Published online Aug 15, 2022. doi: 10.4239/wjd.v13.i8.643
Peer-review started: February 17, 2022
First decision: May 30, 2022
Revised: June 14, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 15, 2022
Processing time: 176 Days and 10.1 Hours
Hypertension (HTN) and type 2 diabetes mellitus (T2DM) are often coincident, and each condition is considered a risk factor for the other. Both occur frequently in the Inner Mongolia region of China. The reasons for differences in risk between Han and Mongolian ethnic groups are not known. The LEPR gene and its polymorphism, rs1137101 (Gln223Arg), are both considered risk factors for HTN and T2DM, but any role of rs1137101 in the occurrence of HTN + T2DM remains unclear for Mongolian and Han populations in the Inner Mongolia region.
To investigate the relationship between rs1137101 and the occurrence of HTN with T2DM in Mongolian and Han populations in Inner Mongolia.
A total of 2652 subjects of Han and Mongolian ethnic origins were enrolled in the current study, including 908 healthy controls, 1061 HTN patients and 683 HTN patients with T2DM.
The association between the rs1137101 polymorphism and HTN with T2DM was analyzed, and differences between Han and Mongolian individuals assessed. There was a significant correlation between rs1137101 and HTN (co-dominant, dominant, over-dominant and log-additive models) and HTN + T2DM (co-dominant, dominant, over-dominant and log-additive models) after adjustment for sex and age in individuals of Mongolian origin. rs1137101 was significantly associated with HTN (co-dominant, recessive and log-additive models) and HTN + T2DM (co-dominant, dominant, over-dominant and log-additive models) in the Han Chinese population.
Mongolian and Han subjects from Inner Mongolia with HTN who had rs1137101 were protected against the development of T2DM. Allele A has the opposite impact on the occurrence of HTN in Mongolian and Han Chinese populations.
Core Tip: Hypertension and type 2 diabetes mellitus are often coincident, and each condition is a risk factor for the other. It is unknown why there are differences in risk between Han and Mongolian ethnic groups. The LEPR gene and its polymorphism, rs1137101 (Gln223Arg), are considered risk factors for the occurrence of hypertension and type 2 diabetes mellitus. The current study investigated the relationship between rs1137101 and the occurrence of hypertension with type 2 diabetes mellitus in Mongolian and Han populations in Inner Mongolia. Differences between the two populations were analyzed. The aim was to inform further research on advanced metabolic disease.
- Citation: Zhao KY, Yuan ML, Wu YN, Cui HW, Han WY, Wang J, Su XL. Association of rs1137101 with hypertension and type 2 diabetes mellitus of Mongolian and Han Chinese. World J Diabetes 2022; 13(8): 643-653
- URL: https://www.wjgnet.com/1948-9358/full/v13/i8/643.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i8.643
The causes of hypertension (HTN) are multifactorial, and the condition is in turn a risk factor for cardiovascular disease and nephropathy[1]. Current estimates put a global figure of 1.3 billion[2,3] on the number of people with high blood pressure, an estimate that is set to rise to 1.6 billion by 2025[2,4]. Advanced age, gender, obesity and genotype are all risk factors for HTN[2]. Diabetes mellitus (DM) is another public health problem that has increased rapidly over recent years with 80%-90% patients having type 2 DM (T2DM)[5,6]. Epidemiological studies have shown that HTN is a major risk factor for T2DM[7]. One-third of HTN patients also have T2DM and are at an increased risk of cardiovascular disease and mortality[8,9].
The leptin (LEP) receptor (LEPR) is a transmembrane protein encoded by the LEPR gene. Several variants have been characterized, and there is widespread expression throughout the body’s tissues[10]. The LEP hormone is known to have roles in the regulation of hunger, energy balance, metabolism, reproduction and insulin secretion mediated by binding to LEPR[11,12]. Binding of LEP to its hypothalamic receptor has been shown to raise blood pressure in mice, and blockade of LEPR resulted in lower values[13,14]. LEPR has roles in insulin secretion, and its activity is relevant to the development of insulin resistance[12,15]. Indeed, a recent study has correlated LEPR polymorphisms with DM and HTN[16,17]. Among the Han Chinese population, the LEPR gene polymorphism, rs13306519, has been associated with DM and rs12037879 with HTN[5]. Moreover, rs1137100 (Arg109Lys) and rs8179183 (Lys656Asn) have been associated with both DM and HTN[15,18].
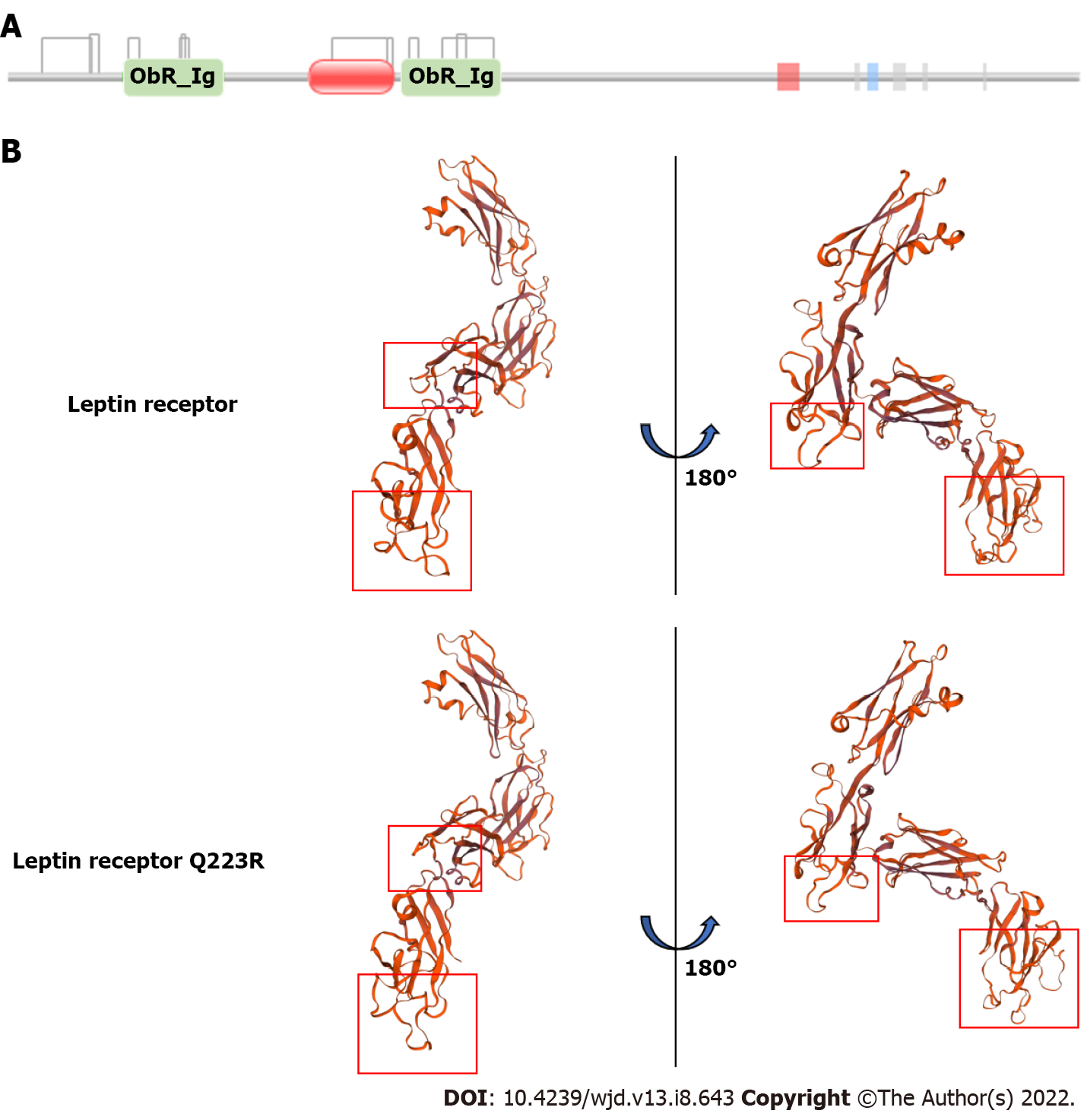
The LEPR gene polymorphism, rs1137101, is located on chromosome 1p31 and involves a substitution of the 223rd amino acid residue, gln (Q) for Arg (R). This mutation affects the ObRIg domain, according to the PFAM database (http://pfam.xfam.org/protein/P48357; Figure 1A and Table 1). Construction of a 3D model of the region including amino acids 126 to 533 using Swiss-model software (https://swissmodel.expasy.org/) revealed a consequent change in protein structure (Figure 1B). These predictions imply that the rs1137101 mutation may influence protein structure and have an impact on protein function. Previous studies have associated rs1137101 (Gln223Arg) with obesity, cancer, HTN and DM[9,19,20]. It also has been shown to be a risk factor for HTN and T2DM in the Chinese population[21,22]. The current study investigated the relationship between rs1137101 and the occurrence of HTN with T2DM in Mongolian and Han populations in Inner Mongolia.
| Source | Domain | Start | End | Gathering threshold (bits) | Score (bits) | E-value | |||
| Sequence | Domain | Sequence | Domain | Sequence | Domain | ||||
| Pfam | ObR_Ig | 126 | 233 | 25.8 | 25.8 | 170.5 | 61.1 | 3.50E-47 | 3.9E-13 |
| Pfam | Lep_receptor_Ig | 329 | 420 | 28.8 | 28.8 | 84.8 | 84.8 | 1.00E-20 | 1.00E-20 |
| Pfam | ObR_Ig | 431 | 533 | 25.8 | 25.8 | 170.5 | 112.7 | 3.50E-47 | 3.40E-29 |
| Transmembrane | NA | 840 | 862 | NA | NA | NA | NA | NA | NA |
| Low_complexity | NA | 849 | 863 | NA | NA | NA | NA | NA | NA |
| Disorder | NA | 924 | 927 | NA | NA | NA | NA | NA | NA |
| Low_complexity | NA | 937 | 946 | NA | NA | NA | NA | NA | NA |
| Disorder | NA | 966 | 967 | NA | NA | NA | NA | NA | NA |
| Disorder | NA | 970 | 973 | NA | NA | NA | NA | NA | NA |
| Disorder | NA | 975 | 976 | NA | NA | NA | NA | NA | NA |
| Disorder | NA | 997 | 1001 | NA | NA | NA | NA | NA | NA |
| Disorder | NA | 1064 | 1065 | NA | NA | NA | NA | NA | NA |
A total of 2652 subjects, including 908 healthy controls, 1061 HTN patients and 683 patients with HTN + T2DM, were randomly selected from adult residents of Mongolia (Hohhot, Wuhai, Xilinhot) and enrolled in the study. Study participants were unrelated, and the ethnic composition was 1347 Han and 1305 Mongolian. All participants provided written informed consent. The study was performed in accordance with the declaration of Helsinki and approved by the ethical committee of the affiliated hospital of Inner Mongolia Medical University.
T2DM and HTN were diagnosed according to the following criteria established by the World Health Organization: HTN: Systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or current prescription for antihypertensive medication[23]. Participants with chronic renal disease, renal artery stenosis, primary hyperaldosteronism, thyroid disease, Cushing syndrome, phaeochromocytoma or other diseases known to cause HTN were excluded; T2DM: Fasting blood sugar (FBS) ≥ 7.0 mmol /L or postprandial blood glucose ≥ 11.1 mmol/L or current definitive diagnosis of T2DM[24]. Participants with T1DM, cancer or other severe metabolic disease were excluded.
Age, weight and medical history were collected by questionnaire. Body mass index was calculated according to the formula: Mass (kg)/height2 (m2). Blood pressure was measured on the right arm using a mercury sphygmomanometer. Blood samples of HTN, T2DM and HTN + T2DM groups were collected after an 8 h fast. Genomic DNA was isolated from whole blood using a Maga bio plus whole blood genomic DNA purification Kit II (Hangzhou Bioer Technology co. Ltd, China) according to the manufacturer’s instructions. FBS, triglyceride, cholesterol, high density lipoprotein and low density lipoprotein were measured after plasmapheresis.
rs1137101 (Gln223Arg) polymorphisms were assessed by PCR amplification. The primers used were forward: 5’-TTCCCCAAAAAGGCAGTTTTCA-3’ and reverse: 5’-AGAAGCCACTCTTAATACCCCCAGT-3’. The target DNA sequences were amplified using a multiplex PCR method. Thermal cycling was performed for the rs1137101 loci in Gene Amp PCR system 9600 (PerkinElmer, Waltham, MA, United States) fluorescent products of ligase detection reaction differentiated by 3130xl genetic analyzer (Applied Biosystems, CA, United States).
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, United States) and SNPStats (https://www.snpstats.net/start.htm)[25] software. Categorical variables were presented as frequencies. Continuous data were reported as the mean ± standard deviation. Student’s t test was used to compare age, weight, height, body mass index, FBS, systolic blood pressure, diastolic blood pressure, triglyceride, cholesterol, high density lipoprotein and low density lipoprotein and statistical hypotheses were tested using the 2-tailed t test. The χ2 test was used to analyze ethnic and gender differences. Logistic regression was used to compute the odds ratio (OR) by adjusting for age and sex and the adjusted OR is presented with 95% confidence interval. Logistic regression, Hardy Weinberg Equilibrium and five genetic models (co-dominant, dominant, recessive, over-dominant and log-additive) were calculated using SNPStats software. A value of P < 0.05 was considered to be significant.
Baseline demographic characteristics of the study population are summarized in Table 2. Significant differences were found in ethnicity, gender, age, weight, height, FBS, Systolic blood pressure, diastolic blood pressure and high density lipoprotein between cases with HTN, those with both HTN + T2DM and controls. No significant deviation from the Hardy Weinberg Equilibrium was detected (Table 3). Allele frequency was not significant in the Han population, but significant differences between Mongolian groups were observed (Table 4).
| Control, n = 908 | HTN, n = 1061 | HTN with T2DM, n = 683 | P value | ||||
| Control vs HTN | Control vs HTN + T2DM | HTN vs HTN + T2DM | |||||
| Ethnic | Han | 455 | 406 | 486 | < 0.0001 | < 0.0001 | < 0.0001 |
| Mongolian | 453 | 655 | 197 | ||||
| Gender | Male | 357 | 601 | 397 | < 0.0001 | < 0.0001 | 0.542 |
| Female | 551 | 460 | 286 | ||||
| Age | 48.11 ± 15.06 | 54.49 ± 15.67 | 63.89 ± 11.17 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Weight (kg) | 66.14 ± 11.06 | 72.32 ± 12.06 | 73.35 ± 12.48 | < 0.0001 | < 0.0001 | 0.2156 | |
| Height (cm) | 163 ± 0.09 | 168 ± 0.08 | 161.26 ± 0.10 | < 0.0001 | 0.6110 | 0.0032 | |
| BMI (kg/m2) | 25.27 ± 8.50 | 25.56 ± 3.63 | 26.48 ± 5.12 | 0.4067 | 0.7588 | 0.8723 | |
| FBS (mmol/L) | 5.06 ± 0.49 | 5.73 ± 0.77 | 8.59 ± 3.37 | 0.0721 | < 0.0001 | < 0.0001 | |
| SBP (mm Hg) | 117.21 ± 14.27 | 151.10 ± 18.94 | 166.47 ± 17.53 | < 0.0001 | < 0.0001 | < 0.0001 | |
| DBP (mm Hg) | 77.20 ± 7.95 | 88.59 ± 12.74 | 100.84 ± 13.31 | < 0.0001 | < 0.0001 | < 0.0001 | |
| TG (mmol/L) | 1.63 ± 1.06 | 2.24 ± 1.52 | 2.59 ± 12.14 | 0.0626 | 0.0051 | 0.4768 | |
| CHO (mmol/L) | 4.53 ± 1.30 | 4.47 ± 3.59 | 4.52 ± 1.26 | 0.8913 | 0.9984 | 0.8885 | |
| HDL (mmol/L) | 1.44 ± 0.54 | 1.75 ± 0.95 | 1.27 ± 0.35 | < 0.0001 | < 0.0001 | < 0.0001 | |
| LDL (mmol/L) | 2.84 ± 1.00 | 2.87 ± 1.38 | 2.94 ± 11.1 | 0.9921 | 0.9225 | 0.9586 | |
| Group | G/G | G/A | A/A | G | A | P value | |
| Han, n = 1347 | Control, n = 455 | 351 | 94 | 10 | 796 | 114 | 0.2 |
| HTN, n = 406 | 312 | 91 | 3 | 715 | 97 | 0.24 | |
| HTN + T2DM, n = 486 | 394 | 84 | 8 | 872 | 100 | 0.21 | |
| Mongolian, n = 1305 | Control, n = 453 | 343 | 101 | 9 | 787 | 119 | 0.68 |
| HTN, n = 655 | 436 | 202 | 17 | 1074 | 236 | 0.29 | |
| HTN + T2DM, n = 197 | 151 | 42 | 4 | 344 | 50 | 0.53 |
| Population | Allele | All subjects count (%) | Control count (%) | HTN count (%) | HTN + T2DM count (%) | P value |
| Han, n = 1347 | G | 2383 (88) | 796 (87) | 715 (88) | 872 (90) | 0.288 |
| A | 311 (12) | 114 (13) | 97 (12) | 100 (10) | ||
| A/A | 21 (2) | 10 (2) | 3 (1) | 8 (2) | 0.153 | |
| G/A | 269 (20) | 94 (21) | 91 (22) | 84 (17) | ||
| G/G | 1057 (78) | 351 (77) | 312 (77) | 394 (81) | ||
| Mongolian, n = 1305 | G | 2205 (84) | 787 (87) | 1074 (82) | 344 (87) | 0.002 |
| A | 405 (16) | 119 (13) | 236 (18) | 50 (13) | ||
| A/A | 30 (2) | 9 (2) | 17 (3) | 4 (2) | 0.006 | |
| G/A | 345 (26) | 101 (22) | 202 (31) | 42 (21) | ||
| G/G | 930 (71) | 343 (76) | 436 (67) | 151 (77) |
The correlation between the LEPR gene polymorphism, rs1137101, and HTN in ethnic Han and Mongolian Chinese subjects was analyzed. A total of 861 subjects of Han origin (control = 455; HTN = 406) and 1108 subjects of Mongolian origin (control = 453; HTN = 655) were assessed. Logistic regression analysis was used to evaluate whether rs1137101 was independently associated with HTN after adjusting for sex and age (Table 5). Use of five inheritance models, codominant, dominant, recessive, over-dominant and log-additive, gave the following results: Co-dominant (A/G) model: OR = 0.88 (0.62-1.27); co-dominant (A/A) model: OR = 0.21 (0.05-0.80); and recessive (A/A) model: OR = 0.21 (0.05-0.82) for hypertensive Han subjects compared with controls. Results for Mongolian subjects were: Co-dominant (A/G) model: OR = 1.49 (1.12-1.97); co-dominant (A/A) model: OR = 1.47 (0.64-3.34); dominant (A/G-A/A) model: OR = 1.49 (1.13-1.95); over-dominant (A/A) model: OR = 1.47 (1.11-1.95); and log-additive model: OR = 1.40 (1.10-1.79). An association between rs1137101 and HTN was established for subjects of Mongolian ethnic origin.
| Model | Genotype | Han, n = 861 | Mongolian, n = 1108 | ||||||
| Control, n (%) | HTN, n (%) | OR (95%CI) | P value | Control, n (%) | HTN, n (%) | OR (95%CI) | P value | ||
| Co-dominant | G/G | 351 (77.1) | 312 (76.8) | 1 | 0.041 | 343 (75.7) | 436 (66.6) | 1 | 0.016 |
| A/G | 94 (20.7) | 91 (22.4) | 0.88 (0.62-1.27) | 101 (22.3) | 202 (30.8) | 1.49 (1.12-1.97) | |||
| A/A | 10 (2.2) | 3 (0.7) | 0.21 (0.05-0.80) | 9 (2.0) | 17 (2.6) | 1.47 (0.64-3.34) | |||
| Dominant | G/G | 351 (77.1) | 312 (76.8) | 1 | 0.23 | 343 (75.7) | 436 (66.6) | 1 | 0.004 |
| A/G-A/A | 104 (22.9) | 94 (23.1) | 0.81 (0.57-1.15) | 110 (24.3) | 219 (33.4) | 1.49 (1.13-1.95) | |||
| Recessive | G/G-A/G | 445 (97.8) | 403 (99.3) | 1 | 0.015 | 444 (98) | 638 (97.4) | 1 | 0.51 |
| A/A | 10 (2.2) | 3 (0.7) | 0.21 (0.05-0.82) | 9 (2.0) | 17 (2.6) | 1.32 (0.58-2.99) | |||
| Over-dominant | G/G-A/A | 361 (79.3) | 315 (77.6) | 1 | 0.63 | 352 (77.7) | 453 (69.2) | 1 | 0.0064 |
| A/G | 94 (20.7) | 91 (22.4) | 0.91 (0.64-1.31) | 101 (22.3) | 202 (30.8) | 1.47 (1.11-1.95) | |||
| Log-additive | - | - | - | 0.75 (0.55-1.04) | 0.082 | - | - | 1.40 (1.10-1.79) | 0.0059 |
The association of rs1137101 with HTN + T2DM was analyzed. A total of 683 subjects, composed of 197 Mongolian and 486 Han, were included. The same five genetic models, codominant, dominant, recessive, over-dominant and log-additive, were used to analyze associations between HTN + T2DM as described above for HTN. OR (adjusted for sex and age) for the five genetic models in Mongolian subjects were: Co-dominant (A/G): 0.70 (0.44-1.11); co-dominant (A/A): 1.06 (0.27-4.25); dominant (A/G-A/A): 0.72 (0.46-1.13); recessive (G/G-A/G):1.15 (0.29-4.57); over-dominant (A/G): 0.70 (0.44-1.11); and log-additive: 0.78 (0.52-1.16). OR (adjusted for sex and age) for the five genetic models in Han subjects were: Co-dominant (A/G): 0.59 (0.40-0.87); co-dominant (A/A): 0.38 (0.14-1.08); dominant (A/G-A/A): 0.56 (0.39-0.82); recessive (G/G-A/G): 0.43 (0.15-1.21); over-dominant (A/G): 0.61 (0.41-0.89); and log-additive: 0.60 (0.43-0.83). No significant differences were found in Mongolian subjects, but the genotypes GA and AA significantly decreased the risk of HTN + T2DM in Han subjects (Table 6). Thus, the LEPR polymorphism is associated with the occurrence of HTN + T2DM in Han Chinese populations but not in Mongolian Chinese.
| Model | Genotype | Han, n = 941 | Mongolian, n = 650 | ||||||
| Control, n (%) | HTN with T2DM, n (%) | OR (95%CI) | P value | Control, n (%) | HTN with T2DM, n (%) | OR (95%CI) | P value | ||
| Co-dominant | G/G | 351 (77.1) | 394 (81.1) | 1 | 0.0075 | 343 (75.7) | 151 (76.7) | 1 | 0.3 |
| A/G | 94 (20.7) | 84 (17.3) | 0.59 (0.40-0.87) | 101 (22.3) | 42 (21.3) | 0.70 (0.44-1.11) | |||
| A/A | 10 (2.2) | 8 (1.6) | 0.38 (0.14-1.08) | 9 (2.0) | 4 (2.0) | 1.06 (0.27-4.25) | |||
| Dominant | G/G | 351 (77.1) | 394 (81.1) | 1 | 0.0024 | 343 (75.7) | 151 (76.7) | 1 | 0.15 |
| A/G-A/A | 104 (22.9) | 92 (18.9) | 0.56 (0.39-0.82) | 110 (24.3) | 46 (23.4) | 0.72 (0.46-1.13) | |||
| Recessive | G/G-A/G | 445 (97.8) | 478 (98.3) | 1 | 0.11 | 444 (98.0) | 193 (98.0) | 1 | 0.84 |
| A/A | 10 (2.2) | 8 (1.6) | 0.43 (0.15-1.21) | 9 (2.0) | 4 (2.0) | 1.15 (0.29-4.57) | |||
| Over-dominant | G/G-A/A | 361 (79.3) | 402 (82.7) | 1 | 0.01 | 352 (77.7) | 155 (78.7) | 1 | 0.12 |
| A/G | 94 (20.7) | 84 (17.3) | 0.61 (0.41-0.89) | 101 (22.3) | 42 (21.3) | 0.70 (0.44-1.11) | |||
| Log-additive | - | - | - | 0.60 (0.43-0.83) | 0.0018 | - | - | 0.78 (0.52-1.16) | 0.22 |
A comparison was made between patients with HTN and those with HTN + T2DM to analyze the correlation between the LEPR polymorphism and the occurrence of these disorders in Mongolian and Han populations. OR (95% confidence interval) (adjusted for sex and age) for Han subjects for the same five genetic models were: Co-dominant (A/G): 0.65 (0.46-0.92); co-dominant (A/A): 1.61 (0.41-6.28); dominant (A/G-A/A): 0.68 (0.49-0.96); recessive (A/A): 1.77 (0.46-6.87); over-dominant (A/G): 0.65 (0.46-0.91); and log-additive: 0.75 (0.55-1.02). All values were non-significant. For Mongolian subjects, OR (adjusted for sex and age) were: Co-dominant (A/G): 0.54 (0.36-0.81); co-dominant (A/A): 0.55 (0.17-1.79); dominant (A/G-A/A): 0.54 (0.36-0.80); recessive (A/A): 0.65 (0.20-2.11); over-dominant (A/G): 0.55 (0.37-0.82); and log-additive: 0.59 (0.41-0.84). The co-dominant A/G model, dominant A/G-A/A model, over-dominant A/G model and log-additive model were all associated with a significantly decreased risk of HTN + T2DM in Mongolian and Han patients (Table 7).
| Model | Genotype | Han, n = 892 | Mongolian, n = 852 | ||||||
| Control, n (%) | HTN with T2DM, n (%) | OR (95%CI) | P value | Control, n (%) | HTN with T2DM, n (%) | OR (95%CI) | P value | ||
| Co-dominant | G/G | 312 (76.8) | 394 (81.1) | 1 | 0.034 | 436 (66.6) | 151 (76.7) | 1 | 0.0067 |
| A/G | 91 (22.4) | 84 (17.3) | 0.65 (0.46-0.92) | 202 (30.8) | 42 (21.3) | 0.54 (0.36-0.81) | |||
| A/A | 3 (0.7) | 8 (1.6) | 1.61 (0.41-6.28) | 17 (2.6) | 4 (2.0) | 0.55 (0.17-1.79) | |||
| Dominant | G/G | 312 (76.8) | 394 (81.1) | 1 | 0.026 | 436 (66.6) | 151 (76.7) | 1 | 0.0016 |
| A/G-A/A | 94 (23.1) | 92 (18.9) | 0.68 (0.49-0.96) | 219 (33.4) | 46 (23.4) | 0.54 (0.36-0.80) | |||
| Recessive | G/G-A/G | 403 (99.3) | 478 (98.3) | 1 | 0.39 | 638 (97.4) | 193 (98.0) | 1 | 0.46 |
| A/A | 3 (0.7) | 8 (1.6) | 1.77 (0.46-6.87) | 17 (2.6) | 4 (2.0) | 0.65 (0.20-2.11) | |||
| Over-dominant | G/G-A/A | 315 (77.6) | 402 (82.7) | 1 | 0.012 | 453 (69.2) | 155 (78.7) | 1 | 0.0028 |
| A/G | 91 (22.4) | 84 (17.3) | 0.65 (0.46-0.91) | 202 (30.8) | 42 (21.3) | 0.55 (0.37-0.82) | |||
| Log-additive | - | - | - | 0.75 (0.55-1.02) | 0.067 | - | - | 0.59 (0.41-0.84) | 0.0024 |
HTN and T2DM are major risk factors for cardiovascular and cerebrovascular diseases, and both conditions are known to result from interactions between genetics and environment[26,27]. The LEPR gene has been widely studied with respect to T2DM and HTN. We have previously demonstrated an association between rs1137101 and HTN in Han subjects and an association between rs7555955 and HTN in Mongolian subjects[28]. No association was found between rs1137101 and HTN or other metabolic traits in Mexican children[29] nor with HTN or cardiovascular disease in Iranian subjects[17]. A meta-analysis did show an association between rs1137101 and T2DM[30], and a Brazilian study suggested a relationship between T2DM and being overweight[31]. Furthermore, rs1137101 was correlated with T2DM, insulin change and being overweight among the Punjabi population of North India[32]. These findings indicate that associations are very dependent on the origins of the population under study. Inner Mongolia is a vast territory with demarcation of urban, agricultural, pastoral and part-farming/part-pastoral areas. Each region has a unique lifestyle with specific eating habits, all of which have an impact on rates of HTN. Overlain on these variations are traditional risk factors, such as smoking, drinking and salt intake[33,34] plus environmental factors[35,36]. Results of the current study were not in accord with those of previous studies and discrepancies may be due to population and lifestyle differences.
The current study focused on the conditions of HTN and HTN + T2DM in ethnic Han and Mongolian populations in Inner Mongolia. There was a significant association between rs1137101 and HTN and HTN + T2DM in Han Chinese subjects. The genotypes, AA and GA, may decrease risk of HTN and HTN + T2DM for control and HTN groups. Whereas rs1137101 was associated with a significantly increased risk of HTN for control subjects, it was associated with a decreased risk of developing T2DM for HTN patients. Further investigations involving larger study populations with further data relating to environmental and lifestyle factors are required to substantiate interactions between genetics and the environment.
The current study investigated the impact of the polymorphism rs1137101 on HTN in Mongolian subjects. Mongolian and Han subjects with HTN who had rs1137101 were protected against the development of T2DM, and rs1137101 decreased the risk of HTN and HTN + T2DM for the Han Chinese population of Inner Mongolia. In contrast with its protective role in the Han population, rs1137101 increased the risk of HTN for the Mongolian population.
Hypertension (HTN) and type 2 diabetes mellitus (T2DM) are each considered a risk factor for the other. Both occur frequently in the Inner Mongolia region of China. rs1137101 is a potential risk factor for the occurrence of HTN and T2DM but the association between rs1137101 and HTN + T2DM in the Mongolian and Han population in Inner Mongolia remains unknown.
The association between rs1137101 and occurrence of HTN + T2DM has not been fully elucidated for Mongolian and Han populations in the Inner Mongolia region.
To investigate the relationship between rs1137101 and the occurrence of HTN with T2DM in Mongolian and Han populations in Inner Mongolia. To illuminate the association between the rs1137101 polymorphism and HTN with T2DM by analyzing differences between Han and Mongolian Chinese.
Data relating to blood samples, blood pressure, weight, height and other body indices among Chinese populations in Inner Mongolia. The rs1137101 polymorphism was measured. Data was analyzed by SPSS 22.0 and SNPstats software (https://www.snpstats.net/start.htm) to correlate rs1137101 with HTN + T2DM in Mongolian and Han populations in Inner Mongolia.
The association between the rs1137101 polymorphism and HTN with T2DM was analyzed, and differences between Han and Mongolian individuals were assessed. There was a significant correlation between rs1137101 with both HTN after adjustment for sex and age in individuals of Mongolian origin. rs1137101 was significantly associated with HTN and HTN + T2DM in the Han Chinese population.
There was significant correlation between rs1137101 and control and HTN/HTN + T2DM in Han and Mongolian subjects. Mongolian and Han subjects with HTN who had rs1137101 were protected against the development of T2DM. rs1137101 decreased the risk of HTN and HTN + T2DM for the Han Chinese population of Inner Mongolia. By contrast, rs1137101 increased the risk of HTN for the Mongolian population.
The current study analyzed the association between rs1137101 and HTN/HTN + T2DM by comparing control, HTN and HTN + T2DM groups and found rs1137101 to be associated with HTN and HTN + T2DM in Mongolian and Han populations in Inner Mongolia. Further investigations involving larger study populations with further data relating to environmental and lifestyle factors are required to substantiate interactions between genetics and the environment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Javor E, Croatia; Mahmoud MZ, Saudi Arabia; Mrzljak A, Croatia S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Saju MD, Allagh KP, Scaria L, Joseph S, Thiyagarajan JA. Prevalence, Awareness, Treatment, and Control of Hypertension and Its Associated Risk Factors: Results from Baseline Survey of SWADES Family Cohort Study. Int J Hypertens. 2020;2020:4964835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Ojha U, Ruddaraju S, Sabapathy N, Ravindran V, Worapongsatitaya P, Haq J, Mohammed R, Patel V. Current and Emerging Classes of Pharmacological Agents for the Management of Hypertension. Am J Cardiovasc Drugs. 2022;22:271-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Ott C, Schmieder RE. Diagnosis and treatment of arterial hypertension 2021. Kidney Int. 2022;101:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Gelaw S, Yenit MK, Nigatu SG. Self-Care Practice and Associated Factors among Hypertensive Patients in Debre Tabor Referral Hospital, Northwest Ethiopia, 2020. Int J Hypertens. 2021;2021:3570050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Jiang B, Liu Y, Fang F, Wang X, Li B. Association of four insulin resistance genes with type 2 diabetes mellitus and hypertension in the Chinese Han population. Mol Biol Rep. 2014;41:925-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Xi Y, Xu PF. Diabetes and gut microbiota. World J Diabetes. 2021;12:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 7. | Alsaadon H, Afroz A, Karim A, Habib SH, Alramadan MJ, Billah B, Shetty AN. Hypertension and its related factors among patients with type 2 diabetes mellitus - a multi-hospital study in Bangladesh. BMC Public Health. 2022;22:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Bazalar-Palacios J, Jaime Miranda J, Carrillo-Larco RM, Gilman RH, Smeeth L, Bernabe-Ortiz A. Aggregation and combination of cardiovascular risk factors and their association with 10-year all-cause mortality: the PERU MIGRANT Study. BMC Cardiovasc Disord. 2021;21:582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Abaturov A, Nikulina A. Obesity in Children with Leptin Receptor Gene Polymorphisms. Acta Medica (Hradec Kralove). 2021;64:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Stefan N, Vozarova B, Del Parigi A, Ossowski V, Thompson DB, Hanson RL, Ravussin E, Tataranni PA. The Gln223Arg polymorphism of the leptin receptor in Pima Indians: influence on energy expenditure, physical activity and lipid metabolism. Int J Obes Relat Metab Disord. 2002;26:1629-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, Mera A, Lago F, Gómez R, Gualillo O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 12. | Seoane-Collazo P, Martínez-Sánchez N, Milbank E, Contreras C. Incendiary Leptin. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Bełtowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Kim LJ, Shin MK, Pho H, Otvos L Jr, Tufik S, Andersen ML, Pham LV, Polotsky VY. Leptin Receptor Blockade Attenuates Hypertension, but Does Not Affect Ventilatory Response to Hypoxia in a Model of Polygenic Obesity. Front Physiol. 2021;12:688375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Wauters M, Mertens I, Rankinen T, Chagnon M, Bouchard C, Van Gaal L. Leptin receptor gene polymorphisms are associated with insulin in obese women with impaired glucose tolerance. J Clin Endocrinol Metab. 2001;86:3227-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Cao X, Huo P, Li W, Li P, He L, Meng H. Interactions among moderate/severe periodontitis, ADIPOQ-rs1501299, and LEPR-rs1137100 polymorphisms on the risk of type 2 diabetes in a Chinese population. Arch Oral Biol. 2019;103:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Nowzari Z, Masoumi M, Nazari-Robati M, Akbari H, Shahrokhi N, Asadikaram G. Association of polymorphisms of leptin, leptin receptor and apelin receptor genes with susceptibility to coronary artery disease and hypertension. Life Sci. 2018;207:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Salopuro T, Pulkkinen L, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Tuomilehto J, Laakso M, Uusitupa M; Finnish Diabetes Prevention Study Group. Genetic variation in leptin receptor gene is associated with type 2 diabetes and body weight: The Finnish Diabetes Prevention Study. Int J Obes (Lond). 2005;29:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Illangasekera YA, Kumarasiri PVR, Fernando DJ, Dalton CF. Association of the leptin receptor Q223R (rs1137101) polymorphism with obesity measures in Sri Lankans. BMC Res Notes. 2020;13:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Rong G, Tang W, Wang Y, Qiu H, Chen S. Investigation of leptin receptor rs1137101 G>A polymorphism with cancer risk: evidence from 35936 subjects. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Gu P, Jiang W, Chen M, Lu B, Shao J, Du H, Jiang S. Association of leptin receptor gene polymorphisms and essential hypertension in a Chinese population. J Endocrinol Invest. 2012;35:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Zhang L, Qin Y, Liang D, Li L, Liang Y, Chen L, Tong L, Zhou J, Li H, Zhang H. Association of polymorphisms in LEPR with type 2 diabetes and related metabolic traits in a Chinese population. Lipids Health Dis. 2018;17:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Al-Makki A, DiPette D, Whelton PK, Murad MH, Mustafa RA, Acharya S, Beheiry HM, Champagne B, Connell K, Cooney MT, Ezeigwe N, Gaziano TA, Gidio A, Lopez-Jaramillo P, Khan UI, Kumarapeli V, Moran AE, Silwimba MM, Rayner B, Sukonthasan A, Yu J, Saraffzadegan N, Reddy KS, Khan T. Hypertension Pharmacological Treatment in Adults: A World Health Organization Guideline Executive Summary. Hypertension. 2022;79:293-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 198] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 24. | Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2021;12:344-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (12)] |
| 25. | Solé X, Guinó E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1541] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 26. | Weng Z, Liu Q, Yan Q, Liang J, Zhang X, Xu J, Li W, Xu C, Gu A. Associations of genetic risk factors and air pollution with incident hypertension among participants in the UK Biobank study. Chemosphere. 2022;299:134398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Teo KK, Rafiq T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can J Cardiol. 2021;37:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 28. | Yuan M, Bi L, Su X. [Association of single nucleotide polymorphisms of LEPR gene with essential hypertension among ethnic Mongolian and Han Chinese from Inner Mongolia region]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2018;35:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Vashi N, Stryjecki C, Peralta-Romero J, Suarez F, Gomez-Zamudio J, Burguete-Garcia AI, Cruz M, Meyre D. Genetic markers of inflammation may not contribute to metabolic traits in Mexican children. PeerJ. 2016;4:e2090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Niu T. A meta-analysis of associations of LEPR Q223R and K109R polymorphisms with Type 2 diabetes risk. PLoS One. 2018;13:e0189366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Queiroz EM, Cândido AP, Castro IM, Bastos AQ, Machado-Coelho GL, Freitas RN. IGF2, LEPR, POMC, PPARG, and PPARGC1 gene variants are associated with obesity-related risk phenotypes in Brazilian children and adolescents. Braz J Med Biol Res. 2015;48:595-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Bains V, Kaur H, Badaruddoza B. Association analysis of polymorphisms in LEP (rs7799039 and rs2167270) and LEPR (rs1137101) gene towards the development of type 2 diabetes in North Indian Punjabi population. Gene. 2020;754:144846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Yu P, Ning Y, Gao Y, Zhao Y, Tie L, Wu L, Zhang L, Zhang R, Cui M, Pang H, Wu Q, Wang Z, Chen L, Zhao L. Hypertension among Mongolian adults in China: A cross-sectional study of prevalence, awareness, treatment, control, and related factors: Hypertension among Mongolian adults in China. J Clin Hypertens (Greenwich). 2021;23:1786-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Sun Z, Zheng L, Xu C, Li J, Zhang X, Liu S, Hu D, Sun Y. Prevalence of prehypertension, hypertension and, associated risk factors in Mongolian and Han Chinese populations in Northeast China. Int J Cardiol. 2008;128:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 409] [Article Influence: 51.1] [Reference Citation Analysis (1)] |
| 36. | Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 740] [Article Influence: 185.0] [Reference Citation Analysis (0)] |