Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1784
Peer-review started: August 8, 2023
First decision: September 19, 2023
Revised: October 18, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: December 15, 2023
Processing time: 128 Days and 6.9 Hours
The diagnosis of peripheral arteriopathy in the diabetic foot is complicated by diabetes and its advanced complications. It has been found that diabetic foot can be categorized into arterial stenosis and non-arterial stenosis, both of which have significant differences in hemodynamic characteristics.
To evaluate the early hemodynamic changes in diabetic foot patients with nonarterial stenosis and arterial stenosis treated by tibial transverse transport (TTT) using high-frequency color Doppler ultrasonography (HFCDU) and a laser Doppler flowmeter.
Twenty-five patients with Wagner grades 3-5 diabetic foot ulcers were treated with TTT, and the wound healing time and rate were recorded. Patients were grouped according to the results of preoperative lower-extremity ultrasonography. Cases with ≥ 50% stenosis in any of the femoral, popliteal, posterior tibial, anterior tibial, and peroneal arteries of the affected limb were classified as the arterial stenosis group (n = 16); otherwise, they were classified as the nonarterial stenosis group (n = 9). Before and one month after surgery, HFCDU was used to evaluate the degree of lower limb artery lesions and hemodynamic changes in patients. The degree of femoral-popliteal atherosclerotic stenosis, the degree of vascular stenosis and occlusion of the lower-knee outflow tract, and the degree of medial arterial calcification were scored; the three scores were added together to obtain the total score of lower extremity arteriopathy. PeriScanPIM3, a laser Doppler flowmeter system, was used to detect alterations in plantar microcirculation before and 1 mo after surgery. Wound healing and hemodynamic indices were compared between the two groups.
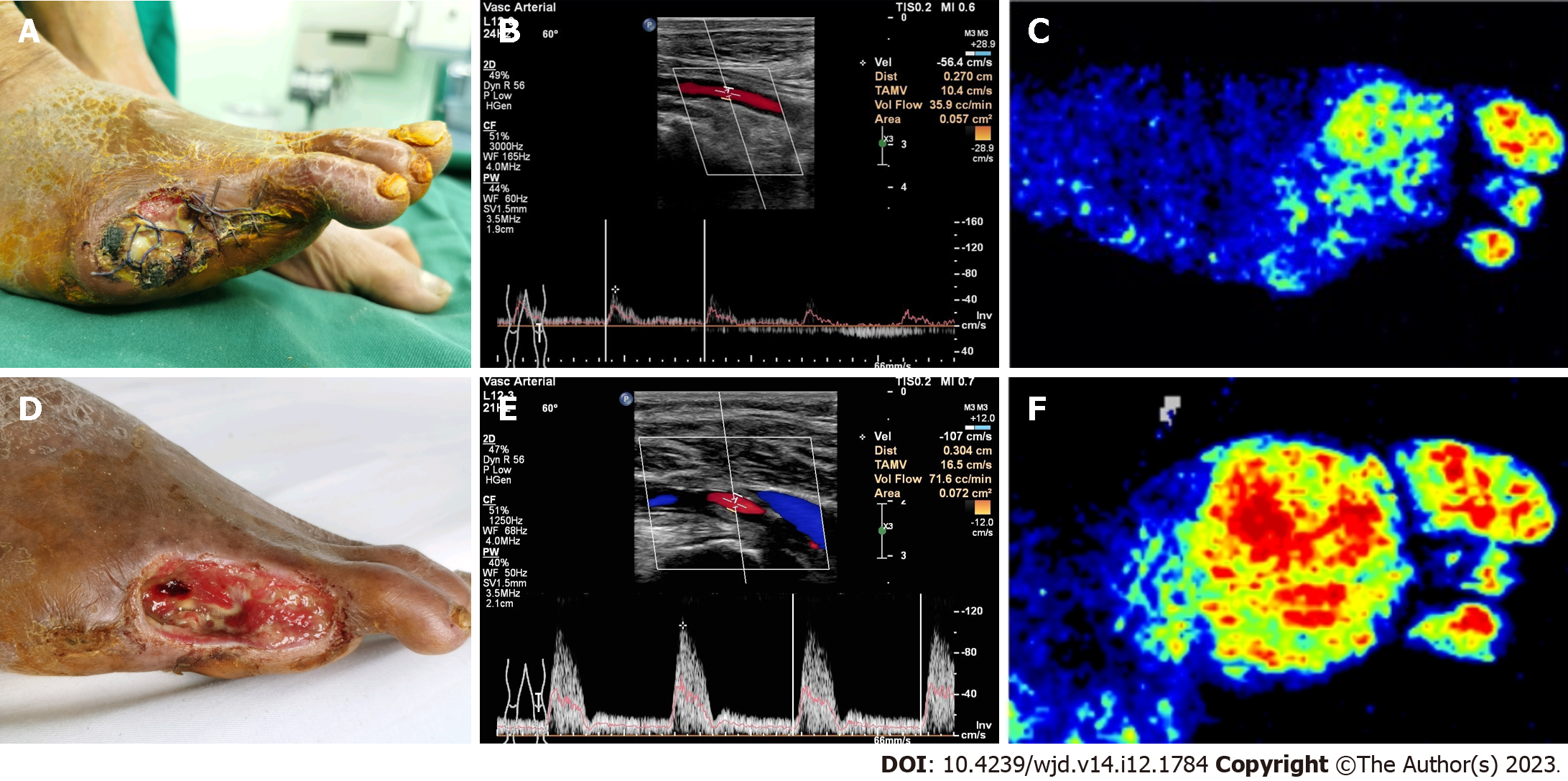
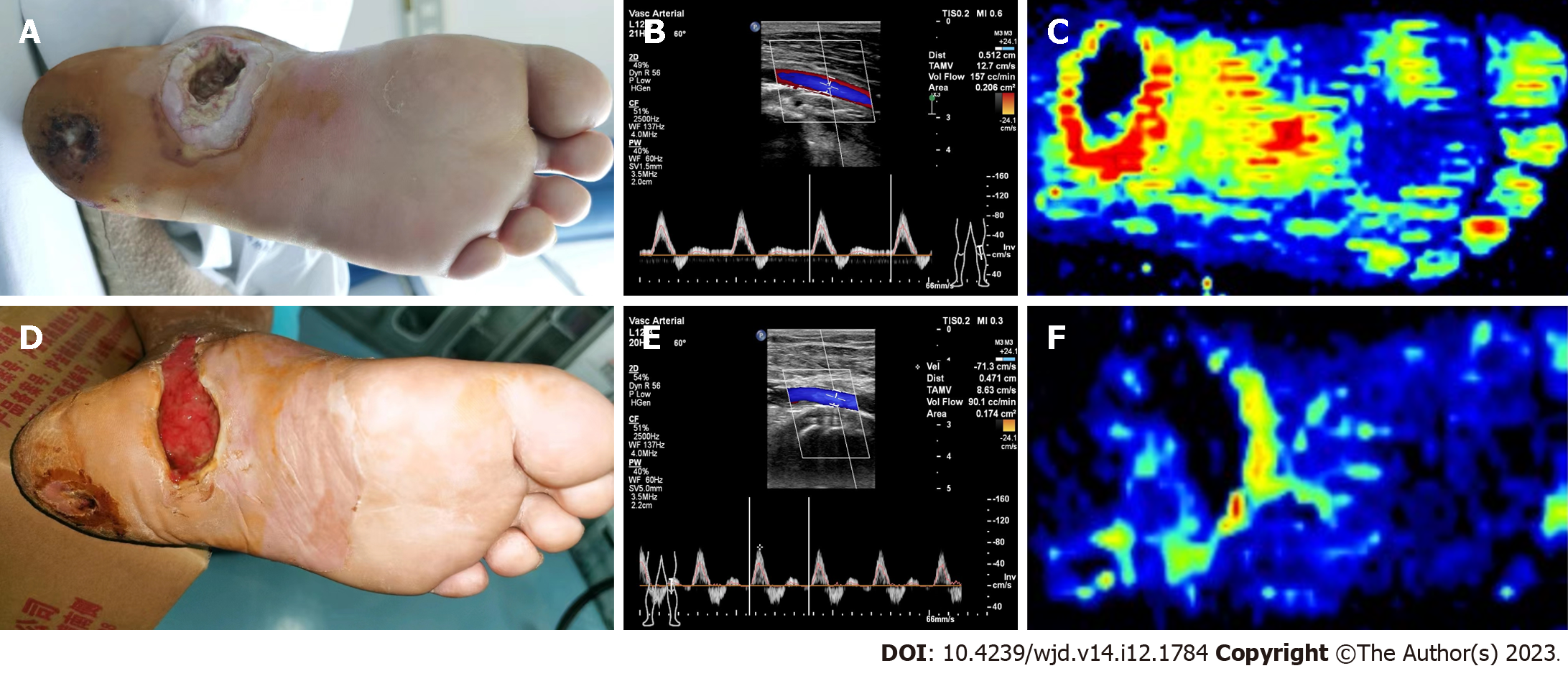
The wound healing time of the diabetic foot was significantly shorter in the nonarterial stenosis group than in the arterial stenosis group (47.8 ± 13 vs 85.8 ± 26, P < 0.05), and the wound healing rate of both groups was 100%. The preoperative total lower extremity arteriopathy scores were lower in the nonarterial stenosis group than those in the arterial stenosis group (18.89 ± 8.87 vs 24.63 ± 3.52, P < 0.05). The nonarterial stenosis group showed higher preoperative popliteal artery (POA) blood flow than the arterial stenosis group (204.89 ± 80.76 cc/min vs 76.75 ± 48.49 cc/min, P < 0.05). Compared with the baseline (before surgery), the postoperative POA blood flow of the affected limb in the nonarterial stenosis group decreased one month after surgery (134.11 ± 47.84 cc/min vs 204.89 ± 80.76 cc/min, P < 0.05), while that in the arterial stenosis group increased (98.44 ± 30.73 cc/min vs 61.69 ± 21.70 cc/min, P < 0.05). Although the POA blood flow in the arterial stenosis group was obviously improved one month after surgery, it was still lower than that in the nonarterial stenosis group (98.44 ± 30.73 cc/min vs 134.11 ± 47.84 cc/min, P < 0.05). The nonarterial stenosis group had higher preoperative plantar microcirculation than the arterial stenosis group (56.1 ± 9.2 vs 33.2 ± 7.5, P < 0.05); compared with the baseline, the plantar microcirculation in the arterial stenosis group was significantly improved one month after surgery (51.9 ± 7.2, P < 0.05), while that in the nonarterial stenosis group was reduced (35.9 ± 7.2, P < 0.05).
Based on preoperative HFCDU findings, diabetic foot patients can be divided into two categories: Those with nonarterial stenosis and those with arterial stenosis, with obvious differences in hemodynamic changes in the early postoperative period between them. In the early stage after TTT, the blood flow volume and velocity and the plantar microcirculation perfusion of the affected limb of the diabetic foot with nonarterial stenosis decreased compared with the baseline, while those of the diabetic foot with arterial stenosis improved significantly compared with the baseline, although both had smoothly healed diabetic foot ulcers.
Core Tip: Diabetes foot can be categorized into arterial stenosis and non-arterial stenosis, which were significantly different in hemodynamics characteristics. This study tended to explore the hemodynamic findings and comparison of non-arterial stenosis group diabetic foot and arterial stenosis group diabetic foot after tibial transverse transport (TTT). In the early stage after TTT, the blood flow volume and velocity and the plantar microcirculation perfusion of the affected limb of the diabetic foot with nonarterial stenosis decreased compared with the baseline, while those of the diabetic foot with arterial stenosis improved significantly compared with the baseline, although both had smoothly healed diabetic foot ulcers.
- Citation: Liao MM, Chen S, Cao JR, Wang MW, Jin ZH, Ye J, Ren YJ, Guo RQ. Early hemodynamics after tibial transverse transport in patients with nonarterial stenosis and arterial stenosis diabetic foot. World J Diabetes 2023; 14(12): 1784-1792
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1784.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1784
Diabetic foot, a serious diabetes-related complication, has a high overall mortality rate of approximately 50% within 5 years[1]. At present, there are many methods to treat diabetic feet, with blood sugar control and local wound mana-gement as the primary treatment goals. For most patients with Wagner grade 1 to 2 diabetic foot ulcers, wound healing can be achieved by controlling blood sugar, dressing changes, negative pressure wound therapy, etc. However, for diabetic ulcers categorized as Wagner grade 3 and above, traditional treatment methods are less effective. In recent years, Chinese orthopedic surgeons have taken the lead in treating diabetic foot with the tibial transverse transport (TTT) technique, especially for cases graded as Wagner 3 and above, with excellent results achieved. Since 2019, our hospital has conducted clinical research on the treatment of diabetic foot with TTT and has also achieved encouraging effects.
During the treatment, we observed that the skin sensation and temperature of the affected foot were obviously improved, and the wounds healed smoothly after TTT therapy. Owing to its convenience, noninvasiveness and cost-effectiveness, high-frequency color Doppler ultrasonography (HFCDU) was used to dynamically observe the changes in blood circulation in the affected limb. It was found that diabetic foot can be divided into arterial stenosis and nonarterial stenosis categories that are significantly different in terms of hemodynamic characteristics. Here, we summarize and report our research results.
Twenty-five patients with diabetic foot who underwent TTT in the Department of Orthopedics of Renmin Hospital of Wuhan University from January 2021 to August 2022 were selected. There were 20 males and 5 females aged 51 to 78 (mean: 63 ± 9.4) years, with Wagner grades 3, 4, and 5 confirmed in 4, 18, and 3 cases, respectively. Inclusion criteria: (1) Age: 18-80 years; (2) Wagner grade 3-5 diabetic foot; and (3) Good compliance and willingness to receive TTT therapy. Exclusion criteria: (1) > 75% stenosis in the lumen of the femoral popliteal artery (POA) on the affected side; (2) New cardio-cerebrovascular accidents within the last 3 mo; and (3) Inability to receive anesthetics due to contraindications to anesthesia. The Ethics Committee of Renmin Hospital of Wuhan University (Review Number: WDRY2022-K200) granted approval for this research, and informed consent was obtained from the subjects.
Ultrasonography: Instrument selection: A Philips EPIQ5 color ultrasonic diagnostic instrument was selected, with a linear array probe and a frequency range of 5.0-12 MHz. The common femoral artery (CFA), deep femoral artery, superficial femoral artery (SFA), POA, anterior tibial artery (ATA), posterior tibial artery (PTA), peroneal artery (PA), and dorsalis pedis artery were examined successively. The arterial inner diameter (ID), intima-media thickness (IMT), peak flow velocity (PFV), resistance index, and blood flow were measured. Atherosclerotic plaques of various blood vessels and medial arterial calcification (MAC) were observed. The lumen stenosis rate and the calcification rate were calculated, of which the stenosis rate was assessed by referring to the ultrasound criteria for the diagnosis of lower limb arterial disease established by Cossman et al[2]. Patients with ≥ 50% vascular stenosis in any of the femoral arteries, POA, PTA, ATA or PA of the affected limb were classified as the arterial stenosis group; otherwise, they were classified as the nonarterial stenosis group.
The score of femoral and popliteal atherosclerosis severity was based on less than 75% stenosis in the CFA, SFA or POA. The CFA, SFA and POA were scored according to the following criteria to assess the degree of femoral popliteal atherosclerosis. Scoring criteria: (1) IMT: Not thick (< 1 mm), 0 points; mild thickening (1-1.2 mm), 1 point; moderate and severe thickening (> 1.2 mm), 2 points; (2) Arterial plaques: 0 for normal (not found), 1 for single, 2 for multiple, and 3 for diffuse; and (3) Arterial stenosis: 0 for normal, 1 for 30%-49% stenosis, and 2 points for 50%-75% stenosis.
Below-knee artery outflow tract score: ATA, PTA, and PA were scored according to the outflow tract standard of the Society for Vascular Surgery[3]: ATA, PTA and PA were assigned scores according to the severity of the lesion, with 3 points for complete occlusion, 2.5 points for partial occlusion, 2 points for > 50% stenosis, 1 point for < 50% stenosis, and 0 for no stenosis. The outflow tract integral was the sum of the scores of these three arteries plus the base value of 1.
Lower-limb MAC score: Score was based on the length of the artery wall involved, with no calcification deposit, calcification range < 1/3, calcification range of 1/3-2/3, and calcification range > 2/3 being assigned 0, 1, 2, and 3 points, respectively. The MAC scores of the CFA, SFA, POA, ATA, PTA and PA were assessed, and the total score of each segment was summed. The POA blood flow and the ID and PFV of the ATA, PAT, PA and dorsalis pedis artery were measured.
Plantar microcirculation detection: PeriScanPIM3, a laser Doppler flowmeter system, was used to detect changes in plantar microcirculation before and 1 mo after surgery. The patient lay flat with the affected foot properly fixed, and a laser Doppler probe was placed on the sole to map plantar skin blood perfusion. Then, the anterior foot blood perfusion image was examined to determine the blood perfusion value of the corresponding area, which was the value of plantar microcirculation.
TTT: The operation was performed under nerve block or general anesthesia. After successful anesthesia, an about 10 cm arc incision was created in the medial middle of the tibia to separate the subcutaneous tissue of the periosteum. Then, the periosteum was cut along the medial tibia and peeled off to both sides of the tibia to determine the range of the bone window for tibial transport, which was 5 cm long and 1.5 cm wide. Next, two 2 mm external fixation screws were screwed into the bone window to move the bone block, followed by separation and removal of the bone block with a drill and a pendulum saw. During this process, attention was given not to damage the bone marrow in the medullary cavity, so that it formed a movable bone flap. Two external fixation screws with a diameter of 4 mm were then screwed into the proximal and distal tibia sides of the bone window, after which the lateral tibial transport frame was installed, adjusted and tightened, the bone transport direction was marked, and the periosteum, subcutaneous tissue and skin were closed layer by layer. During the operation, the ulcer lesions were thoroughly debrided, the abscess cavity was opened, the necrotic and inactivated skin and tendons were removed, the damaged tarsal bone and the blackened toe that lost blood supply were cut off, and the wound was bandaged with sterile dressing. The wound dressing was applied postoperatively. Bone transport was performed one week later, and wound healing was recorded.
The data were analyzed by SPSS 22.0 statistical software. Continuous variables, described as the mean ± SD, were compared between groups by one-way ANOVA and within the same group before and one month after surgery by the paired t test. In all tests, a significance level of 5% (P < 0.05) was adopted.
All 25 patients underwent bone transport 1 wk after surgery, with the external fixator removed 4 wk later. The wound healing time of the diabetic foot was significantly shorter in the nonarterial stenosis group than in the arterial stenosis group (47.8 ± 13 vs 85.8 ± 26, P < 0.05), and the wound healing rate of both groups was 100%, as shown in Table 1.
| Male/female | Age (yr) | Wagner grade | Non-arterial stenosis (n = 9) | Arterial stenosis (n = 16) | ||||
| 3 | 4 | 5 | ||||||
| Patients (n = 25) | 20/5 | 63.0 ± 9.4 | 4 | 18 | 3 | The wound healing time (d) | 47.8 ± 13 | 85.8 ± 26 |
| t value | 4.090 | |||||||
| P value | 0.0004 | |||||||
The total lower extremity arteriopathy scores were markedly lower in the nonarterial stenosis group than those in the arterial stenosis group (18.89 ± 8.87 vs 24.63 ± 3.52, P < 0.05). In addition, the nonarterial stenosis group showed higher preoperative POA blood flow (cc/min) than the arterial stenosis group (204.89 ± 80.76 vs 76.75 ± 48.49, P < 0.05); one month after surgery, the POA blood flow (cc/min) was lower than the baseline in the nonarterial stenosis group (134.11 ± 47.84 vs 204.89 ± 80.76, P < 0.05), while the POA blood flow was higher than the baseline in the arterial stenosis group (98.44 ± 30.73 vs 76.75 ± 48.49, P < 0.05). One month after surgery, the POA blood flow in the arterial stenosis group was obviously improved, but it was still lower than that in the nonarterial stenosis group (98.44 ± 30.73 vs 134.11 ± 47.84, P < 0.05), as shown in Table 2.
The nonarterial stenosis group had better plantar microcirculation than the arterial stenosis group before surgery (56.1 ± 9.2 vs 33.2 ± 7.5, P < 0.05). One month after surgery, plantar microcirculation was significantly improved in the arterial stenosis group compared with the baseline (51.9 ± 7.2, P < 0.05), while it was reduced in the nonarterial stenosis group (35.9 ± 7.2, P < 0.05), as shown in Table 3. Typical cases are shown in Figures 1 and 2.
The development of the TTT technology is considered one of the milestones of orthopedic surgery in the 20th century and was originally created by Professor Ilizarov in accordance with the “Law of tension-stress” of tissue regeneration and the concept of “Natural Reconstruction”. Scholars observed significant improvement in the blood circulation of the distal limbs of patients who underwent TTT[4], so they tried to use this technique to treat ischemic diseases of the lower limbs. In 2014, inspired by the use of TTT for the treatment of lower extremity ischemic disease, the team led by Professor Hua Qi-kai from the First Affiliated Hospital of Guangxi Medical University took the lead in attempting to treat diabetic feet using TTT, and the early clinical effect was satisfactory.
In TTT-related animal and clinical studies, scholars have confirmed through angiography and histology that in the process of bone transport, a large number of new blood vessels are generated around the transported bone block, including the end of the limb, constituting collateral circulation. In an animal experiment of TTT, Ilizarov[5,6] found that with longitudinal bone transport, damage to the bone marrow led to osteogenesis due to the influence of the lateral tensile stress vector, and capillaries, mainly continuous capillaries and sinusoidal capillaries, began to form 7 d after bone transport; the growth rate of blood vessels exceeded the transport rate on day 21 after bone transport, and the blood vessels became tortuous; angiography showed that a rich capillary network was formed around the bone transport block, and the density of blood vessels at the distal end of bone transport was also significantly increased. Cao et al[7] conducted experiments of TTT in dogs and confirmed that with the distraction of the external fixator, obvious microvascular regeneration could be seen in the bone transport area, extending distally along the trunk; moreover, both digital subtraction angiography and tissue hematoxylin and eosin staining confirmed the formation of a large number of microvascular networks around some of the main blood vessels. Through computed tomography angiography and perfusion imaging, some studies found that compared with preoperative patients, diabetic foot patients showed angiogenesis and increased blood perfusion within 3 mo after TTT[8,9], consistent with the growth of granulation tissue during ulcer healing[9]. These results are consistent with those observed in the arterial stenosis group in this study, suggesting that promoting angiogenesis and improving microcirculation in ischemic limbs or lesions is an important mechanism of TTT in the treatment of diabetic feet.
In this study, there was no obvious stenosis in the main artery of the affected limb in the nonarterial stenosis group preoperatively and no obvious abnormalities in the POA blood flow volume or velocity despite abundant plantar microcirculation, but diabetic foot ulcers still persisted and did not heal, suggesting that vascular lesions or hemodynamic factors may not be the main cause of diabetic foot ulcers in such patients. B-ultrasound showed that the POA blood flow decreased one month after surgery compared with the baseline (before surgery), and correspondingly, the perfusion of plantar microcirculation decreased significantly. After TTT treatment, the granulation of the injured foot wound was fresh, the wound surface was obviously reduced, and finally, the ulcer healed smoothly, demonstrating that TTT can effectively treat diabetic foot ulcers without vascular stenosis. The significantly decreased rather than increased blood perfusion of the affected limb suggests that angiogenesis and increased blood perfusion may not be the main mechanism of TTT in the treatment of nondiabetic diabetic feet.
Depending on etiology, diabetic foot ulcers can usually be divided into three types: Neuropathic, neuroischemic, and simple ischemic ulcers[10,11]. Most diabetic foot patients have neuropathy, and approximately 15% to 20% suffer from both neuropathy and peripheral artery diseases[12]. Neuropathy is one of the most common pathogenic factors of diabetic foot[11,13]. Pecoraro et al[12] attributed up to 82% of amputations in patients with diabetes to neuropathy, highlighting neuropathy as one of the main causes of diabetic foot ulcers. Diabetic neuropathy can involve motor, sensory, and autonomic nerve fibers, and its manifestation includes three symptoms. First, motor neuropathy causes muscle atrophy and weakness, resulting in an imbalance of strength in the foot flexor and extensor muscles. This imbalance can lead to a protrusion of the metatarsal bones and increased pressure on the local skin, resulting in a “claw toe” appearance. Second, sensory neuropathy causes a reduction in or loss of sensation, including pain, temperature, vibration and other stimuli, and repeated trauma to the feet, which contributes to ulcer formation[14]. Third, autonomic neuropathy induces autonomic nerve dysfunction, thereby impairing the sweating function of the lower limbs and causing dry and cracked skin, which makes the foot skin prone to repeated minor injuries[14]. Deformities and repeated injuries of the foot cause the skin of the foot to become swollen, predisposing it to ulcers.
In clinical practice, diabetic foot wounds recover normal skin coverage after TTT treatment instead of scar filling, suggesting that TTT may activate the tissue regeneration ability of the body through a certain mechanism that also facilitates skin regeneration[15]. In a study of TTT in the treatment of severe diabetic feet, the authors found that patients with diabetic foot experienced a significant reduction in numbness and improved skin sensation 2-3 mo after surgery and began to develop sensation after 6 mo, suggesting that nerve fibers may have regenerated in the affected limb during TTT treatment[16]. In related basic research, some researchers found that the local stretching effect of TTT can activate stem cells, improve peripheral blood levels of stromal cell-derived factor-1, vascular endothelial cell growth factor and other related factors, and regulate and mobilize the activation of stem cells. Moreover, it can improve the polarization of macrophages in distant wound tissue and balance the degree of inflammatory reaction by reducing the ratio of M1/M2 and regulating its composition to improve the “microenvironment” of wound healing and promote skin stem cells to participate in the regeneration and healing of ulcers[9,17]. Fos/Jun-related genes, FAK, the Wnt pathway and the Hippo pathway are activated during distraction osteogenesis, thus stimulating the regeneration potential of bone tissue. All the above pathways are closely associated with embryonic bone development or cell differentiation, suggesting that stimulating endogenous regeneration potential during embryonic development is the core principle of the Ilizarov technique for tissue regeneration[18]. Therefore, it can be inferred that TTT may help to regenerate tissue of the affected limb through some mechanism, and this regeneration may be include the vascular, nerve and skin systems, but further exploration regarding the regeneration mechanism is needed.
In clinical work, patients with diabetic foot can be divided into those with arterial stenosis and those with nonarterial stenosis according to the extent of stenosis of the femoral POA and three inferior genicular arteries on HFCDU images, and the hemodynamic changes in the early postoperative period between the two categories are obviously different. Compared with the preoperative conditions, the postoperative hemodynamic indices were significantly improved in patients with arterial stenosis and decreased in patients with nonarterial stenosis, although both had smoothly healed diabetic foot ulcers. It is suggested that TTT may have other possible mechanisms in addition to promoting angiogenesis and improving the microcirculation of the affected limb. TTT may achieve tissue regeneration of the affected limb through some mechanism, and this regeneration may be comprehensive, involving vascular, nerve, and skin regeneration. However, this regeneration mechanism needs to be further explored.
However, there are still some limitations in this study. First, this is a single-center, nonrandomized observational study with an insufficient sample size, thus further validation of the phenomena observed and the inferences expressed is warranted. Second, all 25 patients with diabetic foot were treated with TTT. Although encouraging results have been achieved, the choice of this method is largely based on the clinical experience of physicians, and no randomized controlled trials have been conducted. Finally, the pathological and molecular biological mechanisms of TTT in treating diabetic foot need further in-depth research.
At present, there are many methods to treat diabetic feet, with blood sugar control and local wound management as the treatment principle. For most patients with Wagner grade 1 to 2 diabetic foot ulcers, wound healing can be achieved by controlling blood sugar, dressing change, negative pressure wound therapy, etc. But for Wagner grade 3 and above, the traditional treatment methods are less effective.
During the treatment, we observed that the skin sensation and temperature of the affected foot were obviously improved and the wounds healed smoothly after the tibial transverse transport (TTT) therapy. It was found that the diabetic foot can be divided into arterial stenosis and non-arterial stenosis categories that were significantly different in hemodynamic characteristics.
To evaluate the early hemodynamic changes in patients with non-arterial stenosis and arterial stenosis diabetic foot treated by TTT.
Twenty-five patients with Wagner grade 3-5 diabetic foot ulcers were treated with TTT, and the wound healing time and rate were recorded. Patients were grouped according to the results of preoperative lower-extremity ultrasonography, classified as arterial stenosis group (n = 16); otherwise, they were classified as non-arterial stenosis group (n = 9). Before and one month after surgery, high-frequency color Doppler ultrasonography (HFCDU) was used to evaluate the degree of lower limb artery lesions and hemodynamic changes of patients. The degree of femoral-popliteal atherosclerotic stenosis, the degree of vascular stenosis and occlusion of the lower-knee outflow tract, and the degree of medial arterial calcification were scored; the three scores were added together to obtain the total score of lower extremity arteriopathy. Alterations in plantar microcirculation before and 1 mo after surgery were detected. Wound healing and hemodynamic indexes were compared between the two groups.
The wound healing time of diabetic foot was significantly shorter in non-arterial stenosis group than in arterial stenosis group, and the wound healing rate of both groups was 100%. Non-arterial stenosis group showed higher preoperative popliteal artery (POA) blood flow than arterial stenosis group. Although the POA blood flow in arterial stenosis group was obviously improved one month after surgery, it was still lower than that in non-arterial stenosis group. Non-arterial stenosis group had higher preoperative plantar microcirculation than arterial stenosis group.
Patients with diabetic foot can be divided into arterial stenosis and non-arterial stenosis according to the stenosis of femoral POA and three inferior genicular arteries by HFCDU, and the hemodynamic changes in the early postoperative period between the two categories are obviously different.
TTT may achieve tissue regeneration of the affected limb through some mechanism, and this regeneration may be comprehensive, involving vascular, nerve, and skin regeneration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Groop PH, Finland; Mrozikiewicz-Rakowska B, Poland S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: A systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2023;25:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 94] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 2. | Cossman DV, Ellison JE, Wagner WH, Carroll RM, Treiman RL, Foran RF, Levin PM, Cohen JL. Comparison of contrast arteriography to arterial mapping with color-flow duplex imaging in the lower extremities. J Vasc Surg. 1989;10:522-8; discussion 528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2587] [Cited by in RCA: 2573] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 4. | Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994;124-131. [PubMed] |
| 5. | Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;249-281. [PubMed] |
| 6. | Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;263-285. [PubMed] |
| 7. | Cao XJ, Jia ZW, Guo XS, Wang XD, Yu JP, Niu JW, Liu JY, Wei J. [Experimental study on the improvement of Ilizarov transverse tibial bone transport and microcirculation reconstruction technique]. Zhonghua Yi Xue Za Zhi. 2019;99:3592-3596. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zeng Z, Dong Y, Hua Q, Kuang X, Li K, Deng X, Qiu S. Computed tomography perfusion study evaluating the curative effect of tibial transverse transport in patients with severe diabetic foot. J Orthop Translat. 2019;19:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Kuang X, Zhou J, Zhen P, Zeng Z, Lin Z, Gao W, He L, Ding Y, Liu G, Qiu S, Qin A, Lu W, Lao S, Zhao J, Hua Q. Proximal Tibial Cortex Transverse Distraction Facilitating Healing and Limb Salvage in Severe and Recalcitrant Diabetic Foot Ulcers. Clin Orthop Relat Res. 2020;478:836-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA; IWGDF Editorial Board. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 11. | Cheng B, Jiang Y, Fu X, Hao D, Liu H, Liu Y, Huang Z, Tan Q, Wang L, Hu D, Yang Y, Han C, Cheng Z, Ran X, Li Y. Epidemiological characteristics and clinical analyses of chronic cutaneous wounds of inpatients in China: Prevention and control. Wound Repair Regen. 2020;28:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 936] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 13. | Sela Y, Grinberg K, Cukierman-Yaffe T, Natovich R. Relationship between cognitive function in individuals with diabetic foot ulcer and mortality. Diabetol Metab Syndr. 2022;14:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Speight S, Morriss-Roberts C. What is the Lived Experience of the 'Three Great Pathologies' of Diabetic Foot Disease? An Interpretative Phenomenological Analysis of the Independent Thinking of Podiatrists in Diabetes Secondary Care. Inquiry. 2022;59:469580221088622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Ou S, Xu C, Yang Y, Chen Y, Li W, Lu H, Li G, Sun H, Qi Y. Transverse Tibial Bone Transport Enhances Distraction Osteogenesis and Vascularization in the Treatment of Diabetic Foot. Orthop Surg. 2022;14:2170-2179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 16. | Zhen P, Chen Y, Gao W, Lin Z, Zhong Z, Teng Z, He L, Hua Q. [The effectiveness of Ilizarov technique-based transverse tibial bone transport on treatment of severe diabetic foots complicated with systemic inflammation response syndrome]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Ding X, Zhu Y, Jia Z, Qi Y, Chen M, Lu J, Kuang X, Zhou J, Su Y, Zhao Y, Lu W, Zhao J, Hua Q. Effect of tibial cortex transverse transport in patients with recalcitrant diabetic foot ulcers: A prospective multicenter cohort study. J Orthop Translat. 2022;36:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Jordan CJ, Goldstein RY, McLaurin TM, Grant A. The evolution of the Ilizarov technique: part 1: the history of limb lengthening. Bull Hosp Jt Dis (2013). 2013;71:89-95. [PubMed] |