Published online Sep 15, 2022. doi: 10.4239/wjd.v13.i9.776
Peer-review started: May 26, 2022
First decision: June 20, 2022
Revised: July 1, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: September 15, 2022
Processing time: 106 Days and 5.9 Hours
Gestational diabetes mellitus (GDM) refers to abnormal glucose tolerance during pregnancy, and it is often accompanied by obvious changes in glucose and lipid metabolism, and associated with adverse pregnancy outcomes. The incidence of fetal distress, polyhydramnios, puerperal infection, premature delivery, and macrosomia in pregnant women with GDM are higher than in those without GDM.
To analyze the relationship between age of pregnant women with GDM and mode of delivery and neonatal Apgar score.
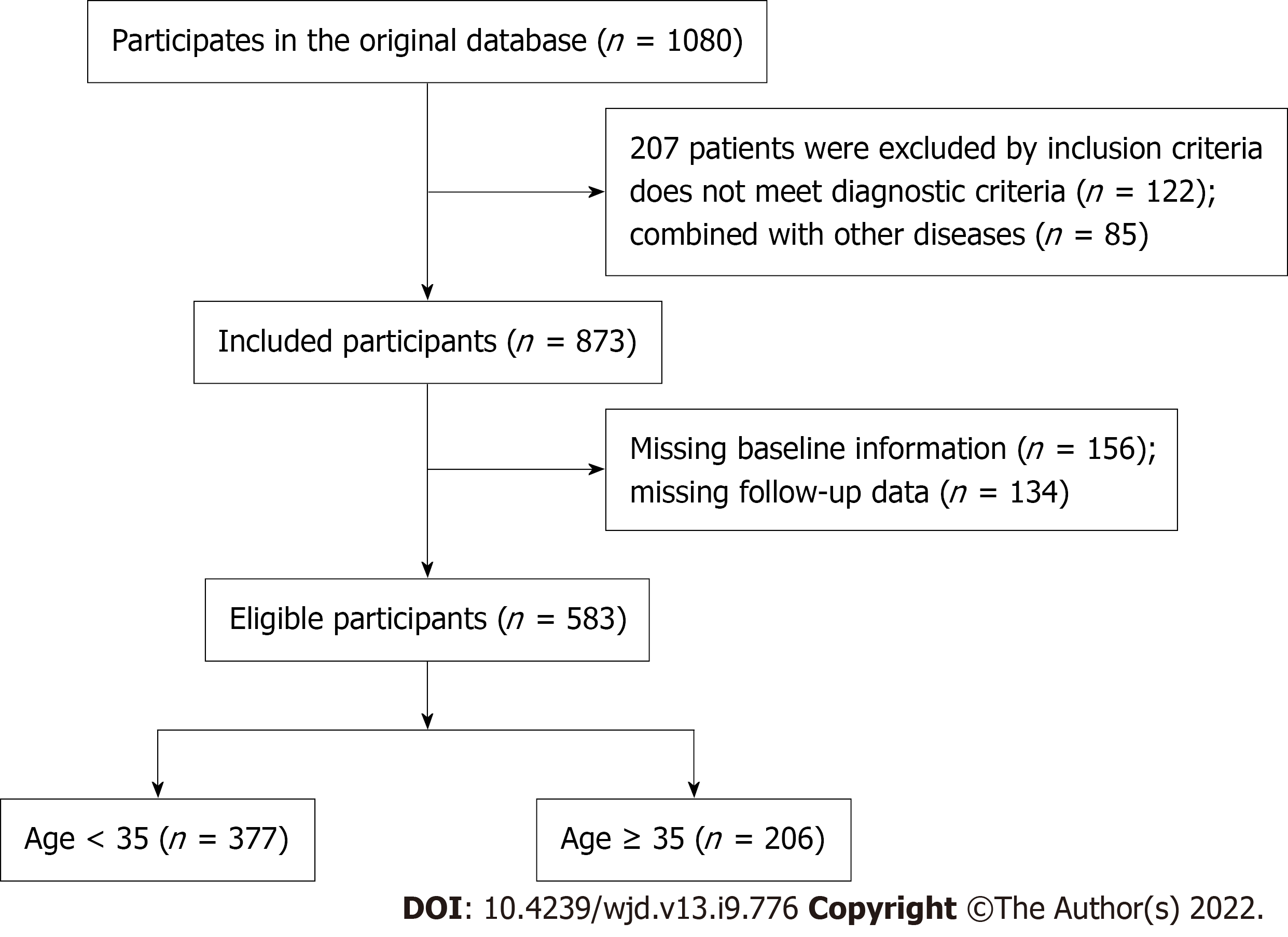
A total of 583 pregnant women with GDM who delivered in the Department of Obstetrics at our hospital between March 2019 and March 2022 were selected. Among them, 377 aged < 35 years were selected as the right age group and 206 aged > 35 years were selected as the older group. The clinical data of the two groups were collected, and the relationship between age of the pregnant women with GDM and mode of delivery, maternal and neonatal outcomes, and neonatal Apgar score were compared. In the older group, 159 women were classed as the adverse outcome group and 47 as the good outcome group according to whether they had adverse maternal and infant outcomes. The related factors of adverse maternal and infant outcomes were analyzed through logistic regression.
The number of women with assisted pregnancy, ≤ 37 wk gestation, ≥ 2 pregnancies, one or more deliveries, and no pre-pregnancy blood glucose screening in the older group were all higher than those in the right age group (P < 0.05). The natural delivery rate in the right age group was 40.85%, which was higher than 22.33% in the older group (P < 0.05). The cesarean section rate in the older group was 77.67%, which was higher than 59.15% in the right age group (P < 0.05). The older group had a higher incidence of polyhydramnios and postpartum hemorrhage, and lower incidence of fetal distress than the right age group had (P < 0.05). There was no significant difference in neonatal weight between the two groups (P > 0.05). The right age group had higher Apgar scores at 1 and 5 min than the older group had (P < 0.05). Significant differences existed between the poor and good outcome groups in age, education level, pregnancy mode, ≤ 37 wk gestation, number of pregnancies, and premature rupture of membranes (P < 0.05). Logistic regression showed that age, education level and premature rupture of membranes were all risk factors affecting the adverse outcomes of mothers and infants (P < 0.05).
Delivery mode and Apgar score of pregnant women with GDM are related to age. Older age increases the adverse outcome of mothers and infants.
Core Tip: This study analyzed the relationship between the age of pregnant women with gestational diabetes mellitus (GDM) and mode of delivery and neonatal Apgar score. Pregnant women with GDM were divided into right age and older groups. Compared with the older group, the natural delivery rate in the right age group was higher, but the cesarean section rate was lower. Moreover, age, education level and premature rupture of membranes were associated with the adverse outcomes of mothers and infants. Age was related to the delivery mode and Apgar score of pregnant women with GDM.
- Citation: Gao L, Chen CR, Wang F, Ji Q, Chen KN, Yang Y, Liu HW. Relationship between age of pregnant women with gestational diabetes mellitus and mode of delivery and neonatal Apgar score. World J Diabetes 2022; 13(9): 776-785
- URL: https://www.wjgnet.com/1948-9358/full/v13/i9/776.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i9.776
Gestational diabetes mellitus (GDM) is one of the specific diseases of pregnant women. It refers to abnormal glucose tolerance in different degrees during pregnancy, often accompanied by obvious changes in glucose and lipid metabolism, and is closely related to adverse pregnancy outcomes. The incidence rate of GDM is increasing annually in China. GDM results in a high-risk pregnancy, which can induce complications, such as abortion, premature delivery, amniotic fluid and infection[1,2]. The incidence of fetal distress, polyhydramnios, puerperal infection, premature delivery, and macrosomia in pregnant women with GDM are higher than in those without GDM[3,4]. Pregnancy at the right age is the key to reduce the risk of adverse outcomes. With the implementation of China’s three-child policy, the number of pregnant women with advanced maternal age is gradually increasing, and the incidence of pregnancy complications is significantly higher than that in right-age pregnant women. In recent years, with the rapid development of medical technology, significant progress has been made in the treatment of birth defects and premature infants. However, there are no effective measures to avoid the adverse outcomes of older-age pregnancies, especially those with GDM. Previous studies have shown that body mass index (BMI) may have an impact on maternal and neonatal outcomes in pregnant women with GDM[5,6]. However, pregnancy is still a risk factor for adverse maternal and neonatal outcomes and is affected by many factors. The clinical data of 583 pregnant women with GDM who delivered in the Department of Obstetrics at our hospital between March 2019 and March 2022 were retrospectively analyzed. The delivery mode and neonatal Apgar score of pregnant women with GDM at different ages were compared, to improve the pregnancy outcome of the older pregnant women, and provide a reference for ensuring the effect of eugenics and prenatal care.
The clinical data of 583 pregnant women with GDM who delivered in the Department of Obstetrics at our hospital between March 2019 and March 2022 were retrospectively analyzed. This study was approved by the Ethics Committee of our hospital. Inclusion criteria were: (1) All women conformed to the clinical diagnostic criteria for GDM[7]; (2) regular pregnancy examination; (3) successful delivery; (4) no hereditary diseases of coagulation system; (5) complete clinical medical records; and (6) pregnant women and family members gave informed consent to participate in this study.
Exclusion criteria were: (1) Women were diagnosed with diabetes mellitus or impaired glucose regulation before pregnancy; (2) women with other pregnancy-related diseases; (3) heart, liver or kidney dysfunction; (4) hematological diseases; (5) other diseases that may affect blood glucose; and (6) mental illness or retardation. Among the selected pregnant woman, 377 aged < 35 years were selected as the right age group and 206 aged > 35 years were selected as the older group. The data flow chart is shown in Figure 1.
The clinical data of the two groups were collected, including: age; pregnancy mode; educational level; BMI; fasting blood glucose; gestational weeks of delivery; number of pregnancies; number of deliveries; pre-pregnancy blood glucose screening; maternal and infant outcomes (preterm birth, polyhydramnios, oligohydramnios, fetal distress, macrosomia, umbilical cord around the neck, neonatal death events, neonatal hospitalization, neonatal aspiration pneumonia, neonatal hypoglycemia, neonatal jaundice, and postpartum hemorrhage); and Apgar scores at 1 and 5 min after birth. The clinical data of pregnant women in the two groups were retrospectively analyzed. In the older group, 159 women were classed as the adverse outcome group and 47 as the good outcome group according to whether they had adverse maternal and infant outcomes.
GDM was diagnosed by the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria. The IADPSG recommends testing to be routinely carried out between 24 and 28 wk of gestation or at the first prenatal visit in high-risk women. Based on the results of a 75-g, 2-h oral glucose tolerance test, a woman was diagnosed with GDM when one or more of her plasma glucose concentrations were equivalent to or exceeded the following levels: fasting, 92 mg/dL; 1 h, 180 mg/dL; or 2 h, 153 mg/dL.
The data in this study were analyzed using SPSS 21.0. The measurement data were expressed as mean ± SD, and were compared using the independent sample t test between two groups. The enumeration data were expressed as n (%), and were compared using the χ2 test between two groups. Factors related to adverse maternal and infant outcomes in older pregnant women were analyzed using logistic regression analysis. P < 0.05 was regarded as statistically significant.
The number of women with assisted pregnancy, ≤ 37 wk gestation, ≥ 2 pregnancies, one or more deliveries, and no pre-pregnancy blood glucose screening in the older group were all higher than those in the right age group (P < 0.05) (Table 1).
| General data | Right age group (n = 377) | Older group (n = 206) | t/χ2 | P | |
| Pregnancy mode | Natural pregnancy | 343 (90.98) | 170 (82.52) | 9.018 | 0.003 |
| Assisted pregnancy | 34 (9.02) | 36 (17.48) | |||
| Education level | Primary school and below | 4 (1.06) | 5 (2.43) | 5.054 | 0.080 |
| Junior high school | 80 (21.22) | 57 (27.67) | |||
| College degree or above | 293 (77.72) | 144 (69.90) | |||
| BMI before pregnancy (kg/m2) | 22.30 ± 3.77 | 22.80 ± 3.75 | 1.534 | 0.126 | |
| Gestational weight gain (kg) | 10.83 ± 15.21 | 9.20 ± 16.15 | 1.210 | 0.227 | |
| FBG (mmol/L) | 4.93 ± 1.14 | 4.89 ± 0.69 | 0.460 | 0.646 | |
| ≤ 37 wk gestation | 110 (29.18) | 84 (40.78) | 8.072 | 0.004 | |
| No. of Pregnancies | 1 | 134 (35.54) | 24 (11.65) | 38.493 | < 0.001 |
| ≥ 2 | 243 (64.46) | 182 (88.35) | |||
| Delivery times | 0 | 226 (59.95) | 59 (28.64) | 52.441 | < 0.001 |
| 1 | 129 (34.22) | 123 (59.71) | |||
| ≥ 2 | 22 (5.84) | 24 (11.65) | |||
| Pre-pregnancy blood glucose screening | Yes | 159 (42.18) | 67 (32.52) | 5.227 | 0.022 |
| No | 218 (57.82) | 139 (67.48) |
The right age pregnant women had a higher natural delivery rate of 40.85% compared with 22.5% in the older group (P < 0.05). The older group had a higher cesarean section rate of 77.67% compared with 59.15% in the right age group (P < 0.05) (Table 2).
| Groups | Cases | Natural delivery | Cesarean section rate |
| Right age group | 377 | 154 (40.85) | 223 (59.15) |
| Older group | 206 | 46 (22.33) | 160 (77.67) |
| χ2 | 20.271 | ||
| P | < 0.001 | ||
The older group had a higher incidence of polyhydramnios and postpartum hemorrhage, and lower incidence of fetal distress than the right age group had (P < 0.05) (Table 3).
| Adverse outcomes | Right age group (n = 377) | Older group (n = 206) | t/χ2 | P |
| Preterm birth | 40 (10.61) | 26 (12.62) | 0.37 | 0.464 |
| Polyhydramnios | 5 (1.32) | 8 (3.88) | 3.996 | 0.046 |
| Oligohydramnios | 32 (8.49) | 10 (4.85) | 2.631 | 0.105 |
| Fetal distress | 40 (10.61) | 10 (4.85) | 5.628 | 0.018 |
| Macrosomia | 8 (2.12) | 2 (0.97) | 1.047 | 0.306 |
| Umbilical cord around the neck | 125 (33.16) | 59 (28.64) | 1.258 | 0.262 |
| Neonatal death events | 3 (0.80) | 1 (0.49) | 0.188 | 0.664 |
| Neonatal hospitalization | 4 (0.27) | 1 (0.49) | 0.519 | 0.471 |
| Neonatal aspiration pneumonia | 3 (0.80) | 2 (0.97) | 0.048 | 0.827 |
| Neonatal Hypoglycemia | 4 (0.27) | 2 (0.97) | 0.011 | 0.918 |
| neonatal jaundice | 5 (1.32) | 2 (0.97) | 0.142 | 0.706 |
| Postpartum hemorrhage (mL) | 318.62 ± 97.02 | 362.20 ± 175.92 | 3.861 | 0.001 |
No significant difference existed in neonatal weight between the two groups (P > 0.05). The right age group had higher Apgar scores at 1 and 5 min after birth than the older group had (P < 0.05) (Table 4).
| Groups | Cases | Neonatal weight (g) | 1 min Apgar score | 5 min Apgar score |
| Right age group | 377 | 3107.66 ± 467.26 | 9.69 ± 0.06 | 9.93 ± 0.05 |
| Older group | 206 | 3102.07 ± 508.40 | 9.67 ± 0.08 | 9.89 ± 0.04 |
| χ2 | 0.134 | 3.408 | 9.882 | |
| P | 0.894 | 0.001 | < 0.001 |
Significant differences existed between the poor (n = 159) and good (n = 47) outcome groups for age, education level, pregnancy mode, ≤ 37 wk gestation weeks, number of pregnancies, and premature rupture of membranes (P < 0.05) (Table 5).
| Factors | Poor outcome group (n = 112) | Good outcome group (n = 94) | t/χ2 | P | |
| Age | 38.75 ± 1.26 | 37.26 ± 1.78 | 7.011 | < 0.001 | |
| Education level | Primary school and below | 0 (0.00) | 5 (5.32) | 6.257 | 0.044 |
| Junior high school | 33 (29.46) | 24 (25.53) | |||
| College degree or above | 79 (70.54) | 65 (69.15) | |||
| Pregnancy mode | Natural pregnancy | 87 (77.68) | 83 (88.30) | 3.996 | 0.046 |
| Assisted reproduction | 25 (22.32) | 11 (11.70) | |||
| ≤ 37 wk gestation | 53 (47.32) | 31 (32.98) | 4.354 | 0.037 | |
| No. of pregnancies | 1 | 18 (16.07) | 6 (6.38) | 4.661 | 0.031 |
| ≥ 2 | 94 (83.93) | 88 (93.62) | |||
| No. of deliveries | 0 | 36 (32.14) | 23 (24.47) | 1.543 | 0.462 |
| 1 | 63 (56.25) | 60 (63.83) | |||
| ≥ 2 | 13 (11.61) | 11 (11.70) | |||
| Mode of delivery | Natural labor | 25 (22.32) | 21 (22.34) | 0.000 | 0.997 |
| Cesarean section | 87 (77.68) | 73 (77.66) | |||
| Premature rupture of membranes | 36 (32.14) | 0 (0.00) | 36.613 | < 0.001 | |
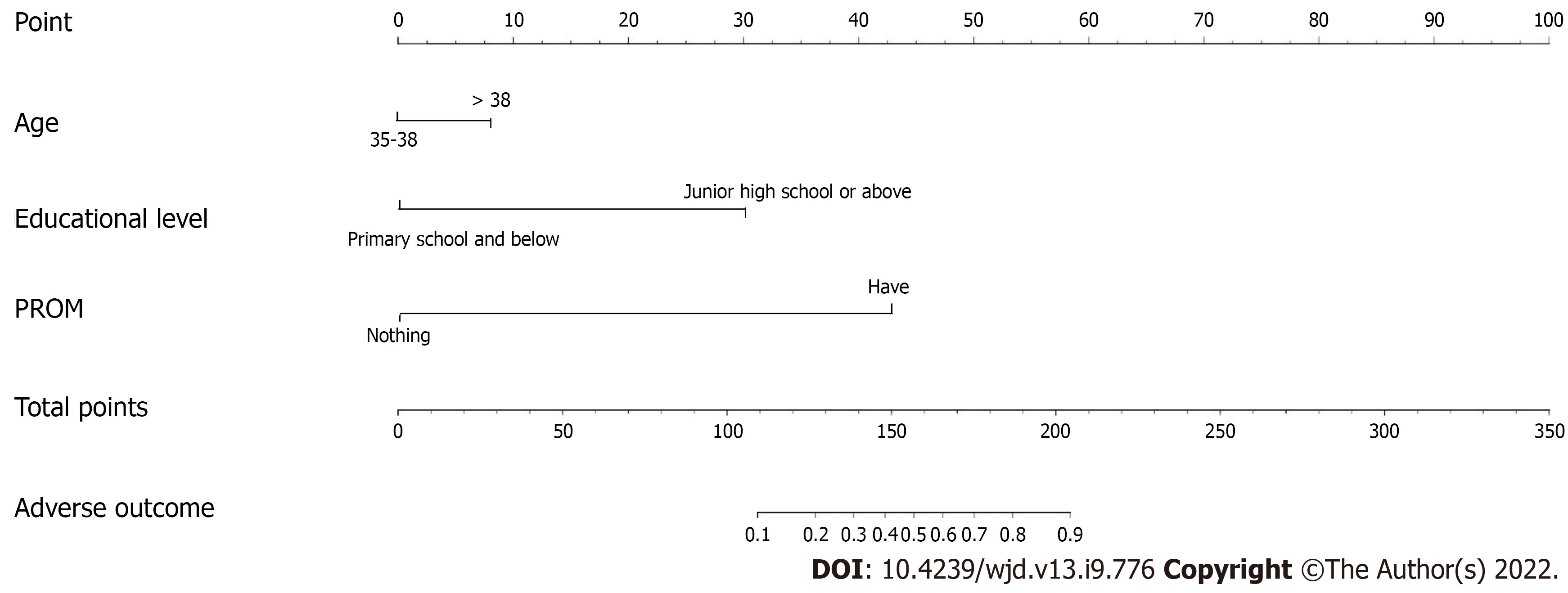
The interference between the various indicators was controlled and the correlation between these indicators and maternal and infant adverse outcomes in older women with GDM was analyzed by logistic regression analysis. The analysis was conducted using significant factors in Table 5 as independent variables and adverse maternal and infant outcomes as the dependent variable. The regression model was established by selecting the indexes such as age, education level, pregnancy mode, ≤ 37 wk gestation, number of pregnancies, and premature rupture of membranes. Logistic regression showed that age, education level and premature rupture of membranes were risk factors for maternal and infant adverse outcomes in older women with GDM (P < 0.05) (Table 6 and nomogram analysis in Figure 2).
| Risk factors | B | SE | Wald χ2 | P | OR | 95%CI |
| Age | 0.485 | 0.074 | 15.090 | 0.005 | 1.254 | 1.002-4.056 |
| Education level | 0.650 | 0.112 | 20.482 | 0.019 | 1.234 | 1.051-5.573 |
| Pregnancy mode | 0.253 | 0.145 | 2. 774 | 0.045 | 1.254 | 0.976-1.780 |
| ≤ 37 wk gestation | 0.504 | 0.256 | 3.157 | 0.086 | 1.643 | 0.949-2.954 |
| No. of pregnancies | 0.784 | 0.165 | 5.48 | 0.097 | 1.262 | 0.758-1.985 |
| Premature rupture of membranes | 0.864 | 0.142 | 16.751 | 0.011 | 1.318 | 1.185-9.254 |
GDM is a special type of diabetes with a morbidity of 17.5%-18.9%, and the morbidity increases with age[8]. GDM increases the morbidity of pregnancy-related complications, which could lead to adverse pregnancy outcomes such as premature delivery, cesarean section, macrosomia and premature rupture of membranes, thus attracting the attention of the majority of medical staff and pregnant women[9,10]. Some scholars have found that gestational age > 35 years, pre-pregnancy BMI, and family history of diabetes are all risk factors for GDM, which increasing the incidence of adverse pregnancy outcomes such as premature delivery, macrosomia and fetal distress[11,12]. Previous studies have mainly focused on the high-risk factors of GDM[13-15], and have confirmed that advanced age is a risk factor for GDM. However, there has been less research on older women with GDM. In this study, we retrospectively analyzed the clinical data of older and right-age pregnant women with GDM. The general situation, delivery mode, and maternal and neonatal outcomes were compared, and the delivery characteristics of women with GDM at different ages were discussed, aiming to improve pregnancy outcome in the older age group and providing a reference for ensuring the effect of eugenics in China.
The number of women with assisted pregnancy, ≤ 37 wk gestation, ≥ 2 pregnancies, one or more deliveries, and no pre-pregnancy blood glucose screening in the older group were all higher than those in the right-age group. The ovarian function of older women decreased significantly, and the ovarian reserve and egg quality decreased gradually, resulting in a decline in successful pregnancy rate. Assisted reproductive technology helps a large number of infertile older women conceive successfully, but the complications during pregnancy are higher than those of pregnant women who conceive naturally. With the implementation of the three-child policy in China, the number of older pregnant women is increasing gradually. Older women have a higher incidence of pregnancy complications, such as GDM, and higher risk of maternal and infant deaths than pregnant women at the right age have. Therefore, we should attach importance to the healthcare of older women during pregnancy and in the perinatal period. The results of the present study showed that the older group had a higher cesarean section rate of 77.67%, compared with 59.15% in the right-age group. The right age group had higher Apgar scores at 1 and 5 min after birth compared with the older group. In recent years, the rate of cesarean section has increased due to incorrect fetal position or prevention of fetal distress. There are many pregnancy complications in older women, uterine contraction is weaker, and the total labor process is prolonged. Therefore, natural delivery can increase maternal and neonatal risks. This is one of the reasons why older women choose cesarean section, but this increases the risk of maternal and neonatal infection and thromboembolism[8,16-18]. With increasing age, pregnant women are prone to obesity, and various bodily functions gradually decrease. In older pregnant women with GDM, the incidence of fetal macrosomia is high. We showed that older women had a higher incidence of polyhydramnios and postpartum hemorrhage than the right-age women had, which confirms that GDM is closely related to maternal and infant adverse outcomes. According to previous studies, older age pregnancy has a higher risk of GDM[19-21]. Older age can also increase the probability of gestational hypertension, perinatal complications and other diseases. The present study found that the older group had a lower incidence of fetal distress than the right-age group had, which may be related to the high rate of cesarean section in the older group. The incidence of fetal distress was reduced due to the high proportion of women with ≤ 37 wk gestation in the older group.
To control the interference between the various indicators and analyze the correlation between related indicators and maternal and infant adverse outcomes in older women with GDM, logistic regression analysis was conducted. Logistic regression showed that age, education level and premature rupture of membranes were risk factors for maternal and infant adverse outcomes in older women with GDM. Pregnant women aged > 35 years old are more likely to have pregnancy complications during pregnancy or delivery. Laura estimated the risk of adverse outcome over a pregnancy cycle of 3-24 mo based on the maternal age at the initial birth (20-34 years and ≥ 35 years)[20]. The risk of maternal mortality or serious morbidity at 6 mo of pregnancy was increased compared to the 18-mo pregnancy cycle of women aged ≥ 35 years. Women aged 20-34 years had an increased risk of spontaneous preterm birth within 6 mo of pregnancy. In the present study, with the increase in academic qualifications, the proportion of maternal and infant adverse outcomes in older pregnant women with GDM increased, and there was a significant difference compared with the good outcome group. Education level is a risk factor for maternal and infant adverse outcomes in older women with GDM. The reason may be that the higher the educational background is, the later the childbearing age is, which affects fertility and reproductive quality. Premature rupture of membranes induces serious adverse effects in both the mother and fetus. In premature rupture of membranes, the reproductive tract loses its protective barrier, and the amniotic fluid gradually decreases, which affects the blood circulation of the placenta and increases proneness to adverse outcomes such as fetal distress[22,23]. Obstetric medical staff should focus on monitoring pregnant women with high-risk factors of premature rupture of membranes and implement timely intervention measures.
The limitation of this study was that the subjects were from a single institution, which limits the extrapolation of research results. More eligible samples will be included in future studies and more disease-related data will be analyzed, to enhance the reliability and validity of the results and provide a basis for treatment.
In summary, the delivery mode and neonatal Apgar score are related to the age of pregnant women with GDM and advanced age increases the adverse outcomes in mothers and infants. Therefore, to improve the pregnancy outcome and reduce the incidence of complications in pregnant women with GDM, it is suggested that pregnant women with a family planning plan should have pre-pregnancy eugenics health examination and pregnancy health care.
Gestational diabetes mellitus (GDM) is one of the serious pregnancy complications, which severely threatens the health of pregnant women and newborns. In recent years, with the increased childbearing age and proportion of overweight people, the incidence of GDM has an upward trend. The detrimental effect of GDM on the prognosis has been recognized, which can increase the incidence of dystocia, cesarean section and macrosomia, and 17%-63% of pregnant women and infants will develop type 2 diabetes in the long term.
We analyzed the risk factors affecting the adverse outcomes of mothers and infants, we also put forward targeted prevention and control measures to provide reference for formulating GDM early prevention and intervention policies.
To explore the relationship between the age of GDM pregnant women and the delivery mode and neonatal Apgar score, so as to provide a theoretical basis for reducing the incidence of adverse pregnancy outcomes.
We used the latest diagnostic criteria of GDM to investigate pregnant women who met the inclusion criteria, and collected their general conditions before and during pregnancy and related clinical data. The women were divided into right age group and older group according to whether they were older than 35 years old. Logistic regression analysis was used to analyze the related risk factors affecting the delivery outcome of GDM pregnant women.
The older group had a higher cesarean section rate, higher incidence of polyhydramnios and postpartum hemorrhage, and lower incidence of fetal distress than the right age group. The right age group had higher Apgar scores at 1 and 5 min after birth than the older group. Moreover, age, education level and premature rupture of membranes were risk factors for adverse pregnant outcomes in older GDM women. Our results showed that gestational age greater than 35 years old will increase the incidence of gestational diabetes and adverse pregnancy outcomes, but it needs to be confirmed by large samples and studies involving patients in multiple research centers.
The age of pregnant women with GDM affects the delivery mode and neonatal Apgar score, and advanced age increases the adverse pregnancy outcomes. Therefore, it is suggested that pregnant women should have pre-pregnancy eugenics health examination and pregnancy health care.
In the future research, we want to explore the effective means of screening GDM in pregnancy physical examination, as well as the means of early intervention and treatment for pregnant women with high-risk factors of GDM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elshazly AHM; Karalliedde J, United Kingdom; Ortega AL, Spain S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Dall TM, Yang W, Gillespie K, Mocarski M, Byrne E, Cintina I, Beronja K, Semilla AP, Iacobucci W, Hogan PF. The Economic Burden of Elevated Blood Glucose Levels in 2017: Diagnosed and Undiagnosed Diabetes, Gestational Diabetes Mellitus, and Prediabetes. Diabetes Care. 2019;42:1661-1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Drehmer M, Silveira L, Bracco P, Schmidt MI. Postpartum weight retention and pregnancy weight gain in women with Gestational Diabetes Mellitus (GDM). Int J Epidemiol. 2021;50:1311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Li H, Song L, Shen L, Liu B, Zheng X, Zhang L, Wang Y, Cao Z, Xu S. Height and Risk of Gestational Diabetes Mellitus: Results from the Healthy Baby Cohort Study. J Diabetes Res. 2018;2018:4679245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Remneva OV, Rozhkova OV, Cherkasova TM, Korenovskiy YV, Gurevich NL. Clinical, metabolic and neurological disorders in full-term newborns from mothers with gestational diabetes mellitus. Rossiyskiy Vestnik Perinatologii i Pediatrii (Russian Bulletin of Perinatology and Pediatrics). 2021;66:46-51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Corrado F, Pintaudi B. Diagnosis of Gestational Diabetes Mellitus: Italian Perspectives on Risk Factor-Based Screening. Nutrition and Diet in Maternal Diabetes. 2018;87-97. [DOI] [Full Text] |
| 6. | KhushBakht D, Mazhar S, Bhalli A, Rashid A, Khan K, Jahanzaib U. Correlation Between Neck Circumference and Gestational Diabetes Mellitus and Associated Risk Factors During Pregnancy. Cureus. 2018;10:e2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Harreiter J, Simmons D, Desoye G, Corcoy R, Adelantado JM, Devlieger R, van Assche A, Galjaard S, Damm P, Mathiesen ER, Jensen DM, Andersen LL, Dunne F, Lapolla A, Dalfra MG, Bertolotto A, Mantaj U, Wender-Ozegowska E, Zawiejska A, Hill D, Jelsma JG, Snoek FJ, Worda C, Bancher-Todesca D, van Poppel MN, Kautzky-Willer A; DALI Core Investigator group. IADPSG and WHO 2013 Gestational Diabetes Mellitus Criteria Identify Obese Women With Marked Insulin Resistance in Early Pregnancy. Diabetes Care. 2016;39:e90-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Karasneh RA, Migdady FH, Alzoubi KH, Al-Azzam SI, Khader YS, Nusair MB. Trends in maternal characteristics, and maternal and neonatal outcomes of women with gestational diabetes: A study from Jordan. Ann Med Surg (Lond). 2021;67:102469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Yoles I, Sheiner E, Wainstock T. First pregnancy risk factors and future gestational diabetes mellitus. Arch Gynecol Obstet. 2021;304:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Bayoumi MAA, Masri RM, Matani NYS, Hendaus MA, Masri MM, Chandra P, Langtree LJ, D'Souza S, Olayiwola NO, Shahbal S, Elmalik EE, Bakry MS, Gad AI, Agarwal R. Maternal and neonatal outcomes in mothers with diabetes mellitus in qatari population. BMC Pregnancy Childbirth. 2021;21:651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hiersch L, Berger H, Okby R, Ray JG, Geary M, McDonald SD, Murray-Davis B, Riddell C, Halperin I, Hasan H, Barrett J, Melamed N; DOH-NET (Diabetes, Obesity and Hypertension in Pregnancy Research Network); SOON (Southern Ontario Obstetrical Network) Investigators. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am J Obstet Gynecol. 2019;220:102.e1-102.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Shin D, Lee KW. High pre-pregnancy BMI with a history of gestational diabetes mellitus is associated with an increased risk of type 2 diabetes in Korean women. PLoS One. 2021;16:e0252442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Nath J. Gestational diabetes mellitus and perinatal complications - A clinical study. Indian J Obstet Gynaecol. 2017;4:120-122. [DOI] [Full Text] |
| 14. | Ushanova FO, Lobanova KG, Perekhodov SN. Gestational diabetes mellitus: peculiarities of course and pregnancy outcomes in real clinical practice. Meditsinskiy sovet = Medical Council. 2021;184-191. [DOI] [Full Text] |
| 15. | Balagopalan N, Pathak R, Islam F, Nigam A, Agarwal S. Diagnostic accuracy of diabetes in pregnancy study group of india criteria for the screening of gestational diabetes mellitus in primary care setting. Indian J Commun and Fam Med. 2021;7:25. [DOI] [Full Text] |
| 16. | Bianchi C, de Gennaro G, Brocchi A, Minaldi E, Del Prato S, Bertolotto A. Risk factors associated with postpartum impaired glucose regulation in women with previous gestational diabetes. J Diabetes Complications. 2021;35:107854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Getaneh T, Asres A, Hiyaru T, Lake S. Adverse perinatal outcomes and its associated factors among adult and advanced maternal age pregnancy in Northwest Ethiopia. Sci Rep. 2021;11:14072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Biagioni EM, May LE, Broskey NT. The impact of advanced maternal age on pregnancy and offspring health: A mechanistic role for placental angiogenic growth mediators. Placenta. 2021;106:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021;70:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Schummers L, Hutcheon JA, Hernandez-Diaz S, Williams PL, Hacker MR, VanderWeele TJ, Norman WV. Association of Short Interpregnancy Interval With Pregnancy Outcomes According to Maternal Age. JAMA Intern Med. 2018;178:1661-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Yeim KO, Snmez E. Impact of maternal age on performance of the progeny in Galleria mellonella (L. 1758) (Lepidoptera: Pyralidae). Turkish J Ent. 2021;45:33-38. [DOI] [Full Text] |
| 22. | Ruth H, Janine Z, Jana P, Yvonne H, Ekkehard S. Placenta inflammation, vaginal microbiome and early-onset neonatal sepsis (eons) after preterm premature rupture of membranes (pprom). Placenta. 2021;112:e66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Puhl A, Weiss C, Schneid A, Zahn E, Kraft K, Pretscher J, Faschingbauer F, Beckmann MW, Kehl S. [Does Induction of Labor for Preterm Premature Rupture of Membranes at 34 Weeks of Gestation Increase the Risk for Cesarean Section? Z Geburtshilfe Neonatol. 2020;224:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |