Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.447
Peer-review started: September 24, 2022
First decision: November 27, 2022
Revised: December 9, 2022
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 15, 2023
Processing time: 233 Days and 6.5 Hours
Gastric emptying (GE) exhibits a wide inter-individual variation and is a major determinant of postprandial glycaemia in health and diabetes; the rise in blood glucose following oral carbohydrate is greater when GE is relatively more rapid and more sustained when glucose tolerance is impaired. Conversely, GE is influenced by the acute glycaemic environment acute hyperglycaemia slows, while acute hypoglycaemia accelerates it. Delayed GE (gastroparesis) occurs frequently in diabetes and critical illness. In diabetes, this poses challenges for management, particularly in hospitalised individuals and/or those using insulin. In critical illness it compromises the delivery of nutrition and increases the risk of regurgitation and aspiration with consequent lung dysfunction and ventilator dependence. Substantial advances in knowledge relating to GE, which is now recognised as a major determinant of the magnitude of the rise in blood glucose after a meal in both health and diabetes and, the impact of acute glycaemic environment on the rate of GE have been made and the use of gut-based therapies such as glucagon-like peptide-1 receptor agonists, which may profoundly impact GE, in the management of type 2 diabetes, has become commonplace. This necessitates an increased understanding of the complex inter-relationships of GE with glycaemia, its implications in hospitalised patients and the relevance of dysglycaemia and its management, particularly in critical illness. Current approaches to management of gastroparesis to achieve more personalised diabetes care, relevant to clinical practice, is detailed. Further studies focusing on the interactions of medications affecting GE and the glycaemic environment in hospitalised patients, are required.
Core Tip: Gastric emptying (GE) is a major determinant of postprandial glycaemia in health, diabetes and critical illness. Acute hyperglycaemia slows GE while insulin-induced hypoglycaemia accelerates it. Gastroparesis occurs frequently in diabetes and critical illness with a weak correlation between gastrointestinal symptoms and GE. Accordingly, diagnosis of gastroparesis should ideally be made after measuring GE with an optimal technique. Glucagon-like peptide-1 receptor agonists, commonly used in the treatment of type 2 diabetes and increasingly in obesity, may profoundly impact GE. We explore the rationale for current glycaemic targets and the implications of dysglycaemia and its management in hospitalised and critically ill populations.
- Citation: Arunachala Murthy T, Chapman M, Jones KL, Horowitz M, Marathe CS. Inter-relationships between gastric emptying and glycaemia: Implications for clinical practice. World J Diabetes 2023; 14(5): 447-459
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/447.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.447
In recent years there has been increasing interest regarding the relevance of gastrointestinal (GI) function, particularly gastric emptying (GE), to post-prandial glycaemia. GE is now recognised as a major determinant of the magnitude of the rise in blood glucose after a meal in both health and diabetes[1,2]. Moreover, in the past decade, use of gut-based therapies such as glucagon-like peptide-1 (GLP-1) receptor agonists (RAs), which may profoundly impact GE, in the management of type 2 diabetes, has become commonplace. On the other hand, it is also clear that the acute glycaemic environment impacts the rate of GE. This review focuses on two inter-related areas: Current knowledge of GE, including the pathophysiology of gastroparesis, and the inter-relationships between GE and glycaemia, including the clinical implications of these insights in hospitalised patients with diabetes, and for critical illness.
Although GI symptoms occur frequently in the general community[3], they are much more prevalent in people with diabetes and the consequences are generally underappreciated, despite impacting quality of life negatively[4]. These symptoms can be classified based on their apparent predominant site of origin in the GI tract, such as from the oesophagus (reflux, dysphagia), stomach (nausea/vomiting, bloating, abdominal distension, early satiety, abdominal pain and discomfort) or the intestines (diarrhoea, constipation, faecal incontinence)[5]. Epidemiological studies are indicative of a wide, but consistently high, prevalence (between 40% to 80%) of upper GI symptoms in people with diabetes, particularly females, the obese, those with Helicobacter Pylori infection and the elderly[5]. It is uncertain whether the prevalence differs between type 1 and type 2 diabetes. The natural history of GI symptoms remains poorly characterized, but a substantial turnover (i.e., appearance and disappearance of symptoms over time) has been observed. The latter may be to the order of 25% over a 24-mo period, such that the overall prevalence appears to be relatively constant[6]. A number of validated questionnaires for the assessment of GI symptoms, including the Patient Assessment of Upper Gastrointestinal Symptom Severity Index[7] and the Diabetes Bowel Symptom Questionnaire, are available but unfortunately, many clinical trials, particularly those related to glucose-lowering therapies[8] continue to report GI symptoms/adverse effects relying solely on participant self-reporting, which is known to be unreliable[9]. An important concept that is still poorly appreciated is that the association of upper GI symptoms with GI motility, including the rate of GE is generally weak in people with diabetes[9-11]. Therefore, a diagnosis of GI dysmotility (including gastroparesis) should not rely on symptoms alone and necessitates objective measurement.
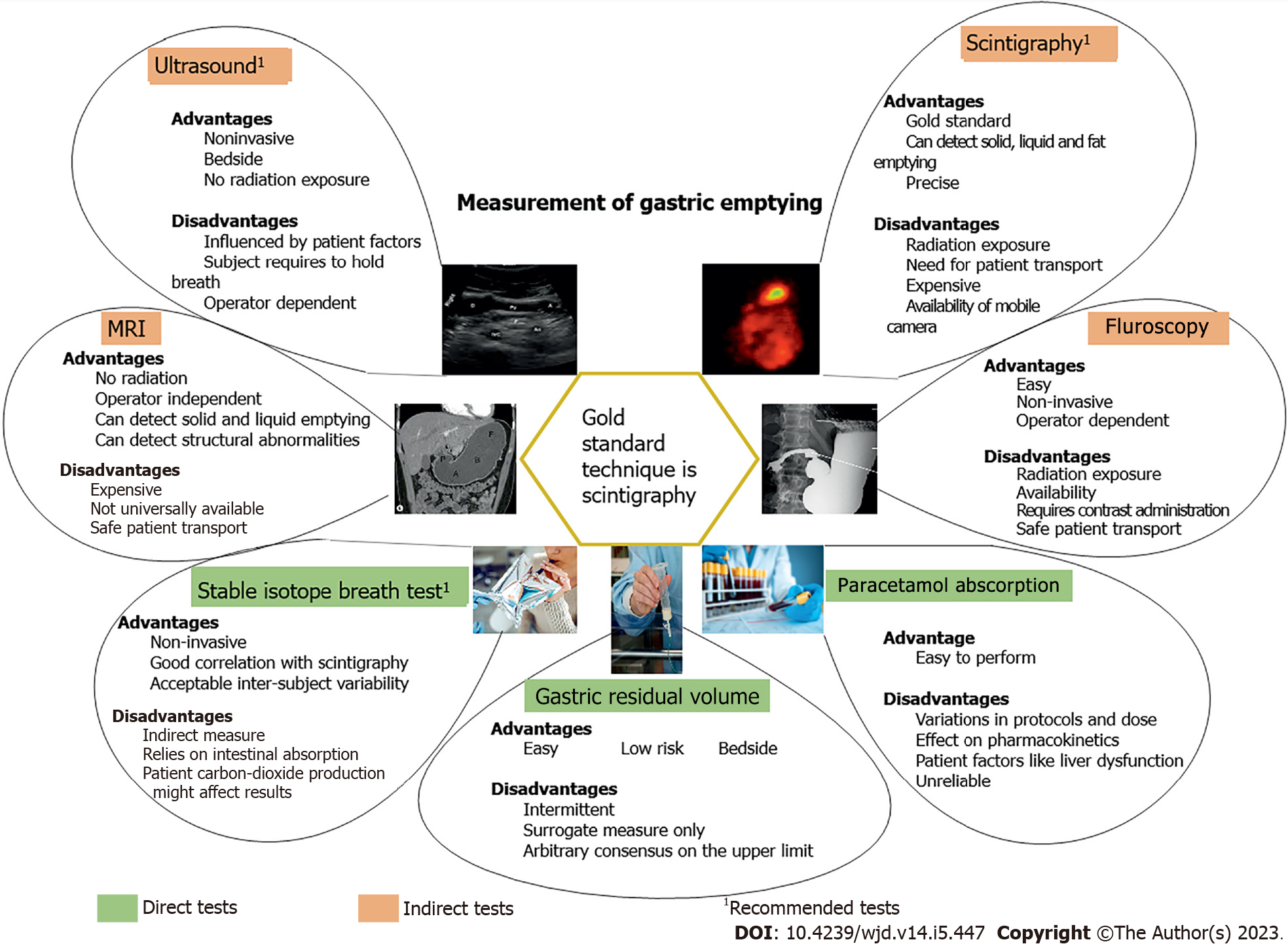
GE exhibits a wide inter-individual variability (approximately 1-4 kcal/min) in health, which is even greater in type 2 diabetes. A substantial proportion of people with longstanding, complicated type 2 diabetes (40%) have gastroparesis whereas, in uncomplicated type 2 diabetes[12] and adolescents with type 1 diabetes[13], GE is often abnormally accelerated. It should be appreciated that in patients with gastroparesis, the magnitude of the delay in GE is often modest[14]. The prevalence of delayed GE in ambulant people with diabetes remains uncertain, particularly as the diagnosis has been based primarily on the presence of significant upper GI symptoms, but diabetes appears to be the most common cause of gastroparesis[15]. The techniques currently available for measurement of GE are summarized in Figure 1.
The principal function of the stomach is transient storage, breakdown and transportation of ingested food. Patterns of gastroduodenal motility are distinct between the fasting and fed states. In the fasted state, a characteristic pattern is observed, referred to as the migratory motor complex (MMC) which has a ‘house-keeping’ role to propagate residual or undigested food through the GI tract[16]. The MMC, which lasts approximately 85-110 min comprises four, distinct phases: The first phase is quiescent (approximately 45-60 min) in which there are no contractions, the second involves initiation of intermittent and irregular contractions, the latter become stronger and more regular with bursts in the third phase, with each burst lasting for 5-15 min and occurring periodically every approximately 90-120 min. The fourth is a transitory period of irregular contractions between the third phase and the quiescent first phase. Thus, the MMC prepares the stomach for the arrival of food, by clearing its content[17]. The MMC continues until nutrients (liquid or solid) are ingested, when it is replaced by continuous post-prandial contractile activity. An important function of the stomach is to ‘accommodate’ the ingested food from the oesophagus with minimal increase in intra-gastric pressure, facilitated by a reduction in gastric tone and increase in compliance after meal ingestion[18]. As food moves from the proximal to the distal stomach, larger solid food particles are ground, predominantly in the antrum, into a fine chyme (partly digested semi-solid contents of the stomach) consisting of particles 1-2 mm in size which are delivered into the small intestine[19].
The rate of GE is regulated primarily by inhibitory feedback arising from the interaction with receptors in the small intestine, rather than intragastric factors[20], The magnitude of this feedback is dependent on both the region and length of small intestine exposed. GE involves a coordinated interplay of the extrinsic nervous system (mediated by the vagus), intrinsic or enteric nervous system (comprising Auerbach’s or myenteric, which controls the rate of peristalsis and Meissner’s plexus located below the level of the musculature, which controls secretion into the lumen of digestive tract), neurotransmitters [both excitatory e.g., acetylcholine and substance-P and inhibitory e.g., nitric oxide (NO) and VIP], the interstitial cells of Cajal (ICCs), mesenchymal cells including platelet-derived growth factors-alpha + cells, fibroblasts, haem-oxygenase 1, macrophages etc.[14,21], immune and smooth muscle cells. Gastric accommodation is mediated, at least in part, by the inhibitory neurotransmitter NO, while antral contractility is modulated by the excitatory neurotransmitter acetylcholine[22,23]. The ICCs are densely located in the corpus and antrum of the stomach, within the Auerbach plexus and regarded as ‘pacemakers’ for GI motility[24] by generating slow-waves responsible for contractions[25] and acting as mechanosensors by affecting the resting membrane potential through nitrergic and cholinergic transmission[26]. The ICCs act as a bridge between the extrinsic nervous system and the enteric nervous system to facilitate smooth muscle contraction.
Abnormally delayed GE, or gastroparesis, is generally a chronic disorder which can be defined as delayed emptying of nutrients from the stomach in the absence of mechanical obstruction[25]. The most common causes of gastroparesis are diabetes, post-surgical and idiopathic. The pathophysiology of disordered GE is, not surprisingly, multifactorial. Significant advances have been made in the last decade and a half, in part, due to concerted efforts of the National Institutes of Health funded, Gastroparesis Clinical Research Consortium (GpCRC). Autonomic neuropathy is mainly responsible for gastroparesis and vagal dysfunction is believed to contribute[27]. At the cellular level, a hallmark feature of gastroparesis is a reduction in the ICC[14]. GpCRC data indicates that in 50% of those with diabetic gastroparesis there is a reduction in ICC[28] and even when there is not a reduction, there are abnormalities in the ICC[28], so that the majority of these cells show signs of apoptosis, with increased mast cells and altered nerve endings which are either large or empty[29]. Expression of neuronal NO synthase[30] is reduced in diabetic gastroparesis[29]. The Kit receptor, tyrosine kinase, is expressed in ICC and loss of the receptor is characteristic in delayed GE[14]. In some studies this has been observed to be associated with a reduction in macrophages and their expression of Haeme-oxygenase 1, potentially affecting the capacity for repair and anti-inflammatory response in these cells[14] as well as increasing their susceptibility to oxidative damage. The heterogenous nature of the dysfunctions in gastroparesis has major implications for effective management.
The rate of GE is both a determinant of, as well as determined by, acute changes in glycaemia. Accordingly, studies exploring the impact on glycaemia have tended to control the rate of GE (e.g., by use of naso-duodenal infusions) and those exploring the impact on rate of emptying have controlled the glycaemic level (usually withglucose-insulin clamps). These studies are thus experimental in nature and the conclusions should be regarded as ‘proof-of-principle’. There is less information about the impact of spontaneous fluctuations in blood glucose.
The impact of GE on glycaemia: There are number of determinants of post-prandial glycaemia, including pre-prandial glycaemia, endogenous glucose production (hepatic and renal), intestinal glucose absorption and its disposal by the liver, hormone secretion (incretins, insulin) and insulin sensitivity[31]. GE, is now recognised to account for almost 35% of the variance in the initial post-prandial glycaemic response in both health[1] and type 2 diabetes[32]. In individuals with normal glucose tolerance, GE of a 75 g oral glucose drink is directly related to the ‘initial’ i.e. 30 min, plasma glucose, not 60 min and inversely related to the blood glucose at 120 min[1]. In contrast, in individuals with impaired glucose tolerance and type 2 diabetes, the rate of GE is related directly to glycaemia at 30 and 60 min and, particularly in type 2 diabetes, there is also a direct relationship at 120 min (the blood glucose level used in the diagnosis of diabetes) with a relatively faster GE associated with an increased glycaemic response, indicative of a ‘rightward’ shift[33,34].
There is evidence that in insulin-treated patients delayed GE/gastroparesis predisposes to post-prandial hypoglycaemia by inducing a mismatch in the coordination of nutrient delivery with the systemic availability of insulin we have proposed the term “gastric hypoglycaemia” to describe this phenomenon[35]. A Japanese study reported that in type 1 patients with gastroparesis, on continuous subcutaneous (SC) insulin infusion therapy, there was a reduction in the post-prandial insulin requirement in the first 120 min, and a greater requirement between 180-240 min[36]. A community study from Israel reported that GE was delayed in the majority of patients (approximately 80%) with unexplained hypoglycaemia[37]. The effect of accelerating/normalising GE on glycaemic control in these groups is not known and warrants evaluation.
The impact of glycaemia on GE: As mentioned, studies evaluating the impact of acute changes in glycaemia on emptying have largely relied on experimental models, particularly the so-called glucose-insulin ‘clamp’ technique. These have shown that acute hyperglycaemia slows GE of nutrient containing meals in health and type 1 diabetes, an effect which is dependent on the level of glycaemia[38-41]. Even so-called “physiological” hyperglycaemia (i.e. approximately 8 mmol/L), compared to 4 mmol/L slows GE in health[42] and type 1 diabetes[41]. Hebbard et al[43] studied regional stomach motility in health and showed that acute hyperglycaemia (15 mmol/L) affected proximal gastric motor function[43] while Samsom et al[40] studied antroduodenal motility using manometry in patients with type 1 diabetes and evidence of autonomic neuropathy and demonstrated a reduction in post-prandial antral contractility during hyperglycaemia (16-19 mmol/L)[40]. Acute hyperglycaemia also appears to delay GE in type 2 diabetes[44] and critically ill[45,46]. In contrast, spontaneous fluctuations in glycaemia has none, or a lesser effect on GE [47].
The impact of chronic glycaemia, as assessed by glycated haemoglobin (HbA1c) on GE is poorly defined, including the effect of improved glycaemic control. Analysis of the data from the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) study[48] indicates that delayed GE is associated with abnormal measures of longer-term hyperglycaemia, such as HbA1c[49]. The impact of intensive glucose lowering on GE is uncertain. Laway et al[50] studied asymptomatic women with newly diagnosed type 2 diabetes and reported a substantial acceleration of GE with improved glycaemic control, but the design of the study was uncontrolled[50]. Other studies failed to find any effect of improved glycaemic control[51]. Bharucha et al[49], followed up participants from the DCCT[49] and its subsequent follow-up (DCCT-EDIC)[48] and found that those with a longer duration of diabetes and worse glycaemic control at baseline, tended to have delayed GE. However, because GE was not quantified at baseline, the impact of intensive glucose-lowering on GE could not be evaluated. The outcomes of other retrospective studies evaluating the relationship of chronic glycaemia (based on HbA1c) and GE are inconsistent[52-54]. Accordingly, further studies are required.
While there is less information about the effects of acute insulin-induced hypoglycaemia on GE, the outcomes are more consistent. Hypoglycaemia is the most common and feared symptom of insulin, and sulfonylurea, treated diabetes and represents a major limiting factor in achieving optimal glucose control[55]. In response to an acute reduction in blood glucose, a predictable sequence of protective (counter-regulatory) mechanisms are elicited in health[56]. Most widely recognised are the hormonal counter-regulatory responses (early response modulated by glucagon and catecholamines and later responses by cortisol and GH)[57,58]. It is not well appreciated that acute hypoglycaemia also accelerates GE markedly. As early as 1924, i.e. within 3 years of the commercial availability of insulin, Bulatao and Carson[59] reported increased contractions of the fasting canine stomach after insulin administration and attributed this effect to hypoglycaemia. In the 1990s and 2000s, acceleration of GE was confirmed employing the ‘gold standard’ technique of scintigraphy to measure GE, in both health and type 1 diabetes. We recently showed that the magnitude of acceleration of GE is also dependent on the level of the hypoglycaemia in health GE was accelerated during both mild; Approximately 3.6 mmol/L (approximately 20% difference) and marked; Approximately 2.6 mmol/L (40% difference) hypoglycaemia when compared to euglycaemia; approximately 6 mmol/L, but was faster during marked compared with mild hypoglycaemia[42]. This acceleration of GE, which is still evident in type 1 patients with gastroparesis or cardiovascular autonomic neuropathy[56]. This acceleration of GE, which is still evident in type 1 patients with gastroparesis and/or cardiovascular autonomic neuropathy[60], is likely to be an important counter-regulatory mechanism which supports more rapid intestinal glucose absorption[57]. Studies evaluating the effects of hypoglycaemia on GE in the critically ill are, not surprisingly, lacking because of the established harmful effects of hypoglycaemia in this population[61,62].
The management of dysglycaemia and its consequences in hospitalised patients is of more relevance due to increasing prevalence of diabetes in this group. The implications of the use of the newer anti-diabetic medications in this group is also of substantial interest.
Dysglycaemia is a major issue in hospitalised patients and associated with poor outcomes, including increased length of stay, morbidity and mortality[63].The prevalence of diabetes is markedly higher in hospitalised patients when compared to the community ranging from 22%-46%[60,64]. While hyperglycaemia is a well-recognised poor prognostic indicator, hypoglycaemia has been reported to occur in about 6% of hospitalised patients[64]. There is only limited information about the relationship of GE to dysglycaemia in this group.
GE is seldom measured using an optimal technique in the hospital setting unless gastroparesis is suspected. Iatrogenic aetiologies (due to medications or post-surgery) are also common. Nevertheless, the prevalence of delayed GE measured with scintigraphy has been estimated to be between 17% to 30%[65] in hospitalised patients with diabetes. Kojecky et al[65] found that female gender, nausea and early satiety were associated with a higher probability of delayed GE[65]. The impact of medications affecting GI motility (e.g., anticholinergics, sympathomimetic vasopressors, GLP-1RAs, opioids, prokinetics etc.) on drug and nutrient absorption during hospitalization is not known. While it is intuitively likely that undiagnosed gastroparesis will increase morbidity in hospitalised patients, there is lack of information about this.
The gut-derived incretin hormones (GIP and GLP-1) account for about 50% of the post-prandial insulin response in health[66,67] and are responsible for the ‘incretin effect’ [the amplified insulin secretory response to oral compared with intravenous (IV) glucose]. GIP is the dominant incretin in health[68] but its insulinotropic capacity is markedly attenuated in type 2 diabetes[69], unlike GLP-1, which largely retains the insulin stimulating and glucagon supressing properties. The rate of GE impacts the secretion of incretin hormones. Studies employing intraduodenal glucose infusion, an experimental model for estimating the impact of GE on incretin secretion by bypassing the gastric pylorus, suggest that there may be a ‘threshold’ rate of emptying at which significant GLP-1 release is observed following a carbohydrate containing meal[70]. Increasing the rate of intraduodenal glucose infusion from 1 to 4 kcal/min results in a proportionate increase in GIP release; in contrast there is minimal, if any GLP-1 release with an infusion rate < 2 kcal/min, with sustained responses at 3 and 4 kcal/min[71].
Native GLP-1, located primarily in the distal small intestine and triggered following macronutrient exposure, is degraded within minutes in vivo, by the ubiquitous enzyme, dipeptidyl peptidase IV (DPP-IV). Two strategies: (1) DPP-IV inhibition which prevents degradation of the enzyme; and (2) GLP-1RAs have been developed to exploit GLP-1 pharmaceutically. Both classes of medication are widely available but the use of GLP-1RA’s, in particular, is expanding rapidly (approximately $11.3 billion global sales in 2019, projected to grow to approximately $18.2 billion by 2027). Recent, large-scale, cardiovascular and renal outcome studies have shown positive benefits of these agents particularly in individuals with diabetes and concomitant ischaemic heart disease or cardiac failure[72].
GLP-1RAs are, in nearly all cases, administered by SC injection either daily or weekly. GLP-1RAs, especially the ‘short acting’ agents, such as exenatide BD and lixisenatide, primarily act by delaying GE and thereby reducing post-prandial glycaemia[73], while the effect of ‘long-acting’ GLP-1RA’s (e.g., dulaglutide, semaglutide) has been poorly characterised due to the use of suboptimal methodology (paracetamol absorption)[74,75]. It had been assumed that they had no effect with sustained use due to tachyphylaxis, but it is now clear that both the exenatide once weekly preparation and liraglutide do slow GE[76,77] and there are anecdotal reports of retained gastric content at endoscopy with these drugs[78]. The effects of these drugs on small intestinal transit, which may affect carbohydrate absorption are poorly studied. Long-acting GLP-1RAs are used increasingly to induce weight loss in obese individuals.
A fundamental issue with these agents is their current essentially empirical use. Given its central importance, the effect of these drugs on GE should be characterised; it is likely that they all slow GE; patients taking long-acting GLP-1RAs for type 2 diabetes or obesity (higher dose) should be, accordingly, regarded at increased risk for delayed GE (i.e., gastroparesis), until this is shown not to be the case, whereas the effect of short-acting GLP-1RA’s should be transient, reflecting their plasma half-life. The impact of GLP-1RA on GE in different glycaemic environments (such as acute hyperglycaemia or hypoglycaemia) is not known. While GLP-1RA by themselves seldom cause hypoglycaemia (i.e., their actions are glucose-dependent)[79], in combination with insulin or sulphonylureas, there is an increased risk of hypoglycaemia. There is need for further studies evaluating the effect of long-acting GLP-1RAs in the presence of other medications that affect GE (prokinetics, oral opioid pain medications etc.). In contrast, DPP-IV inhibitors have minimal or no impact on GE[80], presumably because of the more modest elevation in GLP-1. However, the rate of GE influences the post-prandial glycaemic response to DPP-IV inhibitors[81].
Dysglycaemia is also common in critically ill patients, can present as hyperglycaemia, hypoglycaemia or glycaemic variability and is associated with increased mortality[82,83], infection[84,85] and other complications[86,87]. Hyperglycaemia during critical illness can be attributed to pre-existing diabetes (both type 1 and type 2; 13%-20% of patients)[61,83], incidental/unrecognised diabetes (defined as HbA1c > 6.5% identified for the first time during acute illness; 5%-15%)[88-90] or stress hyperglycaemia (defined as a peak blood glucose concentration that, in health, would lead to a diagnosis of diabetes; 17%-50%)[91-94]. The underlying mechanisms of acute hyperglycaemia in the critically ill include increased insulin resistance[95] and relative insulin insufficiency[96]. Long-term consequences of stress hyperglycaemia include a higher rate of subsequent diabetes and its associated complications[97,98]. Exogenous insulin used to achieve glycaemic control can cause hypoglycaemia and increased glycaemic variability, both of which have an impact on mortality[61,99,100].
In the critically ill, nutrition is most commonly delivered via the nasogastric route and success is, accordingly, dependant on intact gut function. Delayed GE is common, (50%-80%), as indicated by large gastric residual volumes (GRVs), and associated with early cessation of enteral nutrition, increased infection, increased length of stay and increased mortality[101-103]. Surprisingly, pre-existing type 2 diabetes does not appear to be a risk factor for delayed GE[104], suggesting that the delayed GE in critical illness is mechanistically unrelated. We have reported that the rate and extent of glucose absorption following intragastric administration is markedly reduced in about 1/3rd of intensive care unit patients[46] and is dependent on the rate of GE[105]. Thus, GE is a major determinant of postprandial glycaemia in this group[1,13] and may predispose to increased glycaemic variability[106]. Furthermore, delayed GE in patients treated with insulin may represent a risk factor for hypoglycaemia[37]. Likewise, acute hyperglycaemia has been associated with delayed GE in the critically ill[46]. Due to the interdependent relationships and extent of glycaemic variability noted in many studies there are likely to be multiple factors affecting this relationship in both directions. Thus, interventions aimed at overcoming delayed GE, for example the use of prokinetics, post-pyloric tubes and parenteral nutrition, may have as yet unidentified effects on glycaemia. Prokinetic therapy can improve critical illness gastroparesis and has been associated with better clinical outcomes[107], but its impact on glycaemic variability is uncertain[108-110].
The macronutrient composition of feed formulae is likely to have both direct and indirect effects on glycaemia, the latter by affecting the rate of GE. Energy dense and high lipid feed formulae are associated with slower GE (i.e., emptying proceeds at a specific caloric rate (kcal/min) and is, accordingly prolonged) with no significant improvement in glycaemic control[111]. The large, multi-centre TARGET trial, reported that the administration of a high density formula (additional calories from additional lipid and carbohydrate) resulted in both hyperglycaemia requiring higher insulin doses[112] and larger GRVs. The additional carbohydrate is likely to account for the higher blood glucose and the increased lipid could contribute to the slower GE. As these parameters are interrelated, it is impossible to determine from this study whether, and by how much, hyperglycaemia per se is causing the slowing of GE or vice versa. Rugeles et al[113] reported less hyperglycaemia with high-protein hypocaloric feeds. In another pilot RCT (FEED trial) comparing the effect of two protein doses (1.2 g/kg/day vs 0.75 g/kg/day) on muscle mass, no difference in feed intolerance (GRV > 300 mL) was evident[114]. In another pilot study investigating the feasibility of delivering higher protein doses (1.52 ± 0.52 vs 0.99 ± 0.27 g/kg/d), there was no difference in glycaemia and mean daily GRVs were less[115]. High protein feed formulae may, accordingly, potentially result in less GI intolerance and dysglycaemia, but this requires confirmation in larger studies.
Insulin remains the most frequently used medication to treat hyperglycaemia in critically ill patients. Most other oral anti-antidiabetic medications are withheld in intensive care patients due to their unpredictable absorption and concerns about their impact on glycaemic variability and variable nutrition intake. Long-acting insulin is sometimes used in patients tolerating enteral nutrition for sustained glycaemic control during the recovery phase of illness due to the convenience of administration. However, in the acute phase of critical illness, short-acting, continuously infused, IV insulin is generally used. This carries the risks of increased glycaemic variability and hypoglycaemia, necessitating intensive monitoring. Thus, other medications that can normalise elevated blood glucose levels and reduce glycaemic variability and the risk of hyperglycaemia are being explored.
Gut-based antidiabetic therapies (e.g., incretin hormones) may offer a safe yet effective alternative to insulin. Our group has published ‘proof of concept’ studies over the past decade in which we have demonstrated that exogenous GLP-1 infusion attenuates, but does not normalize, hyperglycaemia induced by enteral nutrition in critically ill patients with both type 1 diabetes[116] and stress hyperglycemia. The slowing of GE by GLP-1 appears to be a plausible contributory mechanism[105]. IV GLP-1 may also reduce glycaemic variability, although in this small study it did not appear to impact IV insulin requirements or the frequency of hypoglycaemia)[117]. This study was also limited by the dosing of the medication (FDA mandate limiting GLP-1 dose to 1.5 pmol/kg/min) and the magnitude of glucose lowering (desired glucose range of 4.44–6.11 mmol/L was achieved in only a minority of patients)[117]. The use of GLP-1RAs is of interest, mainly due to the low risk of hypoglycaemia, given the glucose-dependency of the insulinotropic effect and glucagon suppression in comparison to currently used IV insulin therapy. The impact of GLP1-RAs on glycaemic management, GE, nutrition delivery and medium and longer term clinical outcomes in critically ill patients is not known. A potential limitation related to their current SC, rather than IV, use and the lack of safety data in the critically ill. It should be appreciated that GLP-1RAs have cardiac and renal protective effects with longer-term use which may be of relevance[118].
GE has an important and inter-dependent relationship with the acute glycaemic environment in health, diabetes, and critical illness, which is relevant to clinical practice. Abnormally delayed GE, or gastroparesis, is common in type 1 and type 2 diabetes, and in critical illness. Recent insights have led to a better understanding of the pathophysiology of diabetic gastroparesis, especially at the cellular level. Glucose-lowering medications such as GLP-1RAs that act primarily by slowing GE, are used widely today in the management of type 2 diabetes but their actions on GE under various glycaemic conditions are not known and their place in the management of dysglycaemia in critical illness remains uncertain. Advantages of reduced hypoglycaemia and glycaemic variability will need to be balanced against the potentially adverse impact of slowing of GE on nutrition delivery and the risk of aspiration. Further studies building on these insights and focusing on the interactions of medications affecting GE and glycaemic environment in hospitalised patients are required.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Soriano-Ursúa MA, Mexico S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 335] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Thompson DG, Wingate DL, Thomas M, Harrison D. Gastric emptying as a determinant of the oral glucose tolerance test. Gastroenterology. 1982;82:51-55. [PubMed] |
| 3. | Maleki D, Locke GR 3rd, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, Melton LJ 3rd. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal Symptoms in Diabetes: Prevalence, Assessment, Pathogenesis, and Management. Diabetes Care. 2018;41:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Quan C, Talley NJ, Jones MP, Howell S, Horowitz M. Gastrointestinal symptoms and glycemic control in diabetes mellitus: a longitudinal population study. Eur J Gastroenterol Hepatol. 2008;20:888-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Olausson EA, Brock C, Drewes AM, Grundin H, Isaksson M, Stotzer P, Abrahamsson H, Attvall S, Simrén M. Measurement of gastric emptying by radiopaque markers in patients with diabetes: correlation with scintigraphy and upper gastrointestinal symptoms. Neurogastroenterol Motil. 2013;25:e224-e232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, Gerich JE. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36:2945-2951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Annese V, Bassotti G, Caruso N, De Cosmo S, Gabbrielli A, Modoni S, Frusciante V, Andriulli A. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J Clin Gastroenterol. 1999;29:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Ziegler D, Schadewaldt P, Pour Mirza A, Piolot R, Schommartz B, Reinhardt M, Vosberg H, Brösicke H, Gries FA. [13C]octanoic acid breath test for non-invasive assessment of gastric emptying in diabetic patients: validation and relationship to gastric symptoms and cardiovascular autonomic function. Diabetologia. 1996;39:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 12. | Goyal RK, Cristofaro V, Sullivan MP. Rapid gastric emptying in diabetes mellitus: Pathophysiology and clinical importance. J Diabetes Complications. 2019;33:107414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Perano SJ, Rayner CK, Kritas S, Horowitz M, Donaghue K, Mpundu-Kaambwa C, Giles L, Couper JJ. Gastric Emptying Is More Rapid in Adolescents With Type 1 Diabetes and Impacts on Postprandial Glycemia. J Clin Endocrinol Metab. 2015;100:2248-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Ye Y, Yin Y, Huh SY, Almansa C, Bennett D, Camilleri M. Epidemiology, Etiology, and Treatment of Gastroparesis: Real-World Evidence From a Large US National Claims Database. Gastroenterology. 2022;162:109-121.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 16. | Takahashi T. Mechanism of interdigestive migrating motor complex. J Neurogastroenterol Motil. 2012;18:246-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. 2012;9:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Schwizer W, Steingötter A, Fox M, Zur T, Thumshirn M, Bösiger P, Fried M. Non-invasive measurement of gastric accommodation in humans. Gut. 2002;51 Suppl 1:i59-i62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Weisbrodt NW, Wiley JN, Overholt BF, Bass P. A relation between gastroduodenal muscle contractions and gastric empyting. Gut. 1969;10:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Horowitz M, Dent J. Disordered gastric emptying: mechanical basis, assessment and treatment. Baillieres Clin Gastroenterol. 1991;5:371-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Boeckxstaens G, Camilleri M, Sifrim D, Houghton LA, Elsenbruch S, Lindberg G, Azpiroz F, Parkman HP. Fundamentals of Neurogastroenterology: Physiology/Motility - Sensation. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Camilleri M. Gastrointestinal hormones and regulation of gastric emptying. Curr Opin Endocrinol Diabetes Obes. 2019;26:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 24. | Mazet B, Raynier C. Interstitial cells of Cajal in the guinea pig gastric antrum: distribution and regional density. Cell Tissue Res. 2004;316:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, Low PA, Park SY, Parkman HP, Stanghellini V. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 26. | Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913-14918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Minami H, McCallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology. 1984;86:1592-1610. [PubMed] |
| 28. | Farrugia G. Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am. 2015;44:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Ünalp-Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA, Pasricha PJ; NIDDK Gastroparesis Clinical Research Consortium. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575-85.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 30. | Giudice G, Maruccia M, Vestita M, Nacchiero E, Annoscia P, Bucaria V, Elia R. The medial-central septum based mammaplasty: A reliable technique to preserve nipple-areola complex sensitivity in post bariatric patients. Breast J. 2019;25:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectr. 2004;17:183-190. |
| 32. | Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643-1648. [PubMed] |
| 33. | Marathe CS, Horowitz M, Trahair LG, Wishart JM, Bound M, Lange K, Rayner CK, Jones KL. Relationships of Early And Late Glycemic Responses With Gastric Emptying During An Oral Glucose Tolerance Test. J Clin Endocrinol Metab. 2015;100:3565-3571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Jalleh RJ, Wu T, Jones KL, Rayner CK, Horowitz M, Marathe CS. Relationships of Glucose, GLP-1, and Insulin Secretion With Gastric Emptying After a 75-g Glucose Load in Type 2 Diabetes. J Clin Endocrinol Metab. 2022;107:e3850-e3856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Horowitz M, Jones KL, Rayner CK, Read NW. 'Gastric' hypoglycaemia--an important concept in diabetes management. Neurogastroenterol Motil. 2006;18:405-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Ishii M, Nakamura T, Kasai F, Onuma T, Baba T, Takebe K. Altered postprandial insulin requirement in IDDM patients with gastroparesis. Diabetes Care. 1994;17:901-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Lysy J, Israeli E, Strauss-Liviatan N, Goldin E. Relationships between hypoglycaemia and gastric emptying abnormalities in insulin-treated diabetic patients. Neurogastroenterol Motil. 2006;18:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Jones KL, Horowitz M, Wishart MJ, Maddox AF, Harding PE, Chatterton BE. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med. 1995;36:2220-2228. [PubMed] |
| 39. | Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 341] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Samsom M, Akkermans LM, Jebbink RJ, van Isselt H, vanBerge-Henegouwen GP, Smout AJ. Gastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitus. Gut. 1997;40:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Schvarcz E, Palmér M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Murthy TA, Grivell J, Hatzinikolas S, Chapple LS, Chapman MJ, Stevens JE, Malbert CH, Rayner CK, Horowitz M, Jones KL, Marathe CS. Acceleration of Gastric Emptying by Insulin-Induced Hypoglycemia is Dependent on the Degree of Hypoglycemia. J Clin Endocrinol Metab. 2021;106:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Hebbard GS, Sun WM, Dent J, Horowitz M. Hyperglycaemia affects proximal gastric motor and sensory function in normal subjects. Eur J Gastroenterol Hepatol. 1996;8:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 273] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Deane AM, Chapman MJ, Fraser RJ, Summers MJ, Zaknic AV, Storey JP, Jones KL, Rayner CK, Horowitz M. Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med. 2010;38:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Chapman MJ, Fraser RJ, Matthews G, Russo A, Bellon M, Besanko LK, Jones KL, Butler R, Chatterton B, Horowitz M. Glucose absorption and gastric emptying in critical illness. Crit Care. 2009;13:R140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Horowitz M, Wu T, Rayner CK, Marathe CS, Jones KL. Spontaneous or Deliberate: Effects of Acute Variations in Glycemia on Gastric Emptying in Type 1 Diabetes. Diabetes Care. 2021;44:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care. 2016;39:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 49. | Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, Driscoll M, Harth J, Larkin M, Christofi M, Bayless M, Wimmergren N, Herman W, Whitehouse F, Jones K, Kruger D, Martin C, Ziegler G, Zinsmeister AR, Nathan DM; Diabetes Control and Complications Trial–Epidemiology of Diabetes Interventions and Complications Research Group. Delayed Gastric Emptying Is Associated With Early and Long-term Hyperglycemia in Type 1 Diabetes Mellitus. Gastroenterology. 2015;149:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 50. | Laway BA, Malik TS, Khan SH, Rather TA. Prevalence of abnormal gastric emptying in asymptomatic women with newly detected diabetes and its reversibility after glycemic control-a prospective case control study. J Diabetes Complications. 2013;27:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, Zinsmeister AR. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13:466-476.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Izzy M, Lee M, Johns-Keating K, Kargoli F, Beckoff S, Chun K, Tokayer A. Glycosylated hemoglobin level may predict the severity of gastroparesis in diabetic patients. Diabetes Res Clin Pract. 2018;135:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Reddy S, Ramsubeik K, Vega KJ, Federico J, Palacio C. Do HbA1C Levels Correlate With Delayed Gastric Emptying in Diabetic Patients? J Neurogastroenterol Motil. 2010;16:414-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Merio R, Festa A, Bergmann H, Eder T, Eibl N, Stacher-Janotta G, Weber U, Budka C, Heckenberg A, Bauer P, Francesconi M, Schernthaner G, Stacher G. Slow gastric emptying in type I diabetes: relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care. 1997;20:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Wright AD, Cull CA, Macleod KM, Holman RR; UKPDS Group. Hypoglycemia in Type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J Diabetes Complications. 2006;20:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Russo A, Stevens JE, Chen R, Gentilcore D, Burnet R, Horowitz M, Jones KL. Insulin-induced hypoglycemia accelerates gastric emptying of solids and liquids in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2005;90:4489-4495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Marathe CS, Marathe JA, Rayner CK, Kar P, Jones KL, Horowitz M. Hypoglycaemia and gastric emptying. Diabetes Obes Metab. 2019;21:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Alsahli M, Gerich JE. Hypoglycemia. Endocrinol Metab Clin North Am. 2013;42:657-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Bulatao E, Carlson AJ. Contributions to the physiology of the stomach: influence of experimental changes in blood sugar level on gastric hunger contractions. Am J Phys-Lega Conte. 1924;69:107-115. |
| 60. | Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, Van den Berghe G; Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 747] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 61. | van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7077] [Cited by in RCA: 6183] [Article Influence: 257.6] [Reference Citation Analysis (2)] |
| 62. | Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 1881] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 63. | Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 287] [Article Influence: 31.9] [Reference Citation Analysis (1)] |
| 64. | Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 65. | Kojecky V, Bernatek J, Horowitz M, Zemek S, Bakala J, Hep A. Prevalence and determinants of delayed gastric emptying in hospitalised Type 2 diabetic patients. World J Gastroenterol. 2008;14:1564-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 565] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | O'Donovan DG, Doran S, Feinle-Bisset C, Jones KL, Meyer JH, Wishart JM, Morris HA, Horowitz M. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3431-3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Gasbjerg LS, Helsted MM, Hartmann B, Jensen MH, Gabe MBN, Sparre-Ulrich AH, Veedfald S, Stensen S, Lanng AR, Bergmann NC, Christensen MB, Vilsbøll T, Holst JJ, Rosenkilde MM, Knop FK. Separate and Combined Glucometabolic Effects of Endogenous Glucose-Dependent Insulinotropic Polypeptide and Glucagon-like Peptide 1 in Healthy Individuals. Diabetes. 2019;68:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 69. | Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 927] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 70. | MacIntosh CG, Horowitz M, Verhagen MA, Smout AJ, Wishart J, Morris H, Goble E, Morley JE, Chapman IM. Effect of small intestinal nutrient infusion on appetite, gastrointestinal hormone release, and gastric myoelectrical activity in young and older men. Am J Gastroenterol. 2001;96:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Marathe CS, Rayner CK, Bound M, Checklin H, Standfield S, Wishart J, Lange K, Jones KL, Horowitz M. Small intestinal glucose exposure determines the magnitude of the incretin effect in health and type 2 diabetes. Diabetes. 2014;63:2668-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Muskiet MH, Smits MM, Morsink LM, Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10:88-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 73. | Rizzo M, Rizvi AA, Spinas GA, Rini GB, Berneis K. Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin Investig Drugs. 2009;18:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 75. | Jelsing J, Vrang N, Hansen G, Raun K, Tang-Christensen M, Knudsen LB. Liraglutide: short-lived effect on gastric emptying -- long lasting effects on body weight. Diabetes Obes Metab. 2012;14:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | Trujillo J. Safety and tolerability of once-weekly GLP-1 receptor agonists in type 2 diabetes. J Clin Pharm Ther. 2020;45 Suppl 1:43-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 77. | van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38:784-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 78. | Stark JE, Cole JL, Ghazarian RN, Klass MJ. Impact of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA) on Food Content During Esophagogastroduodenoscopy (EGD). Ann Pharmacother. 2022;56:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 79. | Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 959] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 80. | Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, Deacon CF, Foley JE, Rizza RA, Camilleri M. The effect of dipeptidyl peptidase-4 inhibition on gastric volume, satiation and enteroendocrine secretion in type 2 diabetes: a double-blind, placebo-controlled crossover study. Clin Endocrinol (Oxf). 2008;69:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Stevens JE, Buttfield M, Wu T, Hatzinikolas S, Pham H, Lange K, Rayner CK, Horowitz M, Jones KL. Effects of sitagliptin on gastric emptying of, and the glycaemic and blood pressure responses to, a carbohydrate meal in type 2 diabetes. Diabetes Obes Metab. 2020;22:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 83. | NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3409] [Cited by in RCA: 3185] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 84. | Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 704] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 85. | van Vught LA, Wiewel MA, Klein Klouwenberg PM, Hoogendijk AJ, Scicluna BP, Ong DS, Cremer OL, Horn J, Bonten MM, Schultz MJ, van der Poll T; Molecular Diagnosis and Risk Stratification of Sepsis Consortium. Admission Hyperglycemia in Critically Ill Sepsis Patients: Association With Outcome and Host Response. Crit Care Med. 2016;44:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med. 2012;40:3180-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Bellaver P, Schaeffer AF, Dullius DP, Viana MV, Leitão CB, Rech TH. Association of multiple glycemic parameters at intensive care unit admission with mortality and clinical outcomes in critically ill patients. Sci Rep. 2019;9:18498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Hoang QN, Pisani MA, Inzucchi S, Hu B, Honiden S. The prevalence of undiagnosed diabetes mellitus and the association of baseline glycemic control on mortality in the intensive care unit: a prospective observational study. J Crit Care. 2014;29:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Wexler DJ, Nathan DM, Grant RW, Regan S, Van Leuvan AL, Cagliero E. Prevalence of elevated hemoglobin A1c among patients admitted to the hospital without a diagnosis of diabetes. J Clin Endocrinol Metab. 2008;93:4238-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Carpenter DL, Gregg SR, Xu K, Buchman TG, Coopersmith CM. Prevalence and Impact of Unknown Diabetes in the ICU. Crit Care Med. 2015;43:e541-e550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Siegelaar SE, Hickmann M, Hoekstra JB, Holleman F, DeVries JH. The effect of diabetes on mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2011;15:R205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Deane AM, Horowitz M. Dysglycaemia in the critically ill - significance and management. Diabetes Obes Metab. 2013;15:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798-1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 970] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 94. | Plummer MP, Bellomo R, Cousins CE, Annink CE, Sundararajan K, Reddi BA, Raj JP, Chapman MJ, Horowitz M, Deane AM. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014;40:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 95. | Saberi F, Heyland D, Lam M, Rapson D, Jeejeebhoy K. Prevalence, incidence, and clinical resolution of insulin resistance in critically ill patients: an observational study. JPEN J Parenter Enteral Nutr. 2008;32:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Clowes GH Jr, Martin H, Walji S, Hirsch E, Gazitua R, Goodfellow R. Blood insulin responses to blood glucose levels in high output sepsis and spetic shock. Am J Surg. 1978;135:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Ali Abdelhamid Y, Kar P, Finnis ME, Phillips LK, Plummer MP, Shaw JE, Horowitz M, Deane AM. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and meta-analysis. Crit Care. 2016;20:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 98. | Kar P, Plummer MP, Ali Abdelhamid Y, Giersch EJ, Summers MJ, Weinel LM, Finnis ME, Phillips LK, Jones KL, Horowitz M, Deane AM. Incident Diabetes in Survivors of Critical Illness and Mechanisms Underlying Persistent Glucose Intolerance: A Prospective Cohort Study. Crit Care Med. 2019;47:e103-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, Hoekstra JB. Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med. 2006;34:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 100. | Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2385] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 101. | Adike A, Quigley EM. Gastrointestinal motility problems in critical care: a clinical perspective. J Dig Dis. 2014;15:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Retief I. The management of motility disorders in critical illness. S Afr J Clin Nutr. 2011;24:15-18. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 103. | Govil D, Pal D. Gastrointestinal Motility Disorders in Critically Ill. Indian J Crit Care Med. 2020;24:S179-S182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Nguyen NQ, Chapman M, Fraser RJ, Ritz M, Bryant LK, Butler R, Davidson G, Zacharakis B, Holloway RH. Long-standing type II diabetes mellitus is not a risk factor for slow gastric emptying in critically ill patients. Intensive Care Med. 2006;32:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Deane A, Chapman MJ, Fraser RJ, Horowitz M. Bench-to-bedside review: the gut as an endocrine organ in the critically ill. Crit Care. 2010;14:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Clain J, Ramar K, Surani SR. Glucose control in critical care. World J Diabetes. 2015;6:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 107. | Arunachala Murthy T, Chapple LS, Lange K, Marathe CS, Horowitz M, Peake SL, Chapman MJ. Gastrointestinal dysfunction during enteral nutrition delivery in intensive care unit (ICU) patients: Risk factors, natural history, and clinical implications. A post-hoc analysis of The Augmented versus Routine approach to Giving Energy Trial (TARGET). Am J Clin Nutr. 2022;116:589-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 108. | Rayner CK, Su YC, Doran SM, Jones KL, Malbert CH, Horowitz M. The stimulation of antral motility by erythromycin is attenuated by hyperglycemia. Am J Gastroenterol. 2000;95:2233-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Jones KL, Berry M, Kong MF, Kwiatek MA, Samsom M, Horowitz M. Hyperglycemia attenuates the gastrokinetic effect of erythromycin and affects the perception of postprandial hunger in normal subjects. Diabetes Care. 1999;22:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 110. | Braden B, Enghofer M, Schaub M, Usadel KH, Caspary WF, Lembcke B. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 111. | Chapple LS, Summers MJ, Weinel LM, Abdelhamid YA, Kar P, Hatzinikolas S, Calnan D, Bills M, Lange K, Poole A, O'Connor SN, Horowitz M, Jones KL, Deane AM, Chapman MJ. Effects of Standard vs Energy-Dense Formulae on Gastric Retention, Energy Delivery, and Glycemia in Critically Ill Patients. JPEN J Parenter Enteral Nutr. 2021;45:710-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | TARGET Investigators; for the ANZICS Clinical Trials Group; Chapman M, Peake SL, Bellomo R, Davies A, Deane A, Horowitz M, Hurford S, Lange K, Little L, Mackle D, O’Connor S, Presneill J, Ridley E, Williams P, Young P. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. N Engl J Med. 2018;379:1823-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 113. | Rugeles SJ, Rueda JD, Díaz CE, Rosselli D. Hyperproteic hypocaloric enteral nutrition in the critically ill patient: A randomized controlled clinical trial. Indian J Crit Care Med. 2013;17:343-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 114. | Fetterplace K, Deane AM, Tierney A, Beach L, Knight LD, Rechnitzer T, Forsyth A, Mourtzakis M, Presneill J, MacIsaac C. Targeted full energy and protein delivery in critically ill patients: a study protocol for a pilot randomised control trial (FEED Trial). Pilot Feasibility Stud. 2018;4:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Chapple LS, Summers MJ, Bellomo R, Chapman MJ, Davies AR, Ferrie S, Finnis ME, Hurford S, Lange K, Little L, O'Connor SN, Peake SL, Ridley EJ, Young PJ, Williams PJ, Deane AM; TARGET Investigator Collaborative and the ANZICS Clinical Trials Group. Use of a High-Protein Enteral Nutrition Formula to Increase Protein Delivery to Critically Ill Patients: A Randomized, Blinded, Parallel-Group, Feasibility Trial. JPEN J Parenter Enteral Nutr. 2021;45:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 116. | Deane AM, Summers MJ, Zaknic AV, Chapman MJ, Fraser RJ, Di Bartolomeo AE, Wishart JM, Horowitz M. Exogenous glucagon-like peptide-1 attenuates the glycaemic response to postpyloric nutrient infusion in critically ill patients with type-2 diabetes. Crit Care. 2011;15:R35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Galiatsatos P, Gibson BR, Rabiee A, Carlson O, Egan JM, Shannon RP, Andersen DK, Elahi D. The glucoregulatory benefits of glucagon-like peptide-1 (7-36) amide infusion during intensive insulin therapy in critically ill surgical patients: a pilot study. Crit Care Med. 2014;42:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Greco EV, Russo G, Giandalia A, Viazzi F, Pontremoli R, De Cosmo S. GLP-1 Receptor Agonists and Kidney Protection. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |