Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1551
Peer-review started: August 1, 2023
First decision: August 16, 2023
Revised: August 22, 2023
Accepted: September 6, 2023
Article in press: September 6, 2023
Published online: October 15, 2023
Processing time: 69 Days and 10.5 Hours
The trend of prediabetes progressing to type 2 diabetes mellitus (T2DM) is prominent, and effective intervention can lead to a return to prediabetes. Exploring the factors influencing the outcome of prediabetes is helpful to guide clinical intervention. The weight change in patients with prediabetes has not attracted much attention.
To explore the interaction between body weight and the factors affecting the progression of prediabetes to T2DM.
We performed a retrospective analysis of 236 patients with prediabetes and 50 with normal glucose tolerance (NGT), and collected clinical data and follow-up results of all patients. Based on natural blood glucose outcomes, we classified 66 patients with progression to T2DM into the disease progression (DP) group, and 170 patients without progression to T2DM into the disease outcome (DO) group. We analyzed the factors that influenced prediabetes outcome and the influence of body weight on prediabetes blood glucose outcome by unconditional logistic regression. A general linear model (univariate) was used to analyze the inter-action between body weight and independent influencing factors.
There were 98 cases of impaired fasting glucose (IFG), 90 cases of impaired glucose tolerance (IGT), and 48 cases of coexistent IFG and IGT. The body weight, waist circumference, body mass index, fasting blood glucose, and 2 h plasma glucose of patients with IFG, IGT, and coexistent IFG and IGT were higher than those in patients with NGT (P < 0.05). Logistic regression analysis showed that body weight, glycosylated hemoglobin, uric acid, fasting insulin, and homeostatic model assessment for insulin resistance were independent factors affecting progression of prediabetes to T2DM (P < 0.05). Receiver operating characteristic curve analysis showed that the area under the curve predicted by the above indicators combined was 0.905 [95% confidence interval (CI): 0.863-0.948], which was greater than that predicted by each indicator alone. Logistic regression analysis with baseline body weight as an independent variable showed that compared with body weight 1, the odds ratio (95%CI) of body weight 3 was 1.399 (1.142-2.126) (P = 0.033). There was a multiplicative interaction between body weight and uric acid (β = 1.953, P = 0.005).
High body weight in patients with prediabetes is an independent risk factor for progression to T2DM, and the risk of progression is increased when coexisting with high uric acid level.
Core Tip: Progression of prediabetes to type 2 diabetes mellitus (T2DM) is severe, but effective interventions can delay or even reverse progression. High body weight is a common phenomenon in people with prediabetes. Unilateral weight-loss intervention may not be sufficient. We analyzed the interaction between body weight and the factors affecting prediabetes progression to T2DM and explored the influence of body weight and other factors, to better guide clinical intervention and reduce progression of prediabetes to T2DM.
- Citation: Li YY, Tong LP, Wu XD, Lin D, Lin Y, Lin XY. Analysis of influencing factors and interaction of body weight and disease outcome in patients with prediabetes. World J Diabetes 2023; 14(10): 1551-1561
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1551.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1551
Diabetes is a serious threat to public health, and its prevention and treatment are increasingly challenging[1]. Type 2 diabetes mellitus (T2DM) and its complications are increasing worldwide. According to the latest data released by the International Diabetes Federation in 2017, there were 425 million patients worldwide, and this is expected to exceed 629 million by 2045[2]. The latest epidemiological data in China show that the prevalence of DM is 11.2%, and approximately 30% of nonendocrine inpatients have DM[3]. Prediabetes, also known as impaired glucose regulation, is a state of abnormal glucose metabolism between normal glucose metabolism and DM, including impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and three states of both, with an incidence of 35.7%[4]. Clinical practice has proved that blood glucose regulation in the prediabetes stage is reversible. Early intervention can prevent prediabetes from progressing to T2DM and delay its development, which can reduce the risk of progression by 58%[5,6]. At present, clinical intervention is mainly through dietary adjustment, weight control, moderate exercise, and other ways to delay the progression of prediabetes to T2DM, and its effectiveness has been confirmed[7]. Obesity [with body mass index (BMI) as an evaluation index] is an independent risk factor for the conversion of prediabetes to T2DM, and obese prediabetes patients can benefit from weight loss. However, whether weight and its influencing factors can lead to an increase in the risk of adverse outcomes of the disease under the combined influence of prediabetes with T2DM risk factors is currently unknown and has rarely been studied. In this study, by understanding the weight status of prediabetes patients, we analyzed the factors influencing disease outcome (DO) and their interaction to provide a basis for later intervention.
In this retrospective analysis, we selected 236 patients with prediabetes admitted to the Department of General Practice of the First People’s Hospital of Wenling City from February 2019 to January 2021. Based on natural blood glucose outcomes, we classified 66 patients with progression to T2DM into the disease progression (DP) group, and 170 patients without progression to T2DM into the DO group. All patients met the diagnostic criteria for prediabetes in the Chinese Guidelines for Diabetes Prevention and Treatment[8]: (1) IFG: Fasting blood glucose (FBG) 6.1-6.9 mmol/L and oral glucose tolerance test (OGTT) 2h plasma glucose (PG) < 7.8 mmol/L; (2) IGT: FBG < 6.1 mmol/L and OGTT 2h PG 7.8-11.1 mmol/L; and (3) IFG and IGT coexistent: FBG 6.1-6.9 mmol/L and OGTT 2h PG 7.8-11.1 mmol/L.
Inclusion criteria: (1) FBG 5.6-6.9 mmol/L, with or without 2h PG 7.8-11.0 mmol/L; (2) No serious cardiovascular, liver, kidney, and lung diseases; and (3) Complete clinical and follow-up data. Exclusion criteria: (1) Combined with severe systemic diseases, such as heart, liver, kidney or lung disease, mental illness, connective tissue disease, and bone and joint injury; (2) Pregnancy, lactation or pregnancy preparation; and (3) Lack of follow-up data. Another 50 patients with normal glucose tolerance (NGT) were selected. NGT: FBG < 6.1 mmol/L with or without OGTT 2h PG < 7.8 mmol/L.
We consulted patients’ electronic medical records, and collected clinical data and relevant test indicators at admission, including sex, age, blood glucose status, family history of DM, smoking, alcohol consumption, body weight, waist circumference, BMI, FBG, 2h PG, systolic blood pressure, diastolic blood pressure, glycosylated hemoglobin, total cholesterol (TC), triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid, anti-soluble liver antigen antibodies (SLA), fasting insulin (FINS), and homeostatic model assessment for insulin resistance (HOMA-IR).
Body weight was measured by a general practitioner (GP) using a height and weight scale. Patients were measured with an empty stomach. They removed their shoes, wore thin clothing, stood upright, feet together, shoulders and hips close to the scale. The GP lowered the horizontal plate of the scale to the top of the patient’s head to read the results of height and weight. Height readings were accurate to 0.5 cm and weight readings to 0.5 kg. BMI was calculated as weight (kg)/height (m2)[9].
Waist measurement was performed on patients with an empty stomach by a GP with an inelastic tape measure with a minimum scale of 0.1 cm. During the measurement, the patient remained upright with arms hanging down naturally and feet 25-30 cm apart. The tape measure was placed at the midpoint of the line between the anterior superior iliac spine and the line along the lower margin of the costal arch, and ran horizontally around the abdomen. The tape measure was close to the skin but not tight. The results were read at the end of the breath and were accurate to 0.1 cm.
Systolic and diastolic blood pressure was measured by a GP using a uniform mercury sphygmomanometer. Patients were prohibited from smoking and drinking coffee 30 min before the measurement and rested for 5-10 min in a quiet environment. Venous blood samples were collected in the fasting state in the morning (no intake of caloric food for at least 8 h). The blood glucose indices were determined by a HITACHI 7600-020 automatic biochemical analyzer. FPG, FINS, serum uric acid and blood lipids (TC, TG, HDL-C and LDL-C) were also measured. The homeostasis insulin resistance index (HOMA-IR) was calculated as follows[10]: HOMA-IR = (FPG × FINS)/22.5.
OGTT 2h PG: The patients took 75 g of anhydrous glucose dissolved in 250 mL water within 5 min. During the test, they did not drink any beverages, swallow, or perform strenuous exercise. The time was measured from the first mouthful of sugar water, and the venous blood was drawn 2 h after taking the sugar and was quickly examined by the hexokinase method. Glycosylated hemoglobin was determined by a Bole D-10 glycosylated swimming protein meter (high-pressure liquid chromatography).
T2DM[11]: FBG ≥ 7.0 mmol/L with or without OGTT 2h PG ≥ 11.1 mmol/L. According to the blood glucose outcomes of prediabetes patients after a 2-year follow-up, 66 patients with progression of T2DM were classified into the DP group, and 170 patients without progression of T2DM were classified into the DO group.
Epidata3.0 software was used for double data entry and SPSS 21.0 statistical software was used for statistical analysis. The measurement data with normal distribution were expressed as mean ± SD, and the least significant difference-t test was used for pairwise comparison between groups. Numerical data were represented by case number (percentage) and χ2 tests. The factors influencing prediabetes DO and the influence of body weight on prediabetes blood glucose outcome were analyzed by unconditional logistic regression. The multiplication model was used to analyze the interaction between the two influencing factors. Beta level: α = 0.05.
Among 236 patients with prediabetes, there were 98 cases of IFG, 90 of IGT, and 48 of coexistent IFG and IGT. The body weight, waist circumference, BMI, FBG, and 2h PG of patients with IFG, IGT and coexistent of IFG and IGT were higher than those of patients with NGT (all P < 0.05) (Table 1).
| Index | NGT (50 cases) | IFG (98 cases) | IGT (90 cases) | IFG and IGT coexistent (48 cases) |
| Age (yr) | 52.55 ± 14.67 | 54.68 ± 13.27a | 52.38 ± 11.62a | 57.75 ± 13.68a |
| Weight (kg) | 61.52 ± 12.13 | 66.38 ± 13.35a | 70.26 ± 10.62a | 72.26 ± 11.62a |
| Waistline (cm) | 90.37 ± 9.98 | 104.25 ± 12.23a | 100.22 ± 8.56a | 107.35 ± 11.07a |
| BMI (kg/m2) | 25.87 ± 5.46 | 31.57 ± 4.06a | 30.75 ± 3.39a | 32.27 ± 3.38a |
| FBG (mmol/L) | 4.46 ± 0.62 | 6.27 ± 0.75a | 5.58 ± 0.36a | 6.48 ± 0.82a |
| 2h PG (mmol/L) | 5.16 ± 1.08 | 5.87 ± 0.77a | 8.82 ± 1.17a | 9.35 ± 1.12a |
Of 236 patients with prediabetes, 66 (27.97%) developed T2DM (DP group). There were no significant differences in gender, age, family history of diabetes, FBG, diastolic blood pressure, TC, HDL-C and LDL-C between the DP and DO groups (P > 0.05). In addition, glucose status, history of smoking, drinking, body weight, waist circumference, BMI, 2h PG, systolic blood pressure, glycosylated hemoglobin, TG, uric acid, SLA, FINS, and HOMA-IR difference were statistically significant (P < 0.05) (Table 2).
| Data | DP group (n = 66) | DO group (n = 170) | t/χ2/Z | P value |
| Gender | 0.143 | 0.705 | ||
| Male | 39 (59.09) | 105 (61.76) | ||
| Female | 27 (40.91) | 65 (38.24) | ||
| Age | < 0.001 | 0.988 | ||
| < 60 yr | 42 (63.64) | 108 (63.53) | ||
| ≥ 60 yr | 24 (36.36) | 62 (36.47) | ||
| Blood glucose status | 14.639 | 0.001 | ||
| IFG | 16 (24.24) | 82 (48.24) | ||
| IGT | 28 (42.42) | 62 (36.47) | ||
| IFG and IGT coexistent | 22 (33.33) | 26 (15.29) | ||
| Family history of diabetes | 0.279 | 0.598 | ||
| Yes | 6 (9.09) | 12 (7.06) | ||
| No | 60 (90.91) | 158 (92.94) | ||
| Smoking history | 4.561 | 0.033 | ||
| Yes | 20 (30.30) | 30 (17.65) | ||
| No | 46 (69.70) | 140 (82.35) | ||
| Drinking history | 5.457 | 0.019 | ||
| Yes | 26 (39.39) | 41 (24.12) | ||
| No | 40 (60.61) | 129 (75.88) | ||
| Weight, kg (mean ± SD) | 74.59 ± 11.19 | 66.91 ± 11.86 | 4.535 | < 0.001 |
| Waist circumference, cm (mean ± SD) | 106.84 ± 10.31 | 101.98 ± 10.95 | 3.110 | 0.002 |
| BMI, kg/m2 (mean ± SD) | 32.37 ± 3.58 | 30.41 ± 3.75 | 0.074 | 0.941 |
| FBG, mmol/L (mean ± SD) | 5.98 ± 0.73 | 6.08 ± 0.75 | 0.926 | 0.355 |
| 2h PG, mmol/L (mean ± SD) | 8.12 ± 1.83 | 7.54 ± 1.84 | 2.177 | 0.031 |
| Systolic blood pressure (mmHg) | 128 ± 21 | 122 ± 20 | 2.040 | 0.043 |
| Diastolic blood pressure (mmHg) | 81 ± 11 | 80 ± 10 | 0.670 | 0.503 |
| Glycosylated hemoglobin (%) | 6.28 ± 0.53 | 5.89 ± 0.33 | 6.794 | < 0.001 |
| TC (mmol/L) | 4.76 ± 1.17 | 4.64 ± 1.06 | 0.758 | 0.449 |
| TG (mmol/L) | 1.58 ± 0.32 | 1.42 ± 0.47 | 2.544 | 0.012 |
| HDL-C (mmol/L) | 1.25 ± 0.27 | 1.21 ± 0.32 | 0.899 | 0.370 |
| LDL-C (mmol/L) | 1.88 ± 0.53 | 1.85 ± 0.41 | 0.463 | 0.644 |
| Uric acid (μmol/L) | 3.43 ± 0.76 | 3.20 ± 0.87 | 2.067 | 0.040 |
| SLA (μmol/L) | 325.10 ± 75.04 | 300.22 ± 80.23 | 2.176 | 0.031 |
| FINS (μU/mL) | 5.96 ± 1.57 | 5.11 ± 1.19 | 6.016 | < 0.001 |
| HOMA-IR | 1.67 ± 0.45 | 1.21 ± 0.39 | 7.782 | < 0.001 |
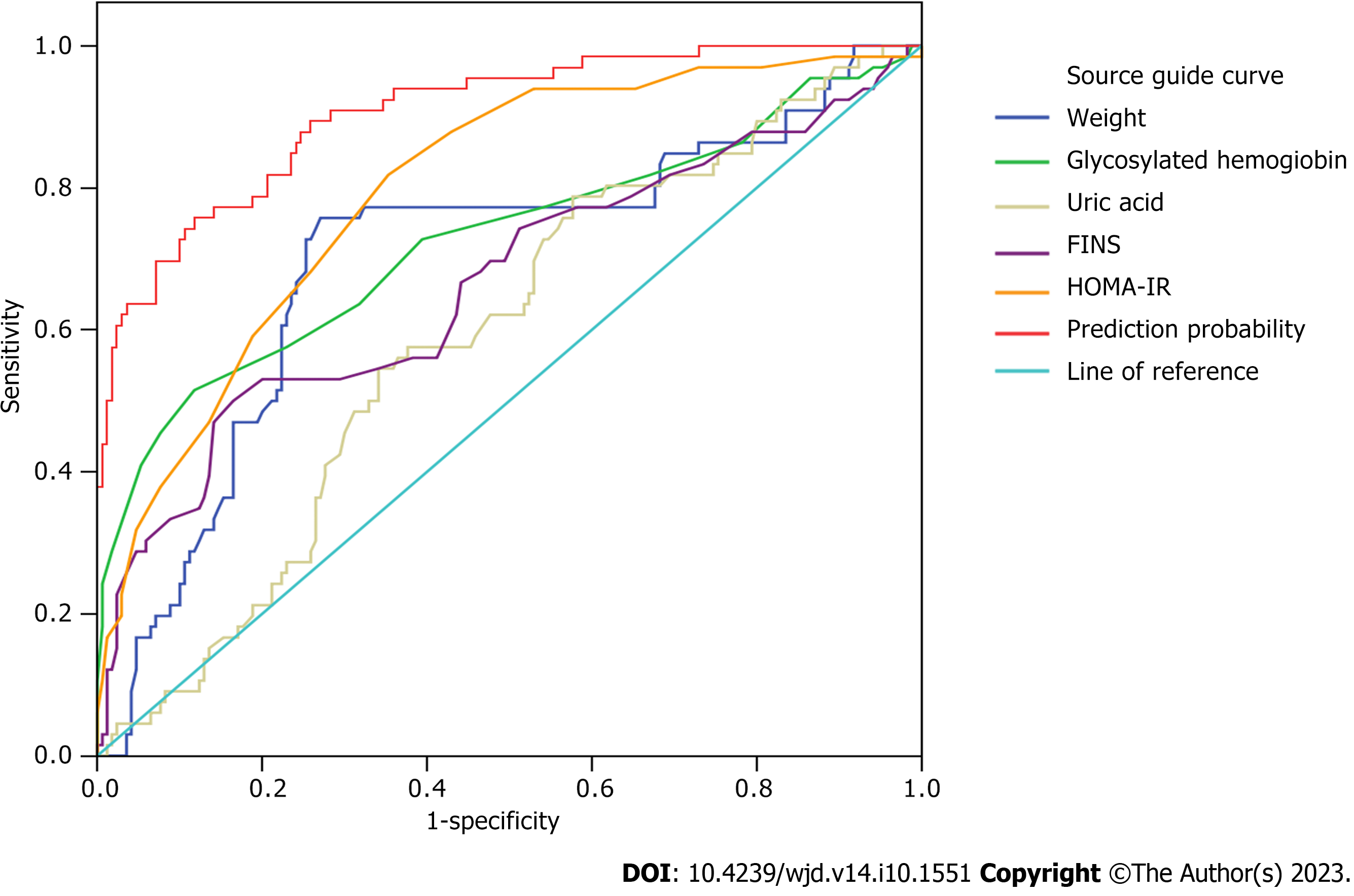
We took whether to progress to T2DM as the dependent variable and the index of P > 0.05 in the above single-factor analysis as the independent variable, which were inserted into the logistic regression model for analysis. Table 3 shows the assignment of the data in the model. Body weight, glycosylated hemoglobin, uric acid, FINS, and HOMA-IR were independent factors affecting progression of prediabetes to T2DM (P < 0.05) (Table 4). Receiver operating characteristic (ROC) curve analysis of body weight, glycosylated hemoglobin, uric acid, FINS, and HOMA-IR predicted prediabetes progression to T2DM, and showed that the area under the curve (AUC) predicted by the above indices combined was 0.905 [95% confidence interval (CI): 0.863-0.948], which was higher than predicted by each index separately (Figure 1, Table 5).
| Independent variable | Assignment |
| Blood glucose status | 1 = IFG, 2 = IGT, 3 = IFG and IGT coexist |
| Smoking history | 1 = Yes, 2 = No |
| Drinking history | 1 = Yes, 2 = No |
| Weight | Measured value |
| Waist circumference | Measured value |
| BMI | Measured value |
| 2h PG | Measured value |
| Systolic blood pressure | Measured value |
| Glycosylated hemoglobin | Measured value |
| TG | Measured value |
| Uric acid | Measured value |
| SLA | Measured value |
| FINS | Measured value |
| HOMA-IR | Measured value |
| Independent variable | β | SE | Wals | P value | OR | 95%CI |
| Blood glucose status | 1.138 | 0.566 | ||||
| Blood glucose status (1) | 0.034 | 0.958 | 0.001 | 0.972 | 1.035 | 0.158-6.771 |
| Blood glucose status (2) | 0.588 | 0.648 | 0.825 | 0.364 | 1.801 | 0.506-6.415 |
| Smoking history | 0.916 | 0.486 | 3.553 | 0.059 | 2.499 | 0.964-6.479 |
| Drinking history | 0.341 | 0.457 | 0.555 | 0.456 | 1.406 | 0.574-3.445 |
| Weight | 0.066 | 0.020 | 11.128 | 0.001 | 1.068 | 1.028-1.110 |
| Waist circumference | 0.041 | 0.022 | 3.427 | 0.064 | 1.042 | 0.998-1.089 |
| BMI | 0.006 | 0.060 | 0.010 | 0.920 | 1.006 | 0.895-1.131 |
| 2h PG | 0.150 | 0.204 | 0.538 | 0.463 | 1.162 | 0.778-1.734 |
| Systolic blood pressure | 0.015 | 0.010 | 2.213 | 0.137 | 1.016 | 0.995-1.036 |
| Glycosylated hemoglobin | 2.542 | 0.599 | 18.014 | < 0.001 | 12.705 | 3.928-41.092 |
| TG | 0.527 | 0.528 | 0.997 | 0.318 | 1.694 | 0.602-4.772 |
| Uric acid | 0.007 | 0.003 | 7.217 | 0.007 | 1.007 | 1.002-1.012 |
| SLA | 0.001 | 0.003 | 0.226 | 0.635 | 1.001 | 0.996-1.007 |
| FINS | 0.503 | 0.169 | 8.872 | 0.003 | 1.653 | 1.188-2.301 |
| HOMA-IR | 2.224 | 0.547 | 16.561 | < 0.001 | 9.245 | 3.167-26.984 |
| Constant (quantity) | -38.654 | 6.686 | 33.420 | < 0.001 | < 0.001 | - |
| Independent variable | AUC | 95%CI | Specificity | Sensitivity | Optimum break value |
| Weight | 0.699 | 0.620-0.778 | 0.729 | 0.758 | 71.75 |
| Glycosylated hemoglobin | 0.726 | 0.645-0.806 | 0.882 | 0.515 | 6.25 |
| Uric acid | 0.590 | 0.512-0.668 | 0.424 | 0.788 | 289.5 |
| FINS | 0.666 | 0.583-0.750 | 0.835 | 0.500 | 6.25 |
| HOMA-IR | 0.798 | 0.736-0.859 | 0.647 | 0.818 | 1.35 |
| Collaborative forecasting | 0.905 | 0.863-0.948 | - | - |
Whether progressed to T2DM was used as the dependent variable, and baseline body weight [body weight 1 (35-60 kg), body weight 2 (60-80 kg), body weight 3 (80-100 kg)] was the independent variable. Logistic regression analysis was performed by adjusting blood glucose status, smoking history, drinking history, waist circumference, BMI, 2h PG, systolic blood pressure, glycosylated hemoglobin, triglycerides, uric acid, SLA, FINS and HOMA-IR. Body weight was a risk factor for prediabetes progression to T2DM. Compared with body weight 1, the odds ratio (OR) (95%CI) of body weight 3 was 1.399 (0.142-1.126) (P = 0.083) (Table 6).
| Model | Weight 1 | Weight 2 | Weight 3 | ||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Model 1 | 1 | 1.210 (1.082-2.532) | 0.001 | 1.366 (1.180-2.743) | 0.005 |
| Model 2 | 1 | 1.233 (1.085-2.640) | 0.005 | 1.357 (1.166-3.768) | 0.008 |
| Model 3 | 1 | 1.164 (1.041-1.662) | 0.011 | 1.399 (1.142-2.126) | 0.033 |
Taking the best cut-off value of each index as the boundary, we converted the data of body weight, glycosylated hemoglobin, uric acid, FINS and HOMA-IR into binary variables. Whether progressed to T2DM was used as the dependent variable and weight by glycosylated hemoglobin, weight by uric acid, weight by FINS, and weight by HOMA-IR as the fixed factors, the interaction analysis showed that there was a multiplicative interaction between weight and uric acid (β = 1.953, P = 0.005) (Table 7).
| Independent variable | β | SE | Wals | P value | OR | 95%CI |
| Weight | 2.664 | 0.52 | 26.23 | < 0.001 | 14.356 | 5.179-39.795 |
| Glycosylated hemoglobin | 2.772 | 0.606 | 20.948 | < 0.001 | 15.987 | 4.878-52.390 |
| Weight by glycosylated hemoglobin | -0.828 | 0.82 | 1.021 | 0.312 | 0.437 | 0.088-2.178 |
| Constant | -3.082 | 0.457 | 45.408 | < 0.001 | 0.046 | - |
| Weight | 2.453 | 0.565 | 18.86 | < 0.001 | 11.625 | 3.842-35.174 |
| Uric acid | 1.887 | 0.578 | 10.664 | 0.001 | 6.6 | 2.127-20.484 |
| Weight by uric acid | 1.953 | 0.719 | 1.758 | 0.005 | 0.386 | 0.094-1.577 |
| Constant | -2.923 | 0.459 | 40.545 | 0 | 0.054 | - |
| Weight | 2.883 | 0.565 | 26.066 | < 0.001 | 17.86 | 5.906-54.009 |
| FINS | 2.578 | 0.622 | 17.189 | < 0.001 | 13.174 | 3.894-44.570 |
| Weight by FINS | -0.797 | 0.833 | 0.916 | 0.339 | 0.451 | 0.088-2.305 |
| Constant | -3.229 | 0.51 | 40.113 | < 0.001 | 0.04 | - |
| Weight | 3.31 | 1.08 | 9.39 | 0.002 | 27.375 | 3.296-227.343 |
| HOMA-IR | 3.067 | 1.049 | 8.55 | 0.003 | 21.471 | 2.749-167.718 |
| Weight by HOMA-IR | -1.463 | 1.15 | 1.619 | 0.203 | 0.231 | 0.024-2.205 |
| Constant | -4.29 | 1.007 | 18.159 | < 0.001 | 0.014 | - |
There has been significant progression of prediabetes to T2DM in recent years[12]. According to our study, 27.97% of prediabetes patients developed T2DM. The progression to T2DM was highest in patients with coexistent IFG and IGT, which is consistent with the findings of Wu et al[13]. We found that most patients with prediabetes had IFG or IGT, and coexistent IFG and IGT accounted for 20%. Further analysis showed that body weight, waist circumference, BMI, FBG and 2h PG were higher in patients with IFG, IGT, and coexistent IFG and IGT than in patients with NGT, which is similar to previous results[14].
Our logistic regression analysis showed that body weight (> 71.75 kg), glycosylated hemoglobin (> 6.25%), uric acid (> 289.5 mmol/L), FINS (> 6.25 mL/mL) and HOMA-IR (> 1.35) were independent risk factors for prediabetes progression to T2DM (P < 0.05). ROC curve analysis showed that the AUC predicted by combination of the above indicators was 0.905 (95%CI: 0.863–0.948), which was greater than that predicted by each indicator alone, indicating that the above indicators had high efficacy in predicting the progression of prediabetes to T2DM, suggesting that high levels of these indicators increase the risk of prediabetes progression to T2DM. Our logistic regression analysis, with T2DM progression as the dependent variable and baseline body weight as the independent variable, showed that the OR (95%CI) of those with weight 80-100 kg was 1.399 (1.142-2.126) (P = 0.033), compared with those with weight ranging from 35 to 60 kg. The risk of prediabetes progression to T2DM was increased by 1.399 times with high body weight based on low body weight.
Prediabetes is an early stage of glucose metabolism disorder, in which glucose regulation function is impaired, accompanied by insulin resistance and lipid metabolism disorder[15]. Being overweight or obese increases the risk of diabetes, and our study did not show the risk effect of BMI on progression to T2DM, which may be because BMI did not reflect the actual content and distribution of body fat. Although BMI may be normal, the body may have ectopic fat deposition and metabolic disorders[16]. Being overweight or obese is an important factor in predicting self-underestimation of body mass in prediabetes, and overweight people tend to underestimate self-body mass[17]. Only 10% of overweight DM patients judged their body weight correctly[18]. High body weight in prediabetes patients with improper control of body mass increases the risk of progression to T2DM. Obesity is associated with metabolic dysfunction and overnutrition. Weight gain often means increased BMI, which is closely related to changes in adipocyte secretion, release of inflammatory mediators, chronic inflammation, and insulin resistance[19]. For patients with DM, 5%-10% weight loss can improve their health status, reduce the level of glycosylated hemoglobin, improve cardiovascular disease risk factors, and reduce the use of antidiabetic, antihypertensive and lipid-regulating drugs[20].
The progression of prediabetes is reversible, and effective intervention is important for the outcome of patients with prediabetes[21]. Weight loss can reduce or even reverse ectopic deposition and reduce progression of prediabetes to T2DM. At present, the main clinical intervention for prediabetes is lifestyle intervention, supplemented by drug intervention. Lifestyle intervention mainly includes diet, exercise, body mass, and dietary intervention is divided into diet control and nutritional supplementation[22]. Through long-term control of total dietary calories and restriction of various types of energy intake, such as fat and sugar, weight loss can be achieved and postprandial hyperglycemia can be reduced, thus reducing the burden on pancreatic islet beta cells, improving the function of beta cells, and improving HOMA-β, to correct the disorder of glucose metabolism[11]. In recent years, many patients with DM in western countries have been affected by various dietary programs, such as low-carbohydrate diet, a very low-carbohydrate diet, and a Mediterranean diet to reduce weight and improve blood sugar. In particular, a low-carbohydrate diet has the most obvious effect on weight loss, which has been widely confirmed in some clinical experiments, including obesity, metabolic disorders, and risk of cardiovascular events[23]. There are also many domestic and foreign studies on low-carbon diets, and it has been preliminarily confirmed that a low-carbon diet can reduce body weight and the level of glycosylated hemoglobin[24]. Griauzde et al[18] showed that carbohydrate-restricted diets benefit patients with obesity and T2DM and can be used as a potential tool to support individual patients’ weight loss and metabolic health. Therefore, we suggest that dietary intervention programs represented by a low-carbon diet can reduce body weight and body fat accumulation in prediabetes patients with high body weight. At the same time, regular monitoring of blood glucose and adjustment of the program are conducive to better weight loss, and to delay or even reverse the course of prediabetes. However, it should be noted that weight loss intervention is not appropriate in patients with severe organ diseases, malnutrition, and age > 55 years.
Our interaction analysis showed that there was a multiplying interaction between body weight and uric acid, but no interaction between body weight and glycosylated hemoglobin, FINS and HOMA-IR, suggesting that for prediabetes patients with T2DM, the higher the body weight, the higher the uric acid level. The levels of glycosylated hemoglobin, FINS and HOMA-IR were not related to body weight. Obesity is a condition in which body weight exceeds the normal standard and body fat is accumulated excessively or distributed abnormally. Obesity is associated with elevated blood uric acid, and hyperuricemia is associated with obesity[25]. Obesity caused by high body weight is related to genetic and environmental factors. Such people often have an unreasonable diet and lack of exercise, resulting in accumulation of body fat, which can be accompanied by disorders of purine metabolism or uric acid excretion, increasing uric acid levels, the end product of purine metabolism. In addition, regular intake of too much high-purine food, such as seafood, animal viscera, or long-term drinking of beer, may lead to increased purine metabolism in the body, resulting in increased uric acid level, and thus manifested as disorders of uric acid metabolism[26]. Uric acid is associated with obesity[27]. Therefore, we suggest that prediabetes patients with high body weight can undertake comprehensive treatment through behavior, diet, and exercise, adopt a healthy lifestyle and reasonable eating habits, to reduce weight, avoid elevated uric acid, and reduce the possibility of prediabetes progressing to T2DM. However, this study has its limitations, that is, the sample size in this study is insufficient, and there may be selective bias leading to biased research results. In addition, we selected the study sample from a single center, and the research results can only reflect the population of this center. It is unknown whether it is widely applicable to the population of other centers. Therefore, multi-center and larger sample size studies are needed to further verify these findings.
High body weight in patients with prediabetes is an independent risk factor for progression to T2DM, and high body weight coexisting with high uric acid level increases the risk of T2DM progression. Clinically, patients with high body weight and high uric acid should be vigilant, and timely clinical intervention measures should be taken to reduce the risk of prediabetes progressing to T2DM.
The high incidence of diabetes mellitus (DM) is a serious threat to public health. There have been many reports on its influencing factors, but few studies on the influence of body weight on the progression from prediabetes to type 2 diabetes mellitus (T2DM), and the interaction between body weight and various influencing factors has not been reported.
The phenomenon of high weight, waist circumference, and body mass index is common in prediabetes patients, and there are many factors affecting the progression of prediabetes to T2DM. Unilateral weight control cannot reduce this risk, and it is necessary to understand the interaction between weight and other factors.
The purpose of this study was to explore the weight status of patients with prediabetes and analyze the interaction between weight and other disease outcome (DO) factors, so as to guide clinical intervention and reduce the risk of prediabetes progressing to T2DM.
A retrospective analysis of 236 patients with prediabetes and 50 patients with normal glucose control was performed. Clinical data and follow-up results of all patients were collected. The influencing factors (including body weight) of prediabetes DO were analyzed by logistic regression, and the interaction between body weight and independent influencing factors was analyzed by a general linear model (univariate).
Body weight, glycosylated hemoglobin, uric acid, fasting insulin (FINS), and homeostatic model assessment for insulin resistance (HOMA-IR) were independent factors affecting the progression of prediabetes to T2DM (P < 0.05). There was a multiplicative interaction between weight and uric acid (β = 1.953, P = 0.005).
Body weight has a significant effect on prediabetes progression to T2DM, and coexistent high body weight and high uric acid increase the risk of progression to T2DM.
From the perspective of high body weight as a risk factor for prediabetes progression to T2DM, the interaction between body weight and other risk factors (including glycosylated hemoglobin, uric acid, FINS and HOMA-IR) was discussed, and low carbon diet and weight loss were proposed to reduce the risk of progression and guide clinical intervention.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Debelee TG, Germany; Popova PV, Russia; Horowitz M, Australia S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Chen YX
| 1. | Geng T, Zhu K, Lu Q, Wan Z, Chen X, Liu L, Pan A, Liu G. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PLoS Med. 2023;20:e1004135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 2. | Tinajero MG, Malik VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol Metab Clin North Am. 2021;50:337-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 227] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 3. | Migdal AL, Fortin-Leung C, Pasquel F, Wang H, Peng L, Umpierrez GE. Inpatient Glycemic Control With Sliding Scale Insulin in Noncritical Patients With Type 2 Diabetes: Who Can Slide? J Hosp Med. 2021;16:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Jindra M. New ways and new hopes for IGR development. J Pestic Sci. 2021;46:3-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Selenius JS, Wasenius NS, Kautiainen H, Salonen M, von Bonsdorff M, Eriksson JG. Impaired glucose regulation, depressive symptoms, and health-related quality of life. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Jiang Q, Li JT, Sun P, Wang LL, Sun LZ, Pang SG. Effects of lifestyle interventions on glucose regulation and diabetes risk in adults with impaired glucose tolerance or prediabetes: a meta-analysis. Arch Endocrinol Metab. 2022;66:157-167. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Pan XF, Chen J, Xia L, Cao A, Zhang Y, Wang J, Li H, Yang K, Guo K, He M, Pan A. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020;63:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 8. | Zhao NJ, Yan B, Piao CL, Lu Y, Yang SY. [Application of traditional Chinese medicine on prevention and treatment of diabetes:interpretation of the traditional Chinese medicine section of national guidelines for the prevention and control of diabetes in primary care (2022)]. Zhonghua Nei Ke Za Zhi. 2022;61:1297-1299. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, Zhou H, Yi X, Zhu L, Zhu S. TyG Index Performs Better Than HOMA-IR in Chinese Type 2 Diabetes Mellitus with a BMI < 35 kg/m2: A Hyperglycemic Clamp Validated Study. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Abdesselam A, Zidoum H, Zadjali F, Hedjam R, Al-Ansari A, Bayoumi R, Al-Yahyaee S, Hassan M, Albarwani S. Estimate of the HOMA-IR Cut-off Value for Identifying Subjects at Risk of Insulin Resistance Using a Machine Learning Approach. Sultan Qaboos Univ Med J. 2021;21:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 11. | Zhou C, Wang M, Liang J, He G, Chen N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Pan X, Kaminga AC, Wen SW, Liu A. Chemokines in Prediabetes and Type 2 Diabetes: A Meta-Analysis. Front Immunol. 2021;12:622438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Wu T, Li X, Zhang D, Gong LG. Early impairment of right ventricular systolic function in patients with prediabetes and type 2 diabetes mellitus: An analysis of two-dimensional speckle tracking echocardiography. Echocardiography. 2023;40:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Li Y, Feng D, Esangbedo IC, Zhao Y, Han L, Zhu Y, Fu J, Li G, Wang D, Wang Y, Li M, Gao S, Willi SM. Insulin resistance, beta-cell function, adipokine profiles and cardiometabolic risk factors among Chinese youth with isolated impaired fasting glucose versus impaired glucose tolerance: the BCAMS study. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Liu J, Shao S, Yang Y, Qi D, Wang C, Lin Q, Liu Y, Tu J, Wang J, Ning X, Cui J. Sex Differences in the Prevalence of and Risk Factors for Abnormal Glucose Regulation in Adults Aged 50 Years or Older With Normal Fasting Plasma Glucose Levels. Front Endocrinol (Lausanne). 2020;11:531796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Chen X, Han Y, Gao P, Yang M, Xiao L, Xiong X, Zhao H, Tang C, Chen G, Zhu X, Yuan S, Liu F, Dong LQ, Kanwar YS, Sun L. Disulfide-bond A oxidoreductase-like protein protects against ectopic fat deposition and lipid-related kidney damage in diabetic nephropathy. Kidney Int. 2019;95:880-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Bjerggaard M, Philipsen A, Jørgensen ME, Charles M, Witte DR, Sandbæk A, Lauritzen T, Færch K. Association of self-perceived body image with body mass index and type 2 diabetes-The ADDITION-PRO study. Prev Med. 2015;75:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A Pragmatic Approach to Translating Low- and Very Low-Carbohydrate Diets Into Clinical Practice for Patients With Obesity and Type 2 Diabetes. Front Nutr. 2021;8:682137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Deng K, Shuai M, Zhang Z, Jiang Z, Fu Y, Shen L, Zheng JS, Chen YM. Temporal relationship among adiposity, gut microbiota, and insulin resistance in a longitudinal human cohort. BMC Med. 2022;20:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Churuangsuk C, Hall J, Reynolds A, Griffin SJ, Combet E, Lean MEJ. Diets for weight management in adults with type 2 diabetes: an umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia. 2022;65:14-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 21. | Farag HFM, Elrewany E, Abdel-Aziz BF, Sultan EA. Prevalence and predictors of undiagnosed type 2 diabetes and pre-diabetes among adult Egyptians: a community-based survey. BMC Public Health. 2023;23:949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Dos Santos Quaresma MVL, Guazzelli Marques C, Nakamoto FP. Effects of diet interventions, dietary supplements, and performance-enhancing substances on the performance of CrossFit-trained individuals: A systematic review of clinical studies. Nutrition. 2021;82:110994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Echeverría G, Tiboni O, Berkowitz L, Pinto V, Samith B, von Schultzendorff A, Pedrals N, Bitran M, Ruini C, Ryff CD, Del Rio D, Rigotti A. Mediterranean Lifestyle to Promote Physical, Mental, and Environmental Health: The Case of Chile. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Liu J, Xiao L, Nie H, Pan Y, Liu Y, Zhang Z, Lin X, Zhang Y, Cai J, Yang M, Zhang L, Xu A, Zhu C. Microecological preparation combined with an modified low-carbon diet improves glucolipid metabolism and cardiovascular complication in obese patients. Diabetol Metab Syndr. 2021;13:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Andres-Hernando A, Cicerchi C, Kuwabara M, Orlicky DJ, Sanchez-Lozada LG, Nakagawa T, Johnson RJ, Lanaspa MA. Umami-induced obesity and metabolic syndrome is mediated by nucleotide degradation and uric acid generation. Nat Metab. 2021;3:1189-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Gherghina ME, Peride I, Tiglis M, Neagu TP, Niculae A, Checherita IA. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 216] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 27. | Tang H, Mo J, Chen Z, Xu J, Wang A, Dai L, Cheng A, Wang Y. Uric Acid Contributes to Obesity-Paradox of the Outcome of Ischemic Stroke. Front Neurol. 2019;10:1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |