INTRODUCTION
Obesity and type 2 diabetes mellitus rates are rising globally. Obesity is the commonest form of malnutrition in the developed world, and is rapidly increasing in developing countries[1-3]. Obesity is strongly associated with insulin resistance and the development of type 2 diabetes. By 2050, it is predicted that half a billion men, women, and children will have type 2 diabetes, of whom three quarters will be from low and middle income countries (LMIC)[4]. Diabetes and its complications including kidney disease, heart disease, stroke, retinopathy and neuropathy, lead to premature mortality, morbidity, disability and reduced quality of life in affected individuals. At present, someone dies due to diabetes-related complications every 7 s[5]. Furthermore, it also leads to decreased work-force productivity, increased healthcare utilization and escalating healthcare costs[6]. Ten percent of the global health expenditure is spent on diabetes-related care[4].
Obesity is the main driver of type 2 diabetes. Obesity refers to the excess accumulation of body fat to an extent that it is harmful to an individual’s health. The fundamental cause of obesity is an imbalance between energy intake and expenditure, with excess energy being stored as fat in adipose tissue. This predisposes adipocytes to secrete more pro-inflammatory adipocytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), causing a state of low-grade inflammation and insulin resistance. An increase in insulin resistance necessitates a compensatory increase in insulin secretion from pancreatic β cells, and failure to achieve this demand results in diabetes. This, however, is a gradual process, and it may take years for diabetes to manifest clinically. Thus, early identification and modification of risk factors at the onset of the above trajectory could help prevent type 2 diabetes[5].
The etiology of obesity and type 2 diabetes is multifactorial and involves complex interactions between genetic, environmental and behavioral factors[3,7]. The rapid rise in obesity is mostly attributed to the unhealthy lifestyle associated with urbanization and technical advancement over the last three to four decades[8]. The present generations live within an obesogenic environment, with energy imbalance arising from excessive energy intake due to high fat, high-sugar, energy-dense processed foods, and a reduction in occupational, household and leisure-time physical activity[3,7,9]. However, there is evidence of an additional factor leading to increases in obesity and type 2 diabetes. This is the impact of the prenatal and early-life environment on long-term health via fetal programming.
The Developmental Origins of Health and Disease (DOHaD) concept states that early-life environmental influences at sensitive periods of development lead to lifelong effects on health and chronic disease risk[10]. There is evidence that exposure to an abnormal in utero environment disturbs the metabolic programming of the growing fetus, increasing the lifelong risk of chronic diseases including type 2 diabetes[11-15] This process is described as fetal or developmental programming[16]. Fetal programming is now recognized as a key factor contributing to the rapid rise in obesity and type 2 diabetes mellitus rates worldwide. Research in humans and animals over the past two decades has provided considerable evidence supporting ‘developmental programming’ by the intrauterine environment[17].
Fetal programming helps explain certain aspects of the obesity epidemic that cannot be fully explained by genetic and environmental factors. The relatively short time over which obesity and type 2 rates have escalated precludes genetic change as a major attributor[15,18]. Furthermore, energy homeostasis and body weight are regulated by biological systems established in early life. Thus, it is difficult to explain how lifestyle changes alone, can override these biological homeostatic mechanisms to bring about obesity[15,18]. Fetal programming is the most plausible reason for this phenomenon. Dysregulation of biological mechanisms maintaining body weight by early life fetal programming also helps explain why reversal of obesity is difficult[19].
FETAL PROGRAMMING OF OBESITY AND TYPE 2 DIABETES
Epidemiological, clinical, and basic sciences research suggest that the foundations of an individual’s lifelong health, including predisposition towards obesity and type 2 diabetes is largely established during the ‘first 1000 days of life’ from day of conception to completion of the 2nd year of life. This is a highly sensitive period of growth and development in humans, where biological systems are formed and developed[10,20,21].
It is difficult to separate out effects of in utero exposure from genetic and nurturing influences in humans. However, studies in small mammals and other animal models have shown that prenatal exposure to an adverse in utero environment associated with maternal overnutrition results in developmental programming of obesity and other disorders in offspring[22-25]. For example, in genetically-modified obesity-prone rats, greater postnatal adiposity was observed in offspring born to over-nourished dams, compared to normally nourished dams[22]. Furthermore, offspring of over-nourished dams developed greater body weight and body fat compared to offspring of lean dams, even when both groups were fostered by lean dams after birth[23]. These studies indicate that in utero exposure to maternal obesity per se increases susceptibility to obesity in later life, beyond genetics or nurturing practices.
The prenatal environment in humans appears to be influenced by maternal body composition, metabolism, stress and diet from conception and throughout pregnancy. Paternal influences are also being recognized. Thus, parental lifestyle appears to influence the health of the offspring prior to birth, via fetal programming. From the maturation of gametes through to early embryonic development, parental lifestyle can adversely influence long-term risks of offspring metabolic, cardiovascular, immune, and neurological morbidities[26].
FACTORS PREDISPOSING TO DEVELOPMENTAL PROGRAMMING OF OBESITY/TYPE 2 DIABETES AND POTENTIAL MECHANISTIC PATHWAYS
The field of developmental programming has begun to move beyond associations to potential causal mechanisms for developmental programming. Studies in humans and animal models are helping unravel underlying biological mechanisms underpinning fetal programming, including epigenetic, cellular, physiological, and metabolic processes[26]. We have however, still not gained a complete understanding of the complex ways in which the maternal genome, metabolome, and microbiome relate throughout pregnancy and lactation to increase the offspring’s disease risk across the life span[25]. Determining mechanisms of fetal programming has been complicated by rapid changes in the social environment and human behavior. Thus, more studies are needed to help better delineate the pathophysiological mechanisms underpinning fetal programming[25].
Epigenetics, and mechanisms of epigenetic modification have led to increased understanding of developmental programming, and how environmental, genetic and epigenetic factors inter-relate to cause lasting effects on offspring size, adiposity and future metabolic outcomes. Neonatal methylation markers associated with birth weight from several gene loci, have shown significant associations with the prenatal environment, as well as longitudinal associations with offspring size and/or adiposity in early childhood, providing evidence that developmental pathways to adiposity begin before birth and are influenced by environmental, genetic and epigenetic factors[16]. Disruption of the gut microbiome observed in maternal obesity, antibiotic use in pregnancy, delivery and early infancy, and cesarean section have also been implicated in increased childhood obesity risk. Disruption of microbiome colonization during critical periods of early development can predispose offspring to obesity, asthma, allergy and diabetes. This may occur due to cesarean delivery, and the use of prophylactic antibiotics during cesarean section, as well as maternal exposure to antibiotics in the second and third trimesters of pregnancy, and use of antibiotics in the offspring in early infancy. Furthermore, increased maternal body mass index (BMI) per se is associated with altered intestinal microbial community structure of infants’ stool up to 2 years of age[25]. Future research in epigenetics and the gut microbiome could yield greater insights into the mechanistic pathways as well as potential methods of modulating fetal programming.
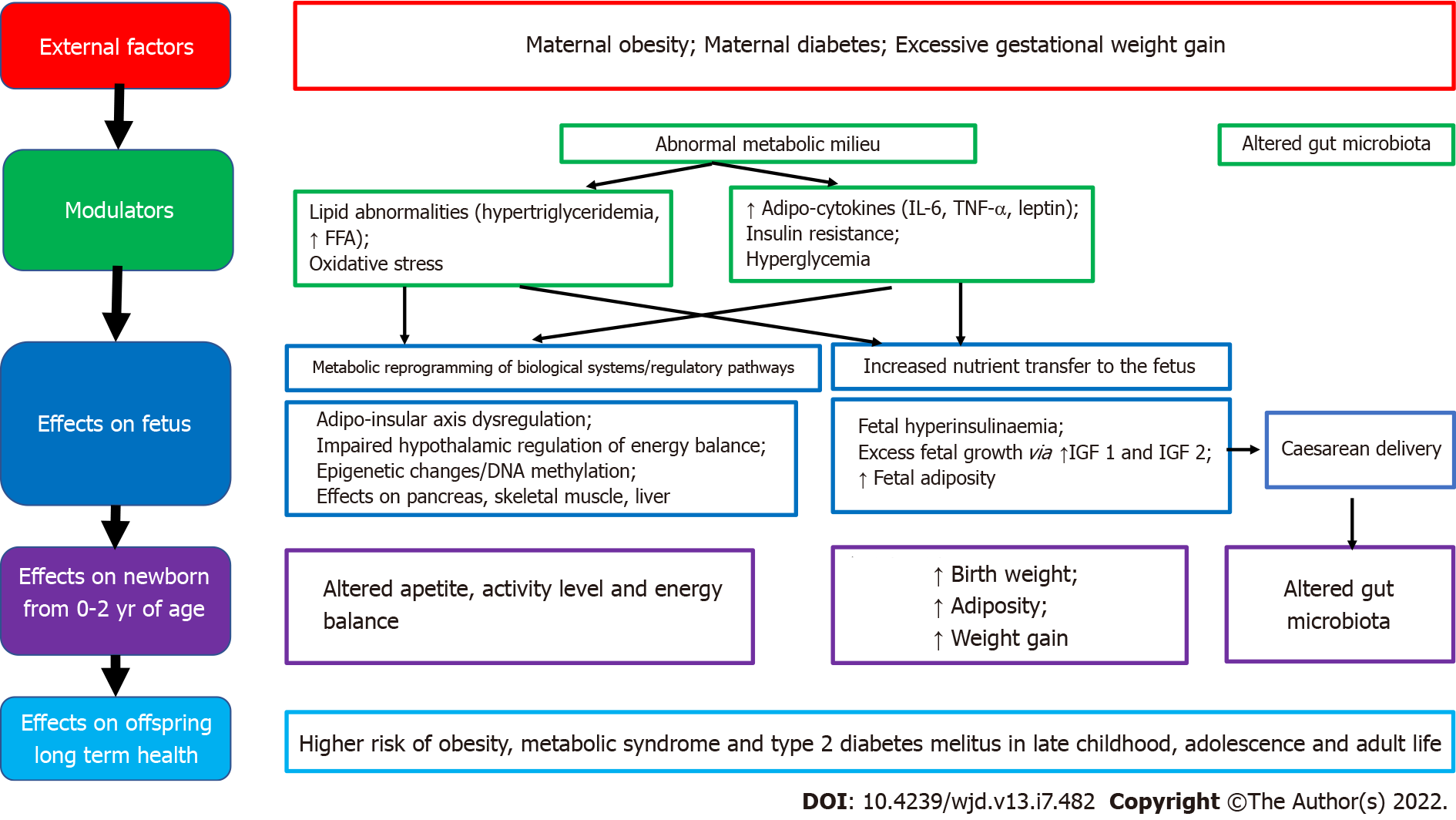
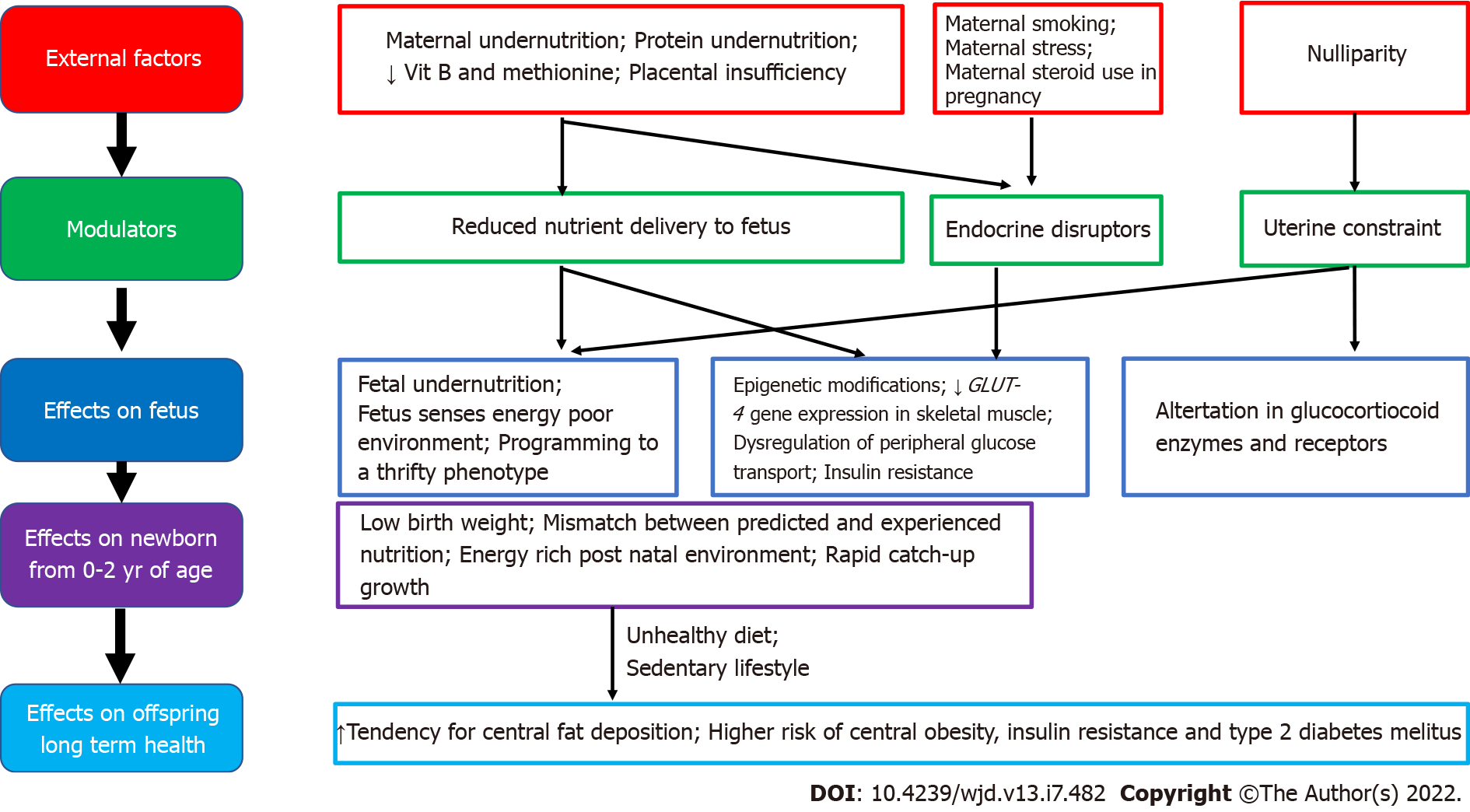
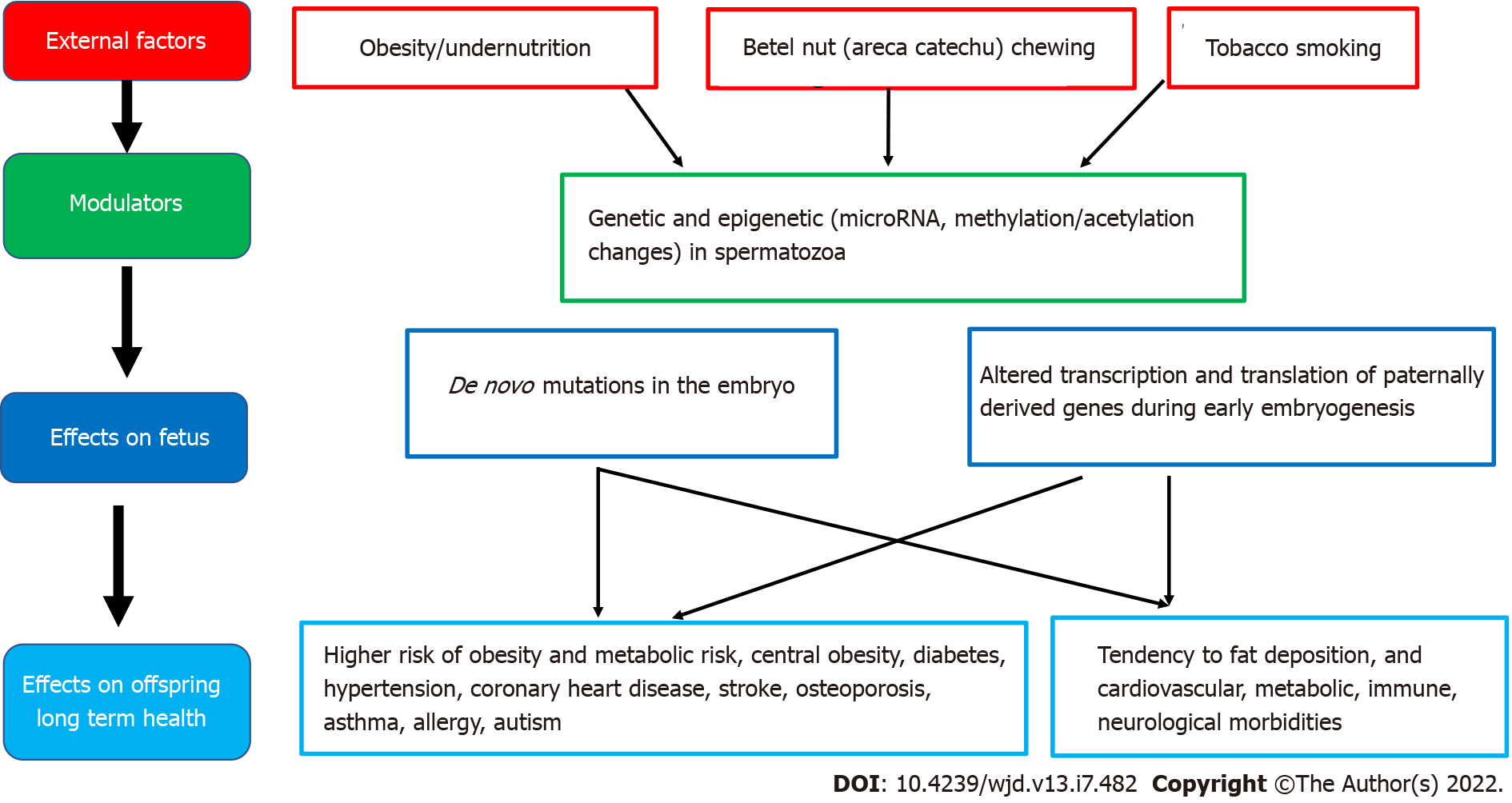
Good maternal nutrition prior to and during pregnancy is important for optimizing offspring long-term health. Fetal programming, initially described in relation to fetal undernutrition, was associated with a higher risk of central obesity, diabetes, hypertension, coronary heart disease and stroke in adult life[12]. Fetal growth is influenced by the in utero environment, and there is trouble at both ends of the birthweight spectrum, with a ‘J’ shaped relationship between birth weight and future obesity risk[27]. It is proposed that the fetus ‘senses’ its future nutritional status via in utero signals from the mother, and responds in ways which establish lasting influences on weight and appetite control[28]. Many umbilical cord blood metabolites and hormones are associated with birth weight and adiposity in human infants[25]. Paradoxically, both a nutritionally limited or nutritionally excessive in utero environment can lead to later obesity and associated co-morbidities[29]. More recent evidence emphasizes the adverse developmental programming effects of fetal overnutrition, and its association with increased risk of obesity in childhood and adulthood[29,30]. In addition, paternal factors are also now being recognized to play a role in fetal programming. The effects of maternal overnutrition, maternal undernutrition/ stress and paternal factors on fetal programming of obesity/type 2 diabetes including potential modulatory pathways and effects on the offspring are shown in Figures 1-3.
Figure 1 Associations between maternal overnutrition and fetal programming of obesity/type 2 diabetes mellitus including potential modulating factors and effects on offspring health.
FFA: Free fatty acid; TNF-α: Tumor necrosis factor alpha; IL-6: Interleukin-6; IGF: Insulin-like growth factor.
Figure 2 Associations between maternal undernutrition and fetal programming of obesity/type 2 diabetes mellitus including potential modulating factors and effects on offspring health.
Figure 3 Associations between paternal health factors and fetal programming of obesity/type 2 diabetes mellitus including potential modulating factors and effects on offspring health.
Maternal overnutrition
Concurrent with the global epidemic of obesity, the prevalence of overweight and obesity in women of reproductive age has risen rapidly over the last three decades[7,31,32]. There is now compelling evidence from human as well as animal studies that maternal obesity, diabetes and increased gestational weight gain all increase offspring birth weight and lead to fetal programming of obesity in the offspring[33]. It is thought that offspring obesity is programmed by the ‘obesogenic’ maternal metabolic environment the fetus is exposed to in utero during development, setting in an ‘obesity cycle’, where maternal obesity leads to neonatal obesity which continues to childhood and adulthood, propagating obesity in the next generation[34,35]. Thus, an increase in overweight and obesity among women of reproductive age should be considered an important modulator of the global obesity epidemic, which is further propagating obesity in future generations.
Children born to overweight/obese women have increased birth weight and an increased risk of obesity and metabolic dysregulation throughout life[34,36-38]. At birth, these babies have increased birth weight and adiposity[39], and thus, an increased risk of assisted delivery as well[40,41]. Exposure to maternal obesity and diabetes accelerates adipogenesis and impairs energy sensing, affecting neurodevelopment, liver, pancreas, and skeletal muscle development in the offspring, creating a lifelong impact on multiple systems[25]. The influence of maternal obesity on the risk of offspring obesity starts manifesting from early life[36,42]. These children show increased weight for age and length, in comparison to offspring of normal weight women, as early as six months of age[42], and their risk of obesity is increased two-fold as preschoolers, even after controlling for birth weight and other confounding factors[36]. They also have an increased risk of metabolic syndrome by late childhood[43]. Furthermore, high maternal BMI in pregnancy is an independent predictor of obesity in the adult offspring, at 30 years of age[37].
If the mother is obese during pregnancy, there is excess transfer of nutrients to the fetus, stimulating increased fetal insulin secretion, fetal overgrowth and increased adiposity. It is hypothesized that this tendency for fat accrual then tends to persist during childhood and adulthood. Furthermore, the metabolic milieu of overweight/obese mothers differs from normal weight mothers, with obese pregnancy being associated with higher insulin resistance, pro-inflammatory adipokines (leptin, IL-6, TNF-α) and lipid abnormalities. In utero exposure to this abnormal metabolic milieu is also implicated in fetal programming[31,35,44]. Factors associated with developmental programming of obesity in offspring of mothers who have obesity/diabetes in pregnancy include high glucose levels, triglycerides, free fatty acids, adiponectin, leptin, hypoxia, oxidative stress, inflammation, and the microbiome[25]. It is proposed that fetal exposure to this abnormal metabolic milieu leads to dysregulation of the offspring adipo-insular axis (leptin and insulin) causing alterations in the central nervous system regulation of appetite, activity level, energy balance and in adipocyte metabolism[14,34,44,45].
Maternal diabetes
Maternal diabetes during pregnancy is also strongly associated with fetal programming of obesity in the offspring. At present, approximately 20 million live births are affected by hyperglycemia in pregnancy, globally[4]. There is evidence that offspring of mothers with gestational diabetes mellitus have an increased risk of developing obesity, insulin resistance, type 2 diabetes, hypertension and cardio-vascular complications at a relatively young age[46]. In the follow-up of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, 4160 children aged between 10-14 years, whose mothers had a 75-g oral glucose tolerance test at 28 wk of gestation demonstrated that exposure to higher maternal glucose spectrum levels in utero was significantly associated with childhood glucose levels and insulin resistance, independent of maternal and childhood BMI and family history of diabetes[47]. Studies have also shown associations between the sex of the fetus and maternal blood glucose concentrations during pregnancy, suggesting that fetal programming could be influenced by offspring gender as well[48,49].
Maternal undernutrition
Maternal undernutrition during critical periods of fetal development has been linked with fetal programming of central obesity, insulin resistance, metabolic syndrome and type 2 diabetes in later life, especially when exposed to an energy-rich diet postnatally. Adipogenesis, which begins in utero and accelerates in neonatal life, is a major candidate for developmental programming. According to the thrifty phenotype hypothesis, maternal undernutrition during critical periods leads to compensatory changes in the fetus, including tendency to store fat, which causes central obesity in later life, when there is a mismatch between the predicted and experienced postnatal nutritional environment[50,51].
Epigenetic pathways in fetal programming from in utero undernutrition described include histone modifications in skeletal muscle that directly decrease GLUT-4 gene expression, which leads to metabolic dysregulation of peripheral glucose transport and insulin resistance, which can contribute to the development of type 2 diabetes in later life. These fetal programming changes, combined with the effects of obesity, ageing and physical inactivity, are the most important factors in determining type 2 diabetes in those born with low birthweight[51]. Furthermore, specific maternal nutrient deficiencies during pregnancy, including low maternal protein consumption, and poor vitamin B and methionine status are also associated with an increased risk of metabolic derangements and type 2 diabetes in later life. Evidence from animal studies show that a protein-restricted diet in utero programs susceptibility to obesity, when exposed to overnutrition in postnatal life[50,52].
Prenatal stress could also be a modulating factor for fetal programming of obesity in severe maternal malnutrition[53]. During fetal development, the hypothalamic-pituitary-adrenal axis is extremely susceptible to programming, and alterations in the expression and function of glucocorticoid receptors and major glucocorticoid regulatory enzymes are observed in those exposed to undernourishment in early life[54]. Other factors associated with fetal programming include maternal exposure to endocrine disruptors, maternal infection and smoking and nulliparity[17,55]. Nulliparity is potentially associated with subtle adverse metabolic outcomes in overweight/obese mothers and their offspring, through uterine constraint effects[55].
Paternal factors
Epidemiological and animal studies suggest that many factors, including paternal under- and over-nutrition, exposure to environmental toxins, father's health conditions such as diabetes, and even grandfather's nutritional status can program diseases in the offspring via germ cell-mediated transmission[56]. High paternal BMI has been linked with newborn adiposity[57]. Furthermore, paternal overweight/obesity appears to induces paternal programming of offspring phenotypes, through genetic and epigenetic changes in spermatozoa. Both human and rodent models have established that paternal obesity impairs sex hormones, basic sperm function, and molecular composition, which can result in perturbed embryo development and increase subsequent offspring disease burden[57]. Theories for the origin of male obesity-induced paternal programming include the accumulation of sperm DNA damage resulting in de novo mutations in the embryo and changes in sperm epigenetic marks (microRNA, methylation, or acetylation) altering the access, transcription, and translation of paternally derived genes during early embryogenesis[57].
Postnatal factors
In keeping with the concept of “the first 1000 days of life”, postnatal factors from the time of birth to the second birthday of a child, could also contribute towards adverse programming increasing the risk of obesity and type 2 diabetes in later life. Our present state of knowledge includes mainly early life nutritional practices, including breastfeeding duration, timing of introducing complementary feeding, and protein rich foods[58]. The underlying mechanisms are yet unclear, but there is emerging evidence that it is associated with altered neuro-endocrine programming, and modified by breastfeeding duration and maternal pre-pregnancy overweight[58,59]. Breastfeeding including longer duration of exclusive breastfeeding and longer duration of partial breastfeeding have been associated with a reduced risk of later life obesity and obesity-related complications. Breastfeeding for greater than 40 wk has been associated with lower weight gain by 1 year, and longer duration of breastfeeding with lower odds of developing hypercholesterolemia, hypertension, obesity and type 2 diabetes in later life[60]. Furthermore, mothers who are overweight and obese appear to breastfeed their babies for a shorter duration and introduce complementary foods earlier than mothers of normal weight, which could play a role in their offspring having increased weight and BMI from early childhood[59]. Exclusive breastfeeding for 6 mo or longer, and delaying the introduction of complementary feeding until 5th month of age, are also associated with lower risk of overweight at 5-6 years of age[61]. In addition, social factors including poor nurturing practices and role modeling by parents, early introduction of highly processed high fat, high sugar snacks/meals and exposure to unhealthy food advertising, are early life factors associated with increased offspring obesity.
PREVENTION OF FETAL PROGRAMMING
Primary and secondary prevention of obesity are at the foundation of diabetes prevention programs. While several medical and lifestyle strategies have shown promising effects in slowing progression to and minimizing complications of type 2 diabetes, implementing community measures to prevent obesity/type 2 diabetes are bound to be more cost-effective and beneficial to the community at large, compared to the cost of screening, treating and managing complications of established obesity/type 2 diabetes.
There is now increasing focus on primary prevention of obesity/type 2 diabetes targeting the first 1000-d of life[5]. The first 1000-d of life offers a unique and critical window of opportunity to shape long-term health at the population level, which can have a lasting effect on a country’s health and prosperity. Firstly, however, it is prudent to consider the important fundamental question of whether fetal programming of obesity can be minimized by interventions which improve the in utero environment of the fetus, in humans. The most promising research findings on preventing adverse fetal programming have come from animal models under experimental conditions. Whether these interventions could be applied in clinical practice, and their effectiveness remain uncertain. However, there are emerging data that improvement in fetal overnutrition and risk of obesity can be achieved via maternal interventions. Perhaps the best evidence available to date, is improvement in long-term health outcomes observed in offspring born to severely obese women, after maternal weight loss following bariatric surgery[62]. Studies comparing offspring pairs born to morbidly obese women conceived before and after substantial weight loss following bariatric surgery found that children conceived after surgery had a lower risk of macrosomia (birth weight > 4 kg) at birth, and continued to have better health outcomes in childhood and adolescence including a 50% lower risk of obesity, three-fold lower risk of severe obesity, and better insulin sensitivity and lipid profile, compared to their older siblings[62-64]. These findings confirm that pre-conception weight loss in severely obese mothers can lower fetal overnutrition and reduce the risk of obesity and metabolic complications in the offspring. However, weight reduction by bariatric surgery prior to conception is not an easily available or feasible option for most overweight and obese women of reproductive age, making it necessary to consider alternative interventions which could potentially improve offspring health outcomes.
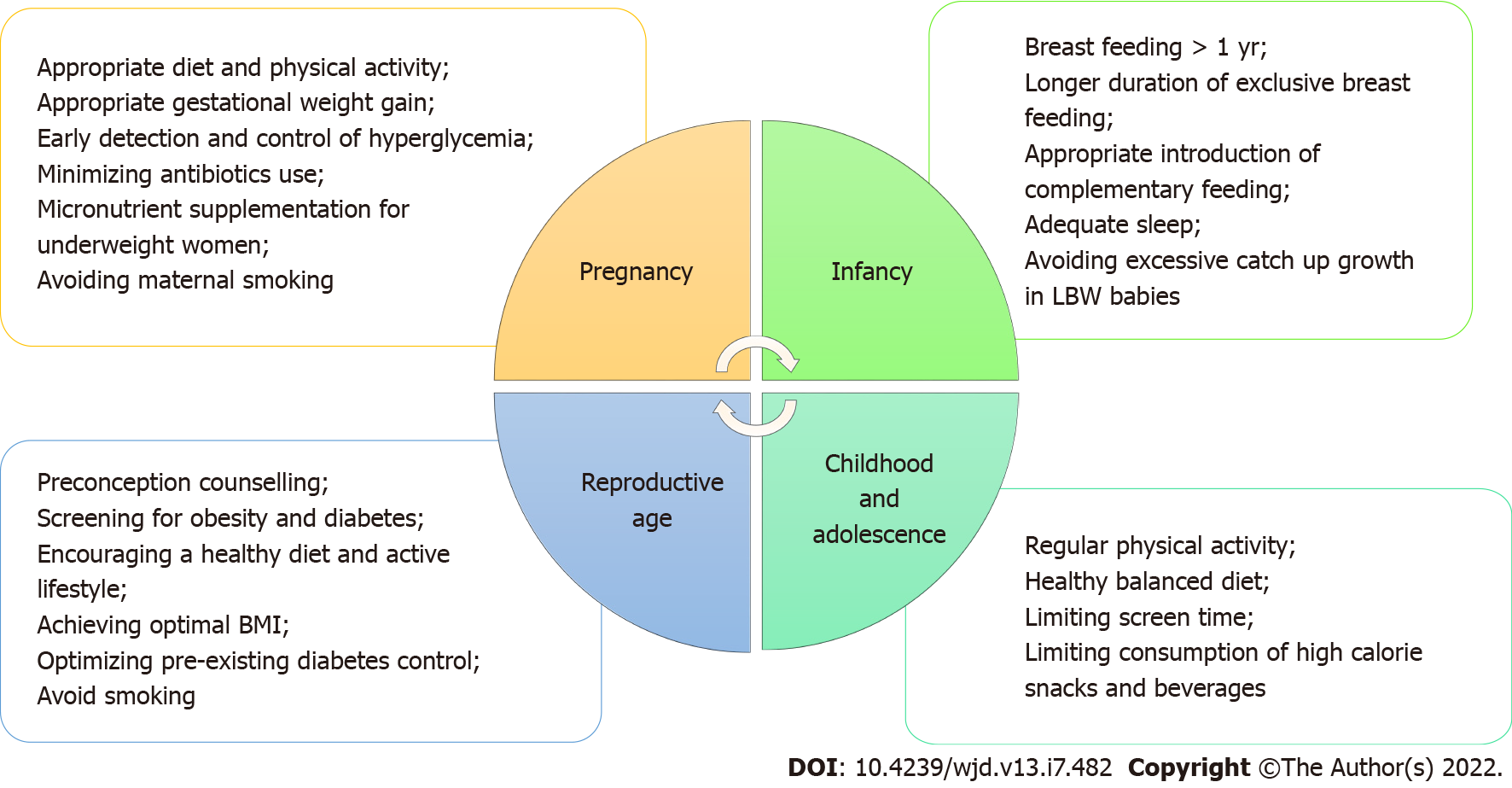
The World Health Organization, having recognized and acknowledged the potential impact of fetal programming on the obesity epidemic, now advocates a life-course approach for the prevention and control of non-communicable diseases including obesity. This life cycle approach starts with maternal health including preconception, antenatal and postnatal care, and maternal nutrition[65,66]. Potential measures that can be taken at various stages of the life cycle to reduce adverse effects of fetal programming of obesity and type 2 diabetes in future generations are shown in Figure 4.
Figure 4 Potential measures that can be taken at various stages of the lifecycle to reduce adverse effects of fetal programming of obesity and type 2 diabetes mellitus in future generations.
LBW: Low birth weight; BMI: Body mass index.
Lifestyle interventions during pregnancy
When considering the impact of intrauterine overnutrition and macrosomia on obesity risk in the next generation, public health measures for healthy maternal weight throughout the reproductive years is justified. Ideally body weight should be optimized to a healthy BMI in all women planning a pregnancy, but this is easier said than done. Pregnancy itself, however, can be an opportune period to commence healthy lifestyle changes, if heath care providers consider it as a "teachable moment" to educate pregnant women on the potential benefits to the baby as well as the mother, and utilize regular and frequent contact with heath care services during this time to provide encouragement and guidance to institute lifestyle interventions[67].
Lifestyle interventions during pregnancy and postpartum appear to reduce gestational weight gain, pregnancy-induced hypertension, need for cesarean section and neonatal respiratory distress syndrome, without any risk of harm to the mother or neonate, across all maternal BMI categories[68]. Thus, a healthy diet and regular exercise for all healthy women during pregnancy and postpartum is a low-cost and feasible intervention which has been advocated as a global health policy[68,69].
Antenatal lifestyle intervention in maternal obesity
Given the rising rates of obesity in women of reproductive age, first in developed, and subsequently in developing countries over the past few decades, there is an urgent need for effective interventions to reduce adverse fetal programming due to maternal obesity[70]. There is expert consensus, that antenatal lifestyle interventions in overweight and obese pregnant women could alter adverse fetal programming and improve offspring health[71,72]. It is postulated that modifying the obesogenic in utero environment by lifestyle changes such as increased antenatal physical activity or improved dietary intake during pregnancy could reduce harmful programming effects in the offspring[72]. Antenatal nutrition-al/lifestyle interventions in overweight/obese pregnant women could potentially be effective by preventing excessive maternal gestational weight gain, and by reducing the risk of developing gestational diabetes, and improving the unhealthy maternal metabolic milieu (insulin resistance, hyperinsulinemia, hyperglycemia, hyperlipidemia, and increased inflammatory markers) which lead to adverse fetal programming[73,74].
Several studies have investigated if lifestyle interventions in overweight and obese mothers during pregnancy can attenuate offspring programming of obesity. In overweight/obese women, multi-component interventions with both a diet and physical activity component have shown some promise, with reduction in gestational weight gain, pregnancy-induced hypertension, macrosomia and neonatal respiratory distress syndrome[68]. Diet was associated with greater reductions in the risk of gestational diabetes mellitus, pregnancy-induced hypertension and preterm birth, compared with any other intervention[68]. However, the effects of these interventions on long-term offspring health are unclear. Studies such as the LIMIT study in Australia reported that providing pregnant women who were overweight or obese with an antenatal dietary and lifestyle intervention improved maternal diet and physical activity during pregnancy, but did not alter 6-mo infant growth and adiposity, or childhood dietary intake, growth and adiposity at 3-5 years of age.
Prenatal exercise has been considered a potential intervention to reduce adverse fetal programming, especially in pregnancies complicated by obesity and/or diabetes[70,75]. Previously, there were concerns regarding the safety of exercise during pregnancy, due to fears regarding teratogenicity from exercise-induced hyperthermia, and fetal hypoxia and intrauterine growth retardation from redistribution of blood flow and nutrients away from the utero-placental circulation during exercise[76]. However, studies on maternal antenatal exercise over the past 20 years have demonstrated that mild-to-moderate intensity antenatal exercise in healthy well-nourished women does not cause observable harm to the fetus[77-80]. There has since been a gradual change of opinion that moderate antenatal exercise is not only safe, but may also be beneficial to offspring health[71,78]. Detailed small scale studies have shown that offspring of physically active lean women who engaged in regular vigorous exercise during pregnancy had lower birthweight and subcutaneous fat at birth, and continued to have lower weight and subcutaneous fat in childhood[81].
However, the effects of antenatal exercise during pregnancy on offspring health appear to vary depending on exercise intensity and frequency, as well as its timing in relation to the period of gestation[82-85]. Commencing exercise in early pregnancy appeared to stimulate feto-placental growth, and increase birth weight, while exercising in the second half of pregnancy appeared to reduce birth weight[83,86]. Furthermore, while it is postulated that regular antenatal exercise during the second half of pregnancy may lead to a reduction in birth weight and adiposity in the offspring, which may be protective against obesity in later life[87], it does not appear to be effective in practice, especially in overweight and obese women[88]. The results of clinical trials targeting antenatal exercise in overweight and obese women have led to varying/inconclusive findings on birthweight and other markers of fetal programming[89,90]. Many trials on supervised antenatal exercise interventions in overweight and obese women have reported a lack of effect on birth weight, or other markers of fetal programming[88,89,91,92]. One explanation for this could be that obese women, who are generally less physically active, tend to further reduce activity levels during pregnancy[70].
Thus, at present, lifestyle interventions during pregnancy in women with obesity/diabetes have not shown much effect on infant or childhood outcomes. However, many such clinical trials started later in pregnancy, and it is possible that developmental programming occurs much earlier and interventions focusing on healthy lifestyle interventions in pregnant humans are missing the crucial time period for effectiveness[25].
Interventions for pregnancies complicated by gestational diabetes/pre-existing maternal diabetes
In woman with diabetes in pregnancy, tight glycemic control will help minimize adverse fetal programming of obesity and diabetes in the offspring. For women with both pre-existing and gestational diabetes, offspring outcomes can be optimized by ensuring appropriate gestational weight gain, and optimal glycemic control via close monitoring of blood glucose levels, and appropriate medical and nutritional therapy and exercise, throughout the pregnancy[93].
For women with pre-existing type 2 diabetes, insulin has long been considered the gold standard managing diabetes during pregnancy[93]. Careful blood glucose monitoring and titration of insulin doses are important as total daily insulin requirement increases linearly with advancing pregnancy[93]. For women with pre-existing diabetes, it is also important to provide preconception counseling, to achieve optimal pre-conceptional body weight and glycemic control, prior to pregnancy whenever possible. The onus is on health care providers to educate and counsel women with diabetes, particularly on the importance of these aspects not only for their own health status but also to protect their unborn baby from the risks of fetal programming.
For women with gestational diabetes, both insulin and metformin can be used to maintain blood glucose levels if lifestyle interventions are inadequate to achieve adequate glycemic control. Furthermore, for women with a previous history of gestational diabetes, post-partum weight reduction prior to pregnancy could potentially help reduce gestational diabetes mellitus and its associated complications in subsequent pregnancies[94]. The MiG TOFU study, a prospective longitudinal follow-up study in Australia and New Zealand which randomized pregnant women with gestational diabetes mellitus to either metformin or insulin therapy, found that mothers on metformin, had higher glycemia in pregnancy and higher rates of babies with birth weight > 90th percentile, compared to those on insulin therapy, while offspring had similar adiposity at 2 years of age, and similar total and abdominal percentage of body fat and metabolic measures at 7-9 years of age[95].
Interventions for maternal undernutrition
Due to a paucity of evidence from long-term follow-up studies, current recommendations to reduce adverse fetal programming effects of maternal undernutrition, presume that interventions helping to optimize pregnancy outcomes and promote healthy infant growth and development will also help improve the long-term risk of chronic diseases such as central obesity and type 2 diabetes. These recommendations include optimizing maternal nutrition prior to pregnancy, ensuring adequate micronutrient intake in the preconception period and throughout pregnancy before birth, and encouraging breastfeeding and high quality complementary foods to the offspring after birth[96]. Maternal multiple micronutrient supplementation including vitamin and mineral supplementation during the preconception period and early pregnancy have shown some benefit in reducing fetal undernutrition and other adverse fetal programming effects in undernourished mothers[21].
Balanced protein-energy supplementation also appears to be an effective intervention to reduce the prevalence of low birthweight and small-for-gestational-age births, especially in undernourished women[97]. Thus, ensuring appropriate and adequate intake of micronutrients, essential fats and protein supplementation in mothers with undernutrition during pregnancy, could improve the nutritional condition of the mother, and confer a protective benefit to the offspring by reducing fetal growth restriction and low birth weight in developing countries with high rates of maternal undernutrition[96].
Interventions in offspring in infancy and childhood
Intervention strategies to reduce adverse effects of fetal programming of later life obesity may be more effective if they target multiple modifiable factors, focusing on the first 1000-d of life.
Breastfeeding appears to protect against obesity in childhood, and could be a modifying factor to mitigate the adverse effects of fetal programming in utero. Promoting longer duration of full breastfeeding and partial breastfeeding, and delaying the introduction of complementary feeding could protect the offspring from obesity. Exclusive breastfeeding for 6 mo or longer, and delaying the introduction of complementary feeding until 5th month of age, has been associated with a lower risk of overweight at 5-6 years of age[61]. The protective effects of breastfeeding on the offspring of diabetic mothers in very early life appears somewhat conflicting, with one study suggesting a potential negative long-term influence on the risk of becoming overweight in offspring exposed to breast milk from mothers with diabetes (type 1 or gestational diabetes) during the first week of life[60]. However, overall, the benefits of breastfeeding appear to be beneficial, and protect infants from the adverse effects of fetal programming of obesity and type 2 diabetes. Women who were overweight or obese before pregnancy, appear to breastfeed their offspring for a shorter time and introduce complementary feeding earlier than normal weight women, which could contribute towards their children being heavier and having a higher BMI by end of infancy[59]. Thus, it is especially important to take measures to encourage and support longer duration of breastfeeding in women who are overweight or obese.
Further protective measures that could be helpful in optimizing long-term health during infancy include ensuring adequate sleep and minimizing antibiotic use. Early antibiotic use before 2 years of age has been associated with disruption of the gut microbiota, and a higher risk of childhood overweight and obesity[98]. Recent evidence on the associations with gut microbiota and infant weight gain or child weight status, suggest that dietary manipulation with human milk and pre/probiotic formulations holds promise for preventing obesity[99].
Furthermore, as short sleep duration increased the risk of childhood obesity, public health efforts that encourage children to have sufficient sleep time are also important in combating obesity[100]. Project Viva prospectively studied the cumulative number of modifiable early-life risk factors associated with programming of obesity/type 2 diabetes in mother-offspring pairs including: maternal smoking and consumption of high sugar-sweetened beverages during pregnancy, excessive gestational weight gain; breastfeeding for less than 1 year; complementary food introduction before 4 mo; and infant sleep duration less than 12 h daily. When reassessed in early adolescence, they found that offspring with 5-6 risk factors had a 2.5 higher rate of obesity and metabolic syndrome, compared to those with 0-1 risk factors[101]. Thus, it appears that promoting exclusive breastfeeding for at least the first 4 mo of life, and continuation of breastfeeding beyond the first year of life, as well as ensuring adequate sleep for infants, could potentially reduce the risk of further life obesity in infants who have already been exposed to risk factors for adverse fetal programming in utero.
Other potential strategies to reduce the adverse impact of fetal programming include identifying and targeting young children at higher risk of fetal programming of obesity/diabetes such as offspring of mothers with obesity/diabetes/undernutrition during pregnancy, especially those being reared in highly urbanized obesogenic environments, for healthy lifestyle interventions during early childhood to encourage them towards a healthier lifestyle, and prevent adverse metabolic health outcomes in later life[46]. Pairing breastfeeding with healthy weaning foods is likely to promote healthy weight trajectories.
A recent review reported that several multicomponent trials promoting breastfeeding, responsive feeding, and a healthy diet (increased fruit and vegetables, and limiting sugar sweetened beverages and unhealthy snacks) through home visits or education at baby health clinics over 1-2 years duration, showed relative reductions in BMI in offspring at the end of the intervention, although early benefits were not maintained in the two trials reporting follow-up 1 year to 3 years later[102]. Thus, there is some evidence that nutrition or feeding interventions in the first two years of life can have a positive impact on a child's BMI, but maintaining this benefit may require continued intervention and sustainable environmental change[102].
Observational studies suggest that rapid weight gain in infancy also increases the long-term risk of obesity and type 2 diabetes in infants from both low-and high-income countries, among infants born preterm or at term, with normal or low birth weight for gestation[103]. Furthermore, it has been hypothesized that the increased risk of adverse long-term outcomes including central obesity and type 2 diabetes in low birth weight infants may be driven by accelerated postnatal catch-up growth. While some studies on health outcomes in babies with low birth weight have reported that increased catch up growth was associated with higher BMI or higher serum cholesterol levels in early adolescence, the quality and quantity of the evidence is limited[102]. Thus, it is prudent to recommend “striking a healthy balance”, especially for low and middle income countries, until more information on underlying mechanisms and suitable interventions on minimizing adverse effects of catch up growth in low birth weight infants become available[104].
Beyond infancy, promising interventional approaches for pre-school age children include age appropriate health and nutrition education for preschoolers, combined with teaching parents behavioral change strategies and increasing parenting skills[105]. For school children, school-based interventions have been shown to be effective in reducing excessive weight gain in children[106]. Programs involving both school and family and lasting ≤ 1 year were the most efficacious for primary school children aged between 6 and 12 years; while family-based interventions have been effective in children < 6 years old[107].
Preconception care
Healthy lifestyle behaviors during the preconception period are important to optimize maternal and child outcomes. Community nurses and midwifery professions who are active across both preconception and pregnancy could play an important role in such interventions[108]. Many women of reproductive age do not appear to have optimal preconception lifestyle behaviors, and a recent systematic review identified the absence of knowledge on healthy behaviors as the most common barrier[109]. The need for further studies on how to best improve preconception women's capability, opportunity, and motivation to modify their lifestyle behaviors has been emphasized[109]. At present, there is a lack of international consensus guidelines on weight management preconception, and its impact on fertility, pregnancy and subsequent maternal and infant outcomes.
The reversibility of obesity-induced parental programming has only recently received attention. These programmed changes to offspring health may be partially restored via diet/exercise interventions in obese fathers, prior to conception, via improvement in sperm DNA integrity. Promising results in animal models utilizing diet and exercise interventions have shown improvements in sperm function and molecular composition, resulting in restorations of both embryo and fetal health and subsequent male offspring fertility[57]. However, it is noteworthy, that most data surrounding paternal obesity and offspring phenotypes have come from rodent models, and implications for clinical practice warrants further research[57].