Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.222
Peer-review started: September 22, 2022
First decision: December 26, 2022
Revised: January 8, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 15, 2023
Processing time: 174 Days and 7.9 Hours
Advanced glycation end products (AGEs) are diabetic metabolic toxic products that cannot be ignored. Nε-(carboxymethyl)lysine (CML), a component of AGEs, could increase macrophage lipid uptake, promote foam cell formation, and thereby accelerate atherosclerosis. The receptor for AGEs (RAGE) and cluster of differentiation 36 (CD36) were the receptors of CML. However, it is still unknown whether RAGE and CD36 play key roles in CML-promoted lipid uptake.
Our study aimed to explore the role of RAGE and CD36 in CML-induced mac-rophage lipid uptake.
In this study, we examined the effect of CML on lipid uptake by Raw264.7 macrophages. After adding 10 mmol/L CML, the lipid accumulation in macro-phages was confirmed by oil red O staining. Expression changes of CD36 and RAGE were detected with immunoblotting and quantitative real-time polymerase chain reaction. The interaction between CML with CD36 and RAGE was verified by immunoprecipitation. We synthesized a novel N-succinimidyl-4-18F-fluorobenzoate-CML radioactive probe. Radioactive receptor-ligand binding assays were performed to test the binding affinity between CML with CD36 and RAGE. The effects of blocking CD36 or RAGE on CML-promoting lipid uptake were also detected.
The study revealed that CML significantly promoted lipid uptake by macro-phages. Immunoprecipitation and radioactive receptor-ligand binding assays indicated that CML could specifically bind to both CD36 and RAGE. CML had a higher affinity for CD36 than RAGE. ARG82, ASN71, and THR70 were the potential interacting amino acids that CD36 binds to CML Anti-CD36 and anti-RAGE could block the uptake of CML by macrophages. The lipid uptake promotion effect of CML was significantly attenuated after blocking CD36 or RAGE.
Our results suggest that the binding of CML with CD36 and RAGE promotes macrophage lipid uptake.
Core Tip: Nε-(carboxymethyl)lysine (CML), a toxic metabolism product in diabetes mellites, is a causative factor of many diseases. CML has been reported to promote lipid uptake in macrophages and foam cell formation. The receptor for advanced glycation end products (RAGE) and cluster of differentiation 36 (CD36) are receptors of CML. However, the roles of RAGE and CD36 in CML-induced lipid uptake in macrophages are currently unclear. Moreover, the relationship and difference between RAGE and CD36 in this process are also worth exploring. The in vitro model was constructed with Raw264.7 cells. Under the stimulation of CML, the lipid uptake by cells was significantly increased. Inhibition of CD36 or RAGE antagonized CML-induced lipid uptake. We synthesized a probe of 18F-CML to explore the relationship of CML to CD36 and to RAGE. CML could bind to CD36 and to RAGE, and CML has a significantly stronger affinity for CD36 than for RAGE. The exploration of the pathogenic mechanism of CML may provide evidence for a deeper understanding of diseases related to metabolic disorders.
- Citation: Wang ZQ, Yao HP, Sun Z. Nε-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products. World J Diabetes 2023; 14(3): 222-233
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/222.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.222
Metabolic disorders caused by diabetes can affect the functions of the bone, brain, blood vessels and other organs and have become an important cause of many diseases[1-5]. Nε-(carboxymethyl)lysine (CML), a representative of advanced glycation end products (AGE), is one of the key active components of toxic metabolites in diabetes mellites[6]. Studies have reported that CML is considered a potential hazard to human health[6,7], but its pathogenic mechanism is still unclear.
CML was firstly identified in 1985. it is also the first glycoxidation product discovered[8]. It can be formed by oxidation of the Amadori product or by direct reaction of glyoxal with the ε-amino group of lysine[9,10]. Exposure to CML may be hazardous to health and increase the risk of many diseases. CML works in two main ways: Non-receptor-dependent and receptor-dependent[11]. In the receptor-independent pathway, CML cross-links with collagen and elastin fibers, which alters extracellular matrix stiffness. In receptor-dependent pathways, CML binds to its receptors to activate a series of signals. Previous studies have found that CML can promote atherosclerotic plaques[12,13], but the mechanisms remain unclear.
Cluster of differentiation 36 (CD36) a highly glycosylated 80kD membrane protein responsible for lipid transport and lipid sense[14]. The amino terminus of CD36 contains a domain that binds to fatty acids, low-density lipoprotein, and phosphatidylcholine. Lysine 164 and 166 are key sites[15]. The receptor for AGE (RAGE) is a multi-ligand receptor. These ligands bind to the extracellular V-C1-C2 domains of RAGE and mediate pathogenic signaling in a variety of diseases[16]. RAGE is abnormally elevated under high-fat conditions. RAGE-related signals further aggravate lipid metabolism disorders and lipid accumulation[17]. Macrophages are the principal effector cells in atherosclerosis. It takes up lipids to form foam cells, which are the basis of atherosclerotic plaque formation[18,19]. RAGE and CD36 have been reported to play an important role in macrophage lipid uptake[20,21]. It is necessary to explore whether CML could promote macrophage lipid uptake via RAGE and CD36 and the binding affinities of CML to RAGE and CD36.
Lipid metabolism in macrophages includes three processes: Lipid uptake, esterification and efflux[22]. When these processes are disturbed, macrophages will form lipid-rich foam cells. Previous studies reported that CML could promote the formation of foam cells[23]. We speculated that CD36 and RAGE might be involved in CML-promoted lipid uptake. Further, this study was intended to compare the relationship and difference between CD36 and RAGE in this process. In this study, a Raw264.7 macrophage lipid uptake model was constructed. The effects of CML on lipid uptake in macrophages were explored. Radioactive receptor-ligand binding assays were performed to determine the binding affinities of CML to CD36 and RAGE. The findings of this study are expected to provide clues for the prevention and treatment of atherosclerosis.
The Raw264.7 cell line was purchased from Procell Life Science & Technology (Wuhan, China). As previously described[13], cells were cultured in Dulbecco's Modified Eagle media supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37°C with 20% CO2. Oxidized low-density lipoprotein (oxLDL) (40 μg/mL) supplemented with or without CML (10 mmol/L) was added to Raw264.7 and incubated for 12 h to detect lipid uptake by Raw264.7. Cells were preincubated with Anti-CD36 (20 μg/mL), anti-RAGE (20 μg/mL) or maleylated-bovine serum albumin (malBSA) (400 nM) to block CD36, RAGE or scavenger receptor, respectively.
The staining was performed as previously reported[23], cells were fixed in 4% paraformaldehyde for 1 h and then washed with phosphate buffered saline (PBS). After immersion in 60% isopropanol for 30s, samples were dyed with Oil Red O working solution (Oil Red O stock solution: Double distilled water 3:2) (Solarbio, Beijing, China) for 30min. The staining was imaged with microscopy (Olympus, Tokyo, Japan). For the quantification of Oil Red O staining, isopropanol was used to completely dissolve Oil Red O. The optical density value at 520 nm was then measured.
For immunoblotting[13], protein samples were prepared with radio-immuno precipitation assay lysis buffer (Beyotime, Shanghai, China). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. After blocking with 5% milk, the membranes were incubated with primary and secondary antibodies. Images were acquired with a chemiluminescence system (Amersham Imager 600). Quantitative analysis was performed using ImageJ software. The primary antibodies used in immunoblotting were anti-CD36 [1:1000, sc-7309; Santa Cruz, CA, United States (USA)], anti-RAGE (1:1000, ab216329; Abcam, USA), anti-CML (1:2000; ab125145, Abcam) and anti-β-actin (1:1000, ab8227, Abcam). For immunoprecipitation, a Protein A/G Immunoprecipitation Kit was used (Beaverbio, Suzhou, China). All operating steps followed the kit instructions. Samples obtained from immunoprecipitation were detected by immunoblotting. Antibodies used in immunoprecipitation were anti-CD36 (1:500, sc-7309; Santa Cruz) and anti-RAGE (1:30, ab216329; Abcam).
As previously described[24], RNA samples were prepared with guanidinium-phenol-based reagent (Invitrogen, Carlsbad, CA, USA). mRNA was reversed to cDNA using a Reverse Transcriptase Kit (Vazyme Biotech, Nanjing, China). The SYBR Quantitative polymerase chain reaction Master Mix Kit (Vazyme Biotech) was then used to detect cDNA levels. β-actin was used as a control. The experimental operation was carried out according to the instructions. The primer sequences were as follows: CD36 F: TCTTCCAGCCAACGCCTTT, R: CTTCTTTGCACTTGCCATGTCC; RAGE F: ACATGTGTGTCTGAGGGAAGC, R: AGCTCTGACCGCAGTGTAAAG; ATP binding cassette transporter A1 (ABCA1) F: TGGACATCCTGAAGCCAG, R: TTCTTCCCACATGCCCT; ATP binding cassette transporter G1 (ABCG1) F: GCTGGGAAGTCCACACTC, R: GATACGGCACGAGATTGG; β-actin F: TCTTGGGTATGGAATCCTGTG, R: ATCTCCTTCTGCATCCTGTCA.
As described in our previous study[25], ethyl 4-trimethylammonium triflate benzoate [10 mg, 31.2 μmol, dissolved in 0.2 mL anhydrous acetonitrile (CH3CN)] was added to a reaction flask containing dry 18F and heated at 80 ℃ for 10 min. After cooling, 0.5 mL NaOH (0.5 mol/L) was added and reacted at 90 ℃ for 5 min. After cooling, 0.7 mL HCl (1 mol/L) was added for neutralization. The reaction solution was loaded onto a Waters Sep-Pak cartridge. Next, the cartridge was washed with 2 mL HCl (0.01 mol/L), blown dry with N2 and eluted with 3 mL CH3CN. The elution fraction was mixed with 20 μL of 10% aqueous (CH3)4NOH solution. The mixture was dried at 100 ℃ and then reacted with 12 mg O-(N-succinimidyl)-N, N, N’, N’-tetramethyluronium tetrafluoroborate (dissolved in 0.25 mL CH3CN) at 80 ℃ for 5 min. Then, the mixture was acidified with 3 mL of 5% aqueous acetic acid and purified on a YMC J’Sphere ODS-H80 column with a mobile phase of CH3CN/H2O (55%/45%) followed by final dilution with 6 mL of water. The above solution was loaded onto a Sep-Pak C18 cartridge. The cartridge was then dried and eluted to obtain N-succinimidyl-4-18F-fluorobenzoate (18F-SFB).
As previously described[25], the 18F-SFB was dissolved in 1 mL of acetonitrile. 300μL of CML (1mg/mL) dissolved in a carbonate buffer solution (pH = 8.4) was added to 18F-SFB and incubated at 65°C for 30 min until most of the SFB had reacted. The final product was purified by C18 reversed-phase chromatography (detection mode was radioactive mode and the ultraviolet absorption was at 254 nm).
AutoDock 4.2.6 software was used to simulate the docking of CML and CD36[26]. The CML structure in standard delay format (obtained from PubChem) was converted to pdbqt format with Open Babel 2.3.1 software. The crystal structure of the CD36 extracellular segment was obtained from the Protein Data Bank (PDB ID: 4Q4B). AutoDock tools were used to remove water molecules and other heteroatoms, add hydrogen atoms, and combine all non-polar hydrogens. The 4Q4B file was then saved as pdbqt file format. The root of CML was calculated with Ligand-Torsion. Rotatable chemical bonds were identified through Torsion Tree. The 4Q4B files were imported through the Input of Flexible Residues. 4Q4B and CML are set as Macromolecule and Ligand in Grid, respectively. Docking box size, coordinates, number of grid points, and distance between points were set in GridBox. Receptor-ligand docking files are saved in gpf file format. The amino acid docking with CML was then calculated. A 3D grid for 4Q4B was created in AutoGrid. The CD36 extracellular segment was set as rigid. Docking simulation was performed with the Lamarckian Genetic Algorithm. After running AutoDock, the most stable bound conformation was determined based on the binding energy. The Interaction button in Display was used to calculate and display the amino acids closest to and interacting with CML.
96-well plates were embedded with different concentrations of recombinant CD36 or RAGE protein (0, 1.6, 3.2, 6.4, 12.8, 25.6, 51.2 and 102.4 mg/L). For total binding (TB), 18F-SFB-CML (100 μci, 50 μl) and PBS (150 μL) were added into plates and incubated at 37 ℃ for 30 min. After the plates were washed twice with PBS, 1M NaOH containing 1% sodium dodecyl sulfate was added to dissolve radioactive material. The solution was then transferred into a radiosensitive tube and detected with a γ counter. For non-specific binding (NB), 96-well plates were also embedded with recombinant protein along concentration gradient. 18F-CML (2 μCi, 50 μL) was added, followed by the addition of a 100-fold concentration of CML and 150 μL PBS. After incubation of 30min, the solution was detected with a γ counter. Specific binding (SB) = TB - NB
All data were expressed as means ± SD. Differences between groups were tested by one-way Analysis of Variance and Student's t-test. P < 0.05 represents a statistically significant difference. All data were analyzed using SPSS 22.0 software.
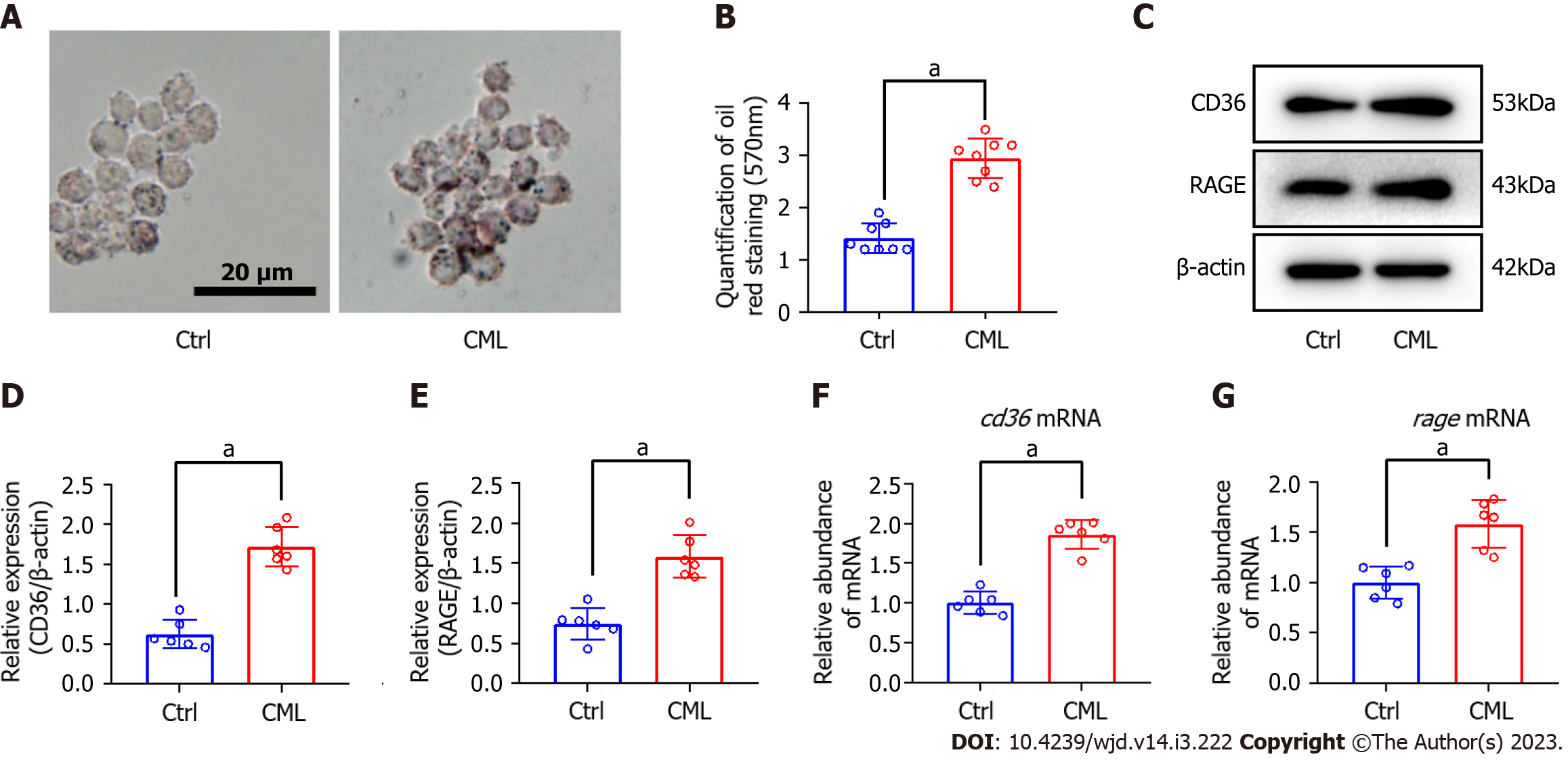
The macrophage lipid uptake model was constructed with oxLDL and RAW264.7 cells. Oil red staining revealed that in response to CML stimulation, macrophages took up more lipids, 2.1 times as much as in the control (Ctrl) group (2.95 ± 0.38 vs 1.41 ± 0.28, P < 0.05) (Figure 1A and B). CML induced a 2.2 and 1.8-fold increase in the protein level of CD36 and RAGE, respectively (Figure 1C-E). CML also significantly increased of Cd36 and RAGE mRNA levels (Figure 1F and G).
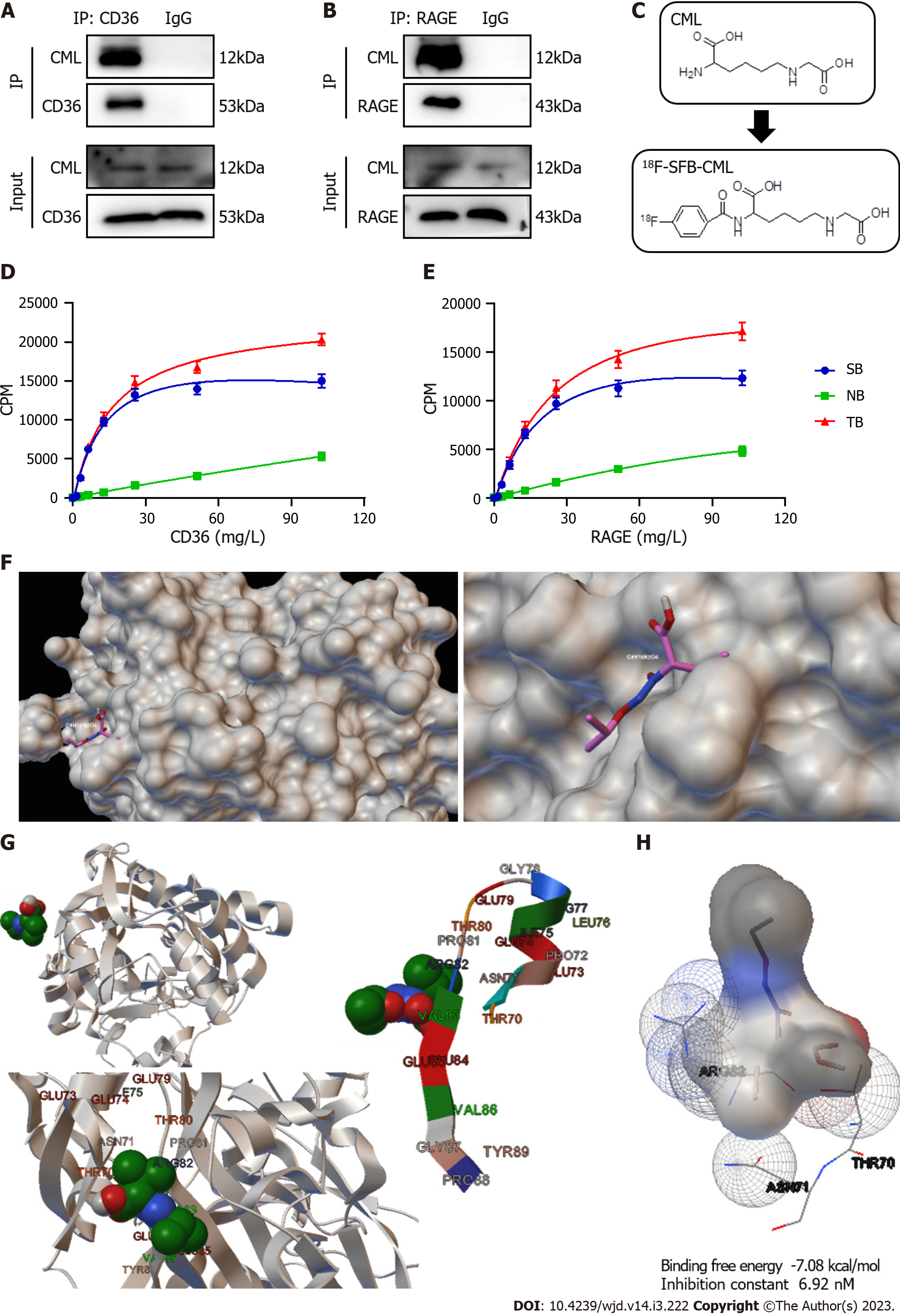
It is unknown whether the binding affinities of the two receptors to CML are different. Immunoprecipitation showed that CML could interact with CD36 and RAGE (Figure 2A and B). We then constructed 18F-labeled CML probes (18F-SFB-CML) for radioreceptor ligand binding assays to test the affinity between CML and its receptors (Figure 2C). The results revealed the specific binding of the CML to both CD36 and RAGE (Figure 2D and E). TB and NB increased with the concentration of CD36 and RAGE. SB was also calculated. The maximum binding capacity between CML and CD36 was not different from that of RAGE (22886 ± 1792 vs 21725 ± 2597). The equilibrium dissociation constant of CD36 was significantly lower than that of RAGE (14.2 ± 2.21 vs 24.48 ± 4.433, P < 0.05), suggesting that CD36 has a higher affinity for CML than RAGE. The molecular docking mode of the CD36 extracellular segment (PDB ID: 4Q4B) and CML was then simulated with Autodock software. CML can be embedded in the active pocket of the CD36 extracellular segment (Figure 2F). Analysis of the binding site identified ARG82, ASN71, and THR70 as interacting amino acids that CD36 binds to CML (Figure 2G). The binding free energy of CD36 and CML was -7.08 kcal/mol and the inhibition constant was 6.92 nM (Figure 2H).
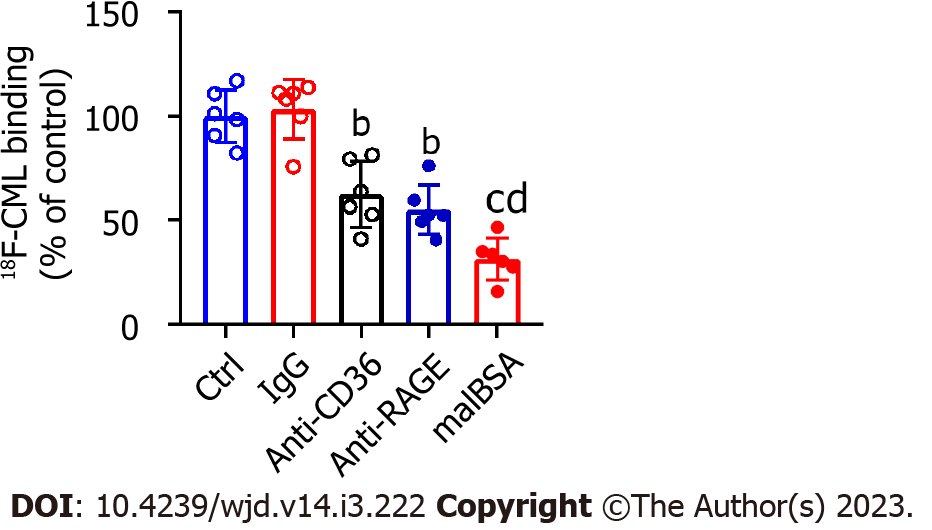
In an in vitro cell-free system, we demonstrated that CML could bind specifically to CD36 and RAGE. In macrophages, we attempted to explore the effects of CD36 and RAGE on the capture of CML by cells. CD36 and RAGE in macrophages were blocked in advance with anti-CD36 and anti-RAGE antibodies. Global scavenger receptors were blocked with malBSA. Cells were incubated with 18F-SFB-CML to detect the capacity of capture CML. Anti-CD36 or anti-RAGE significantly inhibited the capture of CML by macrophages, but there was no significant difference between the inhibitory effects of anti-CD36 and anti-RAGE (Figure 3). Compared with anti-CD36 and anti-RAGE, malBSA showed a more substantial inhibitory effect on the capture of CML.
The studies above have demonstrated that CML could promote macrophage lipid uptake and specifically bind to CD36 and RAGE. Furthermore, we explored whether CD36 and RAGE are involved in the promotion of lipid uptake. Oil red O staining showed that both anti-CD36 and anti-RAGE significantly inhibited CML-induced macrophage lipid uptake (Figure 4A and B). Anti-CD36 and anti-RAGE reduced lipid uptake by 28.4% and 38.7%, respectively. In addition, we also found that the intracellular lipid content in CML + Anti-RAGE group was significantly lower than that in CML + Anti-CD36 group (1.83 ± 0.18 vs 2.14 ± 0.18, P < 0.05), suggesting that the inhibitory effect of anti-RAGE on lipid uptake may be stronger than that of anti-CD36. We also found that CML significantly upregulated Cd36 and downregulated Abca1 and Abcg1. It is speculated that CML enhances the capacity of macrophage lipid uptake and inhibits cholesterol efflux (Figure 4C-E). Furthermore, compared with CML + IgG group, the level of Cd36 mRNA in CML + Anti-RAGE group was significantly decreased, while Abca1 and Abcg1 were markedly increased (Figure 4C-E). RAGE could partially reverse the increase of macrophage lipid uptake induced by CML. However, anti-CD36 did not affect the expression of Abca1 and Abcg1 as anti-RAGE did (Figure 4C-E).
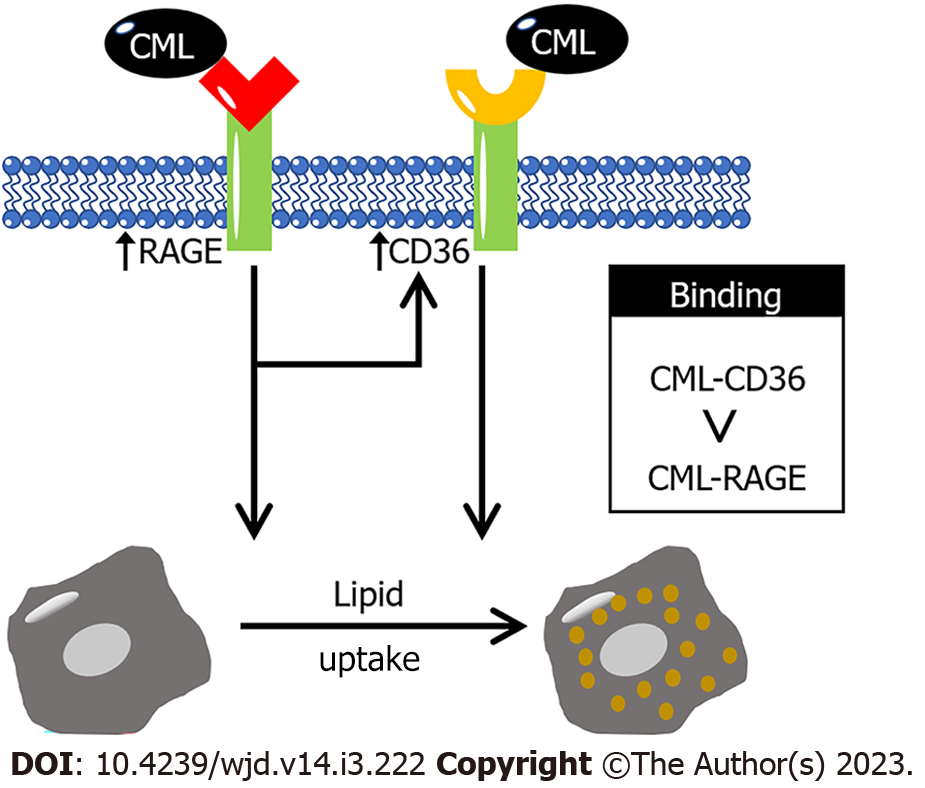
This study investigated the effect of CML on macrophage lipid uptake and revealed the role of CD36 and RAGE in this process (Figure 5). The present study found that CML significantly promotes the intracellular lipid accumulation of macrophages. Previous literatures have confirmed that CD36 and RAGE are closely related to lipid metabolism. So, we speculate that CD36 and RAGE may also be involved in CML-promoted lipid uptake of macrophages. This study revealed that the binding of CML to CD36 and RAGE has a great impact on the lipid uptake by macrophages. To clarify the specific binding affinity of CML to its receptors CD36 and RAGE, radioactive receptor-ligand binding assays were performed with 18F-SFB-CML in a cell-free system. The binding affinity of CD36 for CML was higher than that of RAGE. Our study provides a deeper understanding of the pathogenesis of CML and brings new thinking for the prevention and treatment of atherosclerosis.
RAGE is composed of an extracellular domain, a transmembrane protein and a highly charged cytoplasmic domain consisting of 43 amino acids[27]. Elevated RAGE expression and activation of AGEs-RAGE signaling have been found in a variety of diseases[28,29]. Our previous studies have confirmed that RAGE accelerated the progression of diabetic calcification[30]. The lack of RAGE significantly delayed the development of diabetic atherosclerosis[31]. Leerach et al[20] found that inhibition of AGEs/RAGE signaling attenuated macrophage-derived foam cell formation. The present study also suggested that lipid uptake by macrophages was significantly reduced after the blocking RAGE with anti-RAGE. The AGEs/RAGE signals not only enhance the lipid uptake of macrophages but also have other regulatory effects on macrophages. Qin et al[32] found that AGEs/RAGE could promote macrophage migration. The AGEs/RAGE axis also activated inflammatory signals that induce pro-inflammatory phenotypic transformation of macrophages[33]. The highly plastic macrophages determined their important roles in various pathophysiological environments. Our previous studies on vascular smooth muscle cells (VSMCs) found that the foam cell phenotype of VSMCs could accelerate their osteogenic differentiation[34]. Therefore, we speculate that there is also a correlation between different phenotypes of macrophages, and the AGEs/RAGE signal may serve as a key bridge in phe-notypic transformation.
CD36 is an important member of the scavenger receptor family, which has two transmembrane domains and a glycosylated extracellular domain[35]. Zhu et al[36] reported that AGEs promoted artery thrombus formation in a CD36-dependent manner[36]. Moreover, the stability of CD36 has been previously reported to affect foam cell formation[37]. This study also demonstrated that preincubation with anti-CD36 significantly decreased macrophage lipid uptake. In addition, our results also revealed that CML could not only bind to CD36 but also promote its expression. Besides CML/RAGE axis which has been verified in this study, there may be other ways to upregulate CD36 expression. Previous studies have pointed out that AGEs could activate FoxO1 signaling[38], while FoxO1 has also been reported to promote CD36 expression[39]. Therefore, we speculated that the AGEs/FoxO1 axis might also be involved in the transcriptional regulation of CD36.
RAGE and CD36 have been reported to play important roles in atherosclerosis[40]. At the same time, both RAGE and CD36 are multi-ligand receptors. Ligands for RAGE also include amyloid beta, high mobility group protein B1, lipopolysaccharide, macrophage-1 antigen, phosphatidylserine and S100 calcium binding protein[41]. OxLDL, thrombospondin-1 and free fatty acid are also ligands for CD36[42]. Whether these ligands can bind to RAGE or CD36 more closely than AGEs has not been reported. The binding sites of these ligands to receptors appear to be different. OxLDL binds to a large hydrophobic pocket of CD36 extracellular loop, while thrombospondin-1 binds to the CD36 LIMP-II Emp sequence homology domain of CD36. Zhu et al[36] found that NO2+LDL, a ligand of CD36, could inhibit the binding of AGEs to CD36, but the inhibition was not complete. Therefore, we speculate that the binding affinities of different ligands may be different, and even there are synergistic or inhibitory effects between them. However, due to the different binding sites, the ligand-receptor binding remains relatively independent.
Although both RAGE and CD36 are receptors for CML, their differences are currently unclear. Therefore, we also explored the affinity of both receptors for CML for the first time. We constructed radioactive 18F-SFB-CML, the biological activity of which has been previously reported[25]. In the previous study, the mice were subjected to a dynamic PET scan for 2 h after intravenous injection. Radioactive accumulation in the heart area was observed at 5 min, followed by a concentrated area of perfusion in the liver. After 60 min, no other organs could obtain the high signal area except the kidney and bladder. In this study, radioactive receptor-ligand binding assays suggested that the affinity between CML and CD36 was higher. In the molecular docking model, we also simulated the binding mode between CML and CD36. Interestingly, RAGE and CD36 are not completely isolated. We observed that Cd36 mRNA decreased significantly when RAGE was blocked. Consistent with our study, Xu et al[43] and Yashima et al[44] also found that anti-RAGE inhibited CD36 expression. These results suggest that RAGE may be upstream of CD36. Anti-RAGE exerted stronger inhibition on macrophage lipid uptake than anti-CD36, which might be due to the fact that anti-RAGE not only blocked RAGE but also affected the activity of CD36. Although these previous studies have reported the role of RAGE and CD36 in macrophages, the focus of these studies was different. In our study, we compared the affinity of RAGE and CD36 for CML, the key active component of AGEs, and explored the difference between RAGE and CD36, which has not been reported in previous studies. To study the binding of CML with RAGE and CD36, a novel radioactive probe 18F-SFB-CML was constructed. Based on the radioreceptor ligand binding experiments, the binding affinity between receptors and ligands can be more effectively reflected.
In addition, we also observed that both Abca1 and Abcg1 were upregulated after the blockade of RAGE. ABCA1 and ABCG1 are key molecules in mediating cholesterol efflux of macrophages[45,46]. ABCA1 can transport cholesterol and phosphatidylcholine to apolipoprotein A-I. ABCG1 is mainly responsible for the transfer of cholesterol, phosphatidylcholine and sphingomyelin to nascent HDL. However, anti-CD36 did not have the same inhibitory effect on the expression of ABCA1 and ABCG1 as anti-RAGE. Consistent with our study, the study by Xia et al[47] also suggested that changes in CD36 expression did not affect ABCA1 and ABCG1. Previous studies have demonstrated that CD36 enhances the internalization of oxidized lipids in macrophages and promotes foam cell formation[48]. CD36 may increase macrophage intracellular lipid accumulation in another way, rather than regulating lipid efflux.
Our study explored the interaction between CML with CD36 and RAGE. CML has a higher binding affinity for CD36 than for RAGE. We also found that CD36 and RAGE play key roles in CML promoting lipid uptake of macrophages. Blocking CD36 or RAGE significantly inhibited CML-induced lipid uptake by macrophages. This study may provide clues for the pathogenic mechanism of glucotoxic metabolites.
Nε-(carboxymethyl)lysine (CML) is a representative of advanced glycation end products. CML could promote the lipid uptake of macrophages. The receptor for advanced glycation end products (RAGE) and cluster of differentiation 36 (CD36) are receptors of CML. It is necessary to explore the relationship between RAGE and CD36 and their roles in the lipid uptake of macrophages.
CML was reported an atherogenic factor. CML can promote the lipid uptake of macrophages and the formation of foam cells. RAGE and CD36 are important for the pathogenesis of CML. RAGE and CD36 have also been reported to promote atherosclerosis. Therefore, RAGE and CD36 may be involved in the lipid uptake of macrophages. The different roles of RAGE and CD36 are also worth exploring.
We aimed to clarify the role of RAGE and CD36 in CML-induced macrophage uptake and to explore the relationship and difference between RAGE and CD36 in this process.
Raw264.7 cells were divided into control group and CML group. Cells of the CML group were incubated with oxidized low-density lipoprotein and CML to test the effect of CML on lipid uptake. The expressions of RAGE and CD36 were determined by western blot and quantitative polymerase chain reaction. Immunoprecipitation and radioactive receptor-ligand binding assays were performed to exam the binding of CML to RAGE and CD36. A receptor-ligand binding model was constructed to predict binding sites. The uptake of lipid and CML was tested after the cells were preincubated with Anti-RAGE or Anti-CD36.
CML significantly promotes lipid uptake by macrophages. The expression of CD36 and RAGE was also significantly upregulated under CML stimulation. Immunoprecipitation showed that CML could interact with CD36 and RAGE. Radioreceptor ligand binding assay also confirmed the binding of CML to CD36 and RAGE. Moreover, the affinity of CML with CD36 was significantly higher than that of RAGE. The receptor-ligand binding model found that the binding sites of CML and CD36 may be ARG82, ASN71, and THR70. The binding of CML to cells was significantly reduced after pre-incubation with Anti-CD36 or Anti-RAGE. Furthermore, Anti-CD36 and Anti-RAGE significantly inhibited lipid uptake by macrophages.
CML promotes lipid uptake by macrophages, and its binding to CD36 and RAGE plays a key role in this process.
The current study provides clues for the pathogenesis of metabolic toxic product CML, and also suggests a promising intervention target for the prevention and treatment of atherosclerosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park JH, South Korea; Tzeng IS, Taiwan S-Editor: Liu GL L-Editor: A P-Editor: Chen YX
| 1. | Yamamoto M, Sugimoto T. Advanced Glycation End Products, Diabetes, and Bone Strength. Curr Osteoporos Rep. 2016;14:320-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 2. | Yamagishi S, Matsui T, Ueda S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and cardiovascular disease (CVD) in diabetes. Cardiovasc Hematol Agents Med Chem. 2007;5:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 3. | Theodorou K, Boon RA. Endothelial Cell Metabolism in Atherosclerosis. Front Cell Dev Biol. 2018;6:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Wang ZQ, Jing LL, Yan JC, Sun Z, Bao ZY, Shao C, Pang QW, Geng Y, Zhang LL, Li LH. Role of AGEs in the progression and regression of atherosclerotic plaques. Glycoconj J. 2018;35:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Schalkwijk CG, Micali LR, Wouters K. Advanced glycation endproducts in diabetes-related macrovascular complications: focus on methylglyoxal. Trends Endocrinol Metab. 2023;34:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Han L, Li L, Li B, Zhao D, Li Y, Xu Z, Liu G. Review of the characteristics of food-derived and endogenous ne-carboxymethyllysine. J Food Prot. 2013;76:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Farhangi MA, Dehghan P, Namazi N. Prebiotic supplementation modulates advanced glycation end-products (AGEs), soluble receptor for AGEs (sRAGE), and cardiometabolic risk factors through improving metabolic endotoxemia: a randomized-controlled clinical trial. Eur J Nutr. 2020;59:3009-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261:4889-4894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 591] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Chen G. Dietary N-epsilon-carboxymethyllysine as for a major glycotoxin in foods: A review. Compr Rev Food Sci Food Saf. 2021;20:4931-4949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Jia W, Guo A, Zhang R, Shi L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023;404:134541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 11. | Luévano-Contreras C, Gómez-Ojeda A, Macías-Cervantes MH, Garay-Sevilla ME. Dietary Advanced Glycation End Products and Cardiometabolic Risk. Curr Diab Rep. 2017;17:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Yan J, Li L, Liu N, Liang Y, Yuan W, Chen X. Effects of Nε-carboxymethyl-Lysine on ERS-mediated apoptosis in diabetic atherosclerosis. Int J Cardiol. 2014;172:e478-e483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wang Z, Bao Z, Ding Y, Xu S, Du R, Yan J, Li L, Sun Z, Shao C, Gu W. Nε-carboxymethyl-lysine-induced PI3K/Akt signaling inhibition promotes foam cell apoptosis and atherosclerosis progression. Biomed Pharmacother. 2019;115:108880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Li Y, Huang X, Yang G, Xu K, Yin Y, Brecchia G, Yin J. CD36 favours fat sensing and transport to govern lipid metabolism. Prog Lipid Res. 2022;88:101193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 15. | Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:281-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 465] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 16. | Egaña-Gorroño L, López-Díez R, Yepuri G, Ramirez LS, Reverdatto S, Gugger PF, Shekhtman A, Ramasamy R, Schmidt AM. Receptor for Advanced Glycation End Products (RAGE) and Mechanisms and Therapeutic Opportunities in Diabetes and Cardiovascular Disease: Insights From Human Subjects and Animal Models. Front Cardiovasc Med. 2020;7:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 17. | Asadipooya K, Lankarani KB, Raj R, Kalantarhormozi M. RAGE is a Potential Cause of Onset and Progression of Nonalcoholic Fatty Liver Disease. Int J Endocrinol. 2019;2019:2151302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Poznyak AV, Nikiforov NG, Starodubova AV, Popkova TV, Orekhov AN. Macrophages and Foam Cells: Brief Overview of Their Role, Linkage, and Targeting Potential in Atherosclerosis. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Xu Z, Li X, Ding Z, Zhang Y, Peng Z, Yang X, Cao W, Du R. LRPPRC inhibits autophagy and promotes foam cell formation in atherosclerosis. FEBS J. 2022;289:7545-7560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Leerach N, Munesue S, Harashima A, Kimura K, Oshima Y, Kawano S, Tanaka M, Niimura A, Sakulsak N, Yamamoto H, Hori O, Yamamoto Y. RAGE signaling antagonist suppresses mouse macrophage foam cell formation. Biochem Biophys Res Commun. 2021;555:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Fernando S, Salagaras T, Schwarz N, Sandeman L, Tan JTM, Xie J, Zareh J, Jensen K, Williamson A, Dimasi C, Chhay P, Toledo-Flores D, Long A, Manavis J, Worthington M, Fitridge R, Di Bartolo BA, Bursill CA, Nicholls SJ, Proud CG, Psaltis PJ. Eukaryotic elongation factor 2 kinase regulates foam cell formation via translation of CD36. FASEB J. 2022;36:e22154. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 22. | Maguire EM, Pearce SWA, Xiao Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vascul Pharmacol. 2019;112:54-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 23. | Xu S, Li L, Yan J, Ye F, Shao C, Sun Z, Bao Z, Dai Z, Zhu J, Jing L, Wang Z. CML/CD36 accelerates atherosclerotic progression via inhibiting foam cell migration. Biomed Pharmacother. 2018;97:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Sun Z, Li L, Yan Z, Zhang L, Zang G, Qian Y, Wang Z. Circadian rhythm disorders elevate macrophages cytokines release and promote multiple tissues/organs dysfunction in mice. Physiol Behav. 2022;249:113772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Xu H, Wang Z, Wang Y, Hu S, Liu N. Biodistribution and elimination study of fluorine-18 labeled Nε-carboxymethyl-lysine following intragastric and intravenous administration. PLoS One. 2013;8:e57897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Morris GM, Huey R, Olson AJ. Using AutoDock for ligand-receptor docking. Curr Protoc Bioinformatics. 2008;Chapter 8:Unit 8.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 621] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 27. | Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D'Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457-34468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Prasad K. AGE-RAGE Stress and Coronary Artery Disease. Int J Angiol. 2021;30:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Liang B, Zhou Z, Yang Z, Liu J, Zhang L, He J, Li H, Huang Y, Yang Q, Xian S, Wang L. AGEs-RAGE axis mediates myocardial fibrosis via activation of cardiac fibroblasts induced by autophagy in heart failure. Exp Physiol. 2022;107:879-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 30. | Wang Z, Li L, Du R, Yan J, Liu N, Yuan W, Jiang Y, Xu S, Ye F, Yuan G, Zhang B, Liu P. CML/RAGE signal induces calcification cascade in diabetes. Diabetol Metab Syndr. 2016;8:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Jandeleit-Dahm K, Watson A, Soro-Paavonen A. The AGE/RAGE axis in diabetes-accelerated atherosclerosis. Clin Exp Pharmacol Physiol. 2008;35:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Qin Q, Niu J, Wang Z, Xu W, Qiao Z, Gu Y. Heparanase induced by advanced glycation end products (AGEs) promotes macrophage migration involving RAGE and PI3K/AKT pathway. Cardiovasc Diabetol. 2013;12:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Feng Z, Zhu L, Wu J. RAGE signalling in obesity and diabetes: focus on the adipose tissue macrophage. Adipocyte. 2020;9:563-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Xu SN, Zhou X, Zhu CJ, Qin W, Zhu J, Zhang KL, Li HJ, Xing L, Lian K, Li CX, Sun Z, Wang ZQ, Zhang AJ, Cao HL. Nϵ-Carboxymethyl-Lysine Deteriorates Vascular Calcification in Diabetic Atherosclerosis Induced by Vascular Smooth Muscle Cell-Derived Foam Cells. Front Pharmacol. 2020;11:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Yang M, Silverstein RL. CD36 signaling in vascular redox stress. Free Radic Biol Med. 2019;136:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Zhu W, Li W, Silverstein RL. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood. 2012;119:6136-6144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Xia X, Hu T, He J, Xu Q, Yu C, Liu X, Shao Z, Liao Y, Huang H, Liu N. USP10 deletion inhibits macrophage-derived foam cell formation and cellular-oxidized low density lipoprotein uptake by promoting the degradation of CD36. Aging (Albany NY). 2020;12:22892-22905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Alikhani M, Maclellan CM, Raptis M, Vora S, Trackman PC, Graves DT. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol. 2007;292:C850-C856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Chistiakov DA, Orekhov AN, Bobryshev YV. The impact of FOXO-1 to cardiac pathology in diabetes mellitus and diabetes-related metabolic abnormalities. Int J Cardiol. 2017;245:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Singh S, Siva BV, Ravichandiran V. Advanced Glycation End Products: key player of the pathogenesis of atherosclerosis. Glycoconj J. 2022;39:547-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 41. | Byun K, Yoo Y, Son M, Lee J, Jeong GB, Park YM, Salekdeh GH, Lee B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol Ther. 2017;177:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 42. | Cho S. CD36 as a therapeutic target for endothelial dysfunction in stroke. Curr Pharm Des. 2012;18:3721-3730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Xu L, Wang YR, Li PC, Feng B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016;15:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Yashima H, Terasaki M, Sotokawauchi A, Matsui T, Mori Y, Saito T, Osaka N, Kushima H, Hiromura M, Ohara M, Fukui T, Yamagishi SI. AGE-RAGE Axis Stimulates Oxidized LDL Uptake into Macrophages through Cyclin-Dependent Kinase 5-CD36 Pathway via Oxidative Stress Generation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Matsuo M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J Pharmacol Sci. 2022;148:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 46. | Yu XH, Tang CK. ABCA1, ABCG1, and Cholesterol Homeostasis. Adv Exp Med Biol. 2022;1377:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Xia X, Xu Q, Liu M, Chen X, Liu X, He J, Hu T, Yu C, Huang H, Liu S, Liu N. Deubiquitination of CD36 by UCHL1 promotes foam cell formation. Cell Death Dis. 2020;11:636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014;46:e99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |