Published online Sep 15, 2022. doi: 10.4239/wjd.v13.i9.738
Peer-review started: March 27, 2022
First decision: May 11, 2022
Revised: June 6, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 15, 2022
Processing time: 166 Days and 5.3 Hours
Diabetes mellitus is one of the most common comorbid conditions encountered in patients with severe acute respiratory syndrome coronavirus 2 infection accompanied by significantly increased mortality, prolonged hospital stay, and requirement of invasive mechanical ventilation. This review aims to present the effectiveness and safety profile of available coronavirus disease 2019 (COVID-19) vaccines in people with diabetes as a potential cause of hesitancy for vaccination. Data from published research proves a robust immune response following immunization for COVID-19 in diabetic patients with substantial production of virus-neutralizing antibodies; however, the observed immune response was unequivocally weaker than that in individuals without diabetes. This observation was further enhanced by the findings that worse glycemic control was associated with more suppressed antibody production. In contrast, individuals with optimal glycemic control performed similarly to healthy controls. In addition to the need for strict glucose monitoring and adequate diabetes treatment, those findings reinforce the concept of diabetes-induced secondary immune deficiency and necessitate the application of booster doses to diabetic patients with priority. Nevertheless, after vaccination, reported adverse events were not different from those in the general population. No increase in severe adverse events was documented. While single case reports detected transient increases in blood glucose post-vaccination, more extensive trials could not replicate such a relationship.
Core Tip: Diabetes mellitus is a crucial contributor to coronavirus disease 2019 (COVID-19). This review highlights published research on the effectiveness of vaccination against COVID-19 and related adverse events. Despite data of a notable decrease in the immune response to vaccination of diabetic patients, studies point out the importance of strict glycemic control to achieve adequate immunity against severe acute respiratory syndrome coronavirus 2 and the need to prioritize people with diabetes for the administration of booster doses. Regarding adverse events, none were increased in frequency in the diabetic population, except sporadic transient hyperglycemia observed post-vaccination.
- Citation: Vasilev G, Kabakchieva P, Miteva D, Batselova H, Velikova T. Effectiveness and safety of COVID-19 vaccines in patients with diabetes as a factor for vaccine hesitancy. World J Diabetes 2022; 13(9): 738-751
- URL: https://www.wjgnet.com/1948-9358/full/v13/i9/738.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i9.738
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the novel coronavirus causing coronavirus disease 2019 (COVID-19) encountered since December 8, 2019 when the first cases of pneumonia of unknown origin etiology were described in Wuhan, Hubei Province, China[1]. Since then, the World Health Organization has reported more than 472 million confirmed cases of SARS-CoV-2 infection and more than 6 million deaths[2]. Closely related to SARS-CoV-2, the SARS-CoV pathogen causing a pandemic in 2002-2003 was also established to have originated in bats that probably serve as the natural reservoir host for those two viruses[3]. Another related betacoronavirus, the Middle East respiratory syndrome virus, is the cause of frequently emerging local outbreaks. It was first hypothesized that the Middle East respiratory syndrome coronavirus originated in bats. Still, it was then unanimously proved that the reservoir host was dromedary camels causing spillovers to humans[4]. Zoonotic transmission of novel coronaviruses to humans has been suggested to continue as more and more viruses are detected in bats and spill over to humans[5]. The fatality risk has been estimated to be 0.5% to 1.3% of all confirmed COVID-19 cases, with significantly higher rates in advanced age groups, reaching 18.4% in hospitalized patients over 80 years old[6,7].
Diabetes mellitus is a chronic metabolic disease characterized by impairеd glucose metabolism and increased blood sugar levels as a result of absolute insulin deficiency due to autoimmune destruction of the pancreatic beta-cells (type 1 diabetes and latent autoimmune diabetes of adulthood) or impaired insulin action and a progressive loss of adequate β-cell insulin secretion due to insulin resistance in the target tissues (type 2 diabetes)[8]. Besides increased blood glucose levels, diabetes is associated with numerous chronic microvascular and macrovascular complications, determining diabetes as a cardiovascular disease[8]. Moreover, diabetes is associated with chronic low-grade inflammation[9] and an increased risk of thrombotic events[10], dyslipidemia[11], and metabolic syndrome[12]. Finally, it is a common disease that affects approximately 537 million adults. More than 240 million adults live with undiagnosed diabetes[13]. Collectively, these facts are a prerequisite for determining diabetes as a major risk factor for COVID-19[14]. It is considered that diabetic patients are a high-risk population with a calculated six-times greater risk for hospitalization and twelve-times greater risk for death than the healthy population[15]. Therefore, guidelines clearly state that patients with diabetes are strongly recommended to be vaccinated against COVID-19[16].
It is now widely accepted that diabetes, ischemic heart disease, hypertension, and cerebrovascular disease are the most common chronic comorbid conditions in people suffering from severe COVID-19 and requiring intensive care unit admission[17]. Many studies suggest that prolonged hyperglycemia is related to the increased frequency and severity of any infection, not just COVID-19. A matched cohort study among English primary care patients found that 6% of infection-related hospital admissions and 12% of SARS-CoV-2-related deaths were attributable to diabetes. The incidence rate ratios were highest for soft tissue, bone, and joint infections and sepsis[18].
A retrospective observational study of clinical outcomes in 1122 confirmed cases of COVID-19 found that the mortality rate in patients with diabetes and/or uncontrolled hyperglycemia was 28.8%, significantly higher compared to 6.2% in patients without diabetes or hyperglycemia. Furthermore, the median length of stay was also longer among discharged survivors in diabetic or hyperglycemic patients. Both findings imply the need for meticulous hyperglycemia management in hospitalized COVID-19 patients[19].
Both type 1 and type 2 diabetes mellitus were associated with worse rates of all-cause mortality owing to the COVID-19 pandemic in a large population-based cohort study encompassing more than 98% of primary care practice patients in England[20]. It identified a steep and sizable relationship between HbA1c values and death outcomes. Compared to individuals with optimal HbA1c values (6.5%-7.0%), those with HbA1c > 10% had a dramatically increased in-hospital all-cause mortality (hazard ratio: 2.23; 95% confidence interval: 1.50–3.30; P < 0.0001 in type 1 diabetes and hazard ratio: 1.61; 95% confidence interval: 1.47–1.77; P < 0.0001 in type 2 diabetes), independent from other risk factors[20].
Obesity, being strongly associated with diabetes mellitus type 2, has been investigated as a critical factor in the immune dysregulation that accompanies severe COVID-19. For example, in a French center, the relative risk for the need for invasive mechanical ventilation was seven-fold greater in patients with a body mass index > 30 kg/m2 than those within the normal range of body mass index[21].
Similarly, it has been discovered that obesity alters the immune response to influenza infection and vaccination. Compared to vaccinated normal-weight individuals, vaccinated obese adults demonstrated double the relative risk for influenza or influenza-like infection, despite evidence of seroconversion. The hyperleptinemia and hyperinsulinemia accompanying the obese state contribute to T cell dysfunction, leading to impaired immune response[22].
Monocytes and macrophages appear to have a hallmark role in the dysregulated immune state of severe COVID-19 infections. Experimental data show that higher extracellular glucose concentrations promote sustained monocyte glycolysis, increased SARS-CoV-2 replication in antigen-presenting cells, and proinflammatory cytokine expression, leading to T-cell dysfunction and lung epithelial damage[23]. Therefore, it has been hypothesized that mitochondrial reactive oxygen species generation stimulated by SARS-CoV-2 leads to hypoxia-inducible factor alpha synthesis. Hypoxia-inducible factor alpha increased the expression of ACE2 (the entry point of SARS-CoV-2 into lung epithelial cells), interleukin-1β, tumor necrosis factor-α, interleukin-6, and interferons α, β, and λ in infected monocytes. Those findings suggest that hypoxia-inducible factor alpha is necessary to induce glycolysis and the consequent proinflammatory state of SARS-CoV-2-infected monocytes[23].
In addition to proinflammatory cytokines and coagulation factor modulation, SARS-CoV-2–induced reactive oxygen species production and viral activation of the renin-angiotensin system (leading to increased angiotensin II expression) lead to insulin resistance, hyperglycemia, and vascular endothelial injury, contributing to acute respiratory distress syndrome as well as cardiovascular, cerebrovascular thromboembolic complications, and disseminated intravascular coagulation[24].
Diabetes patients (both type 1 and type 2) are at an increased risk of significant complications from vaccine-preventable illnesses, including hospitalizations and death. Even properly managed diabetes could be associated with second immune deficiency and increased susceptibility to infections due to impaired cellular immune function. Diabetes patients are more likely to die from pneumonia, bacteremia, and meningitis. In line with this, immunization offers the most effective protection against vaccine-preventable illnesses. Therefore, in the next paragraph, we provide information about the routine and recommended vaccination of diabetes patients to emphasize the solid background behind the vaccines that can be used to improve patient and doctor confidence in the vaccines while decreasing vaccine hesitancy.
Moreover, vaccine side effects are often minor and resolve on their own. Severe adverse effects are quite uncommon. Given all the information above and the usually immunocompromised status of diabetes patients, many routine vaccines are officially recommended. For example, the National Health Service in Great Britain recommends the inactivated intramuscular vaccine against seasonal influenza for patients with diabetes types 1 and 2. This is because the risk of severe disease is higher for them than for people without diabetes[25].
The Centers for Disease Control and Prevention (CDC) gives the same recommendation according to seasonal influenza. All patients with diabetes from 6 mo are recommended for the inactivated intramuscular vaccine against the disease. The CDC does not recommend the live attenuated influenza vaccine, also known as the nasal spray, for people with diabetes types 1 and 2[26].
A multicenter, randomized, and controlled study from the Republic of Korea demonstrated the safety and effectiveness of trivalent subunit inactivated intramuscular influenza vaccine, which contained an A/California/7/2009 (H1N1)-like strain, an A/Victoria/361/2011 (H3N2)-like strain, and a B/Brisbane/60/2008-like strain[27]. The World Health Organization recommended the strains during the 2012-2013 influenza season. The scientists observed similar results of seroprotection rates against the A/H3N2 and the B strains in the diabetic and non-diabetic groups. However, the diabetic group had significantly lower rates than those in the non-diabetic controls for the A/H1N1 strain. In both groups, 1 mo after vaccination, the geometric mean titers and seroprotection rates had increased dramatically for all three virus strains (P < 0.001)[27]. According to this study, 6 mo after vaccination, differences in the immunogenicity profiles between the diabetic and the non-diabetic groups were proven, with the seroprotection rate much lower in the elderly diabetes group than in the elderly control group. The safety of the trivalent subunit inactivated intramuscular influenza vaccine was established during the study, and all the participants confirmed that the vaccine was well tolerated. The post-vaccination reactions were mild to moderate, with tenderness at the injection site being the most frequent local reaction. In the diabetes group, 34.3% of the patients reported this local adverse event compared to 45.3% in the control group (P < 0.001). From the systemic reactions, myalgia was most reported, followed by tiredness, headache, malaise, chills, and arthralgia[27].
Another highly recommended vaccine for patients with diabetes is the vaccine against pneumococcal disease. The CDC recommends the pneumococcal vaccine for all children younger than 2 years and all adults 65 years or older. In addition, adults aged 19 through 64 are recommended for vaccination if they have chronic illnesses (including diabetes), human immunodeficiency virus/acquired immunodeficiency syndrome, or cancer or smoke cigarettes[28].
In a randomized controlled trial among elderly adults with comorbidities, the 13-valent pneumococcal conjugate vaccine showed significantly higher vaccine efficacy among subjects with diabetes mellitus[29].
A German retrospective cohort study proved the effectiveness of the 23-valent polysaccharide vaccine for invasive pneumococcal disease provoked by Streptococcus pneumoniae 22/23 serotypes. Therefore, scientists have recommended increasing the vaccination coverage of 23-valent polysaccharide vaccine among elderly adults in Germany[30].
Herpes zoster is an infection that occurs after reactivation of the varicella-zoster virus and is most common in people older than 50 years who have age-related fading of the immune function and concomitant comorbidities[31]. Herpes zoster is more prevalent among people with diabetes mellitus[32]. There are currently two vaccines against herpes zoster, a live-attenuated vaccine and a recombinant zoster vaccine. The effectiveness of both vaccines resulted in a significant decrease in the incidence of the disease in the older adult population[33-35].
Regarding immune responses after vaccination, diabetes patients mounted an adequate B-cell immune response after influenza and the 23-valent polysaccharide vaccine[36]. However, they had a lower response to the hepatitis B vaccine[37]. All findings support the notion that vaccines for vaccine-preventable illnesses should be administered in a timely manner to diabetic patients, given that this population are susceptible to infection and have a higher risk of diabetes deterioration during infections.
Autoimmune inflammatory diseases are characterized by an abnormal immune response to self antigens[38]. The interactions between people with autoimmune diseases and SARS-CoV-2 infection are generally unexpected. The mechanisms underlying the possible complications and fatal outcomes are not fully understood. COVID-19, like other viral infections, has the potential to cause a flare, including in diabetes patients[39,40].
Although preliminary findings revealed that autoimmune diseases did not enhance the incidence of SARS-CoV-2 infection and severe disease[41], autoimmune disorders are associated with organ damage, chronic cardiovascular, metabolic and respiratory comorbidities, susceptibility to bacterial infections, and sometimes, B cell depletion therapy and usage of high-dose glucocorticoids. All these factors may enhance the risk of a poor prognosis for patients during the COVID-19 course[42]. As a result, COVID-19 preventive methods should focus on the unique group of autoimmune disease patients, with immunization against SARS-CoV-2 being one of the most promising approaches. Nonetheless, because of growing findings, their safety and efficacy should be primarily and regularly assessed in different patient populations, including patients with diabetes[43].
We will focus on vaccine hesitancy in patients with diabetes later. Still, aside from the fear of immunization-related autoimmunity, there is considerable evidence that the development of autoimmune diseases is influenced by a variety of other variables. In fact, because autoimmune illnesses can develop without immunizations, it is impossible to conclude that vaccines alone induce autoimmunity[44].
Also, people with autoimmune disorders are most concerned about whether the risk of disease flare or aggravation increases following immunization. However, more than 5000 studies confirmed that those with autoimmune illnesses were not at risk of aggravation or worsening conditions[45].
The approved COVID-19 vaccines have played the most significant role in the battle with the SARS-CoV-2 virus to reduce disease severity and mortality among those affected, especially those with diabetes. As of March 16, 2022, more than 10 billion doses of different COVID-19 vaccines have been administered worldwide, including booster doses[46]. So far, we know that although the COVID-19 vaccinations’ immunogenicity and efficacy in the autoimmune disease patient population may be lower than in healthy controls, they are typically comparable. Furthermore, data on the vaccines’ effectiveness in the autoimmune disease population of adults and children are lacking since only a few studies follow up on the duration of protection and different modalities of immune responses after immunization[47].
Vaccination is recommended as a priority for people with diabetes. The aim is to elicit a sustained immune response in the target population. There is evidence that glycemic control in diabetes significantly affects the immune response[48]. Therefore, it is important to determine whether glycemic disturbances occur before or after vaccination against COVID-19 in people with diabetes.
Monitoring blood glucose levels became critical during the COVID-19 pandemic because the data show two to three times higher hospitalizations and double the mortality rate among patients with simultaneous diabetes and COVID-19[49-51]. It also turned out that emerging diabetes, hyperosmolar hyperglycemic syndrome, and diabetic ketoacidosis could accompany post-COVID syndrome[52,53].
Very few studies have been conducted on how vaccination affects blood sugar levels. However, some effects of COVID-19 on glycolytic metabolism are already known[54]. Several cases of hyperglycemia followed by vaccination against COVID-19 were reported[53,55,56]. One diabetic woman and two diabetic men had post-vaccine hyperglycemia within 6 d of receiving the first dose of the Covishield vaccine (AstraZeneca). Hyperglycemia passed after about a month in the woman after treatment with a higher dose of metformin. At the same time, the two men achieved glycemic control in 3-15 d without an additional medication[56]. However, no association between vaccination and disturbed glycemic control was proven.
Another study reported hyperglycemia between the 20th and 36th days after the first dose of the AstraZeneca vaccine[55]. Similar conditions have also been reported following mRNA vaccines, Comirnaty (Pfizer/BioNTech) and Spikevax (Moderna)[53]. This case report demonstrated remarkably high blood sugar and HbA1c levels after vaccination in a patient with previously reasonable blood glucose control. However, this patient probably had undiagnosed diabetes since his two parents had type 2 diabetes and the patient himself had a clinical picture of insulin resistance[53,57].
Another retrospective study examined 96 adults over the age of 18 with type 1 diabetes before and after their first COVID-19 vaccination[58]. Fifty-nine percent of them had a significant deviation in blood glucose levels, which were controlled within 7-10 d after vaccination. Again, the data show no difference in the effects between the AstraZeneca and Pfizer vaccines.
There could be many reasons for fluctuations in blood glucose. Regarding existing studies, no excipients and/or adjuvants to vaccines have been reported to cause hyperglycemia, so that the condition could be related to the antigens in the vaccine against COVID-19. A possible mechanism for its occurrence is stimulating the immune system, which leads to a stress response. However, it is milder than usually occurs with COVID-19 infection. Different changes in the glycolytic pathway occur in COVID-19 infection in response to stress and lead to increased glucose levels in cells[54,59]. Stress also increases hormones such as adrenaline, cortisol, and/or glucagon that cause metabolism changes[60]. In addition, they affect the immune system by reducing the activity and number of natural killer cells and lymphocytes, decreasing antibodies and reactivating latent viral infections[61].
More research and patient results need to be analyzed to provide a clear and definitive answer about this temporary instability of blood glucose levels after the COVID-19 vaccination. Understanding changes in the glycolytic pathway associated with COVID-19 and/or after vaccination could help find a new treatment for this disease.
Clinical data support a strong response of the neutralizing antibodies in patients with diabetes after COVID-19[36]. However, patients should be consulted and prepared for possible hyperglycemia after the COVID-19 vaccination[60].
There is still no data on the effects on glucose levels after the second COVID-19 vaccination or booster dose. These studies are underway. The question remains if the immunity to vaccination against COVID-19 in people with diabetes would change or decrease.
Although there is a high incidence of diabetes among populations, during the COVID-19 vaccine studies, patients with diabetes are usually rolled out. Therefore, we rely on the data from real-life studies from vaccinations after the vaccine approval.
Soetedjo et al[62], in their systematic review, managed to cover eight studies with a total of 64468 patients and 5156 patients with diabetes[62]. The vaccines included were BNT162b2 vaccine (Pfizer/BioNTech), CoronaVac (Sinovac Life Sciences), Covishield™ (ChAdOx1-nCOV), and Covaxin™ (BBV-152). The effectiveness studies showed lower seropositivity and antibody responses following vaccination in diabetes patients than in healthy controls. This was observed from 1-4 wk after full COVID-19 vaccination.
The studies on the immunogenicity of COVID-19 patients with diabetes are presented in Table 1[63-72].
| Ref. | Type of study | Type and name of the vaccine | Participants | Efficacy/effectiveness | Adverse effects |
| Nomura et al[63] | Observational study | BNT162b2 | 12 from a total of 252, at a mean age of 43.9 yr | Lower antibody titers compared to non-diabetic subjects 3 mo post-vaccination | N/A |
| Lustig et al[64] | Longitudinal cohort study | BNT162b2 | 139 from a total of 2498, at a mean age of 47.7 yr; mostly healthcare workers | Substantial antibody response after 2 doses, but overall lower concentrations of IgG and IgA in diabetics compared to healthy adults | N/A |
| Van Praet et al[65] | Case-control study | BNT162b2 | 25 from a total of 75, at a mean age of 85 yr | Decreased cellular immune response only in individuals with diabetes or active malignancy in the studied population | N/A |
| Ali et al[66] | Cohort study | BNT162b2 | 81 | The BNT162b2 vaccine induced robust IgG and neutralizing antibody responses in people with and without T2DM. On average, diabetics had 13.86 BAU/mL less IgG and 4.42% less neutralizing antibodies compared to non-diabetics | N/A |
| Marfella et al[67] | Prospective observational study | BNT162b2, mRNA-1273, ChAdOx1-S | 251, of which 134 with optimal glycemic control and 117 with poor glycemic control | 21 d after the second dose, neutralizing antibody titers and CD4 Th1 cytokine responses were weaker in individuals with HbA1c > 7% compared to those with HbA1c < 7% whose titers were indistinguishable from those of healthy subjects | N/A |
| Singh et al[68] | Cross-sectional study | ChAdOx1-nCOV (Covishield), BBV-152 (Covaxin) | 52 from a total of 463 at a mean age of 44.8 yr | Amongst all studied comorbidities, people with T2DM had lower seropositivity rates compared to those without (84.6% vs 96.1%) | N/A |
| Sauré et al[69] | Surveillance study | CoronaVac, BNT162b2 | 4626 from a total of 59987 people from Chile’s population | IgG seropositivity was significantly lower in diabetics receiving the CoronaVac vaccine compared to healthy subjects | N/A |
| Piccini et al[70] | Retrospective cohort study | mRNA-1273BNT162b2 | 39 | In adolescents and young adults with T1DM, vaccination with either product was safe and did not influence glycemic control | No serious adverse events were reported |
| Watanabe et al[71] | Observational study | Pfizer/BioNTech BNT162b2 vaccine | 2 from a total of 66 at a mean age of 29 yr; mostly healthcare workers | Undetectable titers of anti-SARS-CoV-2 antibodies | N/A |
| Karamese and Tutuncu[72] | Cross-sectional study | CoronaVac | 49 from a total of 186 people, at a mean age of 70.4 yr | Significantly lower levels of anti-SARS-CoV-2 antibodies in diabetes patients than in the controls | N/A |
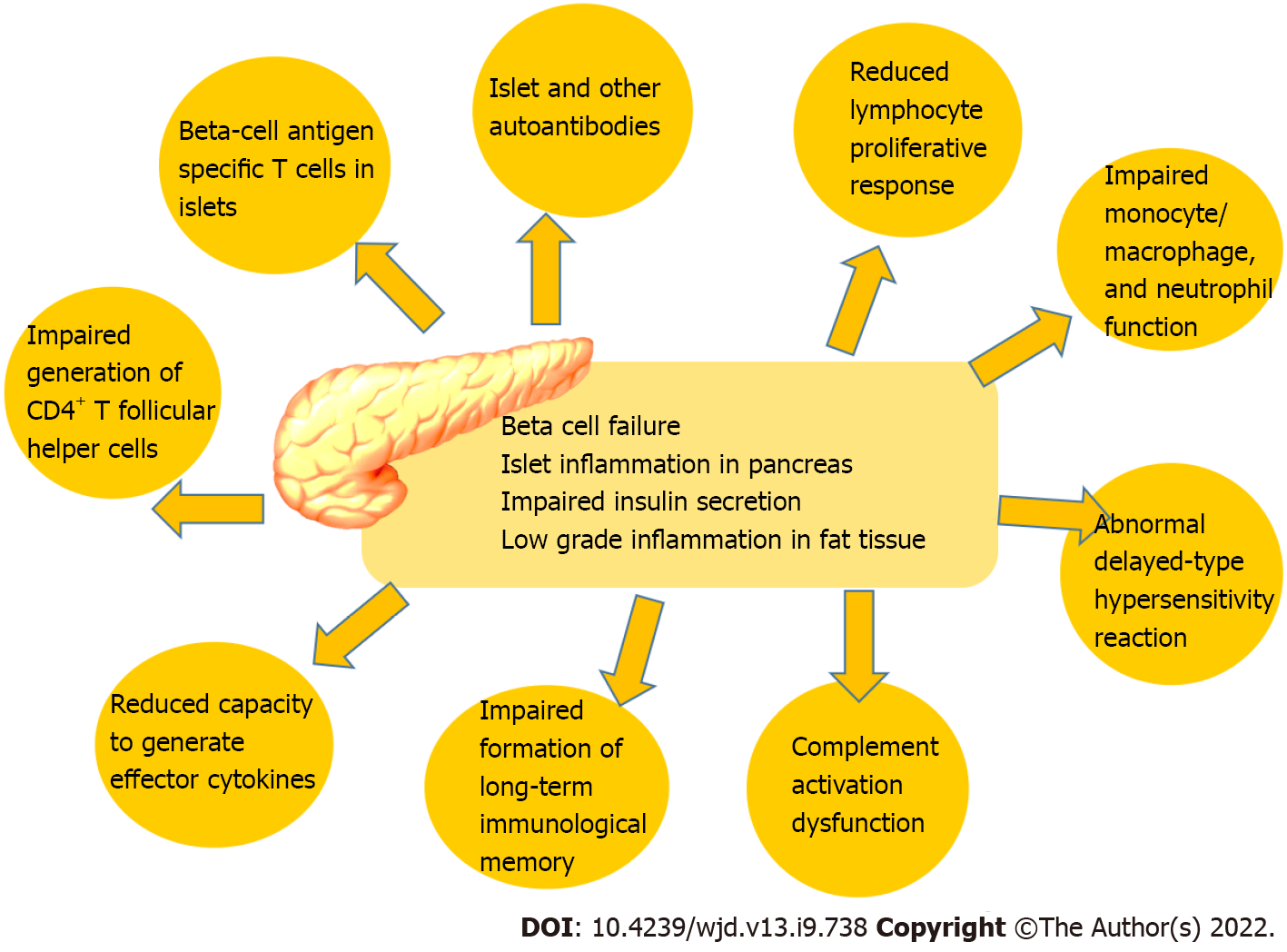
We can assume from the data that the seroconversion rates in diabetes patients following COVID-19 vaccination is lower, including lower antibody titers. However, the underlying reasons for that are not fully understood. It was proposed that impaired adaptive immune response in diabetes patients contributes to altered vaccination response[37]. Additionally, patients with diabetes had some immune alterations such as reduced circulating CD4+ cells, lymphocyte proliferation, and antigen presentation[67]. Immunological alterations in patients with diabetes are shown in Figure 1.
As we stated above, hyperglycemia at immunization may reduce the immune response. As a result, having sufficient glycemic control during the post-vaccination interval increases immunological response because strict glycemic control may predispose to a favorable immune response to the SARS-CoV-2 vaccine[67]. The host’s ability to respond to infections and the formation of long-term immunological memory, including correct responses to immunizations, are both influenced by the immune system’s steady degradation. Among other things, the adaptive immune system can be compromised by poor proliferation in response to antigenic stimulation, impaired generation of CD4+ T follicular helper cells, and a reduced capacity to generate effector lymphokines. Additionally, it is well-known that hyperglycemia induced glycosylated receptors on the immune cells lead to impaired immune cell function[67]. Immunological features and alterations of diabetes are shown in Figure 1.
In line with this, the leading cause of reduced immune response and protection after vaccination in diabetes patients remains relative immune deficiency. Other factors, such as poorly controlled diabetes, may indirectly impact the vaccines’ efficacy and effectiveness. Thus, we must pay attention to hyperglycemia, which can influence clinical COVID-19 results and vaccination efficacy. This leads us to assume that maintaining proper glycemic control after immunization increases immunological response. Also, we anticipate that strict glycemic management will support the favorable immunological response to the SARS-CoV-2 vaccination. Therefore, glycemic management should be the standard during pandemics, which strengthens the role of diabetologists in vaccination program effectiveness[67].
Additionally, different vaccines elicited comparable results, as shown in Table 1. This is also valid for the adverse effects demonstrated in the next section of the paper.
To sum up, the vaccines’ overall effectiveness could also be evaluated by the re-infection rate among patients with diabetes who were immunized against SARS-CoV-2. Generally, patients with diabetes were among the population of people with a higher risk of re-infection, both after natural infection or vaccination[73]. However, no particular numbers or percentages were cited for the re-infection rate after COVID-19 vaccination in diabetes patients, although the risk ratio for hospitalization due to re-infection was declared at 1.6[74]. The re-infection was less likely to occur in naturally infected or vaccinated people than in naïve patients. Therefore, the disease course was expected to be less severe in vaccinated diabetes patients.
Although the benefits of vaccination against COVID-19 in diabetic patients are undeniable, we will try to systematize the information gathered in the literature on the side effects of COVID-19 vaccines. A recent study analyzing the side effects of the two mRNA COVID-19 vaccines (BNT162b2 mRNA and mRNA-1273) among 1245 healthcare workers described general and organ-specific symptoms after the first and/or second dose of mRNA vaccines in the United States. The common endocrine symptoms were decreased appetite (5.73%), heat/cold intolerance (3.24%), increased thirst (1.12%), increased appetite (0.87%), and increased urine production (0.25%)[75]. Importantly, there are no reported symptoms associated with glucose metabolism; nevertheless, there is no information about diabetic participants in this study. Commonly reported symptoms were soreness, fatigue, myalgia, headache, chills, fever, joint pain, nausea, muscle spasm, sweating, dizziness, flushing, feeling of relief, brain fogging, anorexia, localized swelling, decreased sleep quality, itching, tingling, diarrhea, nasal stuffiness, and palpitations. Despite this extended list of symptoms, 79.7% of participants did not violate daily activities. In comparison, around 98.0% of them planned to have the second dose, and 92.9% had already received it[75].
An anaphylactic reaction is another reported side effect post-vaccination. The CDC reported in January 2021 that anaphylaxis might occur more frequently after the BNT162b2 mRNA vaccine than other vaccines[76]. According to this report, 11.1 per million was the estimated rate of anaphylaxis from 1893360 first doses of the Pfizer-BioNTech COVID-19 vaccine. The total reported adverse events after vaccination were 4393 (0.2%). Of them, only 175 cases were identified as potentially life-threatening allergic reactions, and 21 were reported as anaphylaxis. Most of the observed allergic reactions have manifested within the first 30 min of vaccination. Anaphylaxis usually occurs in individuals with a history of allergies or a previous anaphylactic episode. Again, there is no information related to glucose disturbances or predisposition for allergic reactions among diabetes patients[76].
Another CDC report on the side effects of the two mRNA vaccines showed that the most frequently reported symptoms after vaccination were headache, fatigue, and dizziness. The rate of anaphylaxis was defined as rare (4.5 reported cases per million doses administered). There were no data on side effects associated with glucose metabolism and no evidence that vaccine symptoms were more pronounced in diabetics[77].
Increased risk of myocarditis and pericarditis has been reported after mRNA COVID-19 vaccination (Pfizer-BioNTech and Moderna)[78-82] and rarely after adenovirus vector-related vaccine[83,84]. Detailed analyses of these cases showed that myocarditis and pericarditis were more often in adolescents and young adult males. In addition, they were associated with multiple comorbidities, including obesity and hyperlipidemia[85].
An Italian study reported that the most frequent adverse events observed post-vaccination were vagal response (30%), anxiety reaction (24%), and dizziness (21%) among a total of 314671 vaccinated subjects. These side effects were predominantly observed in women and people with comorbidities; however, it is unclear whether diabetes was included[86].
Another study analyzed the adverse effects among 447346 reports 2 wk after vaccination with one of following three COVID-19 vaccines: 19462 Ad26.COV2.S (Janssen COVID-19 vaccine), 120580 mRNA-1273 (Moderna COVID-19 vaccine), and 100752 BNT162b2 (Pfizer-BioNTech COVID-19 vaccine). Headache, joint-related symptoms, muscle pain, musculoskeletal and connective tissue pain, nausea or vomiting, dermal and epidermal conditions, and febrile disorders were common post-vaccination complaints. They were associated with delayed recovery in people with underlying diseases, including diabetes[36].
Theoretically, vaccination could be followed by mild to moderate elevation of blood glucose levels[87]. However, a few studies have already reported worsened glucose control after the COVID-19 vaccination[53,56]. However, a recent study showed that COVID-19 vaccination was not associated with impairment in glucose management. The study analyzed the short-term effects of COVID-19 immunization in patients with type 1 and type 2 diabetes who were vaccinated with one of the following three COVID-19 vaccines: BNT162b2, mRNA-1273, and AZD1222 (Oxford-AstraZeneca). The study collected and analyzed 49200 continuous glucose monitoring data from 74 participants in the study and showed that there were no changes in time spent in the target glycemic range (70−180 mg/dL) on the days of follow-up (2 d before and 3 d after vaccination). However, patients with type 1 diabetes and more pronounced post-vaccine side effects spent more time above the target range (> 180 mg/dL); no such observations were found in patients with type 2 diabetes. Additionally, the study reported no need for adjustment in the insulin bolus dose and changes in carbohydrate intake around the vaccination in patients with both diabetes types[88].
To sum up, the most common systemic side effects are headache, chills, fever, flu-like symptoms, nausea, and fatigue. The local effects are pain, redness, and swelling at the injection site on the arm. Patients with diabetes are not more prone to have pronounced side effects of COVID-19 vaccination than healthy people. However, most of them are mild and disappear a few days after vaccine administration. Although it is possible to have increased blood sugar levels after vaccination, it is rather not associated with a significant impact on glycemic control. Therefore, it does not require any changes in diabetic therapy. However, we must remember that COVID-19 may exert deteriorating effects in patients with autoimmune diseases[89], including type 1 diabetes. On the other hand, therapies for type 2 diabetes that target cytokines can also change the course of infection[90].
Diabetes patients were not excluded from the COVID-19 vaccine trials due to the higher prevalence of the disease amongst the populations. Thus, we obtained much more data on the evidence for the long-term safety and efficacy of the COVID-19 vaccine in patients with diabetes, in contrast to other autoimmune diseases where many gaps in the knowledge still exist. However, despite the considerable information, physicians and patients still fear exacerbation of the disease and the adverse effects of vaccination, which increases the hesitancy to vaccination.
Even though COVID-19 vaccination has emerged as the sole practical approach to improving clinical outcomes, vaccine hesitancy remains a barrier to obtaining significant levels of vaccine coverage. This poses a particular concern to individuals suffering from autoimmune illnesses, who are already at a higher risk of hospitalization and poor clinical outcomes due to COVID-19 infection. While long-term safety and effectiveness data for COVID-19 immunization in individuals with autoimmune illnesses are lacking, existing research clearly shows that the advantages of vaccination exceed the risks of side effects and disease flare-ups.
The COVAD study group demonstrated some causes for vaccination hesitancy, which was reported in around half of the patients with autoimmune illnesses in the study’s pilot findings[91]. Of all the respondents who did not receive any dose of the COVID-19 vaccine, 16.94% reported not getting the vaccine due to long-term safety concerns or other fears, such as disease exacerbation and delayed adverse effects, and 27.45% stated that they plan to wait until more data are available on the safety of the vaccine before vaccination. Other reasons given by the respondents for not vaccinating are the lack of the vaccine in some parts of the world (32.00%), planning for vaccination at a later date (11.67%), and postponing vaccination due to recent COVID-19 infection (7.30%). Some patients also reported not getting the vaccine because they had been advised to do so by their doctor (5.40%)[91].
However, there are no medical recommendations against vaccination. Only 35% of those vaccinated had mild side effects (fever/headache/myalgia). Furthermore, patients with autoimmune diseases had fewer side effects than healthy controls. Recent international studies show a negligible risk of severe side effects or disease exacerbation after vaccination[91].
Wang et al[91] showed that more than half of 483 Chinese diabetes patients experienced vaccine hesitancy (58.2%). Of them, 41.8% were unwilling to get the COVID-19 vaccine. Although patients were aware of the severity of COVID-19 in diabetes patients, they were concerned about vaccine safety. Interestingly, the vaccination status of their relatives did not influence the patients’ decisions, but disagreement with their physician on the ability of the vaccine to reduce the severity of COVID-19 correlated with vaccine hesitancy[92].
The five factors associated with vaccine hesitancy in diabetes patients are the false belief that diabetes is not a high risk factor for severe COVID-19 and a lack of confidence in vaccine efficacy to prevent infection. However, diabetes patients were convinced that diabetes worsens COVID-19 prognosis and that vaccination may reduce the transmission risk. The third factor associated with vaccine hesitancy was the fear of adverse effects, and the fourth and fifth were the dependence on the opinion of others, including vaccines to be administered to a large group of people, and the influence of social media on them[92].
Similarly, Aldossari et al[92] showed that 34.7% of Saudi diabetes patients in the survey were willing to be vaccinated, and 79.0% supported COVID-19 vaccination. However, they showed signs of fear and uncertainty[92]. Therefore, the key to a successful vaccination campaign for these patients remains the accurate information provided and the fight against misinformation. Some factors related to vaccine hesitancy were relatively quick development, beliefs that the trials were insufficient, fears and uncertainty of components, and especially the mRNA behavior after vaccination. In addition, an enormous impact is the anti-vaccination movements in social and traditional media. Furthermore, social media misinformation has led to increased anxiety and vaccine hesitancy.
Since diabetes mellitus is a significant contributor to COVID-19 mortality, patients with disturbed glucose metabolism should be protected from SARS-CoV-2 infection. Few studies have established data on the effectiveness and safety of the COVID-19 vaccine in patients with diabetes. Despite the significant reduction in the immune response to vaccination in diabetes individuals, they should be prioritized for complete vaccination and booster dose delivery. Also, glycemic management in achieving sufficient immunity against SARS-CoV-2 is mandatory. Data also showed that COVID-19 vaccines presented an excellent safety profile with adverse effects following vaccination similar to the healthy population and no increase in the incidence of adverse events in the diabetic group. Finally, vaccine hesitancy among diabetes patients could be overcome with proper information and patient care.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casaca W, Brazil; Wong YP, Malaysia S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12966] [Article Influence: 2593.2] [Reference Citation Analysis (1)] |
| 2. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [cited 20 March 2022]. Available from: https://covid19.who.int/. |
| 3. | Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1310] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 4. | Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Godeke GJ, Jonges M, Farag E, Diab A, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, Al Romaihi HE, Al Khal A, Bermingham A, Osterhaus AD, AlHajri MM, Koopmans MP. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 522] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 5. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281-292.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6146] [Article Influence: 1229.2] [Reference Citation Analysis (0)] |
| 6. | Perez-Saez J, Lauer SA, Kaiser L, Regard S, Delaporte E, Guessous I, Stringhini S, Azman AS; Serocov-POP Study Group. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2021;21:e69-e70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 7. | Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2689] [Cited by in RCA: 2220] [Article Influence: 444.0] [Reference Citation Analysis (0)] |
| 8. | Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14:491-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 9. | Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2178] [Cited by in RCA: 2617] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 10. | Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1841] [Cited by in RCA: 1824] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 11. | Bahiru E, Hsiao R, Phillipson D, Watson KE. Mechanisms and Treatment of Dyslipidemia in Diabetes. Curr Cardiol Rep. 2021;23:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4495] [Article Influence: 224.8] [Reference Citation Analysis (0)] |
| 13. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4721] [Article Influence: 1573.7] [Reference Citation Analysis (36)] |
| 14. | Vasbinder A, Anderson E, Shadid H, Berlin H, Pan M, Azam TU, Khaleel I, Padalia K, Meloche C, O'Hayer P, Michaud E, Catalan T, Feroze R, Blakely P, Launius C, Huang Y, Zhao L, Ang L, Mikhael M, Mizokami-Stout K, Pennathur S, Kretzler M, Loosen SH, Chalkias A, Tacke F, Giamarellos-Bourboulis EJ, Reiser J, Eugen-Olsen J, Feldman EL, Pop-Busui R, Hayek SS; ISIC Study Group:, Hayek SS, Blakely P, Berlin H, Azam TU, Shadid H, Pan M, O'Hayer P, Meloche C, Feroze R, Padalia KJ, Anderson E, Perry D, Bitar A, Kaakati R, Huang Y, Zhao L, Reiser J, Samelko B, Hlepas A, Patel PP, Wang X, Eugen-Olsen J, Altintas I, Stauning M, Baltzer Houlind M, Lindstrøm MB, Gamst-Jensen H, Hartmann LJ, Nehlin JO, Kallemose T, Parvaiz I, Rasmussen C, Andersen O, Tingleff J, Giamarellos-Bourboulis EJ, Adami ME, Solomonidi N, Tsilika M, Saridaki M, Lekakis V, Loosen SH, Luedde T, Keitel V, Chalkias A, Arnaoutoglou E, Pantazopoulos I, Laou E, Kolonia K, Skoulakis A, Tacke F, Tober-Lau P, Mohr R, Kurth F, Sander LE, Jochum C. Inflammation, Hyperglycemia, and Adverse Outcomes in Individuals With Diabetes Mellitus Hospitalized for COVID-19. Diabetes Care. 2022;45:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 977] [Article Influence: 195.4] [Reference Citation Analysis (0)] |
| 16. | CDC COVID-19 Vaccine Breakthrough Case Investigations Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC - United States, January 1-April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:792-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 281] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 17. | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1236] [Article Influence: 247.2] [Reference Citation Analysis (0)] |
| 18. | Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care. 2018;41:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 417] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 19. | Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 484] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 20. | Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, Sattar N, Young B, Valabhji J. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 611] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 21. | Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M; LICORN and the Lille COVID-19 and Obesity study group. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020;28:1195-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1263] [Cited by in RCA: 1576] [Article Influence: 315.2] [Reference Citation Analysis (0)] |
| 22. | Green WD, Beck MA. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann Am Thorac Soc. 2017;14:S406-S409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo Dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:437-446.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 535] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 24. | Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 591] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 25. | Flue vaccination and Diabetes. NHS. [cited 20 March 2022]. Available from: https://www.england.nhs.uk/south/wp-content/uploads/sites/6/2019/10/Diabetes-and-Flu-Vaccine-Importance.pdf. |
| 26. | CDC. Influenza (Flu) Diabetes. [cited 20 March 2022]. Available from: https://www.cdc.gov/flu/highrisk/diabetes.htm#anchor_1537288125609. |
| 27. | Seo YB, Baek JH, Lee J, Song JY, Lee JS, Cheong HJ, Kim WJ. Long-Term Immunogenicity and Safety of a Conventional Influenza Vaccine in Patients with Type 2 Diabetes. Clin Vaccine Immunol. 2015;22:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | CDC. Pneumococcal vaccine–Diabetes. [cited 20 March 2022]. Available from: https://www.cdc.gov/pneumococcal/vaccination.html. |
| 29. | Huijts SM, van Werkhoven CH, Bolkenbaas M, Grobbee DE, Bonten MJM. Post-hoc analysis of a randomized controlled trial: Diabetes mellitus modifies the efficacy of the 13-valent pneumococcal conjugate vaccine in elderly. Vaccine. 2017;35:4444-4449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Perniciaro S, van der Linden M. Pneumococcal vaccine uptake and vaccine effectiveness in older adults with invasive pneumococcal disease in Germany: A retrospective cohort study. Lancet Reg Health Eur. 2021;7:100126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Chuanchaiyakul N, Thongtang N, Rattanaumpawan P. Cumulative incidence of and risk factors for herpes zoster among patients with diabetes mellitus: Results from a 10-year nested case-control study. J Diabetes Complications. 2022;36:108168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 395] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 33. | Salzberger B, Nitschmann S. [Herpes zoster vaccination efficacy in adults 70 years of age or older : ZOE-70 study]. Internist (Berl). 2017;58:522-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Willer DO, Oostvogels L, Cunningham AL, Gervais P, Gorfinkel I, Hyung Kim J, Talarico C, Wascotte V, Zahaf T, Colindres R, Schuind A; ZOE 50/70 study groups. Efficacy of the adjuvanted recombinant zoster vaccine (RZV) by sex, geographic region, and geographic ancestry/ethnicity: A post-hoc analysis of the ZOE-50 and ZOE-70 randomized trials. Vaccine. 2019;37:6262-6267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Godeaux O, Kovac M, Shu D, Grupping K, Campora L, Douha M, Heineman TC, Lal H. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate vaccine in adults ≥ 50 years of age with a prior history of herpes zoster: A phase III, non-randomized, open-label clinical trial. Hum Vaccin Immunother. 2017;13:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15:505-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 37. | Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes Care. 2012;35:2690-2697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 396] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 39. | Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 40. | Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020;39:2055-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 41. | Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41:509-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 42. | Ahmed S, Gasparyan AY, Zimba O. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int. 2021;41:243-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Sen P, Gupta L, Lilleker JB, Aggarwal V, Kardes S, Milchert M, Gheita T, Salim B, Velikova T, Gracia-Ramos AE, Parodis I, O'Callaghan AS, Nikiphorou E, Tan AL, Cavagna L, Saavedra MA, Shinjo SK, Ziade N, Knitza J, Kuwana M, Cagnotto G, Nune A, Distler O, Chinoy H, Aggarwal R; COVAD Study Group. COVID-19 vaccination in autoimmune disease (COVAD) survey protocol. Rheumatol Int. 2022;42:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 44. | Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Salemi S, D'Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol. 2010;29:247-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Johns Hopkins. Johns Hopkins Coronavirus research center. [cited 20 March 2022]. Available from: https://coronavirus.jhu.edu/map.html. |
| 47. | De Martino M, Chiappini E, Galli L. Vaccines and autoimmunity. Int J Immunopathol Pharmacol. 2013;26:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Sakowicz-Burkiewicz M, Kocbuch K, Grden M, Maciejewska I, Szutowicz A, Pawelczyk T. High glucose concentration impairs ATP outflow and immunoglobulin production by human peripheral B lymphocytes: involvement of P2X7 receptor. Immunobiology. 2013;218:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 50. | Gregory JM, Slaughter JC, Duffus SH, Smith TJ, LeStourgeon LM, Jaser SS, McCoy AB, Luther JM, Giovannetti ER, Boeder S, Pettus JH, Moore DJ. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic's Impact in Type 1 and Type 2 Diabetes. Diabetes Care. 2021;44:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 186] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 51. | Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ, Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 663] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 52. | Samuel SM, Varghese E, Triggle CR, Büsselberg D. COVID-19 Vaccines and Hyperglycemia-Is There a Need for Postvaccination Surveillance? Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 53. | Abu-Rumaileh MA, Gharaibeh AM, Gharaibeh NE. COVID-19 Vaccine and Hyperosmolar Hyperglycemic State. Cureus. 2021;13:e14125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 54. | Santos AF, Póvoa P, Paixão P, Mendonça A, Taborda-Barata L. Changes in Glycolytic Pathway in SARS-COV 2 Infection and Their Importance in Understanding the Severity of COVID-19. Front Chem. 2021;9:685196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Edwards AE, Vathenen R, Henson SM, Finer S, Gunganah K. Acute hyperglycaemic crisis after vaccination against COVID-19: A case series. Diabet Med. 2021;38:e14631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Mishra A, Ghosh A, Dutta K, Tyagi K, Misra A. Exacerbation of hyperglycemia in patients with type 2 diabetes after vaccination for COVID19: Report of three cases. Diabetes Metab Syndr. 2021;15:102151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Lee HJ, Sajan A, Tomer Y. Hyperglycemic Emergencies Associated With COVID-19 Vaccination: A Case Series and Discussion. J Endocr Soc. 2021;5:bvab141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 58. | Heald AH, Stedman M, Horne L, Rea R, Whyte M, Gibson JM, Livingston M, Anderson SG, Ollier W. Analysis of Continuous Blood Glucose Data in People with Type 1 Diabetes (T1DM) After COVID-19 Vaccination Indicates a Possible Link Between the Immune and the Metabolic Response. J Diabetes Sci Technol. 2021;15:1204-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 60. | Mifsud S, Schembri EL, Gruppetta M. Stress-induced hyperglycaemia. Br J Hosp Med (Lond). 2018;79:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 356] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 62. | Soetedjo NNM, Iryaningrum MR, Lawrensia S, Permana H. Antibody response following SARS-CoV-2 vaccination among patients with type 2 diabetes mellitus: A systematic review. Diabetes Metab Syndr. 2022;16:102406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 63. | Nomura Y, Sawahata M, Nakamura Y, Kurihara M, Koike R, Katsube O, Hagiwara K, Niho S, Masuda N, Tanaka T, Sugiyama K. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 64. | Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, Indenbaum V, Mandelboim M, Doolman R, Amit S, Mendelson E, Ziv A, Huppert A, Rubin C, Freedman L, Kreiss Y. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 267] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 65. | Van Praet JT, Vandecasteele S, De Roo A, Vynck M, De Vriese AS, Reynders M. Dynamics of the cellular and humoral immune response after BNT162b2 mRNA Covid-19 vaccination in Covid-19 naive nursing home residents. J Infect Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Ali H, Alterki A, Sindhu S, Alahmad B, Hammad M, Al-Sabah S, Alghounaim M, Jamal MH, Aldei A, Mairza MJ, Husain M, Deverajan S, Ahmad R, Cherian P, Alkhairi I, Alkandari A, Abubaker J, Abu-Farha M, Al-Mulla F. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front Immunol. 2021;12:752233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 67. | Marfella R, D'Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, Romano C, Messina V, Turriziani F, Siniscalchi M, Maniscalco M, Boccalatte M, Napolitano G, Salemme L, Marfella LV, Basile E, Montemurro MV, Papa C, Frascaria F, Papa A, Russo F, Tirino V, Papaccio G, Galdiero M, Sasso FC, Barbieri M, Rizzo MR, Balestrieri ML, Angelillo IF, Napoli C, Paolisso G. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes Metab. 2022;24:160-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 68. | Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, Sharma A. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492-6509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 69. | Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2022;22:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 70. | Piccini B, Pessina B, Pezzoli F, Casalini E, Toni S. COVID-19 vaccination in adolescents and young adults with type 1 diabetes: Glycemic control and side effects. Pediatr Diabetes. 2022;23:469-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, Caputi A, Rossetti R, Spoltore ME, Filippi V, Gangitano E, Manfrini S, Mariani S, Lubrano C, Lenzi A, Mastroianni C, Gnessi L. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38:e3465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 72. | Karamese M, Tutuncu EE. The effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on antibody response in participants aged 65 years and older. J Med Virol. 2022;94:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 73. | Ren X, Zhou J, Guo J, Hao C, Zheng M, Zhang R, Huang Q, Yao X, Li R, Jin Y. Reinfection in patients with COVID-19: a systematic review. Glob Health Res Policy. 2022;7:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 74. | Rahman S, Rahman MM, Miah M, Begum MN, Sarmin M, Mahfuz M, Hossain ME, Rahman MZ, Chisti MJ, Ahmed T, Arifeen SE, Rahman M. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci Rep. 2022;12:1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 75. | Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 76. | CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 318] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 77. | Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, Lindsey N, Zhang B, Licata C, Jazwa A, Sotir M, Shimabukuro T. First Month of COVID-19 Vaccine Safety Monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 78. | Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, Zoabi M, Aisman M, Goldschmid N, Berar Yanay N. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790-3793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 79. | Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142-2145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 80. | Bautista García J, Peña Ortega P, Bonilla Fernández JA, Cárdenes León A, Ramírez Burgos L, Caballero Dorta E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed). 2021;74:812-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 81. | Ammirati E, Cavalotti C, Milazzo A, Pedrotti P, Soriano F, Schroeder JW, Morici N, Giannattasio C, Frigerio M, Metra M, Camici PG, Oliva F. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Heart Vasc. 2021;34:100774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 82. | Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessy R, Caron R, Fuss C, Corbin KJE, Emuren L, Faherty E, Hall EK, Di Pentima C, Oster ME, Paintsil E, Siddiqui S, Timchak DM, Guzman-Cottrill JA. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 266] [Article Influence: 66.5] [Reference Citation Analysis (2)] |
| 83. | D'Angelo T, Cattafi A, Carerj ML, Booz C, Ascenti G, Cicero G, Blandino A, Mazziotti S. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? Can J Cardiol. 2021;37:1665-1667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 84. | Nassar M, Nso N, Gonzalez C, Lakhdar S, Alshamam M, Elshafey M, Abdalazeem Y, Nyein A, Punzalan B, Durrance RJ, Alfishawy M, Bakshi S, Rizzo V. COVID-19 vaccine-induced myocarditis: Case report with literature review. Diabetes Metab Syndr. 2021;15:102205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 85. | Gianfredi V, Minerva M, Casu G, Capraro M, Chiecca G, Gaetti G, Mantecca Mazzocchi R, Musarò P, Berardinelli P, Basteri P, Bertini B, Ferri C, Odone A, Signorelli C, Alberti VF, Gastaldi G. Immediate adverse events following COVID-19 immunization. A cross-sectional study of 314,664 Italian subjects. Acta Biomed. 2021;92:e2021487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 86. | Son CS, Jin SH, Kang WS. Propensity-Score-Matched Evaluation of Adverse Events Affecting Recovery after COVID-19 Vaccination: On Adenovirus and mRNA Vaccines. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Aberer F, Moser O, Aziz F, Sourij C, Ziko H, Lenz J, Abbas F, Obermayer AM, Kojzar H, Pferschy PN, Müller A, Unteregger C, Leitner M, Banfic T, Eckstein ML, Wachsmuth N, Kaser S, Mader JK, Tripolt NJ, Sourij H. Impact of COVID-19 Vaccination on Glycemia in Individuals With Type 1 and Type 2 Diabetes: Substudy of the COVAC-DM Study. Diabetes Care. 2022;45:e24-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 88. | Georgiev T, Angelov AK. Complexities of diagnosis and management of COVID-19 in autoimmune diseases: Potential benefits and detriments of immunosuppression. World J Clin Cases. 2020;8:3669-3678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Velikova TV, Kabakchieva PP, Assyov YS, Georgiev TА. Targeting Inflammatory Cytokines to Improve Type 2 Diabetes Control. Biomed Res Int. 2021;2021:7297419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 90. | Sen P, Lilleker JB, Agarwal V, Kardes S, Milchert M, Gheita T, Salim B, Velikova T, Gracia-Ramos AE, Parodis I, O'Callaghan AS, Nikiphorou E, Tan AL, Cavagna L, Saavedra MA, Shinjo SK, Ziade N, Knitza J, Kuwana M, Cagnotto G, Nune A, Distler O, Chinoy H, Aggarwal R, Gupta L. COVAD Study Group. Vaccine Hesitancy in Patients with Autoimmune Diseases- Data from the COVID-19 Vaccination in Autoimmune Diseases (COVAD) Study'. Indian J Rheumatol. 2022;17:188-191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Wang Y, Duan L, Li M, Wang J, Yang J, Song C, Li J, Jia J, Xu J. COVID-19 Vaccine Hesitancy and Associated Factors among Diabetes Patients: A Cross-Sectional Survey in Changzhi, Shanxi, China. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 92. | Aldossari KK, Alharbi MB, Alkahtani SM, Alrowaily TZ, Alshaikhi AM, Twair AA. COVID-19 vaccine hesitancy among patients with diabetes in Saudi Arabia. Diabetes Metab Syndr. 2021;15:102271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |