Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.110
Peer-review started: September 16, 2022
First decision: November 18, 2022
Revised: November 26, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: February 15, 2023
Processing time: 151 Days and 9.9 Hours
In recent years, studies have found that the occurrence and development of diabetic cardiomyopathy (DCM) is closely related to an increase in polyadenosine diphosphate-ribose polymerase-1 (PARP-1) activity. PARP-1 activation could be involved in the pathophysiological process of DCM by promoting oxidative stress, the inflammatory response, apoptosis and myocardial fibrosis.
To investigate the mechanism of liraglutide in improving myocardial injury in type 2 diabetic rats, further clarified the protective effect of liraglutide on the heart, and provided a new option for the treatment of DCM.
Forty healthy male SD rats aged 6 wk were randomly divided into two groups, a normal control group (n = 10) and a model group (n = 30), which were fed an ordinary diet and a high-sugar and high-fat diet, respectively. After successful modeling, the rats in the model group were fed a high-glucose and high-fat diet for 4 wk and randomly divided into a model group and an intervention group (further divided into a high-dose group and a low-dose group). The rats were fed a high-glucose and high-fat diet for 8 wk and then started drug intervention. Blood samples were collected from the abdominal aorta to detect fasting blood glucose and lipid profiles. Intact heart tissue was dissected, and its weight was used to calculate the heart weight index. Haematoxylin and eosin staining was used to observe the pathological changes in the myocardium and the expression of PARP-1 in the heart by immunohistochemistry.
The body weight and heart weight index of rats in the model group were significantly increased compared with those in the normal control group, and those in the intervention group were decreased compared with those in the model group, with a more obvious decrease observed in the high-dose group (P < 0.05). In the model group, myocardial fibers were disordered, and inflammatory cells and interstitial fibrosis were observed. The cardiomyopathy of rats in the intervention group was improved to different degrees, the myocardial fibers were arranged neatly, and the myocardial cells were clearly striated; the improvement was more obvious in the high-dose group. Compared with the normal control group, the expression of PARP-1 in myocardial tissue of the model group was increased, and the difference was statistically significant (P < 0.05). After liraglutide intervention, compared with the model group, the expression of PARP-1 in myocardial tissue was decreased, and the reduction was more obvious in the high-dose group (P < 0.05) but still higher than that in the normal control group.
Liraglutide may improve myocardial injury in type 2 diabetic rats by inhibiting the expression of myocardial PARP-1 in a dose-dependent manner.
Core Tip: Low-dose streptozotocin combined with a high-glucose and high-fat diet can successfully establish a rat model of type 2 diabetes mellitus. After 4 wk of continuous feeding, myocardial injury can occur, which is consistent with diabetic cardiomyopathy. Liraglutide reduced the body weight of type 2 diabetic rats and significantly improved the fasting blood glucose and lipid profile in a dose-dependent manner. Liraglutide may improve myocardial injury in type 2 diabetic rats by inhibiting the expression of myocardial polyadenosine diphosphate-ribose polymerase-1 in a dose-dependent manner.
- Citation: Xue DD, Zhang X, Li DW, Yang YL, Liu JJ. Protective effect of liraglutide on the myocardium of type 2 diabetic rats by inhibiting polyadenosine diphosphate-ribose polymerase-1. World J Diabetes 2023; 14(2): 110-119
- URL: https://www.wjgnet.com/1948-9358/full/v14/i2/110.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i2.110
Diabetic cardiomyopathy (DCM) is a specific type of cardiomyopathy independent of hypertension, coronary heart disease, and congenital heart disease. The early manifestation of DCM is reduced left ventricular diastolic function, which can be combined with end-stage systolic dysfunction, increasing the risk of heart failure in patients with diabetes. Diabetes seriously affects the patient's life, and its incidence is increasing year by year. Its specific pathogenesis is complex and has not been fully elucidated[1-3]. Polyadenosine diphosphate ribose polymerase-1 (PARP-1) is a nuclear protein widely present in the nuclei of most eukaryotes. After activation, PARP-1 catalyzes nicotinamide adenine dinucleotide (NAD+) to form a poly-adenosine diphosphate ribose (PAR) chain and glycosylate and regulate the functions of histones, topoisomerases, DNA polymerases, p53, nuclear transcription factor (NF-κB) and other proteins, which are involved in DNA damage repair and other cellular functions. In recent years, studies have found that the occurrence and development of DCM is closely related to an increase in PARP-1 activity. PARP-1 activation could be involved in the pathophysiological process of DCM by promoting oxidative stress, the inflammatory response, apoptosis and myocardial fibrosis[4-6].
To date, it has been confirmed that glucagon-like peptide-1 (GLP-1) can reduce the apoptosis of islet microcirculation endothelial cells and protect islet tissue through the PARP-1/iNOs pathway[7], but whether GLP-1 can protect cardiomyocytes by inhibiting the expression of PARP-1 is still unclear. This experiment studied different doses of the liraglutide in rats with diabetic cardiomyopathy after treatment. Blood glucose, blood lipids and pathological changes in the heart were recorded in the observation group, and an immunohistochemical method was used to observe the expression of PARP-1 in cardiac tissue to further clarify the etiology and related mechanism of liraglutide in myocardial injury in type 2 diabetes.
Forty 6-week-old SPF male SD rats were purchased from the Animal Center of Shanxi Provincial People's Hospital [experimental animal license number: SYXK (Jin) 2019-0003], weighing 160-240 g, with 3-4 rats/cage. The feeding room was well ventilated, the room temperature was maintained at 18-22 °C, and the light was set in an alternating 12 h day and night cycle. The rats ate and drank freely. Animal experiments were conducted in strict accordance with the relevant regulations of experimental animal ethics.
Firstly, establishment of animal models: (1) Forty healthy male SD rats weighing 160-240 g were randomly divided into a normal control group (n = 10) and an experimental group (n = 30). They were fed an ordinary diet and a high-sugar and high-fat diet for 4 wk, respectively; (2) At the end of the fourth week of feeding, the rats in the experimental group fasted without water for 12 h overnight, and the next day, after fasting and weighing, 1% streptozotocin (STZ) was injected intraperitoneally at a dose of 40 mg/kg once, and the rats were fed a high-sugar and high-fat diet to ensure adequate drinking water and dry padding. Normal control rats were intraperitoneally injected with the same dose of normal saline. Three days after injection, if blood glucose of a random draw was more than 16.7 mmol/L and/or fasting blood glucose (FBG) was more than 11.1 mmol/L and polydipsia, polyuria, and hypereating were observed, the rats were considered type 2 diabetic rats. In the modeling process, 2 died, and 2 failed; and (3) After 4 wk of feeding with high glucose and high fat, the rats in the experimental group were randomly divided into a model group and an intervention group (further divided into a high-dose group and a low-dose group) for the follow-up experiment. Four rats died during feeding.
Secondly, intervention with drugs: The normal control group, the model group and the intervention group continued to receive the normal diet or the high-glucose and high-fat diet as before, and blood glucose level was monitored at random daily. The rats with a blood glucose level outside of the successful range of the model were excluded, and the drug intervention was started after blood glucose level had stabilized. Rats in the high-dose liraglutide group and low-dose liraglutide group were subcutaneously injected with 200 µg/kg and 100 µg/kg liraglutide, respectively, once a day for 8 wk. Rats in the normal control group and model group were subcutaneously injected with 100 µg/kg normal saline. During the intervention, the rats in the model group and the intervention group continued to be fed a high-glucose and high-fat diet, while the rats in the normal control group continued to be fed an ordinary diet.
Finally, specimen collection: (1) At the end of the 8th week of liraglutide intervention, tail venous blood was collected to measure the fasting blood glucose of rats in each group with a blood glucose meter, and the rats were weighed. Then, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g according to body weight), and the blood and heart tissues were collected; (2) Blood lipid test: The blood samples were left for half an hour and centrifuged at low speed for 15 min (4 °C, 3000 rpm), and the upper layer of serum was collected and stored in a -20 °C refrigerator. Serum was collected for the determination of serum lipids and lipoprotein-associated phospholipase A2 by an automatic multifunctional biochemical analyzer; (3) HE staining was used to observe the morphology of the myocardium: Cardiac pathological sections were prepared, and the sections were placed in xylene for 20 min for dewaxing and then placed in 100%, 95%, 85%, 75%, and 0% ethanol for 3 min each for hydration. After hydration, the sections were stained with hematoxylin for approximately 10 min, washed with water, differentiated with alcohol hydrochloric acid for a few seconds, washed with water, immersed in eosin for approximately 1 min and then washed again. Finally, the slices were dehydrated and cleared for a second time, and then the slices were sealed with glycerin gelatin. The morphological changes in the myocardium were observed under a microscope; and (4) The expression of PARP-1 in myocardial tissue was detected by immunohistochemistry: The heart wax block was sliced and heated at 60 °C for 2 h. Dewaxing and hydration were performed. For antigen repair, the sections were placed in 3% hydrogen peroxide solution for 15 min to block endogenous peroxidase activity and rinsed with phosphate buffer saline (PBS) 3 times for 2 min each time. A drop of blocking solution was added, excess liquid was removed, and the samples sat at room temperature for 20 min without washing. A 1:100 diluted primary antibody (rabbit IgG) was added by pipette, and the samples were stored at 4 °C overnight. After equilibration to room temperature for 20 min, the samples were washed 3 times with PBS (2 min each time), polymerized HRP-labeled anti-rabbit IgG was added, and the samples were incubated at 37 °C for 30 min and washed 3 times with PBS (5 min each time). Color development was followed by dehydration, transparency, sealing, and observation. The sections were observed under a light microscope, and images were collected.
One-way analysis of variance was used for comparisons between different groups, and the LSD t test was used for pairwise comparisons within groups. P < 0.05 was considered statistically significant. SPSS 23.0 was used for the above statistical analysis. Origin 8.0 software was used for mapping.
During the feeding period, the rats in the normal control group had good mental acuity and easy activity. In the model group, the overall reaction was sluggish; the rats exhibited less activity, listless spirit, dry hair, and increased food and water intake, and the bedding material was often wet and needed to be replaced daily. Compared with the model group, the state of rats in the intervention group was slightly improved, and the symptoms of polydipsia, hypereating and polyuria were slightly reduced; this improvement was more pronounced in the high-dose group. During the modeling and feeding process, the blood glucose level of 2 rats was lower than the modeling standard, so they were excluded from the group, and 6 rats died, all manifesting as hypertonia and shallow rapid breathing, and immediate blood glucose could not be measured. The cause of death may be acute complications of diabetes.
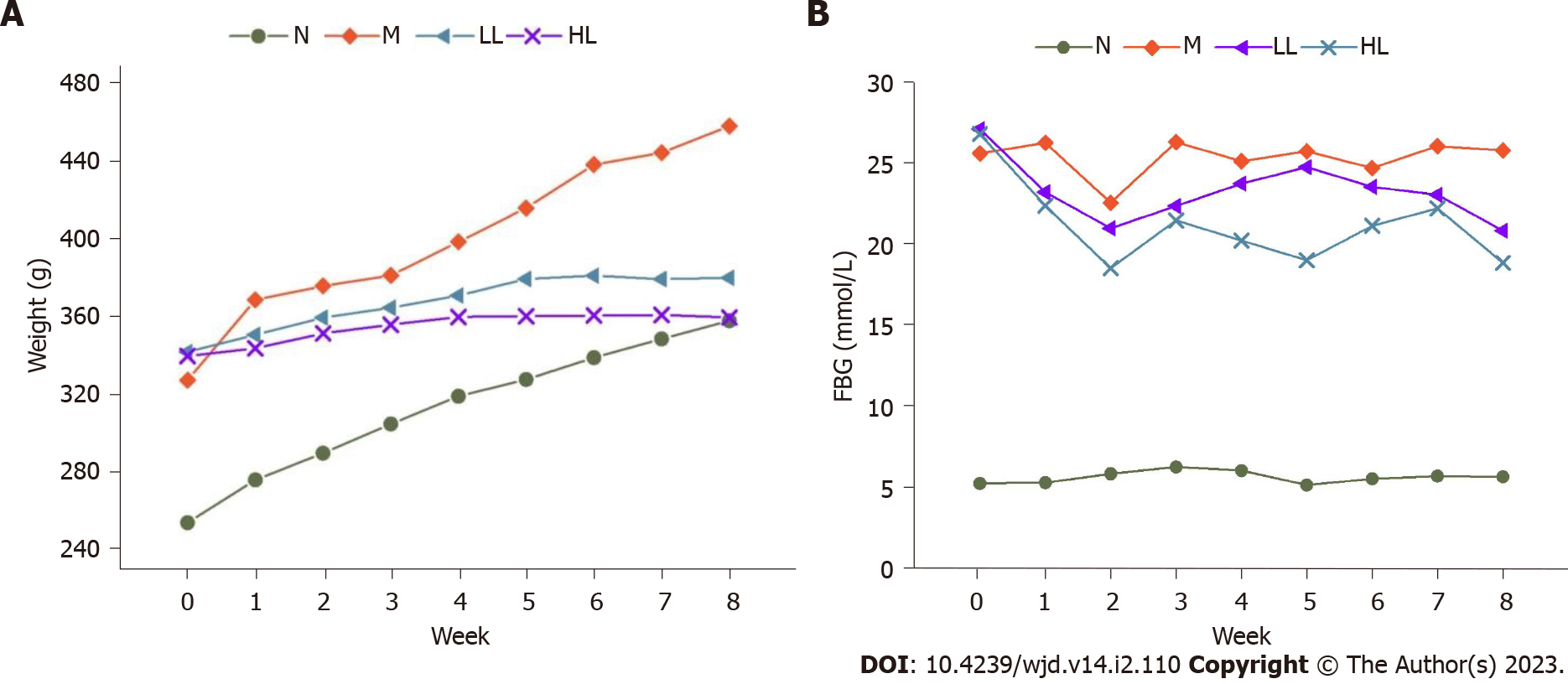
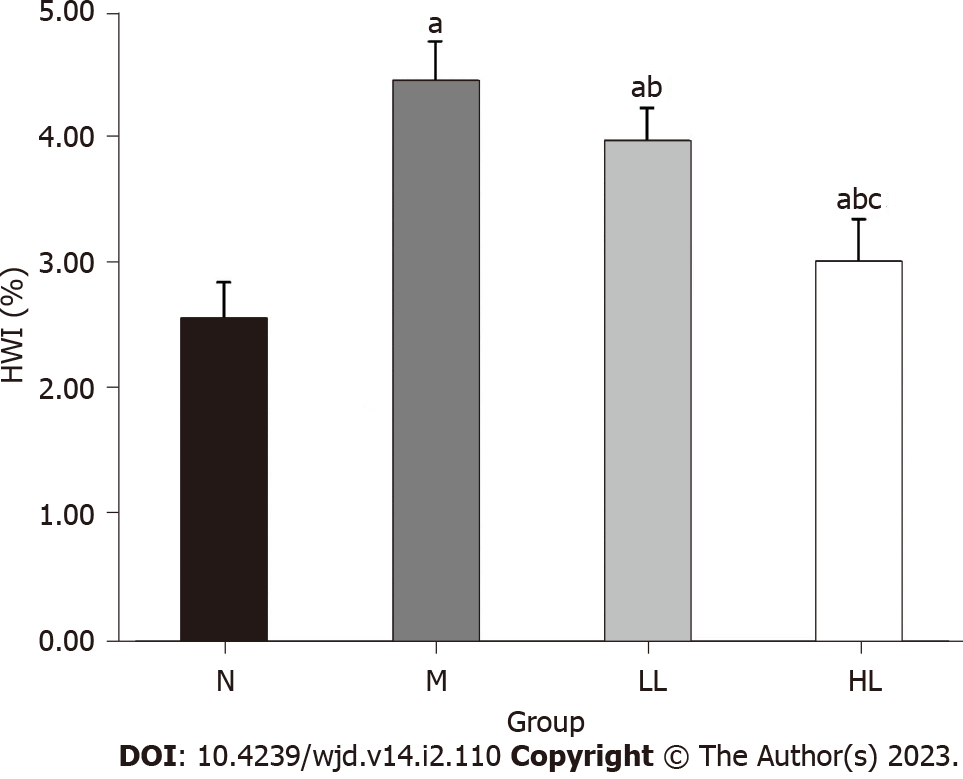
After liraglutide intervention, the body weight of the rats in each group was recorded weekly, and the body weight of rats in the model group increased significantly compared with that in the normal control group. After liraglutide intervention, compared with the model group, the body weight of rats in the intervention group increased slowly or even decreased, and the body weight of rats in the high-dose group increased slower and more obviously (Figure 1A). After the rats were sacrificed at the end of the 8th week, their hearts were weighed, and compared with that in the normal control group, the heart weight index of the model group was significantly increased. After liraglutide intervention, the index was decreased in the intervention compared with that in the model group, and the decrease was more obvious in the high-dose group (Figure 2). Specific values are shown in Table 1.
After liraglutide intervention, the FBG of rats in each group was recorded weekly. Compared with the that in normal control group, the FBG of rats in the model group was significantly increased, and the FBG of rats at different doses of liraglutide intervention was decreased; the decrease was more obvious in the high-dose group (Figure 1B). Compared with those in the normal control group, the levels of total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C) and lipoprotein phospholipase A2 (LP-PLA2) in the model group were significantly increased, and the differences were statistically significant (P < 0.05). Compared with those in the model group, the levels of FPG, TC, TG, LDL-C and LP-PLA2 in the intervention group were significantly decreased (P < 0.05), and the decrease was more obvious in the high-dose group but still higher than that in the normal control group. There was no significant difference in high density lipoprotein cholesterol (HDL-C) levels between groups before and after liraglutide intervention (P > 0.05). See Table 2 for details.
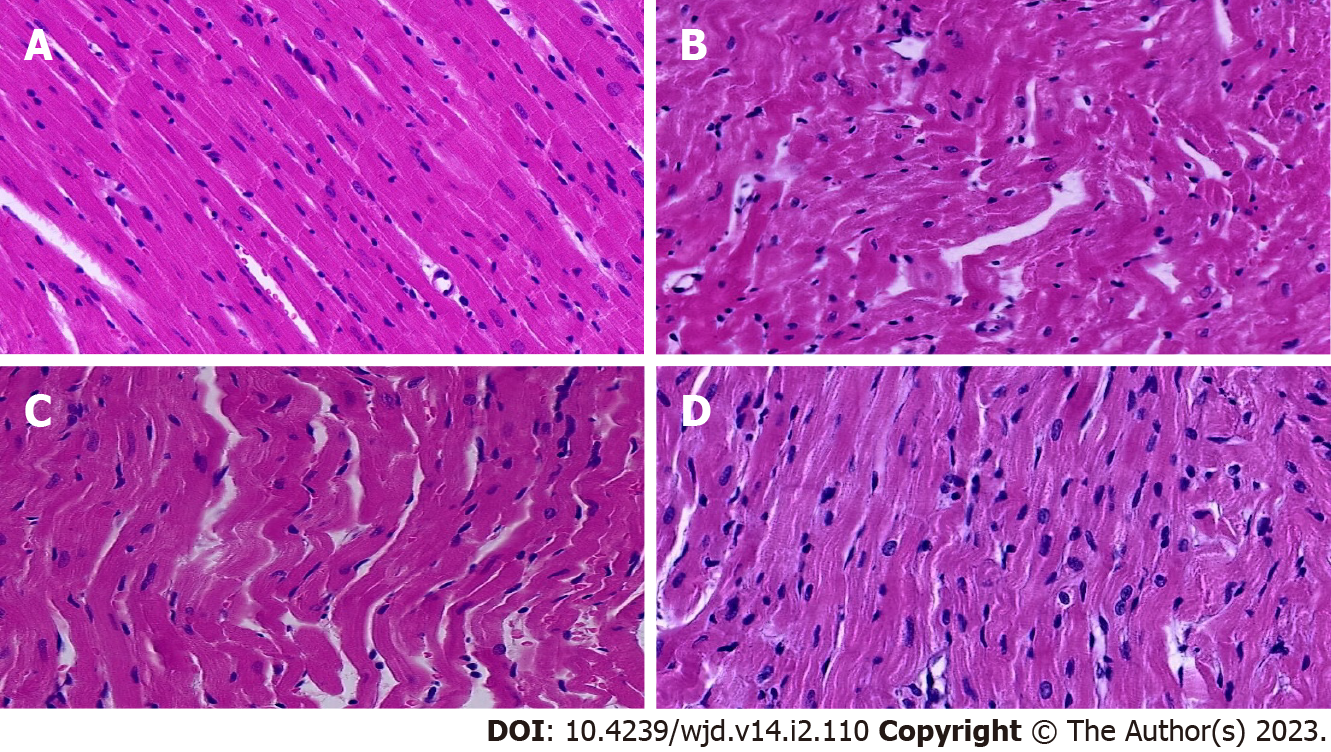
After HE staining, the samples were observed under a light microscope. The myocardial structure found in the rats in the normal control group was normal; the myocardial cells were closely arranged, the nuclei were clearly visible, the size was consistent, and the myocardial fibers were arranged neatly. In the model group, the myocardial fibers were disordered or even broken, the number of normal cardiomyocytes was reduced, the cells were hypertrophic, and the edges of the nuclei were unclear. The myocardial structural injury of rats in the intervention group was improved to different degrees, the myocardial fibers were arranged neatly, and the morphology of myocardial cells was normal; the improvement was more obvious in the high-dose group. Pathological results are shown in Figure 3.
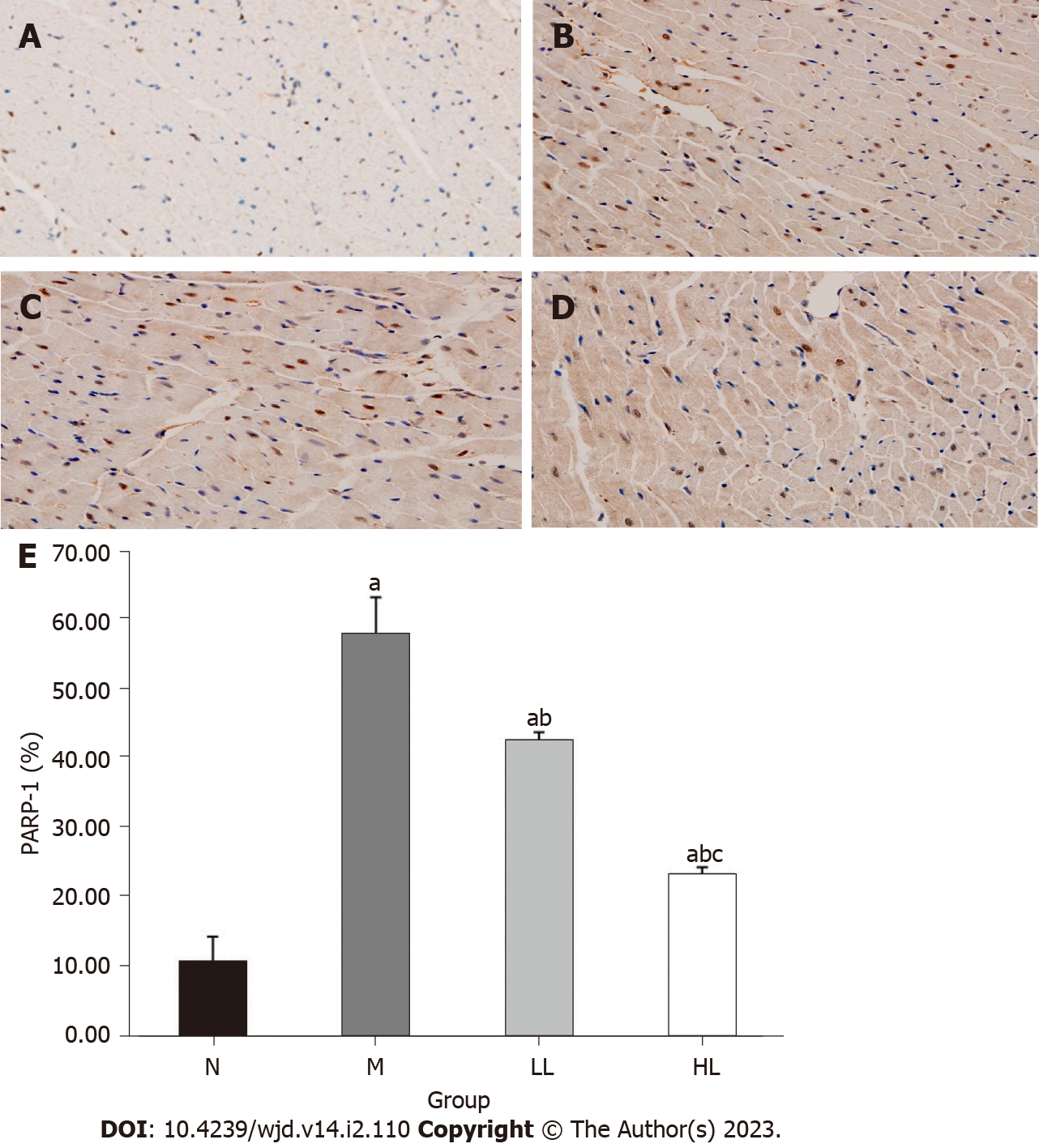
PARP-1 was expressed in the nucleus of the myocardium, and the positive expression of PARP-1 was indicated by brown-yellow particles after immunohistochemical staining. Compared with that in the normal control group, the expression of PARP-1 in the myocardium of the model group was significantly increased (P < 0.05). Compared with that in the model group, the expression of PARP-1 in the intervention group was significantly decreased, and the expression of PARP-1 in the myocardium of the high-dose group was more significantly decreased than that of the low-dose group (P < 0.05). See Table 3 and Figure 4.
Type 2 diabetes mellitus (T2DM) is a chronic and progressive metabolic disease that can lead to multisystem and multiorgan damage. Cardiovascular complications of diabetes mellitus are one of the main causes of death in patients with diabetes, and the trend is increasing every year. The fasting blood glucose, total cholesterol, low-density lipoprotein cholesterol, triglyceride, and heart weight index of rats in the experimental model group were significantly increased, and myocardial fiber arrangement disorder, interruption, cardiomyocyte hypertrophy, and loose shape indicated that T2DM causes myocardial injury[8,9]. Cardiac histopathological changes were consistent with T2DM and DCM, indicating successful modeling. At present, there are no specific and effective drugs for the treatment of diabetic cardiomyopathy, the progress of which can be slowed by improving lifestyle and controlling blood glucose.
DCM is a multifactorial disease that is closely related to cardiomyocyte apoptosis, oxidative stress, the inflammatory response and myocardial fibrosis. However, the exact molecular mechanism of DCM has not been thoroughly studied. Hyperglycemia can increase oxidative stress and nitrosation stress in cells; induce DNA strand breaks; over activate PARP-1; mediate the activation of PKC, the hexosamine pathway, and the polyol pathway; activate the transcription factor NF-κB; promote the expression of genes related to the inflammatory response; and lead to apoptosis and inflammation of cardiomyocytes[10]. This causes structural and functional changes in the heart. In vitro experiments have shown that PARP-1 activity in cardiomyocytes in a high-glucose environment is significantly increased, and in vivo experiments have shown that PARP-1 gene deletion in mice in a high-glucose environment reduced cardiomyocyte apoptosis and inflammation compared with that in wild-type mice[11]. HE staining analysis of the myocardial tissues of the two groups of mice also indicated that the myocardial fibrosis of PARP-1 gene null mice was significantly improved compared with that of wild-type mice[2]. Zakaria et al[12] showed that PARP-1 activity in type 2 diabetic rats was significantly increased compared with that in the control group. After 10 wk of PARP-1 inhibitor (4-AB) treatment, myocardial oxidative stress and inflammation were alleviated, and myocardial fibrosis and microvascular activity were further improved. Therefore, PARP-1 plays an important role in the development of diabetic cardiomyopathy. The results of this study also showed that the expression of PARP-1 in cardiomyocytes of the model group was significantly increased compared with that of the normal control group, suggesting that PARP-1 was involved in high glucose-induced myocardial injury.
Liraglutide, as a GLP-1 agonist, can increase insulin secretion by inhibiting glucagon secretion, promoting proliferation and reducing apoptosis of isletβ-cells, thus smoothly lowering glucose. In recent years, GLP-1 receptor agonists have attracted much attention because of their extensive pharmacological effects. GLP-1 receptors are widely distributed in tissues and organs throughout the body. Studies have found that GLP-1 receptors are distributed in coronary arteries, cardiomyocytes and vascular endothelial cells, regulating and maintaining the normal physiological structure and function of the myocardium[13]. Many studies have shown that GLP-1 can reduce oxidative stress injury and apoptosis of cardiomyocytes induced by high glucose and has a protective effect on cardiomyocytes. Some researchers have shown that liraglutide, a GLP-1 receptor agonist, can reduce the inflammatory response and oxidative stress of vascular endothelial cells by inhibiting the NF-κB signaling pathway in vascular endothelial cells and reducing the activity of NADPH oxidase. In a rat model of heart failure, GLP-1 significantly improved cardiac ejection function and the survival rate of rats after myocardial infarction compared with those in the control group. In addition, the LEADER and SUSTAIN-6 studies have confirmed that liraglutide can clinically reduce cardiovascular morbidity and mortality in patients with T2DM and high cardiovascular risk and has a comprehensive cardiovascular protective effect. The possible mechanisms include reduction of atherosclerosis, systolic blood pressure, and pulmonary capillary pressure; improvement of endothelial function; and increase of myocardial rescue rate after myocardial infarction. In conclusion, liraglutide can protect cardiomyocytes and improve cardiac function, and PARP-1 is closely related to high glucose-induced myocardial injury. Therefore, we speculated that liraglutide could inhibit the expression of cardiac PARP-1 and thereby delay the progression of DCM. In this experiment, we found that after liraglutide intervention, the myocardial injury of rats in the intervention group was significantly reduced compared with that in the model group, and the expression of PARP-1 was significantly reduced, suggesting that the protective effect of liraglutide on cardiomyocytes was related to the reduction of PARP-1 activity. In animal experiments, the current intelligent animal experiment method based on deep learning can obtain the adaptation degree of animals in various environments and the posture and state of animals after intervention, according to fitness of each experiment area, and posture and state of experiment body, which is more conducive to the establishment of animal models and the prediction and evaluation of the effects of drug intervention. This technology belongs to the frontier field at present. It is very helpful for the follow-up research of this experiment. In the future, large-scale in-depth research on animal intervention experiments will be carried out.
In conclusion, through the detection of heart weight index, blood lipids, and PARP-1 expression and observation of cardiac pathological changes in this experiment, we found that liraglutide can delay the occurrence and development of DCM by reducing the expression of cardiac PARP-1, which provides evidence for its clinical application in DCM. However, there were still some shortcomings in this experiment. For example, the TUNEL method was not used to detect cardiomyocyte apoptosis, and there was insufficient evidence of pathological changes in cardiomyocyte morphology based only on cardiac HE staining. In addition, in recent years, a study found that excessive expression of insulin-like growth factor 1 (IGF-1) can reduce myocardial infarction and myocardial cell apoptosis but can also reduce the nonocclusive coronary artery stenosis of genetically modified mice and myocardial cell death after myocardial infarction. IGF-1r cascades the activation of the PI3K/Akt signaling pathway and promotes cell proliferation. However, due to the limited time frame of this study, whether liraglutide can activate the IGF-1/PI3K/Akt pathway by inhibiting PARP-1 to protect cardiomyocytes remains unclear, and further studies are needed.
Low-dose STZ combined with a high-glucose and high-fat diet can successfully establish a rat model of T2DM. After 4 wk of continuous feeding, myocardial injury can occur, which is consistent with DCM. Liraglutide reduced the body weight of type 2 diabetic rats and significantly improved the fasting blood glucose and lipid profile in a dose-dependent manner. Liraglutide may improve myocardial injury in type 2 diabetic rats by inhibiting the expression of myocardial PARP-1 in a dose-dependent manner.
Glucagon-like peptide-1 (GLP-1) can reduce the apoptosis of islet microcirculation endothelial cells and protect islet tissue through the polyadenosine diphosphate-ribose polymerase-1 (PARP-1)/iNOs pathway.
Whether GLP-1 can protect cardiomyocytes by inhibiting the expression of PARP-1 is still unclear.
This study investigated the mechanism of liraglutide in improving myocardial injury in type 2 diabetic rats, further clarified the protective effect of liraglutide on the heart, and provided a new option for the treatment of diabetic cardiomyopathy (DCM).
After successful modeling, the rats were fed a high-glucose and high-fat diet for 8 wk and then started drug intervention. Blood samples were collected from the abdominal aorta to detect fasting blood glucose and lipid profiles. Intact heart tissue was dissected, and its weight was used to calculate the heart weight index. Hematoxylin and eosin staining was used to observe the pathological changes in the myocardium and the expression of PARP-1 in the heart by immunohistochemistry.
After liraglutide intervention, compared with the model group, the expression of PARP-1 in myocardial tissue was decreased, and the reduction was more obvious in the high-dose group (P < 0.05) but still higher than that in the normal control group.
Liraglutide may improve myocardial injury in type 2 diabetic rats by inhibiting the expression of myocardial PARP-1 in a dose-dependent manner.
Through the detection of heart weight index, blood lipids, and PARP-1 expression and observation of cardiac pathological changes in this experiment, we found that liraglutide can delay the occurrence and development of DCM by reducing the expression of cardiac PARP-1, which provides evidence for its clinical application in DCM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemical research methods
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Javor E, Croatia; Lee KS, South Korea S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Kuchmerovska T, Guzyk M, Tykhonenko T, Yanitska L, Pryvrotska I, Diakun K. The parp-1 and bax genes as potential targets for treatment of the heart functioning impairments induced by type 1 diabetes mellitus. Endocr Regul. 2021;55:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Waldman M, Nudelman V, Shainberg A, Abraham NG, Kornwoski R, Aravot D, Arad M, Hochhauser E. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1α axis. Exp Cell Res. 2018;373:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Qin WD, Liu GL, Wang J, Wang H, Zhang JN, Zhang F, Ma Y, Ji XY, Li C, Zhang MX. Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget. 2016;7:35618-35631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Nam DH, Han JH, Lee TJ, Shishido T, Lim JH, Kim GY, Woo CH. CHOP deficiency prevents methylglyoxal-induced myocyte apoptosis and cardiac dysfunction. J Mol Cell Cardiol. 2015;85:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Ivanović-Matić S, Bogojević D, Martinović V, Petrović A, Jovanović-Stojanov S, Poznanović G, Grigorov I. Catalase inhibition in diabetic rats potentiates DNA damage and apoptotic cell death setting the stage for cardiomyopathy. J Physiol Biochem. 2014;70:947-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ivanović-Matić S, Mihailović M, Dinić S, Martinović V, Bogojević D, Grigorov I, Poznanović G. The absence of cardiomyopathy is accompanied by increased activities of CAT, MnSOD and GST in long-term diabetes in rats. J Physiol Sci. 2010;60:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | Chiu J, Farhangkhoee H, Xu BY, Chen S, George B, Chakrabarti S. PARP mediates structural alterations in diabetic cardiomyopathy. J Mol Cell Cardiol. 2008;45:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 9. | Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Szabo C. PARP as a Drug Target for the Therapy of Diabetic Cardiovascular Dysfunction. Drug News Perspect. 2002;15:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Zhang ZH, Zhang ZG, Chen MW, Yang Y, Li RJ, Xu JJ, Yang C, Li YY, Chen HW, Liu SX, Li YL, Luo P, Liu YJ, Chen WB, Shan ZG, Huang ZR. Inhibition of GSDMD Activates Poly(ADP-ribosyl)ation and Promotes Myocardial Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2022;2022:1115749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Zakaria EM, El-Bassossy HM, El-Maraghy NN, Ahmed AF, Ali AA. PARP-1 inhibition alleviates diabetic cardiac complications in experimental animals. Eur J Pharmacol. 2016;791:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Gerö D, Szoleczky P, Chatzianastasiou A, Papapetropoulos A, Szabo C. Modulation of poly(ADP-ribose) polymerase-1 (PARP-1)-mediated oxidative cell injury by ring finger protein 146 (RNF146) in cardiac myocytes. Mol Med. 2014;20:313-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |