Published online Jan 15, 2023. doi: 10.4239/wjd.v14.i1.48
Peer-review started: July 5, 2022
First decision: July 31, 2022
Revised: August 16, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: January 15, 2023
Processing time: 188 Days and 19.6 Hours
Cytokines are essential in autoimmune inflammatory processes that accompany type 1 diabetes. Tumor necrosis factor alpha plays a key role among others in modulating enteric neuroinflammation, however, it has a dual role in cell degeneration or survival depending on different TNFRs. In general, TNFR1 is believed to trigger apoptosis, while TNFR2 promotes cell regeneration. The importance of the neuronal microenvironment has been recently highlighted in gut region-specific diabetic enteric neuropathy, however, the expression and alterations of different TNFRs in the gastrointestinal tract has not been reported.
To investigate the TNFR1 and TNFR2 expression in myenteric ganglia and their environment in different intestinal segments of diabetic rats.
Ten weeks after the onset of hyperglycemia, gut segments were taken from the duodenum, ileum and colon of streptozotocin-induced (60 mg/body weight kg i.p.) diabetic (n = 17), insulin-treated diabetic (n = 15) and sex- and age-matched control (n = 15) rats. Myenteric plexus whole-mount preparations were prepared from different gut regions for TNFR1/HuCD or TNFR2/HuCD double-labeling fluorescent immunohistochemistry. TNFR1 and TNFR2 expression was evaluated by post-embedding immunogold electron microscopy on ultrathin sections of myenteric ganglia. TNFRs levels were measured by enzyme-linked immun-osorbent assay in muscle/myenteric plexus-containing (MUSCLE-MP) tissue homogenates from different gut segments and experimental conditions.
A distinct region-dependent TNFRs expression was detected in controls. The density of TNFR1-labeling gold particles was lowest, while TNFR2 density was highest in duodenal ganglia and a decreased TNFRs expression from proximal to distal segments was observed in MUSCLE-MP homogenates. In diabetics, the TNFR2 density was only significantly altered in the duodenum with decrease in the ganglia (0.32 ± 0.02 vs 0.45 ± 0.04, P < 0.05), while no significant changes in TNFR1 density was observed. In diabetic MUSCLE-MP homogenates, both TNFRs levels significantly decreased in the duodenum (TNFR1: 4.06 ± 0.65 vs 20.32 ± 3.1, P < 0.001; TNFR2: 11.72 ± 0.39 vs 15.91 ± 1.04, P < 0.01), which markedly influenced the TNFR2/TNFR1 proportion in both the ganglia and their muscular environment. Insulin treatment had controversial effects on TNFR expression.
Our findings show diabetes-related region-dependent changes in TNFR expression and suggest that TNFR2 is more affected than TNFR1 in myenteric ganglia in the duodenum of type 1 diabetic rats.
Core Tip: Our findings demonstrate an intestinal segment-specific expression of TNFRs in myenteric ganglia and their muscular environment in type 1 diabetic rats. TNFR2 density was significantly altered only in the duodenum of diabetics with a decrease in the ganglia, while no significant changes in TNFR1 were observed. In diabetic muscle/myenteric plexus homogenates, the levels of both TNFRs decreased significantly in the duodenum, which markedly influenced the TNFR2/TNFR1 proportion in both the ganglia and muscular environment. These results highlight that TNFR2 is more affected than TNFR1 in myenteric ganglia in the duodenum of diabetic rats. Insulin treatment had controversial effects on TNFR expression.
- Citation: Barta BP, Onhausz B, AL Doghmi A, Szalai Z, Balázs J, Bagyánszki M, Bódi N. Gut region-specific TNFR expression: TNFR2 is more affected than TNFR1 in duodenal myenteric ganglia of diabetic rats. World J Diabetes 2023; 14(1): 48-61
- URL: https://www.wjgnet.com/1948-9358/full/v14/i1/48.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i1.48
In the last decade, the individual milieu of the different intestinal regions has been increasingly highlighted in health and disease[1,2]. Numerous studies emphasize the key role of gut microbial dysbiosis[3,4], involving regionally distinct alterations in the intestinal microbiota in type 1 diabetic rats[5]. Diabetes-related gut region-dependent accumulation of reactive oxygen species and segment-specific activation of endogenous antioxidant defense system[6,7] have clearly outlined the imbalance between antioxidative and oxidative mechanisms[8]. Strong shift in balance between anti-inflammatory and pro-inflammatory processes[9-11] has also been characterized in type 1 diabetes. All these region-dependent molecular dysfunctions contribute to the regional diabetic damage affecting the enteric nervous system and have functional consequences, such as disturbed gut motility[12-14].
Type 1 diabetes is accompanied by autoimmune inflammatory processes, therefore different cytokines, such as inflammatory mediators, also play an essential role in enteric neuroinflammation[15]. Naturally, as cytokines have global effects on intestinal homeostasis in healthy and pathophysiological conditions[16-18], it may be of great interest to study the role and alterations of different cytokines in different segments of the gastrointestinal tract.
The variety of ligands and receptors that belong to tumor necrosis factor (TNF) superfamily is characterized by a conserved TNF homology domain at the C-terminal[19]. Tumor necrosis factor alpha
Despite that TNFα is primarily thought to be a powerful pro-inflammatory cytokine[17], it has a dual role in cell survival/cell death depending on which TNFR it binds to. TNFR1 contains an intracellular death domain through which it triggers cell degeneration and apoptosis, while TNFR2 contributes to cell regeneration and survival[23,25]. Therefore, selective TNFR1 inhibitors obstruct the pro-inflammatory TNFR1 signaling without losing the regulatory effect of TNFR2 in the treatment of autoimmune diseases[26]. Blocking the TNFR1-mediated TNF signaling pathway significantly protects rats from obesity and obesity-induced insulin resistance[27]. TNFR2 facilitates the differentiation of induced regulatory T cells, while TNFR1 mediates the inflammatory T cell differentiation in mice[28]. TNFR2 promotes oligodendrocyte regeneration and neuronal survival in an ischemic model[29] and provides protection against oxidative stress[30].
The primary aim of this study was to evaluate the effects of chronic hyperglycemia and immediate insulin treatment on TNFR1 and TNFR2 distribution in myenteric neurons along the duodenum-colon axis. Furthermore, we set out to investigate whether the TNFR expression is affected in muscular environment of enteric neurons in different gut segments.
Adult male Wistar rats (Toxi-Coop Zrt., Hungary) weighing 200-300 g, kept on standard laboratory chow (Innovo Kft., Hungary) and with free access to drinking water, were used throughout the experiments. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity) for 2 wk prior to experimentation. The rats were divided randomly into three groups: streptozotocin (STZ)-induced diabetics (diabetics; n = 17), insulin-treated STZ-induced diabetics (insulin-treated diabetics; n = 15) and sex- and age-matched controls (n = 15). Hyperglycemia was induced as described previously[7,10,12]. The animals were considered diabetic if the non-fasting blood glucose concentration was higher than 18 mmol/L. From this time on, the insulin-treated group of hyperglycaemic rats received a subcutaneous injection of insulin (Humulin M3, Eli Lilly Nederland, Netherlands) each morning (2 IU) and afternoon (3 IU). Equivalent volumes of saline were given subcutaneously to the diabetic and the control rats. The blood glucose level and weight of each animal were measured weekly. Those diabetic animals which recovered spontaneously, or their blood glucose level decreased below 18 mmol/L during the 10-wk experimental period were excluded from the study. In all procedures involving experimental animals, the principles of the National Institutes of Health (Bethesda, MD, United States) guidelines and the EU directive 2010/63/EU for the protection of animals used for scientific purposes were strictly followed, and all the experiments were approved by the National Scientific Ethical Committee on Animal Experimentation (National Competent Authority), with the license number XX./1636/2019.
Ten weeks after the onset of hyperglycemia, the animals were killed by cervical dis-location under chloral hydrate anesthesia (375 mg/kg i.p.). The gut segments of diabetic, insulin-treated diabetic and control rats were dissected and rinsed in 0.05 mol/L phosphate buffer (PB; pH 7.4). Samples were taken from the duodenum (1 cm distal to the pylorus), the ileum (1 cm proximal to the ileo-caecal junction) and the proximal colon, and processed for fluorescent immunohistochemistry (n = 5 animals per group), quantitative electron microscopy (n = 5 animals per group) and enzyme-linked immunosorbent assay (ELISA, control group n = 10 animals, insulin-treated diabetic group n = 12 animals, diabetic group n = 10 animals). For double-labeling fluorescent immunohistochemistry, the intestinal samples from different gut segments were cut along the mesentery, fixed in 4% paraformaldehyde and whole-mount preparations were made. For post-embedding electron microscopy, small pieces (2-3 mm) of the gut segments were fixed in 2% paraformaldehyde and 2% glutaraldehyde solution and then further fixed for 1 h in 1% OsO4. After rinsing in buffer and dehydrating in increasing ethanol concentrations and acetone, they were embedded in Embed812 (Electron Microscopy Sciences, United States). For the ELISA, the 3-cm-long gut segments were cut along the mesentery and pinched flat. After removing the layer of mucosa and submucosa, the residual tissue containing the intestinal smooth muscle layers and the myenteric plexus in between were snap-frozen in liquid nitrogen and stored at -80 °C until use.
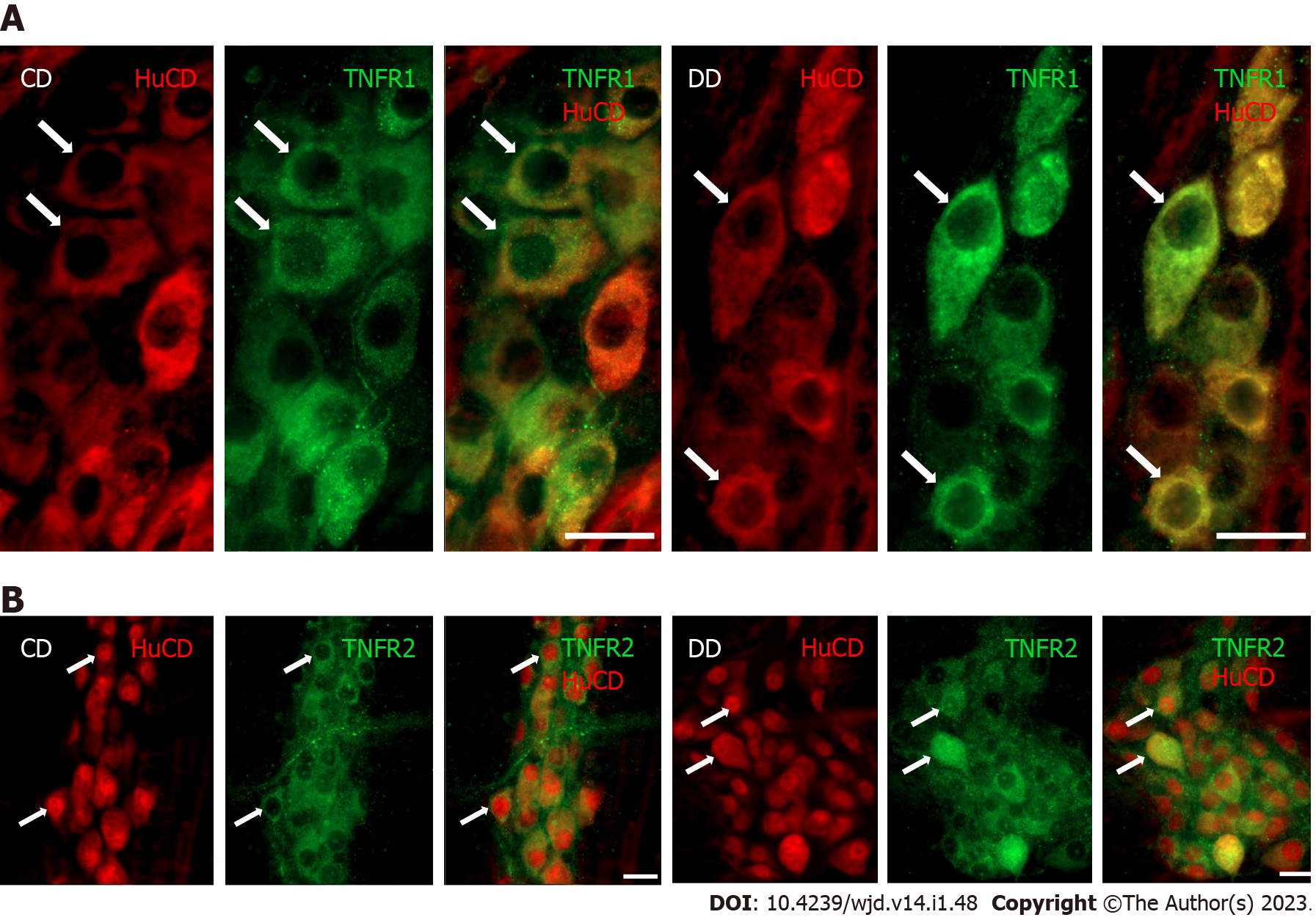
For double-labeling immunohistochemistry, whole-mount preparations derived from different gut segments were immunostained with HuCD and TNFR1 or TNFR2. HuCD as a pan-neuronal marker is suitable for labeling of neuronal cell body without the projections. Briefly, after blocking in tris(hydroxymethyl)aminomethane-buffered saline (TBS) containing 1% bovine serum albumin and 10% normal goat serum, the samples were incubated overnight with pan-neuronal anti-HuCD (mouse monoclonal IgG; A-21271, Invitrogen, United States; final dilution 1:50) and anti-TNFR1 (rabbit polyclonal, SAB4502988, Sigma-Aldrich, Hungary; final dilution 1:100) or anti-TNFR2 (rabbit poly-clonal, SAB4502989, Sigma-Aldrich, Hungary; final dilution 1:200) primary antibodies at 4 °C. After washing in TBS with 0.025% Triton X-100, whole-mounts were incubated with anti-mouse CyTM3 (Jackson ImmunoResearch Laboratories, United States; final dilution 1:200) and anti-rabbit Alexa Fluor 488 (Life Technologies Corporation, Molecular Probes, United States; final dilution 1:200) secondary antibodies for 1 h at room temperature. Negative controls were performed by omitting the primary antibody when no immunoreactivity was observed. Whole-mount preparations were mounted on slides in FluoromountTM Aqueous Mounting Medium (Sigma-Aldrich, Hungary), observed and photographed with a Zeiss Imager Z.2 fluorescent microscope equipped with an Axiocam 506 mono camera.
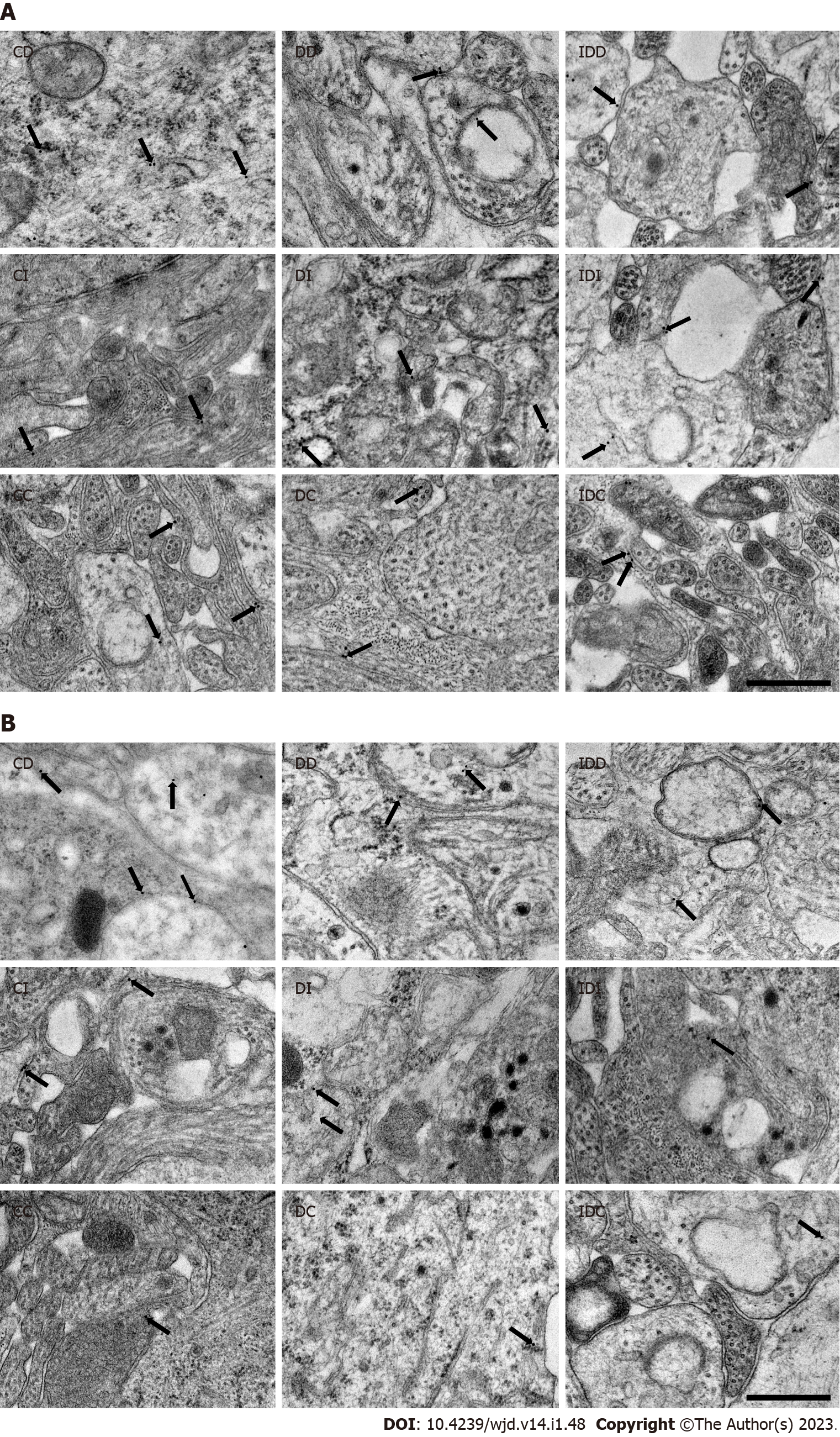
Five Embed blocks originating from each intestinal segment and condition were used to prepare ultrathin (70 nm) sections, which were mounted on nickel grids and processed for TNFR immunogold labeling. Ultrathin sections (three grids per block) were incubated overnight in anti-TNFR1 (rabbit polyclonal, SAB4502988, Sigma-Aldrich, Hungary; final dilution 1:100) or anti-TNFR2 (rabbit polyclonal, SAB4502989, Sigma-Aldrich, Hungary; final dilution 1:200) primary antibodies, followed by colloidal gold conjugated anti-rabbit IgG (conjugated to 18 nm gold particles; Jackson ImmunoResearch, United States; final dilution 1:20) secondary antibody for 3 h. The specificity of the immunoreaction was assessed in all cases by omitting the primary antibodies in the labeling protocol and incubating the sections only in the gold conjugated secondary antibody. Sections were counterstained with uranyl acetate (Merck, Germany) and lead citrate (Merck, Germany), and were examined and photographed with a JEOL JEM 1400 transmission electron microscope. The quantitative features and the subcellular distributions of the gold particles labeling TNFR1 or TNFR2 were determined in the myenteric ganglia. Fifty digital photographs of ten myenteric ganglia per intestinal segment per condition were made at a magnification of 20000 × with the AnalySIS 3.2 program (Soft Imaging System GmbH, Germany). The intensity of the labeling was expressed as the total number of gold particles per unit area (μm2).
The intestinal tissue samples, including the intestinal smooth muscle/myenteric plexus (MUSCLE-MP), were frozen in liquid nitrogen, crushed into powder in a mortar and homogenized in 500 μL homogenizing buffer (100 μL Protease Inhibitor Cocktail (Sigma-Aldrich, Hungary) in 20 mL 0.05 mol/L PB). Tissue homogenates were centrifuged at 5000 rpm for 20 min at 4 °C. The TNFR1 and TNFR2 levels of the MUSCLE-MP tissue samples were determined by means of quantitative ELISA according to the manufacturer’s instructions (TNFR1: GA-E3995RT, TNFR2: GA-E3997RT, GenAsia Bio-tech Co., China). Optical density was measured at 450 nm (Benchmark Microplate Reader; Bio-Rad, Hungary). Tissue TNFR concentrations were expressed as ng/mg protein.
A commercial protein assay kit was used for the determination of protein content in tissue samples. Bradford reagent was added to each sample. After mixing and following 10 min incubation, the samples were assayed spectrophotometrically at 595 nm. Protein level was expressed as mg protein/mL.
Statistical analysis was performed with one-way analysis of variance (ANOVA) and Newman-Keuls test, Kruskal-Wallis test and Dunn’s multiple comparisons test. All analyses were carried out with GraphPad Prism 6.0 (GraphPad Software, United States). A probability of P < 0.05 was set as the level of significance. All data were expressed as means ± SE.
The general characteristics of the diabetic, insulin-treated diabetic and control rats were monitored during the 10-wk experiment and are summarized in Table 1. Diabetic rats were characterized by a long-lasting chronic hyperglycemia, their blood glucose concentration was 5 times that of the control group (25.64 ± 0.73 mmol/L vs 5.89 ± 0.13 mmol/L). Immediate insulin treatment inhibited extremely high glucose levels, however, the values were still higher than in the control group (10.29 ± 0.76 mmol/L). All the animals gained weight during the experimental period, but the final body weight of diabetic rats was significantly lower as compared to insulin-treated diabetic and control animals.
| Body weight (g) | Blood glucose concentration (mmol/L) | |||
| Initial | Final | Initial | Final (average) | |
| Controls (n = 15) | 222.7 ± 9.76 | 429.9 ± 11.53a | 4.83 ± 0.32 | 5.89 ± 0.13 |
| Diabetics (n = 17) | 231.9 ± 13.4 | 330.4 ± 11.5a,b | 4.99 ± 0.26 | 25.64 ± 0.73a,b |
| Insulin-treated diabetics (n = 15) | 227.5 ± 10.76 | 404.3 ± 14.79a,c | 4.81 ± 0.31 | 10.29 ± 0.76a,b,c |
Double-labeling fluorescent immunohistochemistry revealed TNFR immunoreactivity in myenteric ganglia. Both the TNFR1- and TNFR2-immunoreactive myenteric neurons were clearly visible on whole-mount preparations (Figure 1).
Quantitative evaluation of TNFRs expression was carried out by post-embedding immunogold electron microscopy on ultrathin sections of myenteric ganglia (Figure 2). The 18 nm gold particles labeling TNFR1 or TNFR2 were often located at the plasma membranes or intracellular membranes in all the investigated gut segments and experimental conditions (Figure 3).
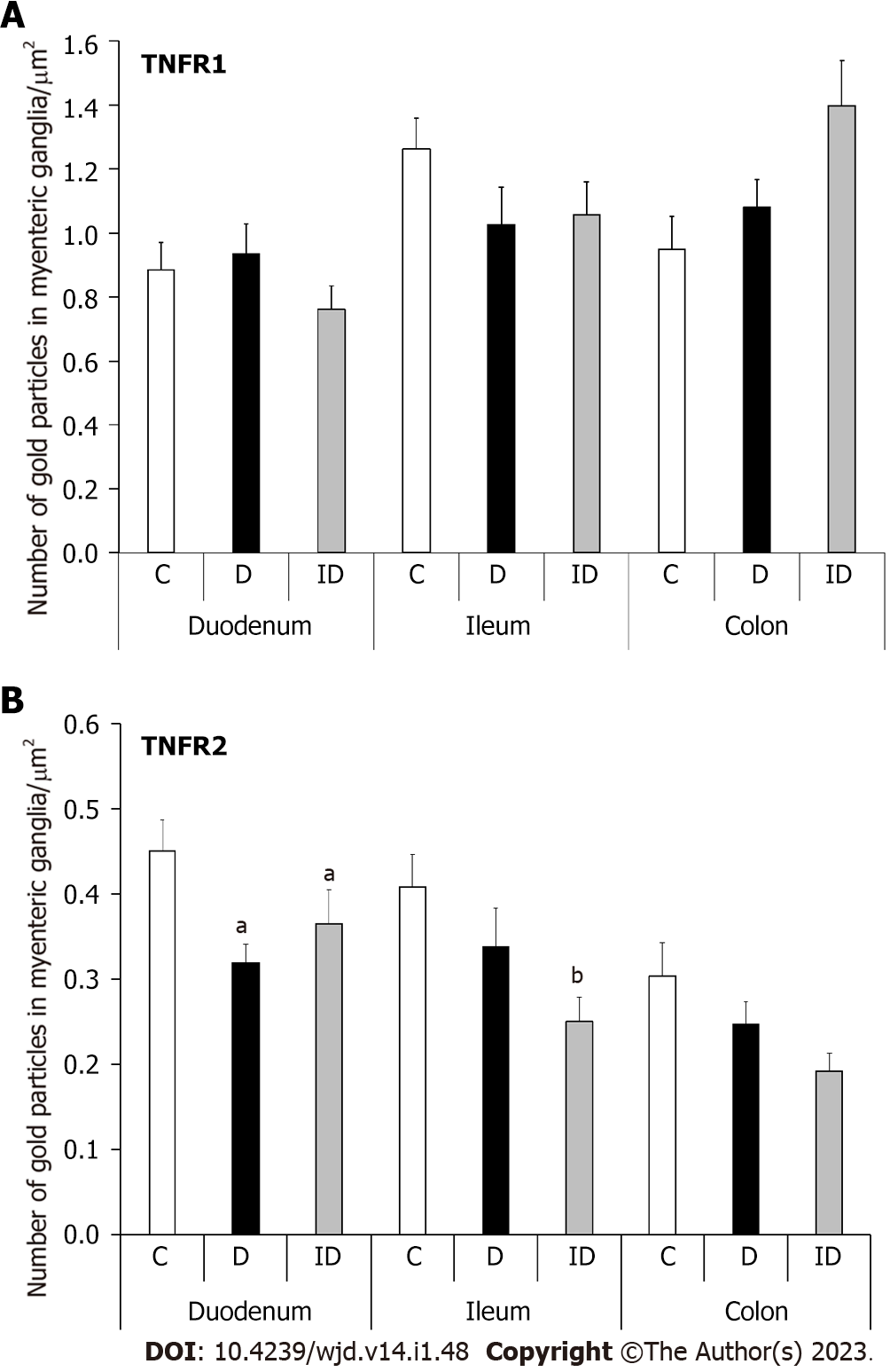
In healthy controls, the baseline density of TNFR1-labeling gold particles was higher than that of TNFR2 in all investigated gut segments. In contrast, TNFRs distribution differed in intestinal regions: TNFR1-labeling gold particle density was lowest in the duodenum (0.88 ± 0.08) and highest in the ileum (1.26 ± 0.09), while TNFR2 density was highest in the duodenum (0.45 ± 0.03) and exhibited a significant decreasing tendency along the duodenum-colon axis (Figure 4).
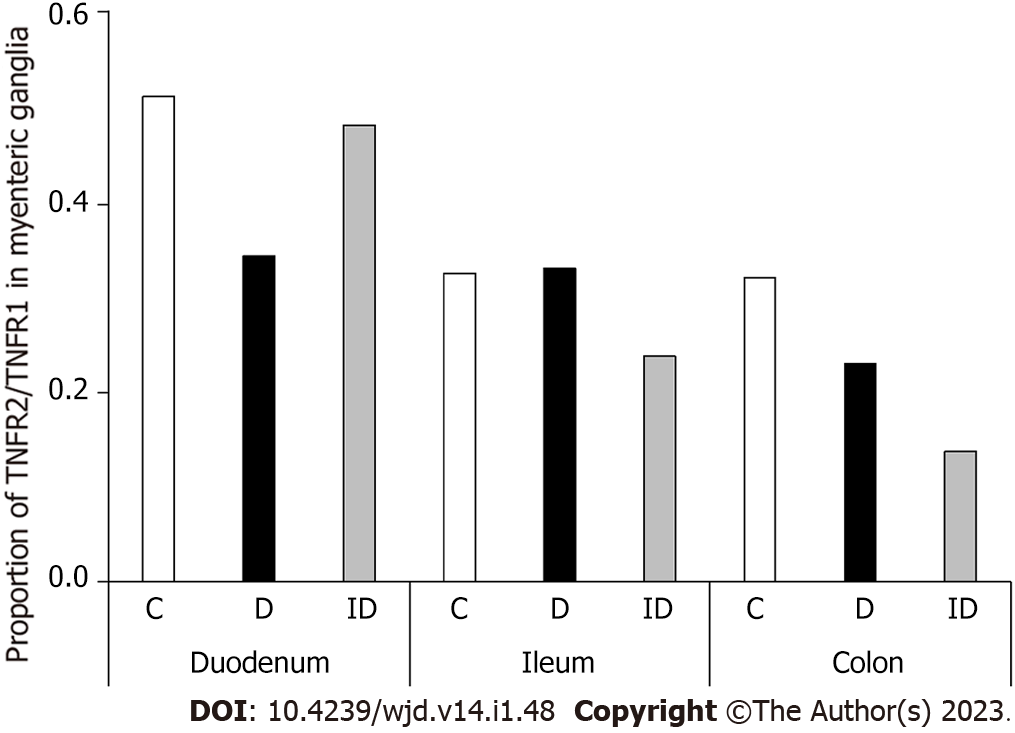
TNFR1 density did not change significantly in neither the diabetic nor the insulin-treated animals in any investigated gut segments (Figure 4A). However, TNFR2 density decreased significantly only in myenteric ganglia of the diabetic duodenum (P < 0.05; Figure 4B). Insulin treatment did not prevent this decrease and resulted in a significantly lower TNFR2 density in the ileal ganglia compared to controls (Figure 4B).
Consequently, in the duodenum of diabetics, the proportion of TNFR2/TNFR1 was decreased by more than 30% (Figure 5). This ratio remained unchanged in the ileum and decreased also in the colonic ganglia of diabetics. In insulin-treated rats, the TNFR2/TNFR1 ratio was close to the control level in the duodenum, and decreased in ileal and colonic ganglia (Figure 5).
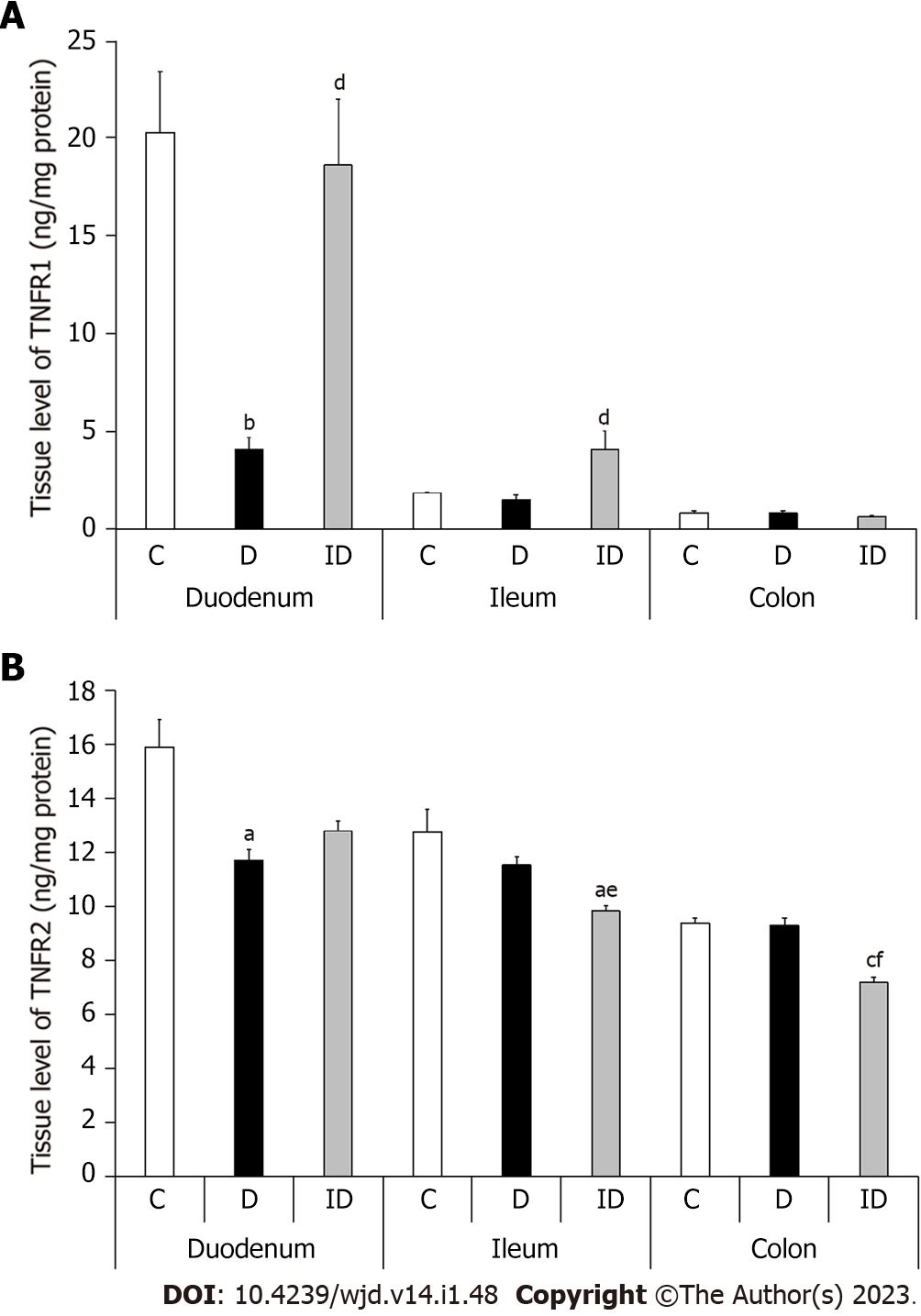
In controls, the level of both TNFRs in MUSCLE-MP homogenates, which included the circular and longitudinal smooth muscle layers of the gut wall as well as the myenteric plexus, displayed a definite decrease from duodenum to colon. While the TNFR1 level was 20.32 ± 3.1 ng/mg in the duodenum, the ileum contained only a tenth of this value (1.85 ± 0.04; P < 0.05) and the colon even less (0.85 ± 0.11; P < 0.0001; Figure 6A). The TNFR2 tissue level was also highest in the duodenal homogenates of controls (15.91 ± 1.04) and significantly lower in the ileum (12.76 ± 0.86; P < 0.05) and colon (9.38 ± 0.21; P < 0.0001; Figure 6B).
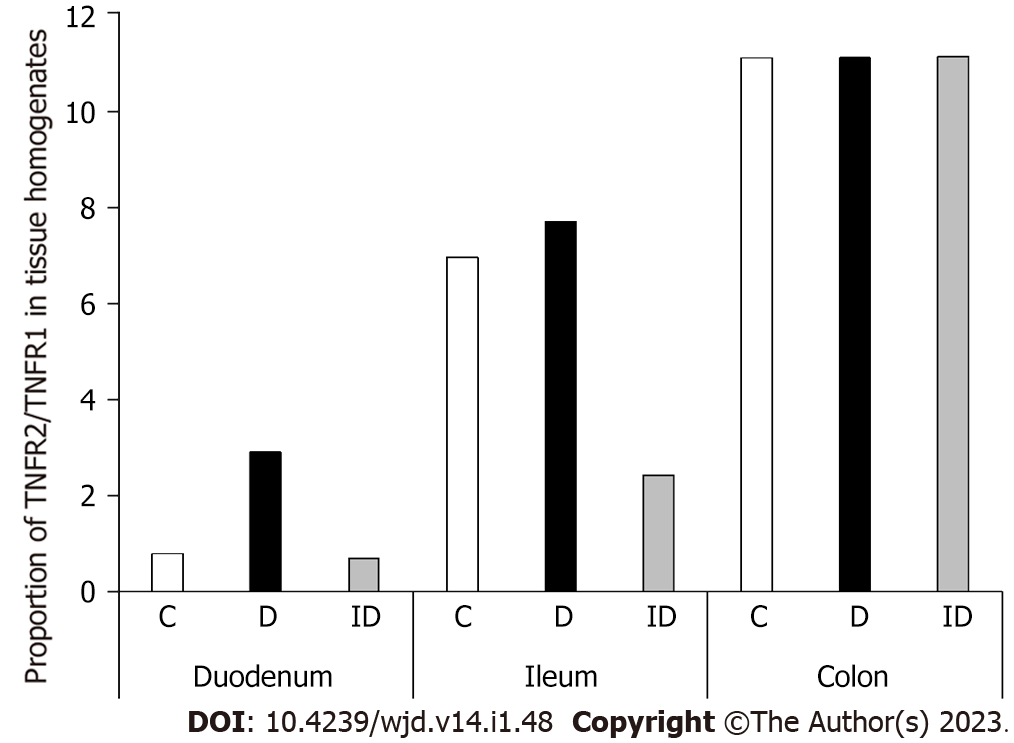
In diabetic homogenates, both TNFR levels decreased markedly in the duodenum compared to control samples (Figure 6). The TNFR2 level was decreased by 25% in these samples (11.72 ± 0.39 vs 15.91 ± 1.04; P < 0.01), but the greatest decrease was observed in the case of TNFR1, which was only a fifth of the control value (4.06 ± 0.65 vs 20.32 ± 3.1; P < 0.001). Therefore, the proportion of TNF-R2/TNFR1 was quadrupled in duodenal tissue homogenates of diabetics as compared to controls (Figure 7). However, there were no changes in either the TNFR1 and TNFR2 tissue level or in their proportion in other diabetic gut segments (Figures 6 and 7). Insulin treatment only prevented themarked TNFR1 decrease in the duodenum, but not the TNFR2 change. In addition, the insulin significantly altered the TNFR levels in the other intestinal segments (Figure 6).
In this study, an intestinal segment-specific TNFR distribution has been demonstrated both in healthy control and type 1 diabetic rats. Enteric neuronal TNFR1 and TNFR2 expression was also shown by other studies[31,32]. The presence of TNFRs on myenteric neurons is supportive of their modulatory impact on neuronal activity, which is triggered by cytokines[32]. Moreover, the regionality of their base level suggests distinct significance in cell fate regulation in different gut segments. Though TNFR1 displayed higher expression than TNFR2 in myenteric ganglia in all gut segments, this does not necessarily prioritize the TNFα-TNFR1 signaling pathway, because the receptors’ affinity to different forms of TNFα is not equal. Recent studies have shown that the transmembrane form of TNFα has higher affinity for TNFR2, while the released soluble form has higher affinity for TNFR1[33,34]. Considering the regional differences along the duodenum-colon axis, the TNFR2 expression was highest in duodenal ganglia, while the TNFR1 density was the lowest there, which suggests that TNFR2 may have stronger influence on duodenal myenteric neurons than TNFR1. Furthermore, control duodenal MUSCLE-MP homogenates displayed the highest TNFR levels, which greatly differed from that of other segments, suggesting a privileged role for TNFRs also in the duodenal microenvironment around myenteric ganglia.
In the colon and ileum of diabetics, neither the TNFR1 nor the TNFR2 density was found to be significantly different in myenteric ganglia. Furthermore, tissue levels of different TNFRs and their proportion also remained unchanged in the MUSCLE-MP homogenates from these segments. Since the myenteric and mucosal/submucosal TNFα expression has been shown to decrease in the distal gut regions of diabetic rats[10] presumably as a result of heme oxygenase induction[6,35], we suppose that the crucial mechanism involved in diabetic loss of myenteric neurons[12] and environmental damage[5,7] in the colon and ileum is not the result of TNFα-TNFRs signaling. Therefore, further investigation is necessary regarding the impact of other cytokines in diabetic enteric neuropathy. Immediate insulin treatment was not preventive, but rather it had confusing effect on TNFR expression. Studies have shown that increased TNFα is fundamentally involved in the disturbed insulin signaling and contributes to the development of insulin resistance[36,37]. Direct exposure to TNFα induces insulin resistance in myocytes and brown adipocytes due to impairment of the insulin receptor substrate proteins[38]. High TNFR1 and TNFα levels are also related to insulin resistance and impaired β-cell function in pregnant women[39]. Diabetes-induced insulin insensitivity is reversed by aryl-hydrocarbon receptor ligands, such as tryptophan, which also decreased the TNFα expression in Kupffer cells and plasma and lead to reversed intestinal barrier function in insulin-dependent diabetic mice[40].
In myenteric ganglia of the diabetic duodenum, a decrease in the number of TNFR2-labeling gold particles was revealed without any significant alterations in TNFR1 density. This suggests that the TNFα-TNFR2 signaling is more affected by the diabetic state in the duodenum. Recently, regionally distinct TNFα production has been demonstrated in myenteric ganglia of different gut segments and intestinal layer-dependent differences in TNFα levels have been revealed in type 1 diabetic rats[10]. A significantly increased TNFα expression has been observed only in the duodenal ganglia of diabetics[10]. TNFR2-deficient mice have decreased sensitivity to TNFα or greater resistance to TNFα-induced cell death[41], therefore decreased TNFR2 levels may protect neurons. This is in agreement with our earlier findings that the duodenum was the only gut segment where diabetic loss of myenteric neurons has not been demonstrated[6,12], despite of the increased TNFα expression in the duodenal ganglia of type 1 diabetic rats[10]. TNFR2 downregulation increased Schwann cell apoptosis in culture, but also triggered the apoptosis-induced proliferation of Schwann cells[42]. In human and murine macrophages, the TNF-induced TRAF2 degradation was blocked in the absence of TNFR2[43]. Renal TNFR2 deficit protects from glomerulonephritis by reducing macrophage infiltration[44]. Furthermore, TNFR2 deficiency reduces the production of immunosuppressive factors, like nitric-oxide and interleukin 6[45]. TNFα/TNFR2 signaling is a crucial checkpoint in immunomodulation[46], it influences the T cell responses in autoimmunity[28,47-49]. TNFR2 signaling has an important role in protecting neurons against glutamate-induced excitotoxicity through the nuclear factor-kappa B (NF-kappa B)-dependent pathway[22,50]. It has been shown that TNFα can be neurotrophic for enteric neurons since it promotes the glial-derived neurotrophic factor expression of intestinal smooth muscle cells and facilitates the neurite outgrowth also via NF-kappa B-related pathway[32]. Therefore, evaluating the possible involvement of NF-kappa B in region-dependent diabetic enteric neuropathy may be an interesting future prospective. Both TNFR1 and TNFR2 tissue levels decreased in diabetic duodenal MUSCLE-MP homogenates. As the intestinal smooth muscle represents in a larger amount in these tissue samples, differences in the results of electron microscopic and ELISA can better reflect different alterations of TNFRs in the myenteric neurons than the muscular environment. It should also be noted that TNFR1 and TNFR2 tissue levels decreased to a very different extent in diabetic duodenal MUSCLE-MP samples. This may result in the complete reversal of the TNFRs proportion in the neuronal muscular environment compared to myenteric ganglia. Shift in the TNFR2/TNFR1 balance may be essential in TNF signaling and considering that TNFR2 in different cell types may be endowed with opposing functions[51], the importance of environmental changes in neuronal loss or survival is further emphasized.
The present study revealed an intestinal segment-specific pattern of TNFRs expression in myenteric ganglia and their muscular environment in type 1 diabetes. Our results support that TNFR2 is more affected than TNFR1 in myenteric ganglia in the duodenum of diabetic rats. The diabetes-related regional changes in TNFRs expression may contribute to region-dependent myenteric neuropathy, however, further experiments are required to conclude its functional consequences.
Tumor necrosis factor alpha plays an essential role in inflammatory modulation of enteric neurons, however, it can promote apoptosis or cell survival depending on its different receptors (TNFR1 and TNFR2).
Distinct neuronal microenvironment is critical in gut segment-specific diabetic enteric neuropathy, therefore, the region-dependent expression and alterations of different TNFRs may also be important in therapy targeting motility disturbances in type 1 diabetes.
To investigate the TNFR1 and TNFR2 expression in myenteric ganglia and their environment in different intestinal regions of diabetic rats.
Double-labeling fluorescent immunohistochemistry, post-embedding immunogold electron microscopy and enzyme-linked immunosorbent assay were applied to evaluate the TNFR1 and TNFR2 expression in myenteric ganglia and muscle/myenteric plexus tissue homogenates from different gut segments of streptozotocin-induced diabetic, insulin-treated diabetic and control rats.
TNFRs expression displayed a strictly region-specific pattern even in control animals. However, among all the investigated gut segments, only the duodenum showed significant alterations of TNFR expression in diabetic rats. The TNFR2 density was decreased in the myenteric ganglia, while no significant changes in TNFR1 density were revealed. Moreover, the TNFR2/TNFR1 proportion was markedly influenced in both the ganglia and their muscular environment of diabetics. Insulin had controversial effects on TNFR expression.
Diabetes-related region-specificity in TNFRs expression shows that TNFR2 is more affected than TNFR1 in duodenal myenteric ganglia of type 1 diabetic rats.
Nuclear factor-kappa B has a key role in TNFR2 signaling pathway. Therefore, evaluation of the involvement of nuclear factor-kappa B in region-dependent diabetic enteric neuropathy may be important in the future.
We thank Németh E and Arany K for the excellent technical assistance. We remember our colleague Dr. Rázga Z with thanks and gratitude.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: He Z, China; Wu QN, China S-Editor: Gao CC L-Editor: Ma JY-MedE P-Editor: Gao CC
| 1. | Brown H, Esterházy D. Intestinal immune compartmentalization: implications of tissue specific determinants in health and disease. Mucosal Immunol. 2021;14:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Kaiko GE, Stappenbeck TS. Host-microbe interactions shaping the gastrointestinal environment. Trends Immunol. 2014;35:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1102] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 5. | Wirth R, Bódi N, Maróti G, Bagyánszki M, Talapka P, Fekete É, Bagi Z, Kovács KL. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS One. 2014;9:e110440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Chandrakumar L, Bagyánszki M, Szalai Z, Mezei D, Bódi N. Diabetes-Related Induction of the Heme Oxygenase System and Enhanced Colocalization of Heme Oxygenase 1 and 2 with Neuronal Nitric Oxide Synthase in Myenteric Neurons of Different Intestinal Segments. Oxid Med Cell Longev. 2017;2017:1890512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Jancsó Z, Bódi N, Borsos B, Fekete É, Hermesz E. Gut region-specific accumulation of reactive oxygen species leads to regionally distinct activation of antioxidant and apoptotic marker molecules in rats with STZ-induced diabetes. Int J Biochem Cell Biol. 2015;62:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Korbut E, Brzozowski T, Magierowski M. Carbon Monoxide Being Hydrogen Sulfide and Nitric Oxide Molecular Sibling, as Endogenous and Exogenous Modulator of Oxidative Stress and Antioxidative Mechanisms in the Digestive System. Oxid Med Cell Longev. 2020;2020:5083876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 427] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 10. | Bódi N, Chandrakumar L, Al Doghmi A, Mezei D, Szalai Z, Barta BP, Balázs J, Bagyánszki M. Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Tu W, Wang H, Li S, Liu Q, Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 466] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 12. | Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, Bagyánszki M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Klinge MW, Sutter N, Mark EB, Haase AM, Borghammer P, Schlageter V, Lund S, Fleischer J, Knudsen K, Drewes AM, Krogh K. Gastric Emptying Time and Volume of the Small Intestine as Objective Markers in Patients With Symptoms of Diabetic Enteropathy. J Neurogastroenterol Motil. 2021;27:390-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26:611-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | Bessac A, Cani PD, Meunier E, Dietrich G, Knauf C. Inflammation and Gut-Brain Axis During Type 2 Diabetes: Focus on the Crosstalk Between Intestinal Immune Cells and Enteric Nervous System. Front Neurosci. 2018;12:725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Jarret A, Jackson R, Duizer C, Healy ME, Zhao J, Rone JM, Bielecki P, Sefik E, Roulis M, Rice T, Sivanathan KN, Zhou T, Solis AG, Honcharova-Biletska H, Vélez K, Hartner S, Low JS, Qu R, de Zoete MR, Palm NW, Ring AM, Weber A, Moor AE, Kluger Y, Nowarski R, Flavell RA. Enteric Nervous System-Derived IL-18 Orchestrates Mucosal Barrier Immunity. Cell. 2020;180:50-63.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 17. | Ruder B, Atreya R, Becker C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Wei HX, Wang B, Li B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front Immunol. 2020;11:1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 19. | Vanamee ÉS, Faustman DL. Structural principles of tumor necrosis factor superfamily signaling. Sci Signal. 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 20. | Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1143] [Cited by in RCA: 1067] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 21. | Coquenlorge S, Duchalais E, Chevalier J, Cossais F, Rolli-Derkinderen M, Neunlist M. Modulation of lipopolysaccharide-induced neuronal response by activation of the enteric nervous system. J Neuroinflammation. 2014;11:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869-32881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 23. | Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front Immunol. 2013;4:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Ihnatko R, Kubes M. TNF signaling: early events and phosphorylation. Gen Physiol Biophys. 2007;26:159-167. [PubMed] |
| 25. | Gough P, Myles IA. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front Immunol. 2020;11:585880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 26. | Zhang N, Wang Z, Zhao Y. Selective inhibition of Tumor necrosis factor receptor-1 (TNFR1) for the treatment of autoimmune diseases. Cytokine Growth Factor Rev. 2020;55:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Liang H, Yin B, Zhang H, Zhang S, Zeng Q, Wang J, Jiang X, Yuan L, Wang CY, Li Z. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-alpha signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology. 2008;149:2943-2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Yang S, Xie C, Chen Y, Wang J, Chen X, Lu Z, June RR, Zheng SG. Differential roles of TNFα-TNFR1 and TNFα-TNFR2 in the differentiation and function of CD4+Foxp3+ induced Treg cells in vitro and in vivo periphery in autoimmune diseases. Cell Death Dis. 2019;10:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 775] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 30. | Maier O, Fischer R, Agresti C, Pfizenmaier K. TNF receptor 2 protects oligodendrocyte progenitor cells against oxidative stress. Biochem Biophys Res Commun. 2013;440:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Chandrasekharan B, Jeppsson S, Pienkowski S, Belsham DD, Sitaraman SV, Merlin D, Kokkotou E, Nusrat A, Tansey MG, Srinivasan S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis. 2013;19:2535-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Gougeon PY, Lourenssen S, Han TY, Nair DG, Ropeleski MJ, Blennerhassett MG. The pro-inflammatory cytokines IL-1β and TNFα are neurotrophic for enteric neurons. J Neurosci. 2013;33:3339-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Benoot T, Piccioni E, De Ridder K, Goyvaerts C. TNFα and Immune Checkpoint Inhibition: Friend or Foe for Lung Cancer? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993;268:18542-18548. [PubMed] |
| 35. | Namkoong S, Sung J, Yang J, Choi Y, Jeong HS, Lee J. Nobiletin Attenuates the Inflammatory Response Through Heme Oxygenase-1 Induction in the Crosstalk Between Adipocytes and Macrophages. J Med Food. 2017;20:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Akash MSH, Rehman K, Liaqat A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem. 2018;119:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 409] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 37. | Fernandez-Real JM, Lainez B, Vendrell J, Rigla M, Castro A, Peñarroja G, Broch M, Pérez A, Richart C, Engel P, Ricart W. Shedding of TNF-alpha receptors, blood pressure, and insulin sensitivity in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;282:E952-E959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci. 2008;86:E94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Xie J, Dai L, Tang XL. Study on the correlation between the changes of TNFR1, TNF-alpha, and adiponectin in patients with gestational diabetes mellitus and insulin resistance. Eur J Inflamm. 2019;17. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Liu WC, Chen PH, Chen LW. Supplementation of endogenous Ahr ligands reverses insulin resistance and associated inflammation in an insulin-dependent diabetic mouse model. J Nutr Biochem. 2020;83:108384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 449] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 42. | Gao Z, Min C, Xie H, Qin J, He X, Zhou S. TNFR2 knockdown triggers apoptosis-induced proliferation in primarily cultured Schwann cells. Neurosci Res. 2020;150:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Ruspi G, Schmidt EM, McCann F, Feldmann M, Williams RO, Stoop AA, Dean JL. TNFR2 increases the sensitivity of ligand-induced activation of the p38 MAPK and NF-κB pathways and signals TRAF2 protein degradation in macrophages. Cell Signal. 2014;26:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115:1199-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Polz J, Remke A, Weber S, Schmidt D, Weber-Steffens D, Pietryga-Krieger A, Müller N, Ritter U, Mostböck S, Männel DN. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun Inflamm Dis. 2014;2:121-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Beldi G, Khosravi M, Abdelgawad ME, Salomon BL, Uzan G, Haouas H, Naserian S. TNFα/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res Ther. 2020;11:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 47. | Atretkhany KN, Mufazalov IA, Dunst J, Kuchmiy A, Gogoleva VS, Andruszewski D, Drutskaya MS, Faustman DL, Schwabenland M, Prinz M, Kruglov AA, Waisman A, Nedospasov SA. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc Natl Acad Sci U S A. 2018;115:13051-13056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 48. | Chatzidakis I, Mamalaki C. T cells as sources and targets of TNF: implications for immunity and autoimmunity. Curr Dir Autoimmun. 2010;11:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Ye LL, Wei XS, Zhang M, Niu YR, Zhou Q. The Significance of Tumor Necrosis Factor Receptor Type II in CD8+ Regulatory T Cells and CD8+ Effector T Cells. Front Immunol. 2018;9:583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Dolga AM, Granic I, Blank T, Knaus HG, Spiess J, Luiten PG, Eisel UL, Nijholt IM. TNF-alpha-mediates neuroprotection against glutamate-induced excitotoxicity via NF-kappaB-dependent up-regulation of K2.2 channels. J Neurochem. 2008;107:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG, Madsen PM, Bixby JL, Lemmon VP, Lambertsen KL, Brambilla R. Opposing Functions of Microglial and Macrophagic TNFR2 in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Cell Rep. 2017;18:198-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |