Published online Sep 15, 2022. doi: 10.4239/wjd.v13.i9.765
Peer-review started: April 29, 2022
First decision: May 29, 2022
Revised: June 9, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: September 15, 2022
Processing time: 133 Days and 13.4 Hours
The pancreatic islet microcirculation adapts its metabolism to cope with limited oxygen availability and nutrient delivery. In diabetes, the balance between oxygen delivery and consumption is impaired. Insulin has been proven to exert complex actions promoting the maintenance of homeostasis of the pancreas under glucotoxicity.
To test the hypothesis that insulin administration can improve the integrated pancreatic microcirculatory oxygen profile and bioenergetics.
The pancreatic microcirculatory partial oxygen pressure (PO2), relative hemoglobin (rHb) and hemoglobin oxygen saturation (SO2) were evaluated in nondiabetic, type 1 diabetes mellitus (T1DM), and insulin-treated mice. A three-dimensional framework was generated to visualize the microcirculatory oxygen profile. Ultrastructural changes in the microvasculature were examined using transmission electron microscopy. An Extracellular Flux Analyzer was used to detect the real-time changes in bioenergetics by measuring the oxygen consumption rate and extracellular acidification rate in islet microvascular endothelial cells (IMECs).
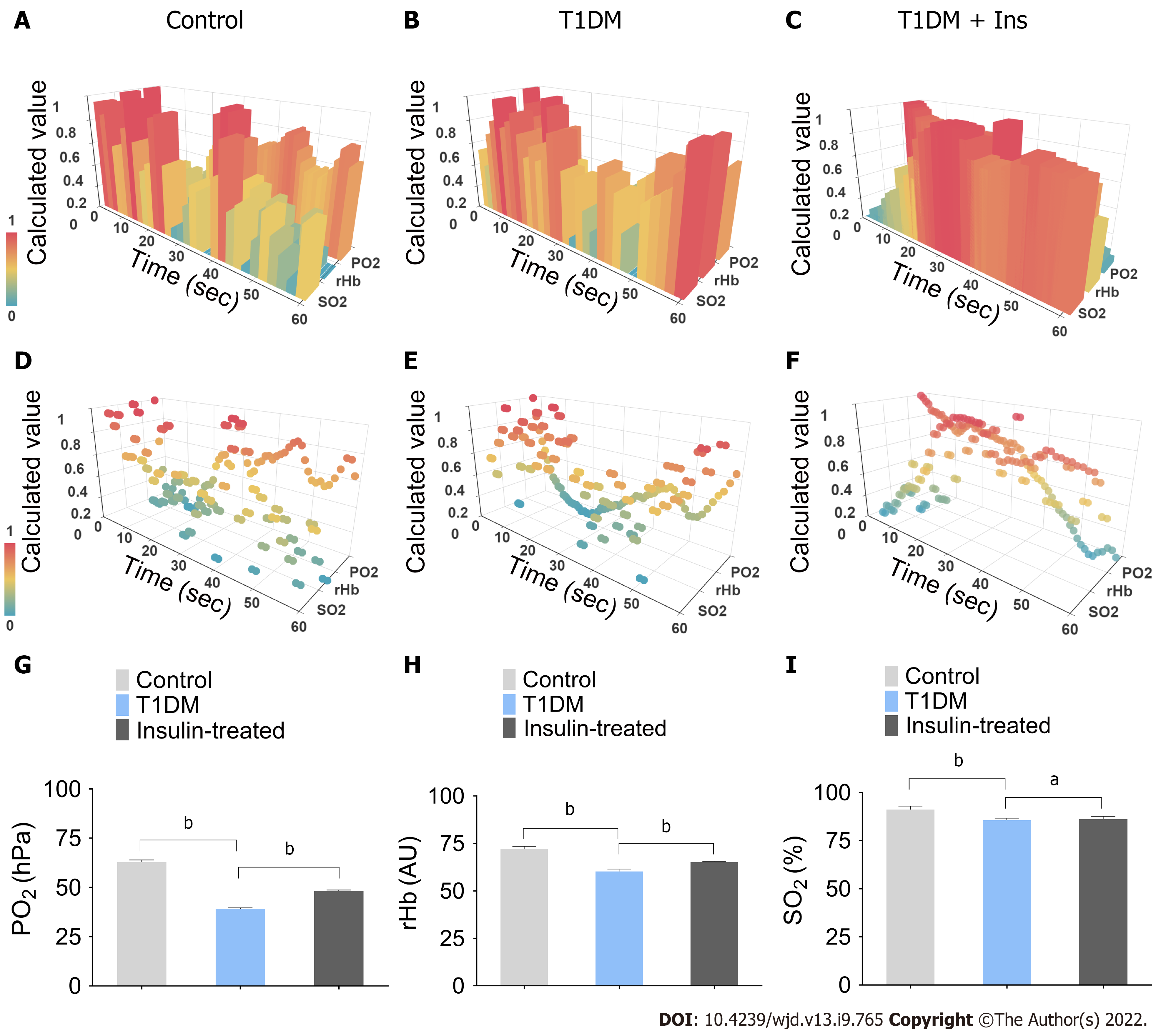
Significantly lower PO2, rHb, and SO2 values were observed in T1DM mice than in nondiabetic controls. Insulin administration ameliorated the streptozotocin-induced decreases in these microcirculatory oxygen parameters and improved the mitochondrial ultrastructural abnormalities in IMECs. Bioenergetic profiling revealed that the IMECs did not have spare respiratory capacity. Insulin-treated IMECs exhibited significantly greater basal respiration than glucotoxicity-exposed IMECs (P < 0.05). An energy map revealed increased energetic metabolism in insulin-treated IMECs, with significantly increased ATP production, non-mitochondrial respiration, and oxidative metabolism (all P < 0.05). Significant negative correlations were revealed between microcirculatory SO2 and bioenergetic parameters.
Glucotoxicity deteriorates the integrated pancreatic microcirculatory oxygen profile and bioenergetics, but this deterioration can be reversed by insulin administration.
Core Tip: The pancreatic islet microvasculature adapts its metabolism to cope with limited oxygen availability and nutrient delivery. Insulin has been proven to exert complex actions promoting the maintenance of homeostasis under glucotoxicity. Our findings demonstrate that insulin ameliorates the suppression of the integrated microcirculatory oxygen profile in type 1 diabetes mellitus mice and improves mitochondrial ultrastructural abnormalities in islet microvascular endothelial cells (IMECs). Additionally, insulin administration restores glucotoxicity-induced microcirculatory failure by increasing the mitochondrial basal respiration and glycolytic capacity of IMECs.
- Citation: Li BW, Li Y, Zhang X, Fu SJ, Wang B, Zhang XY, Liu XT, Wang Q, Li AL, Liu MM. Role of insulin in pancreatic microcirculatory oxygen profile and bioenergetics. World J Diabetes 2022; 13(9): 765-775
- URL: https://www.wjgnet.com/1948-9358/full/v13/i9/765.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i9.765
The concept of pancreatic islet microcirculation, which is currently under the spotlight[1,2], is responsible for coupling metabolic demands with glucose distribution and oxygen delivery in a manner involving microvascular endothelium-dependent vasodilation. Emerging evidence, including ours, indicates that the integrated pancreatic microcirculation in islets is necessary to maintain endocrine function and is involved in the pathogenesis of diabetes, partially through impairment of microcirculatory blood perfusion[3,4].
As part of a highly specialized microvascular system[5], pancreatic islets are richly vascularized and have 5-10-fold higher blood flow than the exocrine pancreas[6]. Pancreatic islet microvascular endothelial cells (IMECs) are therefore responsible for maintaining substance exchange and contribute to the dynamic regulation of glucose metabolism. Malfunction of IMECs is not only the culprit of deteriorated pancreatic islet microvascular blood perfusion and oxygen supply but also a victim of imbalanced energetic homeostasis.
Metabolic abnormalities in glucose are generally related to alterations in energy metabolism, especially at the onset of diabetes. The main organelle of IMECs responsible for energetic homeostasis is the mitochondrion, which plays a critical role in IMEC survival and death by regulating ATP synthesis through glucose metabolism, ROS generation, and apoptosis[7,8]. Furthermore, the metabolic profile of IMECs links the microcirculatory phenomena to the occurrence of pathological phenotypes. It is therefore important and rational to investigate the metabolic states of IMECs to clarify the microcirculatory pathological changes that occur under glucotoxicity.
Several reports have suggested the involvement of microcirculatory endothelial dysfunction in diabetes. However, knowledge surrounding the bioenergetics of IMECs related to insufficient microcirculatory oxygen is rather limited. We have established a new microcirculatory monitoring approach that integrates pancreatic microcirculatory partial oxygen pressure (PO2), relative hemoglobin (rHb) and hemoglobin oxygen saturation (SO2) using a multimodal device[9]. Thus, the purpose of this study was to describe the integrated pancreatic microcirculatory oxygen under glucotoxicity and to determine the impact of insulin on the microcirculatory oxygen and bioenergetic profile of IMECs.
BALB/c mice were obtained from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (CAMS). The mice were bred and housed at 22 ± 1 °C with 55%-65% humidity under a 12 h:12 h light:dark cycle. The mice were randomly divided into three groups, including a type 1 diabetes mellitus (T1DM) model group, an insulin-treated group and a nondiabetic control group (all n = 3). T1DM was established by intraperitoneal administration of streptozotocin (STZ, 40 mg/kg) into the mice for five consecutive days. A level of blood glucose higher than 200 mg/dL was considered to indicate diabetes. Insulin was subcutaneously injected (1.5 IU/day) into the mice in the insulin-treated group to maintain the blood glucose within the normal range[10]. The animal experiments in this study were permitted by the Laboratory Animal Welfare and Ethics Committee, Institute of Microcirculation, CAMS (China).
To assess pancreatic microcirculatory oxygen, we employed a multimodal auxiliary microcirculatory monitoring system established with a fiber-optic oxygen sensor (Precision Sensing, Regensburg, Germany) and an Oxygen to See device (LEA Medizintechnik, Giessen, Germany) to determine the SO2, rHb, and PO2. After anesthesia, the pancreas was gently exposed by a median abdominal incision, and the microcirculatory oxygen profile, including SO2, rHb, and PO2, was subsequently captured. These parameters were measured at three random sites of the pancreas in each mouse.
Python (ver 3.7.4) and Apache ECharts (ver 4.2.0-rc.2) were used to generate a three-dimensional framework to visualize the pancreatic microcirculatory oxygen profile. In the 3-D framework, the X-, Y-, and Z-axes represented the time course, microcirculatory oxygen variables, and calculated values of the microcirculatory oxygen profile, respectively. The outliers were adjusted by the box plot algorithm. Additionally, the least common multiple algorithm was used to adjust the time granularity. Min-max normalization was conducted to transform the dimensions of multiple parameters.
ScreenToGif (version 2.19.3) was used to capture the dynamic 3-D framework. Each video was recorded in an MPEG4 file. The bitrate was 2000 Kbps with a 1920 × 1080 resolution ratio.
Ultrastructural changes in the pancreatic islet microvasculature were examined using transmission electron microscopy (TEM). Fresh pancreatic tissue was fixed in 3% glutaraldehyde and 1% osmic acid and then passed through a graded series of dehydration and embedding solutions. Ultrathin sections (70 nm) were made after resin polymerization and uranyl acetate/Lead citrate staining. The samples were examined using a JEM-1400Plus transmission electron microscope (JEOL Ltd., Tokyo, Japan). The mitochondrial ultrastructure of IMECs was assessed.
The IMECs were purchased from ATCC (MS1, Manassas, VA, United States) and routinely cultured in DMEM supplemented with 10% FBS, 5.6 mmol/L glucose, 2% HEPES and 100 U of penicillin–streptomycin (Gibco, Carlsbad, CA, United States). After IMECs grew to confluence, the cells were divided into three groups, the control, glucotoxicity-exposed (HG, 25 mmol/L glucose), and insulin-treated (HG + Ins, 25 mmol/L glucose plus 10-8 mol/L insulin[10]) groups, and were treated for 24 h (n = 4 each group).
To investigate the effects of the high concentration of glucose with or without insulin on the bioenergetics of IMECs, an Extracellular Flux Analyzer (XFe24, Seahorse Bioscience, Billerica, MA, United States) was used to detect the real-time changes in energy pathways by directly probing the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). Briefly, IMECs were seeded in XFe24 culture plates at 1 × 104 cells per well. The cells were treated according to the abovementioned grouping for 24 h in DMEM with 0.5% FBS. The medium was subsequently replaced by Seahorse XF assay medium, and the cells were incubated without CO2 for 1 h until detection.
For the mitochondrial stress test, mitochondrial function was monitored along with the sequential addition of oligomycin, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) and rotenone/antimycin A (all 0.5 μM). Multiple respiratory indexes, including baseline metabolic functions (basal respiration, proton leak, ATP-linked respiration, non-mitochondrial respiration, and oxidative metabolism) and metabolic stress responses (maximum respiratory capacity [MRC] and spare respiratory capacity [SRC]), were analyzed and compared. In addition, an ECAR value was probed to indicate the basal glycolytic function when 10 mmol/L glucose was preadded into the medium before any pharmacological intervention. The ECAR-associated glycolytic capacity was subsequently reached after the injection of oligomycin. In this study, the values of both OCR and ECAR were normalized to 104 cells.
SPSS software 21.0 (IBM, Armonk, NY) was used to perform the statistical analyses. The data are shown as the mean ± standard error of the mean. The data sets were subjected to one-way ANOVA and post hoc multiple comparisons. A P value under 0.05 was considered to indicate statistical significance. In addition, the correlation between the microcirculatory oxygen profile and bioenergetics of IMECs was analyzed by Pearson's method.
In this study, the efficiency of STZ to induce T1DM mice model was 100%. To analyze the integrated microcirculatory oxygen profile of islet microcirculation, the preprocessed raw data were imported into the common microcirculatory framework. The oscillation of the microcirculatory oxygen profile is shown in histograms of the 3-D module (Figure 1A-C), and the distribution pattern of the microcirculatory oxygen profile was indicated using a scatter plot (Figure 1D-F, Videos 1-6). Loss of microcirculatory oxygen was observed in T1DM mice, which exhibited a significant decrease in PO2, rHb, and SO2 compared with nondiabetic controls. Additionally, insulin administration ameliorated the STZ-induced decreases in these microcirculatory oxygen parameters (Figure 1G-I).
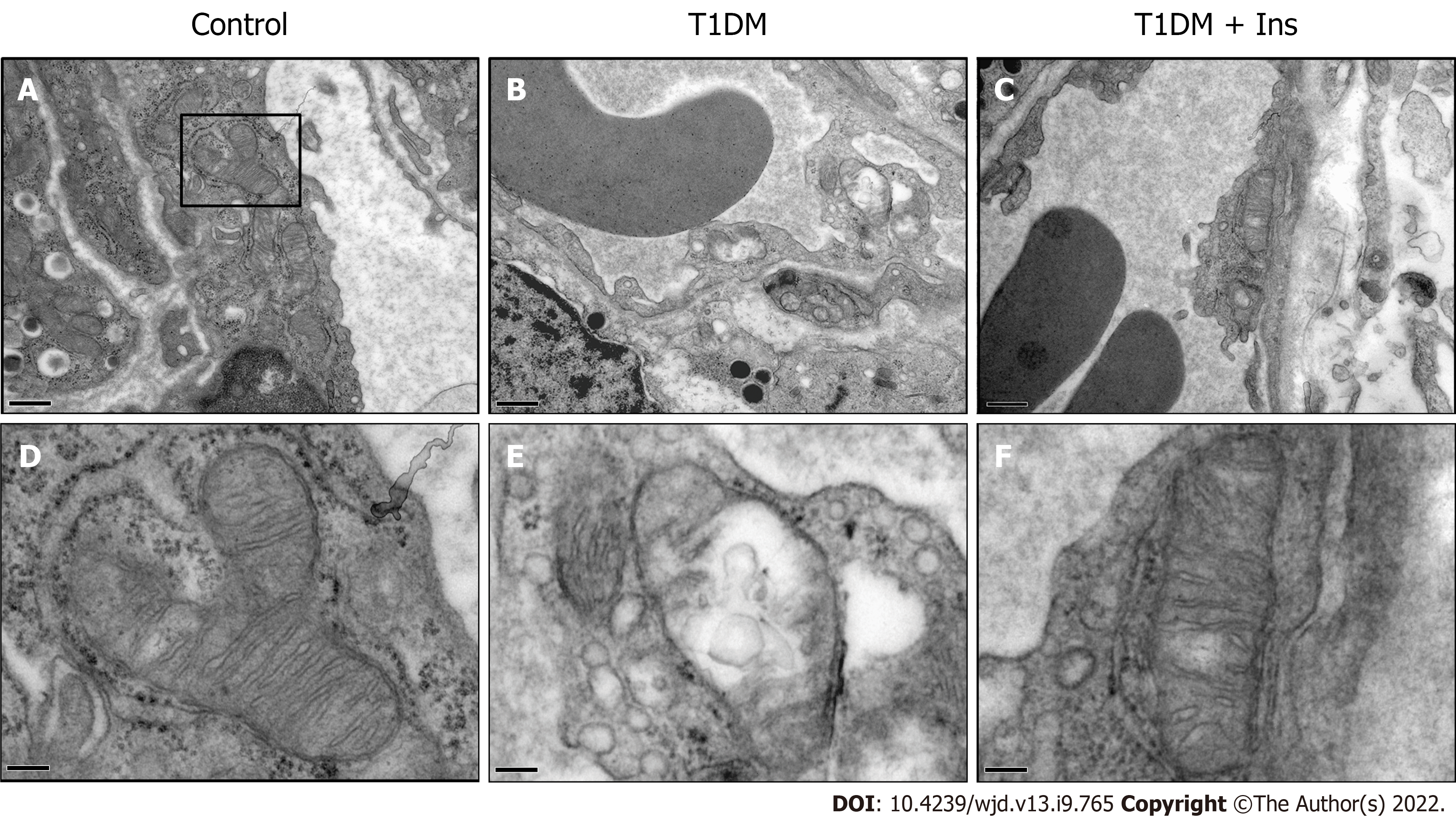
Given that microvessels are responsible for distributing oxygen, we sought to determine whether the mitochondrial ultrastructure of IMECs changes in T1DM mice. TEM revealed the normal architecture of IMECs in the nondiabetic control group, with ovoid nuclei and well-arranged mitochondria in the cytoplasm. In contrast, mice with T1DM showed a narrowed or closed lumen with a contorted and thickened basement membrane, nuclear disaggregation, and mitochondrial swelling, suggesting an impaired ultrastructure of mitochondria in IMECs. The mitochondria in insulin-treated IMECs were restored, with a thin basement membrane, wide capillary lumen, and well-arranged parallel cristae (Figure 2). These data confirm the protective effect of insulin in the microcirculation of T1DM mice.
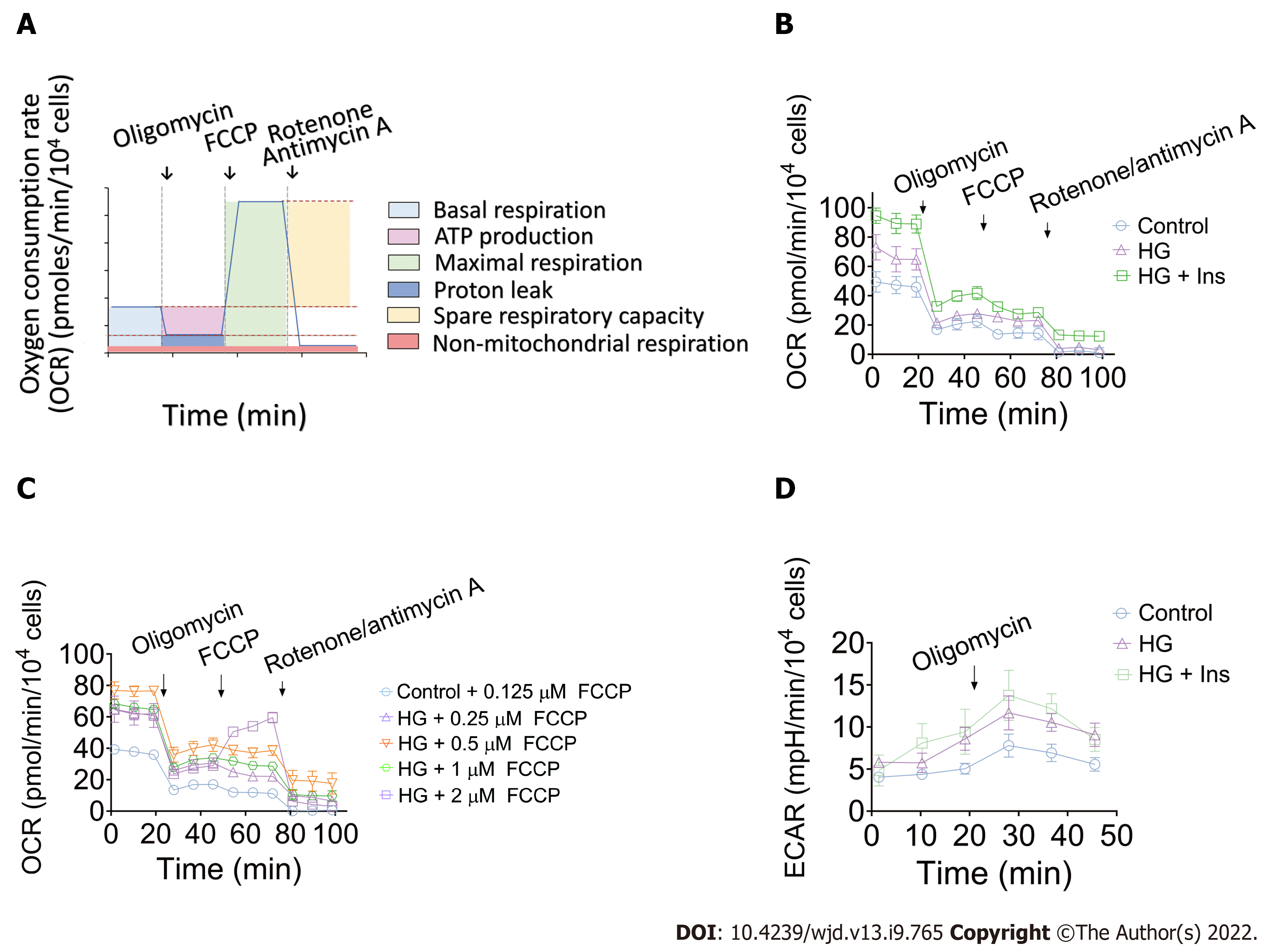
The tight integration between endothelial metabolism and microcirculatory oxygen transport begs for integrative analysis that spans the cellular scale. We then performed real-time analysis of OCR and ECAR to determine energetic metabolic features in IMECs. The OCR of the IMECs was determined before and after receiving interventions of oligomycin, FCCP, and rotenone/antimycin A. A schematic of the real-time analysis of the IMEC OCR is depicted in Figure 3A. Our data revealed comparable mitochondrial maximal respiration in control, glucotoxicity-exposed, and insulin-treated IMECs, which was not induced after the injection of FCCP (Figure 3B).
Subsequently, to determine whether FCCP concentration is responsible for the evaluation of the MRC, the IMECs were incubated with different concentrations of FCCP (0.125, 0.25, 0.5, 1, and 2 μM) in the control and HG groups. Surprisingly, none of the OCR values exceeded the corresponding basal OCR after the FCCP injections, suggesting that the IMECs do not have SRC (Figure 3C). The ECAR values were simultaneously measured to reflect the glycolytic activity of IMECs. After 0.5 μM oligomycin injection, the glycolytic capacity was recorded as the peak value of ECAR (Figure 3D).
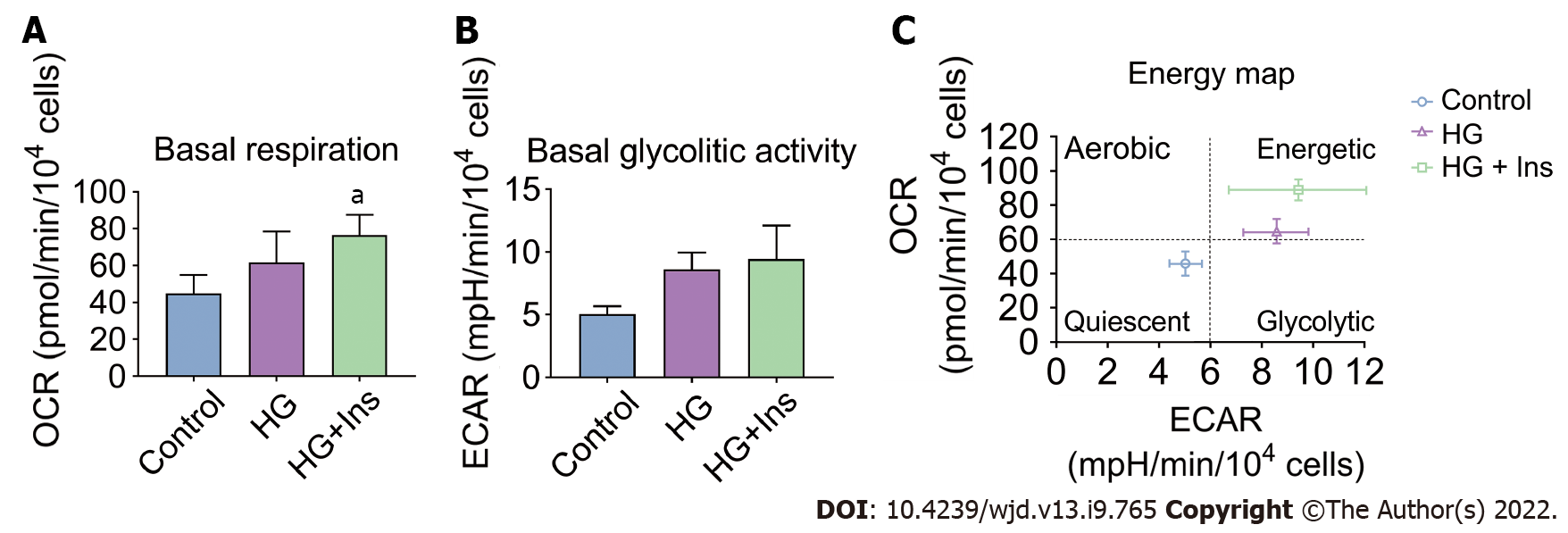
Insulin-treated IMECs exhibited significantly increased basal respiration in comparison with glucotoxicity-exposed IMECs (P < 0.05, Figure 4A). However, there were no significant differences in the basal glycolytic activity among the groups (all P > 0.05, Figure 4B). Moreover, an energy map was plotted based on the basal respiration and glycolytic activity in the IMECs. IMECs in the control group were in the quiescent quadrant (lower left). Glucotoxicity-exposed IMECs shifted to the energetic quadrant (upper right), reflecting increased mitochondrial activity. Insulin-administered IMECs were located in the right upper energetic quadrant, revealing more energetic metabolism (Figure 4C), suggesting that insulin increased the glycolytic activity of glucotoxicity-exposed IMECs when needed.
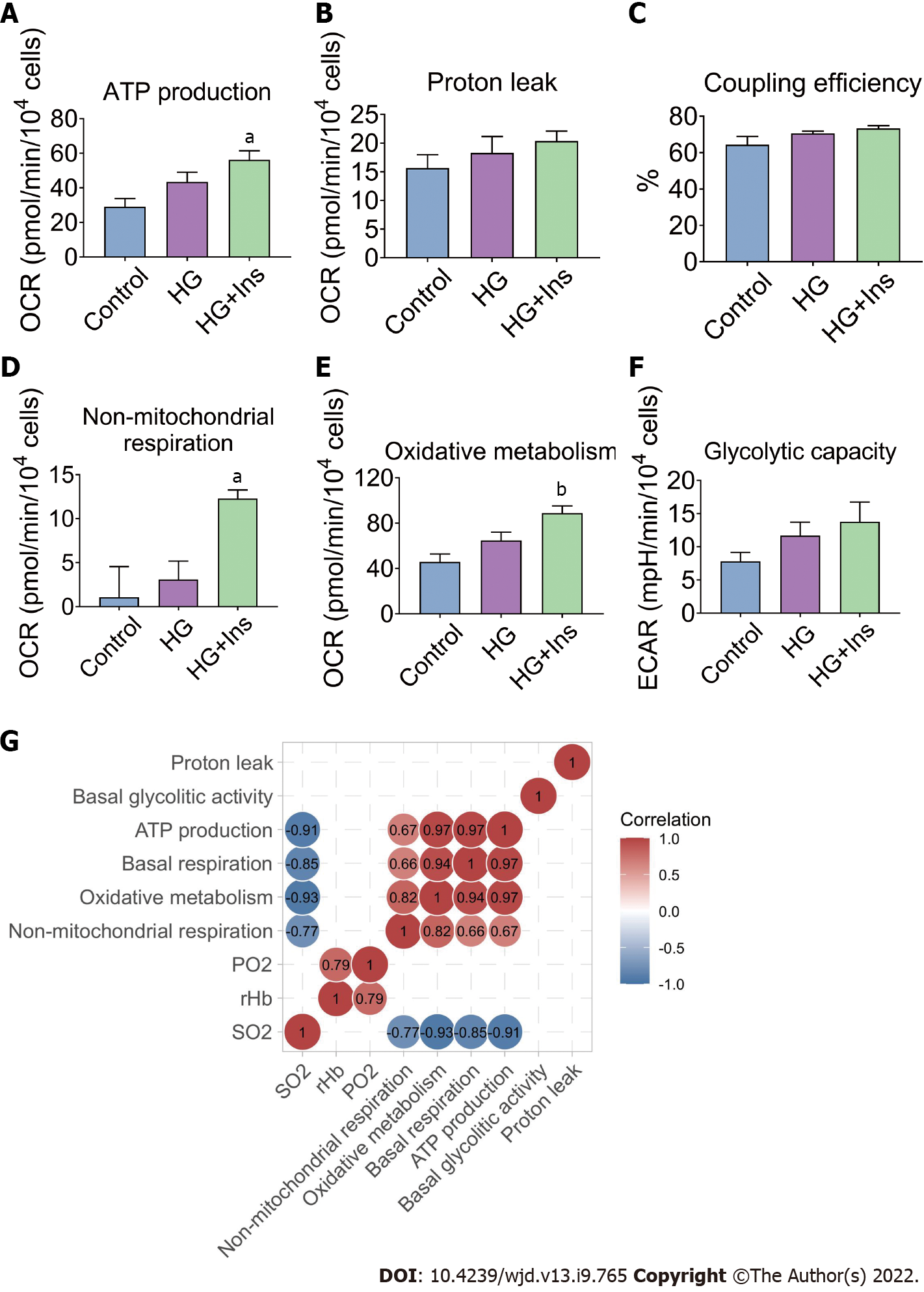
The basal respiration of mitochondria and non-mitochondrial respiration constitute oxidative metabolism in IMECs. Specific mitochondrial and non-mitochondrial functions were subsequently analyzed. ATP production, non-mitochondrial respiration, and oxidative metabolism were significantly increased in insulin-treated IMECs (P < 0.05, Figure 5A, D and E). However, proton leak (Figure 5B), coupling efficiency (Figure 5C), and endothelial glycolytic capacity (Figure 5F) were comparable among the groups.
The correlation between the microcirculatory oxygen profile and bioenergetics of IMECs was then analyzed. Significant negative correlations between microcirculatory SO2 and bioenergetic parameters (ATP production, basal respiration, oxidative metabolism, and non-mitochondrial respiration) were found by Pearson’s correlation analysis (Figure 5G). These lines of evidence further confirmed that glucotoxicity in IMECs was related to pancreatic islet microcirculatory failure that could be reversed by insulin administration.
The influence of microcirculatory disturbance on the development of diabetes mellitus has been highlighted over decades[11]. However, the current data associated with pancreatic microcirculatory oxygen profiles are deficient. Here, we used a computer algorithm-based common microcirculatory framework to reveal integrated pancreatic microcirculatory oxygen profiles among groups. The existence of microcirculatory hypoxia in T1DM was noted.
Considering islet β cells, rather than IMECs, are specific target of STZ. Therefore, STZ-involved T1DM animal models are useful in elucidating the mechanisms of diabetic microvascular endothelial pathogenesis. IMECs are the key determinants in pancreatic islet microcirculation homeostasis. Blood perfusion and oxygen transport requires the coordinated communication of mitochondria with metabolic demands, which is influenced by a variety of factors (including hypoxia)[12]. Coinciding with the impairment in the microcirculatory oxygen profile, pathological alterations in mitochondrial ultrastructure and other subcellular structures have been observed in IMECs of T1DM mice. Earlier studies have reported that defects in mitochondrial function correlate with mal-matching adenosine triphosphate generation[13,14], which interferes with the bioenergetics of pancreatic islet microcirculation. Treatment with insulin during glucotoxicity exposure resulted in restoration of the ultrastructure of IMECs. Thus, our data suggest that insulin can improve the functional status of pancreatic islet microcirculation.
Metabolic capacity is important for energy regulation and the maintenance of cell survival[15]. In parallel with damage to the ultrastructure of IMECs, biogenetic mechanisms act during glucotoxicity exposure to compensate for the decreased blood perfusion and oxygen distribution. IMECs supplied with insulin increase their basal respiration and ATP production and switch to energetic adaptation. Mitochondria are important organelles for ATP production[16]. Dysfunction of mitochondria is one of the key determinants in the pathogenesis of diabetes[17].
Unexpectedly, our results indicated that maximal respiration of the mitochondria was not induced after injection of FCCP. Multiple factors are associated with FCCP-induced maximal respiration of mitochondria[18]. Therefore, to exclude the effect of FCCP concentration, we subsequently tested five FCCP concentrations, but none caused the basal OCR value to be exceeded, suggesting that the IMECs do not have SRC. In addition to the organ- and tissue-specific nature of microvascular endothelial cells[19], one of the possible explanations is that IMECs generate more than 85% of their ATP through glycolysis[20], which does not require an excessive number of mitochondria to obtain energy.
Furthermore, the increased OCR was associated with non-mitochondrial respiration, suggesting the existence of extensive ROS signaling caused by increased enzymatic activity of nitric oxide synthases, NADPH oxidase, and other oxygenases[21,22]. Although the glycolytic metabolism of endothelial cells is a protective strategy against oxidative stress[14], insulin can increase ROS production via activation of non-mitochondrial respiration in vitro. The excessive ROS levels and increased oxidative stress may lead to mitochondrial dysfunction[23] and endothelial dysfunction[24]. In this energy-demanding process, quiescent endothelial cells divide and migrate to form new vessels[25], and excessive ROS synthesis inhibits angiogenesis by inducing excessive ROS synthesis[26].
Similar to basal respiration, the basal glycolytic activity increases when insulin is present, although no significant difference was noted. An in vitro study indicated that insulin, in the context of high glucose, significantly activates oxidative metabolism other than glycolysis in IMECs, although endothelial cells are considered “glycolysis addicted”[27]. The OCR measurements for oxidative metabolism can be divided into three components, including OCR associated with ATP production, proton leak, and non-mitochondrial respiration; the first two indicators together constitute the basal respiration of the mitochondria. Increased ATP production-associated OCR was found in IMECs after insulin treatment, suggesting that mitochondrial energy metabolism participates in the regulatory effects of insulin on microvascular endothelial mitochondrial injury.
The unique role of mitochondria in endothelial cells implies that a cell-regulatory function other than their energy-providing function is dominant[28]. Our previous study indicated that the microvascular blood perfusion of pancreatic islets was significantly decreased in T1DM mice but was partially restored after the administration of insulin[4]. Negative correlations were observed between the microcirculatory oxygen profile and metabolic indexes in the control group. In addition, a relatively low level of mitochondrial metabolism was detected in glycolysis-addicted IMECs, suggesting that glucotoxicity broke the negative correlation due to decreases in microcirculatory perfusion and the oxygen profile.
The current study is the first report on the relationship between pancreatic microcirculatory oxygen profile and microvascular endothelial mitochondrial metabolism. However, there are still several limitations. First, the sample size of mice in each group was limited. Although pancreatic microcirculatory oxygen profile was measured at three random sites of the pancreas in each mouse, large sample size is preferred to ensure the data are representative. Second, in an interdependent functional relationship with β cells, IMECs are involved not only in the delivery of oxygen, but affect adult β cell function and promote β cell proliferation via vasoactive substances. However, the phenotypic and functional crosstalk between IMECs and islet β cells are not involved in our study.
In conclusion, glucotoxicity deteriorates the integrated pancreatic microcirculatory oxygen profile and bioenergetics, but this deterioration can be reversed by insulin administration.
The pancreatic islet microcirculation adapts its metabolism to cope with limited oxygen availability and nutrient delivery. In diabetes, the balance between oxygen delivery and consumption is impaired. Insulin has been proven to exert complex actions promoting the maintenance of homeostasis of the pancreas under glucotoxicity.
We tried to provide new insight into the relationship between pancreatic microcirculatory oxygen profile and microvascular endothelial mitochondrial metabolism.
To test the hypothesis that insulin administration can improve the integrated pancreatic microcirculatory oxygen profile and bioenergetics.
A three-dimensional framework was generated to visualize the pancreatic microcirculatory oxygen profile. The microcirculatory partial oxygen pressure (PO2), relative hemoglobin (rHb) and hemoglobin oxygen saturation (SO2) were evaluated in nondiabetic, type 1 diabetes mellitus (T1DM), and insulin-treated mice. An Extracellular Flux Analyzer was used to detect the real-time changes in bioenergetics by measuring the oxygen consumption rate and extracellular acidification rate in islet microvascular endothelial cells (IMECs).
Insulin administration ameliorated the glucotoxicity-induced decreases in microcirculatory oxygen parameters (PO2, rHb, and SO2) and improved the mitochondrial ultrastructural abnormalities in IMECs. Insulin-treated IMECs exhibited significantly greater basal respiration than glucotoxicity-exposed IMECs. An energy map revealed increased energetic metabolism in insulin-treated IMECs, with significantly increased ATP production, non-mitochondrial respiration, and oxidative metabolism. Significant negative correlations were revealed between microcirculatory SO2 and bioenergetic parameters.
Glucotoxicity deteriorates the integrated pancreatic microcirculatory oxygen profile and bioenergetics, but this deterioration can be reversed by insulin administration.
Our understanding of the physiology and pathology of the pancreas islet microvascular endothelial cell in T1DM has been continually enhanced with the advancement of microcirculatory technology in parallel with rapidly developing bioenergetics that allows us to increase resolution and precision in our investigations.
We thank Xi-Nan Duan for discussions about the computational algorithms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diane A, Qatar; Jovandaric M, Serbia; Trujillo X, Mexico S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Dybala MP, Gebien LR, Reyna ME, Yu Y, Hara M. Implications of Integrated Pancreatic Microcirculation: Crosstalk between Endocrine and Exocrine Compartments. Diabetes. 2020;69:2566-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Dybala MP, Kuznetsov A, Motobu M, Hendren-Santiago BK, Philipson LH, Chervonsky AV, Hara M. Integrated Pancreatic Blood Flow: Bidirectional Microcirculation Between Endocrine and Exocrine Pancreas. Diabetes. 2020;69:1439-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Liu M, Zhang X, Li A, Wang B, Li B, Liu S, Li H, Xiu R. Insulin treatment restores islet microvascular vasomotion function in diabetic mice. J Diabetes. 2017;9:958-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Li Y, Li B, Wang B, Liu M, Zhang X, Li A, Zhang J, Zhang H, Xiu R. Integrated pancreatic microcirculatory profiles of streptozotocin-induced and insulin-administrated type 1 diabetes mellitus. Microcirculation. 2021;28:e12691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Muratore M, Santos C, Rorsman P. The vascular architecture of the pancreatic islets: A homage to August Krogh. Comp Biochem Physiol A Mol Integr Physiol. 2021;252:110846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Jansson L, Carlsson PO. Pancreatic Blood Flow with Special Emphasis on Blood Perfusion of the Islets of Langerhans. Compr Physiol. 2019;9:799-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Wai T, Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol Metab. 2016;27:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 956] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 8. | Bertero E, Maack C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ Res. 2018;122:1460-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 410] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 9. | Li Y, Song X, Liu M, Wang B, Zhang J, Li A, Zhang H, Xiu R. Multimodal Device and Computer Algorithm-Based Monitoring of Pancreatic Microcirculation Profiles In Vivo. Pancreas. 2020;49:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wang B, Zhang X, Liu M, Li Y, Zhang J, Li A, Zhang H, Xiu R. Insulin protects against type 1 diabetes mellitus-induced ultrastructural abnormalities of pancreatic islet microcirculation. Microscopy (Oxf). 2020;69:381-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 12. | Leone TC, Kelly DP. Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb Symp Quant Biol. 2011;76:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36:2187-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 14. | Li X, Sun X, Carmeliet P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019;30:414-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 15. | Yetkin-Arik B, Vogels IMC, Nowak-Sliwinska P, Weiss A, Houtkooper RH, Van Noorden CJF, Klaassen I, Schlingemann RO. The role of glycolysis and mitochondrial respiration in the formation and functioning of endothelial tip cells during angiogenesis. Sci Rep. 2019;9:12608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 16. | Caja S, Enríquez JA. Mitochondria in endothelial cells: Sensors and integrators of environmental cues. Redox Biol. 2017;12:821-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Pinti MV, Fink GK, Hathaway QA, Durr AJ, Kunovac A, Hollander JM. Mitochondrial dysfunction in type 2 diabetes mellitus: an organ-based analysis. Am J Physiol Endocrinol Metab. 2019;316:E268-E285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 18. | Rellick SL, Hu H, Simpkins JW, Ren X. Evaluation of Bioenergetic Function in Cerebral Vascular Endothelial Cells. J Vis Exp. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Minami T, Muramatsu M, Kume T. Organ/Tissue-Specific Vascular Endothelial Cell Heterogeneity in Health and Disease. Biol Pharm Bull. 2019;42:1609-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1133] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 21. | Nickel A, Kohlhaas M, Maack C. Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol. 2014;73:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Lyra-Leite DM, Andres AM, Cho N, Petersen AP, Ariyasinghe NR, Kim SS, Gottlieb RA, McCain ML. Matrix-guided control of mitochondrial function in cardiac myocytes. Acta Biomater. 2019;97:281-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Vandekeere S, Dubois C, Kalucka J, Sullivan MR, García-Caballero M, Goveia J, Chen R, Diehl FF, Bar-Lev L, Souffreau J, Pircher A, Kumar S, Vinckier S, Hirabayashi Y, Furuya S, Schoonjans L, Eelen G, Ghesquière B, Keshet E, Li X, Vander Heiden MG, Dewerchin M, Carmeliet P. Serine Synthesis via PHGDH Is Essential for Heme Production in Endothelial Cells. Cell Metab. 2018;28:573-587.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 24. | Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Brüning U, Scheinok S, Treps L, Cantelmo AR, Dubois C, de Zeeuw P, Goveia J, Zecchin A, Taverna F, Morales-Rodriguez F, Brajic A, Conradi LC, Schoors S, Harjes U, Vriens K, Pilz GA, Chen R, Cubbon R, Thienpont B, Cruys B, Wong BW, Ghesquière B, Dewerchin M, De Bock K, Sagaert X, Jessberger S, Jones EAV, Gallez B, Lambrechts D, Mazzone M, Eelen G, Li X, Fendt SM, Carmeliet P. Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018;28:881-894.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 25. | Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3471] [Cited by in RCA: 4013] [Article Influence: 286.6] [Reference Citation Analysis (0)] |
| 26. | Zhuo W, Song X, Zhou H, Luo Y. Arginine deiminase modulates endothelial tip cells via excessive synthesis of reactive oxygen species. Biochem Soc Trans. 2011;39:1376-1381, suppl 2 p following 1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Pircher A, Treps L, Bodrug N, Carmeliet P. Endothelial cell metabolism: A novel player in atherosclerosis? Atherosclerosis. 2016;253:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Kadlec AO, Beyer AM, Ait-Aissa K, Gutterman DD. Mitochondrial signaling in the vascular endothelium: beyond reactive oxygen species. Basic Res Cardiol. 2016;111:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |