Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.130
Peer-review started: October 12, 2022
First decision: November 6, 2022
Revised: November 26, 2022
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 15, 2023
Processing time: 154 Days and 3.4 Hours
Insulin resistance and pancreatic β-cell dysfunction are major pathological mechanisms implicated in the development and progression of type 2 diabetes (T2D). Beyond the detrimental effects of insulin resistance, inflammation and oxidative stress have emerged as critical features of T2D that define β-cell dysfunction. Predominant markers of inflammation such as C-reactive protein, tumor necrosis factor alpha, and interleukin-1β are consistently associated with β-cell failure in preclinical models and in people with T2D. Similarly, important markers of oxidative stress, such as increased reactive oxygen species and depleted intracellular antioxidants, are consistent with pancreatic β-cell damage in conditions of T2D. Such effects illustrate a pathological relationship between an abnormal inflammatory response and generation of oxidative stress during the progression of T2D. The current review explores preclinical and clinical research on the patho-logical implications of inflammation and oxidative stress during the development of β-cell dysfunction in T2D. Moreover, important molecular mechanisms and relevant biomarkers involved in this process are discussed to divulge a pathological link between inflammation and oxidative stress during β-cell failure in T2D. Underpinning the clinical relevance of the review, a systematic analysis of evidence from randomized controlled trials is covered, on the potential therapeutic effects of some commonly used antidiabetic agents in modulating inflammatory makers to improve β-cell function.
Core Tip: Elevated markers of inflammation and oxidative stress are related to β-cell dysfunction, the intracellular defense (antioxidant) mechanisms responsible for ameliorating some of these effects are significantly depleted during type 2 diabetes (T2D). Thus, beyond lowering glucose levels like most antidiabetic drugs, future research should invest in developing therapeutic agents to ameliorate inflammation and oxidative stress to improve blood control in patients with T2D.
- Citation: Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Basson AK, Pheiffer C, Kengne AP. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes 2023; 14(3): 130-146
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/130.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.130
Type 2 diabetes (T2D) is among the leading causes of death worldwide[1]. Latest global estimates indicate that one in ten adults are currently living with diabetes, of which over 90% of cases are attributed to T2D[2]. Insulin resistance and β-cell dysfunction are considered the major patho-physiological derangements in T2D. Insulin resistance is primarily associated with T2D, however, people with T1D have also been shown to develop insulin resistance mainly because of certain genetic factors or lifestyle modifications[2,3]. Generally, β-cell dysfunction indicates a compromised state of insulin secretion, while insulin resistance refers to the inability of insulin to exert its effects on target organs[3]. In a complex mechanism, insulin resistance and β-cell dysfunction promote elevated blood glucose levels and further drive the pathogenesis of T2D[4]. Changes in β-cell function occur during the early stages of diabetes development (the prediabetic stage) and gradually become worse with disease progression[5,6]. Thus, it has become imperative to delineate the pathological mechanisms driving β-cell dysfunction to alleviate complications linked with T2D, including those that implicate inflammation and oxidative stress.
Inflammation has long been considered the main component of diabetes[7,8]. During T2D, elevated blood glucose levels lead to an undesired inflammatory response, which may be exacerbated by inflammatory intermediaries produced by adipocytes and macrophages in adipose tissue[9]. This process may initiate the low-grade, chronic inflammatory state that induces injury to the pancreatic β-cells, subsequently causing inadequate insulin production, and leading to hyperglycemia[9]. As a result, uncontrolled inflammation has been positioned among the foremost factors in the pathogenesis of T2D[7,8]. Several reviews on the role of inflammation during β-cell dysfunction in T2D have been conducted. For example, Jo and Fang[10] reviewed evidence indicating that malfunctioning of the essential components of the inflammation, including helper T cells, cytotoxic T cells, and regulatory T cells may underpin pancreatic β cell failure in T2D. Sun et al[11] recently discussed that aberrant epigenetic signatures, including DNA methylation, chromatin accessibility, histone alteration, and non-coding RNAs orchestrate β-cell malfunction during embryonic growth and postnatal development, thus contributing to β cell dysfunction. These findings further highlight the pathological link between impaired metabolic function and alterations in molecular mechanisms that may lead to β cell dysfunction in T2D[11-13].
Oxidative stress is another factor that is consistently associated with β-cell destruction during the development of T2D[14,15]. Oxidative stress normally arises due to the excessive production of free radicals, especially reactive oxygen species (ROS) that severely affect the neutralizing capacity of intracellular antioxidants[14,15]. Generally, oxidative stress may induce its destructive effects through causing damage to DNA, proteins, and lipids. In fact, due to the dyslipidemic features of most patients with T2D, uncontrolled oxidative stress is associated with clustering of interconnected plasma lipid and lipoprotein anomalies that may aggravate diabetic complications[16]. Notably, due to the inherent low expression of antioxidant enzymes in pancreatic islets[17], the consequences of oxidative stress can have devastating effects on driving β-cell dysfunction during the development of diabetes[18]. Obesity or excessive fat accumulation within the pancreas are some of the major mechanisms that promote oxidative stress, insulin resistance and β-cell dysfunction in T2D[19,20]. Enhancement of intracellular antioxidants can be targeted to alleviate oxidative stress and improve β-cell function to combat diabetes-associated complications[21,22]. In fact, the pathological relationship between inflammation and oxidative stress can have devastating outcomes leading to the progression of T2D. These complications are distinctively linked with worsening of T2D-related abnormalities, including retinopathy, neuropathy, nephropathy, and damage to tissues[23].
The current review updates and critically discusses literature on the pathological implications of inflammation and oxidative stress during the development of β-cell dysfunction in T2D. Preclinical and clinical research, elucidating the mechanisms that orchestrate the link between inflammation and oxidative stress during the development and progression of T2D are discussed. Firstly, an overview on the link between insulin resistance and β-cell dysfunction is covered to highlight its detrimental effect during the worsening of T2D. Thereafter, different biomarkers of inflammation and oxidative stress are discussed for their relevance in monitoring disease severity. This information also remains important to develop therapeutic targets to alleviate β-cell dysfunction in T2D. To further contribute to the novelty and relevance of the discussed information, a systematic analysis of evidence from randomized controlled trials (RCTs) on the therapeutic effects of antidiabetic agents in modulating inflammatory or oxidative stress to improve β-cell function is also covered.
Findings from preclinical studies, especially animal models, remain important to accurately decipher or characterize pathological mechanisms implicated in the development of disease. Consistent with the main objective of the current review, this infers describing the potential biological processes and molecular mechanisms involving inflammation and oxidative stress during pancreatic β-cell dysfunction in T2D. However, it also remains important to uncover clinical data on the therapeutic effects of commonly used antidiabetic drugs like metformin in modulating inflammation and oxidative stress to protect against β-cell dysfunction in T2D.
Thus, a systematic search of major electronic engines and databases was done from inception until 18 November 2022 for relevant RCTs. To prioritize clinical relevance of the review, the search was restricted to RCTs reporting on the link between inflammation or oxidative stress and β-cell function in T2D. Medical Subject-Heading and text words such as “inflammation”, “oxidative stress”, “β-cells”, and “type 2 diabetes”, including their analogous synonyms and related words were tailored for the individual search engine or database. There was no restriction on the type of antidiabetic drug, with all RCTs reporting on the modulation of these drugs on inflammation or oxidative stress markers in patients with T2D included. The search focused on inflammatory markers such as C-reactive protein (CRP), fibrinogen, interleukins (IL)-6/IL-1β, and tumor necrosis factor alpha (TNF-α) as well as oxidative stress indicators like ROS, glutathione peroxidase (Gpx), superoxide dismutase (SOD), thioredoxin, and catalase (CAT) that have been linked with β-cell dysfunction in T2D.
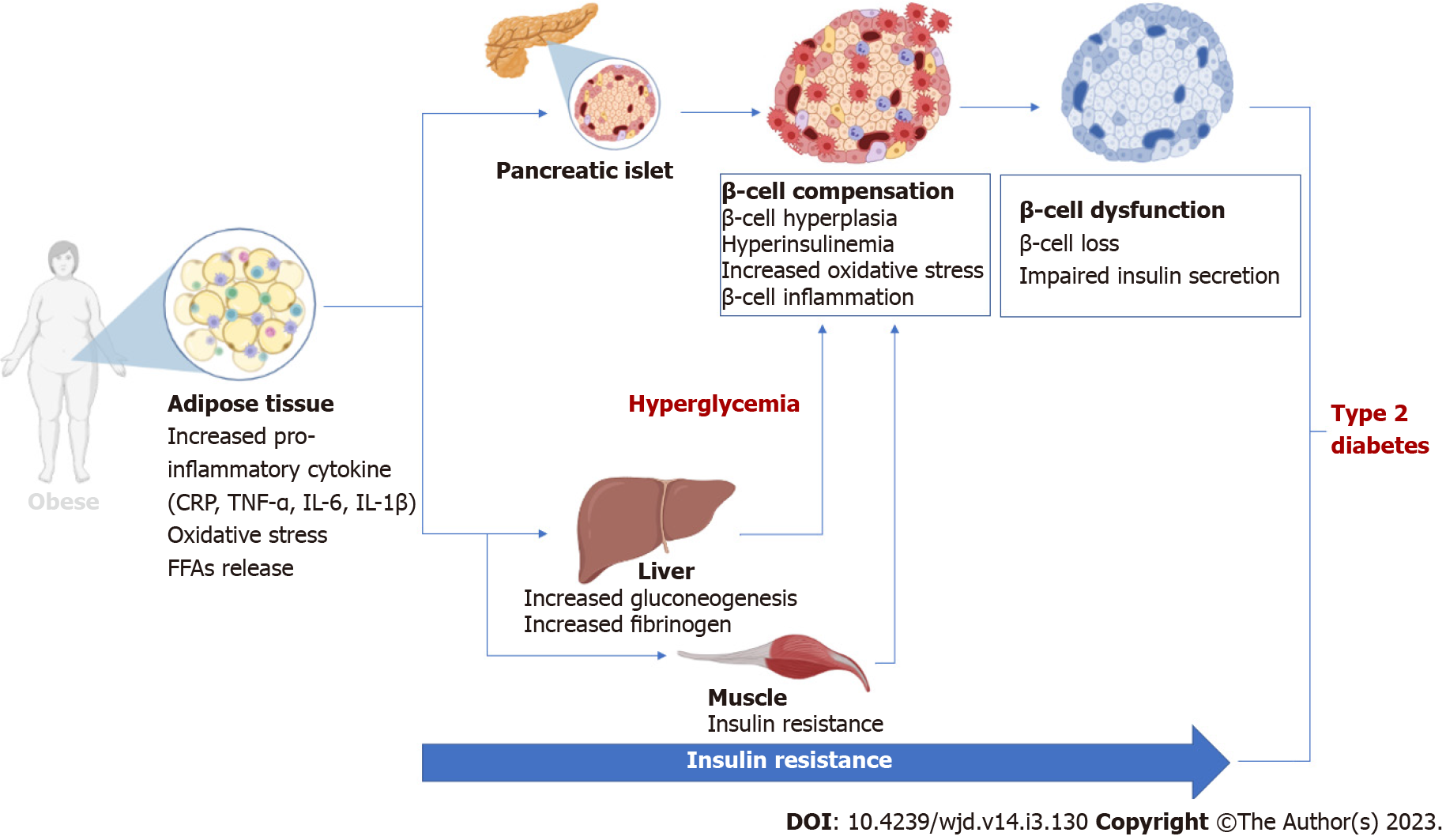
The pancreatic β-cells have the important function of producing and secreting insulin, a vital hormone that is necessary for the regulation of metabolism. Indeed, insulin is critical for the metabolic regulation of key energy substrates such as carbohydrates, lipids, and proteins. Insulin is required for the absorption of glucose from the blood stream into different cells, including cells from adipose tissue, skeletal muscle, and the liver. Thus, exploring the role of insulin in a broad spectrum of physiological processes including its production and regulation has relevance in understanding the development of T2D[24]. Notably, disturbances in insulin signaling through the inhibition of the insulin receptor substrate protein, phosphoinositide-3-kinase, and protein kinase B (AKT) leads to insulin resistance[25]. The latter is consistently associated with obesity, a major risk factor for T2D. Abnormal adipose tissue expansion causes elevated circulating levels of non-esterified fatty acids, glycerol, markers of oxidative stress, and pro-inflammatory cytokines, subsequently leading to the development of insulin resistance in individuals with obesity[26,27]. In fact, there is a close association between insulin resistance, obesity, and T2D[28]. Insulin resistance, obesity and pancreatic β-cell dysfunction are complex pathological mechanisms implicated in the progression of T2D (Figure 1). Both pathogenic states induce hyp-erglycemia and therefore increases insulin demand. Subsequently, β-cell dysfunction arises from insufficient glucose sensing to stimulate insulin secretion, hence increased glucose levels persist. This process leads to the development of insulin resistance, increased glucose concentrations beyond the physiological state, thereby resulting in the manifestation of hyperglycemia. As a result, β-cells compensate for insulin resistance by hypersecretion of insulin, ultimately leading to β-cell failure[29]. β-cell dysfunction follows insulin resistance during the development and progression of T2D. In fact, both pathological states influence each other and likely synergistically worsens diabetes[29]. Maintaining β-cell function and insulin signaling in patients with T2D is vital for controlling glucose homeostasis. As such, alleviating the detrimental effects of inflammation has become a critical feature to protect and maintain β-cell function in conditions of T2D[4,30].
Inflammation is generally classified as a localized response to cellular or tissue injury that is consistent with increased blood flow, leucocyte intrusion, and enhanced production of diverse chemical mediators. This response is necessary to prompt the removal of toxic agents and the restoration of injured tissue. It is now well accepted that chronic inflammation is coupled with insulin resistance and β-cell dysfunction in patients with T2D[4,30]. Adipose tissue expansion is known to play a major role during this process, with inflammation characterized by enhanced levels of macrophages and increased secretion of inflammatory cytokines[31,32]. Briefly, TNF-α, as well as IL-1β, and IL-6 are considered some of the prominent pro-inflammatory markers in the pathogenesis of T2D[31-33]. Beyond the elevated markers of inflammation, immune dysfunction is also an essential component of inflammation that has been implicated in β-cell failure in T2D.
Epidemiologic studies have reported a close relationship between elevated biomarkers of inflammation and the worsening of T2D and its complications[34,35]. Obesity, which is common in T2D, is well-acknowledged to be the main driver of the pathological consequences inflammation[36]. Obesity is responsible for the initiation of chronic systemic inflammation, with this feature characterized by the activation of the innate immune system in adipose tissue. This outcome prompts the systemic acute-phase response which is distinguished by elevation of acute-phase protein levels. For example, CRP is considered one of the predominant markers of systemic inflammation. Indeed, previous research have indicated that elevated levels of high-sensitive CRP (hs-CRP) are strongly associated with advanced β-cell dysfunction and insulin resistance in patients with T2D[37,38]. Although considered of hepatic origin, macrophages and T cells are known to be the main activators CRP levels in response to inflammation. Conventionally exploited as an indicator of infection and cardiovascular events[39], accumulating evidence show that CRP plays a vital role in diverse inflammatory mechanisms including the complement pathway, cell death, autophagy, nitric oxide modulation, and the production of cytokines, especially IL-6 and TNF-α[40-42]. Serum concentrations of CRP and other pro-inflammatory cytokines like IL-6 and TNF-α, have been found to be significantly elevated in rats with T2D[43]. Interestingly, such effects occurred concurrently with damage to islets, islet atrophy and β-cell failure in these diabetic animals. Mechanistically, CRP has been reported to promote tissue injury and accelerate apoptosis, through inducing pro-inflammatory mechanisms involving toll-like receptor 4, nuclear factor kappa B (NF-κB), transforming growth factor-β, and the extracellular signal-regulated kinase (ERK) pathway in preclinical models of diabetes[44,45]. Although not directly reporting on β-cell failure, studies in humans have linked enhanced circulatory levels of CRP with systemic inflammation, development and progression of T2D[46,47]. For example, Weber and colleagues showed that worsened low-grade inflammation (through increased levels of hs-CRP) and poor glycemic control were accompanied by reduced β-cell function in patients with T2D[48].
It has been more than two decades since it was reported that increased plasma levels of fibrinogen are associated with clinical complications of T2D[49]. Fibrinogen is a glycoprotein secreted by the liver and its levels in circulation depict systemic inflammation and tissue injury[50]. The secretion of this protein is crucial for coagulation, revascularization and wound restoration, and this process is mainly facilitated through its enzymatic conversion to fibrin by thrombin. In T2D, fibrinogen levels are positively associated with vascular complications[51-53]. Patients with T2D display elevated levels of fibrinogen, which is linked with poor blood glucose control and increased cardiovascular risk[54,55]. Beyond its role in systemic inflammation[56], fibrinogen levels are increased in rats treated with streptozotocin, a chemical substance known to destroy pancreatic β-cells[57]. Fibrinogen directly promotes profibrogenic and proinflammatory functions in pancreatic stellate cells[58]. These effects are consistent with the activation of pro-inflammatory mechanisms such as activation of NF-κB, three classes of mitogen-activated ERK, c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) in pancreatic cells. Although clinical evidence directly implicating fibrinogen in β-cell dysfunction is limited, enhanced levels of this protein (hyperfibrinogenemia) has been identified in patients with diabetes[59,60].
ILs are known for their diverse biological functions and are of pathological importance during the development of many diseases including T2D. Different interleukin family members have been studied and increasing evidence indicate the significance of these proteins in connecting innate immunity with diverse diseases including inflammatory conditions[41]. Varied IL, including both IL-6 and IL-1β, play a central role in modulating inflammatory responses, especially during the development of metabolic disease[61,62]. Briefly, IL-1β, which is produced by stimulated macrophages is crucial for innate immune regulation (which is considered the first line of defense against invading pathogens). Through its interaction with pattern recognition receptors, the 1β-processing platform is vital for initiating signaling pathways that induce the inflammatory response and regulates adaptive immunity[63]. Notably, IL-6 can induce differentiation of naïve CD4+ T cells, further playing a major role in modulating the acquired immune response[61]. Abnormally regulated levels of both IL-6 and IL-1β are associated with interruption of immunological tolerance and is thus pathologically implicated in the development of autoimmune and chronic inflammatory diseases[62,64-66].
Evidence suggests that IL-6 protects β-cells against oxidative damage through the effective modulation of autophagy and enhancing the antioxidant response. In fact, it was demonstrated that IL-6 couples’ autophagy to antioxidant responses leading to the reduction of ROS in β-cells and human islets[67]; whereas β-cell-specific blockage of IL-6 signaling in vivo causes mice to be more vulnerable to oxidative damage and cell death in response to exposure to selective β-cell toxins such as streptozotocin and alloxan[67]. Similarly, others have reported that pretreating β-cells with IL-6 blocks apoptosis induced by pro-inflammatory cytokines, mainly through effective regulation of autophagy[68]. These results are also consistent with reduced IL-6 pathway signaling in islets from donors with T2D[68]. Although such protective effects are noted, others argue that β-cell specific production of IL-6 is consistent with the development of diabetes, downplaying the potential advantage of targeting IL-6 as a therapeutic target for diabetes[69]. However, unlike IL-6, IL-1β has been associated with the inhibition of β-cell function and activation of fatty acid synthase-triggered apoptosis in part by interacting with the transcription factor NF-κB[70]. IL-1β-secreting β cells were identified in pancreatic sections of patients with T2D but not in nondiabetic control subjects[70].
A landmark study by Hotamisligil et al[71] was instrumental in demonstrating the close relationship between TNF-α and the progression of diabetes. Their results showed that elevated levels of TNF-α within adipose tissue of rats was consistent with the development of insulin resistance, while the inhibition of this pro-inflammatory cytokine was associated with improved glucose control and insulin sensitivity. TNF-α belongs to a superfamily of type II transmembrane proteins comprising of the TNF homology domain and is acknowledged to play a major role in diverse cellular functions, especially the processes of immune response and inflammation. In various preclinical models of diabetes, including the genetically modified mouse model of T2D (db/db), the pancreatic islet cells display increased levels of chemokines and pro-inflammatory cytokines like TNF-α, when compared to nondiabetic controls[72]. In a Kurdish population, genetic polymorphisms of TNF-α have been linked with genetic predisposition to T2D[73]. Recombinant human TNF-α administration has been shown to acutely reduce basal plasma insulin levels but does not affect glucose-stimulated insulin secretion in patients with T2D[74]. Therapeutic targeted reduction in TNF-α levels has been associated with improved insulin sensitivity in patients with T2D[75]. More importantly, it is evident that TNF-α-activated pathways are responsible for inducing apoptotic cell death in pancreatic β-cells. Stephens et al[76] demonstrated that caspase activation is the prevailing mechanism of TNF-α-induced cell death in NIT-1 cells (an insulin-secreting mouse cell line). Others have shown that TNF-α, in combination with another pro-inflammatory cytokine, interferon-γ (IFN-γ) can induce pancreatic β-cell apoptosis by destructing highly controlled B-cell lymphoma 2 member proteins that are essential for efficient mitochondrial function[77]. Preclinical studies have laid an important foundation to clarify the pathological role of TNF-α in causing β-cell dysfunction in T2D[78], and such information is consistent with insulin resistance and progression of diabetes in clinical settings[79,80]. Although evidence on the detrimental effects of IL-1β, together with other pro-inflammatory markers like IFN-γ and TNF-α in pancreatic β-cell dysfunction is acknowledged[78,81].
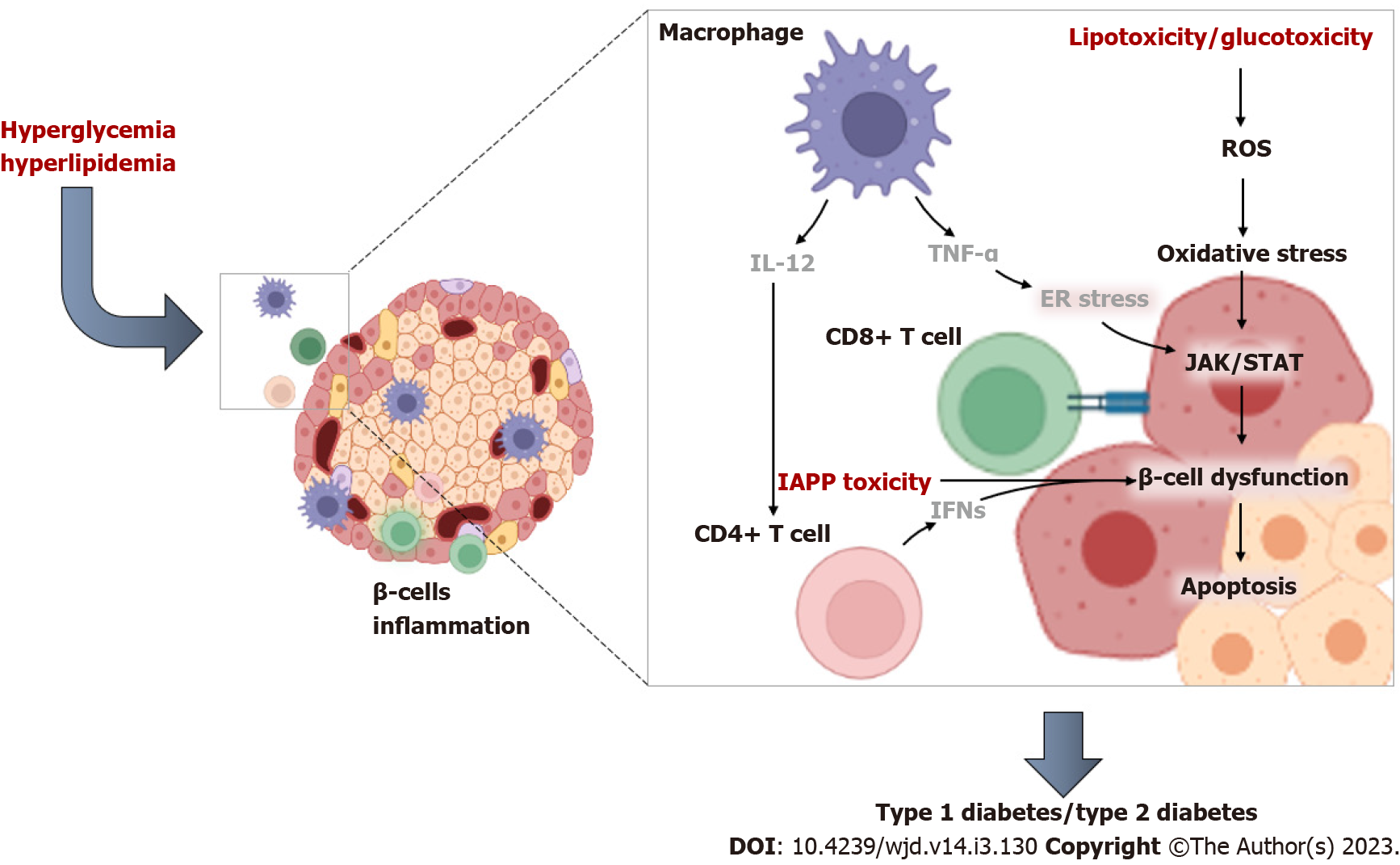
It is acknowledged that both innate and adaptive immune cells play a major role during pancreatic islet inflammation[82]. Innate immune cells produce cytokines that directly and indirectly modulate insulin secretion, whilst also stimulating inflammatory reactions. Macrophages and neutrophils, which physiologically inhabit the pancreatic tissue, can also partake in tissue homeostasis, including harmful activation of immune responses[82,83]. T cells are one of the significant white blood cells of the immune system and are crucial in the adaptive immune response. T-cells consist of two main subtypes, which are CD8+ “killer” and CD4+ “helper” T cells[84]. Regulatory T cells are yet another different subset of T cells that are required to support the mechanism of tolerance, whereby immune cells can recognize and differentiate between parent and invading cells. It is well known that diabetes impairs T cell function[85,86], although the precise mechanisms involved remain to be fully established. In fact, activated T-helper (TH)1 CD4+ T cells and CD8+ cytotoxic T cells have long been implicated in the destruction of pancreatic β-cells in diabetes[87]. Apparently, CD4+ T cells can be activated by IL-12 produced from macrophages and dendritic cells, and this consequence occurs as part of a vicious process involving cytotoxic T cells and recruitment by the pancreatic islets[87]. Notably, CD4+ T cells are instrumental in improving immune responses and their activation can lead to their differentiation into specific subtypes depending on the disease state[84]. Well-known subsets of CD4+ T cells include TH1, which are understood to promote β-cell damage by accelerating apoptosis[88]. Using electron microscopy, it was demonstrated that CD4+ T helper cells exhibit a much higher arrest (a cell jamming process) in the exocrine tissue than islet specific CD8+ T cells in diabetic mice[89]. With the overwhelming evidence supporting the notion that, like autoimmune diabetes, CD4+ (TH1 cell)-mediated inflammation and apoptosis may be the prominent features responsible for β-cell dysfunction and the aggravation T2D-associated complications[86,88,90,91]. Figure 2 highlights some of the pathophysiological mechanisms implicated in immune and T-cell activation during inflammation-mediated β-cell dysfunction in conditions of T2D.
It remains important to decipher the therapeutic mechanisms through which commonly used antidiabetic agents alleviate β-cell insult in conditions of T2D. Table 1 summarizes evidence from RCTs reporting on the effects of antidiabetics and other agents on β-cell function in patients with T2D. Here, the systematic search was focused on establishing the therapeutic link between β-cell function and regulation of circulating levels of prominent inflammatory makers, including hs-CRP, fibrinogen, IL-6, TNF-α, and T-cells. It emerged as early as in 1993 that metformin administration (up to a maximum of 850 mg three times a day for 12 wk) could improve glycemic control and β-cell function but had no effect on plasma fibrinogen concentrations and platelet function in patients with T2D[92]. Metformin is the most used antidiabetic drug with widely reported pleiotropic effects against complications linked with T2D[93,94]. Interestingly, predominantly included RCTs in Table 1 reported on the therapeutic effects of metformin in controlling basic metabolic profiles together with improving β-cell function and modulating inflammatory markers in patients with T2D. Evidence from different research groups published over the years (2006-2018) has indeed confirmed that metformin administration (from a dose between 1700-2000 mg/d) for a treatment duration of at least 6 mo could improve glycemic control, lipid profiles and β-cell function in part by reducing pro-inflammatory markers like hs-CRP and TNF-α in patients with T2D[37,95-99]. Notably, evidence from these RCTs suggest that metformin may be most effective in improving β-cell function in patients with T2D when combined with other antidiabetic drugs such as rosiglitazone, pioglitazone and vildagliptin (Table 1). This highlights the potential enhanced effects of metformin when combined with other therapies in treating compilations of T2D, as discussed elsewhere[100,101].
| Ref. | Study population | Intervention | Findings |
| Nagi and Yudkin[92], 1993 | Patients with T2D (n = 27), with an average age between 48 and 56 yr | Received metformin up to a maximum of 850 mg three times a day, for 12 wk | Improved glycemic control and β-cell function, while ameliorating insulin resistance and risk factors for cardiovascular disease, including plasminogen activator inhibitor-1. But had no effect on plasma fibrinogen concentrations and platelet function |
| Tsunekawa et al[102], 2003 | Patients with T2D (n = 17), with an average age of 67 yr | Received glimepiride started from 1 mg daily and increased up to 6 mg daily for 12 wk | Alleviated insulin resistance by decreasing plasma TNF-α levels and reducing those of adiponectin |
| Dominguez et al[103], 2005 | Patients with T2D (n = 10), with an average age of 53 yr | Received etanercept treatment at 25 mg subcutaneously twice weekly for 4 wk | Reduced plasma levels of CRP and interleukin-6 decreased, while also improving β -cell function |
| Pfützner et al[37], 2006 | Patients with T2D (n = 4270), with an average age of 64 yr | Received a combination therapy of peroxisome proliferator activated receptor g agonists and metformin. Disease duration was 5.4 ± 5.6 yr | Increased hs-CRP levels were associated beta-cell dysfunction but showed no correlation with disease duration or glucose control. Patients receiving combination therapy presented the lowest hs-CRP mean values |
| Hamann et al[95], 2008 | Patients with T2D (n = 294), with an average age of 58 yr | Received maximum tolerated doses of rosiglitazone 8 mg plus metformin 2 g/d during the first 12 wk of double-blind treatment for 52 wk | Fixed-dose combination therapy with rosiglitazone/metformin lowered glycated HbA1c and hs-CRP levels over one year of treatment. This was followed by improved beta-cell function suggest and glycaemic control |
| Pfützner et al[96], 2011 | Patients with T2D (n = 146), with an average age of 59 yr | Received a fixed dose combination of 15 mg of pioglitazone with 850 mg of metformin given twice daily for 24 wk | Improved biomarkers of lipid metabolism, β-cell function, activity of the visceral adipose tissue, and chronic systemic inflammation. This was consistent with reduced hs-CRP and increased adiponectin levels |
| Bellia et al[104], 2012 | Patients with T2D (n = 27), with an average age of 56 yr | Received receive either rosuvastatin 20 mg daily or simvastatin 20 mg daily for 6 mo | Effectively reduced hs-CRP levels, but significantly diminished glycemic control and insulin secretion, without affecting insulin sensitivity |
| Derosa et al[97], 2012 | Patients with T2D (n = 167), with an average age of 53 yr | Received metformin gradually titrated until a mean dosage of 2500 ± 500 mg/d was reached for 8 ± 2 mo. Thereafter, patients were randomly assigned to take, vildagliptin at 50 mg twice a day for 12 mo | A combination of metformin and vildagliptin showed better effect in reducing body weight, glycemic control, Homeostatic Model Assessment for Insulin Resistance and improving β-cell function. However, no significant effect was observed for TNF-α levels |
| Brooks-Worrell and Palmer[105], 2013 | Patients with T2D (n = 26), with an average age of between 54 and 58 yr | Received rosiglitazone at 4 mg once/day and increased to twice/day if glycaemic control (HbA1c 70%) not achieved. Glyburide was at 2.5 mg and increased to twice per day up to a maximum of 10 mg twice/day if glycaemic control not achieved | Rosiglitazone reduced islet-specific T cell responses and improved glucagon-stimulated-β-cell secretion, consistent to decreasing in interferon gamma production. This was accompanied by increased adiponectin levels in comparison to glyburide-treated patients |
| Gagnon et al[106], 2014 | Patients with T2D (n = 35), with an average age of 54 yr | Received a combination of calcium carbonate (1200 mg) and cholecalciferol [2000-6000 IU to target 25(OH)D 0.75 nmol/L] for 6 mo | Treatment did not affect glucose tolerance, inflammatory markers (including hs-CRP levels) and β-cell function in patients with T2D, but improved insulin sensitivity in subjects with prediabetes |
| Zografou et al[98], 2015 | Patients with T2D (n = 64), with an average age between 52 and 56 yr | Received metformin at 1700 mg/d plus vildagliptin at 100 mg/d for 6 mo | A combination of metformin and vildagliptin reduced hs-CRP and improved glycemic control and β-cell function |
| Tao et al[99], 2018 | Patients with T2D (n = 21), with an average of 29 yr | Received metformin at 2000 mg/d or saxagliptin at 5 mg/d for 24 wk | Treatment was comparatively effective at reducing body mass index and hs-CRP levels. This was parallel to improved glycemic control, lipid profiles and β-cell function |
| Zakerkish et al[107], 2019 | Patients with T2D (n = 50), with an average of 55 yr | Received Iranian propolis extract at 1000 mg/d for 90 d (3 mo) | Reduction on hs-CRP corresponded with beneficial effects of the extract in decreasing post prandial blood glucose, serum insulin, insulin resistance, and other inflammatory cytokines like TNF-α |
Furthermore, evidence summarized in Table 1 indicate that other antidiabetic therapies can also improve β-cell function while modulating inflammatory markers in patients with T2D. For example, Tsunekawa et al[102] showed that administration of glimepiride (started from 1 mg daily and increased up to 6 mg daily for 12 wk) could ameliorate insulin resistance by decreasing plasma levels of TNF-α in patients with T2D. Dominguez et al[103] demonstrated that the TNF-α blocker, etanercept (received at 25 mg subcutaneously twice weekly for 4 wk) could reduce plasma levels of CRP and IL-6 to improve β-cell function in patients with T2D. Such potential beneficial effects in improving β-cell function through the modulation of pro-inflammatory markers (especially hs-CRP and TNF-α) were confirmed through daily administration of other therapeutic drugs like rosiglitazone (at 4 mg), rosuvastatin or simvastatin (at 20 mg), a combination of calcium carbonate (at 1200 mg) and cholecalciferol (at 75 nmol/L), and even extracts like Iranian propolis extract (at 1000 mg) in patients with T2D[104-107]. These studies further indicate that interventions that block pro-inflammatory markers, especially hs-CRP and TNF-α levels, are likely to improve β -cell function in patients with T2D.
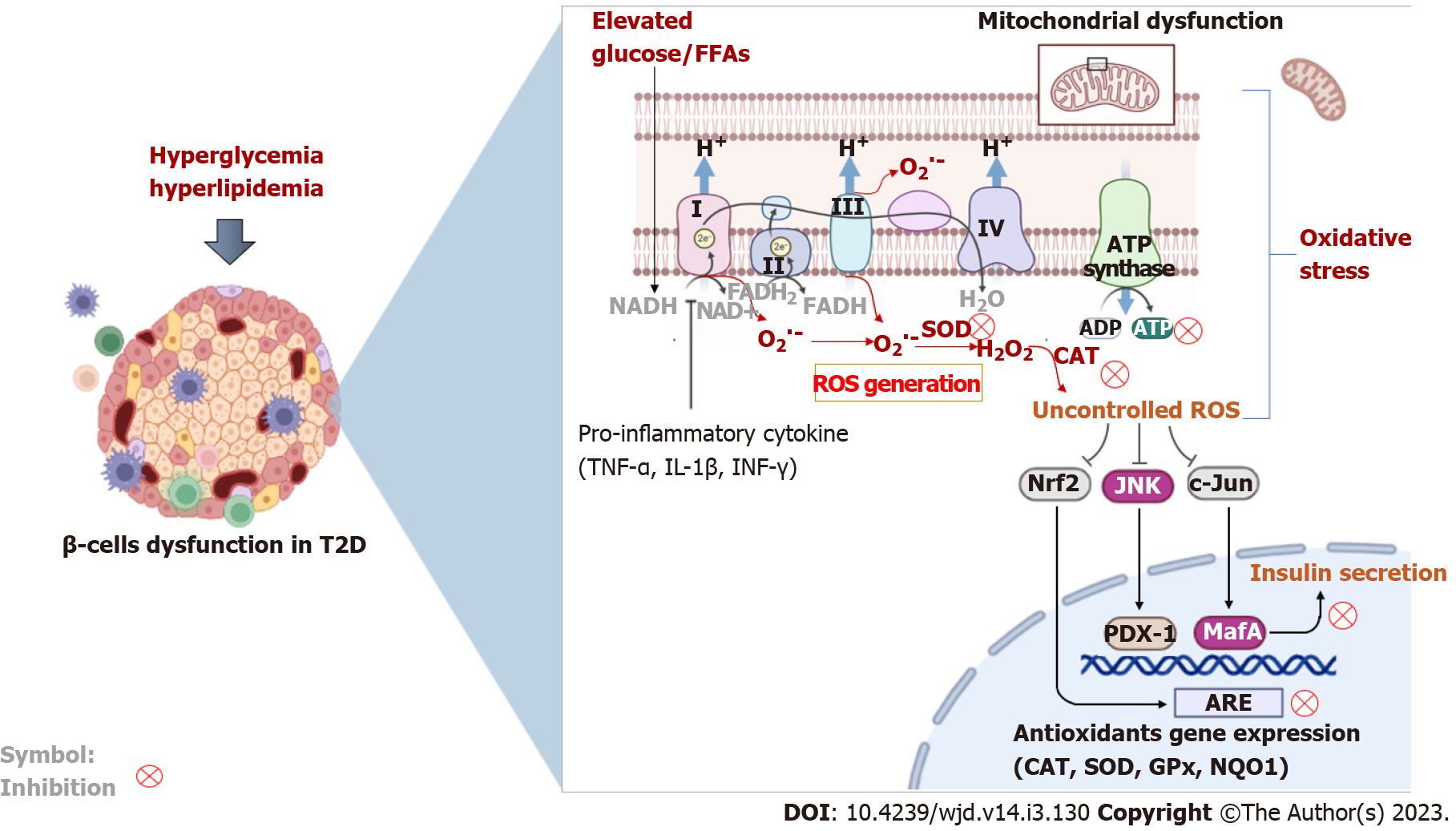
Oxidative stress has emerged as a critical feature involved in health (physiology) or disease (path-ophysiology)[108]. Oxidative stress is caused by the excessive production of free radical molecules (especially ROS) in response to severely diminished intracellular antioxidants. While ROS may be necessary for intracellular signaling[109], even during pathological conditions like cancer[110], this process is associated with unprecedented damage to many cellular processes during conditions like diabetes[23]. If uncontrolled, excess production of ROS can cause damage to DNA, cellular lipids and proteins, resulting in deteriorated metabolic function[14]. Although it can be sourced from different cellular compartments, the mitochondrial electron transport chain remains the major source of ROS within preclinal models and human systems[111]. The pancreatic β-cells contain mitochondria, which is vital for the regulation of glucose-stimulated insulin release by coupling glucose metabolism to insulin exocytosis[112]. Sustained exposure to hyperglycemic conditions has been associated with impaired β-cell dysfunction through diverse biochemical and molecular mechanisms that implicate impaired oxidation phosphorylation, enhanced production of advanced glycation end products, and abnormal activation of protein kinase C, as well as the polyol and hexosamine pathways[14]. Defects in mitochondrial function are consistent with impaired metabolic function, which can ultimately result in accelerated apoptosis and β-cell death[111,113,114]. Likewise, severely depleted levels of intracellular antioxidants, such as Gpx, SOD, thioredoxin, and CAT have been linked with β-cell dysfunction in T2D, as highlighted in Figure 3.
Pancreatic β-cells are susceptible to oxidative damage through enhanced production of ROS. Glucose exposure can significantly increase ROS production and in turn cause damage to cultured β-cell-derived cells[115]. These effects were linked with activation of pro-inflammatory mechanisms involving JNK and MAPKs, leading to accelerated apoptosis and reduced β-cell mass[115]. This outcome further highlights the strong association between oxidative stress and inflammation during β-cell insults in conditions such as glucotoxicity. Diverse ROS molecules are actively studied for their detrimental effects in aggravating diabetes-associated complications, and these include superoxide (O2∙-) and hydroxyl (∙OH) radicals, as well as hydrogen peroxide (H2O2)[14,108]. These ROS can cause chain activation of other free radicals, further driving the pathological features of oxidative stress. Nicotinamide adenine dinucleotide phosphate oxidases (NOX), existing in different isoforms depending on the specific type of tissue, is the prominent enzyme responsible for the generation of ROS, especially O2∙−, although H2O2 can also be produced[116]. Previous evidence indicated that NOX-derived ROS generation was responsible for accelerated apoptosis in cultured pancreatic β-cells (NIT-1 cells)[117]. Consistent with other findings[115], these effects were associated with inflammatory pathways involving activation of JNK and inhibition of AKT, which are required for insulin signaling. In fact, through its interaction with mammalian target of rapamycin complex 1, AKT plays a major role during β-cell cycle development in part by regulating the activity of cyclins D2, D3 and cdk4/cyclin D[118]. Other findings have supported the detrimental effects of ROS in mediating pancreatic β-cell death, especially through the activation of stress-activated protein kinases, mitochondrial dysfunction, p38 and JNK, and MAPKs resulting in reduced glucose-stimulated insulin secretion[119-121].
Several studies have investigated the correlation between markers of oxidative stress and β-cell dysfunction in individuals with T2D or related metabolic complications. To highlight the close association between inflammation and oxidative stress, it was demonstrated that markers of Th1/Th2 cytokines and oxidative stress markers were significantly increased in patients with T2D when compared to controls[122]. These findings were consistent with reduced levels of nuclear factor-erythroid factor 2-related factor 2 (Nrf2) and its downstream targets in peripheral blood mononuclear cells of diabetic patients. In many experimental models of T2D, Nrf2 is considered a master regulator of cellular survival, and its increased levels are instrumental to counteract the damaging effects of oxidative stress[123,124]. Reviewed evidence have further indicated that Nrf2 is essential for maintenance of β-cell mass to support the survival, function, and proliferation of β-cells[125]; whereas activation or upregulation of Nrf2 is necessary to diffuse inflammation, improve insulin sensitivity, decrease body weight, and protect against β-cell insult[125]. Thus, different therapeutic agents are entering clinical trials and being tested for their beneficial effects on β-cell survival and function by lowering markers of oxidative stress and promoting the antioxidant response in patients with metabolic disease and T2D[126-129].
Pancreatic β cells are known to exhibit intrinsically low intracellular antioxidative capacity when compared to other tissues within the body[130]. Notably, Gpx, SOD, thioredoxins, and CAT are some of the prominent intracellular antioxidants that are important in protecting against oxidative insults to pancreatic β-cells[21]. The reduced expression of these antioxidants within the pancreas is pathologically implicated in ROS-induced β-cell damage. Importantly, it has been demonstrated that increasing intrinsic antioxidant defenses, through over-expressing Gpx-1 could protect β-cells from db/db mice against hyperglycemic insult[131]. Gpx is known for its high affinity to neutralize lipid hydroperoxides, with its low serum levels linked with oxidative stress and the progression of T2D[132]. The other isoforms of this enzyme, like Gpx-4, can salvage pancreatic β-cell death by reducing pro-inflammatory cytokines in pancreatic islets isolated from rats[133]. Over-expression of this glutathione-derived enzyme was very useful in alleviating dysregulated islet insulin production and secretion, mainly though acting on pancreatic and duodenal homeobox 1 (PDX-1) and uncoupling protein 2 (UCP-2), in high fat diet-fed mice[134]. PDX-1 is a transcriptional factor necessary for pancreatic development while UCP-2 is important for the detoxification of ROS through improved mitochondrial function. All these findings, highlight the significant role Gpx plays in detoxifying oxidative stress to improve β-cell function and insulin secretion under hyperglycemic conditions[135]. Ultimately, the genetic elimination of both Gpx-1 and SOD-1 can exert different influences on murine islet function and pancreatic integrity[136]. Even worse, this results in significantly reduced plasma insulin concentrations and islet β-cells mass, which also correlate with increased blood glucose, and blocked glucose-stimulated insulin secretion. These effects are orchestrated mainly through elevation of ROS levels within the pancreatic islets, especially the concentrations of O2∙− and H2O2, leading to p53 phosphorylation[136]. While this is related to the diminished role of SOD, known for its neutralizing effects on ROS by eliminating O2∙−; phosphorylation of p53 has evolved the capability to incorporate unique environmental signals that facilitate DNA damage[137].
Beyond the effects of Gpx1 and SOD, the thioredoxin reductase-dependent mechanism is another essential mechanism necessary for the detoxification of H2O2-induced β-cell dysfunction[138]. Thioredoxins mainly act by reducing oxidized cysteine residues, with elevated levels of this enzyme linked to increased levels of circulating non-esterified fatty acids in patients with T2D[139]. Other findings indicate that elevated thioredoxin-interacting protein (TXNIP) levels correlate with increased β-cell apoptosis[140]; whereas TXNIP deficiency safeguards against T2D by promoting β-cell survival[141]. In fact, TXNIP is considered essential for the regulation of pancreatic β-cell function and other complications of T2D[142,143]. Another important enzyme, CAT, has long established to protect transgenic mouse β-cells by neutralizing H2O2[144]. Indeed, CAT mainly acts by converting H2O2 to water and oxygen. Accumulating evidence indicate that increased levels of this enzyme is important for pancreatic β-cell protection, while maintaining insulin secreting function in conditions of T2D[144-146]. Such evidence is consistent with other reviews highlighting the susceptibility of islets to oxidative damage, and the importance of intracellular antioxidant enzymes in protecting β-cells against diabetic insults[21,147].
Currently, diverse pathological mechanisms are acknowledged to be involved in the development and progression of T2D. Although both T1D and T2D are associated with β-cell dysfunction, insulin resistance and obesity are the prominent characteristic features for T2D. During a state of obesity, adipose tissue expansion is linked with the production of an array of pro-inflammatory markers that are involved in accelerating β-cell dysfunction. Such effects are consistent with impaired immune response, further driving insulin resistance and elevated blood glucose levels. Oxidative stress also concurrently occurs with inflammation and can cause havoc in many biochemical processes leading to pancreatic β-cell death. Even worse, while sustainably elevated markers of inflammation and oxidative stress are related to β-cell dysfunction, the intracellular defense (antioxidant) mechanisms responsible for ameliorating some of these effects are significantly depleted during T2D. There is limited clinical evidence supporting the beneficial effects of commonly used antidiabetic therapies in enhancing intracellular antioxidants to protect against β-cell dysfunction in T2D. The systematic analysis of RCTs supports the potential beneficial effects of metformin (especially when used in combination with other antidiabetic therapies like rosiglitazone, pioglitazone and vildagliptin) in improving β-cell function in part by reducing pro-inflammatory markers like hs-CRP and TNF-α in patients with T2D. Thus, beyond improving blood glucose control like with most antidiabetic drugs, future research should invest in developing therapies that can promote intracellular antioxidants and reduce markers of inflammation and oxidative stress to limit pancreatic β-cell failure in patients with T2D.
The work by Khanyisani Ziqubu, reported herein was made possible through partial funding by the South African Medical Research Council through its Division of Research Capacity Development under the Researcher Development Award Programme. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the funders.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: South Africa
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Adela R, India; Saisho Y, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | World Health Organization. The top ten leading causes of death. [cited 10 September 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. |
| 2. | International Diabetes Federation. IDF Diabetes Atlas Tenth Edition. [cited 10 September 2022]. Available from: https://diabetesatlas.org/. |
| 3. | Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J Diabetes Complications. 2018;32:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 4. | Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 543] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 5. | Saisho Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6:109-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 145] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (5)] |
| 6. | Do OH, Gunton JE, Gaisano HY, Thorn P. Changes in beta cell function occur in prediabetes and early disease in the Lepr (db) mouse model of diabetes. Diabetologia. 2016;59:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 689] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 8. | Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 581] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 9. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 517] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 10. | Jo S, Fang S. Therapeutic Strategies for Diabetes: Immune Modulation in Pancreatic β Cells. Front Endocrinol (Lausanne). 2021;12:716692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Sun X, Wang L, Obayomi SMB, Wei Z. Epigenetic Regulation of β Cell Identity and Dysfunction. Front Endocrinol (Lausanne). 2021;12:725131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Cuenco J, Dalmas E. Islet Inflammation and β Cell Dysfunction in Type 2 Diabetes. Handb Exp Pharmacol. 2022;274:227-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Narasimhan A, Flores RR, Robbins PD, Niedernhofer LJ. Role of Cellular Senescence in Type II Diabetes. Endocrinology. 2021;162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3617] [Article Influence: 241.1] [Reference Citation Analysis (0)] |
| 15. | Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24:547-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 854] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 16. | Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 472] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 17. | Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 842] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 18. | Drews G, Krippeit-Drews P, Düfer M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010;460:703-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 986] [Article Influence: 328.7] [Reference Citation Analysis (0)] |
| 20. | Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its clinical relevance: A systematic review, meta-analysis, and meta-regression. Metabolism. 2017;69:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 21. | Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on β cells and diabetes. Antioxid Redox Signal. 2011;14:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Wang J, Wang H. Oxidative Stress in Pancreatic Beta Cell Regeneration. Oxid Med Cell Longev. 2017;2017:1930261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 24. | Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19-39. [PubMed] |
| 25. | Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 992] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 26. | Wondmkun YT. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab Syndr Obes. 2020;13:3611-3616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 27. | Dludla PV, Nkambule BB, Jack B, Mkandla Z, Mutize T, Silvestri S, Orlando P, Tiano L, Louw J, Mazibuko-Mbeje SE. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients. 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 28. | Gołacki J, Matuszek M, Matyjaszek-Matuszek B. Link between Insulin Resistance and Obesity-From Diagnosis to Treatment. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 30. | Böni-Schnetzler M, Meier DT. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019;41:501-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 31. | Cerf ME. Beta Cell Physiological Dynamics and Dysfunctional Transitions in Response to Islet Inflammation in Obesity and Diabetes. Metabolites. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 2020;16:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 33. | Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 34. | Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur Cardiol. 2019;14:50-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 952] [Cited by in RCA: 834] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 35. | Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013;2013:678159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 36. | Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JP, Pinkney JH. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J. 2006;82:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Pfützner A, Standl E, Strotmann HJ, Schulze J, Hohberg C, Lübben G, Pahler S, Schöndorf T, Forst T. Association of high-sensitive C-reactive protein with advanced stage beta-cell dysfunction and insulin resistance in patients with type 2 diabetes mellitus. Clin Chem Lab Med. 2006;44:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | PrayGod G, Filteau S, Range N, Kitilya B, Kavishe BB, Ramaiya K, Jeremiah K, Rehman AM, Changalucha J, Olsen MF, Andersen AB, Friis H, Krogh-Madsen R, Faurholt-Jepsen D. β-cell dysfunction and insulin resistance in relation to pre-diabetes and diabetes among adults in north-western Tanzania: a cross-sectional study. Trop Med Int Health. 2021;26:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Ndevahoma F, Nkambule BB, Dludla PV, Mukesi M, Natanael KN, Nyambuya TM. The effect of underlying inflammation on iron metabolism, cardiovascular risk and renal function in patients with type 2 diabetes. EJHaem. 2021;2:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1708] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 41. | Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 42. | Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204-7218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2209] [Cited by in RCA: 2847] [Article Influence: 406.7] [Reference Citation Analysis (0)] |
| 43. | Sharma AK, Bharti S, Ojha S, Bhatia J, Kumar N, Ray R, Kumari S, Arya DS. Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr. 2011;106:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Sun W, Wu Y, Gao M, Tian Y, Qi P, Shen Y, Huang L, Shi L, Wang Y, Liu X. C-reactive protein promotes inflammation through TLR4/NF-κB/TGF-β pathway in HL-1 cells. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Zhang L, Shen ZY, Wang K, Li W, Shi JM, Osoro EK, Ullah N, Zhou Y, Ji SR. C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/β-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J. 2019;33:6551-6563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3027] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 47. | Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 561] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 48. | Weber KS, Nowotny B, Strassburger K, Pacini G, Müssig K, Szendroedi J, Herder C, Roden M; GDS Group. The Role of Markers of Low-Grade Inflammation for the Early Time Course of Glycemic Control, Glucose Disappearance Rate, and β-Cell Function in Recently Diagnosed Type 1 and Type 2 Diabetes. Diabetes Care. 2015;38:1758-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Barazzoni R, Zanetti M, Davanzo G, Kiwanuka E, Carraro P, Tiengo A, Tessari P. Increased fibrinogen production in type 2 diabetic patients without detectable vascular complications: correlation with plasma glucagon concentrations. J Clin Endocrinol Metab. 2000;85:3121-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Sarangi R, Padhi S, Mohapatra S, Swain S, Padhy RK, Mandal MK, Patro SK, Kumar S. Serum high sensitivity C-reactive protein, nitric oxide metabolites, plasma fibrinogen, and lipid parameters in Indian type 2 diabetic males. Diabetes Metab Syndr. 2012;6:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Emanuele N, Azad N, Abraira C, Henderson W, Colwell J, Levin S, Nuttall F, Comstock J, Sawin C, Silbert C, Marcovina S, Lee HS. Effect of intensive glycemic control on fibrinogen, lipids, and lipoproteins: Veterans Affairs Cooperative Study in Type II Diabetes Mellitus. Arch Intern Med. 1998;158:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Deng Y, Papageorgiou DP, Li X, Perakakis N, Mantzoros CS, Dao M, Karniadakis GE. Quantifying Fibrinogen-Dependent Aggregation of Red Blood Cells in Type 2 Diabetes Mellitus. Biophys J. 2020;119:900-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Le DS, Miles R, Savage PJ, Cornell E, Tracy RP, Knowler WC, Krakoff J. The association of plasma fibrinogen concentration with diabetic microvascular complications in young adults with early-onset of type 2 diabetes. Diabetes Res Clin Pract. 2008;82:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Bembde AS. A study of plasma fibrinogen level in type-2 diabetes mellitus and its relation to glycemic control. Indian J Hematol Blood Transfus. 2012;28:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Chen QF, Cao D, Ye TT, Deng HH, Zhu H. Peripheral Arterial Disease in Type 2 Diabetes Is Associated with an Increase in Fibrinogen Levels. Int J Endocrinol. 2018;2018:3709534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Sarangi S, Mahapatra S, Padhi S. Association of plasma fibrinogen and serum high-sensitivity C-reactive protein in type 2 diabetes mellitus. J Curr Res Sci Med. 2015;1:21-26. |
| 57. | Iwai S, Okazaki M, Nara K, Murakami H, Maruyama M, Kiuchi Y, Oguchi K. Altered fibrinogen and prothrombin mRNA expression in streptozotocin-induced diabetic rats. Showa Univ J Med Sci. 2000;12:295-302. [DOI] [Full Text] |
| 58. | Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Hamada S, Shimosegawa T. Fibrinogen induces cytokine and collagen production in pancreatic stellate cells. Gut. 2009;58:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Ye X, Huai J, Chen R, Ding J, Chen Y, Cai Z. Correlation of fibrinogen-like protein 2 with disease progression in patients with severe acute pancreatitis. Exp Ther Med. 2014;7:85-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 3183] [Article Influence: 289.4] [Reference Citation Analysis (0)] |
| 62. | Mahlangu T, Dludla PV, Nyambuya TM, Mxinwa V, Mazibuko-Mbeje SE, Cirilli I, Marcheggiani F, Tiano L, Louw J, Nkambule BB. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine. 2020;126:154892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Sahoo M, Ceballos-Olvera I, del Barrio L, Re F. Role of the inflammasome, IL-1β, and IL-18 in bacterial infections. ScientificWorldJournal. 2011;11:2037-2050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 64. | Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. 2015;125:2228-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 65. | Mahlangu TJ, Dludla PV, Mxinwa V, Mkandla Z, Tiano L, Louw J, Mutize T, Nyambuya TM, Nkambule BB. Elevated T-helper 2 cytokine levels in high fat diet-fed C57BL/6 mice are attenuated by short-term 6-week treatment with a combination of low-dose aspirin and metformin. Cytokine. 2020;128:154999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Martín-Sánchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J, Spiller D, White M, Daniels MJ, Mortellaro A, Peñalver M, Paszek P, Steringer JP, Nickel W, Brough D, Pelegrín P. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ. 2016;23:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 67. | Marasco MR, Conteh AM, Reissaus CA, Cupit JE 5th, Appleman EM, Mirmira RG, Linnemann AK. Interleukin-6 Reduces β-Cell Oxidative Stress by Linking Autophagy With the Antioxidant Response. Diabetes. 2018;67:1576-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 68. | Linnemann AK, Blumer J, Marasco MR, Battiola TJ, Umhoefer HM, Han JY, Lamming DW, Davis DB. Interleukin 6 protects pancreatic β cells from apoptosis by stimulation of autophagy. FASEB J. 2017;31:4140-4152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Van Belle TL, Pagni PP, Liao J, Sachithanantham S, Dave A, Bel Hani A, Manenkova Y, Amirian N, Yang C, Morin B, Zhang H, Campbell IL, von Herrath MG. Beta-cell specific production of IL6 in conjunction with a mainly intracellular but not mainly surface viral protein causes diabetes. J Autoimmun. 2014;55:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 444] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 71. | Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5334] [Cited by in RCA: 5421] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 72. | Ikeda H. KK mouse. Diabetes Res Clin Pract. 1994;24 Suppl:S313-S316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Darogha SN. Serum levels of TNF-a and IFN-g gene polymorphism in type 2 diabetes mellitus in kurdish patients. Cell Mol Biol (Noisy-le-grand). 2021;67:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Ibfelt T, Fischer CP, Plomgaard P, van Hall G, Pedersen BK. The acute effects of low-dose TNF-α on glucose metabolism and β-cell function in humans. Mediators Inflamm. 2014;2014:295478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Imanparast F, Javaheri J, Kamankesh F, Rafiei F, Salehi A, Mollaaliakbari Z, Rezaei F, Rahimi A, Abbasi E. The effects of chromium and vitamin D3 co-supplementation on insulin resistance and tumor necrosis factor-alpha in type 2 diabetes: a randomized placebo-controlled trial. Appl Physiol Nutr Metab. 2020;45:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Stephens LA, Thomas HE, Ming L, Grell M, Darwiche R, Volodin L, Kay TW. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology. 1999;140:3219-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Barthson J, Germano CM, Moore F, Maida A, Drucker DJ, Marchetti P, Gysemans C, Mathieu C, Nuñez G, Jurisicova A, Eizirik DL, Gurzov EN. Cytokines tumor necrosis factor-α and interferon-γ induce pancreatic β-cell apoptosis through STAT1-mediated Bim protein activation. J Biol Chem. 2011;286:39632-39643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 78. | Wang C, Guan Y, Yang J. Cytokines in the Progression of Pancreatic β-Cell Dysfunction. Int J Endocrinol. 2010;2010:515136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Blank SE, Johnson EC, Weeks DK, Wysham CH. Circulating dendritic cell number and intracellular TNF-α production in women with type 2 diabetes. Acta Diabetol. 2012;49 Suppl 1:S25-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Houjeghani S, Kheirouri S, Faraji E, Jafarabadi MA. l-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-α levels in patients with type 2 diabetes: a double-blind placebo-controlled randomized clinical trial. Nutr Res. 2018;49:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 81. | Alfadul H, Sabico S, Al-Daghri NM. The role of interleukin-1β in type 2 diabetes mellitus: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:901616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 82. | Citro A, Campo F, Dugnani E, Piemonti L. Innate Immunity Mediated Inflammation and Beta Cell Function: Neighbors or Enemies? Front Endocrinol (Lausanne). 2020;11:606332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Hilhorst M, Shirai T, Berry G, Goronzy JJ, Weyand CM. T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol. 2014;5:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Luckheeram RV, Zhou R, Verma AD, Xia B. CD4⁺T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 85. | Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. T-cell activation and cardiovascular risk in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin Immunol. 2020;210:108313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Xia C, Rao X, Zhong J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J Diabetes Res. 2017;2017:6494795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 87. | Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 88. | Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186:299-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Espinosa-Carrasco G, Le Saout C, Fontanaud P, Stratmann T, Mollard P, Schaeffer M, Hernandez J. CD4(+) T Helper Cells Play a Key Role in Maintaining Diabetogenic CD8(+) T Cell Function in the Pancreas. Front Immunol. 2017;8:2001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Almawi WY, Tamim H, Azar ST. Clinical review 103: T helper type 1 and 2 cytokines mediate the onset and progression of type I (insulin-dependent) diabetes. J Clin Endocrinol Metab. 1999;84:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Mxinwa V, Dludla PV, Nyambuya TM, Mokgalaboni K, Mazibuko-Mbeje SE, Nkambule BB. Natural killer cell levels in adults living with type 2 diabetes: a systematic review and meta-analysis of clinical studies. BMC Immunol. 2020;21:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors for cardiovascular disease, and plasminogen activator inhibitor in NIDDM subjects. A study of two ethnic groups. Diabetes Care. 1993;16:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 245] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 93. | Prattichizzo F, Giuliani A, Mensà E, Sabbatinelli J, De Nigris V, Rippo MR, La Sala L, Procopio AD, Olivieri F, Ceriello A. Pleiotropic effects of metformin: Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018;48:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 94. | Dludla PV, Nyambuya TM, Johnson R, Silvestri S, Orlando P, Mazibuko-Mbeje SE, Gabuza KB, Mxinwa V, Mokgalaboni K, Tiano L, Muller CJF, Louw J, Nkambule BB. Metformin and heart failure-related outcomes in patients with or without diabetes: a systematic review of randomized controlled trials. Heart Fail Rev. 2021;26:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 95. | Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with Type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Pfützner A, Schöndorf T, Tschöpe D, Lobmann R, Merke J, Müller J, Lehmann U, Fuchs W, Forst T. PIOfix-study: effects of pioglitazone/metformin fixed combination in comparison with a combination of metformin with glimepiride on diabetic dyslipidemia. Diabetes Technol Ther. 2011;13:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Derosa G, Ragonesi PD, Carbone A, Fogari E, D'Angelo A, Cicero AF, Maffioli P. Vildagliptin action on some adipocytokine levels in type 2 diabetic patients: a 12-month, placebo-controlled study. Expert Opin Pharmacother. 2012;13:2581-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Zografou I, Sampanis C, Gkaliagkousi E, Iliadis F, Papageorgiou A, Doukelis P, Vogiatzis K, Douma S. Effect of vildagliptin on hsCRP and arterial stiffness in patients with type 2 diabetes mellitus. Hormones (Athens). 2015;14:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Tao T, Wu P, Wang Y, Liu W. Comparison of glycemic control and β-cell function in new onset T2DM patients with PCOS of metformin and saxagliptin monotherapy or combination treatment. BMC Endocr Disord. 2018;18:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Prattichizzo F, Ceriello A. Is time ready for combination therapy at diagnosis of type 2 diabetes? Diabetes Metab Res Rev. 2021;37:e3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Dludla PV, Silvestri S, Orlando P, Gabuza KB, Mazibuko-Mbeje SE, Nyambuya TM, Mxinwa V, Mokgalaboni K, Johnson R, Muller CJF, Tiano L, Louw J, Nkambule BB. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-associated Complications: A Systematic Review of Preclinical Studies. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | Tsunekawa T, Hayashi T, Suzuki Y, Matsui-Hirai H, Kano H, Fukatsu A, Nomura N, Miyazaki A, Iguchi A. Plasma adiponectin plays an important role in improving insulin resistance with glimepiride in elderly type 2 diabetic subjects. Diabetes Care. 2003;26:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 104. | Bellia A, Rizza S, Lombardo MF, Donadel G, Fabiano R, Andreadi K, Quon MJ, Sbraccia P, Federici M, Tesauro M, Cardillo C, Lauro D. Deterioration of glucose homeostasis in type 2 diabetic patients one year after beginning of statins therapy. Atherosclerosis. 2012;223:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Brooks-Worrell BM, Palmer JP. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients. Clin Exp Immunol. 2013;171:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, Jean S, Ebeling PR. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and β-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS One. 2014;9:e109607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 107. | Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati AA, Neisi N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci Rep. 2019;9:7289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 108. | Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1959] [Cited by in RCA: 2332] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 109. | Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1501] [Cited by in RCA: 2331] [Article Influence: 333.0] [Reference Citation Analysis (0)] |
| 110. | Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 739] [Article Influence: 123.2] [Reference Citation Analysis (0)] |
| 111. | Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6484] [Cited by in RCA: 6081] [Article Influence: 380.1] [Reference Citation Analysis (0)] |
| 112. | Diane A, Al-Shukri NA, Bin Abdul Mu-U-Min R, Al-Siddiqi HH. β-cell mitochondria in diabetes mellitus: a missing puzzle piece in the generation of hPSC-derived pancreatic β-cells? J Transl Med. 2022;20:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 166] [Reference Citation Analysis (0)] |
| 114. | Bigagli E, Lodovici M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxid Med Cell Longev. 2019;2019:5953685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 115. | Hou N, Torii S, Saito N, Hosaka M, Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 116. | Tarafdar A, Pula G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 261] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 117. | Yuan H, Zhang X, Huang X, Lu Y, Tang W, Man Y, Wang S, Xi J, Li J. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of β-cells via JNK, p38 MAPK and p53 pathways. PLoS One. 2010;5:e15726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 118. | Balcazar Morales N, Aguilar de Plata C. Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb Med (Cali). 2012;43:235-243. [PubMed] |
| 119. | Pi J, Zhang Q, Andersen ME. Reactive Oxygen Species and Antioxidants in Pancreatic β-Cell Function – Yin and Yang. In: Laher I, editor. Systems Biology of Free Radicals and Antioxidants. Berlin, Heidelberg: Springer; 2014. |
| 120. | Ahmed Alfar E, Kirova D, Konantz J, Birke S, Mansfeld J, Ninov N. Distinct Levels of Reactive Oxygen Species Coordinate Metabolic Activity with Beta-cell Mass Plasticity. Sci Rep. 2017;7:3994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab. 2012;23:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 122. | Sireesh D, Dhamodharan U, Ezhilarasi K, Vijay V, Ramkumar KM. Association of NF-E2 Related Factor 2 (Nrf2) and inflammatory cytokines in recent onset Type 2 Diabetes Mellitus. Sci Rep. 2018;8:5126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 123. | Dludla PV, Muller CJ, Joubert E, Louw J, Essop MF, Gabuza KB, Ghoor S, Huisamen B, Johnson R. Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 124. | David JA, Rifkin WJ, Rabbani PS, Ceradini DJ. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J Diabetes Res. 2017;2017:4826724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 125. | Baumel-Alterzon S, Katz LS, Brill G, Garcia-Ocaña A, Scott DK. Nrf2: The Master and Captain of Beta Cell Fate. Trends Endocrinol Metab. 2021;32:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 126. | Constantin A, Dumitrescu M, Nemecz M, Picu A, Smeu B, Guja C, Alexandru N, Georgescu A, Tanko G. Sera of Obese Type 2 Diabetic Patients Undergoing Metabolic Surgery Instead of Conventional Treatment Exert Beneficial Effects on Beta Cell Survival and Function: Results of a Randomized Clinical Study. Obes Surg. 2019;29:1485-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Eckhoff DE, Smyth CA, Eckstein C, Bilbao G, Young CJ, Thompson JA, Contreras JL. Suppression of the c-Jun N-terminal kinase pathway by 17beta-estradiol can preserve human islet functional mass from proinflammatory cytokine-induced destruction. Surgery. 2003;134:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 128. | Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson PA, Moris L, Nilsson PM, Eriksson JW, Lind L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J Clin Lipidol. 2018;12:1390-1403.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 129. | Dandona P, Ghanim H, Abuaysheh S, Green K, Dhindsa S, Makdissi A, Batra M, Kuhadiya ND, Chaudhuri A. Exenatide Increases IL-1RA Concentration and Induces Nrf-2‒Keap-1‒Regulated Antioxidant Enzymes: Relevance to β-Cell Function. J Clin Endocrinol Metab. 2018;103:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 131. | Harmon JS, Bogdani M, Parazzoli SD, Mak SS, Oseid EA, Berghmans M, Leboeuf RC, Robertson RP. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855-4862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 132. | Goyal R, Singhai M, Faizy AF. Glutathione peroxidase activity in obese and nonobese diabetic patients and role of hyperglycemia in oxidative stress. J Midlife Health. 2011;2:72-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Krümmel B, Plötz T, Jörns A, Lenzen S, Mehmeti I. The central role of glutathione peroxidase 4 in the regulation of ferroptosis and its implications for pro-inflammatory cytokine-mediated beta-cell death. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 134. | Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 135. | Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363-12368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 136. | Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, Lei XG. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 2011;14:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 137. | Maclaine NJ, Hupp TR. The regulation of p53 by phosphorylation: a model for how distinct signals integrate into the p53 pathway. Aging (Albany NY). 2009;1:490-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 138. | Stancill JS, Broniowska KA, Oleson BJ, Naatz A, Corbett JA. Pancreatic β-cells detoxify H(2)O(2) through the peroxiredoxin/thioredoxin antioxidant system. J Biol Chem. 2019;294:4843-4853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 139. | Kakisaka Y, Nakashima T, Sumida Y, Yoh T, Nakamura H, Yodoi J, Senmaru H. Elevation of serum thioredoxin levels in patients with type 2 diabetes. Horm Metab Res. 2002;34:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 140. | Panse M, Kluth O, Lorza-Gil E, Kaiser G, Mühlbauer E, Schürmann A, Häring HU, Ullrich S, Gerst F. Palmitate and insulin counteract glucose-induced thioredoxin interacting protein (TXNIP) expression in insulin secreting cells via distinct mechanisms. PLoS One. 2018;13:e0198016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Lei Z, Chen Y, Wang J, Zhang Y, Shi W, Wang X, Xing D, Li D, Jiao X. Txnip deficiency promotes β-cell proliferation in the HFD-induced obesity mouse model. Endocr Connect. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 142. | Wondafrash DZ, Nire'a AT, Tafere GG, Desta DM, Berhe DA, Zewdie KA. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab Syndr Obes. 2020;13:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 143. | Gao Y, Chen S, Peng M, Wang Z, Ren L, Mu S, Zheng M. Correlation Between Thioredoxin-Interacting Protein and Nerve Conduction Velocity in Patients With Type 2 Diabetes Mellitus. Front Neurol. 2020;11:733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Xu B, Moritz JT, Epstein PN. Overexpression of catalase provides partial protection to transgenic mouse beta cells. Free Radic Biol Med. 1999;27:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 145. | Kralik PM, Xu B, Epstein PN. Catalase transfection decreases hydrogen peroxide toxicity in a pancreatic beta cell line. Endocr Res. 1998;24:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Wang N, Zhang J, Qin M, Yi W, Yu S, Chen Y, Guan J, Zhang R. Amelioration of streptozotocininduced pancreatic β cell damage by morin: Involvement of the AMPKFOXO3catalase signaling pathway. Int J Mol Med. 2018;41:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 147. | Karunakaran U, Park KG. A systematic review of oxidative stress and safety of antioxidants in diabetes: focus on islets and their defense. Diabetes Metab J. 2013;37:106-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |