Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1573
Peer-review started: June 8, 2023
First decision: July 18, 2023
Revised: July 22, 2023
Accepted: August 17, 2023
Article in press: August 17, 2023
Published online: October 15, 2023
Processing time: 123 Days and 5.3 Hours
Chiglitazar is an emerging pan-agonist of all peroxisome proliferator activated receptors (PPAR)-α, δ and γ, and has therapeutic potential for type 2 diabetes (T2D). However, to date, no clinical studies or meta-analyses have compared the efficacy and safety of chiglitazar and traditional PPAR-γ agonist thiazolidinediones (TZDs). A meta-analysis concerning this topic is therefore required.
To compare the efficacy and safety of chiglitazar and TZD in patients with T2D.
PubMed, Medline, Embase, the Cochrane Central Register of Controlled Trials, Reference Citation Analysis and Clinicaltrial.gov websites were searched from August 1994 to March 2022. Randomized controlled trials (RCTs) of chiglitazar or TZD vs placebo in patients with T2D were included. Indirect comparisons and sensitivity analyses were implemented to evaluate multiple efficacy and safety endpoints of interest.
We included 93 RCTs that compared TZD with placebo and one that compared chiglitazar with placebo. For efficacy endpoints, the augmented dose of chig-litazar resulted in greater reductions in hemoglobin (Hb)A1c [weighted mean difference (WMD) = -0.15%, 95% confidence interval (CI): -0.27 to -0.04%], triglycerides (WMD = -0.17 mmol/L, 95%CI: -0.24 to -0.11 mmol/L) and alanine aminotransferase (WMD = -5.25 U/L, 95%CI: -8.50 to -1.99 U/L), and a greater increase in homeostasis model assessment-β (HOMA-β) (WMD = 17.75, 95%CI: 10.73-24.77) when compared with TZD treatment. For safety endpoints, the risks of hypoglycemia, edema, bone fractures, upper respiratory tract infection, urinary tract infection, and weight gain were all comparable between the augmented dose of chiglitazar and TZD. In patients with baseline HbA1c ≥ 8.5%, body mass index ≥ 30 kg/m2 or diabetes duration < 10 years, the HbA1c reduction and HOMA-β increase were more conspicuous for the augmented dose of chiglitazar compared with TZD.
Augmented dose of chiglitazar, a pan-activator of PPARs, may serve as an antidiabetic agent with preferable glycemic and lipid control, better β-cell function preserving capacity, and does not increase the risk of safety concerns when compared with TZD.
Core Tip: This is the first indirect meta-analysis comparing efficacy and safety of chiglitazar and thiazolidinediones (TZDs). In patients with type 2 diabetes, compared with TZDs, chiglitazar induced favorable glycemic and lipidemic control, preserved β-cell function, without increasing safety concerns.
- Citation: Lin C, Li ZL, Cai XL, Hu SY, Lv F, Yang WJ, Ji LN. Indirect comparison of efficacy and safety of chiglitazar and thiazolidinedione in patients with type 2 diabetes: A meta-analysis. World J Diabetes 2023; 14(10): 1573-1584
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1573.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1573
Thiazolidinediones (TZDs) are hypoglycemic agents for type 2 diabetes (T2D) that characteristically alleviate insulin resistance (IR) to improve glycemic control[1]. TZDs are able to activate the peroxisome proliferator activated receptors (PPARs), which are mainly distributed in adipose tissue[2]. They also enhance sensitivity to insulin in target tissues through multiple downstream mechanisms including promoting fatty acid storage in adipose tissue and reducing free fatty acids (FFAs)[3], releasing insulin-sensitizing adipokines such as adiponectin[4], and suppressing excretion of IR-inducing cytokines such as tumor necrosis factor (TNF)-α[5]. Therefore, TZDs are effective in patients with traits of IR[6].
In previous clinical trials in patients with T2D, besides the favorable glycemic control[7], TZD also decreased the index of homeostasis model assessment of insulin resistance (HOMA-IR)[8], which indicated improved insulin sensitivity. However, the potential adverse events of TZD (including edema[9], heart failure[10], bone fracture[11], weight gain[2,9] and hepatic injury[12]) raised concerns. It has been reported that TZD lead to overactivation of PPAR-γ, which accelerates weight increase through facilitating adipocyte differentiation[1], and promotes water–sodium retention via more epithelial sodium channel expression in kidney tubules[13]. Other detrimental adverse effects including increased risks of bone fracture and heart failure were also found related to selective and excess PPAR-γ activation[1,13].
Due to the safety concerns, further applications of TZD in T2D treatment are therefore limited and whether the specific benefits of TZD outweigh the risks remains controversial. However, chiglitazar, a pan-agonist of PPAR-α, PPAR-δ and PPAR-γ[14], has been developed as a promising agent with improved therapeutic efficacy and safety by activation of multiple PPARs[15]. PPAR-α is mainly expressed in skeletal muscle and liver which regulates fatty acid metabolism[16], and its activation is associated with improved lipid profiles[17]. PPAR-δ is distributed widely in somatic cells, whose activation participates in elevated insulin sensitivity[18] and reverses metabolic abnormalities[15]. PPAR-α activation might also be associated with a reduced risk of heart failure[19], while PPAR-δ agonists have been reported to alleviate diabetic osteoporosis by promoting macrophage polarization[20].
Subsequently, with comprehensive activation of PPAR subtypes, chiglitazar may outperform TZD in terms of efficacy and safety in the management of T2D. However, to our knowledge, there have been no head-to-head randomized clinical trials (RCTs) directly comparing the efficacy and safety of chiglitazar and TZD. Hence, we conducted an indirect comparison meta-analysis using the data from RCTs comparing chiglitazar and TZD with placebo in patients with T2D.
This systematic review and indirect meta-analysis was conducted in line with the criteria of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) protocol[21]. Registration has been accomplished on International Prospective Register of Systematic Reviews (PROSPERO) platform as CRD42022334206.
In conformation with the recommendations in the Cochrane Handbook for Systematic Reviews for Meta-analysis, we implemented a systematic literature retrieval in Pubmed, Medline, Embase, Cochrane Central Register of Controlled Trials, Reference Citation Analysis (https://www.referencecitationanalysis.com/) and Clinicaltrial.gov websites for RCTs of chiglitazar or TZD treatment with placebo comparator in patients with T2D, which were published between August 1994 and March 2022. The search strings were as follows: Chiglitazar, pioglitazone, rosiglitazone, troglitazone, englitazone, thiazolidinedione, TZD, randomized controlled trial, placebo, efficacy, safety, T2D. The references in retrieved articles were also screened to thoroughly identify available and eligible RCTs.
The inclusion criteria of this indirect meta-analysis were: (1) Studies conducted in patients with T2D; (2) studies comparing chiglitazar or TZD with placebo; and (3) studies with reports of efficacy or safety outcomes. Two investigators (CL and ZL) independently screened articles by titles, abstracts and full text, excluded duplicate and ineligible studies, evaluated the quality and risk of bias with the Cochrane risk of bias tool, and extracted data from eligible studies. The collected data included: Study design (drug exposure, study duration, sample size in experimental and control arms); publication information (first author and publication year); baseline characteristics of patients [age, baseline hemoglobin (Hb)A1c, body mass index (BMI), sex ratio, ethnicity, and diabetes duration]; efficacy parameters [changes in HbA1c, fasting blood glucose (FBG), HOMA-IR, HOMA-β, total triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST)]; and safety parameters (measurements of weight gain; incidence of hypoglycemia, edema, heart failure, bone fracture, upper respiratory tract infection, and urinary tract infection). Required data were primarily abstracted from the original articles or attached supplementary materials. The Clinicaltrials.gov website was subsequently searched if data were not available in articles and supplementary materials. Discrepancies were resolved by reaching a consensus with another joint investigator (XC).
The risk of bias in enrolled RCTs was assessed with the Cochrane Collaboration tool[22]. The evaluating measurements included random sequence generation, allocation concealment, blinding of participants and care-givers, missing outcome data, selective outcomes reporting, and other bias. Each domain was evaluated by degrees of the existing risks of bias, including “definitely yes”, “probably yes”, “definitely no”, “probably no” according to the instruction[22].
The primary efficacy endpoint was defined as indirect comparison of changes in HbA1c after treatment with chiglitazar or TZD in comparison with placebo. The indirect comparisons for other efficacy parameters (including FBG, HOMA-IR, HOMA-β, TG, LDL-C, HDL-C, ALT and AST) were interpreted as exploratory efficacy endpoints. The primary safety endpoint was defined as indirect comparison of the incidence of hypoglycemia after treatment with chiglitazar or TZD in comparison with placebo. Indirect comparisons for the incidence of other adverse events including edema, heart failure, bone fracture, upper respiratory tract infection, and urinary tract infection, and measurement of weight gain were interpreted as exploratory safety endpoints. Subgroup analyses with regard to baseline characteristics including age, baseline HbA1c, BMI, male percentage, predominant ethnicity, diabetes duration, follow-up duration, and monotherapy or combination therapy were performed to further characterize the influences of these potentially associated factors on the outcomes. Caucasian predominance was defined as the percentage of Caucasian > 50% of the participants. Correspondingly, Asian predominance was defined as the percentage of Asian > 50% of the participants. Meanwhile, we also conducted subgroup analyses concerning different TZD subtypes in indirect comparisons for changes in HbA1c and TG to further compare the efficacy between chiglitazar and different subtypes of TZD. Meta-regression analyses evaluating the potential correlation between baseline characteristics (including age, male percentage, BMI, diabetes duration, study duration, and baseline HbA1c) and the study outcomes were also conducted in the TZD treatment group (since the chiglitazar treatment group only involved one RCT, when the meta-regression analysis could not be implemented).
Prior to producing an indirect estimate of the treatment effect of chiglitazar versus TZD, we primarily checked the adequacy of such synthesis[23,24]. Homogeneity of the results from the placebo group as a common comparator for the indirect comparison was first evaluated among included studies. Whether the treatment effects were sufficiently homogeneous to be pooled within each comparison of chiglitazar vs placebo and TZD vs placebo was evaluated. We also qualitatively assessed the trials for patient characteristics and design features for comparability, based on which, the subsequent sensitivity analyses were performed to control the potential confounding effects.
To perform the indirect comparison, we firstly calculated the pooled treatment effect estimates of chiglitazar vs placebo and TZD vs placebo through regular meta-analysis statistical methods. Afterwards, the indirect comparison was implemented by synthesizing the pooled treatment effect estimates of each treatment group compared with placebo. Results of continuous variables in this indirect meta-analysis were presented as the weighted mean difference (WMD) with 95% confidence intervals (CIs). For discontinuous variables, the risk ratios (RRs) with 95%CIs were calculated and rendered. The heterogeneity of the included studies was evaluated by Higgins I2 statistics. I2 ≥ 50% represented a high level of heterogeneity; otherwise, a low level of heterogeneity level was considered. A random-effects model was uniformly adopted for data analyses. Publication bias was assessed with the funnel plot. Statistical significance was considered at P < 0.05. Statistical analyses were principally completed by Review Manager version 5.3 (Nordic Cochrane Center, Copenhagen, Denmark) and STATA version 12.0 (Stata Corp., College Station, TX, United States).
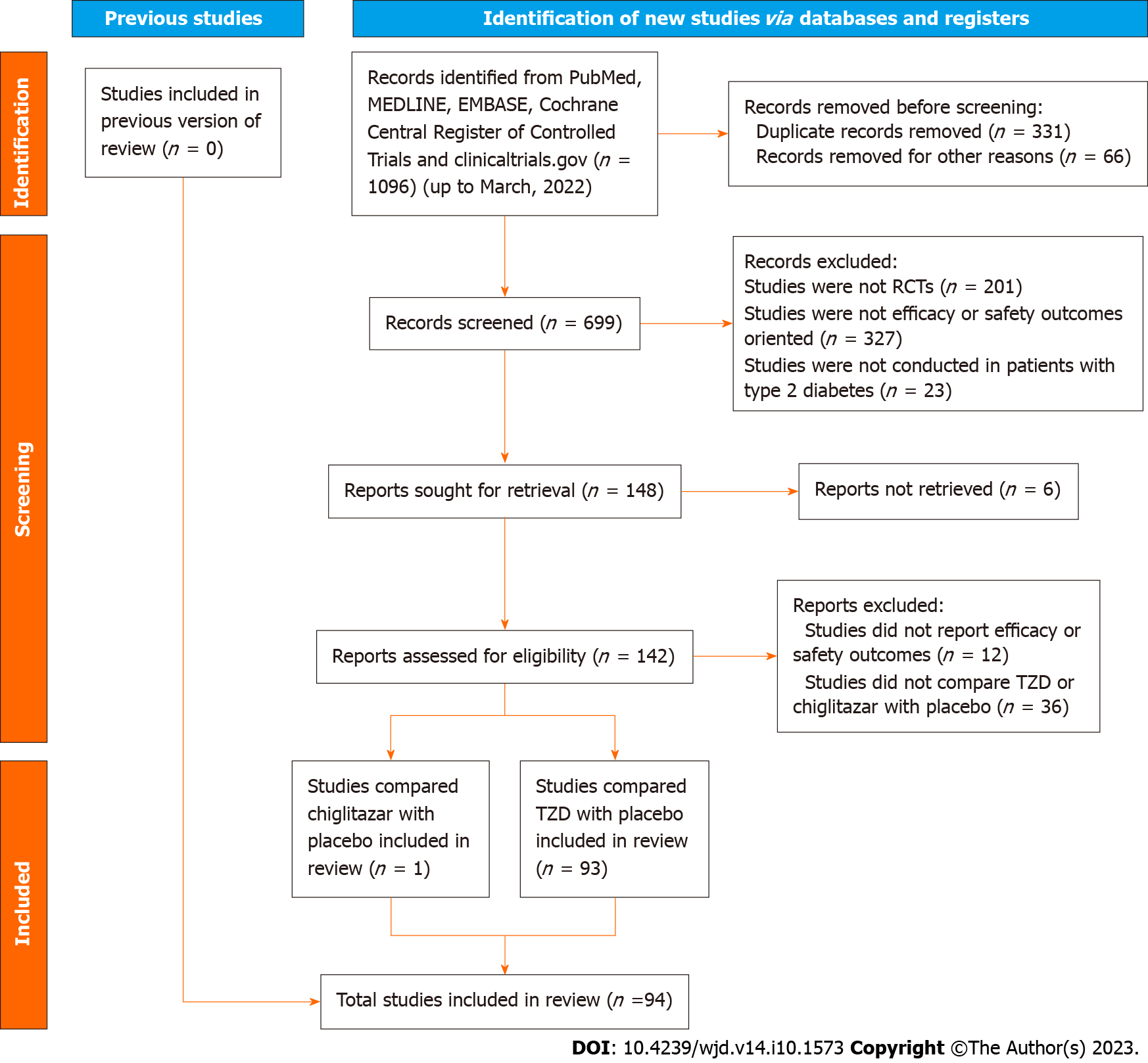
There were 94 RCTs included in this meta-analysis, including one comparing chiglitazar with placebo (166 participants in the chiglitazar arm vs 202 in the placebo arm), and 93 comparing TZD with placebo (15580 participants in the TZD arm vs 14706 in the placebo arm). The RCT of chiglitazar investigated two doses, where 32 mg and 48 mg were defined as the standard and augmented doses, respectively. The TZDs involved in this meta-analysis included pioglitazone, rosiglitazone and troglitazone. The selection and inclusion process of eligible studies is summarized in the flow chart (Figure 1).
Baseline characteristics of included studies are recorded in Supplementary Table 1. The quality assessments were conducted with Cochrane instruments (Supplementary Table 2), which indicated low overall risks of bias in included studies. There was one RCT with high risk of frequent missing data, while all RCTs were with low risks in inadequate randomization sequence generation, inadequate allocation concealment, selective outcome reporting, masking patients and caregivers, and masking outcome assessors. The publication bias was evaluated by funnel plots, which displayed even distributions in most of the endpoints but an asymmetric distribution for the endpoint of edema (Supp
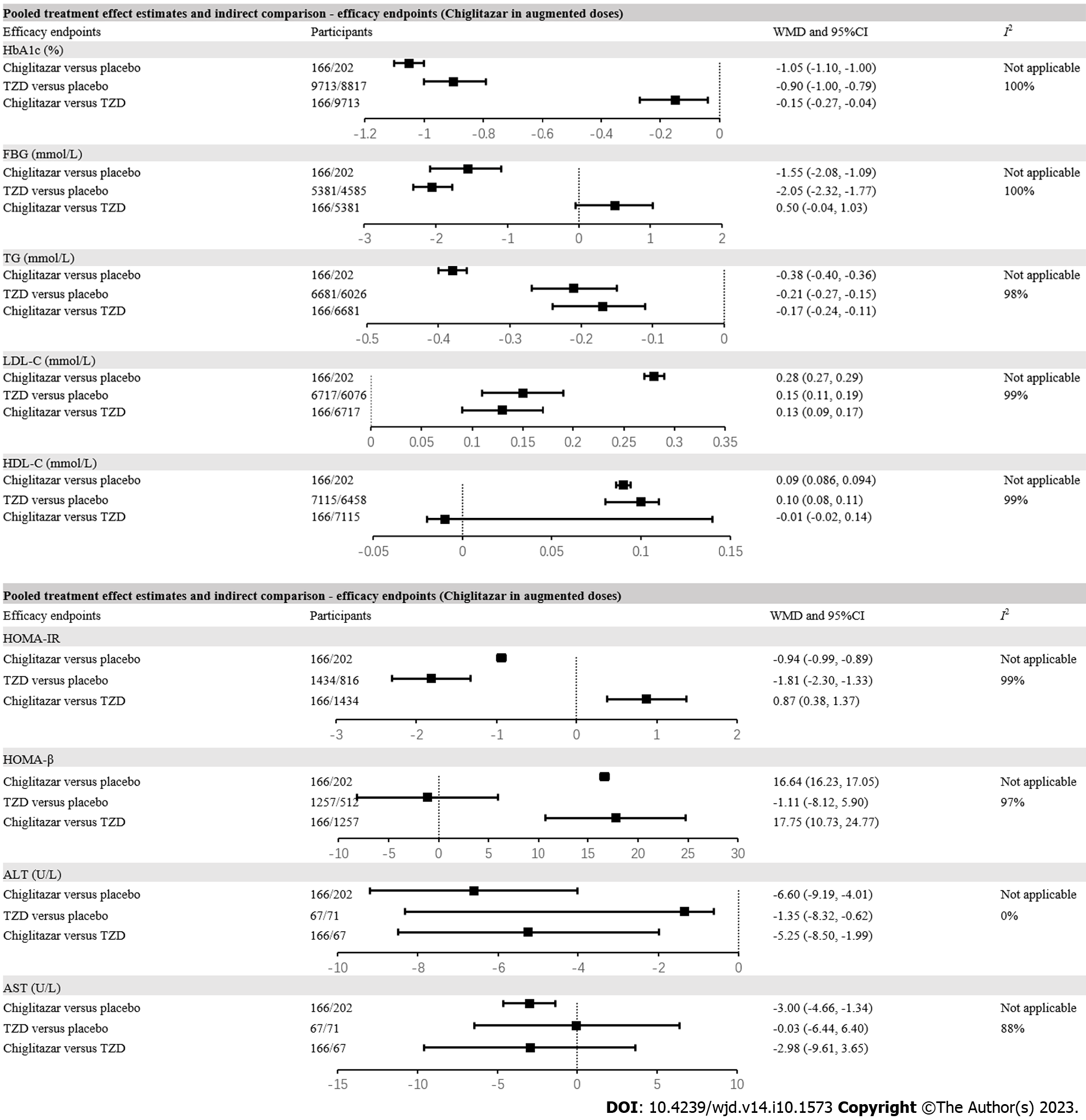
For glycemic control, compared with placebo, chiglitazar (WMD = -1.05%, 95%CI: -1.10 to -1.00%) and TZD (WMD =
With respect to lipid profiles, chiglitazar (WMD = -0.38 mmol/L, 95%CI: -0.40 to -0.36 mmol/L) and TZD treatment (WMD = -0.21 mmol/L, 95%CI: -0.27 to -0.15 mmol/L) were effective in lowering TG levels in patients with T2D compared with placebo. The indirect comparison indicated greater TG reduction with chiglitazar compared with TZD (WMD = -0.17 mmol/L, 95%CI: -0.24 to -0.11 mmol/L). Although chiglitazar and TZD were both associated with increased LDL-C compared with placebo, greater LDL-C elevation was observed in patients with augmented dose chiglitazar compared with TZD (WMD = 0.13 mmol/L, 95%CI: 0.09 to 0.17 mmol/L). Both chiglitazar (WMD = 0.09 mmol/L, 95%CI: 0.086 to 0.094 mmol/L) and TZD (WMD = 0.10 mmol/L, 95%CI: 0.08 to 0.11 mmol/L) contributed to elevated HDL-C levels compared with placebo. Such effects on HDL-C were comparable between augmented dose of chiglitazar and TZD (WMD = -0.01 mmol/L, 95%CI: -0.02 to 0.14 mmol/L).
Although the effectiveness of reducing HOMA-IR index was validated in patients treated with augmented dose chiglitazar (WMD = -0.94, 95%CI: -0.99 to -0.89) and TZD (WMD = -1.81, 95%CI: -2.30 to -1.33) compared with placebo, chiglitazar might underperform with respect to HOMA-IR reduction compared with TZD (WMD = 0.87, 95%CI: 0.38-1.37). However, chiglitazar was associated with a profound elevation in HOMA-β index compared with placebo (WMD = 16.64, 95%CI: 16.23-17.05), which was not observed in patients with TZD treatment compared with placebo (WMD =
For liver enzymes, compared with placebo, chiglitazar treatment was associated with significantly decreased ALT (WMD = -6.60 U/L, 95%CI: -9.19 to -4.01 U/L) and AST level (WMD = -3.00 U/L, 95%CI: -4.66 to -1.34 U/L). TZD was associated with significantly decreased ALT level (WMD = -1.35 U/L, 95%CI: -8.32 to -0.62 U/L) but did not significantly change AST level (WMD = -0.03 U/L, 95%CI: -6.44 to -6.40 U/L) in patients with T2D. By indirect comparison, the augmented dose of chiglitazar outperformed TZD for ALT reduction (WMD = -5.25 U/L, 95%CI: -8.50 to -1.99 U/L), whereas chiglitazar and TZD exhibited similar effects on AST levels (WMD = -2.98 U/L, 95%CI: -9.61 to 3.65 U/L) (Figure 2).
Sensitivity analyses showed that chiglitazar reduced HbA1c more prominently compared with TZD in patients with age ≥ 60 years (WMD = -0.30%, 95%CI: -0.41 to -0.18%), baseline HbA1c ≥ 8.5% (WMD = -0.44%, 95%CI: -0.58 to -0.30%), BMI ≥ 30kg/m2 (WMD = -0.24%, 95%CI: -0.40 to -0.08%), and duration of diabetes < 10 years (WMD = -0.16%, 95%CI: -0.31 to -0.02%) (Supplementary Table 3). The increase in HOMA-β after chiglitazar treatment was significantly greater than that after TZD treatment in patients with baseline HbA1c ≥ 8.5% (WMD = 26.36, 95%CI: 8.80-43.93), BMI ≥ 30 kg/m2 (WMD = 29.42, 95%CI: 19.34-39.50) and duration of diabetes < 10 years (WMD = 26.36, 95%CI: 8.80-43.93) (Supplementary Table 3). Sensitivity analyses of TZD subtypes indicated that the greater reduction in HbA1c in patients treated with augmented dose of chiglitazar vs TZD was mainly shown by comparison between chiglitazar 48 mg once daily and rosiglitazone 4 mg once daily (WMD = -0.39 %, 95%CI: -0.67 to -0.11 %). The greater reduction in TG after treatment by chiglitazar was mainly shown by comparison between chiglitazar 48 mg once daily and rosiglitazone 4 mg once daily (WMD = -0.58 mmol/L, 95%CI: -0.86 to -0.30 mmol/L) as well as comparison between chiglitazar 48 mg once daily and rosiglitazone 8 mg once daily (WMD = -0.22 mmol/L, 95%CI: -0.36 to -0.08 mmol/L) (Supplementary Table 3).
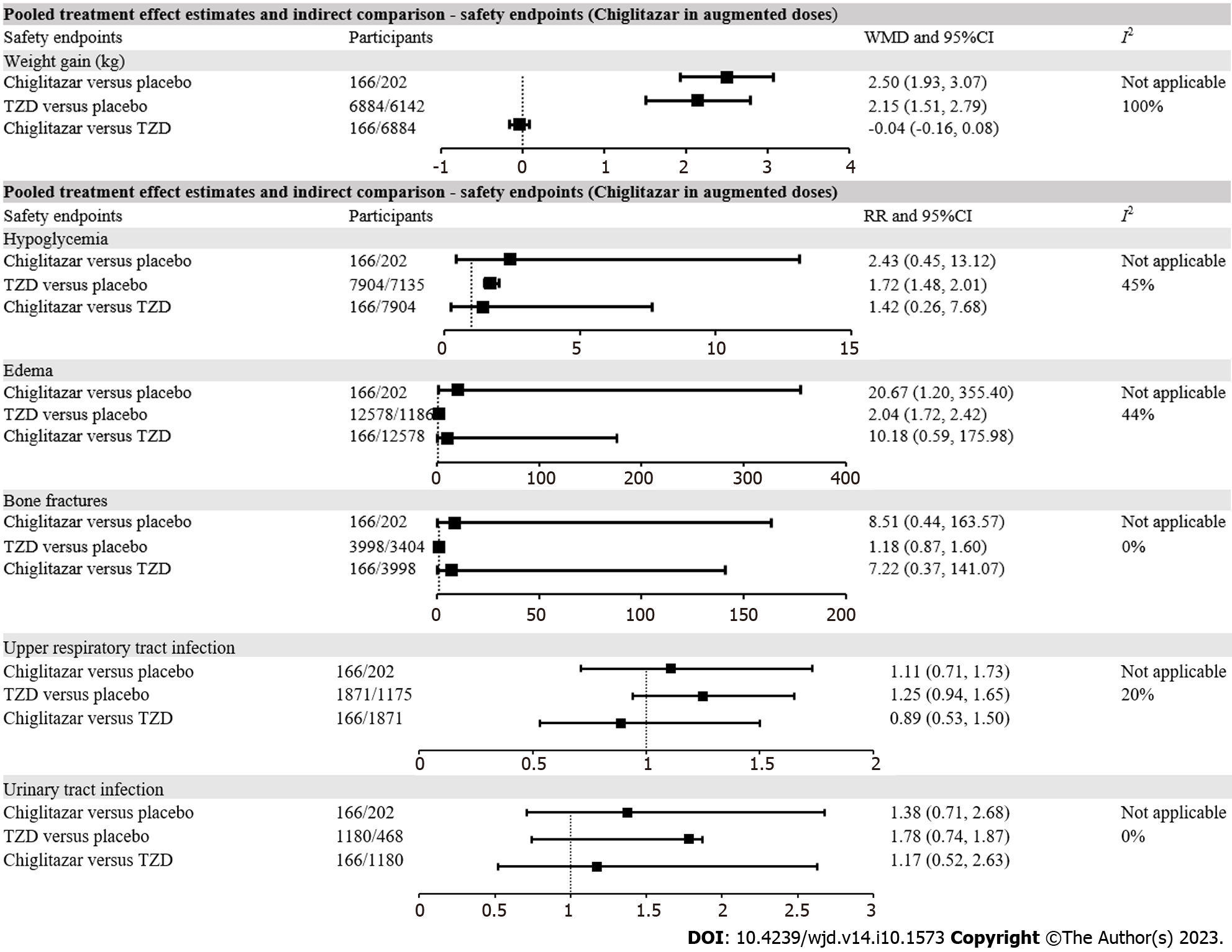
Compared with placebo, chiglitazar did not increase the risk of hypoglycemia (RR = 2.43, 95%CI: 0.45-13.12), which was elevated in patients with TZD treatment (RR = 1.72, 95%CI: 1.48-2.01) (Supplementary Figure 3). However, the indirect comparison suggested a non-significant difference in risk of hypoglycemia between chiglitazar and TZD treatment (RR = 1.42, 95%CI: 0.26-7.68). Both chiglitazar (WMD = 2.50 kg, 95%CI: 1.93-3.07 kg) and TZD (WMD = 2.15 kg, 95%CI: 1.51-2.79 kg) were associated with significantly increased body weight compared with placebo, but the weight gain was comparable between chiglitazar and TZD treatment in patients with T2D (WMD = -0.04 kg, 95%CI: -0.16 to 0.08 kg). Although heart failure was defined as an exploratory safety endpoint in this research, since no case of heart failure was reported in the chiglitazar or placebo treatment arms, we were unable to conduct an indirect comparison of the incidence of heart failure after treatment with chiglitazar or TZD (Figure 3). Compared with placebo, chiglitazar (RR = 20.67, 95%CI: 1.20-355.40) and TZD (RR = 2.04, 95%CI: 1.72-2.42) were both associated with significantly elevated risks of edema in patients with T2D. The risk of edema was comparable between chiglitazar and TZD (RR = 10.18, 95%CI: 0.59-175.98). The incidence of other adverse events, including bone fractures, upper respiratory tract infection and urinary infection, was comparable between chiglitazar/TZD and placebo, when indirect comparison also indicated a non-significant difference between chiglitazar and TZD treatment (Figure 3). Subgroup analyses of safety endpoints also conferred negative findings (Supplementary Table 3).
In patients treated with standard dose of chiglitazar, we observed significantly decreased HbA1c, FBG, TG, HOMA-IR index and ALT, and significantly elevated LDL-C, HDL-C and HOMA-β index compared with placebo, which was consistent with the results of treatment with augmented dose of chiglitazar. However, the indirect comparison suggested comparable change of HbA1c, TG and ALT levels after treatment with chiglitazar or TZD in comparison with placebo in patients with T2D. For safety endpoints, compared with placebo, standard dose of chiglitazar was not associated with increased risk of hypoglycemia. The increased risk of edema with augmented dose of chiglitazar became non-significant after treatment with standard dose of chiglitazar. Indirect comparison indicated comparable risks of safety concerns between standard dose of chiglitazar and TZD treatment, which was consistent with the results of the indirect compassion between augmented dose of chiglitazar and TZD treatment. The detailed results are shown in Supplementary Figure 4.
Meta-regression analyses showed that in patients under TZD treatment, male percentage (β = 0.011, 95%CI: 0.002-0.021, P = 0.019) and baseline HbA1c (β = -0.320, 95%CI: -0.427 to -0.212, P = 0.0001) were significantly correlated with the change in HbA1c, when baseline HbA1c (β = -0.578, 95%CI: -0.768 to -0.388, P = 0.0001) and BMI (β = -0.249, 95%CI: -0.442 to
To our knowledge, this is the first comprehensive meta-analysis comparing the efficacy and safety of chiglitazar and TZD. According to this meta-analysis, augmented doses of chiglitazar outperformed TZD treatment for HbA1c, TG and ALT reduction and HOMA-β index elevation, and conferred greater LDL-C elevation and less HOMA-IR reduction in patients with T2D. For safety endpoints, the risks of hypoglycemia, edema, heart failure, bone fractures, upper respiratory tract infection and urinary tract infection, and weight gain were all comparable between augmented doses of chiglitazar and TZD. Further sensitivity analyses indicated that in patients with age ≥ 60 years, baseline HbA1c ≥ 8.5%, BMI ≥ 30 kg/m2 or diabetes duration < 10 years, the reduction in HbA1c and improvement in HOMA-β were more conspicuous with augmented doses of chiglitazar compared with TZD.
Chiglitazar and TZD, as hypoglycemic agents, both lowered blood glucose level with mutual pivotal mechanisms of activating PPAR-γ[14,25]. PPAR-γ activation could ameliorate hyperglycemia by enhancing glucose transporter-1 and -4 of adipocytes, which facilitated glucose ingestion in adipose tissues[26]. Therefore, PPAR-γ activation mediated glucose lowering effects in both chiglitazar and TZD. However, since chiglitazar acted as a pan-agonist of PPAR-α, PPAR-δ and PPAR-γ, the hypoglycemic capacity of chiglitazar may also be derived from the activation of other PPARs. PPAR-α was distributed widely in liver, skeletal muscle, heart and adipose tissues, and its activation accelerated fatty acid uptake and oxidation and lipoprotein assembly[27], which resulted in decreased FFA and TG levels and fat accumulation. The lipid-modulating effects of PPAR-α activation attenuated lipidic toxicity for β cells[28] and inhibited gluconeogenesis from excess lipids[29], which improved overall glycemic control. PPAR-α activation was also reported to promote glucose metabolism and ketogenesis[27], which increased glucose consumption and thereby lowered blood glucose. Activation of PPAR-δ facilitated glucose metabolism through the pentose phosphate pathway[25] and increased basal metabolic rate[29] to reduce blood glucose. PPAR-α and PPAR-δ activation improved β-cell function[30,31], which lowered glycemia independent of IR remission[27]. The details are elaborated in the next section.
Apart from their hypoglycemic effects, chiglitazar and TZD reduced the serum TG level, for which PPAR-γ activation served as the mutual mechanism. Activation of PPAR-γ was associated with lipid uptake, lipid droplet formation, and adipocyte differentiation[25,44] in adipose tissues, as well as lipid oxidation in skeletal muscle and liver, which resulted in decreased circulating FFA and TG levels[32]. PPAR-γ activation promoted synthesis of bio-active proteins including fat-specific protein 27 and monoacylglycerol O-acyltransferase 1, which participated in lipid uptake and storage[29,33]. PPAR-γ activation also increased preadipocyte differentiation and functionalization, thus accelerating lipogenesis and consumption of lipids[34].
In our study, the augmented doses of chiglitazar outperformed TZD with respect to TG reduction. The enhanced hypolipidemic effects of chiglitazar may also have been derived from activation of PPAR-α and PPAR-δ. PPAR-α was identified as a regulator of lipid metabolism, whose activation increased lipid uptake and transport, fatty acid oxidation, lipoprotein assembly and TG accumulation in the liver[27]. PPAR-α activation also facilitated cytochrome P4504A production, which participated in hydroxylation of fatty acids and thereby reduced TG synthesis[35]. The PPAR-α agonists fibrates lowered TG levels and have been extensively used in patients with dyslipidemia[36], which confirms the hypolipidemic activity of PPAR-α activation. PPAR-δ activation was associated with fatty acid transport, lipid oxidation and decreased fatty acid release[25], and fat combustion and thermogenesis contributed to overall lipid reduction. Mice treated with PPAR-δ agonists had significantly lowered TG levels[37], indicating PPAR-δ activation had the potential to improve TG profiles. Therefore, chiglitazar may result in greater TG reduction compared with TZD, and the additional hypolipidemic effects may be derived from PPAR-α and PPAR-δ activation.
We found that chiglitazar and TZD treatment was associated with elevated LDL-C and HDL-C concentrations, which was more pronounced with augmented doses of chiglitazar compared with TZD. It was indicated that PPAR-α and PPAR-γ activation could facilitate reversed cholesterol transportation and lipoprotein exchange, and therefore increased plasma LDL-C and HDL-C levels[38]. Activation of either PPAR resulted in significant HDL-C elevation in previous in vivo experiments[39-41], whereas the changes in LDL-C under PPAR agonist treatment were inconsistent[42,43]. The underlying mechanisms have also not been fully demonstrated, and further investigations on the correlations between PPARs and cholesterol are required.
The activation of PPAR-α, PPAR-δ and PPAR-γ was associated with ameliorated nonalcoholic fatty liver disease via improved lipidemic and glycemic control[29]. The transfer of fat and lipids from viscera to peripheral tissues was facilitated by PPAR-α activation, which also relieved steatosis of hepatocytes[27]. We observed significantly decreased ALT levels after treatment with augmented doses of chiglitazar compared with TZD. The greater ALT reduction with chiglitazar may also have resulted from alleviated liver injuries with the favorable lipidemic, glycemic control and fat distribution through additional activation of PPAR-α and PPAR-δ.
IR and attenuation of β-cell function have been identified as the central pathophysiology of T2D; therefore, ameliorating IR and postponing β-cell failure have become important strategies in retarding T2D progression[44]. PPAR-γ activation contributed to adipocytes remodeling by virtue of facilitating apoptosis of visceral insulin-resistant adipocytes and generation of subcutaneous insulin-sensitive adipocytes[45]. It was also demonstrated that PPAR-γ activation lowered secretion of adipocytokines and chemokines, which contributed to IR[46]. PPAR-γ activation also prevented β-cell dysfunction by improving glycemic control and lipid metabolism, which attenuated glucotoxicity and lipotoxicity in islets[47,48]. PPAR-γ activation also inhibited the production of inflammatory cytokines, including TNF-α, interleukin (IL)-1 and IL-6, which mitigated islet inflammation and preserved β-cell function[49,50].
PPAR-α and PPAR-δ agonists improved insulin sensitivity in in vitro studies[51-53]. The insulin-sensitizing effects of PPAR-α and PPAR-δ were mostly circuitous and not as well-established as those of PPAR-γ. Since the interactions to PPAR-α, PPAR-δ and PPAR-γ of chiglitazar were generally balanced, the stimulating intensity to PPAR-γ might be relatively decreased when compared with TZD[25]. Therefore, the relief of IR by chiglitazar might also have been attenuated when compared with that of TZD. Although PPAR-γ activation was potentially able to preserve β-cell function as noted above, we observed comparable HOMA-β index alteration between TZD treatment and placebo. However, HOMA-β index was significantly elevated by chiglitazar treatment at both standard and augmented doses when compared with placebo and TZD. According to previous researches, HOMA-β index elevation may be attributed to the activation of PPAR-α and PPAR-δ. PPAR-α activation was associated with islet adaptation to starvation, which enhanced glucose utilization and insulin secretion[54]. Glucose-induced insulin secretion was also promoted by PPAR-α activation[55], especially in response to hyperglycemia[56]. PPAR-α activation stimulated insulin secretion through inhibition of Ca2+ signaling[57]. The islet-preserving effects of PPAR-δ have also received extensive attention. Many studies have indicated that PPAR-δ activation significantly improved islet function in mice, with the potential of elevating β-cell mass[58], alleviating β-cell lipoapoptosis[59], and reducing inappropriate baseline secretion[60]. Favorable glycemic and lipidemic control, and ameliorated chronic inflammatory states derived from PPAR-α and PPAR-δ activation may also participate in preservation of β-cell function[44]. However, the effects of PPAR-γ, PPAR-α and PPAR-δ activation on β-cell function have not been fully characterized. Further research on the specific mechanisms of preservation of β-cell function by chiglitazar and PPAR activation is required.
Although TZD significantly improved glycemic and lipidemic control and relieved IR, the clinical utilization of TZD was limited by the increased risk of adverse events. The adverse events related to TZD were primarily hypoglycemia[10], weight gain[9], edema[9], congestive heart failure[10], and bone fracture[11]. Since chiglitazar may ameliorate the centralized and excess PPAR-γ activation presented in TZD[22], and potentially exert beneficial effects through PPAR-α and PPAR-δ activation, it was expected that the safety risks could be attenuated in chiglitazar treatment in contrast to TZD. However, in this meta-analysis, we observed significantly increased risks of weight gain and edema with both chiglitazar and TZD compared with placebo. Subsequent indirect comparisons exhibited comparable risk of hypoglycemia, weight gain, edema, bone fracture, upper respiratory tract infection and urinary tract infection between chiglitazar and TZD. The safety of PPAR-α and PPAR-δ activation was not shown[61]. Clinical trials of chiglitazar were rare, which made it difficult to thoroughly evaluate safety outcomes. Further researches are required to comprehensively assess the safety features and potential mechanisms in chiglitazar.
A number of baseline characteristics are potentially associated with the effects of chiglitazar and TZD in patients with T2D, including age, sex, glycemic control status (baseline HbA1c), BMI and diabetes duration. According to the meta-regression analysis, male percentage, BMI and baseline HbA1c were linearly associated with several glycemic and lipidemic control outcomes. The potential influence of these baseline characteristics on study results should therefore be cautiously considered when interpreting the outcomes of this study. Meanwhile, in this indirect comparison meta-analysis, reduction in HbA1c and improvement of HOMA-β index were more prominent for treatment with augmented doses of chiglitazar compared with TZD for patients with baseline HbA1c ≥ 8.5% (poorly controlled diabetes), BMI ≥ 30 kg/m2 (obese) or diabetes duration < 10 years (short T2D duration).
In patients with poorly controlled diabetes and frequent hyperglycemia, the systematic metabolic disorders appeared to be more severe[62]. Chiglitazar outperformed TZD in improving lipid profiles and accelerating glucose consumption[49,56]. Therefore, chiglitazar could have achieved better glycemic control and protection of β-cell function through better relief of metabolic disorders, which improved glucose consumption and decreased lipotoxicity to islets.
For patients with obesity, the lipid-modifying effects of chiglitazar may have synergistically improved glycemic control[44]. It would be more effective for chiglitazar to preserve β-cell function in obese patients as their β-cell function was generally better than that in patients who were non-obese[63]. Furthermore, compared with long-established T2D, the severities of metabolic turbulence, glycemic or lipidemic disorder, and deterioration of β-cell function were lower in patients with shorter diabetes duration, which were more reversible with chiglitazar treatment[63].
This study had some limitations. Firstly, this research was based on the statistical approach of indirect comparison. Secondly, since the RCTs had different study designs and populations, the resultant endogenous heterogeneity should not be ignored. To control the heterogeneity, we implemented multiple sensitivity analyses concerning underlying associated factors to minimize the confounding effects. Moreover, there was only one eligible RCT investigating chiglitazar available for the indirect comparison, when the sample size and data abundance were limited. Considering the potential bias, the results and conclusions in this indirect comparison meta-analysis should be interpreted with caution. The comparison should be updated with enriched RCT data of chiglitazar in the future. There was no heart failure event reported in the RCT of chiglitazar; therefore, the indirect comparison of heart failure incidence between chiglitazar and TZD was not possible in this study. More investigations evaluating safety outcomes of chiglitazar, especially heart failure, are still needed.
Through pan-activation of PPAR-α, PPAR-δ and PPAR-γ, chiglitazar may serve as a promising therapeutic agent for T2D with preferable glycemic and lipid control, additional β-cell function preservation, and favorable tolerance for augmented doses when compared with TZD.
Chiglitazar as a pan-agonist of peroxisome proliferator activated receptor (PPAR)-α, δ and γ, has the potential to induce better glycemic and lipidemic control than the PPAR-γ agonist thiazolidinediones (TZDs) in patients with type 2 diabetes (T2D).
Currently, there are no clinical studies or meta-analyses comparing the efficacy and safety of chiglitazar and TZD. A meta-analysis is required to further address this topic.
To compare the efficacy and safety of chiglitazar and TZD in patients with T2D.
Randomized controlled trials (RCTs) of chiglitazar or TZD vs placebo in patients with T2D were retrieved. Indirect comparisons and sensitivity analyses were implemented to evaluate the efficacy and safety endpoints of interest.
We included 93 RCTs comparing TZD with placebo and one comparing chiglitazar with placebo. For efficacy endpoints, the augmented dose of chiglitazar, compared with TZD, resulted in greater reductions in hemoglobin A1c, triglycerides and alanine aminotransferase levels, and greater homeostasis model assessment of β cell function elevation. For safety endpoints, the risks of hypoglycemia, edema, bone fractures, upper respiratory tract infection, urinary tract infection, and weight gain were all comparable between the augmented dose of chiglitazar and TZD.
Chiglitazar, a pan-activator of PPARs, may exhibit preferable glycemic and lipid control, and β-cell function preservation, with no additional safety concerns with augmented doses compared with TZD in patients with T2D.
Chiglitazar has potential for T2D treatment. However, more investigations evaluating safety outcomes of chiglitazar, especially heart failure, are still needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lu XP, China; M Amin KF, Iraq; Horowitz M, Australia S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Davidson MA, Mattison DR, Azoulay L, Krewski D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: past, present and future. Crit Rev Toxicol. 2018;48:52-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Nanjan MJ, Mohammed M, Prashantha Kumar BR, Chandrasekar MJN. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg Chem. 2018;77:548-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 3. | Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1140] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 4. | Liu S, Wu HJ, Zhang ZQ, Chen Q, Liu B, Wu JP, Zhu L. The ameliorating effect of rosiglitazone on experimental nonalcoholic steatohepatitis is associated with regulating adiponectin receptor expression in rats. Eur J Pharmacol. 2011;650:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1451] [Cited by in RCA: 1627] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 6. | Walter H, Lübben G. Potential role of oral thiazolidinedione therapy in preserving beta-cell function in type 2 diabetes mellitus. Drugs. 2005;65:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J; PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3109] [Cited by in RCA: 2958] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 8. | Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE; Pioglitazone 026 Study Group. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Mukherjee K, Chattopadhyay N. Pharmacological inhibition of cathepsin K: A promising novel approach for postmenopausal osteoporosis therapy. Biochem Pharmacol. 2016;117:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Erdmann E, Wilcox RG. Weighing up the cardiovascular benefits of thiazolidinedione therapy: the impact of increased risk of heart failure. Eur Heart J. 2008;29:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Betteridge DJ. Thiazolidinediones and fracture risk in patients with Type 2 diabetes. Diabet Med. 2011;28:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Saha S, New LS, Ho HK, Chui WK, Chan EC. Investigation of the role of the thiazolidinedione ring of troglitazone in inducing hepatotoxicity. Toxicol Lett. 2010;192:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Deeks ED. Chiglitazar: First Approval. Drugs. 2022;82:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | He BK, Ning ZQ, Li ZB, Shan S, Pan DS, Ko BC, Li PP, Shen ZF, Dou GF, Zhang BL, Lu XP, Gao Y. In Vitro and In Vivo Characterizations of Chiglitazar, a Newly Identified PPAR Pan-Agonist. PPAR Res. 2012;2012:546548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Haluzík MM, Haluzík M. PPAR-alpha and insulin sensitivity. Physiol Res. 2006;55:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Aguilar-Recarte D, Palomer X, Wahli W, Vázquez-Carrera M. The PPARβ/δ-AMPK Connection in the Treatment of Insulin Resistance. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Sarma S, Ardehali H, Gheorghiade M. Enhancing the metabolic substrate: PPAR-alpha agonists in heart failure. Heart Fail Rev. 2012;17:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chen M, Lin W, Ye R, Yi J, Zhao Z. PPARβ/δ Agonist Alleviates Diabetic Osteoporosis via Regulating M1/M2 Macrophage Polarization. Front Cell Dev Biol. 2021;9:753194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47111] [Article Influence: 2944.4] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24774] [Article Influence: 1769.6] [Reference Citation Analysis (3)] |
| 23. | Cho YK, Kim YJ, Kang YM, Lee SE, Park JY, Lee WJ, Jung CH. Comparison between sodium-glucose cotransporter 2 inhibitors and pioglitazone as additions to insulin therapy in type 2 diabetes patients: A systematic review with an indirect comparison meta-analysis. J Diabetes Investig. 2018;9:882-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Min SH, Yoon JH, Hahn S, Cho YM. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017;13:279-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 26. | Liao W, Nguyen MT, Yoshizaki T, Favelyukis S, Patsouris D, Imamura T, Verma IM, Olefsky JM. Suppression of PPAR-gamma attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;293:E219-E227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 2017;13:259-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 28. | Lencioni C, Lupi R, Del Prato S. Beta-cell failure in type 2 diabetes mellitus. Curr Diab Rep. 2008;8:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Nakajima T, Gonzalez FJ, Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 30. | Sugden MC, Holness MJ. Potential role of peroxisome proliferator-activated receptor-alpha in the modulation of glucose-stimulated insulin secretion. Diabetes. 2004;53 Suppl 1:S71-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Tang T, Abbott MJ, Ahmadian M, Lopes AB, Wang Y, Sul HS. Desnutrin/ATGL activates PPARδ to promote mitochondrial function for insulin secretion in islet β cells. Cell Metab. 2013;18:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3510] [Cited by in RCA: 3503] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 33. | Lee YJ, Ko EH, Kim JE, Kim E, Lee H, Choi H, Yu JH, Kim HJ, Seong JK, Kim KS, Kim JW. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci U S A. 2012;109:13656-13661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Marion-Letellier R, Savoye G, Ghosh S. Fatty acids, eicosanoids and PPAR gamma. Eur J Pharmacol. 2016;785:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 35. | Yu S, Rao S, Reddy JK. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 2003;3:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2010;210:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Leibowitz MD, Fiévet C, Hennuyer N, Peinado-Onsurbe J, Duez H, Bergera J, Cullinan CA, Sparrow CP, Baffic J, Berger GD, Santini C, Marquis RW, Tolman RL, Smith RG, Moller DE, Auwerx J. Activation of PPARdelta alters lipid metabolism in db/db mice. FEBS Lett. 2000;473:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Ji L, Song W, Fang H, Li W, Geng J, Wang Y, Guo L, Cai H, Yang T, Li H, Yang G, Li Q, Liu K, Li S, Liu Y, Shi F, Li X, Gao X, Tian H, Ji Q, Su Q, Zhou Z, Wang W, Xu Y, Ning Z, Cao H, Pan D, Yao H, Lu X, Jia W. Efficacy and safety of chiglitazar, a novel peroxisome proliferator-activated receptor pan-agonist, in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, phase 3 trial (CMAP). Sci Bull (Beijing). 2021;66:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Chehaibi K, Cedó L, Metso J, Palomer X, Santos D, Quesada H, Naceur Slimane M, Wahli W, Julve J, Vázquez-Carrera M, Jauhiainen M, Blanco-Vaca F, Escolà-Gil JC. PPAR-β/δ activation promotes phospholipid transfer protein expression. Biochem Pharmacol. 2015;94:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Liu ZM, Hu M, Chan P, Tomlinson B. Early investigational drugs targeting PPAR-α for the treatment of metabolic disease. Expert Opin Investig Drugs. 2015;24:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Botta M, Audano M, Sahebkar A, Sirtori CR, Mitro N, Ruscica M. PPAR Agonists and Metabolic Syndrome: An Established Role? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 42. | Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, Gomez EV, Russo JM. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297:1362-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Shim WS, Do MY, Kim SK, Kim HJ, Hur KY, Kang ES, Ahn CW, Lim SK, Lee HC, Cha BS. The long-term effects of rosiglitazone on serum lipid concentrations and body weight. Clin Endocrinol (Oxf). 2006;65:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Wysham C, Shubrook J. Beta-cell failure in type 2 diabetes: mechanisms, markers, and clinical implications. Postgrad Med. 2020;132:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 45. | Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 291] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 46. | Janani C, Ranjitha Kumari BD. PPAR gamma gene--a review. Diabetes Metab Syndr. 2015;9:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 523] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 47. | Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 48. | Kono T, Ahn G, Moss DR, Gann L, Zarain-Herzberg A, Nishiki Y, Fueger PT, Ogihara T, Evans-Molina C. PPAR-γ activation restores pancreatic islet SERCA2 levels and prevents β-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol Endocrinol. 2012;26:257-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Jabbari P, Sadeghalvad M, Rezaei N. An inflammatory triangle in Sarcoidosis: PPAR-γ, immune microenvironment, and inflammation. Expert Opin Biol Ther. 2021;21:1451-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Bonora E. Protection of pancreatic beta-cells: is it feasible? Nutr Metab Cardiovasc Dis. 2008;18:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Wagner N, Wagner KD. The Role of PPARs in Disease. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 52. | Smeets PJ, Teunissen BE, Planavila A, de Vogel-van den Bosch H, Willemsen PH, van der Vusse GJ, van Bilsen M. Inflammatory pathways are activated during cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors PPARalpha and PPARdelta. J Biol Chem. 2008;283:29109-29118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, Larsen TS. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol. 2002;283:H949-H957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Gremlich S, Nolan C, Roduit R, Burcelin R, Peyot ML, Delghingaro-Augusto V, Desvergne B, Michalik L, Prentki M, Wahli W. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021;18:809-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 532] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 56. | Bihan H, Rouault C, Reach G, Poitout V, Staels B, Guerre-Millo M. Pancreatic islet response to hyperglycemia is dependent on peroxisome proliferator-activated receptor alpha (PPARalpha). FEBS Lett. 2005;579:2284-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Ropero AB, Juan-Picó P, Rafacho A, Fuentes E, Bermúdez-Silva FJ, Roche E, Quesada I, de Fonseca FR, Nadal A. Rapid non-genomic regulation of Ca2+ signals and insulin secretion by PPAR alpha ligands in mouse pancreatic islets of Langerhans. J Endocrinol. 2009;200:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Iglesias J, Barg S, Vallois D, Lahiri S, Roger C, Yessoufou A, Pradevand S, McDonald A, Bonal C, Reimann F, Gribble F, Debril MB, Metzger D, Chambon P, Herrera P, Rutter GA, Prentki M, Thorens B, Wahli W. PPARβ/δ affects pancreatic β cell mass and insulin secretion in mice. J Clin Invest. 2012;122:4105-4117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Li J, Xu S, Liu Y, Yan Z, Zhang F, Lv Q, Tong N. Activated PPARβ/δ Protects Pancreatic β Cells in Type 2 Diabetic Goto-Kakizaki Rats from Lipoapoptosis via GPR40. Lipids. 2019;54:603-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Cohen G, Riahi Y, Shamni O, Guichardant M, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S. Role of lipid peroxidation and PPAR-δ in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60:2830-2842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 61. | Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 62. | Home P. The challenge of poorly controlled diabetes mellitus. Diabetes Metab. 2003;29:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Bergman M. The Early Diabetes Intervention Program--is early actually late? Diabetes Metab Res Rev. 2014;30:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |