Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.512
Peer-review started: January 20, 2023
First decision: February 8, 2023
Revised: February 21, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 15, 2023
Processing time: 114 Days and 20.4 Hours
Type 2 diabetes mellitus (T2DM) is a leading risk factor for cardiovascular complications around the globe and one of the most common medical conditions. Atrial fibrillation (AF) is the most common supraventricular arrhythmia, with a rapidly increasing prevalence. T2DM has been closely associated with the risk of AF development, identified as an independent risk factor. Regarding cardio-vascular complications, both AF and T2DM have been linked with high mortality. The underlying pathophysiology has not been fully determined yet; however, it is multifactorial, including structural, electrical, and autonomic pathways. Novel therapies include pharmaceutical agents in sodium-glucose cotransporter-2 inhibitors, as well as antiarrhythmic strategies, such as cardioversion and ablation. Of interest, glucose-lowering therapies may affect the prevalence of AF. This review presents the current evidence regarding the connection between the two entities, the pathophysiological pathways that link them, and the therapeutic options that exist.
Core Tip: Diabetes mellitus (DM) and atrial fibrillation (AF) are interconnected pathological conditions that are associated with excess morbidity and mortality. DM is implicated in AF’s pathophysiology, with mechanisms involving structural remodeling, electrical alterations, autonomic dysfunction, and dysglycemia. The management of this deleterious combination is multifaceted and includes the use of conventional methods such as direct oral anticoagulation, electrical cardioversion, and antiarrhythmic drugs. Sodium-glucose cotransporter-2 inhibitors, catheter ablation, and left atrial appendage occlusion represent appealing modern approaches, whose efficacy in this subgroup of patients needs to be thoroughly examined.
- Citation: Leopoulou M, Theofilis P, Kordalis A, Papageorgiou N, Sagris M, Oikonomou E, Tousoulis D. Diabetes mellitus and atrial fibrillation-from pathophysiology to treatment. World J Diabetes 2023; 14(5): 512-527
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/512.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.512
Type 2 diabetes mellitus (T2DM) is a leading risk factor for cardiovascular complications around the globe and one of the most common medical conditions[1]. Atrial fibrillation (AF) is the most common supraventricular arrhythmia, with a rapidly increasing prevalence[2]. T2DM has been closely associated with the risk of AF development, being identified as an independent risk factor for AF. Furthermore, T2DM has been linked with an increased symptom burden for patients that suffer from AF, leading to impaired life quality and increased hospitalization[3].
The risk of AF development in patients with T2DM has been established by large studies and meta-analyses showing a clear link between AF and T2DM. Based on the association between the two medical conditions and the high risk of cardiovascular morbidity and mortality that their combination presents, literature has concluded that underlying pathophysiology is related to structural, electrical-electromechanical, and autonomic remodeling as well as metabolic parameters[4,5]. Furthermore, their association has highlighted the need for surfacing therapeutic models that can alter the risk of the AF and T2DM combination or lower the risk of AF development in the diabetic population.
In this review, we present the pathophysiologic mechanisms that may combine the two entities, and the therapeutic options that are available for diabetic patients with AF.
The Women's Health Study established T2DM as a significant predictor of risk for AF[6]. Similarly, a 2010 study suggested a 40% higher risk of developing AF, for diabetic patients, with the overall risk increasing by 3% for every year of T2DM[7]. In 2011, the risk of developing AF in patients with T2DM was identified at 34% over the non-diabetic population[8], while in a 2017 meta-analysis, higher serum glycated hemoglobin levels (HbA1c) were associated with incident AF in prospective cohort studies[9]. In a prospective study, T1DM was associated with a modest increase in the risk of AF in men and a 50% increased risk of AF in women; the risk was proportional to worse glycemic control and renal complications[10]. Similarly, in the prospective cross-sectional observational NOMED-AF study, researchers concluded that AF affects one in four patients with T2DM, highlighting the excessive need for AF screening amongst the diabetic population[11]. Interestingly, a recent Swedish cohort revealed an overall 35% higher risk of AF compared to age- and sex-matched controls from the general population for patients with T2DM; renal complications or poor glycemic control increased the risk of AF[12]. In a Danish study, T2DM was associated with a relative 19% increased risk of incident AF, especially in the 18-39-year-old group[13], while a case-control study concluded that T1DM modestly increases the risk of AF in men but elevates the risk for women by 50%, especially in the cases of poor glycemic control and renal complications[10]. Interestingly, prediabetes, a condition that is also associated with heart failure[14], cardiovascular and all-cause mortality[15], may drive the development of AF[16]. While there is significant evidence pointing concerning the high rates of AF among individuals with T2DM, there is no data on the prevalence of T2DM among AF populations. Thus, the bidirectional relationship between those two entities could only be speculated at present.
The presence of both T2DM and AF can present more complications than each individual entity. In 2022, a meta-analysis of 21 studies concluded that AF patients with T2DM run a higher cardiovascular and all-cause mortality risk[17]. Similarly, in the much earlier ADVANCE study, T2DM patients with AF had an increased risk of major cardiovascular and cerebrovascular events, as well as of cardiovascular and all-cause mortality death, when compared to diabetic patients without AF[18]. Similar results were presented by the ORBIT-AF study, as high symptom burden, low life quality, cardiovascular and overall mortality were higher for AF patients with T2DM compared to AF patients without T2DM[3]. The 2021 Swiss-AF study also claimed that AF patients with T2DM are less self-aware of AF symptoms and maybe should be systematically screened for silent AF[19]. Moreover, although individuals with T2DM may exhibit a higher thrombotic risk, the rates of electrical cardioversion and catheter ablation use are significantly lower compared to non-T2DM individuals, as shown in the EORP-AF general pilot registry report[1].
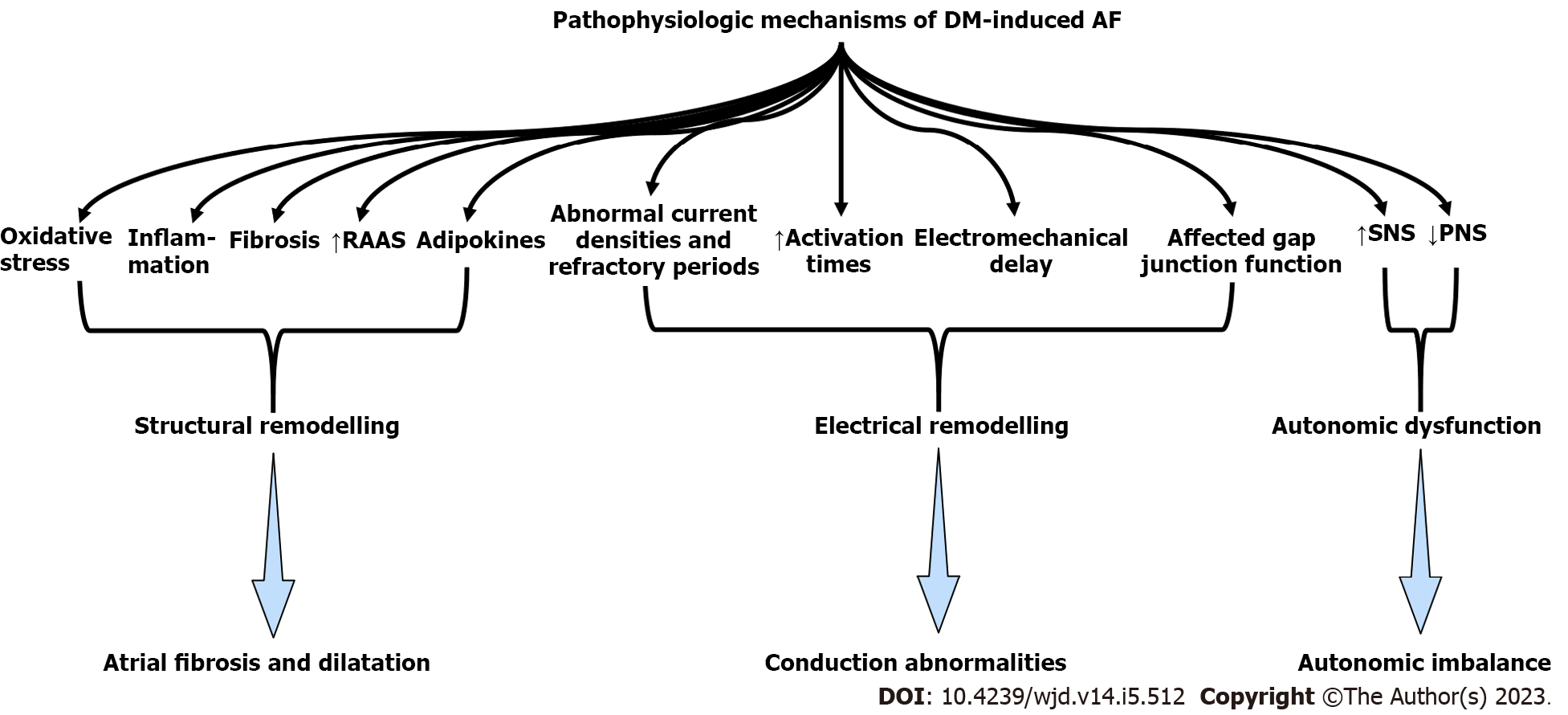
All pathophysiologic mechanisms are depicted in Table 1 and Figure 1. The most prominent structural modification that AF causes is atrial dilatation and fibrosis. Interestingly, atrial dilatation and fibrosis can result in AF development. In this context, as myocardial fibrosis is independently associated with T2DM, diabetic patients have a prominent substrate for developing AF[4,20]. More specifically, the cellular and molecular underlying mechanisms linking T2DM to myocardial fibrosis include inflammation and oxidative stress deriving from prolonged hyperglycemia[20]. Both increased production of reactive oxygen species (ROS) and decreased expression of enzymes that downregulate ROS have been revealed in diabetic patients, suggesting a high oxidative stress burden[21,22]. A high oxidative stress burden can both result in and aggravate pre-existing inflammation and inflammatory markers such as C-reactive protein and tumor necrosis factor-α, associated with left atrial dilatation and increased AF incidence[23-25]. Furthermore, high levels of ROS result in the activation of fibrotic pathways (i.e., nuclear factor-kappaB pathway) that can result in atrial fibrosis[21].
| Involved mechanism | Result | |
| Structural remodelling | Inflammation | Atrial fibrosis and dilatation |
| Oxidative stress | ||
| Expression of profibrotic growth factors | ||
| Enhanced collagen synthesis and high fibroblast activity | ||
| Activation of the (RAAS) system | ||
| Obesity and adiposity | ||
| Electrical remodelling | Longer activation times | Conduction abnormalities |
| Abnormal current densities and refractory periods | ||
| Electromechanical delay | ||
| Affected gap junction function | ||
| Autonomic dysfunction | Downsizing of parasympathetic nervous system | Autonomic imbalance |
| Upregulation of sympathetic stimuli | ||
| Glycemic parameters | Sympathetic activation due to hypoglycaemia | AF susceptibility |
| Remodelling due to chronic hyperglycemia | ||
| Oxidative stress and fibrosis due to glycemic fluctuations | ||
| Fibrosis due to adipokines |
Furthermore, T2DM upregulates the expression of profibrotic growth factors, such as transforming growth factor (TGF)-β, which activates profibrotic pathways[20,26]. In addition, the increased production of advanced glycation end-products (AGE)s and AGE receptors that derive from T2DM also contributes to atrial fibrosis by upregulating connective tissue growth factors[27]. Fibrosis can slow down atrial conduction and create the substrate for re-entry[28]. Notably, diabetic hearts exhibit enhanced levels of collagen synthesis and high fibroblast activity[29]. We should also mention that the levels of myocardial fibrosis biomarkers, including ST2 and galectin-3, could indicate structural remodeling[25].
In addition, the renin-angiotensin-aldosterone system has also been implicated in promoting fibrosis through the TGF-β signaling pathway[4,20]. Angiotensin II is known to induce cardiac fibrosis[30]. Besides the atria, myocardial fibrosis can also occur in the ventricular myocardium of diabetic patients, resulting in stiffening and diastolic dysfunction of the left ventricle, which is associated with left atrium enlargement[31].
Adiposity may also contribute to atrial interstitial fibrosis and concomitant conduction abnormalities[30]. Obesity is associated with T2DM and lipomatous metaplasia of the heart[31]. In an animal model of a high-caloric diet, authors reported left atrial enlargement, bi-atrial conduction abnormalities, and an increased propensity for inducible and spontaneous AF among the findings[32,33].
Another pathway that may lead to the development of AF in diabetic patients is electrical and electromechanical remodeling. Patients with abnormal glucose metabolism may present conduction abnormalities, such as longer activation times[34]. Experimental data from animal studies suggest that T2DM is linked to abnormal electrical current densities, atrial conduction, and refractory periods, all increasing susceptibility to AF[26,35]. In addition to the electrical and conduction remodeling, T2DM can affect the atrial excitation-contraction coupling, resulting in electromechanical delay (EMD) and arrhythmogenesis, as EMD is an independent predictor of both new and recurrent AF[36,37]. Interestingly, diabetic patients tend to have a higher recurrence of AF after ablation, possibly due to a proarrhythmic substrate caused by electrical remodeling[34]. Furthermore, prolonged conduction times were found in patients with abnormal fasting glucose[38], while EMDs in the atrium are higher in patients with T2DM[37].
Atrial action potential morphology altercations due to ionic currents can alter conduction velocity or susceptibility to triggered activity. In addition, gap junction function may also be affected in the atria of diabetic patients, possibly due to changes in the expression or localization of connexins[30].
Autonomic dysfunction can also contribute to the development of AF in diabetic patients. Cardiac autonomic neuropathy caused by T2DM contributes to the downsizing of parasympathetic and upregulation of the sympathetic stimuli, resulting in an autonomic imbalance that can excite an arrhythmia, such as AF[39]. A cross-sectional controlled study of 1992 T2DM patients suggested a strong relationship between autonomic dysfunction and silent AF in T2DM originating from autonomic dysfunction[40].
Patients with T2DM may suffer from hypoglycemia, which can propagate sympathetic activation and overdrive, resulting in an increased risk of AF[41]. The fact that intensive glycemic control does not lower the risk of AF may be attributed to the sympathetic overdrive caused by severe hypoglycemia[42]. On the other hand, chronic hyperglycemia also creates a substrate for atrial remodeling and initiation of AF[4,26]. Hyperglycemia is also associated with enhanced angiotensin II signaling ROS production[43]. Furthermore, high glucose levels can enhance fibrosis through the production of AGEs, which can regulate cardiac fibroblasts by activating their surface receptors[27]. Studies have found, though, that it is actually glycemic fluctuations, rather than chronic hyperglycemia, that may increase the risk of AF, as they can cause oxidative stress and atrial fibrosis[42,44]. Moreover, a 2017 study revealed that long-term glycemic variability is associated with new-onset AF[45]. It has been suggested that AF and T2DM may share thrombotic pathways. Patients with T2DM suffer from insulin resistance as part of their metabolic profile. In itself, insulin resistance is associated with hypercoagulability, platelet hypersensitivity, endothelial dysfunction, and impaired fibrinolysis, all of which result in high thromboembolic risk[46]. Last, adipokines, signaling modules produced in the epicardial fat layer, have been implicated in the pathophysiology of AF in diabetic patients[30]. Leptin has been found to be associated with atrial fibrosis and AF susceptibility[47]. Other adipokines, such as secreted frizzled-related protein 5, may represent important biomarkers in the risk prediction and management of diabetic complications such as heart failure[48], since they are implicated in mitochondrial energetics, oxidative stress, and apoptosis pathways[49]. However, their role in AF has not been thoroughly assessed. Insulin resistance and adiposity are also considered the main contributors to nonalcoholic fatty liver disease development, a condition that is linked to AF development[50].
Regarding the treatment of diabetic patients, medication should aim to lower blood glucose levels and prevent glycemic fluctuations. Various oral medications are currently being used to treat T2DM, several of which have been associated with a lower risk of AF, as shown in Table 2[4]. Metformin is the most commonly prescribed oral medication. By inhibiting hepatic gluconeogenesis, opposing the action of glucagon, and increasing insulin sensitivity, it exerts its glucose-lowering action. Moreover, its use has been associated with a lower risk for new-onset AF[51]. Several mechanisms have been implicated, including the prevention of the structural and electrical remodeling of left atrium via attenuating intracellular ROS, activation of 5′ adenosine monophosphate-activated protein kinase, improvement of calcium homeostasis, attenuation of inflammation, increase in connexin-43 gap junction expression, and restoration of small conductance calcium-activated potassium channels current[52]. Thiazolidinediones (TZD) increase insulin sensitivity by acting on adipose, muscle, and, to a lesser extent, liver to increase glucose utilization and decrease glucose production. Antioxidant effects may be additionally evident, through proliferator-activated receptor-γ agonism and stimulation of catalase[53]. They are also associated with a lower risk of new-onset AF, possibly due to their anti-fibrotic effect[54]; a meta-analysis identified that the risk was reduced by 27% for patients treated with TZDs compared to the control group, especially pioglitazone[55]. On the other hand, sulfonylureas, a widely prescribed second-line hypoglycemic drug category that directly stimulates insulin release from pancreatic beta cells, is not associated with a lower risk for AF[56]. Of interest, sulfonylureas are associated with severe hypoglycemic effects, a substrate for AF development[57]. Insulin therapy has been associated with an increased risk for AF occurrence, possibly due to its hypoglycemic effect[58]. A large study, however, reported no increase in AF incidence with the use of insulin glargine vs standard care[59].
| Ref. | Medication | Study design | Effect |
| Chang et al[51] | Metformin | Non-RCT | Lower risk of new-onset AF (HR: 0.81, 95%CI: 0.76-0.86, P < 0.0001) |
| Zhang et al[55] | TZD | MA | Approximately 30% lower risk of developing AF compared to controls, only in observational studies |
| Chang et al[60] | DPP4i | Non-RCT | DPP4i users were associated with a lower risk of new-onset AF compared with non-DPP4i |
| Monami et al[66] | GLP1-RA | MA | No effect on AF incidence (OR: 0.87, 95%CI: 0.71-1.05, P = 0.15) |
| Zelniker et al[71] | SGLT2i | RCT | Reduced AF risk (HR: 0.81, 95%CI: 0.68-0.95, P = 0.009) |
| Fernandes et al[73] | SGLT2i | MA | Reduced incidence of atrial arrhythmias (OR: 0.81, 95%CI: 0.69-0.95, P = 0.008) |
| Engström et al[77] | SGLT2i | Non-RCT | SGLT2i modestly reduced AF risk compared to GLP1-RA (adjusted HR: 0.89, 95%CI: 0.81-0.96) |
| Lee et al[80] | SGLT2i | Non-RCT | Lower risk of incident AF compared to DPP4i (HR: 0.68, 95%CI: 0.56, 0.83, P = 0.0001) |
Moving to novel antidiabetic agents, dipeptidyl peptidase-4 (DPP-4) inhibitors are glucose-lowering agents that inhibit DPP-4 activity in peripheral plasma, which prevents the inactivation of the incretin hormone glucagon-like peptide-1 (GLP1) in the peripheral circulation. Those agents were found to produce a lower risk of AF when compared to other antidiabetic medications, as shown in a previous study[60]. However, large trials have not revealed a correlation between DPP-4 inhibitors and the incidence of AF[61,62]. Another new class of antidiabetic drugs, GLP1 receptor agonists, are a potent glucose-lowering option by stimulating glucose-dependent insulin release from the pancreatic islets. They exhibit many cardioprotective effects, including antioxidant responses through the upregulation of antioxidant substances (catalase, glutathione peroxidase)[63]. However, they have not been associated with the incidence of AF in large trials; thus, no association between them and AF has been established[64-66].
Sodium-glucose cotransporter-2 (SGLT2) inhibitors lower plasma glucose levels by blocking the reabsorption of filtered glucose at the level of the kidneys. These agents have established cardioprotective effects[67,68], which are dependent on numerous molecular mechanisms, including restoration of beneficial autophagy, antioxidant[63], anti-inflammatory[69,70], and anti-fibrotic responses. SGLT2 inhibitors appear to affect the AF burden. A post-hoc analysis of the DECLARE-TIMI 58 trial reported decreased AF and atrial flutter episodes in individuals with T2DM on dapagliflozin regardless of AF history[71]. Even though the findings from the canagliflozin trial program were neutral[72], recent meta-analyses of randomized controlled trials point to a significant reduction of atrial arrhythmias compared to placebo[73-75]. It also has to be noted that treatment with an SGLT2 inhibitor that was accompanied by a greater than 30% initial decline in the estimated glomerular filtration rate led to a higher risk of AF incidence[76]. In a recently reported Scandinavian cohort study of 79343 new users of SGLT2 inhibitors and 57613 new users of GLP1 receptor agonists, the former was associated with a modestly reduced risk of new-onset AF[77]. Similar findings have also been reported in large registry data analyses[78-80]. Moreover, in elderly individuals with T2DM, the initiation of an SGLT2 inhibitor was accompanied by a lower incidence of AF across the follow-up[81].
Experimental studies have been conducted to assess the antiarrhythmic mechanisms of SGLT2 inhibitors. Shao et al[82] initially demonstrated the reversal of atrial structural and electrical remodeling induced by T2DM in rats following treatment with empagliflozin. This effect was possibly mediated by the peroxisome proliferator-activated receptor-c coactivator 1α/nuclear respiratory factor-1/mitochondrial transcription factor A signaling pathway[82]. Moreover, the administration of canagliflozin in an experimental model of rapid atrial pacing resulted in a diminished atrial refractory period reduction, suppressed AF inducibility, attenuated atrial interstitial fibrosis, and oxidative stress[83]. A decreased inducibility and duration of pacing-induced AF were also reported in a rat model of mitral regurgitation following treatment with dapagliflozin[84]. Overall, the published preclinical and clinical data regarding the effect of SGLT2 inhibitors on AF appears promising, while appropriately designed randomized controlled trials are warranted to provide further insight into their antiarrhythmogenic potential.
Anticoagulants: While AF is independently associated with a high risk of stroke, it seems that DM has an additive effect on the established risk. More specifically, T2DM is associated with a 70% relative increase in the risk of stroke for patients with AF[85]. Of importance, T2DM, as a comorbidity, is included in CHAD2DS2-VASc risk score, which is the pillar of risk assessment and anticoagulation management[86]. A cohort of 37358 diabetic patients with AF demonstrated that elevated HbA1c levels were associated with an increased risk of stroke[87]. A nationwide cohort study concluded that while in AF patients with T2DM, long-lasting T2DM was associated with a higher risk of thromboembolism, it was not associated with a higher risk of anticoagulant-related bleeding[88]. In addition, the duration of T2DM for over three years was independently associated with a high risk of ischemic stroke for AF patients in the ATRIA study[89]. Insulin-dependent patients exhibit a worse prognosis regarding the incidence of stroke or systemic embolism when compared to diabetic patients who do not require insulin therapy[90]. In an observational cohort, prediabetes was also associated with increased risk for stroke for patients with incident non-valvular AF, even after accounting for other CHA2DS2-VASc risk factors[91]. It was also shown that T2DM in AF patients seems to increase the risk of both all-cause and cardiovascular mortality, as well as stroke. Furthermore, HbA1c values of < 6.2% for patients with both conditions predict significantly decreased all-cause and cardiovascular mortality[92].
Based on the CHAD2DS2-VASc risk score, anticoagulant treatment should be considered in every diabetic patient by default. When contemplating the anticoagulant of choice in this patient population, it has been shown that T2DM affects the time of therapeutic range for AF patients that receive warfarin, a fact that raises safety issues[93]. On the other hand, direct oral anticoagulants (DOACs) use resulted in a 20% reduction in stroke incidents and a 43% reduction in intracranial bleeding compared to warfarin[85]. Furthermore, a study showed that DOACs are as safe and efficient for people with T2DM as for non-diabetic people[94]. A study proposed that dabigatran had the lowest risk for T2DM among AF patients compared to warfarin[95]. For patients with T2DM and CHA2DS2-VASc scores ≥ 2, DOACs may be recommended over warfarin[4]. For a CHA2DS2-VASc score of 1 in AF patients with T2DM, the optimal type of coagulation has not yet been determined[4]. A 2021 systematic review examining the safety (hypoglycemia or bleeding) and efficacy (stroke or systemic embolism) of OACs in diabetic patients concluded that DOACs have a better clinical profile than warfarin[96].
Atrial appendage closure: Because of their improved safety and effectiveness profile, DOACs (apixaban, rivaroxaban, dabigatran, edoxaban) have replaced warfarin as the cornerstone of stroke prevention in AF patients. However, alternative treatments must be considered for the subset of individuals at extremely high risk of bleeding. It has long been demonstrated that the great majority (> 90%) of thrombi in nonvalvular AF originate in the left atrial appendage (LAA)[97]. This is a structure of variable form and size with neurohormonal and reservoir functions. Left atrial remodeling with changes in shape, blood flow (stasis), and the presence of trabeculations is thought to be involved in LAA thrombogenesis in AF[98]. T2DM has been associated with adverse LAA remodeling, with important prognostic implications regarding embolic events. Such alterations include the enlargement of the LAA orifice and the reduction of orifice flow velocity, as shown by Yosefy et al[99] in a retrospective study of 242 individuals with AF[99]. Interestingly, this appears to be unrelated to the coexistence of AF, as indicated by the experimental study of the same research group[100]. The reduced LAA flow velocity is proportional to the degree of T2DM control, measured by HbA1c[101].
LAA closure (LAAC) is a therapeutic option that is gaining ground in the field of stroke prophylaxis for AF[102]. Surgical LAAC is a technique with confirmed effectiveness, as demonstrated in the recently completed LAAOS-III randomized trial and a recent meta-analysis, for patients with AF who are having cardiac surgery for another cause[103,104]. However, no subgroup analysis according to T2DM status was made, and no safe conclusions can be drawn based on those studies. Percutaneous LAAC has also gained attention recently due to the safety and efficacy of the Watchman and Amplatzer devices, with noninferior outcomes compared to direct OACs in a randomized trial[105]. When examining the devices separately, the landmark trial comparing the Watchman device to warfarin in nonvalvular AF with CHADS2 score ≥ 1 revealed a decreased rate of the primary endpoint (stroke, systemic embolism, and cardiovascular/unexplained mortality) after a 3.8-year follow-up with the device implantation[106]. However, no subgroup analysis based on the presence of T2DM was performed. An upgraded version, the Watchman FLX, is also available and is associated with superior sealing, together with similar safety[107-109], but limited data on the impact of T2DM. Concerning the Amplatzer devices (Cardiac Plug and Amulet), no dedicated large randomized trials are currently available.
The outcomes of LAAC in patients with T2DM have been inconsistent across the reported cohort studies. Litwinowicz et al[110] demonstrated similar rates of thromboembolism, mortality, and bleeding events after LAAC between T2DM and non-T2DM individuals[110]. However, in a study of 807 patients undergoing LAAC, T2DM emerged as an independent predictor of the incident early mortality[111]. T2DM was also an independent determinant of hospital readmission 30 and 90 d after LAAC[112]. These T2DM-related readmissions could be more likely associated with gastrointestinal bleeding[113]. Additionally, according to a recent report from the National Cardiovascular Data Registry of 36681 patients receiving the Watchman device, T2DM was an independent variable associated with incident ischemic stroke[114]. To our knowledge, no studies with the Amplatzer devices have assessed the role of T2DM in its safety and efficacy.
Electrical and pharmacologic cardioversion: T2DM is associated, as comorbidity, with less efficacy of cardioversion. So far, various studies have shown that T2DM results in a lower cardioversion immediate success rate and lower success of sinus rhythm maintenance at 74.5 d follow-up, while it has also been identified as an independent risk factor for cardioversion failure within 30 d[115-117]. Interestingly, T2DM, higher HbA1c, digoxin treatment, and structural and functional cardiac abnormalities were identified as independent risk factors for cardioversion failure and AF recurrence in a 2018 retrospective outcome analysis[117]. In another study, however, this finding was not confirmed[118]. It should also be noted that although spontaneous cardioversion may be seen in a significant proportion of patients with AF, the rates are significantly lower in individuals with coexisting T2DM[119].
Similarly, antiarrhythmic drugs seem less effective for T2DM patients in experimental studies[120], although the evidence is scarce in the clinical setting. Kriz et al[121] did not detect a significant association between T2DM and the failure of pharmacologic cardioversion in a single-center study of 236 patients with recent-onset AF[121]. Moving to specific drug classes, in a study of 50 consecutive patients with recent-onset AF, the presence of T2DM did not affect the efficacy of cardioversion with propafenone[122]. Regarding dronedarone use in T2DM, it has been favorably associated with a lower rate of cardiovascular hospitalizations and mortality, as well as greater freedom from AF, compared to placebo[123]. At the same time, no data are available for the specific subgroup of AF patients with T2DM who receive amiodarone. However, a previous study has suggested a delayed antiarrhythmic effect of amiodarone in individuals with T2DM, partly attributed to diabetic autonomic neuropathy[124]. Often, due to concomitant QTc prolongation, silent coronary artery disease, or renal failure, patients with T2DM may be at higher risk of developing adverse effects from antiarrhythmic drug therapy[62,125]. Despite that, a study by D’Angelo et al[126] observed that patients with T2DM were less likely to discontinue the prescribed antiarrhythmic regimen[126].
Ablation: Regardless of symptoms, early rhythm management is critical in lowering the burden of AF consequences[127,128]. Percutaneous catheter AF ablation is an appealing technique for rhythm regulation. The most often used ablation treatment in electrophysiology is radiofrequency catheter ablation. It mainly consists of pulmonary vein isolation, which is thought to be a key trigger of paroxysmal AF[129]. Catheter ablation is a well-established treatment for drug-refractory, symptomatic AF with a variety of clinical benefits and better AF control for diabetic patients when compared to antiarrhythmic drugs[130]. Despite that fact, individuals with T2DM may be less likely to receive catheter ablation, as pointed out by the recent study of Quiroz et al[131]. However, the rate of T2DM patients receiving this treatment has increased over the years[132].
There have been reports of a lower efficacy of catheter ablation in individuals with T2DM than in those without T2DM. This could be due to the fact that the induced scar may impair atrial relaxation, promoting a stiff left atrial phenotype in individuals with T2DM[133]. Wang et al[134] highlighted that T2DM was associated with lower arrhythmia-free intervals in patients with T2DM after a median 29.5-mo follow-up[134]. A recent study of 369 patients with AF reported that T2DM was a predictor of AF recurrence in patients with paroxysmal AF[135]. This has not been the case in persistent AF, where the already established fibrotic changes may account for the increased risk of recurrence[136]. The performance of a second-generation, cryoballoon-based procedure may be accompanied by similar success rates in T2DM and non-T2DM patients, as pointed out by the study of Amr et al[137]. Moreover, T2DM is among the variables of the DR-FLASH score that has been utilized to identify individuals with a greater burden of arrhythmogenic substrates that may benefit from extensive ablation beyond the pulmonary veins[138,139]. T2DM is also an independent predictor of pulmonary vein stenosis after catheter ablation, as shown by the ADVICE trial[140]. It should also be mentioned that individuals with T2DM may be less likely to receive catheter ablation, as pointed out by the recent study of Quiroz et al[131]. However, the rate of T2DM patients receiving this treatment has increased over the years[132].
Other studies have concluded that there is no difference in post-ablation recurrence between diabetic and non-diabetic patients[141,142]. The degree of glycemic control might be an important confounding variable. More specifically, a 2015 metanalysis concluded that AF ablation has similar safety and efficacy for diabetic patients as for the general population, especially for younger patients with efficient glycemic control; however, it was shown that higher basal glycated hemoglobin levels were associated with a higher incidence of AF recurrence after catheter ablation[143]. Although the literature has not yet concluded, insufficiently managed T2DM may be a risk factor for AF recurrence following catheter ablation[144]. T2DM has also been correlated with a higher risk of cardioversion failure for early AF recurrence (≤ 7 d) after ablation[115].
Antidiabetic drugs may alter the efficacy of AF ablation in individuals with T2DM. Metformin was recently shown to be independently associated with a lower risk of AF recurrence in T2DM patients after catheter ablation[145]. A randomized trial contemplating the effect of SGLT2 inhibitors on AF following ablation concluded that tofogliflozin exhibited a better profile and less AF recurrence when compared to anagliptin[146]. Previously, dapagliflozin was an independent predictor of longer arrhythmia-free intervals in patients with T2DM undergoing radiofrequency catheter ablation after a mean follow-up of 15.5 mo[147].
DM and AF are widely affiliated entities. DM has been closely associated with the risk of AF development, identified as an independent risk factor for AF. Regarding cardiovascular risk and mortality, the presence of both conditions has been linked with high mortality. Even though the pathophysiology is still not fully determined, structural, electrical, and autonomic pathways have been identified as underlying mechanisms. Regarding therapy, novel antidiabetic agents and revolutionary antiarrhythmic and antithrombotic strategies are being examined concerning the optimal therapeutic plan for diabetic patients with AF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hsieh YS, Taiwan; Huang Y, China; Yang J, China; Horowitz M, Australia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56-e528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 5854] [Article Influence: 975.7] [Reference Citation Analysis (5)] |
| 2. | Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 970] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 3. | Echouffo-Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, Singer DE, Hylek EM, Go AS, Peterson ED, Piccini JP, Fonarow GC. Care Patterns and Outcomes in Atrial Fibrillation Patients With and Without Diabetes: ORBIT-AF Registry. J Am Coll Cardiol. 2017;70:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 5. | Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 6. | Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 9. | Qi W, Zhang N, Korantzopoulos P, Letsas KP, Cheng M, Di F, Tse G, Liu T, Li G. Serum glycated hemoglobin level as a predictor of atrial fibrillation: A systematic review with meta-analysis and meta-regression. PLoS One. 2017;12:e0170955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Dahlqvist S, Rosengren A, Gudbjörnsdottir S, Pivodic A, Wedel H, Kosiborod M, Svensson AM, Lind M. Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population: a prospective case-control study. Lancet Diabetes Endocrinol. 2017;5:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Gumprecht J, Lip GYH, Sokal A, Średniawa B, Mitręga K, Stokwiszewski J, Wierucki Ł, Rajca A, Rutkowski M, Zdrojewski T, Grodzicki T, Kaźmierczak J, Opolski G, Kalarus Z. Relationship between diabetes mellitus and atrial fibrillation prevalence in the Polish population: a report from the Non-invasive Monitoring for Early Detection of Atrial Fibrillation (NOMED-AF) prospective cross-sectional observational study. Cardiovasc Diabetol. 2021;20:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Seyed Ahmadi S, Svensson AM, Pivodic A, Rosengren A, Lind M. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: a Swedish cohort study. Cardiovasc Diabetol. 2020;19:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Pallisgaard JL, Schjerning AM, Lindhardt TB, Procida K, Hansen ML, Torp-Pedersen C, Gislason GH. Risk of atrial fibrillation in diabetes mellitus: A nationwide cohort study. Eur J Prev Cardiol. 2016;23:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, Huang Y. Prediabetes and the risk of heart failure: A meta-analysis. Diabetes Obes Metab. 2021;23:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 15. | Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 16. | Lind V, Hammar N, Lundman P, Friberg L, Talbäck M, Walldius G, Norhammar A. Impaired fasting glucose: a risk factor for atrial fibrillation and heart failure. Cardiovasc Diabetol. 2021;20:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Xu J, Sun Y, Gong D, Fan Y. Impact of preexisting diabetes mellitus on cardiovascular and all-cause mortality in patients with atrial fibrillation: A meta-analysis. Front Endocrinol (Lausanne). 2022;13:921159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, Woodward M, Cooper M, Harrap S, Hamet P, Poulter N, Lip GY, Patel A; ADVANCE Collaborative Group. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Bano A, Rodondi N, Beer JH, Moschovitis G, Kobza R, Aeschbacher S, Baretella O, Muka T, Stettler C, Franco OH, Conte G, Sticherling C, Zuern CS, Conen D, Kühne M, Osswald S, Roten L, Reichlin T; of the Swiss‐Investigators. Association of Diabetes With Atrial Fibrillation Phenotype and Cardiac and Neurological Comorbidities: Insights From the Swiss-AF Study. J Am Heart Assoc. 2021;10:e021800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 352] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 21. | Ziolo MT, Mohler PJ. Defining the role of oxidative stress in atrial fibrillation and diabetes. J Cardiovasc Electrophysiol. 2015;26:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 23. | Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 640] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 24. | Faria A, Persaud SJ. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol Ther. 2017;172:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 25. | Oikonomou E, Zografos T, Papamikroulis GA, Siasos G, Vogiatzi G, Theofilis P, Briasoulis A, Papaioannou S, Vavuranakis M, Gennimata V, Tousoulis D. Biomarkers in Atrial Fibrillation and Heart Failure. Curr Med Chem. 2019;26:873-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Liu C, Fu H, Li J, Yang W, Cheng L, Liu T, Li G. Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu Kardiyol Derg. 2012;12:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Kato T, Yamashita T, Sekiguchi A, Tsuneda T, Sagara K, Takamura M, Kaneko S, Aizawa T, Fu LT. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J Cardiovasc Electrophysiol. 2008;19:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Schotten U, Dobrev D, Platonov PG, Kottkamp H, Hindricks G. Current controversies in determining the main mechanisms of atrial fibrillation. J Intern Med. 2016;279:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Sedgwick B, Riches K, Bageghni SA, O'Regan DJ, Porter KE, Turner NA. Investigating inherent functional differences between human cardiac fibroblasts cultured from nondiabetic and Type 2 diabetic donors. Cardiovasc Pathol. 2014;23:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The Association Between Diabetes Mellitus and Atrial Fibrillation: Clinical and Mechanistic Insights. Front Physiol. 2019;10:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 31. | Tiwari S, Schirmer H, Jacobsen BK, Hopstock LA, Nyrnes A, Heggelund G, Njølstad I, Mathiesen EB, Løchen ML. Association between diastolic dysfunction and future atrial fibrillation in the Tromsø Study from 1994 to 2010. Heart. 2015;101:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J Am Coll Cardiol. 2015;66:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 322] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 33. | Samanta R, Pouliopoulos J, Thiagalingam A, Kovoor P. Role of adipose tissue in the pathogenesis of cardiac arrhythmias. Heart Rhythm. 2016;13:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Chao TF, Suenari K, Chang SL, Lin YJ, Lo LW, Hu YF, Tuan TC, Tai CT, Tsao HM, Li CH, Ueng KC, Wu TJ, Chen SA. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. Am J Cardiol. 2010;106:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Watanabe M, Yokoshiki H, Mitsuyama H, Mizukami K, Ono T, Tsutsui H. Conduction and refractory disorders in the diabetic atrium. Am J Physiol Heart Circ Physiol. 2012;303:H86-H95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | De Vos CB, Weijs B, Crijns HJ, Cheriex EC, Palmans A, Habets J, Prins MH, Pisters R, Nieuwlaat R, Tieleman RG. Atrial tissue Doppler imaging for prediction of new-onset atrial fibrillation. Heart. 2009;95:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Demir K, Avci A, Kaya Z, Marakoglu K, Ceylan E, Yilmaz A, Ersecgin A, Armutlukuyu M, Altunkeser BB. Assessment of atrial electromechanical delay and P-wave dispersion in patients with type 2 diabetes mellitus. J Cardiol. 2016;67:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Ayhan S, Ozturk S, Alcelik A, Ozlu MF, Erdem A, Memioglu T, Ozdemir M, Yazici M. Atrial conduction time and atrial mechanical function in patients with impaired fasting glucose. J Interv Card Electrophysiol. 2012;35:247-52; discussion 252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Rizzo MR, Sasso FC, Marfella R, Siniscalchi M, Paolisso P, Carbonara O, Capoluongo MC, Lascar N, Pace C, Sardu C, Passavanti B, Barbieri M, Mauro C, Paolisso G. Autonomic dysfunction is associated with brief episodes of atrial fibrillation in type 2 diabetes. J Diabetes Complications. 2015;29:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Ko SH, Park YM, Yun JS, Cha SA, Choi EK, Han K, Han E, Lee YH, Ahn YB. Severe hypoglycemia is a risk factor for atrial fibrillation in type 2 diabetes mellitus: Nationwide population-based cohort study. J Diabetes Complications. 2018;32:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, Bigger T, Cushman W, Goff D, Soliman EZ, Thomas A, Papademetriou V. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol. 2014;114:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 43. | Fiaschi T, Magherini F, Gamberi T, Lucchese G, Faggian G, Modesti A, Modesti PA. Hyperglycemia and angiotensin II cooperate to enhance collagen I deposition by cardiac fibroblasts through a ROS-STAT3-dependent mechanism. Biochim Biophys Acta. 2014;1843:2603-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1788] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 45. | Gu J, Fan YQ, Zhang JF, Wang CQ. Impact of long-term glycemic variability on development of atrial fibrillation in type 2 diabetic patients. Anatol J Cardiol. 2017;18:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Li X, Weber NC, Cohn DM, Hollmann MW, DeVries JH, Hermanides J, Preckel B. Effects of Hyperglycemia and Diabetes Mellitus on Coagulation and Hemostasis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 47. | Fukui A, Takahashi N, Nakada C, Masaki T, Kume O, Shinohara T, Teshima Y, Hara M, Saikawa T. Role of leptin signaling in the pathogenesis of angiotensin II-mediated atrial fibrosis and fibrillation. Circ Arrhythm Electrophysiol. 2013;6:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Wu J, Zheng H, Liu X, Chen P, Zhang Y, Luo J, Kuang J, Li J, Yang Y, Ma T, Huang X, Liang G, Liang D, Hu Y, Wu JHY, Arnott C, Mai W, Huang Y. Prognostic Value of Secreted Frizzled-Related Protein 5 in Heart Failure Patients With and Without Type 2 Diabetes Mellitus. Circ Heart Fail. 2020;13:e007054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, Liang J, Cao Y, Hu Y, Huang Y. SFRP2 Improves Mitochondrial Dynamics and Mitochondrial Biogenesis, Oxidative Stress, and Apoptosis in Diabetic Cardiomyopathy. Oxid Med Cell Longev. 2021;2021:9265016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 50. | Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. 2020;40:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 52. | Nantsupawat T, Wongcharoen W, Chattipakorn SC, Chattipakorn N. Effects of metformin on atrial and ventricular arrhythmias: evidence from cell to patient. Cardiovasc Diabetol. 2020;19:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Chung SS, Kim M, Lee JS, Ahn BY, Jung HS, Lee HM, Park KS. Mechanism for antioxidative effects of thiazolidinediones in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2011;301:E912-E921. [PubMed] [DOI] [Full Text] |
| 54. | Kume O, Takahashi N, Wakisaka O, Nagano-Torigoe Y, Teshima Y, Nakagawa M, Yufu K, Hara M, Saikawa T, Yoshimatsu H. Pioglitazone attenuates inflammatory atrial fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats. Heart Rhythm. 2011;8:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Zhang Z, Zhang X, Korantzopoulos P, Letsas KP, Tse G, Gong M, Meng L, Li G, Liu T. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017;17:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 56. | Chen HY, Yang FY, Jong GP, Liou YS. Antihyperglycemic drugs use and new-onset atrial fibrillation in elderly patients. Eur J Clin Invest. 2017;47:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Yu O, Azoulay L, Yin H, Filion KB, Suissa S. Sulfonylureas as Initial Treatment for Type 2 Diabetes and the Risk of Severe Hypoglycemia. Am J Med. 2018;131:317.e11-317.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Liou YS, Yang FY, Chen HY, Jong GP. Antihyperglycemic drugs use and new-onset atrial fibrillation: A population-based nested case control study. PLoS One. 2018;13:e0197245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | ORIGIN Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1176] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 60. | Chang CY, Yeh YH, Chan YH, Liu JR, Chang SH, Lee HF, Wu LS, Yen KC, Kuo CT, See LC. Dipeptidyl peptidase-4 inhibitor decreases the risk of atrial fibrillation in patients with type 2 diabetes: a nationwide cohort study in Taiwan. Cardiovasc Diabetol. 2017;16:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK; CARMELINA Investigators. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 783] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 62. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2570] [Article Influence: 214.2] [Reference Citation Analysis (0)] |
| 63. | Theofilis P, Vordoni A, Kalaitzidis RG. Oxidative Stress Management in Cardiorenal Diseases: Focus on Novel Antidiabetic Agents, Finerenone, and Melatonin. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1204] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 65. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1480] [Article Influence: 185.0] [Reference Citation Analysis (0)] |
| 66. | Monami M, Nreu B, Scatena A, Giannini S, Andreozzi F, Sesti G, Mannucci E. Glucagon-like peptide-1 receptor agonists and atrial fibrillation: a systematic review and meta-analysis of randomised controlled trials. J Endocrinol Invest. 2017;40:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis K, Tousoulis D. Pleiotropic effects of SGLT2 inhibitors and heart failure outcomes. Diabetes Res Clin Pract. 2022;188:109927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Theofilis P, Antonopoulos AS, Katsimichas T, Oikonomou E, Siasos G, Aggeli C, Tsioufis K, Tousoulis D. The impact of SGLT2 inhibition on imaging markers of cardiac function: A systematic review and meta-analysis. Pharmacol Res. 2022;180:106243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 69. | Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis K, Tousoulis D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int Immunopharmacol. 2022;111:109080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 70. | Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis K, Tousoulis D. The Anti-Inflammatory Effect of Novel Antidiabetic Agents. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Budaj A, Kiss RG, Padilla F, Gause-Nilsson I, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation. 2020;141:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 72. | Li C, Yu J, Hockham C, Perkovic V, Neuen BL, Badve SV, Houston L, Lee VYJ, Barraclough JY, Fletcher RA, Mahaffey KW, Heerspink HJL, Cannon CP, Neal B, Arnott C. Canagliflozin and atrial fibrillation in type 2 diabetes mellitus: A secondary analysis from the CANVAS Program and CREDENCE trial and meta-analysis. Diabetes Obes Metab. 2022;24:1927-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Fernandes GC, Fernandes A, Cardoso R, Penalver J, Knijnik L, Mitrani RD, Myerburg RJ, Goldberger JJ. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021;18:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 74. | Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C, Li KHC, Tang Y, Wei Y, Tse G, Xia Y. Protective Effects of Sodium-Glucose Transporter 2 Inhibitors on Atrial Fibrillation and Atrial Flutter: A Systematic Review and Meta- Analysis of Randomized Placebo-Controlled Trials. Front Endocrinol (Lausanne). 2021;12:619586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Li HL, Lip GYH, Feng Q, Fei Y, Tse YK, Wu MZ, Ren QW, Tse HF, Cheung BY, Yiu KH. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021;20:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 76. | Chan YH, Chen SW, Chao TF, Kao YW, Huang CY, Chu PH. Impact of the initial decline in estimated glomerular filtration rate on the risk of new-onset atrial fibrillation and adverse cardiovascular and renal events in patients with type 2 diabetes treated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2021;23:2077-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Engström A, Wintzell V, Melbye M, Hviid A, Eliasson B, Gudbjörnsdottir S, Hveem K, Jonasson C, Svanström H, Pasternak B, Ueda P. Sodium-Glucose Cotransporter 2 Inhibitor Treatment and Risk of Atrial Fibrillation: Scandinavian Cohort Study. Diabetes Care. 2023;46:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 78. | Chan YH, Chao TF, Chen SW, Lee HF, Li PR, Chen WM, Yeh YH, Kuo CT, See LC, Lip GYH. The risk of incident atrial fibrillation in patients with type 2 diabetes treated with sodium glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and dipeptidyl peptidase-4 inhibitors: a nationwide cohort study. Cardiovasc Diabetol. 2022;21:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Hsiao FC, Yen KC, Chao TF, Chen SW, Chan YH, Chu PH. New-Onset Atrial Fibrillation in Patients With Type 2 Diabetes Treated With Novel Glucose-Lowering Therapies. J Clin Endocrinol Metab. 2022;107:2493-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Lee S, Zhou J, Leung KSK, Wai AKC, Jeevaratnam K, King E, Liu T, Wong WT, Chang C, Wong ICK, Cheung BMY, Tse G, Zhang Q. Comparison of Sodium-Glucose Cotransporter-2 Inhibitor and Dipeptidyl Peptidase-4 Inhibitor on the Risks of New-Onset Atrial Fibrillation, Stroke and Mortality in Diabetic Patients: A Propensity Score-Matched Study in Hong Kong. Cardiovasc Drugs Ther. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 81. | Zhuo M, D'Andrea E, Paik JM, Wexler DJ, Everett BM, Glynn RJ, Kim SC, Patorno E. Association of Sodium-Glucose Cotransporter-2 Inhibitors With Incident Atrial Fibrillation in Older Adults With Type 2 Diabetes. JAMA Netw Open. 2022;5:e2235995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 83. | Nishinarita R, Niwano S, Niwano H, Nakamura H, Saito D, Sato T, Matsuura G, Arakawa Y, Kobayashi S, Shirakawa Y, Horiguchi A, Ishizue N, Igarashi T, Yoshizawa T, Oikawa J, Hara Y, Katsumura T, Kishihara J, Satoh A, Fukaya H, Sakagami H, Ako J. Canagliflozin Suppresses Atrial Remodeling in a Canine Atrial Fibrillation Model. J Am Heart Assoc. 2021;10:e017483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 84. | Lin YW, Chen CY, Shih JY, Cheng BC, Chang CP, Lin MT, Ho CH, Chen ZC, Fisch S, Chang WT. Dapagliflozin Improves Cardiac Hemodynamics and Mitigates Arrhythmogenesis in Mitral Regurgitation-Induced Myocardial Dysfunction. J Am Heart Assoc. 2021;10:e019274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 85. | Patti G, Cavallari I, Andreotti F, Calabrò P, Cirillo P, Denas G, Galli M, Golia E, Maddaloni E, Marcucci R, Parato VM, Pengo V, Prisco D, Ricottini E, Renda G, Santilli F, Simeone P, De Caterina R; Working Group on Thrombosis of the Italian Society of Cardiology. Prevention of atherothrombotic events in patients with diabetes mellitus: from antithrombotic therapies to new-generation glucose-lowering drugs. Nat Rev Cardiol. 2019;16:113-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 86. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5167] [Cited by in RCA: 4874] [Article Influence: 541.6] [Reference Citation Analysis (0)] |
| 87. | Saliba W, Barnett-Griness O, Elias M, Rennert G. Glycated hemoglobin and risk of first episode stroke in diabetic patients with atrial fibrillation: A cohort study. Heart Rhythm. 2015;12:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Overvad TF, Skjøth F, Lip GY, Lane DA, Albertsen IE, Rasmussen LH, Larsen TB. Duration of Diabetes Mellitus and Risk of Thromboembolism and Bleeding in Atrial Fibrillation: Nationwide Cohort Study. Stroke. 2015;46:2168-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 89. | Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, Singer DE. Effect of Diabetes and Glycemic Control on Ischemic Stroke Risk in AF Patients: ATRIA Study. J Am Coll Cardiol. 2016;67:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 90. | Patti G, Lucerna M, Cavallari I, Ricottini E, Renda G, Pecen L, Romeo F, Le Heuzey JY, Zamorano JL, Kirchhof P, De Caterina R. Insulin-Requiring Versus Noninsulin-Requiring Diabetes and Thromboembolic Risk in Patients With Atrial Fibrillation: PREFER in AF. J Am Coll Cardiol. 2017;69:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Kezerle L, Tsadok MA, Akriv A, Senderey AB, Bachrach A, Leventer-Roberts M, Haim M. Pre-Diabetes Increases Stroke Risk in Patients With Nonvalvular Atrial Fibrillation. J Am Coll Cardiol. 2021;77:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Papazoglou AS, Kartas A, Samaras A, Vouloagkas I, Vrana E, Moysidis DV, Akrivos E, Kotzampasis G, Baroutidou A, Papanastasiou A, Liampas E, Botis M, Karagiannidis E, Stalikas N, Karvounis H, Tzikas A, Giannakoulas G. Prognostic significance of diabetes mellitus in patients with atrial fibrillation. Cardiovasc Diabetol. 2021;20:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 93. | Nelson WW, Choi JC, Vanderpoel J, Damaraju CV, Wildgoose P, Fields LE, Schein JR. Impact of co-morbidities and patient characteristics on international normalized ratio control over time in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2013;112:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Itzhaki Ben Zadok O, Eisen A. Use of non-vitamin K oral anticoagulants in people with atrial fibrillation and diabetes mellitus. Diabet Med. 2018;35:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Cheung CL, Sing CW, Lau WCY, Li GHY, Lip GYH, Tan KCB, Cheung BMY, Chan EWY, Wong ICK. Treatment with direct oral anticoagulants or warfarin and the risk for incident diabetes among patients with atrial fibrillation: a population-based cohort study. Cardiovasc Diabetol. 2021;20:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Alwafi H, Alotaibi B, Naser AY, Salawati E, Qadus S, Sweiss K, Dairi MS, Hassouneh L, Aldalameh Y, Samannodi M. The safety and efficacy of the use of oral anticoagulant medications in patients with diabetes mellitus: A systematic review. Saudi Pharm J. 2021;29:1374-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 97. | Zhang H, Yu M, Xia Y, Li X, Liu J, Fang P. The differences of atrial thrombus locations and variable response to anticoagulation in nonvalvular atrial fibrillation with ventricular cardiomyopathy. J Arrhythm. 2020;36:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 459] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 99. | Yosefy C, Pery M, Nevzorov R, Piltz X, Osherov A, Jafari J, Beeri R, Gallego-Colon E, Daum A, Khalameizer V. Difference in left atrial appendage remodeling between diabetic and nondiabetic patients with atrial fibrillation. Clin Cardiol. 2020;43:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Yosefy O, Sharon B, Yagil C, Shlapoberski M, Livoff A, Novitski I, Beeri R, Yagil Y, Yosefy C. Diabetes induces remodeling of the left atrial appendage independently of atrial fibrillation in a rodent model of type-2 diabetes. Cardiovasc Diabetol. 2021;20:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Wei Y, Cui M, Liu S, Yu H, Feng J, Gao W, Li L. Increased hemoglobin A1c level associates with low left atrial appendage flow velocity in patients of atrial fibrillation. Nutr Metab Cardiovasc Dis. 2021;31:3176-3183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Theofilis P, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis K, Tousoulis D. Percutaneous Treatment Approaches in Atrial Fibrillation: Current Landscape and Future Perspectives. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 103. | Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, Reents W, Budera P, Baddour AJ, Fila P, Devereaux PJ, Bogachev-Prokophiev A, Boening A, Teoh KHT, Tagarakis GI, Slaughter MS, Royse AG, McGuinness S, Alings M, Punjabi PP, Mazer CD, Folkeringa RJ, Colli A, Avezum Á, Nakamya J, Balasubramanian K, Vincent J, Voisine P, Lamy A, Yusuf S, Connolly SJ; LAAOS III Investigators. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med. 2021;384:2081-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 425] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 104. | Nso N, Nassar M, Zirkiyeva M, Lakhdar S, Shaukat T, Guzman L, Alshamam M, Foster A, Bhangal R, Badejoko S, Lyonga Ngonge A, Tabot-Tabot M, Mbome Y, Rizzo V, Munira MS, Thambidorai S. Outcomes of cardiac surgery with left atrial appendage occlusion versus no Occlusion, direct oral Anticoagulants, and vitamin K Antagonists: A systematic review with Meta-analysis. Int J Cardiol Heart Vasc. 2022;40:100998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, Poloczek M, Stasek J, Haman L, Branny M, Chovancik J, Cervinka P, Holy J, Kovarnik T, Zemanek D, Havranek S, Vancura V, Opatrny J, Peichl P, Tousek P, Lekesova V, Jarkovsky J, Novackova M, Benesova K, Widimsky P, Reddy VY; PRAGUE-17 Trial Investigators. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J Am Coll Cardiol. 2020;75:3122-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 403] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 106. | Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, Gordon N, Holmes D; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 731] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 107. | Paitazoglou C, Meincke F, Bergmann MW, Eitel I, Fink T, Vireca E, Wohlmuth P, Veliqi E, Willems S, Markiewicz A, Grygier M. The ALSTER-FLX Registry: 3-Month outcomes after left atrial appendage occlusion using a next-generation device, a matched-pair analysis to EWOLUTION. Heart Rhythm. 2022;19:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 108. | Vizzari G, Grasso C, Sardone A, Mazzone P, Laterra G, Frazzetto M, Sacchetta G, Micari A, Tamburino C, Contarini M. Real-world experience with the new Watchman FLX device: Data from two high-volume Sicilian centers. The FLX-iEST registry. Catheter Cardiovasc Interv. 2022;100:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Galea R, Mahmoudi K, Gräni C, Elhadad S, Huber AT, Heg D, Siontis GCM, Brugger N, Sebag F, Windecker S, Valgimigli M, Landolff Q, Roten L, Amabile N, Räber L. Watchman FLX vs. Watchman 2.5 in a Dual-Center Left Atrial Appendage Closure Cohort: the WATCH-DUAL study. Europace. 2022;24:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 110. | Litwinowicz R, Bartus M, Ceranowicz P, Brzezinski M, Kapelak B, Lakkireddy D, Bartus K. Left atrial appendage occlusion for stroke prevention in diabetes mellitus patients with atrial fibrillation: Long-term results. J Diabetes. 2019;11:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Mesnier J, Cruz-González I, Arzamendi D, Freixa X, Nombela-Franco L, Peral V, Caneiro-Queija B, Mangieri A, Trejo-Velasco B, Asmarats L, Regueiro A, McInerney A, Mas-Lladó C, Estevez-Loureiro R, Laricchia A, O'Hara G, Rodés-Cabau J. Incidence and Predictors of Early Death in Patients Undergoing Percutaneous Left Atrial Appendage Closure. JACC Clin Electrophysiol. 2022;8:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 112. | Murthi M, Vardar U, Sana MK, Shaka H. Causes and predictors of immediate and short-term readmissions following percutaneous left atrial appendage closure procedure. J Cardiovasc Electrophysiol. 2022;33:2213-2216. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 113. | Morita S, Malik AH, Kuno T, Ando T, Kaul R, Yandrapalli S, Briasoulis A. Analysis of outcome of 6-month readmissions after percutaneous left atrial appendage occlusion. Heart. 2022;108:606-612. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 114. | Price MJ, Slotwiner D, Du C, Freeman JV, Turi Z, Rammohan C, Kusumoto FM, Kavinsky C, Akar J, Varosy PD, Koutras C, Curtis JP, Masoudi FA. Clinical Outcomes at 1 Year Following Transcatheter Left Atrial Appendage Occlusion in the United States. JACC Cardiovasc Interv. 2022;15:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 115. | Ebert M, Stegmann C, Kosiuk J, Dinov B, Richter S, Arya A, Müssigbrodt A, Sommer P, Hindricks G, Bollmann A. Predictors, management, and outcome of cardioversion failure early after atrial fibrillation ablation. Europace. 2018;20:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Grönberg T, Hartikainen JE, Nuotio I, Biancari F, Vasankari T, Nikkinen M, Ylitalo A, Airaksinen KE. Can we predict the failure of electrical cardioversion of acute atrial fibrillation? Pacing Clin Electrophysiol. 2015;38:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | Soran H, Banerjee M, Mohamad JB, Adam S, Ho JH, Ismaeel SM, Dhage S, Syed AA, Abdulla IMA, Younis N, Malik RA. Risk Factors for Failure of Direct Current Cardioversion in Patients with Type 2 Diabetes Mellitus and Atrial Fibrillation. Biomed Res Int. 2018;2018:5936180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Voskoboinik A, Kalman E, Plunkett G, Knott J, Moskovitch J, Sanders P, Kistler PM, Kalman JM. A comparison of early versus delayed elective electrical cardioversion for recurrent episodes of persistent atrial fibrillation: A multi-center study. Int J Cardiol. 2019;284:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Abadie BQ, Hansen B, Walker J, Deyo Z, Biese K, Armbruster T, Tuttle H, Sadaf MI, Sears SF, Pasi R, Gehi AK. Likelihood of Spontaneous Cardioversion of Atrial Fibrillation Using a Conservative Management Strategy Among Patients Presenting to the Emergency Department. Am J Cardiol. 2019;124:1534-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Ito I, Hayashi Y, Kawai Y, Iwasaki M, Takada K, Kamibayashi T, Yamatodani A, Mashimo T. Diabetes mellitus reduces the antiarrhythmic effect of ion channel blockers. Anesth Analg. 2006;103:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 121. | Kriz R, Freynhofer MK, Weiss TW, Egger F, Gruber SC, Eisenburger P, Wojta J, Huber K, Koch J. Safety and efficacy of pharmacological cardioversion of recent-onset atrial fibrillation: a single-center experience. Am J Emerg Med. 2016;34:1486-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 122. | Ergene U, Ergene O, Cete Y, Fowler J, Nazli C, Oktay C. Predictors of success in the conversion of new-onset atrial fibrillation using oral propafenone. Eur J Emerg Med. 1998;5:425-428. [PubMed] |
| 123. | Handelsman Y, Bunch TJ, Rodbard HW, Steinberg BA, Thind M, Bigot G, Konigsberg L, Wieloch M, Kowey PR. Impact of dronedarone on patients with atrial fibrillation and diabetes: A sub-analysis of the ATHENA and EURIDIS/ADONIS studies. J Diabetes Complications. 2022;36:108227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 124. | Iervasi G, Clerico A, Bonini R, Nannipieri M, Manfredi C, Sabatino L, Biagini A, Donato L. Effect of antiarrhythmic therapy with intravenous loading dose of amiodarone: evidence for an altered response in diabetic patients. Int J Clin Pharmacol Res. 1998;18:109-120. [PubMed] |
| 125. | Veglio M, Bruno G, Borra M, Macchia G, Bargero G, D'Errico N, Pagano GF, Cavallo-Perin P. Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J Intern Med. 2002;251:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 126. | D'Angelo RN, Rahman M, Khanna R, Yeh RW, Goldstein L, Yadalam S, Kalsekar I, Tung P, Zimetbaum PJ. Limited duration of antiarrhythmic drug use for newly diagnosed atrial fibrillation in a nationwide population under age 65. J Cardiovasc Electrophysiol. 2021;32:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 127. | Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G; EAST-AFNET 4 Trial Investigators. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. 2020;383:1305-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 1344] [Article Influence: 268.8] [Reference Citation Analysis (0)] |
| 128. | Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Ng GA, Schnabel RB, Suling A, Szumowski L, Themistoclakis S, Vardas P, van Gelder IC, Wegscheider K, Kirchhof P. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 129. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5432] [Cited by in RCA: 5383] [Article Influence: 199.4] [Reference Citation Analysis (0)] |
| 130. | Forleo GB, Mantica M, De Luca L, Leo R, Santini L, Panigada S, De Sanctis V, Pappalardo A, Laurenzi F, Avella A, Casella M, Dello Russo A, Romeo F, Pelargonio G, Tondo C. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol. 2009;20:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 131. | Quiroz JC, Brieger D, Jorm LR, Sy RW, Falster MO, Gallego B. An Observational Study of Clinical and Health System Factors Associated With Catheter Ablation and Early Ablation Treatment for Atrial Fibrillation in Australia. Heart Lung Circ. 2022;31:1269-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 132. | Ngo L, Ali A, Ganesan A, Woodman R, Adams R, Ranasinghe I. Ten-year trends in mortality and complications following catheter ablation of atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2022;8:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 133. | Kim MH, Yu HT, Park YJ, Kim TH, Joung B, Lee MH, Pak HN. Diabetes Mellitus Is an Independent Risk Factor for a Stiff Left Atrial Physiology After Catheter Ablation for Atrial Fibrillation. Front Cardiovasc Med. 2022;9:828478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 134. | Wang A, Truong T, Black-Maier E, Green C, Campbell KB, Barnett AS, Febre J, Loring Z, Al-Khatib SM, Atwater BD, Daubert JP, Frazier-Mills C, Hegland DD, Jackson KP, Jackson LR, Koontz JI, Lewis RK, Pokorney SD, Sun AY, Thomas KL, Bahnson TD, Piccini JP. Catheter ablation of atrial fibrillation in patients with diabetes mellitus. Heart Rhythm O2. 2020;1:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 135. | Uemura T, Kondo H, Sato H, Takahashi M, Shinohara T, Mitarai K, Fukui A, Hirota K, Fukuda T, Kodama N, Miyoshi M, Ogawa N, Wada M, Yamasaki H, Iwanaga K, Uno A, Tawara K, Yonezu K, Akioka H, Teshima Y, Yufu K, Nakagawa M, Takahashi N. Predictors of outcome after catheter ablation for atrial fibrillation: Group analysis categorized by age and type of atrial fibrillation. Ann Noninvasive Electrocardiol. 2022;e13020. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 136. | Guckel D, Isgandarova K, Bergau L, Piran M, El Hamriti M, Imnadze G, Braun M, Khalaph M, Fink T, Sciacca V, Nölker G, Lee-Barkey YH, Tschöpe D, Sommer P, Sohns C. The Effect of Diabetes Mellitus on the Recurrence of Atrial Fibrillation after Ablation. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Amr A, Christian-H H, Kivanc Y, Francesco S, Natale Daniele B, Thomas F, Spyridon L, Ben B, Ahmad K, Huong Lan P, Makoto S, Vanessa S, Evgeny L, Dong AN, Roza MS, Feifan O, Karl-Heinz K, Charlotte E, Julia V, Roland Richard T. Safety and Efficacy of Cryoballoon Ablation for the Treatment of Atrial Fibrillation in Diabetic Patients. J Atr Fibrillation. 2020;12:2285. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 138. | Sato T, Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Okada K, Dohi T, Kitamura T, Sunaga A, Kida H, Oeun B, Egami Y, Watanabe T, Minamiguchi H, Miyoshi M, Tanaka N, Oka T, Okada M, Kanda T, Matsuda Y, Kawasaki M, Masuda M, Inoue K, Sakata Y; Osaka Cardio Vascular Conference (OCVC)‐Arrhythmia Investigators *. DR-FLASH Score Is Useful for Identifying Patients With Persistent Atrial Fibrillation Who Require Extensive Catheter Ablation Procedures. J Am Heart Assoc. 2022;11:e024916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 139. | Kosiuk J, Dinov B, Kornej J, Acou WJ, Schönbauer R, Fiedler L, Buchta P, Myrda K, Gąsior M, Poloński L, Kircher S, Arya A, Sommer P, Bollmann A, Hindricks G, Rolf S. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm. 2015;12:2207-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 140. | Samuel M, Khairy P, Mongeon FP, Andrade JG, Gomes S, Galvan Z, Weerasooriya R, Novak P, Nault I, Arentz T, Deisenhofer I, Veenhuyzen GD, Jaïs P, Parkash R, Verma A, Menon S, Puererfellner H, Scavée C, Talajic M, Guerra PG, Rivard L, Dubuc M, Dyrda K, Thibault B, Mondesert B, Tadros R, Cadrin-Tourigny J, Aguilar M, Tardif JC, Levesque S, Roy D, Nattel S, Macle L. Pulmonary Vein Stenosis After Atrial Fibrillation Ablation: Insights From the ADVICE Trial. Can J Cardiol. 2020;36:1965-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 141. | Bogossian H, Frommeyer G, Brachmann J, Lewalter T, Hoffmann E, Kuck KH, Andresen D, Willems S, Spitzer SG, Deneke T, Thomas D, Hochadel M, Senges J, Eckardt L, Lemke B. Catheter ablation of atrial fibrillation and atrial flutter in patients with diabetes mellitus: Who benefits and who does not? Int J Cardiol. 2016;214:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 142. | Lin KJ, Cho SI, Tiwari N, Bergman M, Kizer JR, Palma EC, Taub CC. Impact of metabolic syndrome on the risk of atrial fibrillation recurrence after catheter ablation: systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;39:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Anselmino M, Matta M, D'ascenzo F, Pappone C, Santinelli V, Bunch TJ, Neumann T, Schilling RJ, Hunter RJ, Noelker G, Fiala M, Frontera A, Thomas G, Katritsis D, Jais P, Weerasooriya R, Kalman JM, Gaita F. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace. 2015;17:1518-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 144. | Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM, Cha YM, Shen WK, Brady PA, Bluhm CM, Haroldson JM, Hammill SC, Packer DL. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol. 2010;21:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 145. | Deshmukh A, Ghannam M, Liang J, Saeed M, Cunnane R, Ghanbari H, Latchamsetty R, Crawford T, Batul SA, Chung E, Bogun F, Jongnarangsin K, Pelosi F, Chugh A, Morady F, Oral E, Oral H. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 146. | Kishima H, Mine T, Fukuhara E, Kitagaki R, Asakura M, Ishihara M. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors on Outcomes After Catheter Ablation for Atrial Fibrillation. JACC Clin Electrophysiol. 2022;8:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 147. | Luo F, Sun L, Wang Z, Zhang Y, Li J, Chen Y, Dong J. Effect of Dapagliflozin on the Outcome of Radiofrequency Catheter Ablation in Patients with Type 2 Diabetes Mellitus and Atrial Fibrillation. Cardiovasc Drugs Ther. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |