Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.234
Peer-review started: November 21, 2022
First decision: January 5, 2023
Revised: January 12, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 15, 2023
Processing time: 114 Days and 12.6 Hours
Peripheral arterial disease (PAD) has become one of the leading causes of disa-bility and death in diabetic patients. Restoring blood supply to the hindlimbs, especially by promoting arteriogenesis, is currently the most effective strategy, in which endothelial cells play an important role. Tongxinluo (TXL) has been widely used for the treatment of cardio-cerebrovascular diseases and extended for diabetes-related vascular disease.
To investigate the effect of TXL on diabetic PAD and its underlying mechanisms.
An animal model of diabetic PAD was established by ligating the femoral artery of db/db mice. Laser Doppler imaging and micro-computed tomography (micro-CT) were performed to assess the recovery of blood flow and arteriogenesis. Endothelial cell function related to arteriogenesis and cellular pyroptosis was assessed using histopathology, Western blot analysis, enzyme-linked immuno-sorbent assay and real-time polymerase chain reaction assays. In vitro, human vascular endothelial cells (HUVECs) and human vascular smooth muscle cells (VSMCs) were pretreated with TXL for 4 h, followed by incubation in high glucose and hypoxia conditions to induce cell injury. Then, indicators of HUVEC pyroptosis and function, HUVEC-VSMC interactions and the migration of VSMCs were measured.
Laser Doppler imaging and micro-CT showed that TXL restored blood flow to the hindlimbs and enhanced arteriogenesis. TXL also inhibited endothelial cell pyroptosis via the reactive oxygen species/nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3/Caspase-1/GSDMD signaling pathway. In addition, TXL restored endothelial cell functions, including maintaining the balance of vasodilation, acting as a barrier to reduce inflammation, and enhancing endothelial-smooth muscle cell interactions through the Jagged-1/Notch-1/ephrin-B2 signaling pathway. Similar results were observed in vitro.
TXL has a pro-arteriogenic effect in the treatment of diabetic PAD, and the mechanism may be related to the inhibition of endothelial cell pyroptosis, restoration of endothelial cell function and promotion of endothelial cell-smooth muscle cell interactions.
Core Tip: Peripheral arterial disease (PAD) has become one of the leading causes of disability and death in diabetic patients. Restoring blood supply to the hindlimbs, especially by promoting arteriogenesis, is currently the most effective strategy, in which endothelial cells play an important role. The present study demonstrated the role of Tongxinluo in promoting arteriogenesis in the treatment of diabetic PAD, and the mechanism may be related to the inhibition of endothelial cell pyroptosis, restoration of endothelial cell function, and promotion of endothelial-smooth muscle cell interaction.
- Citation: Gu JJ, Hou YL, Yan YH, Li J, Wei YR, Ma K, Wang XQ, Zhang JH, Wang DD, Li CR, Li DQ, Sun LL, Gao HL. Tongxinluo promotes endothelium-dependent arteriogenesis to attenuate diabetic peripheral arterial disease. World J Diabetes 2023; 14(3): 234-254
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/234.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.234
Diabetes is a chronic systemic metabolic disease that is genetically determined and associated with environmental factors. The development of diabetes is usually accompanied by various acute and chronic complications, among which peripheral arterial disease (PAD) is one of the most common vascular complications and one of the leading causes of foot ulcers, hindlimb amputation and death[1]. The main pathological feature of diabetic PAD is progressive occlusion of arteries in the hindlimbs. It is characterized by intermittent claudication, dorsalis pedis artery pulse loss, decreased foot skin temperature, skin cyanosis and other clinical signs. Currently, the basic approach to control the progression of diabetic PAD is to restore blood supply to the hindlimbs. Anticoagulant drugs and angioplasty surgery can restore vascular reperfusion to some extent[2], but the adverse side effects associated with these drugs and the high recurrence rate following surgery limit their applications[3].
There is increasing evidence that promoting ischemic hindlimb revascularization, including angiogenesis and arteriogenesis, is an effective way to improve blood supply[4]. Angiogenesis is the expansion of the microvascular system, characterized by capillary sprouting, endothelial cell proliferation and migration, and vessel wall remodeling. Arteriogenesis, also commonly referred to as collateral angiogenesis or therapeutic angiogenesis, involves the enlargement, opening, and remodeling of existing closed collateral arteries. As a method of capillary formation, angiogenesis is not sufficient to replace narrowed or occluded arteries[5], whereas arteriogenesis is essential to enhance perfusion and is critical to rescue ischemic tissue. Arteriogenesis is in turn dependent on multiple functions of endothelial cells[6,7]. Briefly, endothelial cells not only regulate vasodilation and promote vascular remodeling but also act as a vascular barrier to regulate the inflammatory response, both of which play an important role in arteriogenesis. In addition, communication between endothelial cells and smooth muscle cells is necessary in arteriogenesis as a lateral signal across the vessel wall. However, excessive reactive oxygen species (ROS), persistent inflammation, and other pathological factors caused by diabetes contribute to endothelial cell pyroptosis and dysfunction, further accelerating the progression of PAD[8,9]. Therefore, finding safer and more effective therapeutic agents that can enhance endothelium-dependent arteriogenesis has become a high priority to reduce the incidence of PAD and facilitate hindlimb blood flow recovery.
Tongxinluo (TXL) is a Chinese herbal formula consisting of 12 herbs, including five animal agents represented by leech, scorpion, centipede, periostracum cicadae and ground beetle and seven plant agents represented by radix ginseng, radix paeoniaerubra, lignum albergiae odoriferae, dalbergia wood, frankincense, ice chips and spine date seed. The above 12 herbs are concentrated, extracted, lyophilized and standardized into TXL capsules, which are widely used in clinical practice for the treatment of cardio-cerebral vascular diseases[10,11]. Studies have proven that TXL inhibits thrombosis, protects vascular endothelial cells, promotes vasodilation, enhances angiogenesis and inhibits inflammation[12,13,14]. Moreover, studies have found a role for TXL in the intervention of diabetes-related vascular diseases. For example, a previous study showed that TXL protected the diabetic heart from ischemia/reperfusion injury by maintaining endothelial cell barrier integrity[15]. Another study indicated that TXL prevented the transfer of transforming growth factor 1 (TGF-1) from glomerular endothelial cells to glomerular thylakoid cells, thereby inhibiting the progression of diabetic nephropathy[16]. However, scientific evidence related to its efficacy in diabetic-induced hindlimb vasculopathy has not been obtained to date. Therefore, our study aimed to determine the role of TXL in the treatment of diabetic PAD and to explore the mechanisms associated with endothelial-dependent arteriogenesis.
A total of 75 male diabetic db/db (C57BLKS/J-Leprdb/Leprdb) and 25 male nondiabetic db/m (C57BLKS/J-Leprdb/m) mice (aged 4-6 wk, average weight of 28 ± 3 g, specific pathogen-free grade) were purchased from the Experimental Animal Center of Changzhou Kavans Co., Ltd. (Changzhou, China).
All mice were adaptively fed for one week, and db/db mice were randomly assigned to three groups (n = 25 per group): The model group, low-dose TXL (TXL-L) group and high-dose TXL (TXL-H) group. The right femoral artery of db/db mice was ligated as previously described[17]. Specifically, db/db mice were anesthetized by an intraperitoneal injection of 2% pentobarbital sodium and immobilized in a supine position. After making a 5-mm skin incision in the right hindlimb adductor, the femoral artery and vein were fixed and bluntly separated with pointed tweezers. The proximal femoral artery (the proximal end of the superficial caudal epigastric artery and the proximal caudal femoral artery) and the bifurcation of the femoral popliteal artery were ligated with 6-0 sutures. Penicillin was sprayed on the wound to prevent infection, and the wound was closed with 4-0 sutures. Three days after surgery, animals in the model group were gavaged with 5% (w/v) sodium carboxymethylcellulose. TXL ultrafine powder (Yiling Pharmaceutical Co., Inc., Shijiazhuang, China) dissolved in 0.5% sodium carboxymethylcellulose was gavaged at 0.38 and 1.5 g/kg/day to the mice in the TXL-L and TXL-H groups, respectively, according to clinical practical and dose conversions, as well as an earlier study[18]. Additionally, db/m mice without hindlimb ischemia (HLI) were gavaged with 5% (w/v) sodium carboxymethylcellulose and used as normal controls. After 4 wk of administration, all mice were euthanized, and plasma, aortic and ischemic adductor samples were obtained for subsequent measurements.
Limb appearance was independently scored on a scale from 0 to 4 (4=digit/foot autotomy, 3=severe discoloration/gangrene, 2=moderate discoloration, 1=mild discoloration, and 0=normal appearance).
The ratio of blood flow in the ischemic (right) limb to the contralateral nonischemic limb was measured with a Laser Doppler perfusion imaging system (Moor Instruments Limited, Millwey, Axminster, Devon, EX13 5HU, United Kingdom). Images were obtained before and after arterial ligation and on postoperative days 7, 14, 21, and 28.
To visualize and quantify the limb vasculature, hindlimb vascular casting was performed by perfusing Microfil (Flow-Tech Inc, Massachusetts, United States) as described previously[19]. Specifically, the animals were anesthetized as in HLI surgery, and the left third and the fourth ribs were opened. Heparinized saline (5 mL) was then perfused into the descending thoracic aorta until the fluid in the inferior vena cava became clear. Subsequently, diluted Microfil (5 mL) was injected at a rate of 2 mL/min. After perfusion, hindlimb samples were embedded in 10% neutral formalin and refrigerated overnight at 4 °C. Samples were then scanned with a Micro-CAT II (Siemens) at a voltage of 50 kV and a current of 500 µA using a high-resolution scanning mode and 72-µm voxel sizes for imaging. Then, three-dimensional (3D) reconstructions of the hindlimbs were obtained. After thresholding the vessels, the surface area and volume of the vessels were quantified using Analyze (Caliper Analyze).
The ischemic adductors were collected, soaked in 10% neutral buffered formalin for 24 h, and then cut into 4-μm-thick sections for immunostaining. The sections were incubated with primary antibodies against intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Horseradish peroxidase-labeled sheep anti-rabbit IgG (Proteintech, G1213, Wuhan, China) was used as a secondary antibody and incubated with the samples. All images were taken by a fully automatic biological microscope (Leica DM6000B, Wetzlar, Germany) at 400× magnification. The percentage of the positive areas was recorded, and quantitative analysis was performed using ImageJ software.
Ischemic samples were processed in the same way as samples for the immunostaining assay. Capillaries and arterioles were stained with anti-CD31 and anti-α-smooth muscle actin (α-SMA) antibodies, respectively. Macrophages were stained with anti-F4/80 antibodies. Antibodies against inducible nitric oxide synthase (iNOS) and Arginase-1 (Arg-1) were used as specific markers of M1- and M2-type macrophages, respectively. Leukocytes were stained with anti-CD45 antibodies. After the samples were incubated with the primary antibodies overnight at 4 °C, Alexa Flour® 488 goat anti-rabbit IgG and/or Cy3-conjugated goat anti-mouse IgG were added, and the samples were incubated for 60 min. Finally, the sections were sealed with mounting medium containing DAPI (Solarbio, Beijing, China). Images were captured using a Zeiss confocal microscope (Oberkochen, Germany), and the mean fluorescence intensities were analyzed by ImageJ software.
Ischemic muscle sections were incubated for 30 min at room temperature with ROS staining solution (SIGMA, D7008, St. Louis, United States), followed by DAPI restaining of nuclei and sealing with anti-fluorescence quenching sealer. Sections were observed under a fluorescent microscope and images were acquired at 400× magnification.
Collected aortic tissues were processed as described for the immunostaining assay. All samples were stained with a H&E staining kit (Biyuntian, Shanghai, China). All the above sections were observed by a fully automatic biological microscope (Leica DM6000B, Wetzlar, Germany) at 400× magnification.
To detect changes in inflammatory factor levels, blood was harvested after the mice were euthanized and then centrifuged at 3000 r/min for 20 min at 4 °C. The upper serum layer was collected and stored at -80 °C. The levels of interleukin (IL)-1β, IL-18, IL-6 and C-reactive protein (CRP) were measured by ELISA kits (Biyuntian, Shanghai, China) in accordance with the manufacturer’s instructions.
Human vascular endothelial cells (HUVECs) and human vascular smooth muscle cells (VSMCs) were purchased from Yaji Biotechnology Co., Ltd. (Shanghai, China). As per the experiment, both HUVECs and VSMCs were divided into four groups: the normal group, which included cells cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, United States) supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin and streptomycin (Gibco, Gaithersburg, MD, United States), in 5% CO2 at 37 °C; the model group, which included cells incubated with high glucose (HG, DMEM containing 10% FBS, 100 U/mL penicillin, and streptomycin plus 30 mmol/L glucose) medium in hypoxia (1% O2, 5% CO2 and 94% N2) at 37°C for 6 h; the TXL-L group, which included cells pretreated with TXL (200 μg/mL) for 4 h and cultured under the same conditions as the model group for 6 h; and the TXL-H group, which included cells pretreated with TXL (600 μg/mL) for 4 h and cultured under the same conditions as the model group for 6 h (TXL ultrafine powder dissolved in DMEM was sonicated for 1 h and centrifuged at 8000 rpm for 30 minutes. Then the supernatant was filtered (pore diameter 0.22 μm), the weight of the dissolved powder was measured, and the appropriate concentration was selected for in vitro experiments according to previous studies[20]). The prepared cells were then used for further experimentation or coculture.
In the Transwell (Corning, 3413, New York, United States) coculture system, HUVECs and VSMCs were placed in the upper and lower chambers, respectively. Two chambers of culture medium were transported through the insert membrane pores. The medium in the upper and lower chambers of the Transwell culture system was changed daily for at least 3 days.
The morphology of HUVECs was observed using a fully automatic biological microscope at 100× magnification.
HUVEC viability was assessed by performing the cell counting Kit-8 (CCK-8) assay (Biyuntian, Shanghai, China) according to the manufacturer’s protocol.
To examine the role of TXL in HUVEC tube formation, a tube formation assay was performed. First, 60 µl of Matrigel adhesive matrix (Coring, New York, United States) was added to each well of a 96-well plate and solidified for 1 h at 37 °C. HUVECs (5 × 104 cells/mL) were added to each well, and tube formation was observed using a fully automatic biological microscope at 400× magnification.
In our study, pyroptosis was measured by flow cytometry. Briefly, HUVECs were harvested, washed with PBS twice and stained using the caspase-1/propidium iodide (PI) Detection Kit (Life-iLab, AC12L062, Shanghai, China) according to the manufacturer’s instructions. After incubation at room temperature for 15 min in the dark, the stained cells were analyzed on a FACSVerse flow cytometer (BD Biosciences, San Jose, CA, United States). Data acquisition and analysis were performed using FlowJo software (BD Biosciences, San Jose, CA, United States).
Cell migration was assessed by performing a wound healing assay. Briefly, VSMCs were seeded into a 6-well plate and then scratched with a 200 μl pipette tip. Next, VSMCs were observed at 0 and 48 h following in vitro injury. A fully automatic biological microscope was used to obtain images of the wound at different time points. The percentage of wound healing area was calculated.
To further evaluate cell migration, VSMCs were resuspended (1 × 106 cells/mL) in serum-free DMEM. Transwell chambers (Corning, 3599, New York, United States) were placed into a 24-well plate and the cell suspension (20 µl/well) was added to the upper chamber. Subsequently, DMEM supplemented with 10% FBS was added to the lower chamber. After 12 h of incubation, the cell chambers were removed, and the unmigrated cells left on the surface were removed with cotton swabs. Cells that migrated to the bottom surface were fixed with 4% paraformaldehyde for 20 min, followed by staining with 0.1% crystal violet for 15 min. Migrated cells were observed with a fully automatic biological microscope.
Ischemic adductor samples (100 mg) were lysed with RIPA lysis buffer (SEVEN, Beijing, China) supplemented with protease inhibitor (MCE, Shanghai, China) and then centrifuged at 12000 rpm for 10 min at 4 °C to obtain the supernatant. The protein concentrations in the adductor supernatant and HUVECs were determined using a BCA protein assay kit (Biyuntian, Shanghai, China), and appropriate amounts of 5× loading buffer were added to an aliquot of each protein. The proteins were denatured in a metal bath at 100 °C for 5-8 min. For each sample, 20 μg of protein was separated by 4%-20% SurePAGE (Genscript, M00657, New Jersey, United States) and then transferred to PVDF membranes (Millipore, IPVH00010, Billerica, United States). The membranes were blocked with 5% blocking buffer (SEVEN, Beijing, China) and then incubated with primary antibodies against endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF), Caspase-1, Cleaved-caspase-1, nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3), GSDMD, tumor necrosis factor-α (TNF-α), VE-cadherin, β-catenin, Jagged-1, Notch-1, ephrin-B2, matrix metalloproteinase 9 (MMP9), and MMP2 (Table 1) at 4 °C overnight. Goat anti-rabbit IgG was added and incubated for 1 h at 37 °C. Finally, the membranes were scanned with an Odyssey imager (LI-COR, Lincoln United States). GAPDH and α-tubulin were used as internal references. The gray values of the protein bands were analyzed by ImageJ software.
| Antibodies | Company | Dilution |
| CD31 | abcam, ab281583 | 1:300 dilution |
| α-SMA | abcam, ab124964 | 1:300 dilution |
| Caspase-1 | Servicebio, GB11383 | 1:500 dilution |
| Cleaved-caspase-1 | Immunoway, YC002 | 1:1000 dilution |
| NLRP3 | Immunoway, YT5382 | 1:1000 dilution |
| GSDMD | Proteintech, 66387-1-l g | 1:5000 dilution |
| ICAM-1 | abcam, ab222736 | 1:1000 dilution |
| VCAM-1 | abcam, ab134047 | 1:1000 dilution |
| eNOS | abcam, ab5589 | 1:1000 dilution |
| VEGF | abcam, ab52917 | 1:5000 dilution |
| CD45 | Proteintech, 60287-1-l g | 1:200 dilution |
| TNF-α | abcam, ab183218 | 1:1000 dilution |
| VE-cadherin | Proteintech, 27956-1-AP | 1:1000 dilution |
| β-catenin | Proteintech, 51067-2-AP | 1:5000 dilution |
| F4/80 | Servicebio, GB113373 | 1:500 dilution |
| iNOS | abcam, ab15323 | 1:250 dilution |
| Arg-1 | Proteintech, 66129-1-l g | 1:1000 dilution |
| Jagged-1 | Immunoway, YT5401 | 1:1000 dilution |
| Notch-1 | Servicebio, GB111690 | 1:500 dilution |
| ephrin-B2 | Immunoway, YT1586 | 1:1000 dilution |
| MMP9 | Servicebio, GB11132 | 1:1000 dilution |
| MMP2 | Servicebio, GB11130 | 1:1000 dilution |
| GAPDH | Servicebio, G3206-1OD | 1:2000 dilution |
| α-tubulin | Servicebio, GB15139 | 1:500 dilution |
| Goat anti-rabbit IgG | abcam, ab216777 | 1:5000 dilution |
| Goat anti-mouse IgG H&L | Abcam, ab216772 | 1:5000 dilution |
| Alexa Flour® 488 goat anti-rabbit IgG | Servicebio, GB25303 | 1:250 dilution |
| Cy3-conjugated goat | Servicebio, GB25303 | 1:250 dilution |
| anti-mouse IgG |
Total RNA was extracted from ischemic adductor muscles and isolated from the cells using TRIzol reagent (Ambion, United States). RNA (1 μg) was reverse-transcribed by the PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara Clontech, Kyoto, Japan), and qPCR was subsequently performed using TB Green™ Premix Ex Taq™ (Takara Clontech, Kyoto, Japan) according to the manufacturer’s protocol. The fold change in the target gene was calculated using the 2 −ΔΔCt method, and GAPDH mRNA was used as an internal control. The sequences of the primers used are listed in Table 2.
| Gene | Species | Forward sequence (5’-3’) | Reverse sequence (5’-3’) |
| PCNA | Mouse | GTCGGGTGAATTTGCACGTA | CTCTATGGTTACCGCCTCCTC |
| GAPDH | Mouse | CCTCGTCCCGTAGACAAAATG | TGAGGTCAATGAAGGGGTCGT |
| Jagged-1 | Human | TGTGGCTTGGATCTGTTGCTTGG | ACGTTGTTGGTGGTGTTGTCCTC |
| Notch-1 | Human | GTGCCCTGGGCTTCTCTG | GGCACGATTTCCCTGACCA |
| ephrin-B2 | Human | CGTCCAGAACTAGAAGCTGGT | CCGTCTGTGCTAGAACCTGG |
| VEGF | Human | ACAAATGTGAATGCAGACCAAA | ACCAACGTACACGCTCCAG |
| eNOS | Human | GCCGGAACAGCACAAGAGTTA | CCCTGCACTGTCTGTGTTACT |
| VE-Cadherin | Human | CAGCCCAAAGTGTGTGAGAA | CGGTCAAACTGCCCATACTT |
| β-catenin | Human | AGCTTCCAGACACGCTATCAT | CGGTACAACGAGCTGTTTCTAC |
| GAPDH | Human | GGAAGCTTGTCATCAATGGAAATC | TGATGACCCTTTTGGCTCCC |
Nitric oxide (NO) levels in serum and cell supernatants were measured with an NO assay kit (Nanjing Jiancheng, Nanjing, China). The procedure was carried out according to the instructions.
Experimental data are expressed as the mean ± standard deviation (SD) and were analyzed by SPSS 23.0 software. Multiple groups were compared using one-way ANOVA, followed by Tukey’s test. For perfusion recovery, two-way ANOVA followed by Sidak's multiple comparison post-test was performed. P < 0.05 was defined as statistically significant, and P < 0.01 was considered highly statistically significant. All graphs were created by GraphPad Prism (8.0) software.
The primary observation of the present study was arteriogenesis in preexisting collateral arteries that interconnected the deep femoral artery branch with the distal superficial femoral artery. Given that the blood supply of the adductor is predominantly derived from this site, we ligated the proximal end of the femoral artery and the bifurcation of the femoral popliteal artery to observe arteriogenesis in the adductor (Figure 1A and B). TXL was administered to mice three days after ligation of the femoral artery. Laser Doppler images were obtained at weekly intervals, and microsphere perfusion and other biomedical detection analyses were performed 4 wk after surgery (Figure 1C). We found that the hindlimbs of mice in all experimental groups showed different degrees of necrosis after femoral artery ligation (Figure 1D), while the limb appearance scores of mice in the group treated with TXL were lower than those of mice in the model group, especially at Day 28 after HLI (Figure 1E). Laser Doppler images showed a significant decrease in hindlimb perfusion in all experimental groups immediately after surgery, followed by an increase in a time-dependent manner (Figure 1F). Figure 1G shows that by Day 7 after HLI, the mean ratio of ischemic to nonischemic perfusion in the TXL-H group was significantly higher than in the model group. From postoperative Days 14 to 28, TXL increased perfusion recovery in a dose-dependent manner, suggesting that TXL may restore blood flow in the ischemic hindlimb to some extent.
Vascular casting was performed by perfusing Microfil to visualize the hindlimb vasculature. Micro computed tomography (micro-CT) analysis and 3D reconstruction showed that compared to the model group, mice in the TXL-treated group had more corkscrew-like collateral vessels, and even more corkscrew-like collateral vessels in the TXL-H group (Figure 2A; white arrows). Vascular volume and surface area were quantified by micro-CT analysis, which revealed no significant difference between the normal, model and TXL-L groups. However, there was a significant increase in the TXL-H group compared to the model group (Figure 2B and C). These results indicated that high-dose TXL may enhance perfusion by promoting the generation of small arteries during HLI. To further evaluate arteriogenesis, CD31 and α-SMA were labeled to observe capillaries and myogenic vessels, respectively (Figure 2D-F). There was no significant difference in α-SMA expression between the normal and model groups, and CD31 expression was significantly higher in the model group than in the normal group. In addition, CD31 and α-SMA expression was significantly higher in the TXL-H group than in the model group. These results indicated that high-dose TXL promoted capillary formation as well as arteriogenesis.
An increasing number of studies have confirmed the involvement of endothelial cell pyroptosis in the pathogenesis of diabetic vascular complications[21], and pyroptosis may be induced by excessive reactive oxygen species (ROS). To verify whether TXL inhibits endothelial cell pyroptosis through the classical ROS/NLRP3/Caspase-1/GSDMD signaling pathway, we assessed the levels of ROS, the above proteins, and downstream inflammatory factors. The results suggested that the expression levels of ROS, NLRP3, GSDMD, Caspase-1, Cleaved caspase-1, IL-18 and IL-1β were significantly increased in the model group compared with the normal group, while TXL reversed this trend (Figure 3A-I), among which, TXL reduced the expression of ROS, GSDMD, Cleaved caspase-1, IL-1β and IL-18 in a dose-dependent manner. Overall, these results indicated that TXL may inhibit endothelial cell pyroptosis via the ROS/NLRP3/Caspase-1/GSDMD signaling pathway, which in turn inhibits the persistence of inflammation.
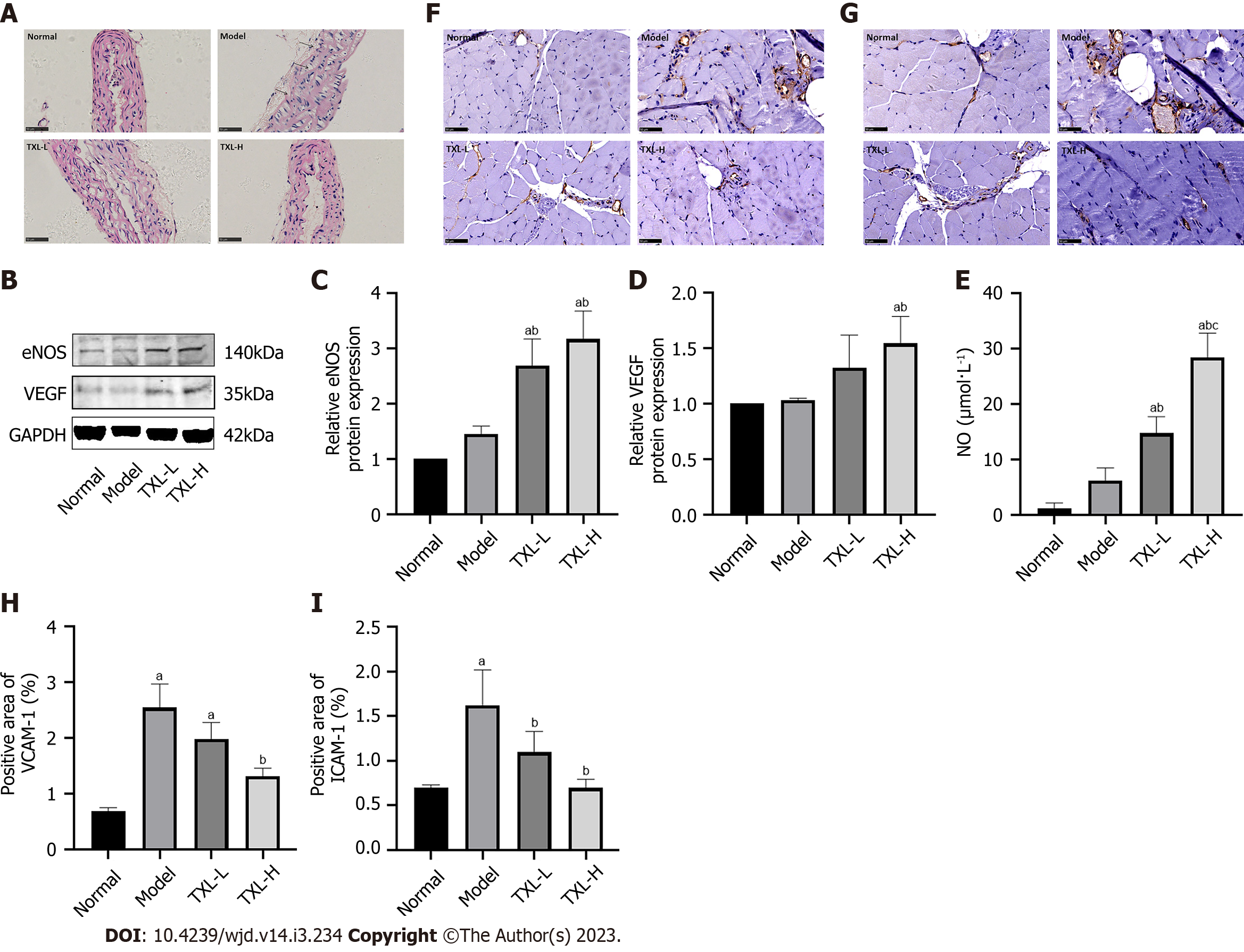
To further demonstrate that the mechanism by which TXL promotes arteriogenesis is associated with endothelial cells, we examined the effect of TXL on the morphology of endothelial cells via H&E staining. As shown in Figure 4A, mice in the normal group had an intact and smooth aortic intima with an orderly arrangement of smooth muscle cells. Notably, the aortas of mice in the model group had thickened lumens, disrupted intimal structures and disturbed smooth muscle arrangement (black arrows). However, these characteristic morphological changes were abated in the TXL group. To assess the effect of TXL on endothelial cell function, the levels of NO, eNOS, VEGF, ICAM-1 and VCAM-1 were evaluated. eNOS and its product NO are involved in endothelial cell functions, including the maintenance of vascular permeability and vasodilator tone. VEGF, on the other hand, is highly correlated with vascular structural remodeling. As shown in Figure 4B-E, there was no significant difference in eNOS, VEGF and NO levels between the normal and model groups. However, a marked increase in eNOS, VEGF and NO levels was observed in the TXL-H group compared with the model groups. In addition, the adhesion molecules VCAM-1 and ICAM-1 can recruit leukocytes and regulate leukocyte adhesion on vascular endothelial cells, which are important indicators of endothelial cell damage. Immunohistochemical assays showed that the levels of ICAM-1 and VCAM-1 were significantly increased in the model group compared with the normal group and decreased after TXL treatment (Figure 4F-I). Thus, these data suggested that TXL restored endothelial cell function to promote arteriogenesis in the context of acute HLI.
It is well known that the inflammatory response plays a crucial role in the progression of diabetic vascular injury[22] and that endothelial cells act as a vascular barrier to inhibit the infiltration of inflammatory cells. To assess the effect of TXL on the barrier function of endothelial cells, we measured barrier-related protein levels in ischemic tissues and inflammatory cytokine levels in the blood. As depicted in Figures 5A-G, the Western blot and ELISA results showed that compared to the model group, the TXL groups had increased expression of the barrier-related proteins VE-cadherin and β-catenin and altered expression of the leukocyte marker CD45 and proinflammatory cytokines TNF-α, IL-6 and CRP. More specifically, TXL decreased CRP levels and increased VE-cadherin levels in a dose-dependent manner (Figure 5B-G). The above results confirmed that there are pathological changes in the vascular barrier in diabetic PAD mice and support the role of TXL in repairing the endothelial cell barrier and reducing inflammation.
The macrophage phenotype may reflect the inflammatory response and revascularization in ischemic muscles. There are two types of macrophages: M1 and M2. The main function of M1-type macrophages is to participate in the inflammatory response, while the main function of M2-type macrophages is to proliferate blood vessels and inhibit the inflammatory response. To observe the polarization of macrophages, we examined the colocalization of F4/80 with the M1-type macrophage marker iNOS (proinflammatory) and the M2-type macrophage marker Arg-1 (anti-inflammatory) in ischemic muscles. Compared with the model group, TXL effectively decreased the Pearson’s coefficient of iNOS co-localization with F4/80 and increased the Pearson coefficient of Arg-1 co-localization with F4/80 (Figure 5H-K).
To further demonstrate that TXL promotes arteriogenesis in an endothelium-dependent manner, we evaluated the crosstalk between endothelial and smooth muscle cells. Studies have shown that signal transduction between Notch (receptor) in smooth muscle cells and Jagged (ligand) in endothelial cells may be a mechanism for cell differentiation and vascular maturation[23,24]. In addition, some studies have indicated that ephrin-B2, a downstream signaling molecule of Notch, contributes to endothelial-smooth muscle cell communication during vessel formation[25]. In our study, Western blot results showed that there was no significant difference in Jagged-1, Notch-1 and ephrin-B2 Levels between the normal and model groups, but all of the above proteins were notably upregulated in the TXL-H group compared to the model group (Figure 6A-D). These results indicated that TXL enhanced the interaction between endothelial and smooth muscle cells, probably through the Jagged-1/Notch-1/ephrin-B2 signaling pathways.
The effect of TXL on the proliferation and migration of smooth muscle cells was assessed by RT–PCR and Western blot assays. Our findings indicated that TXL-H treatment upregulated the mRNA expression of PCNA (Figure 6E), which is a marker of cell proliferation. Similar to PCNA, Western blot analysis showed that the expression of MMP2 and MMP9, which are important indicators of smooth muscle cell migration, was significantly higher in the TXL-H group than in the model group (Figure 6F-H). Taken together, these results indicated that TXL enhanced the interactions between endothelial cells and smooth muscle cells through the Jagged-1/Notch-1/ephrin-B2 signaling pathway and promoted smooth muscle cell proliferation and migration, thereby promoting arteriogenesis.
We next validated the role of TXL in endothelium-dependent arteriogenesis at the cellular level. HUVEC morphology was observed first, and results showed that compared with the normal group, the cell growth of HUVECs in the model group was stagnant, with a decrease in cell number and volume. However, TXL, especially high-dose TXL, significantly increased the number and volume of cells (Figure 7A). The CCK-8 assay suggested that HG/hypoxia induced a decrease in the viability of HUVECs compared to the normal group, and TXL treatment effectively increased the viability of the cells in a dose-dependent manner (Figure 7B).
Subsequently, NO levels were measured by an NO kit and the levels of VEGF, eNOS, VE-cadherin and β-catenin secreted by endothelial cells were detected by polymerase chain reaction (RT-PCR). As shown in Figures 7C-G, compared with the normal group, the levels of VEGF, eNOS, and β-catenin were increased in the model group while NO levels were decreased. High-dose TXL significantly increased the levels of NO and the above proteins, while low-dose TXL only increased the levels of NO and VE-cadherin. In addition, the HUVEC tube formation assay showed that under normal conditions, HUVECs can form a clear honeycomb-like independent tubular structure. However, after HG/hypoxia stimulation, the tube-forming function of HUVECs was severely impaired, and the ability to form independent tube-like structures was almost eliminated. TXL significantly improved the tube-forming ability of HUVECs (Figure 7H), suggesting that TXL promoted capillary formation, which is consistent with the results of animal experiments in which TXL increased the expression of CD31. The above results suggest that TXL restores endothelial cell functions, including secretion, barrier formation, and capillary formation.
To further confirm that TXL suppressed endothelial cell pyroptosis, we counted caspase-1/PI double-positive cells using flow cytometry (Figure 7I-J). The results showed a marked increase in the Caspase-1+/PI+ HUVEC number in the model group compared to that in the normal group. The Caspase-1+/PI+ HUVEC number in the TXL group was significantly reduced compared with that in the model group, and the reduction in the TXL-H group was more pronounced than that in the TXL-L group.
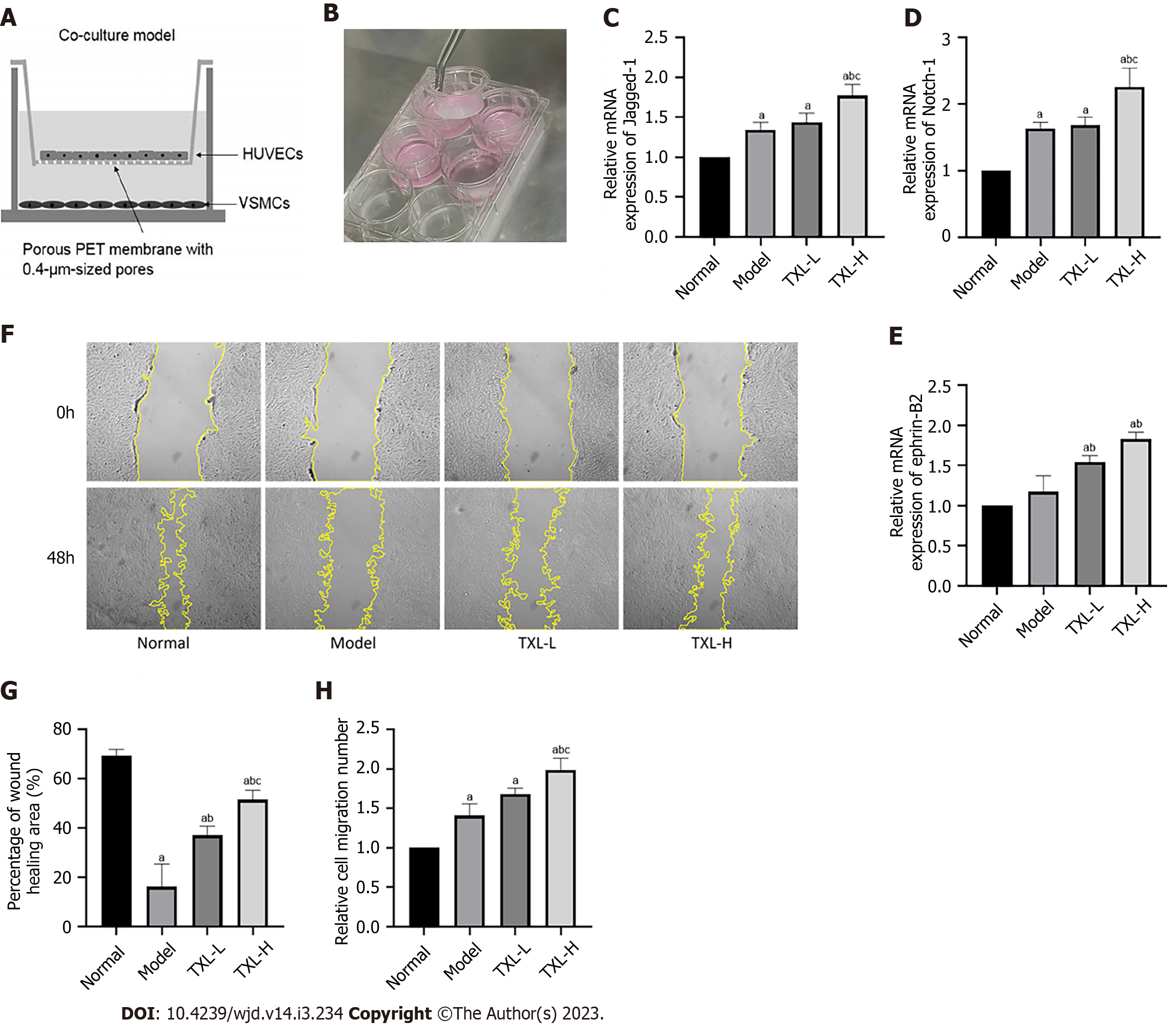
We established a coculture system of HUVECs and VSMCs (Figure 8A-B)[26] and detected the levels of Jagged-1 in HUVECs and Notch-1 and ephrin-B2 in VSMCs by RT-PCR (Figure 8C-E). The results showed that high-dose TXL increased the expression levels of Jagged-1 and Notch-1, and both high and low doses of TXL increased the level of ephrin-B2, thus increasing two-cell communication. Finally, to analyze the role of TXL in the migration of VSMCs, we performed transwell and scratch wound healing assays. We found that the wound healing ability of VSMCs in the model group was significantly decreased compared with that of the control cells. TXL increased the wound healing ability of VSMCs (Figure 8F-G). Similarly, using a Transwell assay, we found that the migration number of VSMCs in the model group was higher than that of cells in the control group. The migratory number of VSMCs was significantly increased in the TXL-H group compared to the model group, which is an essential prerequisite for arteriogenesis (Figure 8H). From the above results, we conclude that TXL promotes the interaction between endothelial cells and smooth muscle cells, which in turn promotes smooth muscle cell migration and further induces arteriogenesis.
Diabetic PAD is characterized by poor blood circulation and abnormal sensation in the hindlimb induced by metabolic abnormalities. Currently, the pathogenesis of diabetic PAD is not fully understood, and there is a great clinical need for new therapeutic options to stimulate collateral revascularization and increase blood flow. Therapeutic arteriogenesis is an attractive strategy to improve the management of these disabling and often lethal diseases. Among all the complex pathophysiological mechanisms of arteriogenesis, the role of endothelial cells has been emphasized[6,7]. In the present study, we first investigated the effect of TXL on arteriogenesis in the treatment of diabetic PAD and explored the mechanism associated with endothelial cells. We established a diabetic mouse model of HLI by ligating the femoral artery of db/db mice and administered TXL by gavage. In terms of observing arteriogenesis, we used laser Doppler and micro-CT to visualize that TXL increased blood flow and collateral vascularity in the hindlimb of mice. We then performed immunofluorescence staining of capillaries (CD31) and muscle arteries (α-SMA) in ischemic muscle, which made it clearer that TXL promotes both capillary formation and arteriogenesis. After clarifying that TXL treatment of diabetic PAD has a pro-arteriogenesis effect, we further investigated the potential mechanisms of arteriogenesis associated with endothelial cells. Furthermore, we found that the inhibition of endothelial cell pyroptosis and restoration of endothelial cell function played an essential role in the TXL-regulated stimulation of arteriogenesis. Finally, we further validated the above experimental results by in vitro experiments.
As mentioned in previous studies, pyroptosis of vascular endothelial cells is a key factor in the pathogenesis of diabetes-related vascular complications[27,28]. It has been reported that hyperglycemia-induced ROS-dependent activation of the NLRP3 inflammasome is an important initiator of endothelial cell injury[29]. Briefly, sustained hyperglycemia induces excess ROS, which are natural activators of inflammasomes. After activation of the NLRP3 inflammasome, GSDMD is cleaved to the N-terminal GSDMD forms by active Caspase-1, leading to oligomerization membrane pore formation[30]. All of the above processes further lead to cell swelling and rupture, triggering the release of cellular contents and proinflammatory factors, such as IL-18 and IL-1β, into the intercellular space[31]. In the present study, hindlimb ischemia and diabetes together led to massive production of ROS in mice, which in turn induced endothelial cell pyroptosis, as manifested by a significant increase in the expression of Caspase-1, Cleaved Caspase-1, NLRP3, and GSDMD proteins and a massive release of the inflammatory factors IL-18 and IL-1β. Subsequently, inflammatory mediators spill over into the systemic circulation, triggering a sustained exacerbation of the systemic inflammatory response. These results are identical to those of Yu et al[32], who reported that the pyroptosis of endothelial cells may play a key role in the mechanism of progression of diabetic PAD. Our results indicated that TXL may inhibit endothelial cell pyroptosis via the ROS/NLRP3/Caspase-1/GSDMD signaling pathway and suppress the persistence of vascular inflammation. We further validated the role of TXL in endothelial cell pyroptosis at the cellular level. In in vivo experiments, we exposed HUVECs to HG/hypoxia to induce cell damage. Flow cytometry analysis showed that TXL significantly reduced the percentage of pyroptotic cells, which was consistent with the results of animal experiments. Furthermore, the repression of endothelial cell pyroptosis by TXL is a prerequisite for restoring endothelial cell function.
With regard to endothelial cell function, our in vivo experiments revealed that TXL upregulated the expression of NO, eNOS and VEGF, allowing endothelial cells to maintain the appropriate level of vascular permeability and tonicity, which is crucial in the process of vascular remodeling. Moreover, TXL reduced the expression of the adhesion molecules ICAM-1 and VCAM-1 to inhibit leukocyte-endothelial adhesion and endothelial inflammation. In terms of inflammation, endothelial cells act as a barrier and are impermeable to cell migration under physiological conditions. However, diabetes leads to adhesion of leukocytes to endothelial cells and transendothelial migration, resulting in increased local inflammation and vascular fragility[33]. In the present study, we found that TXL increased the expression of VE-cadherin and β-catenin, which are important intercellular proteins involved in maintaining endothelial cell barrier integrity. Moreover, our results showed that TXL dramatically downregulated the expression of CD45, CRP, TNF-α and IL-6, which is consistent with a previous study[34]. The above results demonstrated that TXL protected the integrity of the endothelial barrier and inhibited inflammation. Then, we further validated the above results at the cellular level. The results showed that TXL maintained HUVEC structure and enhanced function by increasing NO, eNOS, VEGF, VE-cadherin and β-catenin protein expression. In addition, TXL significantly increased the tube-forming ability of HUVECs, suggesting that TXL plays a role in promoting capillary formation, which is consistent with the results of animal experiments
The transformation of the macrophage phenotype plays a vital role in vascular remodeling and the inflammatory state of ischemic muscle. Macrophages are categorized as M1 type (proinflammatory) and M2 type (anti-inflammatory), which have been demonstrated to be involved in the initial and vascular remodeling phases of HLI, respectively[35]. Our study showed that TXL decreased the expression of the M1-type marker iNOS while increasing the expression of the M2-type marker Arg-1, indicating that TXL promoted the polarization of macrophages toward the M2 type to decrease inflammation and enhance vascular remodeling. Previous studies have shown that the regulatory relationship between endothelial cells and macrophage polarization plays a role in revascularization and perfusion recovery in ischemic muscle[36]. We suppose that the decrease in M1-type macrophages may be related to the protection of endothelial cell barrier function and inhibition of the inflammatory response by TXL. There was no evidence that the increase in M2-type macrophages was related to endothelial cells. Based on previous studies of endothelial cell-macrophage interactions[37,38,39], we speculate that this increase may be related to increased secretion of VEGF promoting the adhesion of macrophages to endothelial cells.
Previous studies have shown that M2-type macrophages increase the secretion of metallomatrix enzymes to promote the proliferation and migration of smooth muscle cells, which is a fundamental requirement for the formation of functional arteries[40]. In this study, we measured the levels of the cell proliferation marker PCNA and the smooth muscle cell migration markers MMP2 and MMP9 to further determine the effect of TXL on arteriogenesis. Our data implied that TXL promoted smooth muscle cell proliferation and migration to generate functional arterioles, which was in accordance with previous studies showing that TXL enhances the migratory capacity of marrow mesenchymal stem cells through upregulation of metallomatrix enzymes and VEGF[41]. In addition, communication between endothelial cells and smooth muscle cells across the vessel wall is essential for the generation of collateral arteries[42]. Currently, strong evidence suggests that Notch signaling plays an important role in endothelial cell-smooth muscle cell crosstalk. In contrast, deletion of Jagged-1, a ligand for Notch, in endothelial cells leads to vascular defects associated with smooth muscle proliferation. This pathway regulates smooth muscle cell proliferation and migration mainly by regulating the downstream ephrin-B2 signaling pathway. Therefore, we examined the Jagged-1/Notch pathway and its downstream target ephrin-B2 to further identify the underlying mechanisms in this study. We found that TXL upregulated the Jagged-1/Notch-1/ephrin-B2 signaling pathway, which was consistent with previous findings that the Notch signaling pathway regulates the ability of MMP2 and MMP9 expression to induce the migration of vascular smooth muscle cells[43]. Thus, this study provides new evidence that TXL enhances endothelial cell-smooth muscle cell interactions and promotes vascular smooth muscle cell proliferation and migration. In other words, TXL promoted endothelium-dependent arteriogenesis.
In the HUVEC and VSMC co-culture system, RT-PCR results demonstrated that TXL enhanced the interaction between HUVECs and VSMCs through the Jagged-1/Notch-1/ephrin-B2 signaling pathway. Furthermore, scratch and Transwell experiments demonstrated that TXL promoted the proliferation and migration of VSMCs, which is a prerequisite for arteriogenesis. All of the above results are consistent with the results of animal experiments.
There are some limitations in this study. As there was no db/db sham group in this study, we could not determine whether diabetes or arterial ligation caused the inflammation and endothelial injury. We believe that the increased inflammatory response in the hindlimb, as well as the endothelial damage, may be induced by the combination of diabetes and arterial ligation. We will further demonstrate this separately in future studies.
In summary, in the current study, it was shown that TXL promoted arteriogenesis in diabetic mice with HLI, which may be achieved by the inhibition of endothelial cell pyroptosis, restoration of endothelial cell function and enhancement of the interaction between endothelial cells and smooth muscle cells. However, our in vitro and animal study findings will have to be validated in clinical studies. This study provides an experimental basis for the clinical application of TXL in the treatment of diabetic PAD, which provides evidence and direction for further research in the future.
Peripheral arterial disease (PAD) has become one of the leading causes of disability and death in diabetic patients. Restoring blood supply to the hindlimbs, especially by promoting arteriogenesis, is currently the most effective strategy, in which endothelial cells play an important role. Tongxinluo (TXL) has been widely used for the treatment of cardio-cerebrovascular diseases and extended for diabetes-related vascular disease.
To investigate the effect of TXL on diabetic PAD and its underlying mechanisms.
In the current study, it was shown that TXL promoted arteriogenesis in diabetic mice with HLI, which may be achieved by the inhibition of endothelial cell pyroptosis, restoration of endothelial cell function and enhancement of the interaction between endothelial cells and smooth muscle cells. This study provides an experimental basis for the clinical application of TXL in the treatment of diabetic PAD, which provides evidence and direction for further research in the future.
An animal model of diabetic PAD was established by ligating the femoral artery of db/db mice. Laser Doppler imaging and micro-computed tomography (micro-CT) were performed to assess the recovery of blood flow and arteriogenesis. Endothelial cell function related to arteriogenesis and cellular pyroptosis was assessed using histopathology, Western blot analysis, enzyme-linked immunosorbent assay and real-time polymerase chain reaction assays. In vitro, human vascular endothelial cells (HUVECs) and human vascular smooth muscle cells (VSMCs) were pretreated with TXL for 4 h, followed by incubation in high glucose and hypoxia conditions to induce cell injury. Then, indicators of HUVEC pyroptosis and function, HUVEC-VSMC interactions and the migration of VSMCs were measured.
Laser Doppler imaging and micro-CT showed that TXL restored blood flow to the hindlimbs and enhanced arteriogenesis. TXL also inhibited endothelial cell pyroptosis via the ROS/NLRP3/Caspase-1/GSDMD signaling pathway. In addition, TXL restored endothelial cell functions, including maintaining the balance of vasodilation, acting as a barrier to reduce inflammation, and enhancing endothelial-smooth muscle cell interactions through the Jagged-1/Notch-1/ephrin-B2 signaling pathway. Similar results were observed in vitro.
TXL promoted arteriogenesis in diabetic mice with HLI, which may be achieved by the inhibition of endothelial cell pyroptosis, restoration of endothelial cell function and enhancement of the interaction between endothelial cells and smooth muscle cells.
Since there was no db/db sham group in this study, we were unable to determine whether diabetes or arterial ligation caused inflammation as well as endothelial injury. We believe that the increased inflammatory response in the hindlimb as well as the endothelial damage may be caused by the combination of diabetes and arterial ligation. We will demonstrate this further in a future study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu Z, China; Ueda H, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Chen YX
| 1. | Cornejo Del Río V, Mostaza J, Lahoz C, Sánchez-Arroyo V, Sabín C, López S, Patrón P, Fernández-García P, Fernández-Puntero B, Vicent D, Montesano-Sánchez L, García-Iglesias F, González-Alegre T, Estirado E, Laguna F, de Burgos-Lunar C, Gómez-Campelo P, Abanades-Herranz JC, de Miguel-Yanes JM, Salinero-Fort MA; on behalf SPREDIA-2 Group. Prevalence of peripheral artery disease (PAD) and factors associated: An epidemiological analysis from the population-based Screening PRE-diabetes and type 2 DIAbetes (SPREDIA-2) study. PLoS One. 2017;12:e0186220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH, Aboyans V, Aksoy M, Alexandrescu VA, Armstrong D, Azuma N, Belch J, Bergoeing M, Bjorck M, Chakfé N, Cheng S, Dawson J, Debus ES, Dueck A, Duval S, Eckstein HH, Ferraresi R, Gambhir R, Gargiulo M, Geraghty P, Goode S, Gray B, Guo W, Gupta PC, Hinchliffe R, Jetty P, Komori K, Lavery L, Liang W, Lookstein R, Menard M, Misra S, Miyata T, Moneta G, Munoa Prado JA, Munoz A, Paolini JE, Patel M, Pomposelli F, Powell R, Robless P, Rogers L, Schanzer A, Schneider P, Taylor S, De Ceniga MV, Veller M, Vermassen F, Wang J, Wang S; GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and World Federation of Vascular Societies (WFVS). Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur J Vasc Endovasc Surg. 2019;58:S1-S109.e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 911] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 3. | Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, Venermo M, Zierler RE, Schaper NC; International Working Group on the Diabetic Foot (IWGDF). Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 4. | Rizzi A, Benagiano V, Ribatti D. Angiogenesis versus arteriogenesis. Rom J Morphol Embryol. 2017;58:15-19. [PubMed] |
| 5. | Simon F, Oberhuber A, Floros N, Düppers P, Schelzig H, Duran M. Pathophysiology of chronic limb ischemia. Gefasschirurgie. 2018;23:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Godo S, Shimokawa H. Endothelial Functions. Arterioscler Thromb Vasc Biol. 2017;37:e108-e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 335] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 7. | Schneckmann R, Suvorava T, Hundhausen C, Schuler D, Lorenz C, Freudenberger T, Kelm M, Fischer JW, Flögel U, Grandoch M. Endothelial Hyaluronan Synthase 3 Augments Postischemic Arteriogenesis Through CD44/eNOS Signaling. Arterioscler Thromb Vasc Biol. 2021;41:2551-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Ruiter MS, van Golde JM, Schaper NC, Stehouwer CD, Huijberts MS. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisms. Clin Sci (Lond). 2010;119:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Hansen NW, Hansen AJ, Sams A. The endothelial border to health: Mechanistic evidence of the hyperglycemic culprit of inflammatory disease acceleration. IUBMB Life. 2017;69:148-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Yang P, Liu P, Yang R. Systematic Review of Tongxinluo Capsule on the Therapeutic Effect and Hemorheology of Patients with Transient Ischemic Attack. Evid Based Complement Alternat Med. 2021;2021:5541768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Xu Y, Li X, Zhang H, Wu Y, Zhang J, Li J, Dou K, Yan H, You S, Yang Y, Liang Y, Xu L, Gao X, Liu C, Dong Q, Zhang W, Song G, Zhang T, Jiang L, Chen G, Tang R, Jin C, Yang J, Yao C, Xian Y, Peterson ED, Gao R. China Tongxinluo Study for myocardial protection in patients with Acute Myocardial Infarction (CTS-AMI): Rationale and design of a randomized, double-blind, placebo-controlled, multicenter clinical trial. Am Heart J. 2020;227:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ma J, Qiao L, Meng L, Ma L, Zhao Y, Liu X, Ni M, Zhang Y. Tongxinluo may stabilize atherosclerotic plaque via multiple mechanisms scanning by genechip. Biomed Pharmacother. 2019;113:108767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Chang C, Liu H, Wei C, Chang L, Liang J, Bei H, Li H, Liu S, Wu Y. Tongxinluo Regulates Expression of Tight Junction Proteins and Alleviates Endothelial Cell Monolayer Hyperpermeability via ERK-1/2 Signaling Pathway in Oxidized Low-Density Lipoprotein-Induced Human Umbilical Vein Endothelial Cells. Evid Based Complement Alternat Med. 2017;2017:4198486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Li Q, Li N, Cui HH, Tian XQ, Jin C, Chen GH, Yang YJ. Tongxinluo exerts protective effects via anti-apoptotic and pro-autophagic mechanisms by activating AMPK pathway in infarcted rat hearts. Exp Physiol. 2017;102:422-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Qi K, Li X, Geng Y, Cui H, Jin C, Wang P, Li Y, Yang Y. Tongxinluo attenuates reperfusion injury in diabetic hearts by angiopoietin-like 4-mediated protection of endothelial barrier integrity via PPAR-α pathway. PLoS One. 2018;13:e0198403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Wu XM, Gao YB, Xu LP, Zou DW, Zhu ZY, Wang XL, Yao WJ, Luo LT, Tong Y, Tian NX, Han ZJ, Dang WY. Tongxinluo Inhibits Renal Fibrosis in Diabetic Nephropathy: Involvement of the Suppression of Intercellular Transfer of TGF-[Formula: see text]1-Containing Exosomes from GECs to GMCs. Am J Chin Med. 2017;45:1075-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Aref Z, de Vries MR, Quax PHA. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Yuan GQ, Gao S, Geng YJ, Tang YP, Zheng MJ, Shelat HS, Collins S, Wu HJ, Wu YL. Tongxinluo Improves Apolipoprotein E-Deficient Mouse Heart Function. Chin Med J (Engl). 2018;131:544-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Aicher BO, Mukhopadhyay S, Lu X, Muratoglu SC, Strickland DK, Ucuzian AA. Quantitative Micro-CT Analysis of Aortopathy in a Mouse Model of β-aminopropionitrile-induced Aortic Aneurysm and Dissection. J Vis Exp. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Kuang X, Yin Y, Han N, Chang L, Wang H, Hou Y, Li H, Li Z, Liu Y, Hao Y, Wei Y, Wang X, Jia Z. Tongxinluo prevents chronic obstructive pulmonary disease complicated with atherosclerosis by inhibiting ferroptosis and protecting against pulmonary microvascular barrier dysfunction. Biomed Pharmacother. 2022;145:112367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Kong H, Zhao H, Chen T, Song Y, Cui Y. Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022;13:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 22. | Silambarasan M, Tan JR, Karolina DS, Armugam A, Kaur C, Jeyaseelan K. MicroRNAs in Hyperglycemia Induced Endothelial Cell Dysfunction. Int J Mol Sci. 2016;17:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Lilly B. We have contact: endothelial cell-smooth muscle cell interactions. Physiology (Bethesda). 2014;29:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Chen H, Feng Z, Li L, Fan L. MicroRNA-9 rescues hyperglycemia-induced endothelial cell dysfunction and promotes arteriogenesis through downregulating Notch1 signaling. Mol Cell Biochem. 2021;476:2777-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Korff T, Braun J, Pfaff D, Augustin HG, Hecker M. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood. 2008;112:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Lin X, He Y, Hou X, Zhang Z, Wang R, Wu Q. Endothelial Cells Can Regulate Smooth Muscle Cells in Contractile Phenotype through the miR-206/ARF6&NCX1/Exosome Axis. PLoS One. 2016;11:e0152959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M, Meng Q, Zhou B, Leng Y, Xia ZY. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxid Med Cell Longev. 2017;2017:9743280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 28. | Al Mamun A, Mimi AA, Zaeem M, Wu Y, Monalisa I, Akter A, Munir F, Xiao J. Role of pyroptosis in diabetic retinopathy and its therapeutic implications. Eur J Pharmacol. 2021;904:174166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, Aung LHH, Li PF, Yu T, Chu XM. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11:776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 308] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 30. | Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2573] [Cited by in RCA: 4542] [Article Influence: 454.2] [Reference Citation Analysis (0)] |
| 31. | Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579-4590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 32. | Yu ZW, Zhang J, Li X, Wang Y, Fu YH, Gao XY. A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240:117138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 33. | Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng Transl Med. 2019;4:75-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Chen GH, Xu CS, Zhang J, Li Q, Cui HH, Li XD, Chang LP, Tang RJ, Xu JY, Tian XQ, Huang PS, Xu J, Jin C, Yang YJ. Inhibition of miR-128-3p by Tongxinluo Protects Human Cardiomyocytes from Ischemia/reperfusion Injury via Upregulation of p70s6k1/p-p70s6k1. Front Pharmacol. 2017;8:775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Zhang H, Zhou M, Yi X, Duan P, Yu A, Qi B. Autologous Fat Grafting Promotes Macrophage Infiltration to Increase Secretion of Growth Factors and Revascularization, Thereby Treating Diabetic Rat Skin Defect. Diabetes Metab Syndr Obes. 2020;13:4897-4908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 36. | Kalucka J, Bierhansl L, Wielockx B, Carmeliet P, Eelen G. Interaction of endothelial cells with macrophages-linking molecular and metabolic signaling. Pflugers Arch. 2017;469:473-483. [PubMed] |
| 37. | Czapla J, Cichoń T, Pilny E, Jarosz-Biej M, Matuszczak S, Drzyzga A, Krakowczyk Ł, Smolarczyk R. Adipose tissue-derived stromal cells stimulated macrophages-endothelial cells interactions promote effective ischemic muscle neovascularization. Eur J Pharmacol. 2020;883:173354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Ganta VC, Choi M, Farber CR, Annex BH. Antiangiogenic VEGF(165)b Regulates Macrophage Polarization via S100A8/S100A9 in Peripheral Artery Disease. Circulation. 2019;139:226-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Mei Y, Thompson MD, Shiraishi Y, Cohen RA, Tong X. Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase C674 promotes ischemia- and hypoxia-induced angiogenesis via coordinated endothelial cell and macrophage function. J Mol Cell Cardiol. 2014;76:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Busti C, Falcinelli E, Momi S, Gresele P. Matrix metalloproteinases and peripheral arterial disease. Intern Emerg Med. 2010;5:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Zhou J, Cao B, Ju W, Liu S, Song J, Liu L. Effects of tongxinluo on angiogenesis in the carotid adventitia of hyperlipidemic rabbits. Mol Med Rep. 2016;14:3832-3840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Méndez-Barbero N, Gutiérrez-Muñoz C, Blanco-Colio LM. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 43. | Delbosc S, Glorian M, Le Port AS, Béréziat G, Andréani M, Limon I. The benefit of docosahexanoic acid on the migration of vascular smooth muscle cells is partially dependent on Notch regulation of MMP-2/-9. Am J Pathol. 2008;172:1430-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |