Published online Apr 15, 2022. doi: 10.4239/wjd.v13.i4.376
Peer-review started: November 9, 2021
First decision: January 12, 2022
Revised: January 20, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 15, 2022
Processing time: 156 Days and 9.4 Hours
The risk of early mortality of patients who start dialysis urgently is high; however, in patients with diabetes undergoing urgent-start peritoneal dialysis (USPD), the risk of, and risk factors for, early mortality are unknown.
To identify risk factors for mortality during high-risk periods in patients with diabetes undergoing USPD.
This retrospective cohort study enrolled 568 patients with diabetes, aged ≥ 18 years, who underwent USPD at one of five Chinese centers between 2013 and 2019. We divided the follow-up period into two survival phases: The first 6 mo of USPD therapy and the months thereafter. We compared demographic and baseline clinical data of living and deceased patients during each period. Kaplan-Meier survival curves were generated for all-cause mortality according to the New York Heart Association (NYHA) classification. A multivariate Cox proportional hazard regression model was used to identify risk factors for mortality within the first 6 mo and after 6 mo of USPD.
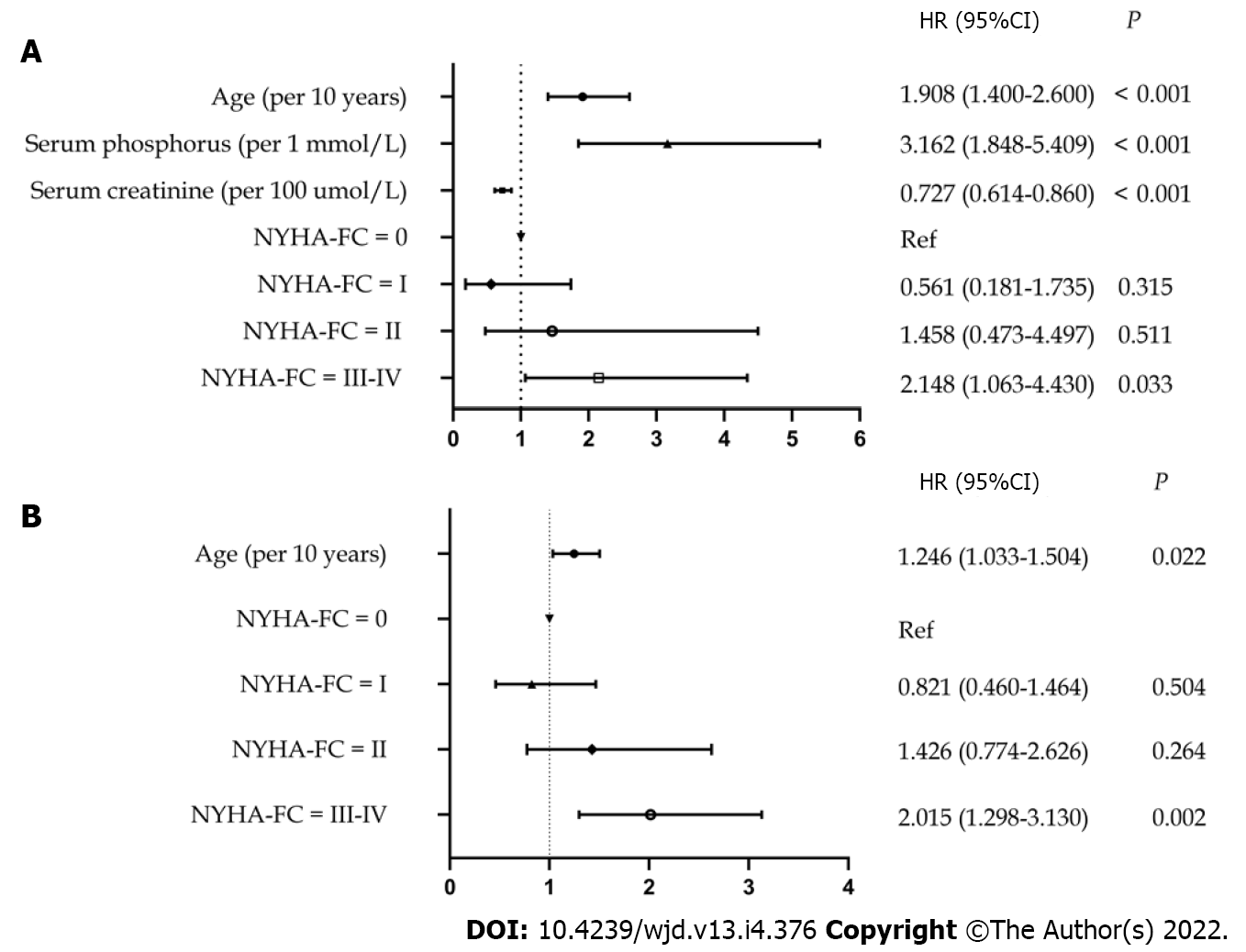
Forty-one patients died within the first 6 mo, accounting for the highest proportion of mortalities (26.62%) during the entire follow-up period. Cardiovascular disease was the leading cause of mortality within 6 mo (26.83%) and after 6 mo (31.86%). The risk of mortality not only within the first 6 mo but also after the first 6 mo was higher for patients with obvious baseline heart failure symptoms than for those with mild or no heart failure symptoms. Independent risk factors for mortality within the first 6 mo were advanced age [hazard ratio (HR: 1.908; 95%CI: 1.400-2.600; P < 0.001), lower baseline serum creatinine level (HR: 0.727; 95%CI: 0.614-0.860; P < 0.001), higher baseline serum phosphorus level (HR: 3.162; 95%CI: 1.848-5.409; P < 0.001), and baseline NYHA class III-IV (HR: 2.148; 95%CI: 1.063-4.340; P = 0.033). Independent risk factors for mortality after 6 mo were advanced age (HR: 1.246; 95%CI: 1.033-1.504; P = 0.022) and baseline NYHA class III-IV (HR: 2.015; 95%CI: 1.298-3.130; P = 0.002).
To reduce the risk of mortality within the first 6 mo of USPD in patients with diabetes, controlling the serum phosphorus level and improving cardiac function are recommended.
Core Tip: The first 6 mo after the initiation of urgent-start peritoneal dialysis is a high-risk period. We identified the following as risk factors for mortality within the first 6 mo in urgent-start peritoneal dialysis recipients with diabetes: Advanced age, lower baseline serum creatinine level, higher baseline phosphorus level, and baseline New York Heart Association class III-IV.
- Citation: Cheng SY, Yang LM, Sun ZS, Zhang XX, Zhu XY, Meng LF, Guo SZ, Zhuang XH, Luo P, Cui WP. Risk factors for mortality within 6 mo in patients with diabetes undergoing urgent-start peritoneal dialysis: A multicenter retrospective cohort study. World J Diabetes 2022; 13(4): 376-386
- URL: https://www.wjgnet.com/1948-9358/full/v13/i4/376.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i4.376
End-stage renal disease (ESRD) requiring dialysis is a global health problem[1,2]. Patients with late-stage chronic kidney disease—who often delay visiting a doctor owing to economic difficulties or other reasons—often need to start dialysis urgently without any preparation[3]. Several studies have documented the safety and feasibility of urgent-start peritoneal dialysis (USPD)[4-7]. USPD has several benefits over urgent-start hemodialysis (USHD), including better quality of life, better preservation of residual kidney function, and cost savings[4,8-11].
Studies on patients undergoing peritoneal dialysis have shown that those with diabetes mellitus (DM) have a poorer prognosis than do those without DM, in addition to a poorer survival rate owing to the high prevalence of cardiovascular diseases[12,13]. Currently, there is only a small, single-center study published on USPD in ESRD patients with diabetes[14]; it mainly compares the characteristics and complications between patients with diabetes treated with USPD and USHD. However, it does not identify the risk factors for mortality in patients with diabetes undergoing USPD.
The first 6 mo after the initiation of urgent dialysis is a high-risk period[5]. Patients with diabetes undergoing USPD are critically ill; hence, we speculated that the risk of mortality within the first 6 mo in these patients is high. Additionally, as the patient’s peritoneal dialysis treatment progresses, the overall patient condition tends to be stable; therefore, we deliberated that the risk factors for mortality within the first 6 mo may be different from those for mortality after 6 mo in patients with diabetes undergoing USPD. However, the distribution of mortalities over time in patients with diabetes undergoing USPD has not been reported, and the risk factors for mortality within the first 6 mo in these patients are not clear. This study examines both the occurrence of and the risk factors for mortality within the first 6 mo of USPD initiation in patients with diabetes.
We screened patients with ESRD who underwent USPD between January 1, 2013 and December 31, 2019 at the following five hospitals: The Second Hospital of Jilin University, Second Part of the First Hospital of Jilin University, Jilin Central Hospital, Jilin First Automobile Work General Hospital, and Xing’anmeng People’s Hospital. Patients with incomplete data, those aged < 18 years, and those without diabetes were excluded. All patients were followed up until mortality, kidney transplantation, technical failure, or the follow-up cutoff date (June 30, 2020). All patients were informed about renal replacement therapy modalities. Although experienced nephrologists guided the choice of modality, the final choice was made by the patient.
This retrospective study was approved by the Ethics Committee of the Second Hospital of Jilin University (design No. 2020031). To identify risk factors for mortality within the first 6 mo of USPD in patients with diabetes, we divided the follow-up time into two survival periods: The first 6 mo and the months thereafter. We compared demographic and baseline clinical data of patients who were living or deceased during each period. To highlight the characteristics of the patients deceased within the first 6 mo, we compared the causes of, and risk factors for, mortality after 6 mo to those of mortality within the first 6 mo.
In the present study, automated peritoneal dialysis (APD) and continuous ambulatory peritoneal dialysis (CAPD) were the two modes of peritoneal dialysis. Peritoneal dialysis for each patient was prescribed based on fluid overload, uremia, hyperkalemia, and acid-base imbalance. A low-volume abdominal cavity (0.5-1.0 L) was initially obtained with the patient in the supine position to avoid dialysate leakage, and the volume was progressively increased to 2 L per cycle within 2 wk. The number of cycles per day was 3-4 for CAPD and 6-9 for APD. The dialysis procedure was performed by a peritoneal dialysis nurse until the patient and/or caregiver could independently perform the process. The patients were followed up every 3-6 mo and peritoneal dialysis dose was adjusted to keep the total Kt/V urea above 1.70 or creatinine clearance above 50 L/week/1.73 m2.
We collected the following data: (1) Basic information, including sex, age, cardiac function classification, and comorbidities such as diabetes, cerebrovascular disease, hypertension, and tumors; (2) Baseline (before peritoneal dialysis within 2 d) laboratory indicators, including hemoglobin, blood albumin, blood white cells, blood phosphorus, blood calcium, blood potassium, blood creatinine, and blood sodium; and (3) Clinical outcomes, including mortality, technical failure, kidney transplantation, and continued dialysis. USPD was commenced within 2 wk of catheter insertion[15]. Technical failure was considered a transition to hemodialysis and its administration to the patient for at least 1 mo[16]. Cardiovascular events included myocardial infarction, stroke, heart failure, unstable angina, peripheral vascular events, fatty pulmonary embolism, sudden mortality, and unknown mortality caused by cardiovascular disease[17]. In accordance with the New York Heart Association (NYHA) categorization[18], patients without symptoms of heart failure were classified as class 0, whereas those with occasional, effort dyspnea were classified as class I; consistent with the traditional classification of cardiac function class, class II was characterized with mildly limited physical activity and general activity that can cause symptoms of heart failure, class III with obviously limited heart function and mild physical lower-than-general activities that can cause symptoms of heart failure, and class IV with symptoms of heart failure that can occur in a resting state.
Baseline characteristics are expressed as median (interquartile range) for continuous data and frequency and percentage for categorical data. For comparisons between groups, the rank-sum test was used for continuous variables, and the chi-square test or exact probability test was used for categorical variables. Kaplan-Meier curves were used to compare the survival rates of patients with different cardiac function classes. A Cox proportional hazard regression model was used to identify the risk factors for mortality during different periods of follow-up. The censored data included switching to HD, renal transplantation, technical failure, loss to follow-up, or still at our PD centers during each period. Additionally, for each selected period, mortalities after the period were censored. Factors with P < 0.1 in a univariate analysis were included in the multivariate analysis. Statistical significance was set at P < 0.05. SPSS 24.0 software (IBM Corp., Armonk, NY, United States) was used for data analysis, and GraphPad 8.0 software (GraphPad Software, San Diego, CA, United States) was used for plotting.
The statistical methods of this study were reviewed by Jin LN from School of Public Health, Jilin University, Changchun, Jilin, China.
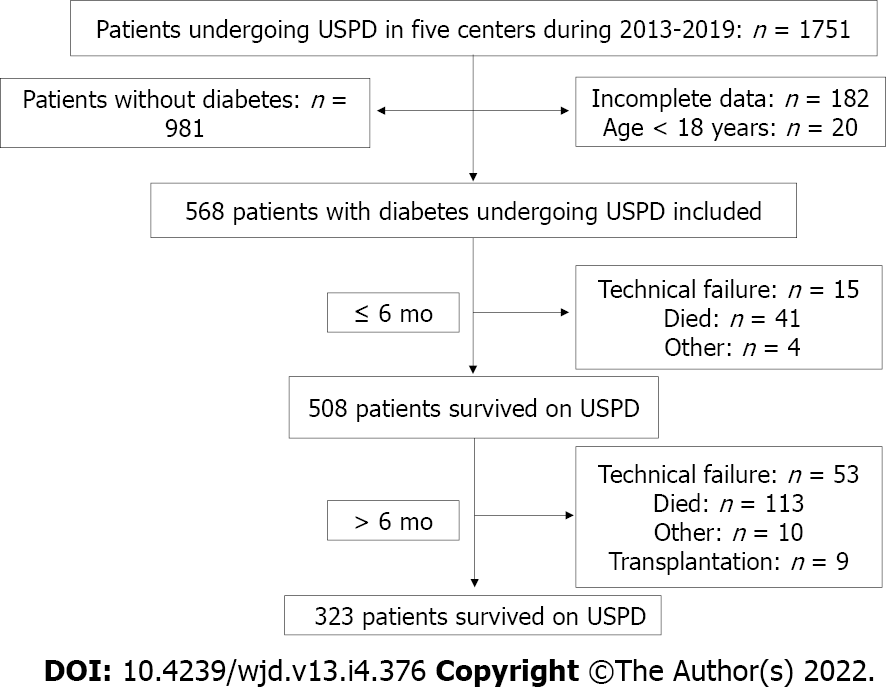
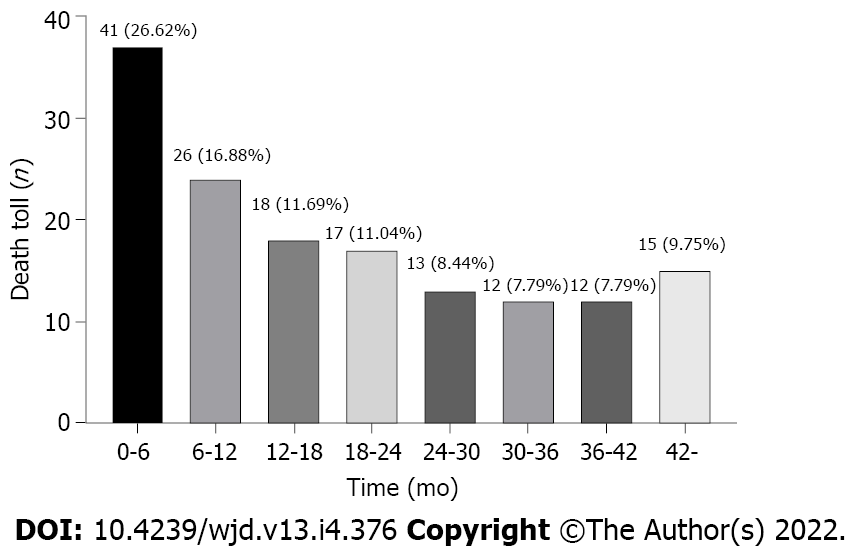
In this study, we screened 1751 patients undergoing USPD in the aforementioned five centers between 2013 and 2019, of which, we ultimately included 568 patients with diabetes undergoing USPD (Figure 1). Figure 2 shows the mortality proportions of patients with diabetes for the entire follow-up period calculated at 6-mo intervals after the initiation of USPD. As shown, the highest proportion (26.62%) of mortalities occurred between 0 and 6 mo. In the first 6 mo, 41 people died, with a mortality rate of 7.2%. A total of 113 people died after 6 mo of USPD, with a mortality rate of 22.38%.
Patient age and blood glucose level were significantly higher in patients who died within the first 6 mo than in those who survived the first 6 mo (P < 0.001, P = 0.011, respectively). The patients who died within the first 6 mo had a lower proportion of those with NYHA class 0-II and a much higher proportion of those with NYHA class III-IV than did patients who survived the first 6 mo (P = 0.009) (Table 1).
| Index | Within the first 6 mo | After 6 mo | ||||||
| Died (n = 41) | Survived (n = 527) | χ2/Z | P | Died (n = 113) | Survived (n = 395) | χ2/Z | P | |
| Demographic characteristics | ||||||||
| Age, yr, M (P25, P75) | 66.0 (57.0, 75.0) | 58.0 (51.0, 66.0) | -4.003 | < 0.001 | 61.0 (55.0, 67.0) | 58.0 (50.0, 64.0) | -3.357 | 0.001 |
| Gender, male, n (%) | 22 (53.7) | 322 (61.1) | 0.882 | 0.348 | 65 (57.5) | 244 (61.8) | 0.666 | 0.414 |
| Abdominal surgery history, n (%) | 3 (7.3) | 64 (12.1) | 0.852 | 0.356 | 13 (11.5) | 47 (11.9) | 0.013 | 0.909 |
| Co-morbidities, n (%) | ||||||||
| Cerebrovascular disease, n (%) | 12 (29.3) | 112 (21.3) | 1.432 | 0.231 | 28 (24.8) | 82 (20.8) | 0.837 | 0.360 |
| Hypertension, n (%) | 40 (97.6) | 512 (97.2) | 0.000 | 1.000 | 110 (97.3) | 384 (97.2) | 0.006 | 0.941 |
| NYHA-FC | - | - | 11.647 | 0.009 | - | - | 9.265 | 0.026 |
| 0 | 14 (34.1) | 254 (48.2) | - | - | 46 (40.7) | 196 (49.6) | - | - |
| I | 4 (9.76) | 97 (18.4) | - | - | 16 (16.2) | 79 (20.0) | - | - |
| II | 4 (9.76) | 55 (10.4) | - | - | 14 (12.4) | 39 (9.9) | - | - |
| III-IV | 19 (46.3) | 121 (23.0) | - | - | 37 (32.7) | 118 (23.2) | ||
| Laboratory test | ||||||||
| WBC (109/L) | 7.24 (6.11, 9.13) | 7.26 (5.70, 8.60) | -0.674 | 0.501 | 6.82 (5.51, 7.75) | 7.40 (5.77, 8.71) | -1.891 | 0.059 |
| Alb (g/L) | 31.20 (28.00, 33.95) | 32.39 (28.80, 36.00) | -1.436 | 0.151 | 32.38 (29.00, 35.50) | 32.30 (28.60, 36.00) | -0.202 | 0.840 |
| Hb (g/L) | 86.0 (74.5, 98.0) | 86.0 (74.0, 97.0) | -0.214 | 0.831 | 85.0 (74.0, 95.0) | 86.0 (74.0, 97.0) | -0.677 | 0.498 |
| K (mmol/L) | 4.57 (3.91, 5.11) | 4.57 (4.00, 5.09) | -0.195 | 0.845 | 4.50 (3.95, 4.92) | 4.61 (4.01, 5.13) | -1.442 | 0.149 |
| Na (mmol/L) | 140.00 (136.60, 142.50) | 140.03 (138.00, 142.30) | -0.909 | 0.363 | 140.10 (137.95, 142.85) | 140.10 (138.00, 142.30) | -0.364 | 0.176 |
| Ca (mmol/L) | 1.95 (1.77, 2.13) | 1.97 (1.84, 2.11) | -0.875 | 0.382 | 1.99 (1.88, 2.11) | 1.96 (1.81, 2.11) | -1.348 | 0.178 |
| P (mmol/L) | 2.00 (1.60, 2.41) | 1.87 (1.48, 2.16) | -1.145 | 0.252 | 1.71 (1.42, 2.05) | 1.88 (1.53, 2.18) | -3.242 | 0.001 |
| Boold glucose (mmol/L) | 6.91 (5.60, 9.96) | 6.16 (4.83, 7.70) | -2.555 | 0.011 | 6.30 (4.87, 8.52) | 6.16 (4.83, 7.56) | -0.547 | 0.584 |
| Scr (μmol/L) | 646.80 (484.45, 806.85) | 710.00 (562.00, 876.10) | -1.855 | 0.064 | 642.00 (532.25, 840.25) | 723.05 (578.80, 887.80) | -2.626 | 0.009 |
Among the patients who were still followed at our PD centers after the first 6 mo, those who died after 6 mo had more advanced age (P = 0.001) and lower levels of baseline serum creatinine and serum phosphorus (P = 0.009, P = 0.001, respectively) than did those who survived throughout follow-up. Compared with the patients who died after 6 mo of USPD, those who survived throughout the follow-up period included a lower proportion of patients with NYHA class III-IV and a higher proportion of patients with NYHA class 0-II (P = 0.026) (Table 1).
The top three known causes of mortality in the 41 patients who died within the first 6 mo were cardiovascular diseases (26.83%), respiratory failure (19.51%), and infectious diseases (9.76%) (Table 2). Furthermore, the top three causes of mortality after 6 mo were the same as those for mortality within the first 6 mo (Table 2).
| Causes | Number of mortalities within the first 6 mo, n (%) | Number of the mortalities after 6 mo, n (%) |
| Infectious diseases | 4 (9.76) | 16 (14.16) |
| Cardiovascular events | 11 (26.83) | 36 (31.86) |
| Cerebrovascular disorder | 3 (7.32) | 10 (8.85) |
| Respiratory failure | 8 (19.51) | 14 (12.39) |
| Malignancy | 3 (7.32) | 3 (2.66) |
| Multiple organ dysfunction | 3 (7.32) | 2 (1.77) |
| Unknown | 9 (21.95) | 32 (28.32) |
| Total | 41 (100.00) | 113 (100.00) |
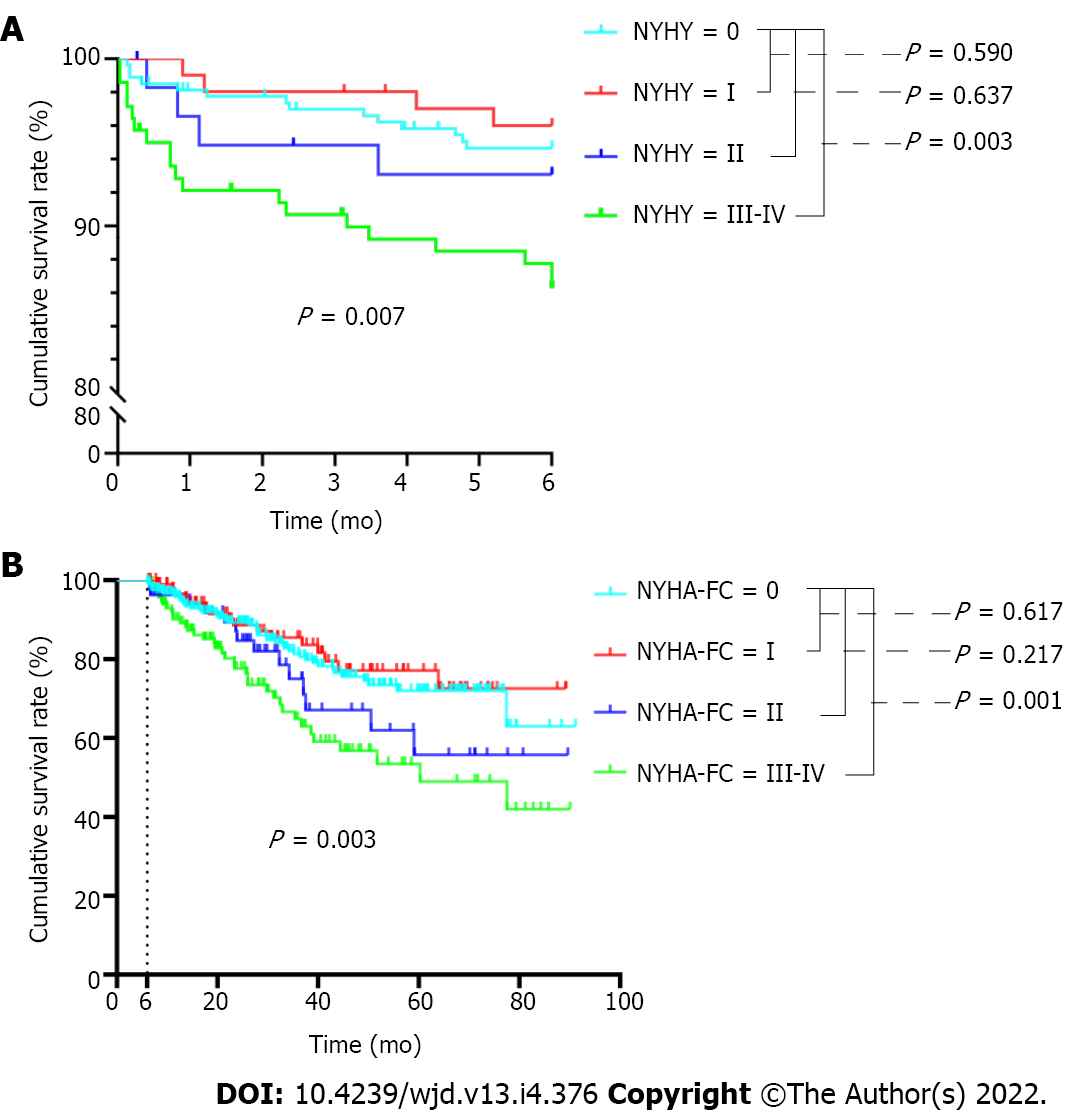
Considering that cardiovascular disease was the main reason for mortality within the first 6 mo and also after 6 mo, we further analyzed the survival of patients with different classes of cardiac function. As shown in Figure 3A, in the first 6 mo, the mortality rate for patients with baseline cardiac function of NYHA III-IV was much higher than that of patients without cardiac function limitation (P = 0.003). Similar results were found for these comparisons after 6 mo (Figure 3B).
After correcting for confounding factors (serum calcium levels and blood glucose levels), multivariate Cox modeling analysis identified the following as independent risk factors for mortality within the first 6 mo in patients with diabetes receiving USPD: Increased age [hazard ratio (HR: 1.908; 95%CI: 1.400-2.600; P < 0.001]; lower levels of baseline serum creatinine (HR: 0.727; 95%CI: 0.614-0.860; P < 0.001); higher levels of baseline serum phosphorus (HR: 3.162; 95%CI: 1.848-5.409; P < 0.001); and NYHA class III-IV at baseline (HR: 2.148; 95%CI: 1.063-4.430; P = 0.033) (Figure 4A). Additionally, after adjusted serum creatinine calcium, phosphorus, and blood glucose levels, we found that advanced age (HR: 1.246; 95%CI: 1.033-1.504; P = 0.022) and baseline NYHA class III-IV (HR: 2.015; 95%CI: 1.298-3.130; P = 0.002) were risk factors for mortality after 6 mo (Figure 4B).
To the best of our knowledge, our study provides the first multicenter evaluation of the risk factors for mortality within the first 6 mo in patients with diabetes undergoing USPD. Advanced age and NYHA class III-IV at baseline were risk factors for mortality within the first 6 mo and after 6 mo; however, higher serum phosphorus levels and lower serum creatinine levels before dialysis were the only independent risk factors for mortality within the first 6 mo. The strength of the study was that it included data from five hospitals, making it representative and comprehensive.
Currently, the only report published on patients with diabetes undergoing USPD included 50 participants and reported an early mortality rate of 4.1%[14], similar to that of the present study. Moreover, we have demonstrated for the first time that the mortality in patients with diabetes undergoing USPD is highest in the first 6 mo. Thus, special attention should be paid to these patients during this time period. As in previous studies[19,20], the leading cause of mortality within the first 6 mo for the USPD recipients with diabetes in our study was cardiovascular disease. Active treatment is therefore required at an early stage to reduce the risk of mortality due to cardiovascular events in these patients.
In agreement with previous reports[4,5,21], we identified advanced age as an independent risk factor not only for mortality within the first 6 mo but also after 6 mo of USPD in patients with diabetes. A reasonable explanation is that advanced age increases the incidence of cardiovascular events and consequently mortality in patients with diabetes[22]. Immune dysfunction and microinflammation in patients with renal failure can easily lead to sepsis, which increases the risk of mortality. The more advanced the age, the worse the immune function and, consequently, the greater the risk of mortality[23].
We found that the baseline serum creatinine level inversely correlated with mortality within the first 6 mo of USPD in patients with diabetes. We believe that the condition of the patient mainly accounts for this linkage. Patients with diabetes who start dialysis urgently often present with severe symptoms rather than biochemical indicators of severe renal failure. A lower serum creatinine level in patients with diabetes before USPD is reflective of an earlier initiation of emergency peritoneal dialysis, which contrarily reflects more severe symptoms in the patient at initial presentation. However, the baseline serum creatinine level was not an independent risk factor for mortality after 6 mo. A possible reason was that as the course of USPD progressed, the patient’s condition improved, and the baseline serum creatinine level did not reflect the disease severity on follow-up; therefore, the results indicated that USPD alleviated the patient’s condition.
For patients with diabetes undergoing USPD, a link between the serum phosphorus level and mortality has not been reported. For these patients, we found that the risk of mortality within the first 6 mo increased by 216.2%% for each 1 mmol/L increase in the baseline serum phosphorus level. Other studies have shown that a high level of serum phosphate correlates with vascular calcification in uremic patients[24-26], and that vascular calcification increases the risk of myocardial infarction[27], coronary artery disease[28], and mortality[29]. Serum phosphate level has also been found to be a powerful independent predictor of coronary heart disease in patients with diabetes[28]. Baseline serum phosphorus level was not a risk factor for mortality after 6 mo, which is similar to findings in other studies[30,31]. In our study, we focused on the risk factors for mortality within the first 6 mo; therefore, we only collected the baseline serum phosphorus levels. We speculated that with the progress of dialysis and the use of phosphorus-reducing drugs during dialysis, serum phosphorus levels would gradually be corrected; therefore, the baseline serum phosphorus level is not reflective of the overall level after 6 mo of treatment. This suggests that it is crucial and beneficial to control the serum phosphorus level of patients in the initial stage of dialysis to reduce early mortality.
Patients with ESRD and diabetes are more likely to develop cardiovascular diseases than are non-diabetic patients with ESRD[32]. Additionally, it was proved that patients on dialysis with poor cardiac function have a very poor prognosis[33]. Therefore, exploration of the relationship between mortality and cardiac function in patients with diabetes undergoing USPD is critical and significant. We found that among patients with diabetes undergoing USPD, those with obvious heart failure symptoms have a higher risk of mortality within the first 6 mo and after 6 mo than do those with mild or no heart failure symptoms. We found that NYHA class III-IV was a risk factor for mortality both within and after the first 6 mo; however, the risk of mortality after 6 mo was lower than that within the first 6 mo for patients with poorer baseline cardiac function. As a possible cause, we speculated that patients with poorer baseline cardiac function are more likely to suffer from complications of heart disease, and after a series of treatments such as dialysis, although their cardiac function improves, it cannot be completely corrected; therefore, the risk of mortality is merely reduced. Thus, routine monitoring of the patient’s cardiac function in the early stages of dialysis is advised. Moreover, when the patient’s cardiac function is not ideal, appropriate measures should be taken to promptly improve it.
Our study had several limitations. First, because it was retrospective, information bias could not be avoided. For example, the laboratory indicators such as cardiac function and serum phosphorus levels at different time points had not been determined, and the cause of mortality could not always be precisely established. Second, our sample size was small, and larger studies are needed to accurately predict mortality within the first 6 mo in diabetic patients undergoing USPD to provide further guidance for clinical applications.
The risk of mortality within the first 6 mo in patients with diabetes was the highest after USPD initiation. We suggest that controlling serum phosphorus levels and improving cardiac function will decrease the risk of mortality within the first 6 mo in these patients.
Many patients with end-stage renal disease have to choose urgent-start peritoneal dialysis (USPD), and patients with diabetes mellitus (DM) who are undergoing USPD have a poorer prognosis than do those without DM. The first 6 mo after the start of urgent dialysis is a high-risk period, and for patients with DM undergoing USPD, we speculate that the mortality risk is high in the first 6 mo after USPD. However, the distribution of mortalities over time and the risk factors for mortality within the first 6 mo in this patient population has not been reported. Thus, it is important to identify the risk factors for mortality within the first 6 mo of USPD initiation in patients with DM.
We hoped to identify the reasons for the poor prognosis of patients with DM undergoing USPD.
The main aim of this study was to identify risk factors for mortality within the first 6 mo in patients with DM undergoing USPD in order to facilitate better management of such patients in clinical practice.
In this multicenter, retrospective cohort study, we screened patients with ESRD who underwent USPD at five hospitals. To highlight the specificity of risk factors within the first 6 mo, we divided the follow-up period into two survival phases: the first 6 mo and the months thereafter. We compared the survival rates of patients with different cardiac function classes in each period using Kaplan-Meier curves. The risk factors for mortality during the different periods were analyzed using a Cox proportional hazard regression model.
We found that the highest proportion (26.62%) of mortalities occurred between 0 and 6 mo. The mortality rate for patients with baseline cardiac function represented by New York Heart Association (NYHA) III-IV was much higher than that for patients without cardiac function limitation, both within the first 6 mo and after 6 mo (all P < 0.05). Increased age (P < 0.001), lower levels of baseline serum creatinine (P < 0.001), higher levels of baseline serum phosphorus (P < 0.001), and NYHA class III-IV at baseline (P = 0.033) were risk factors for mortality within the first 6 mo. The risk factors for mortality after 6 were advanced age (P = 0.022) and baseline NYHA class III-IV (P = 0.002).
This study suggests the importance of controlling serum phosphorus levels and improving cardiac function for decreasing the mortality risk within the first 6 mo in patients with DM undergoing USPD.
Further research is needed to build a model to predict the risk of mortality within the first 6 mo in patients with DM undergoing USPD.
We thank all the staff of the five peritoneal dialysis for providing data, which made this paper possible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Darenskaya MA, Russia; Sateesh J, India S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Kanda H, Hirasaki Y, Iida T, Kanao-Kanda M, Toyama Y, Chiba T, Kunisawa T. Perioperative Management of Patients With End-Stage Renal Disease. J Cardiothorac Vasc Anesth. 2017;31:2251-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1491] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 3. | Ivarsen P, Povlsen JV. Can peritoneal dialysis be applied for unplanned initiation of chronic dialysis? Nephrol Dial Transplant. 2014;29:2201-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Dias DB, Mendes ML, Caramori JT, Falbo Dos Reis P, Ponce D. Urgent-start dialysis: Comparison of complications and outcomes between peritoneal dialysis and haemodialysis. Perit Dial Int. 2021;41:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Koch M, Kohnle M, Trapp R, Haastert B, Rump LC, Aker S. Comparable outcome of acute unplanned peritoneal dialysis and haemodialysis. Nephrol Dial Transplant. 2012;27:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Bitencourt Dias D, Mendes ML, Burgugi Banin V, Barretti P, Ponce D. Urgent-Start Peritoneal Dialysis: The First Year of Brazilian Experience. Blood Purif. 2017;44:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Wojtaszek E, Grzejszczak A, Grygiel K, Małyszko J, Matuszkiewicz-Rowińska J. Urgent-Start Peritoneal Dialysis as a Bridge to Definitive Chronic Renal Replacement Therapy: Short- and Long-Term Outcomes. Front Physiol. 2018;9:1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant. 2006;21 Suppl 2:ii56-ii59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Dias DB, Banin V, Mendes ML, Barretti P, Ponce D. Peritoneal dialysis can be an option for unplanned chronic dialysis: initial results from a developing country. Int Urol Nephrol. 2016;48:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Wong LP, Li NC, Kansal S, Lacson E Jr, Maddux F, Kessler J, Curd S, Lester K, Herman M, Pulliam J. Urgent Peritoneal Dialysis Starts for ESRD: Initial Multicenter Experiences in the United States. Am J Kidney Dis. 2016;68:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | See EJ, Cho Y, Hawley CM, Jaffrey LR, Johnson DW. Early and Late Patient Outcomes in Urgent-Start Peritoneal Dialysis. Perit Dial Int. 2017;37:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Saha HH, Leskinen YK, Salenius JP, Lahtela JT. Peripheral vascular disease in diabetic peritoneal dialysis patients. Perit Dial Int. 2007;27 Suppl 2:S210-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | García-López E, Carrero JJ, Suliman ME, Lindholm B, Stenvinkel P. Risk factors for cardiovascular disease in patients undergoing peritoneal dialysis. Perit Dial Int. 2007;27 Suppl 2:S205-S209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Jin H, Ni Z, Che X, Gu L, Zhu M, Yuan J, Huang J, Gu A, Jin Y, Yan H, Wang Q, Yu Z, Zhou W, Fang W. Peritoneal Dialysis as an Option for Unplanned Dialysis Initiation in Patients with End-Stage Renal Disease and Diabetes Mellitus. Blood Purif. 2019;47:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Tunbridge M, Cho Y, Johnson DW. Urgent-start peritoneal dialysis: is it ready for prime time? Curr Opin Nephrol Hypertens. 2019;28:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC. Determinants of peritoneal dialysis technique failure in incident US patients. Perit Dial Int. 2013;33:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Wheeler DC, London GM, Parfrey PS, Block GA, Correa-Rotter R, Dehmel B, Drüeke TB, Floege J, Kubo Y, Mahaffey KW, Goodman WG, Moe SM, Trotman ML, Abdalla S, Chertow GM, Herzog CA; EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) Trial Investigators. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc. 2014;3:e001363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Postorino M, Marino C, Tripepi G, Zoccali C; Calabrian Registry of Dialysis and Transplantation. Prognostic value of the New York Heart Association classification in end-stage renal disease. Nephrol Dial Transplant. 2007;22:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Chung SH, Han DC, Noh H, Jeon JS, Kwon SH, Lindholm B, Lee HB. Risk factors for mortality in diabetic peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25:3742-3748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Fang W, Yang X, Kothari J, Khandelwal M, Naimark D, Jassal SV, Bargman J, Oreopoulos DG. Patient and technique survival of diabetics on peritoneal dialysis: one-center's experience and review of the literature. Clin Nephrol. 2008;69:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Wick JP, Turin TC, Faris PD, MacRae JM, Weaver RG, Tonelli M, Manns BJ, Hemmelgarn BR. A Clinical Risk Prediction Tool for 6-Month Mortality After Dialysis Initiation Among Older Adults. Am J Kidney Dis. 2017;69:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Gu Y, Cheng LT, Zeng J, Wang T. Increased arterial stiffness in elderly female diabetic peritoneal dialysis patients. Am J Nephrol. 2009;29:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Shang D, Xie Q, Ge X, Yan H, Tian J, Kuang D, Hao CM, Zhu T. Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol. 2015;16:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 818] [Article Influence: 35.6] [Reference Citation Analysis (2)] |
| 26. | Carrillo-López N, Panizo S, Alonso-Montes C, Martínez-Arias L, Avello N, Sosa P, Dusso AS, Cannata-Andía JB, Naves-Díaz M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol Dial Transplant. 2019;34:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Negri AL. Vascular calcifications in chronic kidney disease: are there new treatments? Curr Vasc Pharmacol. 2005;3:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 429] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Frink RJ, Achor RW, Brown AL Jr, Kincaid OW, Brandenburg RO. Significance of calcification of the coronary arteries. Am J Cardiol. 1970;26:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 178] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Gong N, Xiao Z, Zhang F, Zhong X, He Y, Yi Z, Tang D, Yang C, Lin Y, Nie J, Ai J. Duration of Serum Phosphorus Control Associated with Overall Mortality in Patients Undergoing Peritoneal Dialysis. Kidney Dis (Basel). 2020;6:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Liu X, Huang R, Wu H, Wu J, Wang J, Yu X, Yang X. Patient characteristics and risk factors of early and late death in incident peritoneal dialysis patients. Sci Rep. 2016;6:32359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | MacDonald MR, Petrie MC, Hawkins NM, Petrie JR, Fisher M, McKelvie R, Aguilar D, Krum H, McMurray JJ. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J. 2008;29:1224-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 371] [Article Influence: 12.8] [Reference Citation Analysis (0)] |