role of minimally processed whole grains over 2 wk in improving measures of glycemia in free-living adults with T2DM[22]. In addition, beta-glucan is evident to increase the intestinal viscosity, decrease the starch digestion, and reduce the food intake by increasing satiety, reducing hyperglycemia, lowering the lipid profile, and reducing weight[23].
Grains are generally high in magnesium. Magnesium is an essential co-factor for many enzymes, including the enzymes involved in glucose and insulin metabolism. Grain also contains a group of phenolic compounds, the avenanthramides. Avenanthramides are antioxidant and can enhance endothelial functions and anti-inflammatory properties[24]. Another potential antioxidant found in grains is vitamin E. Vitamin E is an intracellular antioxidant, which prevents the oxidative damage of the polyunsaturated fatty acids in cell membranes. Vitamin E also facilitates to remain selenium in a reduced state[25]. Selenium plays an important role as a potent antioxidant. For example, GPx reacts with hydrogen peroxide to prevent harmful free radicals, DNA damage, and the formation of metabolic active carcinogens[26]. High selenium levels may help to reduce the formation of oxidized low-density lipoprotein (LDL) cholesterol and, as a result, reduce the risk of heart disease[27] and inflammation, strengthen the immune system in the body[28], and prevent the incidence of cancer[29]. Collectively, micronutrients in grains have their own beneficial roles to reduce the risk of T2DM complications.
In addition, bioactive compounds present in grains (such as phenolic compounds, phytosterols, betaine, and carotenoids) can help to improve insulin sensitivity and slow the progression of T2DM[30]. Bioactive compounds act by reducing the oxidative stress, inflammatory cytokine transcription, and subclinical inflammation[31] since increased oxidative stress seems to be a harmful component contributing to worsening insulin resistance and beta-cell dysfunction, which may lead to T2DM complications[32]. A previous study showed that a diet rich in polyphenols increased glucose tolerance and insulin sensitivity, and reduced the postprandial triglyceride response[33]. Moreover, phytosterols are known to be effective to reduce LDL cholesterol, as consumption of 2 g of plant sterol from wholegrain resulted in a 5.6% reduction in LDL cholesterol among T2DM patients after 4 wk ingestion[34]. Indirectly, this may reduce the risk of diabetes complications, particularly macrovascular complications.
Grain plays a significant role in reducing the energy intake. It has lower energy density, and the larger starch granules significantly contribute to a greater chewing rate, hence increasing satiation[10,35,36]. Fiber from the grain also increases gastric distension and delay the intestinal transit time, contributing to the stimulation of satiety signals[37] and increasing hormones levels involved in the energy homeostasis and plasma glucose control[38]. This process involves the stimulation of satiety signal in the brain, where body weight regulation hormones, ghrelin, peptide YY, cholecystokinin, gastric inhibitory polypeptide, and glucagon-like peptide 1 are regulated as part of the energy homeostasis and plasma glucose control[39]. This process might have a positive impact due to the change in gut microbiota profile[40,41] and cause a decrease in subclinical inflammation. Similarly, the slower process of carbohydrate digestion, as well as the glucose and free fatty acid absorption in the intestine[42], reduces insulin demand and stimulates fat oxidation, thus contributing to the reduction of fat storage[20]. Collectively, the synergistic mechanisms result in an increase in the hypothalamic satiety signal in the brain[20], which further leads to the body weight reduction and energy homeostasis, as well as glucose control[10,43-45].
Strengths of this study include a randomized controlled trial design, where the covariates could be equally distributed. The multigrain powder is formulated using commonly consumed grains, thus omitting the issues of food safety concern. Regular follow-up on a monthly basis allowed close monitoring of supplement adherence. The trial also included detail measurements of nutritional status, antioxidative status, oxidative stress biomarkers, and QoL, which allowed better result interpretation. These analyses will inform whether any potential effect extends to other metabolic or peripheral parameters. We acknowledge the small sample size of the study as the major limitation for this pilot clinical trial.
Important implications are expected from this research regardless of the findings. In a condition where beneficial effect is supported by evidence of a positive effect on long-term blood glucose levels, public health efforts should be undertaken to encourage the consumption of multigrain as functional foods. Contradictorily, if a beneficial effect is not supported, this could suggest that multigrain does not translate into strong long-term benefits for blood glucose control under daily conditions.
This is a pioneer, pilot clinical trial that aims to evaluate the efficacy of high beta-glucan multigrain supplementation among T2DM patients. Important trial outcomes, such as glycemic control, peripheral antioxidative capacity, cardiometabolic health, nutritional status, QoL, safety, and compliance have been studied extensively. The results of the trial are important to suggest a scientifically driven complementary dietary agent for better management of T2DM.
Type II diabetes mellitus (T2DM) has emerged as a major public health challenge around the world. Diet is a major lifestyle factor that can greatly influence the incidence and progression of T2DM. The notion that foods not only provide basic nutrition but can also prevent diseases and ensure good health and longevity is now attaining greater prominence.
Typically, grains, with its rich non-starch polysaccharides content, are receiving concern among the scientific communities. Multigrain is rich with thiamine, riboflavin, pantothenic acid, iron, zinc, and copper, and it can be prepared using different preparation processes, which usually comprises a high amount of dietary fiber content. Multigrain consumption is indeed a more representative dietary intervention as compared to single grain intake. There is a need to examine whether supplementation with multigrain, a more representative dietary regimen to human routine consumption pattern, would yield better outcomes among T2DM patients.
The objectives of the present study were to evaluate the effects of multigrain supplementation on glycemic control, cardiometabolic profile, oxidative stress, nutritional status, and quality of life (QoL) among T2DM patients. The safety, tolerability, and adherence of the supplementation were evaluated.
Fifty T2DM patients have been randomly assigned to receive either 60 g multigrain supplementation (containing 3.4 g beta-glucan) coupled with prescribed standard medication regimen (n = 25), or standard medication regimen alone (n = 25) for 12 wk. Study outcomes involved the changes of glycemic control, cardiometabolic profile, oxidative stress, nutritional status, and QoL.
No result is provided as this is a pilot study protocol for a human clinical trial.
This is a pioneer, pilot clinical trial that aims to evaluate the efficacy of high beta-glucan multigrain supplementation among T2DM patients. Important trial outcomes, such as glycemic control, peripheral antioxidative capacity, cardiometabolic health, nutritional status, QoL, safety, and compliance, have been studied extensively. The results of the trial are important to suggest a scientifically driven complementary dietary agent for better management of T2DM.
The findings are expected to contribute and expand the fundamental mechanism of the role of multigrain as a complementary management agent in diabetic physiology.
We would like to thank the participation from all patients, technical staff, and staff nurses. Special thanks to Universiti Sains Malaysia for providing the support in the study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Integrative and complementary medicine
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Horowitz M, Australia; Mohammadi S, Iran; Moreno-Gómez-Toledano R, Spain S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 543] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 2. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3402] [Article Influence: 486.0] [Reference Citation Analysis (0)] |
| 3. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2512] [Article Influence: 314.0] [Reference Citation Analysis (0)] |
| 4. | Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus: A review. Int J Health Sci (Qassim). 2017;11:65-71. [PubMed] |
| 5. | National Institutes of Health Ministry of Health Malaysia. National Health and Morbidity Survey 2019: Non-Communicable Diseases: Risk Factors and other Health Problems. 2020. [cited 29 April 2021]. Available from: http://bit.ly/NHMS2019. |
| 6. | Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1394] [Article Influence: 278.8] [Reference Citation Analysis (0)] |
| 7. | American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S33-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 8. | Koithan M, Devika J. New Approaches to Nutritional Therapy. J Nurse Pract. 2010;6:805-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kyrø C, Tjønneland A, Overvad K, Olsen A, Landberg R. Higher Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes among Middle-Aged Men and Women: The Danish Diet, Cancer, and Health Cohort. J Nutr. 2018;148:1434-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Della Pepa G, Vetrani C, Vitale M, Riccardi G. Wholegrain Intake and Risk of Type 2 Diabetes: Evidence from Epidemiological and Intervention Studies. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Wirström T, Hilding A, Gu HF, Östenson CG, Björklund A. Consumption of whole grain reduces risk of deteriorating glucose tolerance, including progression to prediabetes. Am J Clin Nutr. 2013;97:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Niu M, Hou GG, Kindelspire J, Krishnan P, Zhao S. Microstructural, textural, and sensory properties of whole-wheat noodle modified by enzymes and emulsifiers. Food Chem. 2017;223:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Jing Q, Yingguo L, Yuanhui W, Jie C, Panfeng H. Formula and quality study of multigrain noodles. Grain Oil Sci Technol. 2018;1:157-162. [DOI] [Full Text] |
| 14. | Dedeepiya VD, Sivaraman G, Venkatesh AP, Preethy S, Abraham SJ. Potential effects of nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: preliminary findings from the study on three patients from India. Case Rep Med. 2012;2012:895370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Li X, Cai X, Ma X, Jing L, Gu J, Bao L, Li J, Xu M, Zhang Z, Li Y. Short- and Long-Term Effects of Wholegrain Oat Intake on Weight Management and Glucolipid Metabolism in Overweight Type-2 Diabetics: A Randomized Control Trial. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Cheng HH, Huang HY, Chen YY, Huang CL, Chang CJ, Chen HL, Lai MH. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann Nutr Metab. 2010;56:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Acharya LD, Shaista K, Ashan FK, Surulivelrajan M. Development and validation of quality of life assessment instrument for diabetic patients. Asian J Pharm Health Sci. 2014;4:1114-1120. |
| 18. | Vuksan V, Whitham D, Sievenpiper JL, Jenkins AL, Rogovik AL, Bazinet RP, Vidgen E, Hanna A. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care. 2007;30:2804-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Pereira MA, Jacobs DR Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 20. | Bach Knudsen KE, Hartvigsen ML, Hedemann MS, Hermansen K. Mechanisms Whereby Whole Grain Cereals Modulate the Prevention of Type 2 Diabetes. In: Mauricio D. Molecular Nutrition and Diabetes: A Volume in the Molecular Nutrition Series 2016; 87-103. [DOI] [Full Text] |
| 21. | Sobhana PP, Kandlakunta B, Nagaraju R, Thappatla D, Epparapalli S, Vemula SR, Gavaravarapu SRM, Korrapati D. Human clinical trial to assess the effect of consumption of multigrain Indian bread on glycemic regulation in type 2 diabetic participants. J Food Biochem. 2020;44:e13465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Åberg S, Mann J, Neumann S, Ross AB, Reynolds AN. Whole-Grain Processing and Glycemic Control in Type 2 Diabetes: A Randomized Crossover Trial. Diabetes Care. 2020;43:1717-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 616] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 24. | Lyly M, Liukkonen KH, Salmenkallio-Marttila M, Karhunen L, Poutanen K, Lähteenmäki L. Fibre in beverages can enhance perceived satiety. Eur J Nutr. 2009;48:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Hou Q, Li Y, Li L, Cheng G, Sun X, Li S, Tian H. The Metabolic Effects of Oats Intake in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients. 2015;7:10369-10387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Newberne PM, Suphakarn V. Nutrition and cancer: a review, with emphasis on the role of vitamins C and E and selenium. Nutr Cancer. 1983;5:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Furman C, Rundlöf AK, Larigauderie G, Jaye M, Bricca G, Copin C, Kandoussi AM, Fruchart JC, Arnér ES, Rouis M. Thioredoxin reductase 1 is upregulated in atherosclerotic plaques: specific induction of the promoter in human macrophages by oxidized low-density lipoproteins. Free Radic Biol Med. 2004;37:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 30. | Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2008;13:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 343] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 31. | Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, Shu F, Gao Y, Yuan L, Zhang Q, Mi M. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. 2015;47:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 32. | Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 649] [Cited by in RCA: 769] [Article Influence: 76.9] [Reference Citation Analysis (10)] |
| 33. | Belobrajdic DP, Bird AR. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr J. 2013;12:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Bozzetto L, Annuzzi G, Pacini G, Costabile G, Vetrani C, Vitale M, Griffo E, Giacco A, De Natale C, Cocozza S, Della Pepa G, Tura A, Riccardi G, Rivellese AA. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: a controlled randomised intervention trial. Diabetologia. 2015;58:1551-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Clifton P, Keogh J. Cholesterol-Lowering Effects of Plant Sterols in One Serve of Wholegrain Wheat Breakfast Cereal Biscuits-a Randomised Crossover Clinical Trial. Foods. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Salvin J, Green H. Dietary fibre and satiety. Nutr Bull. 2007;32:32-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 37. | Wanders AJ, van den Borne JJ, de Graaf C, Hulshof T, Jonathan MC, Kristensen M, Mars M, Schols HA, Feskens EJ. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev. 2011;12:724-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Lyly M, Ohls N, Lähteenmäki L, Salmenkallio-Marttila M, Liukkonen KH, Karhunen L, Poutanen K. The effect of fibre amount, energy level and viscosity of beverages containing oat fibre supplement on perceived satiety. Food Nutr Res. 2010;54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Sleeth ML, Thompson EL, Ford HE, Zac-Varghese SE, Frost G. Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev. 2010;23:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 40. | Xi Y, Xu PF. Diabetes and gut microbiota. World J Diabetes. 2021;12:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 41. | Bastos RMC, Rangel ÉB. Gut microbiota-derived metabolites are novel targets for improving insulin resistance. World J Diabetes. 2022;13:65-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Nilsson A, Granfeldt Y, Ostman E, Preston T, Björck I. Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr. 2006;60:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Sánchez D, Miguel M, Aleixandre A. Dietary fiber, gut peptides, and adipocytokines. J Med Food. 2012;15:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | McRorie JW Jr. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr Today. 2015;50:82-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Bodnaruc AM, Prud'homme D, Blanchet R, Giroux I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review. Nutr Metab (Lond). 2016;13:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |