Published online Mar 15, 2022. doi: 10.4239/wjd.v13.i3.251
Peer-review started: September 19, 2021
First decision: November 8, 2021
Revised: December 6, 2021
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: March 15, 2022
Processing time: 177 Days and 1.1 Hours
Protein glycosylated hemoglobin, hemoglobin A1c (HbA1c) binds hemoglobin (Hb) in red blood cells to blood glucose. However, the relationship between Hb and HbA1c remains unclear.
To elucidate their relationship in a nondiabetic population aged ≥ 16 years in the United States, using data from the 1999-2018 National Health and Nutrition Examination Survey.
This study was based on data from 44560 adults aged ≥ 16 years, excluding those with diabetes. The relationship was estimated using a multivariate regression. We also used piecewise linear regression for subgroup analysis based on age and sex stratification and analysis of the threshold effects of Hb on HbA1c.
Hb and HbA1c levels were negatively correlated in the unadjusted model (β = -0.01; 95%CI: -0.01, -0.01). The correlation was significantly negative when the regression model was minimally regulated and stratified by age and sex, and remained negative when the model was further regulated (more than 10%) to identify covariates with the HbA1c level influence estimates. In subgroup analyses based on age and sex stratification, the association remained negative when the covariates were controlled. A nonlinear relationship was observed between them when the Hb levels reached the tipping point (13.2 g/dL) (adjusted odds ratio, -0.04; 95%CI: -0.05, -0.03) and when the Hb levels exceeded 13.2 g/dL (adjusted odds ratio, -0.10; 95%CI: -0.10, -0.09).
Our study shows that normal Hb levels are negatively correlated with HbA1c in nondiabetic Americans aged ≥ 16 years.
Core Tip: Our research revealed that hemoglobin (Hb) within the normal values is negatively related to hemoglobin A1c (HbA1c) in non-diabetic American populations aged 16 years and older. HbA1c decreases by 0.08% for every 1g/dL increase in Hb.
- Citation: Bai XF, Wang H, Zhao QL. Hemoglobin within normal range is negatively related to hemoglobin A1c in a nondiabetic American population aged 16 years and older. World J Diabetes 2022; 13(3): 251-259
- URL: https://www.wjgnet.com/1948-9358/full/v13/i3/251.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i3.251
Diabetes mellitus (DM) has a high global incidence. The prevalence and incidence of DM continue to increase annually. DM is a major cause of global morbidity and mortality, and was one of the major causes of death in the United States in 2015. Over 30 million and 86 million Americans suffer from diabetes and prediabetes, respectively, which could increase the occurrence rate of many chronic diseases, especially type 2 DM (T2DM)[1]. Obesity may serve as a major inducement factor for diabetes, and the prevalence of diabetes and obesity are increasing[2]. Diabetes status can be classified into three categories: nondiabetes, prediabetes, and diabetes (T2DM)[3]. Chronic prediabetes and diabetes often cause a series of complications, including renal, ophthalmological, neurological, and vascular complications. It is well known that controlling high blood glucose levels could reduce and postpone the appearance and progression of DM-related complications[4]. Therefore, many prospective ongoing clinical studies are evaluating the efficacy of new and rarely studied diabetes biomarkers.
Hemoglobin (Hb) is a protein molecule that only exists in red blood cells (RBCs) that can bind oxygen. In the bloodstream, Hb is glycated. Hemoglobin A1c (HbA1c) acts as glycosylated hemoglobin (GHb) constructed by the nonenzymatic binding of glucose to valine at the N-terminus of the Hb β chain, which is the most abundant and common Hb in human erythrocytes. The GHb (HbA1c) level represents the percentage of Hb proteins bound to glucose. Glycemic control has been assessed using GHb. The higher the primary environmental level of blood glucose, the higher the HbA1c level[5]. However, the relationship between the Hb and HbA1c levels remains unclear. Hence, our study aimed to reveal the relationship between the normal level of Hb and GHb in a nondiabetic American population aged ≥ 16 years through cross-sectional investigation data obtained from the 1999-2018 National Health and Nutrition Examination Survey (NHANES).
This study analyzed the NHANES data from 1999 to 2018 (20 years). The NHANES participants are representative of the non-institutionalized civilians in America employed by the NHANES multistage stratified sampling design[6].
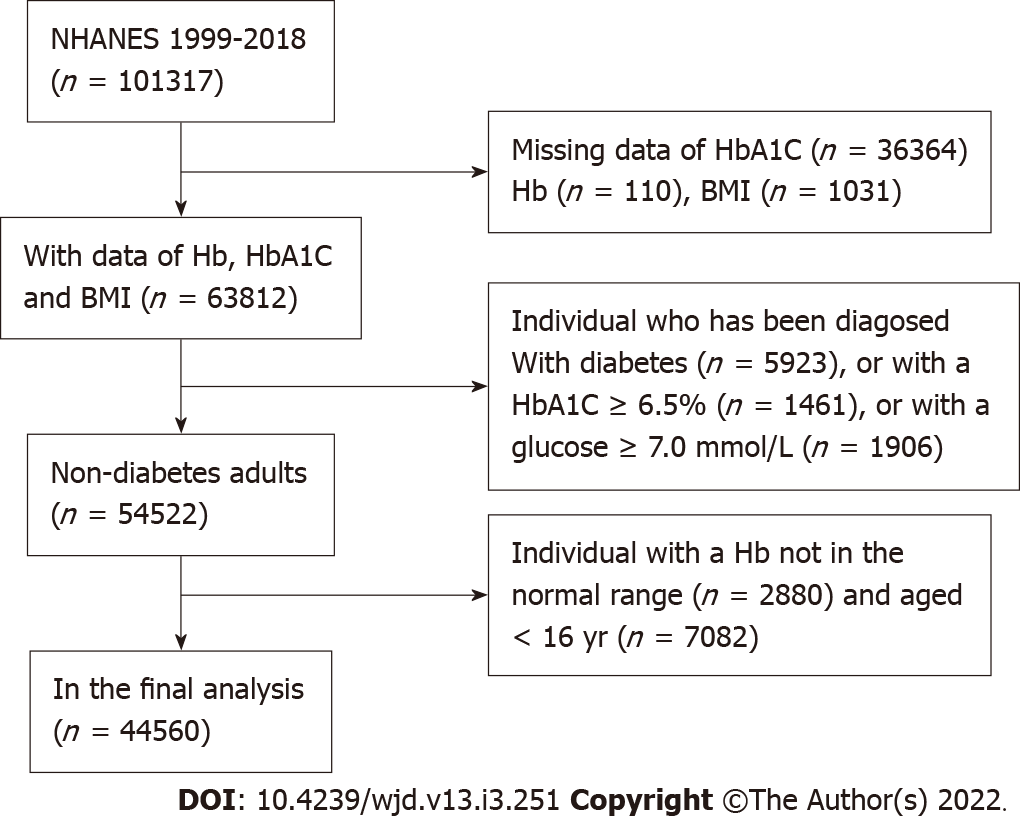
A total of 101317 participants were registered in the NHANES 1999-2018 database. In this research, 44560 adults aged ≥ 16 years with Hb and HbA1c level data were considered available. We excluded the individual cases with missing HbA1c data (n = 36364); with missing Hb data (n = 110); with missing body mass index (BMI) data (n = 1031); aged < 16 years (n = 7,082); diagnosed with diabetes (n = 5923); with an HbA1c level of > 6.5% (n = 1461) or a glucose level of > 7.0 mol/L (n = 1906); and with an abnormal Hb level (n = 2880). Furthermore, 44560 nondiabetic patients were included in the final analysis (Figure 1).
The exposure variable was the Hb level. The method used to derive the complete blood count (CBC) parameters was based on the Beckmann Kurt counting and grading method, combined with an automatic dilution and hybrid device used for sample treatment and a single beam photometer for the determination of the Hb level.
The outcome variable was the HbA1c level. The HbA1c whole blood sample was processed, stockpiled, and transferred to the University of Kansas, Columbia, Missouri.
The multivariate model contained variables that may confuse the association between the HbA1c and Hb. Age; sex; ethnicity; education level; marital status; smoking behavior; BMI; levels of blood glucose, uric acid, total protein, alanine aminotransferase, cholesterol, and serum creatinine; platelet count; and white blood cell and RBC counts were acquired from questionnaires. Smoking behavior was derived from the question “Have you/Has SP smoked more than 100 cigarettes in your/his/her whole life?” The variable name was SMQ020. The SAS label referred to having smoked more than 100 cigarettes in a lifetime. Marital status was defined as follows: unmarried (never married), married (including married and living with partner), divorced (including widowed, divorced, and separated), and unknown (including refused, unknown, and missing). Educational level was quantified as follows: less than high school (including less than the 9th grade and 9th to 11th grade), high school, and above high school (including some university or AA degrees and above university). Race was classified as follows: Mexican Americans, non-Hispanic whites, non-Hispanic blacks, Hispanics, and others, including multiple ethnic groups. BMI was measured at the mobile examination center and calculated as weight/height2. Blood glucose, total protein, uric acid, cholesterol, alanine aminotransferase, and serum creatinine levels and other data were obtained through the Beckman Synchron LX20 standard biochemical curve analysis. The platelet and white blood cell/RBC count data were obtained from a CBC with a 5-part differential. Diabetes was defined as an HbA1c level of > 6.5% or a fasting blood glucose level of > 7 mmol/L, according to the 2019 American Diabetes Association standards[7]. Details of the data are presented in Table 1 and freely available on the NHANES website (www.cdc.gov/nchs/nhanes/).
| Male (n = 22255) | Female (n = 22305) | P value | |
| Age (yr) | 42.12 ± 16.96 | 43.92 ± 17.71 | < 0.0001 |
| Race/ethnicity (%) | < 0.0001 | ||
| Non-Hispanic White | 68.87 | 70.50 | |
| Non-Hispanic Black | 9.90 | 9.51 | |
| Mexican American | 9.07 | 7.61 | |
| Other race/ethnicity | 12.16 | 12.38 | |
| Education level (%) | < 0.0001 | ||
| Less than high school | 15.25 | 13.56 | |
| High school | 22.89 | 21.07 | |
| More than high school | 53.71 | 58.36 | |
| Others | 8.15 | 7.01 | |
| Marital status | < 0.0001 | ||
| Never married | 22.22 | 17.57 | |
| Married | 61.53 | 57.48 | |
| Divorced | 10.22 | 19.85 | |
| Others | 6.03 | 5.10 | |
| Smoked at least 100 cigarettes in life (%) | < 0.0001 | ||
| Yes | 48.27 | 37.03 | |
| No | 44.76 | 56.91 | |
| Others | 6.97 | 6.06 | |
| Body mass index (kg/m2) | 27.91 ± 5.70 | 27.95 ± 7.01 | 0.4535 |
| Alanine aminotransferase (U/L) | 29.29 ± 22.06 | 20.74 ± 20.30 | < 0.0001 |
| Serum creatinine (μmol/L) | 85.93 ± 26.19 | 66.31 ± 18.96 | < 0.0001 |
| Blood glucose (mmol/L) | 5.08 ± 0.61 | 4.95 ± 0.59 | < 0.0001 |
| Total protein (g/L) | 72.34 ± 4.53 | 71.24 ± 4.62 | < 0.0001 |
| Uric acid (μmol/L) | 361.26 ± 72.02 | 277.45 ± 68.64 | < 0.0001 |
| Cholesterol (mmol/L) | 5.0 ± 1.1 | 5.1 ± 1.1 | < 0.0001 |
| White blood cell count (109/L) | 7.10 ± 2.30 | 7.30 ± 2.21 | < 0.0001 |
| Red blood cell count (1012/L) | 4.99 ± 0.41 | 4.48 ± 0.36 | < 0.0001 |
| Platelet count (109/L) | 240.75 ± 57.14 | 266.28 ± 64.99 | < 0.0001 |
| Hemoglobin (g/dL) | 15.28 ± 1.02 | 13.64 ± 0.93 | < 0.0001 |
| Hemoglobin A1c (%) | 5.34 ± 0.36 | 5.32 ± 0.37 | < 0.0001 |
Further subgroup analyses were performed based on sex and age. The quartile classification of the Hb level and sensitivity analysis was performed, and the trend P value was calculated. This was followed by a subgroup analysis sorted by age and sex. We applied smoothing curve fitting and generalized additive models to search for the potential nonlinear relationship between the GHb and Hb levels. Piecewise linear regression was employed to analyze the threshold effect of HbA1c. All analyses incorporated the NHANES sampling weights. Statistical significance was set at P < 0.05.
A total of 44560 participants ≥ 16 years of age were included in this study. The weighted distributions of sex are shown in Table 1. Women were more educated than men and comprised a higher percentage of non-Hispanic whites and older age individuals; they also had higher cholesterol levels and white blood cell and platelet counts, lower percentage of smoking at least 100 cigarettes in a lifetime, lower levels of alanine aminotransferase, total protein, serum uric acid, serum creatinine, blood glucose, Hb, and HbA1c, and a lower RBC count.
The correlation between Hb and HbA1c obtained by multiple regression analysis is shown in Table 2. There was a negative correlation between the Hb and HbA1c levels (β = -0.01; 95%CI: -0.01, -0.01) in the unadjusted model. The correlation remained significant with the smallest adjustment for age and sex in the regression model (β = -0.01; 95%CI: -0.01, -0.00). After further adjusting the covariates with the estimated impact of the HbA1c level in the model exceeding 10%, the correlation remained negative (β = -0.08; 95%CI: -0.08, -0.07). P value was < 0.001 for trend.
| Unadjusted model β (95%CI) | Minimally adjusted model β (95%CI) | Fully adjusted model β (95%CI) | |
| Hemoglobin | -0.01 (-0.01, -0.01)c | -0.01 (-0.01, -0.00)c | -0.08 (-0.08, -0.07)c |
| Hemoglobin (Quartile) | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.01 (0.00, 0.02) | 0.01 (-0.00, 0.01) | -0.06 (-0.07, -0.05)c |
| Q3 | 0.02 (0.01, 0.03)b | 0.01 (-0.00, 0.02) | -0.12 (-0.13, -0.11)c |
| Q4 | -0.04 (-0.05, -0.03)c | -0.02 (-0.03, -0.01)c | -0.23 (-0.24, -0.22)c |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
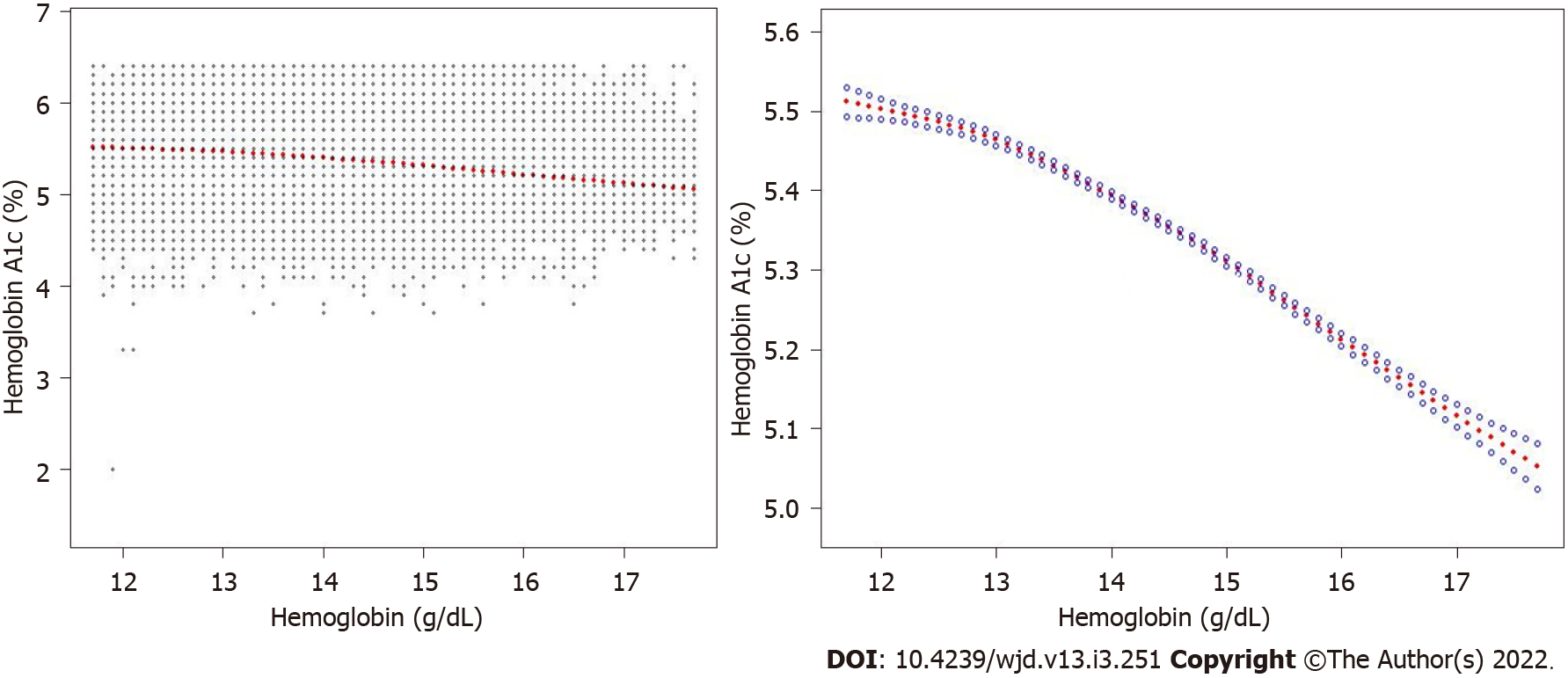
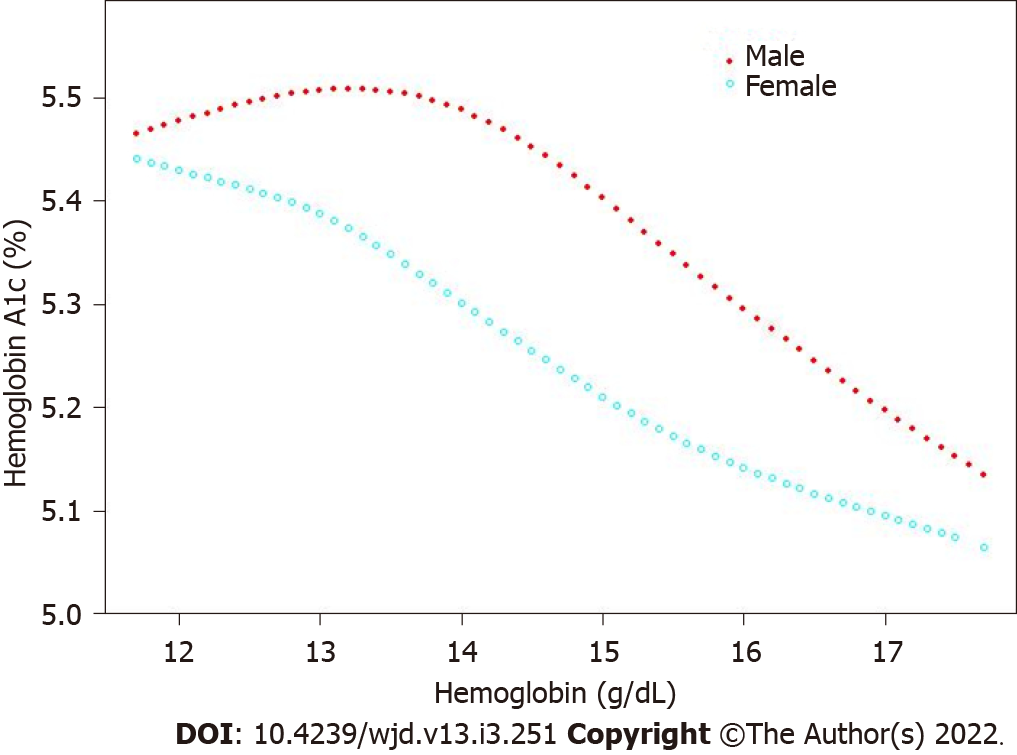
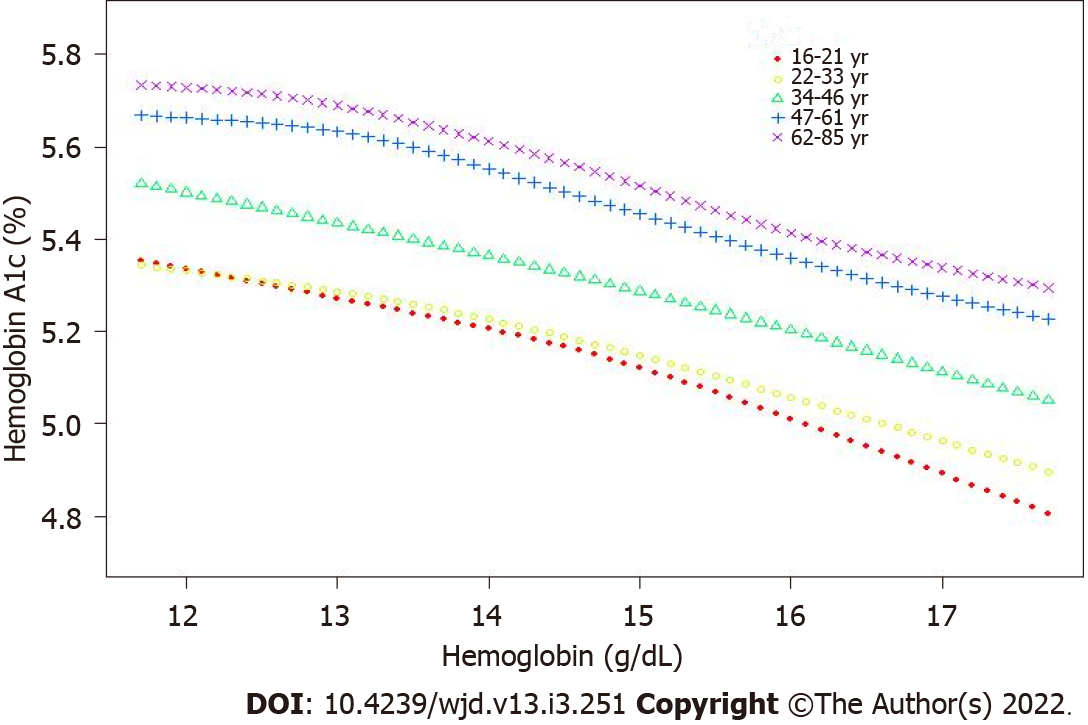
The correlation was still negative in the subgroup analysis classified by age (16-29 years, β = -0.011; 95%CI: -0.015, -0.008; 30-51 years, β = -0.004; 95%CI: 0.008, 0.001; 52-85 years, β = -0.021; 95%CI: -0.025, -0.016) and sex (men, β = -0.057; 95%CI: -0.062, -0.052; women, β = -0.012; 95%CI: -0.017, -0.007) when the covariates were controlled. The results are presented in Table 3. The smooth curve fitting and generalized additive model further verified the negative correlation between the Hb and HbA1c levels (Figures 2-4).
| Unadjusted model β (95%CI) | Minimally adjusted model β (95%CI) | Fully adjusted model β (95%CI) | |
| Stratified by age | |||
| 16-29 | -0.011 (-0.015, -0.008)a | -0.054 (-0.059, -0.048)a | -0.104 (-0.110, -0.097)a |
| 30-51 | -0.004 (-0.008, 0.001) | -0.037 (-0.042, -0.031)a | -0.090 (-0.097, -0.083)a |
| 52-85 | -0.021 (-0.025, -0.016)a | -0.023 (-0.028, -0.017)a | -0.084 (-0.091, -0.077)a |
| Stratified by sex | |||
| Men | -0.057 (-0.062, -0.052)a | -0.031 (-0.036, -0.027)a | -0.085 (-0.091, -0.080)a |
| Women | -0.012 (-0.017, -0.007)a | -0.033 (-0.038, -0.029)a | -0.091 (-0.096, -0.085)a |
A nonlinear relationship between Hb and HbA1c was observed when the Hb levels reached the turning point (13.2 g/dL) [adjusted odds ratio (OR), -0.04; 95%CI: -0.05, -0.03; P < 0.0001) and when the Hb levels exceeded 13.2 g/dL (adjusted OR, -0.10; 95%CI: -0.10, -0.09; P < 0.0001). The results are presented in Table 4.
| Point of hemoglobin (g/dL) | Odd ratio (95%CI) | P value |
| < 13.2 | -0.04 (-0.05, -0.03) | < 0.0001 |
| > 13.2 | -0.10 (-0.10, -0.09) | < 0.0001 |
In the present study, we examined numerous samples of American nondiabetic individuals aged ≥ 16 years to investigate the relationship between the Hb and HbA1c levels in the normal range. Our study showed that the Hb and HbA1c levels were negatively correlated in both men and women.
HbA1c is a GHb, which is a nonenzymatic reaction of glucose binding to Hb. HbA1c is considered as a better marker to evaluate the state of DM compared with blood glucose monitoring, and it is stable and able to represent the average blood glucose level over the past 2-3 mo. The HbA1c levels are affected by a large number of factors, such as race, RBC disorders, and hemoglobinopathies.
A large retrospective cohort study conducted by Grossman et al included 11,352 individuals without diabetes and assessed the correlation between the Hb and HbA1c levels. The fifth highest Hb level of HbA1c individuals was significantly lower than that of the other fifth of individuals, and the correlation between the Hb and HbA1c levels was negligible[8]. However, Lai et al[9] found that in 1659 Chinese nondiabetic adults aged 20-49 years, the normal Hb levels were negatively correlated with HbA1c.
In our study, we found that the correlation between the Hb and HbA1c levels was obviously negative in the unadjusted model. When minimal adjustments to sex and age were made in the regression model and when the model further adjusted for the estimated value of the HbA1c level to exceed 10 covariates, the association was still significant. Our results were generally consistent with those of Lai et al[9]’s studies on Chinese populations.
Since 1999, the NHANES has investigated approximately 5000 people in 15 different counties in America every year[8,10-13]. Each participant represents approximately 65000 people across the country, and such individuals have made significant contributions to the study. Our research had a large-scale, population-based research design; therefore, our results can be extended to the entire American population. However, there are some limitations that should be noted. First, a cause-and-effect relationship between Hb and HbA1c levels could not be established as a consequence of the cross-sectional design of our research. Longitudinal, prospective, large-scale human studies are needed at this point. Second, some covariant data were extracted from self-reporting, which may be easily affected by self-reporting bias. Nevertheless, the data were gathered by skilled interviewers in accordance with standardized agreements. Third, we excluded individuals with diabetes and abnormal Hb levels and those younger than 16 years of age. Therefore, our conclusions do not apply to these groups of people. Fourth, although several potential confounding factors were regulated, other potential confounding factors were not included in this study. Therefore, our study may include biases. Further prospective studies with large sample sizes are needed which include the measurement of these additional variables.
In conclusion, our results show that in the nondiabetic American population aged ≥ 16 years, the Hb levels were negatively correlated with the HbA1c levels within the normal range in both men and women. The Hb levels were independent and negatively related to the HbA1c levels.
The relationship between hemoglobin (Hb) and hemoglobin A1c (HbA1c) remains unclear.
To elucidate the relationship between Hb and HbA1c in a nondiabetic population aged ≥ 16 years in America.
To elucidate the relationship between Hb and HbA1c.
The relationship was estimated using a multivariate regression.
Hb levels are negatively correlated with HbA1c.
Normal Hb levels are negatively correlated with HbA1c in nondiabetic Americans aged ≥ 16 years.
From a clinical point of view, HbA1c decreases by 0.08% for every 1 g/dL increase in Hb.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemical research methods
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Darenskaya MA, Scaramuzza A S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Conway BN, Han X, Munro HM, Gross AL, Shu XO, Hargreaves MK, Zheng W, Powers AC, Blot WJ. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PLoS One. 2018;13:e0190993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Anders S, Schroeter C. Diabetes, diet-health behavior, and obesity. Front Endocrinol (Lausanne). 2015;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Harreiter J, Roden M. [Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019)]. Wien Klin Wochenschr. 2019;131:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 4. | Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 866] [Cited by in RCA: 1017] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 5. | Emerging Risk Factors Collaboration, Di Angelantonio E, Gao P, Khan H, Butterworth AS, Wormser D, Kaptoge S, Kondapally Seshasai SR, Thompson A, Sarwar N, Willeit P, Ridker PM, Barr EL, Khaw KT, Psaty BM, Brenner H, Balkau B, Dekker JM, Lawlor DA, Daimon M, Willeit J, Njølstad I, Nissinen A, Brunner EJ, Kuller LH, Price JF, Sundström J, Knuiman MW, Feskens EJ, Verschuren WM, Wald N, Bakker SJ, Whincup PH, Ford I, Goldbourt U, Gómez-de-la-Cámara A, Gallacher J, Simons LA, Rosengren A, Sutherland SE, Björkelund C, Blazer DG, Wassertheil-Smoller S, Onat A, Marín Ibañez A, Casiglia E, Jukema JW, Simpson LM, Giampaoli S, Nordestgaard BG, Selmer R, Wennberg P, Kauhanen J, Salonen JT, Dankner R, Barrett-Connor E, Kavousi M, Gudnason V, Evans D, Wallace RB, Cushman M, D'Agostino RB Sr, Umans JG, Kiyohara Y, Nakagawa H, Sato S, Gillum RF, Folsom AR, van der Schouw YT, Moons KG, Griffin SJ, Sattar N, Wareham NJ, Selvin E, Thompson SG, Danesh J. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. 2014;311:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care. 2010;33:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 1963] [Article Influence: 327.2] [Reference Citation Analysis (0)] |
| 8. | Grossman A, Gafter-Gvili A, Schmilovitz-Weiss H, Koren-Morag N, Beloosesky Y, Weiss A. Association of glycated hemoglobin with hemoglobin levels in elderly nondiabetic subjects. Eur J Intern Med. 2016;36:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Lai Y, Lin Z, Zhu Z. Association between hemoglobin within the normal range and hemoglobin A1c among Chinese non-diabetes adults. BMC Endocr Disord. 2021;21:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | National Health and Nutrition Examination Survey (NHANES) data. [cited 19 Sep 2021]. Available from: https://www.cdc.gov/nchs/nhanes/index.htm. |
| 11. | Campbell L, Pepper T, Shipman K. HbA1c: a review of non-glycaemic variables. J Clin Pathol. 2019;72:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care. 2017;40:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 13. | English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58:1409-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |