Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.481
Peer-review started: December 14, 2022
First decision: March 14, 2023
Revised: March 21, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 15, 2023
Processing time: 151 Days and 16.8 Hours
Somatic disturbances that occur in parallel with psychiatric diseases are a major challenge in clinical practice. Various factors contribute to the development of mental and somatic disorders. Type 2 diabetes mellitus (T2DM) is a significant health burden worldwide, and the prevalence of diabetes in adults is increasing. The comorbidity of diabetes and mental disorders is very common. By sharing a bidirectional link, both T2DM and mental disorders influence each other in various manners, but the exact mechanisms underlying this link are not yet elucidated. The potential mechanisms of both mental disorders and T2DM are related to immune and inflammatory system dysfunction, oxidative stress, endothelial dysfunction, and metabolic disturbances. Moreover, diabetes is also a risk factor for cognitive dysfunction that can range from subtle diabetes-associated cognitive decline to pre-dementia and dementia. A complex re-lationship between the gut and the brain also represents a new therapeutic approach since gut-brain signalling pathways regulate food intake and hepatic glucose production. The aim of this minireview is to summarize and present the latest data on mutual pathogenic pathways in these disorders, emphasizing their complexity and interweaving. We also focused on the cognitive performances and changes in neurodegenerative disorders. The importance of implementing integrated approaches in treating both of these states is highlighted, along with the need for individual therapeutic strategies.
Core Tip: Mental disorders and type 2 diabetes mellitus (T2DM) are common, chronic, and frequently comorbid diseases that contribute significantly to global disability and mortality. Substantial evidence on the association between mental disorders and T2DM has been gathered over the past decade. In this review, we presented the latest cellular and molecular mechanisms of the shared pathways of T2DM and mental disorders, including neuroendocrine alterations and inflammation, immune response, oxidative stress, gut dysbiosis and gut-brain axis dysregulation, along with the hypothalamic-pituitary-adrenal axis dysregulation. The bidirectional link between mental disorders and T2DM underlines the importance of treating these disorders together rather than separately.
- Citation: Borovcanin MM, Vesic K, Petrovic I, Jovanovic IP, Mijailović NR. Diabetes mellitus type 2 as an underlying, comorbid or consequent state of mental disorders. World J Diabetes 2023; 14(5): 481-493
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/481.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.481
In the era of creating a concept of precision psychiatry[1], it is of utmost importance to acknowledge somatic disturbances that co-occur in mental disorders. Anamnesis vitae does not begin at the very moment of birth, yet it needs to include intrauterine development. Many factors can and do contribute to the future development of mental and somatic disorders. The interrelation of diabetes mellitus (DM) and mental disorders has fascinated both endocrinologists and psychiatrists for years. By sharing a bidirectional association, both DM and mental disorders influence each other in various manners, but the exact mechanisms underlying this link are not yet clear, and there are many questions that need to be addressed. The unique immunometabolic disturbances deserve special discussion because they could be associated with specific mental disorders later in life[2]. In this context, it is important to consider developmental programming or alterations of the intrauterine environment that induce compensatory responses and may persist in later life. Maternal diabetes during pregnancy could lead to neurodevelopmental outcomes, autism spectrum disorder, attention-deficit/hyperactivity disorder, and intellectual disabilities in the offspring, with increased risk for autism spectrum disorder and attention-deficit/hyperactivity disorder in pre-existing forms of diabetes, type 1 DM (T1DM) and type 2 DM (T2DM), but not with significance in gestational DM (GDM). For intellectual disorders, a two-fold increased risk was observed after exposure to T2DM compared to T1DM and GDM[3].
Synergistic effects of various factors could explain the multifactorial etiopathogenesis of mental disorders. T2DM could be seen in conjunction with different mental disorders. It could precede the onset of depression or could follow depressive symptomatology[4]. Anxiety overlaps diabetes microneuropathy[5], while eating disorders are accompanied by metabolic disturbances[6]. As we already discussed, intrauterine programming, lifestyle habits, or antipsychotic treatment could all contribute to diabetes onset in patients with schizophrenia[7]. Considering the worldwide burden of dementia, targeting a healthy lifestyle could prevent cognitive decline and preserve cognitive functions[8,9]. Recently, Dyer et al[10] have explored the precise timing and cascade of inflammatory mechanisms that convert physiological cognitive decline into dementia. A complex relationship between the gut and the brain also opens new therapeutic avenues, as gut-brain signalling pathways regulate food intake and hepatic glucose production. All these data have occupied our attention to explore the importance of T2DM in neuroinflammation and neurodegeneration. In this review, we aimed to enlighten the new concepts of T2DM etiopathogenesis that could contribute to mental disturbances and mental disorders symptomatology.
DM is defined as a complex and heterogeneous disease with a common state of hyperglycemia (Table 1). The American Diabetes Association considers T1DM as autoimmune β-cell destruction with absolute insulin deficiency and progressive loss of β-cells. This process is mediated by activated helper T lymphocytes which trigger effector cells of the immune system to destroy healthy β-cells. Simultaneously, a disruption of regulatory cells with a predominance of pro-inflammatory phenotypes occurs[11,12]. A hallmark of T2DM is significant insulin resistance and chronically increased β-cells engagement. The pathogenesis of this type of diabetes is multifactorial and has been investigated through the effects of various β-cell molecules[13-17].
| Type of diabetes mellitus | Pathophysiology |
| Type 1 diabetes mellitus | Autoimmune β-cell destruction |
| Type 2 diabetes mellitus | Insulin resistance (liver, muscle, adipose tissue) |
| Disorder of insulin secretion and β-cells breakdown | |
| Immune dysregulation and metainflammation | |
| Disorder of incretin production (glucagon-like peptide-1) | |
| Hyperglucagonemia | |
| Gut dysbiosis | |
| Increased glucose apsorption in stomach | |
| Kidney adaptation with increased glucose reabsorption and gluconeogenesis | |
| Decreased dopamine and increased sympathetic tone in brain | |
| Type 3 diabetes mellitus concept | Impaired insulin and insulin-like growth factor-1 signaling |
| Gestational diabetes mellitus | Pregnancy induced glucose intolerance |
GDM is defined as hyperglycemia occurring during pregnancy and registered during the second or third trimester. Although in 80% of cases, the main cause is marked insulin resistance caused by hormonal imbalance, the other 20% of cases are autoimmune in origin or other types caused by various factors that, even if they occur independently, can lead to the onset of the disease. These factors include genetic mutation, diseases of the exocrine pancreas, and drug- or chemical-induced diabetes[11].
According to the World Health Organization, DM is a chronic, metabolic disease characterized by elevated levels of blood glucose, which leads to the development of chronic complications over time[18]. T2DM is one of the most common metabolic disorders worldwide, and it is estimated that the number of patients will increase significantly in the coming decades. Current analyses indicate the dominant representation of patients with T2DM (90%-95%) considering all patients with diabetes[11]. Patients with T2DM are mostly obese or have a higher body fat percentage, distributed predominantly in the central body region. At the same time, they have a 15% increased risk of all-cause mortality compared with people without diabetes[19]. The pathogenesis of T2DM is multifactorial and represents a combination of several simultaneous factors such as insulin resistance and β-cells deterioration, intestinal dysbiosis, and the presence of meta-inflammation (Table 1). The organs involved in T2DM development include the pancreas (β-cells and α-cells), liver, skeletal muscle, brain, kidney, small intestine, and adipose tissue[20,21].
Obesity is strongly associated with energy imbalance, characterized by increased food intake and decreased catabolism, and is associated with a state of chronic, low-grade inflammation, particularly in white adipose tissue[22]. Namely, as a result of long-term stimulation, adipocyte hypertrophy leads to the development of insulin resistance and reduced insulin-responsive glucose uptake in peripheral tissues[23]. Over time, the hypertrophy of adipocytes leads to their apoptosis. Apoptosis of adipocytes facilitates the accumulation of macrophages into adipose tissue, their differentiation toward the M1 phenotype, and subsequent production of proinflammatory cytokines[24].
Insulin resistance occurring in the liver unblocks glucose production in hepatocytes. This phenomenon is accompanied by additional glucogenesis in the fed state and even postprandially, which further leads to additional hyperglycemia[25]. All of the above-mentioned changes and the predominance of the pro-inflammatory response in the fat tissue and liver result in the reduced effect of insulin on peripheral tissues, compensatory hyperinsulinemia, and cause the burden of β-cells. Because of the long-term increase in insulin secretion, the accumulation of amylin takes a significant place in the decay of β-cells. This process is especially pronounced during the early phase of T2DM[26]. The enhanced function of β-cells, their deterioration, and the loss of compensatory hyperinsulinemia result in severe hyperglycaemia[27].
Another important aspect is the role of adipose tissue. Adipose tissue represents an important endocrine organ that regulates metabolism and behaviour through the production of adipokines. Among them, leptin, which is mainly produced in adipocytes, has a powerful influence on eating behaviour. Leptin-gene expression is extremely sensitive to acute energy balance, regardless of the long-term energy balance[28]. Short-term fasting decreases leptin messenger ribonucleic acid (mRNA) levels and plasma concentrations, whereas refeeding quickly restores its mRNA levels[29]. These changes suggest that leptin protects fat reserves against weight loss[30]. Leptin’s access to key neurons in the central nervous system is of critical importance for its action. In obese people, the effect of leptin is weaker or absent[31], suggesting the disruption of its regulatory functions. Regarding the immunological functions of leptin, it has been shown that CD4+ helper T cells cannot differentiate in the direction of T regulatory cells in states of elevated leptin[32]. In T2DM the main determinants of leptin levels are insulin secretion and the degree of insulin resistance[33].
Glucagon-like peptide-1 (GLP-1) is a hormone that regulates islet function, satiety, and gut motility with reduced secretion in patients with T2DM. McLean et al[34] have recently discussed new insights and refined their previous understanding of the GLP-1 function. In addition to the significant effects of GLP-1 on increased insulin production and reduced glucagon production, activation of GLP-1 receptors exerts hypophagic effects in the ventral hippocampus[35]. Numerous studies over the past decade have provided a deeper understanding of GLP-1 action in the brain. The direct link between gut secretion and the brain’s GLP-1 system has not been found. GLP-1 receptor agonists exert their appetite-suppressing effects on cells in the circumventricular organs which transmit the signal to deeper brain structures[34].
During the last decade, it has been shown that the disturbance of intestinal flora, known as dysbiosis, occupies a significant place in the pathogenesis of T2DM. Dysbiosis represents an imbalance of commensal and pathogenic bacteria in the intestines and the production of microbial antigens and metabolites[36]. The occurrence of dysbiosis is accompanied by a disturbance of peripheral immune tolerance in the intestines with a predominance of dysregulated T-cell subpopulations[37]. The state of dysbiosis is accompanied by a disruption of the permeability of the intestinal epithelial barrier with the occurrence of hyperpermeability, also known as a leaky gut syndrome (LGS). LGS is defined as a condition in which intestinal endothelial cells allow microorganisms, their toxins, and antigens to “leak” into the bloodstream above the physiological values, consequently causing systemic reactions[38]. Dysbiosis is also accompanied by intestine inflammation[39]. The intestinal tract may develop an inflammatory response characterized by increased expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), and IL-6 that leads to the development of insulin resistance[40]. In addition to dietary factors, pro-inflammatory cytokines also promote the formation of LGS. Interferon-gamma increases intestinal permeability by redistributing tight junction proteins and restructuring the cell cytoskeleton. TNF-α increases intestinal permeability by inducing apoptosis of endothelial cells[41]. On the other hand, IL-6 enhances intestinal permeability by altering the expression of molecules that play a major role in forming tight junction pores[42]. Alterations in transepithelial transport pathways may induce further translocations of harmful factors because of this vicious circle[43].
It is obvious that T2DM is associated with immune system dysfunction[44]. While T2DM can facilitate immune system activity in some tissues, it also negatively affects the immune response, which is confirmed by the higher incidence of unsuccessful vaccinations and complications of infections[45-47]. It appears that hyperglycaemia and pathologies in obesity, insulin resistance, and inflammation have a strong impact on the immunity of the host[48-50]. Various mechanisms have been proposed to be responsible for this phenomenon. Hyperglycaemia directly disturbs endoplasmic reticulum function, thus facilitating the accumulation of misfolded proteins in the lumen and promoting endoplasmic reticulum stress, which in turn modulates the function of immunocompetent cells[50]. Second, reactive oxidative species, which are abundant in the sera of patients with diabetes, alter innate immune cells activity through the diminished expression of activating receptors[51]. Taking everything into account, both innate and adaptive immune responses are altered in patients with T2DM and are not capable to provide adequate and effective protection against invading pathogens[45]. The logical outcome is a constant and permanent chronic inflammatory reaction in the immune response to pathogens and the resulting constant production of pro-inflammatory cytokines in amounts insufficient to initiate a strong immune response and elimination of pathogens, but still sufficient to induce many consequences in diabetic subjects.
Cognitive impairment and dementia are frequently accompanying and complicating T1 and T2DM[52]. 1.25-1.9-fold higher risk is established for cognitive dysfunction in diabetes[53]. There is increasing evidence that diabetes predisposes to cognitive decline leading to dementia[54,55], with a stronger link confirmed between dementia in T2DM than in T1DM. The risk for dementia progress increases with the aging of patients with diabetes, with a 50% higher risk in patients aged 75 years and over than in patients aged 65-75 years[56]. Diabetes-associated decrements in their mildest stage can occur in all age groups, from young adults and even adolescents with T2DM[57] to the oldest patients[58]. A meta-analysis revealed that the domains of the speed of processing information, attention, concentration, executive functioning, and working memory were mainly influenced in diabetes compared to non-diabetic people[59].
The risk of diabetes-related cognitive decline was significantly increased in more severe clinical presentation and longer duration of T2DM[60,61]. Although the severity of diabetes is a risk factor for developing dementia[62], individuals without diabetes who have higher average glucose levels were also found to be at significant risk for dementia[63]. Diabetes does not act alone, but rather within a broader cluster of cardiometabolic disorders. Cognitive decline was associated with elevated blood sugar levels, a longer duration of diabetes, comorbid hypertension, and a history of a cerebrovascular event or myocardial infarction[64]. The impact of diabetes on the prodromal phase of dementia was demonstrated in the cohort of older adults and showed that poorly controlled diabetes increased the risk and progression of cognitive impairment, which was exacerbated by comorbid heart disease and mediated by systemic inflammation[65]. Hyperglycemia was observed as the main contributor to cognitive decline in metabolic syndrome[66,67]. Numerous epidemiological studies have identified diabetes and obesity measured in later life as risk factors for cognitive impairment[68]. Other comorbidities associated with aging and diabetes also add to the burden of cognitive impairment. Depression has been associated with a greater decline in cognitive function in patients with T2DM[69].
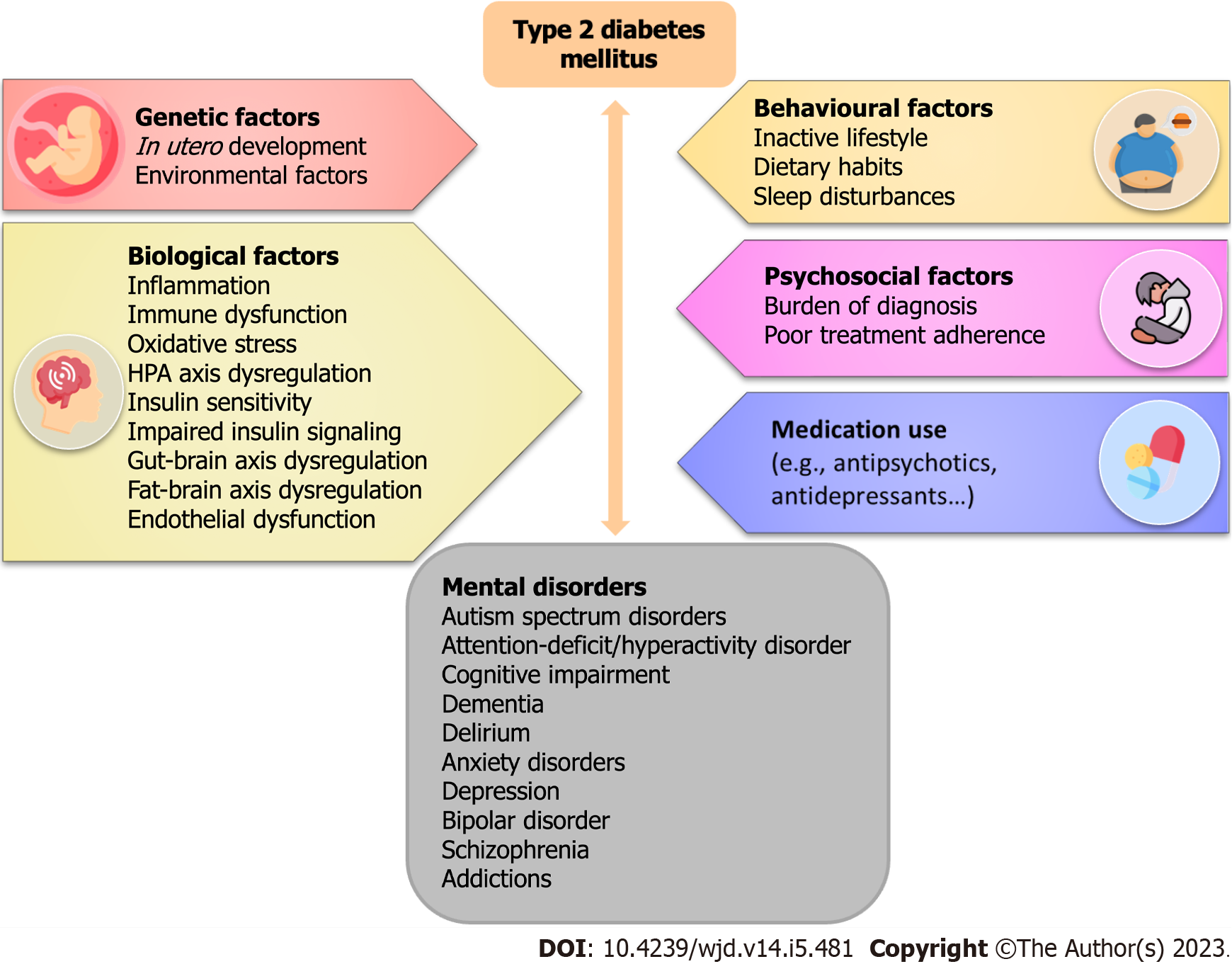
The exact pathogenic mechanisms underlying cognitive impairment in T2DM are not fully understood and are undoubtedly complicated, with numerous interacting factors (Figure 1). The cognitive impairments in diabetic encephalopathy have been associated with structural changes[70] and brain atrophy[71]. Cortical, subcortical, and hippocampal atrophy, particularly in the dentate gyrus, has been detected in T2DM patients by brain magnetic resonance imaging[71-73]. Various endocrinological, metabolic, and vascular abnormalities are DM-related and may precipitate the worsening of cognitive abilities.
Insulin could have a significant role in cognitive processing through the cerebrocortical activity of insulin receptors. They are allocated extensively in the hippocampus, entorhinal cortex, and frontal lobes, localities of the brain whose functions are involved in memory, attention, and executive functioning[74]. Variabilities in signalling pathways of insulin, phosphorylation of insulin receptor substrate 1, and altered signalling of insulin-like growth factor-1 were considered as main contributors to cognitive dysfunction pathogenesis[75,76].
Overexpression of proinflammatory cytokines TNF-α, IL-1, IL-2, and IL-6 in the brain under diabetic conditions indicates that the innate immune system and microglial cells in particular are activated[77,78], and play an important role in neuronal damage in diabetic animals and patients[79,80]. Hyperglycemia, a defective insulin signalling system, and oxidative stress have been linked to neuronal toxicity and apoptosis, neuroinflammation, and the consequential development of neurodegeneration in diabetes[81,82].
There is growing evidence of a strong association between T2DM and neurodegenerative disorders such as Alzheimer’s disease (AD) and neurovascular disorders[83,84]. Metabolic alterations, including central insulin resistance and abnormal glucose metabolism, are obvious in the mild cognitive impairment prodromal phase and in individuals that are still asymptomatic, but at increased genetic risk for AD[85]. Limited autopsy analyses suggest that hyperglycemia may promote AD pathology by inducing more prominent Aβ plaques and tau-positive cells accumulation, and activation of microglia in the comorbidity of AD and T2DM than in those patients with AD and without T2DM[86].
Recently, de la Monte and Wands[87] proposed a new term, type-3 diabetes or ‘Brain-specific type-2 diabetes’, for the neuroendocrine disorder that represents the progression of T2DM to AD[87,88] (Table 1). This state is characterized by decreased insulin production and insulin resistance[89]. The authors found that impairments of insulin-like growth factor signalling lead to these deficits in energy metabolism with increased oxidative stress, neuroinflammation, vascular damage, tau phosphorylation, Aβ accumulation, and neuronal degeneration[87,90]. In T2DM, islet amyloid polypeptide, also known as amylin, is secreted by pancreatic β-cells that modulate insulin and glucagon secretion and contribute to glucose regulation[91]. Islet amyloid polypeptide mainly affects cognitive function and causes blood-brain barrier (BBB) interruption, interacting and aggregating with Aβ peptides and hyperphosphorylates of tau protein within the brains of AD patients. Consequently, this leads to disruption in the neuronal network and neurodegeneration which could also be a link between T2DM and AD[92]. Inflammatory processes play a crucial pathogenic role in T2DM and AD[93]. A crosstalk between peripheral and central inflammation has been described[94]. Patel and Santani[95] showed that nuclear factor kappa B (NF-κβ) is involved in the inflammation of the brain during the progression of diabetes. NF-κβ also upregulates the expression of cytokines that are responsible for the insulin resistance onset, such are TNF-α, IL-1β, and IL-6[96,97]. These inflammatory mediators can cross the disrupted BBB and enter the brain, further promoting neuroinflammation and leading to abnormalities of synapses, insulin resistance and damage of neural tissue, and eventually neurodegeneration[98-100]. Previous studies have reported that these proinflammatory cytokines are elevated in AD and found in amyloid plaques and their related glial cells[101].
T2DM is an established risk factor for neurovascular diseases such as ischemic stroke and cortical and subcortical microinfarcts[102]. Many studies report that cerebral infarcts are significantly associated with increased development of post-stroke cognitive impairment or vascular dementia[103,104]. The alterations in the glucose levels cause dysfunction and damage to the vessel’s endothelium leading to atherosclerosis[105]. T2DM vascular complications affect the circulatory system in the brain by remodelling and stiffening the vascular walls, causing the reduction of vessel calibre with hypo-perfusion[106]. Possible pathways of endothelial damage include oxidative stress and inflammation[107]. Chronic hyperglycemia and the production of reactive oxygen species apparently damage the vessel endothelium and lead to atherosclerosis[108]. In addition, damaged endothelial cells can release danger-associated molecular patterns (DAMP), activate toll-like receptor 4, and further potentiate inflammation[109]. The specific DAMP signals, the advanced glycation end products (AGEs), are proteins or lipids that become glycated as a result of exposure to elevated glucose concentration[110]. These molecules stimulate the receptor for AGEs (RAGE), CD36, and toll-like receptor 4 receptors which in turn stimulate inflammation, vascular injury, and oxidative stress[111]. RAGE is strongly expressed in microglia, astrocytes, and brain endothelial cells in T2DM[112,113]. Inflammatory signals can trigger local thrombotic vascular events leading to brain infarction[114] (all potential mechanisms summarized in Figure 1). The differential and relative contributions of T2DM, cerebrovascular and neurodegenerative disease to cognitive impairment and dementia are still unknown. Understanding the mecha
The study integrating data from transcriptomic meta-analysis of peripheral blood mononuclear cells and systems biology provided new insights into the shared pathogenetic mechanisms of schizophrenia and T2DM. This study showed that 28 genes concordantly dysregulated were included in the “positive regulation of catabolic process” pathway and low-grade inflammation, “membrane trafficking” particularly focused on clathrin-mediated endocytosis and “signalling by interleukins”, transforming growth factor beta and NF-κβ[115]. Schizophrenia as a neurodevelopmental condition is associated with a higher risk of T2DM also by common exposure to early life stress and alteration of fetal mental programming and immune-inflammatory dysregulation[116]. The association between drug-naïve first-episode schizophrenia and pre-diabetes conditions indicates an inherent risk for glucose regulation before antipsychotic treatment[117,118]. Parental history of diabetes was associated with the onset of diabetes in patients with schizophrenia that are treated with clozapine[119]. Treatment with second-generation antipsychotics has a 1.3-fold elevated risk of diabetes compared to first-generation antipsychotics[120].
Depression has also been shown to be nearly three and two times more common in patients with T1DM and T2DM, respectively[121]. When behavioural factors such as dietary habits, physical activity, socioeconomic status, and sleep are altered, they could lead to depression and T2DM. The relationship between a poor intrauterine environment and the risk of depression in adulthood is not clear, and there is no genetic association between T2DM and depression[122]. Habib et al[123] described shared etiological factors for the comorbidity between diabetes and depression, considering hypothalamic-pituitary-adrenal axis dysregulation and cortisol release, hyperactivity of the autonomic nervous system and catecholamines release, inflammatory processes, activation of the polyol pathway, inducing oxidative stress and increasing the formation of AGEs, and also damage via microvascular dysfunction. The bidirectional relationship between depression and diabetes is reflected in the psychological and psychosocial impact of depression, microvascular brain lesions, higher levels of glutamate, poor glycemic control, and medication compliance that could lead to diabetes, and conversely, the stress associated with diabetes management could lead to depression[124] (Figure 1). These mutual interactions are of particular clinical interest in vascular depression, a type of late-life depression that correlates with white matter hypersensitivity, which is also observed in patients with diabetes and associated depression[125].
Increased gut permeability links depression to T2DM when metabolic endotoxemia with lipopolysaccharides induces β-cell damage, and neuroinflammation[126,127]. Immune-inflammatory pathways, sterile inflammation, the release of DAMP, oxidative and nitrosative stress, and glia activation are also shared mechanisms. Non-alcoholic fatty liver disease is more common in people with mental disorders, including schizophrenia, major depressive disorder, and bipolar disorder, and is driven by the same lifestyle factors that put them at risk for T2DM[128].
The co-occurrence of diabetes and depression has more severe negative consequences. Individuals with depression and T2DM have a higher risk of cognitive decline and dementia compared with individuals treated for T2DM alone, which is important in clinical practice[129]. If clear causality is established, mental changes could certainly be prevented and cured. In a large cohort of Taiwanese diabetic patients, 0.8% of deaths were found to be due to suicide (0.14% of all patients)[130], and AbdElmageed and Mohammed Hussein[124] discussed different aspects of how suicide risk increased with elevated blood glucose levels and could be facilitated by patient access to potentially lethal agents such as oral hypoglycemics and insulin.
Martins et al[131] have concluded, based on an extensive literature review, that antidepressants may exert some positive effects on glycemic control in patients with DM. However, it is important to consider a specific subclass of anti-depressants or even different antidepressants of the same class, treatment duration, and the use of combination therapy. That being so, metabolic consequences need to be evaluated individually. Tricyclic antidepressants can worsen glycemic control, monoamine inhibitors may induce weight gain, and selective serotonin reuptake inhibitors are associated with the im-provement in glycemic control. The antidepressant bupropion seems to improve glycemic control[132].
Enhanced release of dopamine by insulin is involved in the modulation of motivation and reward leading to depression symptoms[133]. Endocannabinoid system dysfunction could contribute to the development of depression in T2DM and could also be a therapeutical target[126]. On the other hand, antidiabetic drugs have a positive effect on the treatment of the major depressive disorder, by crossing the BBB and by mediating insulin signalling, inflammatory pathways, and cognitive performance. A group of distinguished authors has recently discussed that metformin may have beneficial effects not only in medical conditions but also in core illness domains in a wide range of psychiatric and neurodegenerative disorders[134]. Metformin, as an antihyperglycemic, appears to promote antidepressant, anxiolytic, and cognitive functions by increasing GLP-1, but also exerts anti-inflammatory effects by lowering C-reactive protein, inhibiting Th17 cell differentiation, and reducing TNF-β, IL-1β, IL-6, and IL-17. It also reduces oxidative and nitrosative stress, leading to an improvement in serotonergic neurotransmission in the hippocampus. The attractive new potential of metformin is to protect the intestinal barrier and modulate BBB function. It is worth noting that leptin crosses the BBB and binds to receptors that are spread in different brain areas and seem to have antidepressant and anxiolytic properties[135].
The relationship between T2DM and psychiatric disorders demonstrates how our mental and physical health are inevitably intertwined. The mechanisms underlying this bidirectional relationship remain unresolved, with various intriguing hypotheses. Common biological mechanisms that may underlie both diabetes and psychiatric disorders represent the basic goals of future research. Shared genetic pathways could be a potential explanation, but data from existing studies are still insufficient to draw definitive conclusions. Of particular interest are the possible overlaps in genetic mechanisms between schizophrenia and T2DM. Intrauterine development represents the initial and unavoidable starting point for the predisposition to numerous pathological conditions after birth. Inflammation is another likely suspect underlying both diabetes and psychiatric disorders. A better understanding of the gut-brain axis and its complex relationship with the gut microbiome is essential for developing new therapeutic strategies to combat both diabetes and psychiatric disorders.
Given the burden of diabetes and concomitant cognitive changes and psychiatric diseases, it is a crucial need to understand the complex multifactorial pathophysiology of DM and to identify molecular targets and pathways that might lead to future therapies. The potential of integrated approaches needs to be thoroughly explored in future trials. In the clinical arena, the early evaluation and accurate quantification of cognitive functions and mental state need to be implemented in the clinical assessment of diabetic patients at the very beginning as well as on follow-up on a regular basis, as it significantly impacts the complete recovery and quality of life these patients. Vice versa approach should also be applied. Translational application of anti-glycemic drugs in the treatment of depression and dementia could be a useful path in the future. All this could jointly direct future interventions to improve the outcome of somatic treatment and better quality of life in persons with mental disorders.
We thank Bojana Mircetic for language editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beg MMA, Kyrgyzstan; Zhao CF, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Zanardi R, Prestifilippo D, Fabbri C, Colombo C, Maron E, Serretti A. Precision psychiatry in clinical practice. Int J Psychiatry Clin Pract. 2021;25:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Pillinger T, D'Ambrosio E, McCutcheon R, Howes OD. Is psychosis a multisystem disorder? Mol Psychiatry. 2019;24:776-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Eletri L, Mitanchez D. How Do the Different Types of Maternal Diabetes during Pregnancy Influence Offspring Outcomes? Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 4. | Alzoubi A, Abunaser R, Khassawneh A, Alfaqih M, Khasawneh A, Abdo N. The Bidirectional Relationship between Diabetes and Depression: A Literature Review. Korean J Fam Med. 2018;39:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Jain R, Jain S, Raison CL, Maletic V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diab Rep. 2011;11:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Himmerich H, Kan C, Au K, Treasure J. Pharmacological treatment of eating disorders, comorbid mental health problems, malnutrition and physical health consequences. Pharmacol Ther. 2021;217:107667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Borovcanin MM, Vesic K, Jovanovic M, Mijailovic NR. Galectin-3 possible involvement in antipsychotic-induced metabolic changes of schizophrenia: A minireview. World J Diabetes. 2021;12:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Mijailovic NR, Vesic K, Borovcanin MM. The Influence of Serum Uric Acid on the Brain and Cognitive Dysfunction. Front Psychiatry. 2022;13:828476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Mijailović NR, Vesic K, Arsenijevic D, Milojević-Rakić M, Borovcanin MM. Galectin-3 Involvement in Cognitive Processes for New Therapeutic Considerations. Front Cell Neurosci. 2022;16:923811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Dyer AH, Batten I, Reddy C, Townsend L, Woods CP, O'Neill D, Gibney J, Kennelly SP, Bourke NM. Neuropsychological decrements in midlife type-2 diabetes are not associated with peripheral NLRP3 inflammasome responsiveness. Front Immunol. 2022;13:1021351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1418] [Article Influence: 472.7] [Reference Citation Analysis (1)] |
| 12. | Wållberg M, Cooke A. Immune mechanisms in type 1 diabetes. Trends Immunol. 2013;34:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Roefs MM, Carlotti F, Jones K, Wills H, Hamilton A, Verschoor M, Durkin JMW, Garcia-Perez L, Brereton MF, McCulloch L, Engelse MA, Johnson PRV, Hansen BC, Docherty K, de Koning EJP, Clark A. Increased vimentin in human α- and β-cells in type 2 diabetes. J Endocrinol. 2017;233:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Mellado-Gil JM, Fuente-Martín E, Lorenzo PI, Cobo-Vuilleumier N, López-Noriega L, Martín-Montalvo A, Gómez IGH, Ceballos-Chávez M, Gómez-Jaramillo L, Campos-Caro A, Romero-Zerbo SY, Rodríguez-Comas J, Servitja JM, Rojo-Martinez G, Hmadcha A, Soria B, Bugliani M, Marchetti P, Bérmudez-Silva FJ, Reyes JC, Aguilar-Diosdado M, Gauthier BR. The type 2 diabetes-associated HMG20A gene is mandatory for islet beta cell functional maturity. Cell Death Dis. 2018;9:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Efrat S. Beta-Cell Dedifferentiation in Type 2 Diabetes: Concise Review. Stem Cells. 2019;37:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Marinkovic M, Petrovic I. The Role of Galectin 3 in the Pathogenesis of Diabetes Mellitus: Focus on Β-Cell Function and Survival. Serbian J Exp Clin Res. 2022;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Cadavez L, Montane J, Alcarraz-Vizán G, Visa M, Vidal-Fàbrega L, Servitja JM, Novials A. Chaperones ameliorate beta cell dysfunction associated with human islet amyloid polypeptide overexpression. PLoS One. 2014;9:e101797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 19. | Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 2861] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 20. | Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1971] [Cited by in RCA: 1956] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 21. | Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1392] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 22. | Poli VFS, Sanches RB, Moraes ADS, Fidalgo JPN, Nascimento MA, Bresciani P, Andrade-Silva SG, Cipullo MAT, Clemente JC, Caranti DA. The excessive caloric intake and micronutrient deficiencies related to obesity after a long-term interdisciplinary therapy. Nutrition. 2017;38:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 24. | Röszer T. Adipose Tissue Immunometabolism and Apoptotic Cell Clearance. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 26. | Chen YC, Taylor AJ, Verchere CB. Islet prohormone processing in health and disease. Diabetes Obes Metab. 2018;20 Suppl 2:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Robertson RP. Beta-cell deterioration during diabetes: what's in the gun? Trends Endocrinol Metab. 2009;20:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Chua S Jr. Leptin Function and Regulation. Compr Physiol. 2017;8:351-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 29. | Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 724] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 30. | Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223:T83-T96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 32. | Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 33. | Wauters M, Considine RV, Yudkin JS, Peiffer F, De Leeuw I, Van Gaal LF. Leptin levels in type 2 diabetes: associations with measures of insulin resistance and insulin secretion. Horm Metab Res. 2003;35:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr Rev. 2021;42:101-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 35. | Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 36. | Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 801] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 37. | Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 38. | Obrenovich MEM. Leaky Gut, Leaky Brain? Microorganisms. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 39. | Slyepchenko A, Maes M, Machado-Vieira R, Anderson G, Solmi M, Sanz Y, Berk M, Köhler CA, Carvalho AF. Intestinal Dysbiosis, Gut Hyperpermeability and Bacterial Translocation: Missing Links Between Depression, Obesity and Type 2 Diabetes. Curr Pharm Des. 2016;22:6087-6106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 453] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 41. | Graziani C, Talocco C, De Sire R, Petito V, Lopetuso LR, Gervasoni J, Persichilli S, Franceschi F, Ojetti V, Gasbarrini A, Scaldaferri F. Intestinal permeability in physiological and pathological conditions: major determinants and assessment modalities. Eur Rev Med Pharmacol Sci. 2019;23:795-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 42. | Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263-31271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 43. | Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 44. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 518] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 45. | Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 1674] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 46. | Bandaru P, Rajkumar H, Nappanveettil G. The Impact of Obesity on Immune Response to Infection and Vaccine: An Insight into Plausible Mechanisms. Endocrinol Metab Syndr. 2013;2:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 469] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 48. | Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 361] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 49. | Wrann CD, Laue T, Hübner L, Kuhlmann S, Jacobs R, Goudeva L, Nave H. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. 2012;302:E108-E116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Berrou J, Fougeray S, Venot M, Chardiny V, Gautier JF, Dulphy N, Toubert A, Peraldi MN. Natural killer cell function, an important target for infection and tumor protection, is impaired in type 2 diabetes. PLoS One. 2013;8:e62418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Choi SW, Benzie IF, Ma SW, Strain JJ, Hannigan BM. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008;44:1217-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Biessels GJ, Nobili F, Teunissen CE, Simó R, Scheltens P. Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol. 2020;19:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 53. | Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 54. | Wong RH, Scholey A, Howe PR. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus--a review with implications for future intervention studies. Curr Diab Rep. 2014;14:547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1522] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 56. | Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2018;53:1149-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 57. | Brady CC, Vannest JJ, Dolan LM, Kadis DS, Lee GR, Holland SK, Khoury JC, Shah AS. Obese adolescents with type 2 diabetes perform worse than controls on cognitive and behavioral assessments. Pediatr Diabetes. 2017;18:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006;49:2015-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 60. | Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Foster JK, Almeida OP, Davis TM. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Roberts RO, Geda YE, Knopman DS, Christianson TJ, Pankratz VS, Boeve BF, Vella A, Rocca WA, Petersen RC. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1486] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 63. | Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 656] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 64. | Varghese SM, Joy N, John AM, George G, Chandy GM, Benjamin AI. Sweet Memories or Not? Front Public Health. 2022;10:822062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (1)] |
| 65. | Dove A, Shang Y, Xu W, Grande G, Laukka EJ, Fratiglioni L, Marseglia A. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement. 2021;17:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 66. | Feinkohl I, Lachmann G, Brockhaus WR, Borchers F, Piper SK, Ottens TH, Nathoe HM, Sauer AM, Dieleman JM, Radtke FM, van Dijk D, Pischon T, Spies C. Association of obesity, diabetes and hypertension with cognitive impairment in older age. Clin Epidemiol. 2018;10:853-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 67. | Dik MG, Jonker C, Comijs HC, Deeg DJ, Kok A, Yaffe K, Penninx BW. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30:2655-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Abbatecola AM, Lattanzio F, Spazzafumo L, Molinari AM, Cioffi M, Canonico R, Dicioccio L, Paolisso G. Adiposity predicts cognitive decline in older persons with diabetes: a 2-year follow-up. PLoS One. 2010;5:e10333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Sullivan MD, Katon WJ, Lovato LC, Miller ME, Murray AM, Horowitz KR, Bryan RN, Gerstein HC, Marcovina S, Akpunonu BE, Johnson J, Yale JF, Williamson J, Launer LJ. Association of depression with accelerated cognitive decline among patients with type 2 diabetes in the ACCORD-MIND trial. JAMA Psychiatry. 2013;70:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Hernández-Fonseca JP, Rincón J, Pedreañez A, Viera N, Arcaya JL, Carrizo E, Mosquera J. Structural and ultrastructural analysis of cerebral cortex, cerebellum, and hypothalamus from diabetic rats. Exp Diabetes Res. 2009;2009:329632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 338] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 72. | Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ, Small SA. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging. 2011;32:1942-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 74. | Freychet P. Insulin receptors and insulin actions in the nervous system. Diabetes Metab Res Rev. 2000;16:390-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Ávila-Funes JA, Aguilar-Salinas CA. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr Diabetes Rev. 2019;15:456-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 76. | Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer's disease. Trends Neurosci. 2003;26:404-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Sima AA, Zhang W, Kreipke CW, Rafols JA, Hoffman WH. Inflammation in Diabetic Encephalopathy is Prevented by C-Peptide. Rev Diabet Stud. 2009;6:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 873] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 80. | Kuhad A, Chopra K. Effect of sesamol on diabetes-associated cognitive decline in rats. Exp Brain Res. 2008;185:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 407] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 82. | Prpić-Križevac I, Canecki-Varžić S, Bilić-Ćurčić I. Hyperactivity of the hypothalamic-pituitary-adrenal axis in patients with type 2 diabetes and relations with insulin resistance and chronic complications. Wien Klin Wochenschr. 2012;124:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Patel VN, Chorawala MR, Shah MB, Shah KC, Dave BP, Shah MP, Patel TM. Emerging Pathophysiological Mechanisms Linking Diabetes Mellitus and Alzheimer's Disease: An Old Wine in a New Bottle. J Alzheimers Dis Rep. 2022;6:349-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 84. | Khan MS, Ikram M, Park TJ, Kim MO. Pathology, Risk Factors, and Oxidative Damage Related to Type 2 Diabetes-Mediated Alzheimer's Disease and the Rescuing Effects of the Potent Antioxidant Anthocyanin. Oxid Med Cell Longev. 2021;2021:4051207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Kim B, Elzinga SE, Henn RE, McGinley LM, Feldman EL. The effects of insulin and insulin-like growth factor I on amyloid precursor protein phosphorylation in in vitro and in vivo models of Alzheimer's disease. Neurobiol Dis. 2019;132:104541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 86. | Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J Alzheimers Dis. 2010;20:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 87. | de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 761] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 88. | Sebastião I, Candeias E, Santos MS, de Oliveira CR, Moreira PI, Duarte AI. Insulin as a Bridge between Type 2 Diabetes and Alzheimer Disease - How Anti-Diabetics Could be a Solution for Dementia. Front Endocrinol (Lausanne). 2014;5:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Kroner Z. The relationship between Alzheimer's disease and diabetes: Type 3 diabetes? Altern Med Rev. 2009;14:373-379. [PubMed] |
| 90. | Tumminia A, Vinciguerra F, Parisi M, Frittitta L. Type 2 Diabetes Mellitus and Alzheimer's Disease: Role of Insulin Signalling and Therapeutic Implications. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 91. | Raimundo AF, Ferreira S, Martins IC, Menezes R. Islet Amyloid Polypeptide: A Partner in Crime With Aβ in the Pathology of Alzheimer's Disease. Front Mol Neurosci. 2020;13:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 92. | Arya S, Claud SL, Cantrell KL, Bowers MT. Catalytic Prion-Like Cross-Talk between a Key Alzheimer's Disease Tau-Fragment R3 and the Type 2 Diabetes Peptide IAPP. ACS Chem Neurosci. 2019;10:4757-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Chatterjee S, Mudher A. Alzheimer's Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front Neurosci. 2018;12:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 94. | Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27:36-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 354] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 95. | Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 96. | Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol. 2006;2:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 97. | Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer's disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 98. | Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 99. | Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269:13623-13628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 246] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 100. | Li J, Cesari M, Liu F, Dong B, Vellas B. Effects of Diabetes Mellitus on Cognitive Decline in Patients with Alzheimer Disease: A Systematic Review. Can J Diabetes. 2017;41:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2009;19:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 102. | Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J Diabetes Investig. 2019;10:780-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 103. | Rost NS, Meschia JF, Gottesman R, Wruck L, Helmer K, Greenberg SM; DISCOVERY Investigators. Cognitive Impairment and Dementia After Stroke: Design and Rationale for the DISCOVERY Study. Stroke. 2021;52:e499-e516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 104. | Einstad MS, Saltvedt I, Lydersen S, Ursin MH, Munthe-Kaas R, Ihle-Hansen H, Knapskog AB, Askim T, Beyer MK, Næss H, Seljeseth YM, Ellekjær H, Thingstad P. Associations between post-stroke motor and cognitive function: a cross-sectional study. BMC Geriatr. 2021;21:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 105. | Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 106. | Spinetti G, Kraenkel N, Emanueli C, Madeddu P. Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res. 2008;78:265-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 107. | Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34:575-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1036] [Article Influence: 148.0] [Reference Citation Analysis (1)] |
| 108. | Maamoun H, Benameur T, Pintus G, Munusamy S, Agouni A. Crosstalk Between Oxidative Stress and Endoplasmic Reticulum (ER) Stress in Endothelial Dysfunction and Aberrant Angiogenesis Associated With Diabetes: A Focus on the Protective Roles of Heme Oxygenase (HO)-1. Front Physiol. 2019;10:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 109. | Li C, Che LH, Ji TF, Shi L, Yu JL. Effects of the TLR4 signaling pathway on apoptosis of neuronal cells in diabetes mellitus complicated with cerebral infarction in a rat model. Sci Rep. 2017;7:43834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 654] [Cited by in RCA: 864] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 111. | Solito E, Sastre M. Microglia function in Alzheimer's disease. Front Pharmacol. 2012;3:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 112. | Otazu GK, Dayyani M, Badie B. Role of RAGE and Its Ligands on Inflammatory Responses to Brain Tumors. Front Cell Neurosci. 2021;15:770472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 113. | Schmidt AM. Diabetes Mellitus and Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2019;39:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 114. | Rawish E, Nording H, Münte T, Langer HF. Platelets as Mediators of Neuroinflammation and Thrombosis. Front Immunol. 2020;11:548631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 115. | Rahman MR, Islam T, Nicoletti F, Petralia MC, Ciurleo R, Fisicaro F, Pennisi M, Bramanti A, Demirtas TY, Gov E, Islam MR, Mussa BM, Moni MA, Fagone P. Identification of Common Pathogenetic Processes between Schizophrenia and Diabetes Mellitus by Systems Biology Analysis. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Garcia-Rizo C, Bitanihirwe BKY. Implications of early life stress on fetal metabolic programming of schizophrenia: A focus on epiphenomena underlying morbidity and early mortality. Prog Neuropsychopharmacol Biol Psychiatry. 2020;101:109910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 117. | Greenhalgh AM, Gonzalez-Blanco L, Garcia-Rizo C, Fernandez-Egea E, Miller B, Arroyo MB, Kirkpatrick B. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naïve patients with nonaffective psychosis. Schizophr Res. 2017;179:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 118. | Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 119. | Fernandez-Egea E, Walker R, Ziauddeen H, Cardinal RN, Bullmore ET. Birth weight, family history of diabetes and diabetes onset in schizophrenia. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Smith M, Hopkins D, Peveler RC, Holt RI, Woodward M, Ismail K. First- v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2008;192:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 121. | Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142 Suppl:S8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 771] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 122. | Samaan Z, Garasia S, Gerstein HC, Engert JC, Mohan V, Diaz R, Anand SS, Meyre D. Lack of association between type 2 diabetes and major depression: epidemiologic and genetic evidence in a multiethnic population. Transl Psychiatry. 2015;5:e618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Habib S, Sangaraju SL, Yepez D, Grandes XA, Talanki Manjunatha R. The Nexus Between Diabetes and Depression: A Narrative Review. Cureus. 2022;14:e25611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | AbdElmageed RM, Mohammed Hussein SM. Risk of Depression and Suicide in Diabetic Patients. Cureus. 2022;14:e20860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 125. | Kumar A, Gupta R, Thomas A, Ajilore O, Hellemann G. Focal subcortical biophysical abnormalities in patients diagnosed with type 2 diabetes and depression. Arch Gen Psychiatry. 2009;66:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 126. | Duarte-Silva E, de Melo MG, Maes M, Filho AJMC, Macedo D, Peixoto CA. Shared metabolic and neuroimmune mechanisms underlying Type 2 Diabetes Mellitus and Major Depressive Disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 127. | Petrovic I, Pejnovic N, Ljujic B, Pavlovic S, Miletic Kovacevic M, Jeftic I, Djukic A, Draginic N, Andjic M, Arsenijevic N, Lukic ML, Jovicic N. Overexpression of Galectin 3 in Pancreatic β Cells Amplifies β-Cell Apoptosis and Islet Inflammation in Type-2 Diabetes in Mice. Front Endocrinol (Lausanne). 2020;11:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 128. | Soto-Angona Ó, Anmella G, Valdés-Florido MJ, De Uribe-Viloria N, Carvalho AF, Penninx BWJH, Berk M. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 2020;18:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 129. | Chow YY, Verdonschot M, McEvoy CT, Peeters G. Associations between depression and cognition, mild cognitive impairment and dementia in persons with diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2022;185:109227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 130. | Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 131. | Martins LB, Braga Tibães JR, Berk M, Teixeira AL. Diabetes and mood disorders: shared mechanisms and therapeutic opportunities. Int J Psychiatry Clin Pract. 2022;26:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 132. | Sarwar H, Rafiqi SI, Ahmad S, Jinna S, Khan SA, Karim T, Qureshi O, Zahid ZA, Elhai JD, Levine JC, Naqvi SJ, Jaume JC, Imam S. Hyperinsulinemia Associated Depression. Clin Med Insights Endocrinol Diabetes. 2022;15:11795514221090244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 133. | Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith ME, Carr KD, Rice ME. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 134. | Dodd S, Sominsky L, Siskind D, Bortolasci CC, Carvalho AF, Maes M, Walker AJ, Walder K, Yung AR, Williams LJ, Myles H, Watson T, Berk M. The role of metformin as a treatment for neuropsychiatric illness. Eur Neuropsychopharmacol. 2022;64:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 135. | Ge T, Fan J, Yang W, Cui R, Li B. Leptin in depression: a potential therapeutic target. Cell Death Dis. 2018;9:1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |