Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1793
Peer-review started: August 8, 2023
First decision: September 29, 2023
Revised: October 20, 2023
Accepted: November 27, 2023
Article in press: November 27, 2023
Published online: December 15, 2023
Processing time: 128 Days and 7.1 Hours
Type 2 diabetes mellitus (T2DM) is associated with periodontitis. Currently, there are few studies proposing predictive models for periodontitis in patients with T2DM.
To determine the factors influencing periodontitis in patients with T2DM by constructing logistic regression and random forest models.
In this a retrospective study, 300 patients with T2DM who were hospitalized at the First People’s Hospital of Wenling from January 2022 to June 2022 were selected for inclusion, and their data were collected from hospital records. We used logistic regression to analyze factors associated with periodontitis in patients with T2DM, and random forest and logistic regression prediction models were established. The prediction efficiency of the models was compared using the area under the receiver operating characteristic curve (AUC).
Of 300 patients with T2DM, 224 had periodontitis, with an incidence of 74.67%. Logistic regression analysis showed that age [odds ratio (OR) = 1.047, 95% confidence interval (CI): 1.017-1.078], teeth brushing frequency (OR = 4.303, 95%CI: 2.154-8.599), education level (OR = 0.528, 95%CI: 0.348-0.800), glycosylated hemoglobin (HbA1c) (OR = 2.545, 95%CI: 1.770-3.661), total cholesterol (TC) (OR = 2.872, 95%CI: 1.725-4.781), and triglyceride (TG) (OR = 3.306, 95%CI: 1.019-10.723) influenced the occurrence of periodontitis (P < 0.05). The random forest model showed that the most influential variable was HbA1c followed by age, TC, TG, education level, brushing frequency, and sex. Comparison of the prediction effects of the two models showed that in the training dataset, the AUC of the random forest model was higher than that of the logistic regression model (AUC = 1.000 vs AUC = 0.851; P < 0.05). In the validation dataset, there was no significant difference in AUC between the random forest and logistic regression models (AUC = 0.946 vs AUC = 0.915; P > 0.05).
Both random forest and logistic regression models have good predictive value and can accurately predict the risk of periodontitis in patients with T2DM.
Core Tip: With the rapid increase in the number of patients with type 2 diabetes mellitus (T2DM), the number of cases complicated by periodontitis has also increased. Without timely intervention, periodontitis can lead to tooth loosening and loss, and a decline in oral function, reducing patient quality of life. We retrospectively analyzed the data of 300 patients with T2DM to determine the factors influencing periodontitis. Random forest and logistic regression models were constructed to provide a theoretical basis for predicting periodontitis in patients with T2DM.
- Citation: Xu HM, Shen XJ, Liu J. Establishment of models to predict factors influencing periodontitis in patients with type 2 diabetes mellitus. World J Diabetes 2023; 14(12): 1793-1802
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1793.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1793
Type 2 diabetes mellitus (T2DM) accounts for more than 90% of cases of diabetes, and occurs mostly in adults over 40 years of age[1,2]. Research has shown that individuals with diabetes are more likely to develop periodontitis than those without[3]. Periodontitis is a chronic inflammation that occurs in periodontal supporting tissues and is characterized by gingival inflammation, formation of a periodontal pocket, resorption and destruction of the alveolar bone, and tooth loosening, displacement, and loss[4]. It may also affect masticatory function and nutritional intake[5]. Many studies have shown a bidirectional relationship between T2DM and periodontitis, and the incidence of periodontitis in patients with T2DM is approximately 2-3 times that of the general population[6]. Many studies have reported on the factors influencing periodontitis in patients with T2DM, but the majority use logistic regression analysis, which cannot intuitively present the importance of the outcome[7,8]. With the advent of the era of big data, predictive models have become useful in predicting the occurrence of diseases, but there are few relevant prediction models for periodontitis in patients with T2DM. Studies have shown that among the multiple machine learning models for predicting the risk of kidney disease in patients with T2DM, the random forest model has better performance and higher accuracy than logistic regression[9]. Therefore, the objective of this study was to retrospectively analyze the factors influencing periodontitis in patients with T2DM, and establish random forest and logistic regression prediction models for this disease.
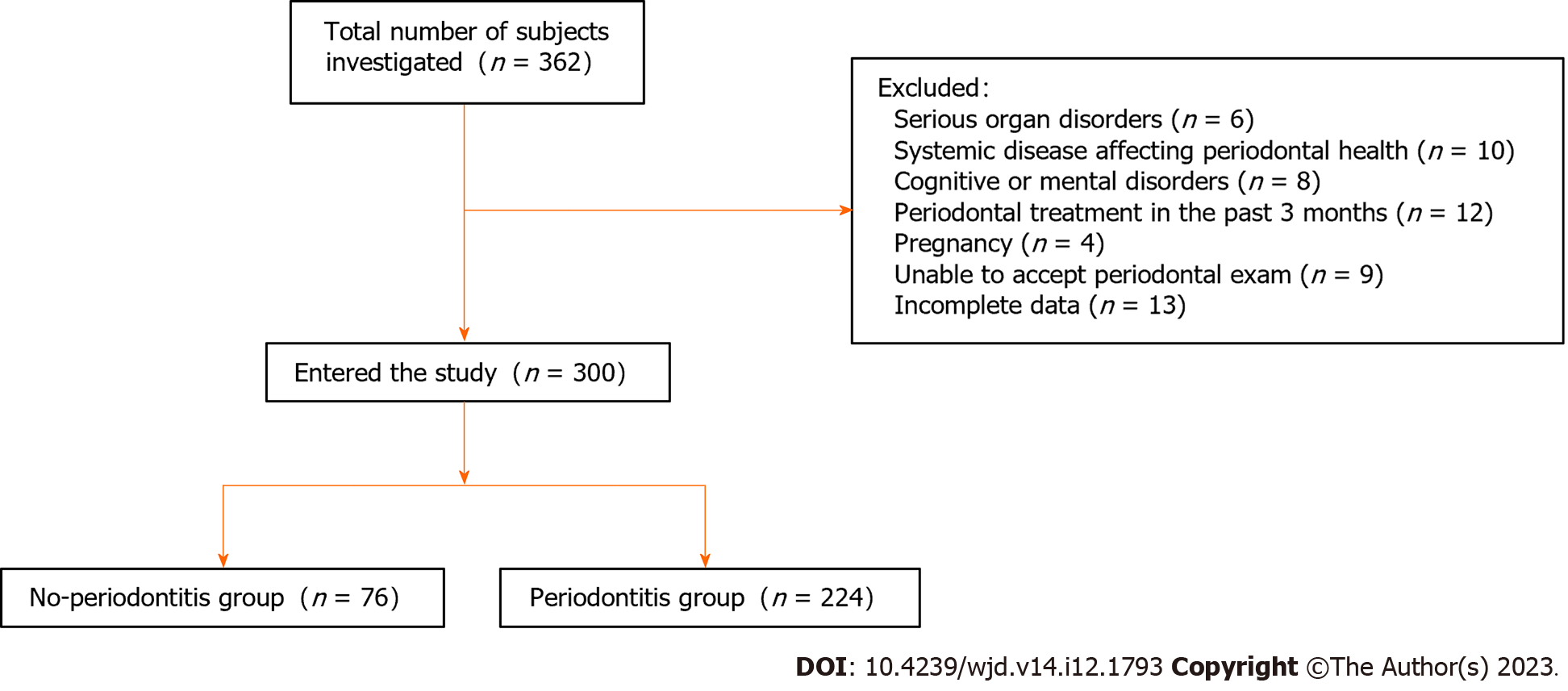
Three hundred patients with T2DM who were hospitalized at the First People’s Hospital of Wenling from January 2022 to June 2022 were selected as research subjects, and their relevant data were collected for this retrospective study (Figure 1). The inclusion criteria were: (1) Age ≥ 18 years old; and (2) T2DM diagnosed at our hospital and without other serious complications. The exclusion criteria were: (1) Patients with other systemic diseases affecting periodontal health; (2) Serious organ disorders; (3) Cognitive or mental disorders; (4) Periodontal treatment in the past 3 mo; (5) Pregnancy; (6) Unable to accept oral periodontal examination; and (7) Incomplete data.
General information and clinical examination data were collected from hospital records. General patient information included monthly income, age, education level, body mass index (BMI), sex, smoking, and alcohol intake. We also asked about daily brushing frequency and exercise habits. Clinical examination data included glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels.
The Diagnostic criteria for periodontitis were: (1) A definite history of periodontitis; (2) Loose teeth in the mouth due to periodontitis without other factors; and (3) Periodontal examination showing that the depth of a periodontal pocket of at least one index tooth was ≥ 4 mm, and the tooth had clinical attachment loss (AL) ≥ 1 mm. According to the diagnostic criteria for periodontitis, the patients were divided into periodontitis and no periodontitis groups. Based on the depth of the periodontal pocket, AL, and alveolar bone resorption, periodontitis was divided into three categories: (1) Mild: Gingival inflammation and bleeding on probing, probing depth of periodontal pocket ≤ 4 mm, AL 1-2 mm, and alveolar bone resorption not more than one third of root length; (2) Moderate: Gingival inflammation, bleeding on probing or pus, depth of periodontal pocket ≤ 6 mm, AL 3-4 mm, alveolar bone resorption more than one third of the length of the root, but not more than half the length of the root, mild tooth loosening, and mild furcation lesions; and (3) Severe: Obvious inflammation accompanied by periodontal abscess, periodontal pocket depth > 6 mm, AL ≥ 5 mm, alveolar bone resorption more than half of the root length, multiple root furcation lesions, and tooth loosening and displacement.
According to BMI [weight (kg)/height (m2)], the patients were classified as: Underweight: BMI < 18.5; normal weight: BMI 18.5-24.0; overweight: BMI 24.0-28; obesity: BMI ≥ 28. According to HbA1c, blood glucose control was classified as: Ideal control: HbA1c < 6.5%; good control: HbA1c 6.5%-7.5%; poor control: HbA1c 7.5%-8.5%; very poor control: HbA1c ≥ 8.5%. The normal levels of TC, TG, HDL-C, and LDL-C are < 5.18 mmol/L, < 1.7 mmol/L, ≥ 1.04 mmol/L, and ≤ 3.37 mmol/L, respectively.
Patient information was analyzed using SPSS 26.0. For univariate analysis, the data that passed the normality test, represented by the mean ± SD, were compared by the t-test. Data that failed the normality test, presented as median (M) and 25% and 75% percentiles (P25, P75), were compared by the rank sum test. Count data, denoted as n (%), were compared by the χ2 test. Statistical significance was set at P < 0.05. The variables that were statistically significant in the univariate analysis were included in the logistic regression to analyze the factors influencing periodontitis in T2DM patients. Logistic regression and random forest prediction models were constructed using the R, and the receiver operating characteristic curves of the training dataset and validation dataset of the two models were drawn. The predictive efficacy of the models was compared according to the area under curve (AUC), and the AUC was compared using the Delong test.
Among the 300 patients with T2DM, 224 had periodontitis, with an incidence of 74.67%. Among them, 83 (37.05%) had mild, 78 (34.82%) had moderate, and 63 (28.13%) had severe periodontitis. One hundred and sixty-four (54.67%) were male and 136 (45.33%) were female, with an incidence based on sex of 58.04% and 44.74%, respectively. The average age was 60.91 ± 9.49 years in the periodontitis group and 54.11 ± 14.69 years in the no periodontitis group. The proportion of patients with education level below high school was highest (43.75%) in the periodontitis group. In terms of monthly income, the highest proportion of monthly income in the periodontitis group was between 2000 and 5000 yuan (44.20%). Univariate analysis showed that there were significant differences in sex, age, and education level between the periodontitis and non-periodontitis groups (P < 0.05), but there was no significant difference in monthly income (P > 0.05; Table 1).
| Factor | Total number (n = 300) | Periodontitis group (n = 224) | Non-periodontitis group (n = 76) | t/χ²/Z | P value |
| Sex, n (%) | 4.050 | 0.044 | |||
| Male | 164 (54.67) | 130 (58.04) | 34 (44.74) | ||
| Female | 136 (45.33) | 94 (41.96) | 42 (55.26) | ||
| Age (yr), (mean ± SD) | 59.18 ± 11.40 | 60.91 ± 9.49 | 54.11 ± 14.69 | -4.646 | < 0.001 |
| Education level, n (%) | -3.626 | < 0.001 | |||
| Below high school education | 118 (39.33) | 98 (43.75) | 20 (26.31) | ||
| High school education | 98 (32.67) | 76 (33.93) | 22 (28.95) | ||
| Above high school education | 84 (28.00) | 50 (22.32) | 34 (44.74) | ||
| Monthly income, n (%) | -1.890 | 0.059 | |||
| < 2000 yuan | 65 (21.67) | 54 (24.11) | 11 (14.47) | ||
| 2000-5000 yuan | 133 (44.33) | 99 (44.20) | 34 (44.74) | ||
| > 5000 yuan | 102 (34.00) | 71 (31.70) | 31 (40.79) |
Of the patients with periodontitis, 23.21% smoked, 17.86% drank alcohol, 51.34% exercised regularly, and 63.39% brushed their teeth less than twice a day. Of the patients without periodontitis, 13.16% smoked, 14.47% drank alcohol, 56.58% exercised regularly, and 27.63% brushed their teeth less than twice a day. Univariate analysis showed that there was a significant difference in brushing frequency between the periodontitis and non-periodontitis groups (P < 0.05), but there were no significant differences in smoking, drinking, or regular exercise (P > 0.05; Table 2).
| Factor | Total (n = 300) | Periodontitis group (n = 224) | Non-periodontitis group (n = 76) | χ² | P value |
| Smoking, n (%) | 3.500 | 0.061 | |||
| No | 238 (79.33) | 172 (76.79) | 66 (86.84) | ||
| Yes | 62 (20.67) | 52 (23.21) | 10 (13.16) | ||
| Alcohol consumption, n (%) | 0.460 | 0.497 | |||
| No | 249 (83.00) | 184 (82.14) | 65 (85.53) | ||
| Yes | 51 (17.00) | 40 (17.86) | 11 (14.47) | ||
| Regular exercise, n (%) | |||||
| No | 142 (47.33) | 109 (48.66) | 33 (43.42) | 0.625 | 0.429 |
| Yes | 158 (52.67) | 115 (51.34) | 43 (56.58) | ||
| Brushing frequency, n (%) | 29.248 | < 0.001 | |||
| ≥ 2 times/d | 137 (45.67) | 82 (36.61) | 55 (72.37) | ||
| < 2 times/d | 163 (54.33) | 142 (63.39) | 21 (27.63) |
Among the 300 patients with T2DM, the overall BMI was 24.96 (22.36, 26.81). The BMI of patients with periodontitis was overweight at 24.86 (22.13, 26.65), and the BMI of patients without periodontitis was overweight at 25.61 (23.26, 27.23). There were 72.77%, 64.29%, and 58.48% of patients in the periodontitis group with normal TG, TC, and HDL-C, respectively. These were lower than the proportions of patients without periodontitis with normal TG, TC, and HDL-C. All patients had normal levels of LDL-C. The periodontitis group had the highest proportion of patients with very poor blood glucose control at 37.50%. The proportion of patients in the non-periodontitis group with good blood glucose control was 40.79%. Univariate analysis showed that there were significant differences in TG, TC, and HbA1c between the two groups (P < 0.05), but there were no significant differences in BMI, LDL-C, or HDL-C (P > 0.05; Table 3).
| Factor | Total number (n = 300) | Periodontitis group (n = 224) | Non-periodontitis group (n = 76) | t/Z | P value |
| BMI (kg/m2), median (P25, P75) | 24.96 (22.36, 26.81) | 24.86 (22.13, 26.65) | 25.61 (23.26, 27.23) | -1.223 | 0.221 |
| TG (mmol/L) | 1.51 ± 0.28 | 1.53 ± 0.28 | 1.44 ± 0.26 | -2.678 | 0.008 |
| < 1.7 | 226 (75.33) | 163 (72.77) | 63 (82.89) | ||
| ≥ 1.7 | 74 (26.67) | 61 (27.23) | 13 (17.11) | ||
| TC (mmol/L) | 4.72 ± 0.77 | 4.86 ± 0.78 | 4.33 ± 0.59 | -5.361 | < 0.001 |
| < 5.18 | 212 (70.67) | 144 (64.29) | 68 (89.47) | ||
| ≥ 5.18 | 88 (29.33) | 80 (35.71) | 8 (10.53) | ||
| HDL-C (mmol/L) | 1.09 ± 0.19 | 1.08 ± 0.20 | 1.12 ± 0.15 | 1.777 | 0.077 |
| < 1.04, n (%) | 114 (38.00) | 93 (41.52) | 21 (27.63) | ||
| ≥ 1.04, n (%) | 186 (62.00) | 131 (58.48) | 55 (72.37) | ||
| LDL-C (mmol/L) | 2.42 ± 0.29 | 2.44 ± 0.29 | 2.39 ± 0.30 | -1.183 | 0.238 |
| < 3.37, n (%) | 300 (100.00) | 224 (100.00) | 76 (100.00) | ||
| ≥ 3.37, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| HbA1c (mmol/L) | 7.86 ± 1.17 | 8.10 ± 1.16 | 7.14 ± 0.85 | -6.596 | < 0.001 |
| < 6.5%, n (%) | 37 (12.33) | 17 (7.59) | 20 (26.32) | ||
| 6.5%-7.5%, n (%) | 83 (27.66) | 52 (23.21) | 31 (40.79) | ||
| 7.5%-8.5%, n (%) | 90 (30.00) | 71 (31.70) | 19 (25.00) | ||
| ≥ 8.5%, n (%) | 90 (30.00) | 84 (37.50) | 6 (7.89) |
Whether patients with T2DM developed periodontitis (no occurrence = 0, occurrence = 1) was used as the dependent variable, and statistically significant variables (sex, age, brushing frequency, education level, HbA1c, TC, and TG) were used as independent variables in the univariate analysis (Table 4). Logistic regression analysis showed that age, brushing frequency, education level, HbA1c, TC, and TG were factors influencing periodontitis in patients with T2DM (P < 0.05). Older age was associated with a higher risk of periodontal disease [odds ratio (OR) = 1.047, 95% confidence interval (CI): 1.017-1.078]. Brushing teeth < 2 times/d was also associated with a higher risk of periodontal disease (OR = 4.303, 95%CI: 2.154-8.599). The higher the education level, the lower the risk of periodontitis (OR = 0.528, 95%CI: 0.348-0.800). Higher HbA1c, TC, and TG levels were associated with a higher risk of periodontal disease (OR = 2.545, 95%CI: 1.770-3.661, OR = 2.872, 95%CI: 1.725-4.781, OR = 3.306, 95%CI: 1.019-10.723, respectively; Table 5).
| Factor | Assignment of value |
| Sex | Female = 0 |
| Male = 1 | |
| Age | Continuous variables |
| Brushing frequency | ≥ 2 times/d = 0 |
| < 2 times/d = 1 | |
| Education level | Below high school education = 0 |
| High school education = 1 | |
| Above high school education = 2 | |
| HbA1c | Continuous variables |
| TC | Continuous variables |
| TG | Continuous variables |
| Factor | β | SE | Wald χ² | P value | OR (95%CI) |
| Age | 0.046 | 0.015 | 9.813 | 0.002 | 1.047 (1.017-1.078) |
| Sex | 0.622 | 0.346 | 3.230 | 0.072 | 1.863 (0.945-3.674) |
| Brushing frequency | 1.459 | 0.353 | 17.073 | < 0.001 | 4.303 (2.154-8.599) |
| Education level | -0.639 | 0.212 | 9.065 | 0.003 | 0.528 (0.348-0.800) |
| HbA1c | 0.934 | 0.185 | 25.392 | < 0.001 | 2.545 (1.770-3.661) |
| TC | 1.055 | 0.260 | 16.451 | < 0.001 | 2.872 (1.725-4.781) |
| TG | 1.196 | 0.600 | 3.967 | 0.046 | 3.306 (1.019-10.723) |
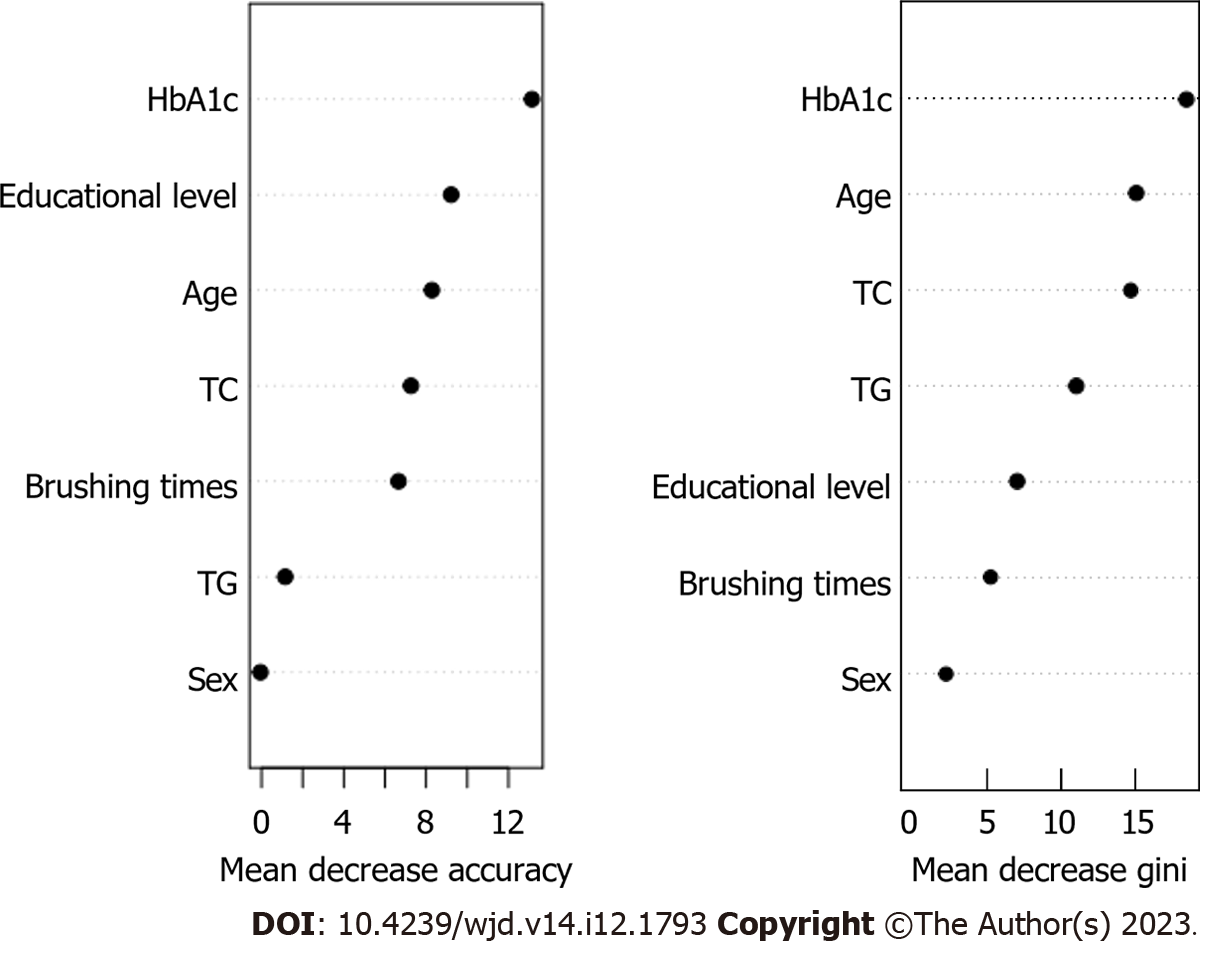
According to the random number method, patients were randomly divided into the training dataset (n = 200) and validation dataset (n = 100) according to 2/3 and 1/3 of the total number of patients, respectively. The seven variables (age, brushing frequency, HbA1c, TC, education level, TG, and gender) that were statistically significant in the univariate analysis were included in the random forest model. As shown in Figure 2, the importance of variables influencing the occurrence of periodontitis in patients with T2DM was ranked as HbA1c, age, TC, TG, educational level, brushing frequency, and sex.
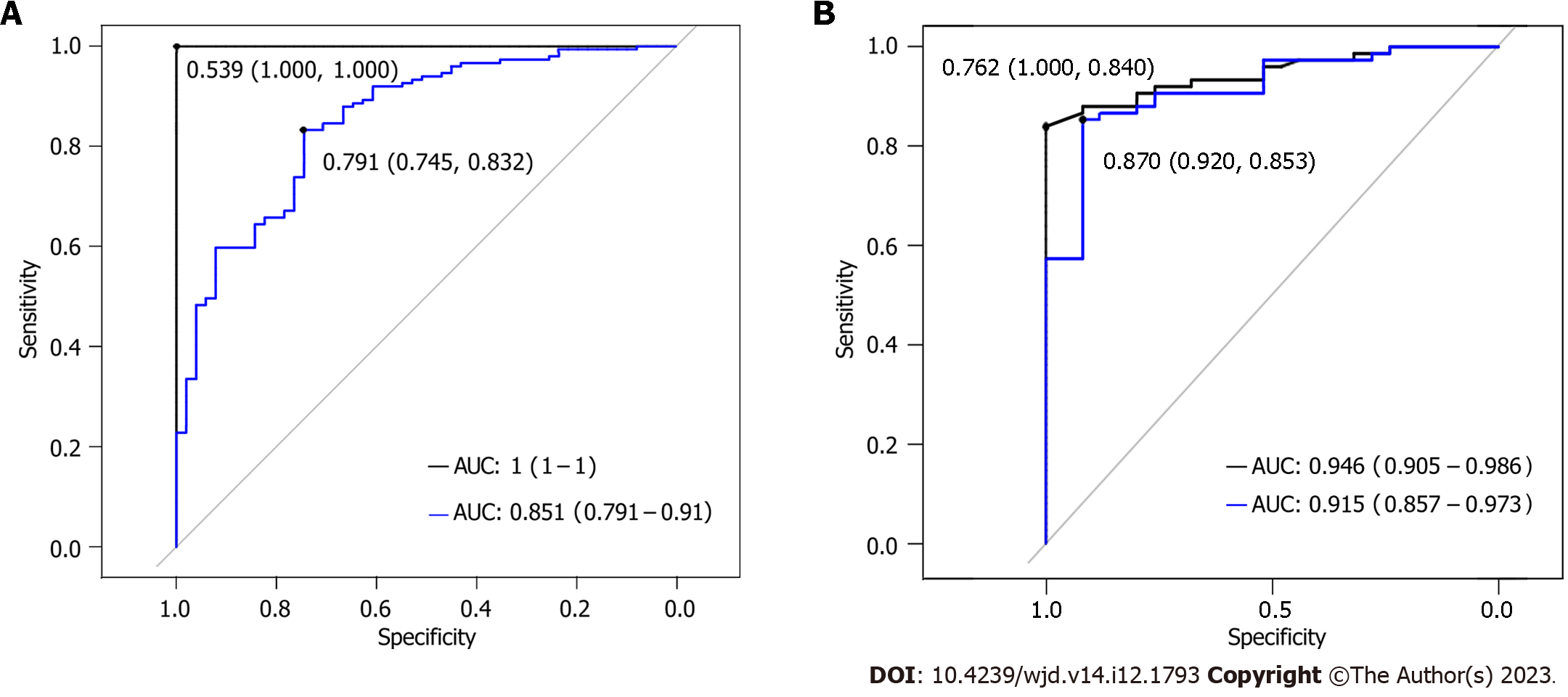
In the training dataset, the overall efficacy of the random forest model in predicting periodontitis in patients with T2DM was higher than that of the logistic regression model. The AUC of the random forest model was significantly higher than that of the logistic regression model (P < 0.05; Figure 3A, Table 6). In the validation dataset, the overall performances of the random forest and logistic regression models were comparable and there was no significant difference in AUC between them (P > 0.05; Figure 3B, Table 7).
| Model | Sensitivity | Specificity | Accuracy | Recall | Precision | AUC (95%CI) |
| Random forest | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 (1.000-1.000) |
| Logistic regression | 0.569 | 0.919 | 0.830 | 0.569 | 0.707 | 0.851 (0.791-0.910) |
| Model | Sensitivity | Specificity | Accuracy | Recall | Precision | AUC (95%CI) |
| Random forest | 0.520 | 0.960 | 0.850 | 0.520 | 0.813 | 0.946 (0.905-0.986) |
| Logistic regression | 0.640 | 0.907 | 0.840 | 0.640 | 0.696 | 0.915 (0.857-0.973) |
Periodontitis is internationally recognized as the sixth most common complication of diabetes. With an increase in the number of diabetic patients, the number of people with periodontitis in the diabetic population is also increasing[10-12]. The results of this study showed that among 300 patients with T2DM treated at our hospital, 74.67% had periodontitis, which is higher than that reported by de Miguel-Infante et al[13] (23.8%) and Hong et al[14] (43.7%). The differences in the incidence of periodontitis may be linked to population differences in different countries and regions or to the lifestyle of people in different regions. The higher incidence of periodontitis in patients with T2DM in this study may be due to the older age of the study population as a whole and the irreversible damage to the periodontal tissue caused by inflammation[15].
Our study found that periodontitis in patients with T2DM was associated with several factors. Logistic regression analysis showed that age, brushing frequency, education level, HbA1c, TC, and TG were the factors significantly influencing periodontitis in patients with T2DM. The importance of the variables was ranked by the random forest model as HbA1c, age, TC, TG, education level, brushing frequency, and sex. The results of the two models were similar, indicating that the prediction results were reliable.
We found that the greater the HbA1c level, the higher the risk of periodontitis (OR = 2.545, 95%CI: 1.770-3.661), which is similar to the results of Wu et al[16], Qureshi et al[17], and Dhir et al[18]. HbA1c is reflective of a patient’s blood glucose control in the past 2-3 mo. Poor control of blood glucose can cause more severe periodontitis symptoms, while good blood glucose control can delay the progression of periodontitis[19,20]. We also found that the risk of periodontal disease increased with age (OR = 1.047, 95%CI: 1.017-1.078). This is similar to the results of a study by de Miguel-Infante et al[13]. Age may be associated with disease as with increasing age, there is generally a weakened immune system making tissues more susceptible to the invasion of anaerobic bacteria and aggravating susceptibility to periodontitis[21]. Blood lipid levels, particularly higher levels of TC and TG, were associated with an increased risk of periodontal disease. This is similar to the results obtained by Dhir et al[18] and Ding et al[22]. High blood lipid levels in patients with T2DM may increase the body’s susceptibility to periodontitis by promoting the expression of inflammatory factors, increasing the oxidative stress response and lipid peroxidation, inhibiting bone formation, and promoting bone resorption[23,24]. Low education level was also associated with a higher risk of periodontitis which has also been reported by de Miguel-Infante et al[13] and Yamamoto et al[25]. People with lower education may have less exposure to information about oral health and less understanding about the risk of periodontitis with T2DM. Unsurprisingly, less frequent brushing was associated with a higher risk of disease which has also been reported by Hong et al[14] and Chang et al[26]. Brushing removes food residue and reduces the reproduction of bacteria in the mouth, thereby decreasing the likelihood of developing periodontitis[27].
The random forest model is widely used, and some studies have used it to predict the risk of nephropathy, peripheral neuropathy, and foot ulcers in patients with T2DM[9,28,29]. We found that the random forest model was significantly better than the logistic regression model for the validation but not the test dataset. Both models had good predictive value with AUCs of 0.946 and 0.915, respectively. The random forest model is an ensemble learning method based on decision trees. Its advantage is that it requires less data, and its modeling process is more convenient and faster than logistic regression. However, the sensitivity, specificity, accuracy, recall, precision, and AUC of the training dataset of the random forest model in this study reached 1, indicating that the model may have been overfitting. A logistic regression model is a commonly used probability prediction model, which is simple to use and has strong predictive ability. Its advantage lies in that it can quantify the risk of disease through the OR value of variables, but it cannot intuitively determine the importance of each independent variable to the model prediction.
This study has some limitations: (1) This was a retrospective study based on the data of patients diagnosed with T2DM at our hospital. The study subjects and sources are from one site and the sample size included in the model is small, so there is a certain sample bias; (2) This study analyzed only a subset of the factors that may influence periodontitis; and (3) The random forest model may have an issue with overfitting. Future studies should include samples from other regions and a more comprehensive analysis of factors that influence periodontitis, and construct a more complete prediction model.
In conclusion, the factors influencing periodontitis in patients with T2DM were identified using logistic regression analysis. In patients with T2DM, the greater the age and HbA1c, TC, and TG levels, the higher the risk of periodontitis. Our predictive models had good predictive value and could effectively predict the risk of periodontitis in patients with T2DM. The random forest and logistic regression prediction models can complement each other and provide a full analysis of the risk of disease and the importance of specific factors. In clinical practice, the results of this study can provide reference for the identification and intervention of early periodontitis in patients with T2DM.
Periodontitis is a complication of type 2 diabetes mellitus (T2DM). With lifestyle changes and the acceleration of the aging process, the prevalence of periodontitis and diabetes is increasing annually.
Periodontitis can lead to tooth loosening and loss, decline in oral function, and reduced living standards.
This study aimed to explore and analyze the factors influencing periodontal disease in patients with T2DM, and construct prediction models for the risk of periodontal disease in patients with T2DM.
We conducted a retrospective study in patients with T2DM hospitalized in our hospital to analyze the factors influencing periodontitis in patients with T2DM. We used random forest and logistic regression prediction models to assess the risk of specific factors in periodontitis.
This study found that the factors influencing periodontal disease in patients with T2DM were age, brushing frequency, education level, and glycosylated hemoglobin, total cholesterol, and triglyceride levels. The prediction models both had good predictive value.
In this study, a random forest model was established and compared to a logistic regression model. The results showed that the random forest and logistic regression models had good predictive value and can accurately predict the risk of periodontitis in patients with T2DM.
In the future, we will expand the sample size, combine samples from multiple regions, and include additional influencing factors to build a more complete prediction model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kazkayasi I, Turkey; Pappachan JM, United Kingdom; Skrlec I, Croatia S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Yan Y, Wu T, Zhang M, Li C, Liu Q, Li F. Prevalence, awareness and control of type 2 diabetes mellitus and risk factors in Chinese elderly population. BMC Public Health. 2022;22:1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 2. | Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, Pino P, Gamonal J. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. 2020;28:e20190248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 3. | Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 782] [Cited by in RCA: 1026] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 4. | Stöhr J, Barbaresko J, Neuenschwander M, Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021;11:13686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: association studies. Periodontol 2000. 2020;83:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 6. | Preshaw PM, Bissett SM. Periodontitis: oral complication of diabetes. Endocrinol Metab Clin North Am. 2013;42:849-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Nitta H, Katagiri S, Nagasawa T, Izumi Y, Ishikawa I, Izumiyama H, Uchimura I, Kanazawa M, Chiba H, Matsuo A, Utsunomiya K, Tanabe H, Takei I, Asanami S, Kajio H, Ono T, Hayashi Y, Ueki K, Tsuji M, Kurachi Y, Yamanouchi T, Ichinokawa Y, Inokuchi T, Fukui A, Miyazaki S, Miyauchi T, Kawahara R, Ogiuchi H, Yoshioka N, Negishi J, Mori M, Mogi K, Saito Y, Tanzawa H, Nishikawa T, Takada N, Nanjo K, Morita N, Nakamura N, Kanamura N, Makino H, Nishimura F, Kobayashi K, Higuchi Y, Sakata T, Yanagisawa S, Tei C, Ando Y, Hanada N, Inoue S. The number of microvascular complications is associated with an increased risk for severity of periodontitis in type 2 diabetes patients: Results of a multicenter hospital-based cross-sectional study. J Diabetes Investig. 2017;8:677-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Mikami R, Mizutani K, Matsuyama Y, Gohda T, Gotoh H, Aoyama N, Matsuura T, Kido D, Takeda K, Saito N, Fujiwara T, Izumi Y, Iwata T. Association of type 2 diabetes with periodontitis and tooth loss in patients undergoing hemodialysis. PLoS One. 2022;17:e0267494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Zou Y, Zhao L, Zhang J, Wang Y, Wu Y, Ren H, Wang T, Zhang R, Wang J, Zhao Y, Qin C, Xu H, Li L, Chai Z, Cooper ME, Tong N, Liu F. Development and internal validation of machine learning algorithms for end-stage renal disease risk prediction model of people with type 2 diabetes mellitus and diabetic kidney disease. Ren Fail. 2022;44:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Bian Y, Liu C, Fu Z. Application value of combination therapy of periodontal curettage and root planing on moderate-to-severe chronic periodontitis in patients with type 2 diabetes. Head Face Med. 2021;17:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Barutta F, Bellini S, Durazzo M, Gruden G. Novel Insight into the Mechanisms of the Bidirectional Relationship between Diabetes and Periodontitis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Romano F, Perotto S, Mohamed SEO, Giraudi M, Bernardi S, Durazzo M, Gruden G, Aimetti M. Type 2 diabetes mellitus and periodontitis: Are diabetic patients aware about this bidirectional association? Acta Diabetol. 2021;58:1277-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | de Miguel-Infante A, Martinez-Huedo MA, Mora-Zamorano E, Hernández-Barrera V, Jiménez-Trujillo I, de Burgos-Lunar C, Cardenas Valladolid J, Jiménez-García R, Lopez-de-Andrés A. Periodontal disease in adults with diabetes, prevalence and risk factors. Results of an observational study. Int J Clin Pract. 2018;e13294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Hong M, Kim HY, Seok H, Yeo CD, Kim YS, Song JY, Lee YB, Lee DH, Lee JI, Lee TK, Ahn HS, Ko YH, Jeong SC, Chae HS, Sohn TS. Prevalence and risk factors of periodontitis among adults with or without diabetes mellitus. Korean J Intern Med. 2016;31:910-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Eke PI, Wei L, Borgnakke WS, Thornton-Evans G, Zhang X, Lu H, McGuire LC, Genco RJ. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontol 2000. 2016;72:76-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 16. | Wu CZ, Yuan YH, Liu HH, Li SS, Zhang BW, Chen W, An ZJ, Chen SY, Wu YZ, Han B, Li CJ, Li LJ. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 17. | Qureshi A, Haque Z, Bokhari SAH, Baloch AA. Evaluation of HbA1c in type-2 diabetes mellitus patients with periodontitis: preliminary findings of three-arm clinical trial. J Pak Med Assoc. 2020;70:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Dhir S, Wangnoo S, Kumar V. Impact of Glycemic Levels in Type 2 Diabetes on Periodontitis. Indian J Endocrinol Metab. 2018;22:672-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Simpson TC, Clarkson JE, Worthington HV, MacDonald L, Weldon JC, Needleman I, Iheozor-Ejiofor Z, Wild SH, Qureshi A, Walker A, Patel VA, Boyers D, Twigg J. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2022;4:CD004714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 20. | Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 21. | Persson GR. Dental geriatrics and periodontitis. Periodontol 2000. 2017;74:102-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Ding C, Du F, Li L, Chen Y. Synergistic effect of blood lipids and uric acid on periodontitis in patients with type 2 diabetes. Am J Transl Res. 2023;15:1430-1437. [PubMed] |
| 23. | Jiménez-Corona M, Falcón-Flores J, Borges-Yáñez A, Castrejón-Pérez R, Jiménez-Corona A. Dyslipidemia and severe periodontitis among patients with type 2 diabetes. Salud Publica Mex. 2021;63:331-332. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Thomas B, Prasad RB, Shetty S, Vishakh R. Comparative Evaluation of the Lipid Profile in the Serum of Patients with Type II Diabetes Mellitus and Healthy Individuals with Periodontitis. Contemp Clin Dent. 2017;8:96-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Yamamoto T, Tanaka M, Kuribayashi N, Okuguchi F, Isotani H, Iwamoto M, Sugimoto H, Nakagawa O, Minabe M, Fuchida S, Mochida Y, Yokoyama H. Low education is associated with poor periodontal status in patients with type 2 diabetes mellitus: A cross-sectional study. Clin Exp Dent Res. 2021;7:419-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia. 2020;63:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 27. | Leite RS, Marlow NM, Fernandes JK, Hermayer K. Oral health and type 2 diabetes. Am J Med Sci. 2013;345:271-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Wang J, Xue T, Li H, Guo S. Nomogram Prediction for the Risk of Diabetic Foot in Patients With Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:890057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 29. | Wu B, Niu Z, Hu F. Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model. Diabetes Metab J. 2021;45:526-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |