Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1721
Peer-review started: July 24, 2023
First decision: August 17, 2023
Revised: August 31, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: December 15, 2023
Processing time: 142 Days and 13.6 Hours
Diabetes mellitus (DM) is a chronic metabolic condition characterized predominantly by hyperglycemia. The most common causes contributing to the patho
Core Tip: Diabetes mellitus (DM) is a chronic metabolic condition characterized by hyperglycemia. Major contributing factors are insufficient insulin secretion, insulin resistance, or both. Global diabetes prevalence has risen from 4% to 6.4% in the past 30 years and may reach 430 million in the future. Age, obesity, and a sedentary lifestyle exacerbate the disease. Developing safe and effective therapies is crucial. Medications like glucagon-like peptide-1 agonists, thiazolidinediones, and others have been available for over a decade. However, clinical observations suggest ongoing research. This review focuses on insulin regulation targets for potentially more efficient and secure diabetes treatments.
- Citation: Sun HY, Lin XY. Analysis of the management and therapeutic performance of diabetes mellitus employing special target. World J Diabetes 2023; 14(12): 1721-1737
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1721.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1721
Mellitus, short for “sugar disease”, pertains to the endocrine conditions known as diabetes. Diabetes mellitus (DM), one of the most common conditions, is presently the seventh leading cause of death, with 5.2 million deaths worldwide[1]. Diabetes that is either untreated or poorly managed is thought to be the cause of 1.5 million deaths annually globally. From 108 million cases (4.7%) in 1980 to 425 million patients (8.5%) in 2017, it is expected that 629 million people will have diabetes by 2045. The cost of treating diabetes worldwide is predicted to be 760 billion USD per year, with costs being the same for men and women[2]. Both inadequate insulin production by the pancreas or elevated glycosylated hemoglobin and improper insulin response by bodily cells contribute to the development of DM[3]. In addition, the development of diabetes can be influenced by a wide variety of factors, including a lack of physical activity, excessive consumption of food and beverages, obesity, stress, and industrialization. Environmental and genetic factors are the primary causes of diabetes[4]. Diabetes can cause many health problems if not treated, such as chronic hyperglycemia, which can cause long-term damage to the blood vessels, heart, eyes, nerves, kidneys, and other organs[5].
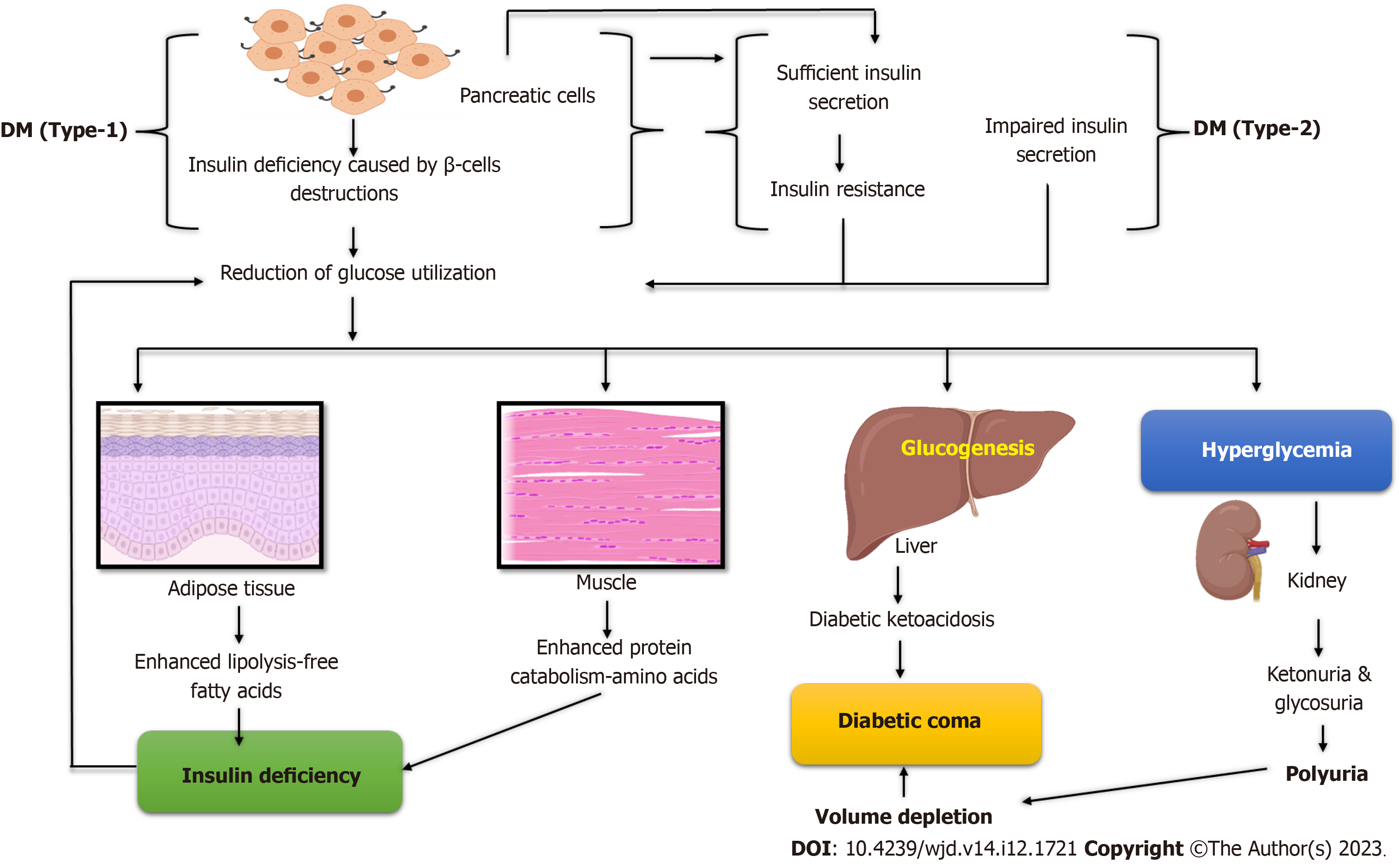
Diabetes is classified into three types: Type 1 diabetes (T1D), type 2 diabetes (T2D), gestational diabetes (GD), and other variants. T1D affects approximately 5%-10% of individuals diagnosed with it, usually young children and teenagers[6]. A complete lack of insulin production brings on type 1 DM (T1DM). Type 2 DM (T2DM), which is much more com
The proportion of the aging population is increasing, and this trend is explained by urbanization, socioeconomic growth, highly processed diets, and a decline in physical exercise. Untreated diabetes typically causes unintentional weight loss, increased excretion, increased thirst, and increased appetite[10]. T1DM symptoms can appear suddenly, whereas T2DM symptoms typically appear much more gradually and may even be nonexistent. About half of the people do not realize they have T2D because there are few symptoms or signs in the early stages of the disease. As a result, symptoms go undetected and lead to complications associated with diabetes[11]. Glycated hemoglobin (HbA1C), fasting plasma glucose level (126 mg/dL), and plasma glucose (200 mg/dL) tests are used to identify DM. Nowadays, there is no validated prophylactic method for T1DM. By maintaining a healthy body weight, getting exercise, and adhering to a wholesome diet, T2DM can be prevented. Higher levels of physical activity (> 90 min/d) reduce diabetes risk by 28%[11].
Diabetes management aims to keep blood sugar levels near normal without lowering them. This is usually accom
Several hormones cooperate to maintain an appropriate amount of glucose in the body. However, two hormones, insulin and glucagon, dominate in regulating glucose homeostasis. When the level of glucose increases, cells in the pancreatic islets of Langerhans produce insulin. Insulin lowers blood sugar levels by preventing the liver’s synthesis of glucose through glycogenolysis and gluconeogenesis[13] or by boosting the liver, muscle, and fat tissues’ glucose uptake, except for soft muscle, where insulin functions via insulin-like growth factor-1. Therefore, all types of DM are caused by insulin deficiency or receptor insensitivity. Insulin has the following effects: Decreasing gluconeogenesis and inhibiting gluconeogenesis, promoting glucose transport into adipose and muscle cells, and raising glycogen storage[14].
Fewer beta cells produce insulin when glucose levels are low, and less glycogen is converted into glucose. The pan
Additionally, low glucose levels in the blood increase hunger, leading to overeating (polyphagia)[17]. Cortisol and catecholamines also raise plasma glucose levels in addition to glucagon. Glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1 (GLP-1), and amylin are additional hormones that help to maintain an average blood glucose level [glucose-dependent insulinotropic polypeptide (GIP)][18].
Along with insulin, amylin is released. It lessens stomach emptying, improving glucose absorption following a meal. Incretins or peptides produced from the gut include GLP and GIP. These incretins help the pancreatic beta cells produce and secrete insulin[19]. Neither the intestine nor cells in need of energy can easily absorb glucose. Therefore, glucose transporters are responsible for delivering glucose to the cells. Sodium-glucose co-transporter (SGLT) and facilitative glucose transporter are two examples of the two kinds of glucose transporters, which are a family of membrane-bound glycoproteins (GLUT)[20]. The interplay of genetic and environmental factors largely determines T2D. The risks increase with increasing levels of overweight or obesity. Hormonal changes that arise during pregnancy are the cause of GD. Hor
The most common forms of monogenic diabetes or maturity-onset diabetes of the young are diabetes at birth and diabetes that develops in early adults. Thick mucus is produced by cystic fibrosis, which prevents the pancreas from producing enough insulin, leading to pancreatic scarring. The body stores an excessive amount of iron due to hemochromatosis. Iron can accumulate in the body and harm other organs, including the pancreas, if the condition is not treated[20]. High hormone production levels in the body are a symptom of some hormonal illnesses, which can lead to insulin resistance and diabetes in some people. Excessive levels of cortisol, also known as the stress hormone, cause Cushing’s syndrome[21]. Too much growth hormone causes acromegaly[22,23]. When the thyroid gland generates too much thyroid hormone, hyperthyroidism develops. Diabetes is caused by pancreatic damage or removal, including pancreatitis, pancreatic cancer, and trauma. These conditions have the potential to cause harm to β-cells or decrease their ability to produce insulin. Diabetes develops if the damaged pancreas is removed due to β-cell loss[24] (Figure 1).
Diabetes management aims to boost output and quality of life for people with diabetes by: (1) Early detection; (2) Long-term and short-term morbidity prevention; (3) Early death prevention are all examples of early diagnosis[25]; (4) Supporting diabetes patients’ freedom and self-care habits; (5) Reduction of the personal, family, and societal burden of diabetes; and (6) Achieving these objectives depends on the facilities and diabetes health care team being successfully established. This involves educating those with diabetes and healthcare professionals[26].
A glucose meter is used to test blood sugar levels, and the results are displayed either in mg/dL or mmol/L of blood. A healthy individual’s average fasting glucose level is 4.5 mmol/L (81 mg/dL), ranging from 65 to 98 mg/dL at its lowest and highest points, respectively[27]. The most effective method to manage diabetes is for each patient to keep track of their blood glucose levels and how exercise and food affect them. Patients can improve their diabetes management by changing their habits[28].
A hypoglycemic episode is a glucose level of 3.8 mmol/L. 55% of cases of severe hypoglycemia occur during sleep in T1D that is well-controlled. 6% of fatalities in people with diabetes under 40 are attributed to nocturnal hypoglycemia (dead-in-bed syndrome)[29]. According to the National Institute of Health data, hypoglycemia accounts for 2% to 4% of all diabetic deaths. After intensive glucose control, 21% of hypoglycemia incidents in children and adolescents were unexplained. In addition to being fatal, hypoglycemia can also cause cerebral damage during severe episodes. Although glucose is typically linked to diabetic nerve disease, hypoglycemia can also start or exacerbate neuropathy in people with diabetes who are actively trying to lower their hyperglycemia[30]. It is essential to carefully monitor levels above 230-270 mg/dL, regarded as high and should be brought down rather than allowed to stay high. Hyperglycemia is the term for high blood sugar levels, which is harder to spot than hypoglycemia and typically develops over days instead of hours or minutes. If unattended, this may cause a diabetic coma and mortality[31].
In medicine, the word “GC” refers to the typical blood sugar levels of a person with DM. Numerous pieces of evidence indicate that years of hyperglycemia cause multiple serious issues of diabetes, especially complications of microvascular origin. Effective glycemic control (GC), in the sense of a “target” for treatment, has become a crucial aim of diabetes care despite recent research suggesting that genetic factors may be accountable for the complications of diabetes[32]. T1D is brought on by the autoimmune condition that first rendered the pancreas incapable of making insulin. Because blood sugar levels vary throughout the day and glucose records are unreliable indicators of these changes, the quantity of HbA1C is a substitute indicator of long-term GC in research studies and clinical therapy for people with diabetes[33].
The hemoglobin A1c test, or HbA1C, measures the average glucose levels over the two to three months prior. By the most popular measures, HbA1C is typically 4%-6% in non-diabetic individuals with average glucose metabolism Blood glucose and HbA1C levels of 11-28 mmol/L and 9%-15% or higher, respectively, over months and years before severe complications develop, are indicative of poor GC[34]. There has been no difference in all-cause mortality, nonfatal stroke, or limb amputation in extensive studies comparing the impact of strict GC to conventional or more relaxed GC in T2D. Still, there has been a 15% decrease in the risk of nonfatal coronary artery disease[35]. Despite being linked to a 2.4-fold higher risk of hypoglycemia, strict glucose control is also related to a lower risk of retinopathy and nephropathy and a lower incidence of peripheral neuropathy[36].
Regular home glucose meter use by patients, especially those with T1D, may improve management and outcomes for both type 1 and 2 diabetes. Keeping tabs on one’s glucose levels is time-consuming and labor-intensive, not to mention costly. Monitoring blood glucose levels helps keep the illness under control and lessen the likelihood of serious complications later on[37]. There are many different kinds of blood monitoring devices, and each one works for every patient. For those with T1D, self-testing is crucial because insulin therapy can result in low blood sugar (hypoglycemia), and home testing allows you to adjust the dosage each time you administer insulin. A new study suggests that self-monitoring does not improve blood glucose control or quality of life, even though its efficacy in T2D has been more controversial. Despite home blood glucose monitoring, type 2 patients are considered to have poor long-term management[38].
Continuous glucose monitoring (CGM) technology has improved to provide data regarding the pace and pattern of glucose changes in people with diabetes. The accuracy of these devices is improving with each new advancement, al
The measurement of blood HbA1c levels is a valuable test typically performed in a laboratory. This is the proportion of HbA1C to overall hemoglobin. The percentage of these molecules increases as plasma glucose levels remain elevated. This test, once thought to assess the average level of diabetic control over about three months, has been suggested to emphasize the most recent 2 to 4 wk[41]. HbA1c levels range from 4.0 to 6.0 in people who don’t have diabetes. People with diabetes whose HbA1c levels stay < 6.5% are said to have reasonable GC[37]. If diet or treatment adjustments have been made within the last six weeks, or if there is a hemoglobinopathy or a disruption in red cell aging, the HbA1c test is inappropriate. The alternative Fructosamine test shows standard control over the previous two to three weeks[42].
People with T2DM can lower their blood sugar levels by sharing their electronic health data with them. It is a method of assisting individuals in understanding their health conditions and actively participating in their administration. About 100000 health-related apps are available on Google Play and the App Store, and the most general category is diabetes applications[43]. Routine self-management activities such as taking medication and insulin, monitoring blood sugar levels, adhering to a diet, and participating in physical exercise present significant challenges. Nevertheless, despite the many applications available, only a relatively small percentage of patients use them[44].
Furthermore, a 2016 study of 65 Android diabetes apps discovered that confidential information, such as insulin and blood sugar levels, “was routinely collected and shared with third parties”. One study investigates how a T2D Android mobile application can integrate supporting hardware such as an exercise bike, a treadmill, a heart-rate sensor, a wearable band, and a glucometer. This mobile program includes drugs, food consumption, exercise, and sleep tracking. Adesina et al[45] examine the effectiveness and applicability of digital tools designed to assist women with GD dietary self-mana
The likelihood of diabetic foot ulcers can be predicted by keeping an eye on a person’s feet. A standard method is using a specialized thermometer to check for hotspots on the foot that could be signs of an ulcer. However, there is scant reliable research on the benefits of tracking foot temperature at home[46]. This technique is intended to supplement, not replace, people who regularly check their feet[47].
A healthy diet with some carbohydrates; over time, consuming a consistent quantity of carbohydrates is beneficial to help T1DM patients better control their blood sugar levels. There is insufficient proof that low-carbohydrate diets benefit individuals with T1D[48]. However, it may be possible for some people to follow a low-carbohydrate diet and carefully manage their insulin doses[49].
In addition to lowering blood sugar levels, exercise can increase insulin sensitivity and lower the chance of diabetes-related heart disease[50]. Numerous studies have demonstrated that exercise aids glycemic management and reduces HbA1c levels by about 4.2 mmol/mol (0.6%). Studies have shown that individuals with T2D who participate in both physical activity and dietary changes have a lower risk of developing impaired glucose tolerance[51]. Physical activity has an impact on T1D glucose management in that near-exercise energy expenditure rises to account for possible hypo
The vast majority of drugs for diabetes work by lowering blood sugar levels in various ways. There is widespread consensus that people with diabetes who maintain tight glucose control and keep their blood glucose levels within normal limits have fewer complications, such as kidney or eye problems. Several distinct types of anti-diabetic medi
Even though biomedicine has made a lot of progress and we are learning more and more about how to treat different diseases, diabetes is still hard to treat. To solve this problem, researchers from various fields are looking for a way to treat diabetes that is both safe and easy[57]. In addition, rigorous evaluation of the drug action mechanisms of known com
In contrast to several extant synthetic medicines, natural biomolecules have a wide range of structural variability and have emerged as a valuable source of active agents for developing newer lead compounds in drug discovery[58]. Anti-diabetic medications like dipeptidyl peptidase 4, thiazolidinedione, sulfonylurea, or metformin inhibitors are currently used to treat DM. However, these drugs cannot entirely limit diabetes, and future studies are required to find a better cure[59].
In biological systems, receptors are chemical structures composed of proteins that receive and transmit signals. These are some of the receptors and medications that are currently used to treat diabetes, including thiazolidinediones, gliptins, GLP-1, glinides, biguanides, insulin, peroxisome proliferator-activated receptors (PPARs), sulphonylureas, β-glucosidase inhibitors, amylin mimics, SGLT-2, and dopamine D-2 agonists[60]. Pro-hormone convertases (PC I and PC 2) and exo-protease carboxypeptidase make insulin from pro-insulin. These enzymes produce insulin and C-peptide[61]. Insulin faci
Exenatide and liraglutide are two examples of GLP-1 analogs that replicate the effects of endogenous GLP-1. They contribute to better blood glucose control and weight management by increasing glucose-dependent insulin secretion, decreasing glucagon secretion, delaying stomach emptying, and promoting satiety[63]. Recent randomized controlled trials have shown that T2D patients who consume GLP-1 analogs experience significant weight loss and a significant drop in HbA1c levels. Liraglutide, for instance, was associated with a 13% relative risk decrease in major cardiovascular events, according to the LEADER trial[64]. Frequently observed adverse effects encompass gastrointestinal issues, while ongoing investigations are being conducted to ascertain the long-term safety implications, particularly concerning pancreatitis and thyroid cancer[65].
PPARs, especially PPAR-gamma agonists like pioglitazone, regulate glucose and lipid metabolism and increase insulin sensitivity in peripheral tissues. In diabetic patients, PPAR-gamma agonists have decreased insulin resistance, lowered HbA1c levels, and minimized cardiovascular risk[66]. The administration of pioglitazone has been correlated with weight gain and elevated susceptibility to heart failure, necessitating the meticulous selection of patients[67].
Although vaspin has gained attention recently, its therapeutic applications are not as well established as those of GLP-1 and PPARs. Several investigations have indicated changes in vaspin levels in individuals with diabetes. However, the precise therapeutic consequences of these alterations have not been completely clarified[68]. Further investigation is required to elucidate the mechanisms by which vaspin may be selectively manipulated to provide therapeutic advantages in diabetes. Concurrently, active clinical trials are being conducted to explore this potential. These targets may represent the diabetes treatments of the future (Table 1).
| Type of agents | Dosing | Formulation | FDA clearance date | Observations |
| Euglycemics: Drugs that lower blood sugar levels to typical levels. These drugs shouldn’t result in glucose | ||||
| Biguanides: Reduces intestinal glucose absorption and hepatic glucose release and enhances insulin sensitivity (increases glucose uptake and utilization) | ||||
| Metformin: Glumetza Fortamet®, Glucophage XR®, Glucophage® | 500 mg, 1000 mg. 500 mg, 750 mg pills. 500 mg, 750 mg pills. 500 mg, 850 mg, and 1000 mg pills | Initial dose: 500 mg once daily. Dose: 2-3 times a day. Range: 500-2550 mg. Initial: 500 mg 2 times daily or 850 mg once a day | December 1994 | SE: Can’t use it if you have problems with your liver or kidneys, take medicine for heart failure, or drink too much alcohol. Consume with food (ER with evening meal) 0.03 cases per 1000 individuals are lactic acidosis. Gastrointestinal complaints (3%) such as diarrhea, nausea, and upset stomach |
| Thiazolidinediones, also known as glitazones or TZDs, are compounds that lower the body’s insulin intolerance (muscle and fat tissues) | ||||
| Rosiglitazone: Avandia® | Tablets of 2 mg (pink), 4 mg (orange), and 8 mg (red-brown) | Initially: 4 mg per day. Range: 4-8 mg. Take it once or twice every day | May 1999 | SE: Bone loss and fractures in women, anemia, edema from fluid retention, weight increase, macular edema (in the eye), and may raise the chance of heart issues, such as angina or heart attacks, which are caused by the heart (myocardial infarction) may lead to or exacerbate cardiac failure. You cannot use this without severe heart failure or liver disease. Liver surveillance is necessary |
| Pioglitazone (preferred over rosiglitazone): Actos® | Tablets, 15 mg, 30 mg, and 45 mg (white to off-white) | 15-30 mg initially; 15-45 mg daily. Dose: One dose per day | July 1999 | SE: Bone loss and fractures in women, anemia, edema from fluid retention, weight increase, macular edema (in the eye), and may lead to or exacerbate cardiac failure. You cannot use this without severe heart failure or liver disease. Liver surveillance is necessary |
| GLP-1 analogs: Make more insulin, stop the liver from releasing glucose after meals, keep the stomach from emptying as quickly, and make people feel full | ||||
| Dulaglutide: Trulicity® | 1.5 mg or 0.75 mg each time. Under the epidermis (subcutaneous/SQ), injected available in single-dose, dose-specific pen instruments | At first: 0.75 mg once per week. Range: If the reaction is insufficient, it may be increased to 1.5 mg once weekly | September 2014 | SE: Sickness, diarrhea, throwing up, stomach pain. It can’t be used if you have multiple endocrine neoplasia syndrome type 2 or if you have a family history of medullary thyroid cancer (MEN2). In patients with a history of medullary thyroid cancer, it is contraindicated; there have been a few cases of pancreatitis (inflammation of the pancreas) |
| Albiglutide: Tanzeum® | 30 mg or 50 mg each time under the epidermis (subcutaneous/SQ), injected calls for rebuilding available in single-dose pens with a particular dose | Initial: 30 mg once weekly. Range: Can increase to 50 mg once weekly if inadequate response | September 2014 | SE: Upper respiratory infection, nausea, and injection site response. Infrequent cases of pancreatitis (inflammation of the pancreas); contraindicated in patients with a history of medullary thyroid cancer |
| SGLT2 inhibitors: Make people pee out more glucose | ||||
| Dapagliflozin: Farxiga® | 5 mg tablets are yellow and round, and 10 mg tablets are yellow and diamond-shaped | 5 mg once every day at first. Up to 10 mg per day | January 2014 | SE: Increased urination, UT infections, genital yeast infections, dizziness, lower blood pressure, increase in blood potassium; rare severe allergic reactions (severe rash; swelling of the pharynx tongue, body or face) (swelling of the tongue, throat, face or body; severe inflammation). If you have kidney difficulties, you cannot use this product |
| Empagliflozin: Jardiance® | Tablets 10 mg (pale, beige, round) and 25 mg (pale, beige rectangular) | Initial: 10 mg once daily. Range: Up to 25 mg daily | August 2014 | SE: Rare severe allergic responses; side effects including frequent urination, low blood pressure, dizziness, genital yeast infections, and urinary tract infections; and a rise in blood potassium (swelling of tongue, throat, face, or body; severe rash). Do not take it if you have renal disease |
| Canagliflozin: Invokana® | Tablets come in two different dosages and pill colors: 100 mg (colored yellow) and 300 mg (colored white) | At first: 100 mg every day. Range: 100-300 mg per day. Dose: One dose per day | March 2013 | SE: Side effects include frequent or urgent urination, low blood pressure, dizziness, genital yeast infections, UTIs, a rise in blood potassium, and severe but uncommon allergic reactions (swelling of the tongue, throat, face, or body, severe rash). Do not take it if you have renal disease |
| DPP-4 inhibitors: Increased insulin production and decreased post-meal liver glucose release are two effects | ||||
| Linagliptin: Tradjenta® | 5 mg (red-light) tablet | At first, 5 mg every day. Dose: One dose per day | May 2011 | SE: No weight gain, nasal congestion, throat pain, rare reports of pancreatitis, extremely rare severe allergic reactions |
| Saxagliptin: Onglyza® | 2.5 mg tablets are pale to light yellow, and 5 mg tablets are pink | Range: 2.5-5 mg daily, starting with 2.5 or 5 mg. Dose: One dose per day | July 2009 | SE: Headache, urinary tract illness, and upper respiratory infection. No gaining weight: If kidney issues exist, lower amounts are used |
| Sitagliptin: Januvia® | Tablets of 25 mg (pink), 50 mg (light brown), and 100 mg (beige) | At first, take 100 mg every day. Daily dose: 25-100 mg. Dosage: Once every day | December 2006 | SE: Symptoms include a runny nose, upper respiratory infection, and uncommon severe allergic responses (swelling of the tongue, throat, face, or body, severe rash). There has been no weight increase. If there are kidney issues, lower doses are used |
| Alogliptin: Nesina® | Tablets of 6.25 mg (light pink), 12.5 mg (yellow), and 25 mg (light red) | Every day, take 25 mg by mouth. Given once a day | January 2013 | SE: Upper respiratory infection, headache, sore throat, stuffy or runny nose, uncommon serious allergic responses (swelling of the tongue, throat, face, or body), and severe rash. Accounts of pancreatitis are uncommon. No weight increase |
| α-glucosidase inhibitors: STARCH blockers are substances that slow down the digestive process and the assimilation of carbohydrates | ||||
| Acarbose: Precose® various generics | Tablets of 25 mg, 50 mg, and 100 mg | Initial: Three times per day, 25 mg, 75 to 300 mg. Maximum 150 mg if under 60 kg. Dose: Three times per day | September 1995 | SE: Defecation. Take with the first mouthful of your meal. To avoid GI intolerance, begin with a modest dose and gradually increase it |
| Stimulators of insulin release (insulin secretagogues): Raise the amount of insulin the liver produces | ||||
| Glinides | ||||
| Nateglinide: Starlix® | Tablets of 60 mg (pink) and 120 mg (yellow) | 120 mg three times every day at first (if A1C is close to goal, use 60 mg). Range: 180-360 mg daily dosage is three times | December 2000 | SE: Syndrome of uncontrolled hypoglycemia protection for the aged. Only 2 h of actual playtime are involved. Take it within 30 min of dinner |
| Repaglinide: Prandin® | Tablets of 0.5 mg (white), 1 mg (yellow), and 2 mg (red) | Starting dose: 1-2 mg daily (0.5 mg if A1C 8%). From 0.5 to 16 mg. The maximum dose is 4 mg per dinner. Given twice, three times, or four times per day | December 1997 | SE: Hypoglycemic. It is safe for older adults. The activity lasts only 4 h. Take 15-30 min after eating |
| SFUs | ||||
| Glimepiride: Amaryl® various generics | Tablets ranging from 1 mg to 4 mg | To start, try 1-2 mg once a day. Between 1 and 8 mg. One daily dose is recommended | November 1995 | SE: Weight increase and hypoglycemia. Only one daily dose is necessary |
| Glyburide, micronized: Glynase PresTab® various generics | Tablets with dosages of 1.5 mg, 3 mg, 4.5 mg, and 6 mg | Initial: 1.5-3 mg/d; permitted range: 0.75-12 mg. Dosage: One or two daily doses (if > 6 mg) | March 1992 | SE: Weight increase and hypoglycemia |
| Glyburide: Micronase®, DiaBeta® various generics | Tablets of 1.25 mg, 2.5 mg, and 5 mg | Initial: 2.5-5 mg everyday. Range: 1.25-20 mg. To be consumed once or twice every day | May 1984 | SE: Hypoglycemia and obesity are possible side effects |
| Glipizide: Glucotrol®, Glucotrol XL® various generics | Tablets of 5 mg and 10 mg. Tablets of 2.5 mg, 5 mg, and 10 mg ER | At first, 5 mg every day. From 2.5 to 40 mg (20 mg for XL). Dosage: once or twice daily (if more than 15 mg) | May 1984. April 1994 | SE: Hypoglycemia and weight increase are symptoms of SE. SFU is preferred by the aged. ER means extended-release/once-daily |
| Oral pills in combination | ||||
| Empagliflozin/metformin, Synjardy® | 12.5 mg/500 mg (pale brownish purple), 12.5 mg/1000 mg (dark brownish purple), 5 mg/500 mg (orange-yellow), 5 mg/1000 mg (brownish yellow). Tablet with an oval sheet coating | Starting dose: 5 mg/500 mg or 5 mg/1000 mg. Maximum dose: 25 mg/2000 mg split into two doses | January 2015 | SE: It’s the same deal with empagliflozin and metformin |
| Empagliflozin/linagliptin, Glyxambi® | Triangular pills, 10 mg/5 mg (pale yellow), 25 mg/5 mg (pale pink) | At first: 10 mg/5 mg once every day. Range: 5 mg once every day up to 25 mg | February 2015 | SE: All the same applies to empagliflozin and linagliptin |
| Dapagliflozin/metformin XR, Xigduo XR® | 10 mg/500 mg (pink), 10 mg/1000 mg (pink to dark pink), and 5 mg/500 mg (orange) (yellow to dark yellow) oval tablets covered in celluloid | Starting dose: The patient’s present regimen up to 10 mg/2000 mg per day | October 2014 | SE: Dapagliflozin and metformin are the same as previously mentioned |
| Canagliflozin/metformin, Invokamet® | Film-coated capsule-shaped pills, 50 mg/500 mg (white), 50 mg/1000 mg (beige), 150 mg/500 mg (yellow), and 150 mg/1000 mg (purple) | Beginning: With 50 mg/500 mg or 50 mg/1000 mg. Range: 300 mg to 2000 mg. Taken in 2 divided quantities | August 2014 | SE: Identical to the preceding, but with metformin and canagliflozin |
| Alogliptin/pioglitazone, Oseni® | The next round of pills is available: 25 mg/45 mg (red), 25 mg/30 mg (peach), 25 mg/15 mg (yellow), 12.5 mg/15 mg (pale yellow), 12.5 mg/30 mg (pale peach), 12.5 mg/45 mg (pale red) | Initial dosage: Once daily, 12.5/15 mg. Range: 25/45 mg and higher ingested with or without food once daily | January 2013 | SE: The same applies to pioglitazone and alogliptin |
| Alogliptin/metformin, Kazano® | Oblong pills, 12.5 mg/1000 mg (pale yellow), 12.5 mg/500 mg (pale yellow) | At first: 12.5 mg/500 mg once or twice every day. Maximum range: 25/2000 taken with meals twice a day | January 2013 | SE: Alogliptin and metformin in the same way as previously |
| Linagliptin/metformin, Jentadueto® | Oval pills with dosages of 2.5 mg/1000 mg (light pink), 2.5 mg/850 mg (light orange), and 2.5 mg/500 mg (golden yellow) | Initial dosage: 2 times a day with food, 2.5 mg/500 mg. Range: Twice daily dosages of up to 2.5 mg/1000 mg food | January 2012 | SE: With linagliptin and metformin, the same as above |
| Sitagliptin/metformin, Janumet XR® | Oval pills, 50 mg/500 mg (light blue), 50 mg/1000 mg (light green), and 100 mg/1000 mg (blue) | At first: 100 mg/1000 mg every day. Maximum daily dose: 100 mg/2000 mg. Dosage: Once every day | February 2012 | SE: As with sitagliptin and metformin, the same rules apply |
| Saxagliptin/metformin XR, Kombiglyze XR® | Capsule-shaped pills contain 2.5 mg/1000 mg (pale yellow to light yellow), 5 mg/1000 mg (pink), and 5 mg/500 mg (golden brown to brown) | Starting dose: 5 mg/500 mg or 5 mg/1000 mg once daily. Maximum dose: 5 milligrammes/2000 mg. Dosage: Once every day | November 2010 | SE: The same holds for metformin and saxagliptin |
Conventional targets are medications used in the market for a while to treat diabetes. Still, their availability is restricted, and they come with several drawbacks, like weight gain, hypoglycemia, and other side effects. They work by keeping the level of glucose in the blood steady. For example, biguanides reduce the amount of glucose produced and increase the amount of glucose used by skeletal muscles and the liver. SGLT-2 antagonists stimulate the kidney’s ability to excrete glucose. A-glucosidase inhibitors reduce intestinal uptake of glucose and free fatty acids (FFA). Pancreatic insulin production and sensitivity are both improved by sulphonyl urease. The release of FFA from adipose cells is reduced by 2,4-thiazolidinediones[32,69].
The pineal gland secretes the neuroendocrine hormone MLT at night. MLT has been identified as a possible therapeutic target for treating T2D because it also regulates glucose and the pancreatic release of insulin. It exerts its pharmacological effects by interacting with the MT1 and MT2 MLT receptors[70]. Recent research has revealed that mice lacking the MLT MT1 receptor exhibit increased insulin resistance and glucose intolerance. MLT’s MT1 receptor is an important the
Transcription factors control gene expression called PPARs, of which there are three types: PPARα, PPARγ, and PPARβ/δ[72]. Thiazolidinediones, PPAR-agonists, turn on the receptor, making the body more sensitive to insulin. After being turned on, they lower the levels of FFA in the blood and change the levels of adipokines. This is possible by increasing insulin release from the pancreas, improving glucose intake in skeletal muscle and adipose tissues, and lowering glucose synthesis in the liver[73].
Muscles, liver, and pancreatic beta cells all contain G-protein coupled receptor 119 (GPR119). Like incretin hormones, the activation of GPR119 may increase insulin production and favor insulin secretion when agonists are attached to its binding site[74]. GPR119 improves glucose homeostasis through two distinct mechanisms: The release of GLP-1 and GIP from enteroendocrine cells and the direct impact of the glucose-activated insulin release in β-cells[75].
One of the incretin hormones, or GIP, is found in the brain, fatty tissue, and β-cells. It enhances the insulin response prompted by the post-prandial rise in glycemia, where it plays a significant part in T2D and other metabolic disorders[76]. By binding to the GIP receptor, GIP exerts its insulinotropic effects by raising intracellular (cAMP) levels. PKA & exchange protein-activated cAMP2 are activated by elevated cAMP (EPAC2)[77]. The depolarization of the voltage-gated Ca2+ channels increase the concentration of Ca2+ within the cell, which in turn initiates the release of Ca2+ from intra
Free fatty acid receptor 1 (FFA1), also known as GPR-40, is a free fatty receptor. FFA1 is primarily present in pancreatic and intestinal cells. Researchers found that the FFA1 receptor affects lipid and glucose metabolism and boosts insulin release from pancreatic β-cells in vivo studies using mouse islets’ β-cell lines. FFA1 impacts blood glucose levels by indi
Although very little knowledge exists about these targets’ roles in diabetes, they can be critical in managing the disease. A new technique for treating DM is gene therapy, which works by repairing or correcting the defective genes that cause the disease[80]. This approach allows for the replacement of the insulin gene and the transfer of genes via viral vectors and non-viral transduction methods to suppress auto-reactive T cells and prevent the destruction of islet cells. According to research, stem cells can be used to treat diabetes because they can readily multiply in culture and act as surrogate β-cells.
Additionally, research has discovered that rodents receiving intrahepatic injections of modified stem cells have low blood glucose levels (Table 2)[81]. Under a fluorescent microscope, the stem cells fluoresce green after the mice have been slaughtered for histopathological examinations. Insulin was found using an anti-human insulin polyclonal antibody to stain the tissue[32]. Mesenchymal stem cells successfully expressed human insulin and maintained blood glucose levels normal, according to a 42-d study. Compared to rodents that were not treated with gene therapy. As a result, as a deve
| Nature | Special targets | Diabetics | Method of activity | Ref. |
| Gene | Gene therapy | Auto-reactive T cells need to be stopped from killing islet cells | Act by fixing or modifying the problematic genes | [81] |
| Glycoprotein in serum | SERPIN A12 or vaspin | KLK7 reduction enhances insulin signaling and lengthens the half-life of insulin, contributing to lower blood sugar levels | Vaspin blocks KLK7 | [89] |
| Adipokine | Metrnl | Enhanced insulin responsiveness | Cause of PPAR pathway upregulation | [92] |
| Hormone | ACRP-30 | Acrp30 increases insulin sensitivity and lowers blood sugar | Low amounts bring on insulin sensitivity | [106] |
| Glucocorticoids | 11β-HSD1 | 11β-HSD inhibition glucose reduction, insulin sensitivity improvement | Increasing amounts lead to glucose sensitivity | [85] |
| Glycoprotein | Fetuin-A | When fetuin-A levels are low, insulin sensitivity will go up | Associated with beta-cell inflammation | [86] |
| Glycoprotein | GPER | Boost insulin production | Through binding with Gi/o and Gs proteins, glucose homeostasis is regulated | [94] |
| Glycoprotein | PEDF | Insulin sensitivity is improved by reducing PEDF levels | Insulin resistance is caused by an upregulated chain of kinase-mediated Serine/threonine phosphorylation of IRS | [99] |
| Protein | Visfatin | Activity that mimics insulin | Receptor for insulin that it binds to | [103] |
| Protein | CCN3/NOV | Improved glucose tolerance and insulin sensitivity | Strong correlation with hs-CRP | [97] |
| Glycoprotein | PTP1B | Inhibits insulin signaling by dephosphorylating insulin receptor kinase | [83] |
Leukocyte antigen-related tyrosine phosphatase and protein tyrosine phosphatase 1B (PTP1B) are critical players in the regulation of insulin signal transductions. An essential stage in the insulin signaling process is tyrosine phosphorylation in the insulin-receptor activation loop[59]. Insulin signaling is negatively regulated by PTP1B, which dephosphorylates phosphor-tyrosine residues in insulin receptor kinase activation regions. More evidence shows that PTP1B, insulin sen
Cortisone, a glucocorticoid, is converted to cortisol, a hormone, by hydroxysteroid dehydrogenase. There are two iso
A glycoprotein called fetuin-A is made in the liver and released into the bloodstream. The main protein needed to transport FFA to the bloodstream is fetuin-A. It also plays a role in-cell irritation and degeneration in the pancreas. Tyrosine kinase is a crucial enzyme for insulin signaling that thoroughly opposes insulin activity and is inhibited by fetuin-A (Table 2)[86]. Tyrosine kinase and insulin work together to maintain a healthy blood sugar level. If the blood’s fetuin-A content rises, it may lead to insulin resistance and eventually diabetes. Studies have shown that rodents with fetuin-A knockout genes have increased insulin sensitivity, demonstrating the negative relationship between fetuin-A and insulin sensitivity in diabetes[87].
Serpin A12, also known as vaspin, is a glycoprotein in serum that belongs to the superfamily “serpin”. It is derived from fatty cells and significantly impacts insulin activity. It has been discovered that as the severity of diabetes rises, the serum levels of vaspin begin to fall. This raises the possibility that increasing the vaspin levels in circulation could aid in managing DM[88]. Vaspin administration in rodents has been linked in studies on rodents to enhanced glucose tolerance and insulin sensitivity. This implies that it might be an option for therapy for managing metabolic disorders such as T2D and obesity. Vaspin can exert its effect by suppressing the insulin-degrading enzyme known as kallikrein 7 (KLK7), which in turn reduces the insulin’s half-life and causes the insulin to be degraded more quickly (Table 2)[89]. Because KLK7 is blocked, insulin signals work better, and insulin’s half-life is lengthened, which helps lower blood glucose levels. It also does a few other things that indirectly reduce blood sugar. For example, it makes you eat less, which lowers your hepatic glucose production via increasing insulin signaling in the liver and reducing hepatic lipid accumulation[90]. It decreases inflammation and boosts insulin signaling in brown adipose tissue and white adipose tissue. It activates the vagus nerve in the central nervous system to reduce appetite[91].
Metrnl is an adipokine derived from the fatty tissues prevalent in the body’s subcutaneous white fat. Metrnl is crucial for sustaining immunological inflammation, cardiovascular function, lipid metabolism, energy metabolism, insulin sensitivity, and its essential role in maintaining glucose homeostasis (Table 2)[92]. According to a report, researchers discovered that it functions by upregulating the PPAR pathway, which increases insulin sensitivity in mice models. Additionally, it has been found to encourage the browning of adipose tissue, increasing energy expenditure and better glucose tolerance[93].
GPER is an orphan 7-transmembrane G-protein-coupled estrogen receptor that helps send signals about estrogen. They are found in the intracellular membranes of cells. Gi/o and Gs protein binding in organisms are crucial for controlling glucose homeostasis[94]. It was discovered that a GPER-deficient female mouse model produces insufficient insulin, leading to DM. A study also found that estrogen levels are high in premenopausal women, which benefits glucose homeostasis, lipid metabolism, and blood pressure[95]. In addition to decreasing inflammation, estrogen levels decline after menopause, making the female population more susceptible to metabolic disorders and insulin resistance, contributing to DM[96]. This data suggests that GPER may be essential for managing diabetes and a valuable drug target for treating diabetes and associated disorders (Table 2).
Cellular communication network 3 (CCN3), known as nephroblastoma overexpressed, is a protein high in cysteine with growth-regulating properties. Numerous human organs and bodily fluids, including the musculoskeletal system, kidneys, and cerebrospinal fluid, have been found to contain them[59]. Hyperlipidemic obese patients have substantially higher than expected plasma levels of CCN3, correlated with high-sensitivity C-reactive protein, body mass index, and fat mass. Dalle et al[97] showed that mice who didn’t have enough CCN3 and ate standard high-fat diets lost much weight and had better glucose tolerance and insulin sensitivity (Table 2). Furthermore, Li et al[98] compared serum CCN3 levels in recently diagnosed T2DM (nT2DM) patients to healthy control subjects. CCN3 levels were significantly higher in T2DM individuals.
The serine protease inhibitor family includes the 50 kDa PEDF, secreted from adipose tissue and the pigment cells of the human eye. It induces the insulin receptor substrate to undergo kinase-mediated serine/threonine phosphorylation, which results in decreased insulin signaling and insulin resistance in body cells (Table 2)[99]. Additionally, the body’s insulin sensitivity causes the production of interleukin-1 and tumor necrosis factor-alpha (TNF-α) in the system. The research discovered that animals’ insulin sensitivity decreased after receiving PEDF but returned to normal after receiving anti-PEDF. PEDF correlates well with insulin resistance in infants and adults[100]. Therefore, if we can lower the amount of PEDF in the blood, it might help the body respond better to insulin. This makes PEDF a possible new way to treat DM and other metabolic syndromes[81].
Visfatin is a protein with many different functions. It is also called nicotinamide phosphoribosyl-transferase. It was founded in 2005. It can be found in several organs and tissues, but most comprise visceral adipose tissue. It has insulin-like properties, which means it helps to restore insulin sensitivity. This suggests that it may also play a role in diabetes, making it a new way to treat DM[101]. It has been demonstrated that serum visfatin concentrations rise alongside the progression of T2DM, establishing a relationship between visfatin and T2DM. Current research has shown that visfatin binds to the insulin receptor at a location different from that of insulin, suggesting that it has properties similar to insulin and stimulates cell growth[102]. Though scientists are investigating the underlying mechanisms of visfatin in DM, it is unclear how visfatin is fully linked to the disease. Nevertheless, there are some visfatin stimulators and inhibitors. With this knowledge, it is possible to conclude that visfatin and diabetes are related in the body, making it an appropriate focus for DM treatment (Table 2)[103].
ACRP 30 or Adipocyte complement protein of 30 kDa, the capacity of adipose tissue to store fat has long been known. However, current studies have demonstrated that it may serve as a reservoir of hormones such as Acrp30, adiponectin, resistin, TNF-α, leptin, or adipsin[104]. TNF-α is a crucial pro-inflammatory mediator responsible for insulin resistance, and serum protein Acrp30 is found to serve a primary part in managing DM. In addition, another report reveals that Acrp30 levels are reduced in numerous obesity and diabetes models[105]. Since mice missing Acrp30 exhibit insulin resistance, which results in the development of DM, high TNF-α levels also demonstrate a negative correlation of this protein with DM (Table 2)[106]. When the concentration of Acrp30 in the blood is raised, insulin sensitivity can also be elevated, making it easier to control blood glucose levels. As a result, Acrp30 will potentially become an additional avenue for the therapy of DM[107].
Current literature increasingly underscores the substantial influence of social determinants on the development, management, and outcomes of DM. This section aims to provide an updated perspective on this critical aspect, incor
Data from Tapager et al[109] emphasize the role of healthcare access in diabetes management. Their findings indicate that individuals with limited access to healthcare services face more significant challenges in managing their diabetes, resulting in health disparities. This observation aligns with the growing awareness of differences in diabetes outcomes based on factors such as race and geography. Moreover, research published by Kanchi et al[110] has shed light on the significance of the food environment in diabetes risk. Their study demonstrated that neighborhoods with limited access to fresh and healthy food options were associated with higher rates of diabetes incidence. This highlights the importance of addressing the food environment as a key social determinant in diabetes prevention and management.
The latest research on psychosocial factors and how they affect diabetes control has revealed significant new information. According to a study by Abate and Gedamu[111], stress and social support networks significantly impact how well people with diabetes manage their blood sugar levels. These results highlight the importance of comprehensive psychosocial support in treating diabetes. Based on these recent findings, our discussion aims to underscore the evolving understanding of how social determinants intricately shape the landscape of DM. These insights emphasize the need for a multifaceted approach that considers clinical factors and the social, economic, and environmental contexts in which diabetes occurs.
DM is a pervasive and challenging health condition affecting a substantial population worldwide. The primary goal of DM therapies is to achieve near-normal blood glucose levels. However, it is essential to acknowledge that current treatments cannot offer a complete cure; they can only manage symptoms and slow the progression of the disease, often accompanied by a range of adverse effects. The quest for innovative solutions to address DM and its consequences is an ongoing endeavor within the scientific community. Researchers are steadfast in their pursuit of compounds that could potentially offer a lasting remedy for DM with minimal side effects. While traditional methods, such as insulin therapy and biguanides, have been relied upon for an extended period, other classes of medications, including sulphonylureas, glinides, thiazolidinediones, gliptins, inhibitors of α-glucosidase, analogs of amylin, SGLT-2 inhibitors, and dopamine D-2 agonists, have also been explored. Unfortunately, these treatments are not without limitations, often presenting adverse effects ranging from bladder cancer to hypoglycemia and weight gain.
In response to these challenges, researchers have been actively investigating alternative targets for diabetic therapy. While targets like PPARs have garnered significant attention over the past decade, the translation from pre-clinical research to clinical studies and commercialization has been limited. This underscores the pressing need for novel, creative pharmacological targets in diabetes management. In light of recent advancements, several receptors, including GPCR 119, GPER, GPCR, GIP, MLT, visfatin, ACRP 30, fetuin-A, PEDF, metrnl, vaspin, and 11-hydroxysteroid dehydrogenase-1, have emerged as promising candidates that play a direct or indirect role in insulin regulation. These receptors hold the potential to be leveraged as therapeutic targets for diabetes management, paving the way for the development of long-term remedies and the mitigation of its complications. Furthermore, it is worth noting that cutting-edge approaches, such as gene therapy and stem cell-based interventions, hold the promise of delivering treatments with increased efficacy and fewer adverse effects. These innovative strategies represent exciting avenues for exploration in the pursuit of more effective and patient-friendly interventions for DM.
In conclusion, while we acknowledge the challenges associated with the existing approaches to diabetes management, we remain optimistic about the future of diabetes research and therapy. Our understanding of the intricacies of this condition continues to evolve, offering fresh perspectives and novel opportunities. We encourage continued exploration into the receptors and innovative therapies discussed here, anticipating that they will contribute significantly to deve
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Wu K, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019;21:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 401] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 2. | Tosh SM, Bordenave N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr Rev. 2020;78:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Yousif Hussin Alimam H, Abdelateif Hussein W, Ibrahim S, Abdelgani S, Alharthi N, Bashier Eltayeb L, Abdelgadir Elmahdi S, Abobakr Abdrabo A. Blood Glucose, HbA1c Level, and its Correlation with VEGF-A (+405G/C) Polymorphism as Biomarker Predicts the Risk of Retinopathy and Nephropathy in Type 2 Diabetic Patients. Rep Biochem Mol Biol. 2022;11:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi'i A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med. 2021;136:104754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 5. | Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC, Maroyi A, Martins N. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front Pharmacol. 2020;11:01021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, Prakash Mishra A, Nigam M, El Rayess Y, Beyrouthy ME, Polito L, Iriti M, Martins N, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-Rad J. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020;11:694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1271] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 7. | Maddaloni E, Bolli GB, Frier BM, Little RR, Leslie RD, Pozzilli P, Buzzetti R. C-peptide determination in the diagnosis of type of diabetes and its management: A clinical perspective. Diabetes Obes Metab. 2022;24:1912-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 8. | Cabello-Olmo M, Araña M, Urtasun R, Encio IJ, Barajas M. Role of Postbiotics in Diabetes Mellitus: Current Knowledge and Future Perspectives. Foods. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Soliman AT, Prabhakaran Nair A, Al Masalamani MS, De Sanctis V, Abu Khattab MA, Alsaud AE, Sasi S, Ali EA, Ola A H, Iqbal FM, Nashwan AJ, Fahad J, El Madhoun I, Yassin MA. Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID-19: A comparative study. Acta Biomed. 2020;91:e2020010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 10. | Blonde L, Umpierrez GE, Reddy SS, McGill JB, Berga SL, Bush M, Chandrasekaran S, DeFronzo RA, Einhorn D, Galindo RJ, Gardner TW, Garg R, Garvey WT, Hirsch IB, Hurley DL, Izuora K, Kosiborod M, Olson D, Patel SB, Pop-Busui R, Sadhu AR, Samson SL, Stec C, Tamborlane WV Jr, Tuttle KR, Twining C, Vella A, Vellanki P, Weber SL. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan-2022 Update. Endocr Pract. 2022;28:923-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 250] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 11. | Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9:174-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 12. | Galindo RJ, Dhatariya K, Gomez-Peralta F, Umpierrez GE. Safety and Efficacy of Inpatient Diabetes Management with Non-insulin Agents: an Overview of International Practices. Curr Diab Rep. 2022;22:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Quispe C, Herrera-Bravo J, Javed Z, Khan K, Raza S, Gulsunoglu-Konuskan Z, Daştan SD, Sytar O, Martorell M, Sharifi-Rad J, Calina D. Therapeutic Applications of Curcumin in Diabetes: A Review and Perspective. Biomed Res Int. 2022;2022:1375892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Wan L, Gao Q, Deng Y, Ke Y, Ma E, Yang H, Lin H, Li H, Yang Y, Gong J, Li J, Xu Y, Liu J, Zhang X, Huang L, Feng J, Zhang Y, Huang H, Wang H, Wang C, Chen Q, Huang X, Ye Q, Li D, Yan Q, Liu M, Wei M, Mo Y, Tang K, Lin C, Zheng F, Xu L, Cheng G, Wang P, Yang X, Wu F, Sun Z, Qin C, Wei C, Zhong H. GP73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat Metab. 2022;4:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Pedersen C, Kraft G, Edgerton DS, Scott M, Farmer B, Smith M, Laneve DC, Williams PE, Moore LM, Cherrington AD. The kinetics of glucagon action on the liver during insulin-induced hypoglycemia. Am J Physiol Endocrinol Metab. 2020;318:E779-E790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Radhakrishnan S, Lakshmy S, Santhosh S, Kalarikkal N, Chakraborty B, Rout CS. Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials. Biosensors (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Bertini A, Gárate B, Pardo F, Pelicand J, Sobrevia L, Torres R, Chabert S, Salas R. Impact of Remote Monitoring Technologies for Assisting Patients With Gestational Diabetes Mellitus: A Systematic Review. Front Bioeng Biotechnol. 2022;10:819697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 548] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 19. | Min T, Bain SC. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021;12:143-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 20. | Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 21. | Kung WJ, Kuo HY, Lee CH, Zen YH, Kong LC, Lin CC. Association between gestational abnormal glucose tolerance and maternal-fetal outcomes. J Obstet Gynaecol Res. 2022;48:2505-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Jones C, Gwenin C. Cortisol level dysregulation and its prevalence-Is it nature's alarm clock? Physiol Rep. 2021;8:e14644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | van Hoek I, Hodgkiss-Geere H, Bode EF, Hamilton-Elliott J, Mõtsküla P, Palermo V, Pereira YM, Culshaw GJ, Ivanova A, Dukes-McEwan J. Associations among echocardiography, cardiac biomarkers, insulin metabolism, morphology, and inflammation in cats with asymptomatic hypertrophic cardiomyopathy. J Vet Intern Med. 2020;34:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Petrov MS, Basina M. DIAGNOSIS OF ENDOCRINE DISEASE: Diagnosing and classifying diabetes in diseases of the exocrine pancreas. Eur J Endocrinol. 2021;184:R151-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399:394-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 302] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 26. | Chen Y, Ran X, Chen Y, Jiang K. Effects of Health Literacy Intervention on Health Literacy Level and Glucolipid Metabolism of Diabetic Patients in Mainland China: A Systematic Review and Meta-Analysis. J Diabetes Res. 2021;2021:1503446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 27. | American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S83-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 419] [Article Influence: 139.7] [Reference Citation Analysis (1)] |
| 28. | Bergman M, Abdul-Ghani M, DeFronzo RA, Manco M, Sesti G, Fiorentino TV, Ceriello A, Rhee M, Phillips LS, Chung S, Cravalho C, Jagannathan R, Monnier L, Colette C, Owens D, Bianchi C, Del Prato S, Monteiro MP, Neves JS, Medina JL, Macedo MP, Ribeiro RT, Filipe Raposo J, Dorcely B, Ibrahim N, Buysschaert M. Review of methods for detecting glycemic disorders. Diabetes Res Clin Pract. 2020;165:108233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 29. | Alarcón-Gómez J, Chulvi-Medrano I, Martin-Rivera F, Calatayud J. Effect of High-Intensity Interval Training on Quality of Life, Sleep Quality, Exercise Motivation and Enjoyment in Sedentary People with Type 1 Diabetes Mellitus. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, Karlsson C, Norjavaara E. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 31. | Porumb M, Stranges S, Pescapè A, Pecchia L. Precision Medicine and Artificial Intelligence: A Pilot Study on Deep Learning for Hypoglycemic Events Detection based on ECG. Sci Rep. 2020;10:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | Kumar S, Behl T, Sachdeva M, Sehgal A, Kumari S, Kumar A, Kaur G, Yadav HN, Bungau S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021;264:118661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 33. | Asbaghi O, Nazarian B, Yousefi M, Anjom-Shoae J, Rasekhi H, Sadeghi O. Effect of vitamin E intake on glycemic control and insulin resistance in diabetic patients: an updated systematic review and meta-analysis of randomized controlled trials. Nutr J. 2023;22:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 34. | Shah VN, Vigers T, Pyle L, Calhoun P, Bergenstal RM. Discordance Between Glucose Management Indicator and Glycated Hemoglobin in People Without Diabetes. Diabetes Technol Ther. 2023;25:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 35. | Tsoutsouki J, Wunna W, Chowdhury A, Chowdhury TA. Advances in the management of diabetes: therapies for type 2 diabetes. Postgrad Med J. 2020;96:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1622] [Cited by in RCA: 1819] [Article Influence: 259.9] [Reference Citation Analysis (0)] |
| 37. | Yapanis M, James S, Craig ME, O'Neal D, Ekinci EI. Complications of Diabetes and Metrics of Glycemic Management Derived From Continuous Glucose Monitoring. J Clin Endocrinol Metab. 2022;107:e2221-e2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 38. | Awad A, Trenfield SJ, Pollard TD, Ong JJ, Elbadawi M, McCoubrey LE, Goyanes A, Gaisford S, Basit AW. Connected healthcare: Improving patient care using digital health technologies. Adv Drug Deliv Rev. 2021;178:113958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 39. | Davis J, Fischl AH, Beck J, Browning L, Carter A, Condon JE, Dennison M, Francis T, Hughes PJ, Jaime S, Lau KHK, McArthur T, McAvoy K, Magee M, Newby O, Ponder SW, Quraishi U, Rawlings K, Socke J, Stancil M, Uelmen S, Villalobos S. 2022 National Standards for Diabetes Self-Management Education and Support. Diabetes Care. 2022;45:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 40. | Twigg S, Lim S, Yoo SH, Chen L, Bao Y, Kong A, Yeoh E, Chan SP, Robles J, Mohan V, Cohen N, McGill M, Ji L. Asia-Pacific Perspectives on the Role of Continuous Glucose Monitoring in Optimizing Diabetes Management. J Diabetes Sci Technol. 2023;19322968231176533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 41. | Wang M, Hng TM. HbA1c: More than just a number. Aust J Gen Pract. 2021;50:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Indyk D, Bronowicka-Szydełko A, Gamian A, Kuzan A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci Rep. 2021;11:13264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 43. | Sriram RD, Reddy SSK. Artificial Intelligence and Digital Tools: Future of Diabetes Care. Clin Geriatr Med. 2020;36:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Rhee SY, Kim C, Shin DW, Steinhubl SR. Present and Future of Digital Health in Diabetes and Metabolic Disease. Diabetes Metab J. 2020;44:819-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Adesina N, Dogan H, Green S, Tsofliou F. Effectiveness and Usability of Digital Tools to Support Dietary Self-Management of Gestational Diabetes Mellitus: A Systematic Review. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, van Netten JJ; International Working Group on the Diabetic Foot. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 47. | Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, Game F. Diabetic foot ulcer classifications: A critical review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 48. | Choi YJ, Jeon SM, Shin S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 49. | Yu J, Lee SH, Kim MK. Recent Updates to Clinical Practice Guidelines for Diabetes Mellitus. Endocrinol Metab (Seoul). 2022;37:26-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Kwan YH, Ong ZQ, Choo DYX, Phang JK, Yoon S, Low LL. A Mobile Application to Improve Diabetes Self-Management Using Rapid Prototyping: Iterative Co-Design Approach in Asian Settings. Patient Prefer Adherence. 2023;17:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 51. | Nyström T, Schwarz E, Dahlqvist S, Wijkman M, Ekelund M, Holmer H, Bolinder J, Hellman J, Imberg H, Hirsch IB, Lind M. Evaluation of Effects of Continuous Glucose Monitoring on Physical Activity Habits and Blood Lipid Levels in Persons With Type 1 Diabetes Managed With MDI: An Analysis Based on the GOLD Randomized Trial (GOLD 8). J Diabetes Sci Technol. 2022;19322968221101916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Riddell MC, Li Z, Beck RW, Gal RL, Jacobs PG, Castle JR, Gillingham MB, Clements M, Patton SR, Dassau E, Doyle Iii FJ, Martin CK, Calhoun P, Rickels MR. More Time in Glucose Range During Exercise Days than Sedentary Days in Adults Living with Type 1 Diabetes. Diabetes Technol Ther. 2021;23:376-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | AlBurno H, Mercken L, de Vries H, Al Mohannadi D, Schneider F. Determinants of healthful eating and physical activity among adolescents and young adults with type 1 diabetes in Qatar: A qualitative study. PLoS One. 2022;17:e0270984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 54. | Dowarah J, Singh VP. Anti-diabetic drugs recent approaches and advancements. Bioorg Med Chem. 2020;28:115263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 55. | Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, Wang X, Wang R, Fu C. Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther. 2022;7:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 874] [Article Influence: 291.3] [Reference Citation Analysis (0)] |
| 56. | Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 858] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 57. | Kaur N, Kumar V, Nayak SK, Wadhwa P, Kaur P, Sahu SK. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem Biol Drug Des. 2021;98:539-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 58. | Semwal DK, Kumar A, Aswal S, Chauhan A, Semwal RB. Protective and therapeutic effects of natural products against diabetes mellitus via regenerating pancreatic β-cells and restoring their dysfunction. Phytother Res. 2021;35:1218-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Kanwal A, Kanwar N, Bharati S, Srivastava P, Singh SP, Amar S. Exploring New Drug Targets for Type 2 Diabetes: Success, Challenges and Opportunities. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Dhankhar S, Chauhan S, Mehta DK, Nitika G, Saini K, Saini M, Das R, Gupta S, Gautam V. Novel targets for potential therapeutic use in Diabetes mellitus. Diabetol Metab Syndr. 2023;15:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 61. | He S, Lim GE. The Application of High-Throughput Approaches in Identifying Novel Therapeutic Targets and Agents to Treat Diabetes. Adv Biol (Weinh). 2023;7:e2200151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Reed J, Bain S, Kanamarlapudi V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab Syndr Obes. 2021;14:3567-3602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 63. | Xu D, Nair A, Sigston C, Ho C, Li J, Yang D, Liao X, Chen W, Kuang M, Li Y, Reid C, Xiao H. Potential Roles of Glucagon-Like Peptide 1 Receptor Agonists (GLP-1 RAs) in Nondiabetic Populations. Cardiovasc Ther. 2022;2022:6820377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 64. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4903] [Article Influence: 544.8] [Reference Citation Analysis (0)] |
| 65. | Sun F, Chai S, Yu K, Quan X, Yang Z, Wu S, Zhang Y, Ji L, Wang J, Shi L. Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Technol Ther. 2015;17:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Ivanova EA, Parolari A, Myasoedova V, Melnichenko AA, Bobryshev YV, Orekhov AN. Peroxisome proliferator-activated receptor (PPAR) gamma in cardiovascular disorders and cardiovascular surgery. J Cardiol. 2015;66:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Devchand PR, Liu T, Altman RB, FitzGerald GA, Schadt EE. The Pioglitazone Trek via Human PPAR Gamma: From Discovery to a Medicine at the FDA and Beyond. Front Pharmacol. 2018;9:1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Yang HW, Huang YG, Gai CL, Chai GR, Lee S. Serum vaspin levels are positively associated with diabetic retinopathy in patients with type 2 diabetes mellitus. J Diabetes Investig. 2021;12:566-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Rizzo MR, Di Meo I, Polito R, Auriemma MC, Gambardella A, di Mauro G, Capuano A, Paolisso G. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol Res. 2022;176:106062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 70. | Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15:105-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 71. | Yu LM, Dong X, Xue XD, Xu S, Zhang X, Xu YL, Wang ZS, Wang Y, Gao H, Liang YX, Yang Y, Wang HS. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J Pineal Res. 2021;70:e12698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 72. | Decara J, Rivera P, López-Gambero AJ, Serrano A, Pavón FJ, Baixeras E, Rodríguez de Fonseca F, Suárez J. Peroxisome Proliferator-Activated Receptors: Experimental Targeting for the Treatment of Inflammatory Bowel Diseases. Front Pharmacol. 2020;11:730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 73. | Wang Y, Nakajima T, Gonzalez FJ, Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 335] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 74. | Pola S, Shah SR, Pingali H, Zaware P, Thube B, Makadia P, Patel H, Bandyopadhyay D, Rath A, Giri S, Patel JH, Ranvir RK, Sundar SR, Kumar J, Jain MR. Discovery of a potent G-protein-coupled receptor 119 agonist for the treatment of type 2 diabetes. Bioorg Med Chem. 2021;35:116071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Papazoglou I, Lee JH, Cui Z, Li C, Fulgenzi G, Bahn YJ, Staniszewska-Goraczniak HM, Piñol RA, Hogue IB, Enquist LW, Krashes MJ, Rane SG. A distinct hypothalamus-to-β cell circuit modulates insulin secretion. Cell Metab. 2022;34:285-298.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 76. | Vieira R, Souto SB, Sánchez-López E, Machado AL, Severino P, Jose S, Santini A, Fortuna A, García ML, Silva AM, Souto EB. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome-Review of Classical and New Compounds: Part-I. Pharmaceuticals (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 77. | Wan W, Qin Q, Xie L, Zhang H, Wu F, Stevens RC, Liu Y. GLP-1R Signaling and Functional Molecules in Incretin Therapy. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Stožer A, Paradiž Leitgeb E, Pohorec V, Dolenšek J, Križančić Bombek L, Gosak M, Skelin Klemen M. The Role of cAMP in Beta Cell Stimulus-Secretion and Intercellular Coupling. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 79. | Oberhauser L, Maechler P. Lipid-Induced Adaptations of the Pancreatic Beta-Cell to Glucotoxic Conditions Sustain Insulin Secretion. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Stauss HJ, Tran MGB. TCR Gene Therapy: Challenges, Opportunities, and Future Directions. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Behl T, Gupta A, Sehgal A, Albarrati A, Albratty M, Meraya AM, Najmi A, Bhatia S, Bungau S. Exploring protein tyrosine phosphatases (PTP) and PTP-1B inhibitors in management of diabetes mellitus. Biomed Pharmacother. 2022;153:113405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 82. | Chellappan DK, Sivam NS, Teoh KX, Leong WP, Fui TZ, Chooi K, Khoo N, Yi FJ, Chellian J, Cheng LL, Dahiya R, Gupta G, Singhvi G, Nammi S, Hansbro PM, Dua K. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Sharma B, Xie L, Yang F, Wang W, Zhou Q, Xiang M, Zhou S, Lv W, Jia Y, Pokhrel L, Shen J, Xiao Q, Gao L, Deng W. Recent advance on PTP1B inhibitors and their biomedical applications. Eur J Med Chem. 2020;199:112376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 84. | Teimouri M, Hosseini H, ArabSadeghabadi Z, Babaei-Khorzoughi R, Gorgani-Firuzjaee S, Meshkani R. The role of protein tyrosine phosphatase 1B (PTP1B) in the pathogenesis of type 2 diabetes mellitus and its complications. J Physiol Biochem. 2022;78:307-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 85. | Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022;18:540-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 86. | Gao D, Jiao J, Wang Z, Huang X, Ni X, Fang S, Zhou Q, Zhu X, Sun L, Yang Z, Yuan H. The roles of cell-cell and organ-organ crosstalk in the type 2 diabetes mellitus associated inflammatory microenvironment. Cytokine Growth Factor Rev. 2022;66:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 87. | Yan S, Jiang Z, Cheng L, Lin Y, Fan B, Luo L, Yan Y, Yang L, Shen X. TLR4 knockout can improve dysfunction of β-cell by rebalancing proteomics disorders in pancreas of obese rats. Endocrine. 2020;67:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Salek Maghsoudi A, Hassani S, Mirnia K, Abdollahi M. Serpin A12 (Vaspin) as a Serine Protease Inhibitor: From Functional Mechanisms to Applications as a Biomarker in Diabetes. In: Biomarkers in Diabetes. Cham: Springer International Publishing. 2022; 24: 1-17. |
| 89. | Weiner J, Zieger K, Pippel J, Heiker JT. Molecular Mechanisms of Vaspin Action - From Adipose Tissue to Skin and Bone, from Blood Vessels to the Brain. Adv Exp Med Biol. 2019;1111:159-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 90. | Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020;472:1273-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 91. | Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021;17:468-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 92. | AlKhairi I, Cherian P, Abu-Farha M, Madhoun AA, Nizam R, Melhem M, Jamal M, Al-Sabah S, Ali H, Tuomilehto J, Al-Mulla F, Abubaker J. Increased Expression of Meteorin-Like Hormone in Type 2 Diabetes and Obesity and Its Association with Irisin. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Yao F, Yu Y, Feng L, Li J, Zhang M, Lan X, Yan X, Liu Y, Guan F, Chen L. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARγ of insulin resistance induced by high-fat diet-associated obesity. Exp Cell Res. 2017;355:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 94. | Masi M, Racchi M, Travelli C, Corsini E, Buoso E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Sharma G, Mauvais-Jarvis F, Prossnitz ER. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. J Steroid Biochem Mol Biol. 2018;176:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 96. | Sachs S, Bastidas-Ponce A, Tritschler S, Bakhti M, Böttcher A, Sánchez-Garrido MA, Tarquis-Medina M, Kleinert M, Fischer K, Jall S, Harger A, Bader E, Roscioni S, Ussar S, Feuchtinger A, Yesildag B, Neelakandhan A, Jensen CB, Cornu M, Yang B, Finan B, DiMarchi RD, Tschöp MH, Theis FJ, Hofmann SM, Müller TD, Lickert H. Targeted pharmacological therapy restores β-cell function for diabetes remission. Nat Metab. 2020;2:192-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 97. | Dalle H, Garcia M, Antoine B, Boehm V, Do TTH, Buyse M, Ledent T, Lamazière A, Magnan C, Postic C, Denis RG, Luquet S, Fève B, Moldes M. Adipocyte Glucocorticoid Receptor Deficiency Promotes Adipose Tissue Expandability and Improves the Metabolic Profile Under Corticosterone Exposure. Diabetes. 2019;68:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Li JY, Wang YD, Qi XY, Ran L, Hong T, Yang J, Yan B, Liao ZZ, Liu JH, Xiao XH. Serum CCN3 levels are increased in type 2 diabetes mellitus and associated with obesity, insulin resistance and inflammation. Clin Chim Acta. 2019;494:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Mancusi C, Izzo R, di Gioia G, Losi MA, Barbato E, Morisco C. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High Blood Press Cardiovasc Prev. 2020;27:515-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 100. | Popov SS, Anufrieva EI, Kryl'skii ED, Shulgin KK, Verevkin AN, Popova TN, Pashkov AN. The effect of methylethylpiridinol addition to the therapy on the level of pigment epithelium-derived factor and oxidative status in patients with diabetic nephropathy: randomized controlled open-label clinical study. J Diabetes Metab Disord. 2021;20:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 101. | Estienne A, Bongrani A, Reverchon M, Ramé C, Ducluzeau PH, Froment P, Dupont J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 102. | Heo YJ, Choi SE, Jeon JY, Han SJ, Kim DJ, Kang Y, Lee KW, Kim HJ. Visfatin Induces Inflammation and Insulin Resistance via the NF-κB and STAT3 Signaling Pathways in Hepatocytes. J Diabetes Res. 2019;2019:4021623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 103. | Romacho T, Valencia I, Ramos-González M, Vallejo S, López-Esteban M, Lorenzo O, Cannata P, Romero A, San Hipólito-Luengo A, Gómez-Cerezo JF, Peiró C, Sánchez-Ferrer CF. Visfatin/eNampt induces endothelial dysfunction in vivo: a role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci Rep. 2020;10:5386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 104. | Otu LI, Otu A. Adiponectin and the Control of Metabolic Dysfunction: Is Exercise the Magic Bullet? Front Physiol. 2021;12:651732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr Physiol. 2018;9:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 106. | Al-Mansoori L, Al-Jaber H, Prince MS, Elrayess MA. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation. 2022;45:31-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 107. | Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2017;40:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 108. | Liu C, He L, Li Y, Yang A, Zhang K, Luo B. Diabetes risk among US adults with different socioeconomic status and behavioral lifestyles: evidence from the National Health and Nutrition Examination Survey. Front Public Health. 2023;11:1197947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 109. | Tapager I, Olsen KR, Vrangbæk K. Exploring equity in accessing diabetes management treatment: A healthcare gap analysis. Soc Sci Med. 2022;292:114550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Kanchi R, Lopez P, Rummo PE, Lee DC, Adhikari S, Schwartz MD, Avramovic S, Siegel KR, Rolka DB, Imperatore G, Elbel B, Thorpe LE. Longitudinal Analysis of Neighborhood Food Environment and Diabetes Risk in the Veterans Administration Diabetes Risk Cohort. JAMA Netw Open. 2021;4:e2130789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Abate TW, Gedamu H. Psychosocial and clinical factors associated with depression among individuals with diabetes in Bahir Dar City Administrative, Northwest Ethiopia. Ann Gen Psychiatry. 2020;19:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |