Published online Feb 15, 2022. doi: 10.4239/wjd.v13.i2.97
Peer-review started: January 1, 2021
First decision: July 28, 2021
Revised: August 10, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 15, 2022
Processing time: 403 Days and 23 Hours
Diabetes mellitus is a metabolic disorder characterized by prolonged elevation of blood glucose due to various causes. Currently, the relationship between diabetic retinopathy (DR) and altered connectivity of brain function is unclear.
To investigate the relationship between this brain activity and clinical manifestations and behaviors of DR patients by using the amplitude of low-frequency fluctuation (ALFF) technique.
Twenty-four DR patients and 24 healthy controls (HCs) matched for age and gender were enrolled. We measured and recorded average ALFF values of DR patients and HCs and then classified them using receiver operating characteristic (ROC) curves.
ALFF values of both left and right posterior cerebellar lobe and right anterior cingulate gyrus were remarkably higher in the DR patients than in the HCs; however, DR patients had lower values in the bilateral calcarine area. ROC curve analysis of different brain regions demonstrated high accuracy in the area under the curve analysis. There was no significant relationship between mean ALFF values for different regions and clinical presentations in DR patients. Neuronal synchronization abnormalities in some brain regions of DR patients were associated with cognitive and visual disorders.
Abnormal spontaneous brain activity was observed in many areas of DR patients’ brains, which may suggest a possible link between clinical manifestations and behaviors in DR patients.
Core Tip: We found that patients with diabetic retinopathy (DR) may have multiple low-frequency amplitude frequency changes in the brain, and the generation of this change may be related to the alteration of patients' visual cortex and anxiety, which may help us to explore the pathological mechanism and disease progression in DR patients.
- Citation: Shi WQ, Zhang MX, Tang LY, Ye L, Zhang YQ, Lin Q, Li B, Shao Y, Yu Y. Altered spontaneous brain activity patterns in patients with diabetic retinopathy using amplitude of low-frequency fluctuation. World J Diabetes 2022; 13(2): 97-109
- URL: https://www.wjgnet.com/1948-9358/full/v13/i2/97.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i2.97
Chronic complications of diabetes are the main causes of death and disability with diabetes, and its morbidity may be related to many factors, among which diabetic retinopathy (DR) is the main cause of blindness and visual impairment[1]. The incidence of DR is increasing worldwide mainly because of the longevity of diabetes patients[2]. A large proportion of adults who are over the age of 40 are affected by DR. Early manifestations of DR are mainly aneurysms, bleeding spots, hard exudation, cotton buds, venous beading, intravascular microvascular abnormalities, and macular edema[3]. According to the presence of retinal neovascularization, we can divide DR into proliferative DR (PDR) and non-proliferative DR (NPDR). In PDR, retinal damage stimulates neovascular growth. Neovascular growth is detrimental to the retina, which can cause fibrosis and even retinal detachment. Neovascularization can also enter the vitreous, which will lead to vitreous hemorrhage[4] (Figure 1). Recently, there has been increasing evidence that similar microvascular lesions happen in the brains of diabetics. Autopsy results in patients with chronic diabetes have shown that their brains developed a severe microvascular disease and neurological disease[5]. Pearce et al[6] pointed out that a diagnosis of DR suggests an increased risk of brain parenchymal disease in diabetic patients. These diabetic vascular and neurological complications interact for a long time, and we believe that the effects of diabetic complications on the brain's microvasculature are often overlooked. The previous gold standard for a DR diagnosis relied mainly on fundus fluorescence imaging, but it is not suitable for people with skin test allergies and poor liver and kidney function. However, there has been little analysis of changes in brain function in DR and its relationship to clinical manifestations in the eye to date. We hypothesized that the presence of DR may suggest central damage to the brain neural network caused by the existence of a microvascular system, while central damage in the brain neural network is an early predictor of DR.
Functional magnetic resonance imaging (fMRI) makes it possible to observe specific differences in the brain neural network[7] and studying these changes can enhance our knowledge of diseases. Thus, fMRI neuroimaging is of great interest for exploring the function of the central nervous system, considering it can monitor neural activity in the brain and can also provide some new explanations for the pathophysiological mechanism and pathogenesis of disease[8]. Resting-state fMRI (rs-fMRI) is a functional magnetic resonance technique used in resting-state functional network research. It explores the human nervous system through magnetic resonance imaging, which is simpler than task-state fMRI[9]. By combining electroneurophysiological records with fMRI, studies have demonstrated that low frequency (0.0-0.08 Hz) fluctuations (LFF) in blood oxygen level dependent (BOLD) fMRI signals are closely associated with spontaneous neuronal activity[10]. ALFF is one of the methods used to evaluate fMRI analysis of resting brain activity[11,12]. It reflects the intensity of local spontaneous brain activity at rest and brain endogenous/background neurophysiological processes. We have used the ALFF method to assess the neurological status and brain changes in some patients with eye diseases; for example, optic neuritis[13], glaucoma and strabismus[14,15], Parkinson’s disease[16], in our previous studies.
We randomly selected 24 DR patients who visited the First Affiliated Hospital of Nanchang University, Nanchang, China. All the subjects met the following inclusion criteria: (1) Diagnosis of type 2 diabetes; (2) Diagnosis of PDR; (3) Fasting blood glucose controlled at approximately 7-10 mmol/L and blood glucose after a meal at 10-14 mmol/L; (4) No abnormality of the cerebral parenchyma on cranial MRI; (5) Without other ocular diseases bilaterally (e.g., retinal detachment, glaucoma, amblyopia, ocular trauma, optic nerve disease); (6) Right-handed; and (7) Untreated PDR. The exclusion criteria were: (1) Smokers; (2) No other eye diseases; (3) Pregnant or lactating women; (4) Other diabetes complications; (5) Congenital systemic disease; (6) Mental illness (such as depression, memory impairment, cognitive impairment, and schizophrenia); and (7) Cerebral infarction diseases or cerebral vascular malformations. Twenty-four healthy controls (HCs) matched in age, educational status, and sex were enrolled. Both the DR group and HCs met the following criteria: (1) MRI showed no obvious damage or deformity of the brain parenchyma; (2) No history of brain infarction, cardiovascular disease or cerebral hemorrhage; (3) No evidence of drug or alcohol addiction; and (4) Were able to tolerate an MRI examination.
This study was conformed with the Declaration of Helsinki and had formal approval from the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University. All the volunteers signed informed consent forms and were allowed to ask questions after learning about the purpose, content, and potential risks of this research.
The International Council of Ophthalmology in Sydney defined the international classification standard of DR in 2002, and the details are as follows: (1) In the early stage of the disease, after mydriasis, ophthalmoscopy revealed diffuse microaneurysms and small petechia in the posterior pole of the retina; some patients exhibited white or yellow exudate with complaints of blurry vision; (2) Retinopathy was found in the fundus under fundus angiography machine; (3) Using fundus fluorescein angiography, the number of microadenomas in the fundus was obviously increased and were beyond the results of fundus microscopy and there was capillary dilation around the retina, increased permeability, and increased abnormalities such as bleeding or neovascularization[17]. A patient with any of these symptoms was diagnosed as DR.
In this research, we performed MRI scans using 3-Tesla MRI scanners (Siemens, Munich, Germany). All participants were asked to close their eyes and maintain natural breathing until the end of the scan. We applied a gradient echo sequence of pulses of the 3D variation to obtain function data, and the parameters were as follows: 176 structural images (acquisition matrix = 256 × 256, field of view = 250 mm × 250 mm, TR = 2 s, TE = 2.26 ms, cycle time = 1900 ms, thickness = 1.0 mm, gap = 0.5 mm, flip angle = 9°). We acquired 240 functional images in total (acquisition matrix = 64 × 64, field of view = 220 mm × 220 mm, thickness = 4.0 mm, gap = 1.2 mm, cycle time = 2000 ms, echo time = 30 ms, flip angle = 90°, 29 axial). A typical scan took 15 min to complete.
Our previous reports described how to analyze fMRI data. First, we used MRIcro software (www.mricro.com) to expel broken data. During magnetization equilibration, the first ten time points were discarded. Data Processing Assistant for the advanced edition of Resting-State fMRI (DPARSFA 4.0, http://rfmri.org/DPARSF) software was used for the form conversion of digital imaging communications in medicine (DICM), the correction of head motion, slice timing, realignment, spatial normalization, full-width smoothing with a Gaussian kernel of 6 mm × 6 mm × 6 mm at half-maximum, detrending, and nuisance covariates regression, based on the rs-fMRI data analysis toolkit (REST, http://www.restfmri.net). The other images were corrected time differences and micromotions during scanning. During the fMRI examination, subjects were excluded if they had more than three degrees of motion or if the maximum excursion in the x, y or z direction exceeded 3 mm.
We used linear regression analysis to remove spurious covariates and their time derivatives from various other sources, including the signal from the region of interest (ROI) to the central white matter region[18]. It should be noted that, in this study, the data showed that the global signal did not shrink, as was the case in our former study[19,20], which may have been caused by the elimination of global signals during preprocessing of the data at rest[21,22]. The fMRI images were unified to the Montreal Neurological Institute spatial standard using a standard echoplanar imaging template after correction for head motion, while the images were resampled to a resolution of 3 mm × 3 mm × 3 mm. To reduce the effect of diversity between participants, we divided the ALFF of each voxel by the average whole-brain ALFF value for each subject.
According to the results of ALFF, REST software was applied to divide different brain areas of these groups into areas of interest. The mean ALFF values in each region of interest were obtained by calculating the average ALFF values of all voxels. Using GraphPad Prism 9.0 (Graph Pad Software Inc., San Diego, CA, United States), the correlation between the average ALFF values of multiple brain regions in the DR group and the clinical data was evaluated (P < 0.05).
We used the SPSS version 22.0 (IBM Corporation, Armonk, NY, United States) with an independent samples t-test to analyze the cumulative clinical variables between HCs and the DR group. A P value < 0.05 showed statistical significance. We compared functional data with a two-sample t-test using REST software. Through Gaussian random field theory, the statistical thresholds for multiple comprehensively compared voxel levels were set as P < 0.001. Alphasim was corrected for cluster sizes > 30 voxels at a P < 0.01 level.
No statistically significant differences were found between the two groups in weight (P = 0.982) or age (P = 0.975). The mean ± SD of DR duration was 253.18 ± 76.22 d (Table 1).
| Condition | DR | HC | t | P value |
| Male/female | 10/14 | 10/14 | N/A | > 0.99 |
| Age (yr) | 54.31 ± 5.96 | 53.08 ± 5.33 | 0.093 | 0.975 |
| Weight (kg) | 53.67 ± 9.64 | 59.86 ± 9.93 | 0.095 | 0.982 |
| Handedness | 24R | 24R | N/A | > 0.99 |
| Duration of DR (d) | 253.18 ± 76.22 | N/A | N/A | N/A |
| Best-corrected VA-right eye | 0.19 ± 0.08 | 1.12 ± 0.36 | -0.883 | 0.009 |
| Best-corrected VA-left eye | 0.22 ± 0.09 | 1.09 ± 0.42 | -0.743 | 0.014 |
| IOP-R (mmHG) | 15.63 ± 2.93 | 17.56 ± 2.74 | 0.092 | 0.815 |
| IOP-L (mmHG) | 16.13 ± 2.32 | 16.54 ± 2.63 | 0.088 | 0.843 |
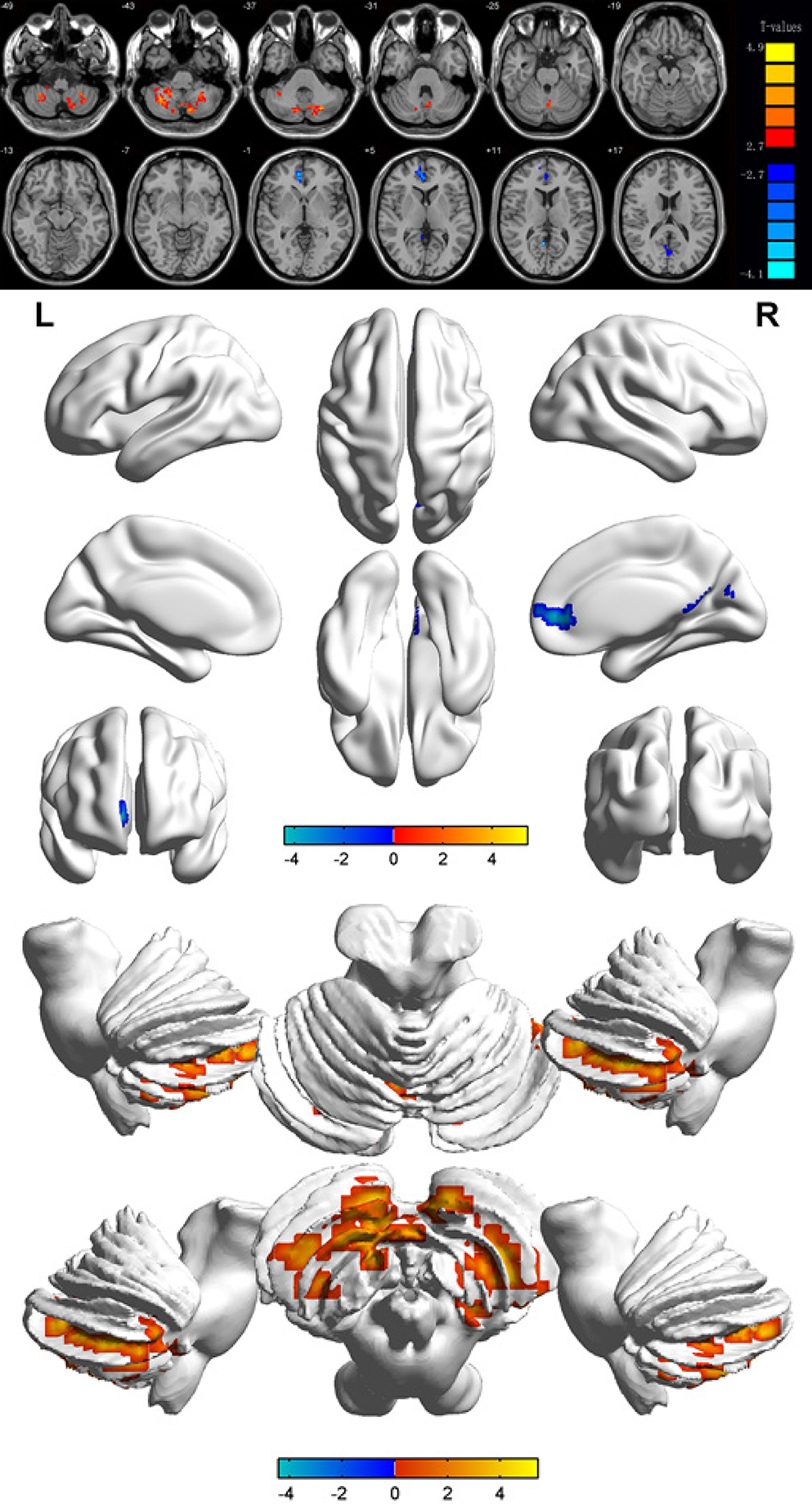
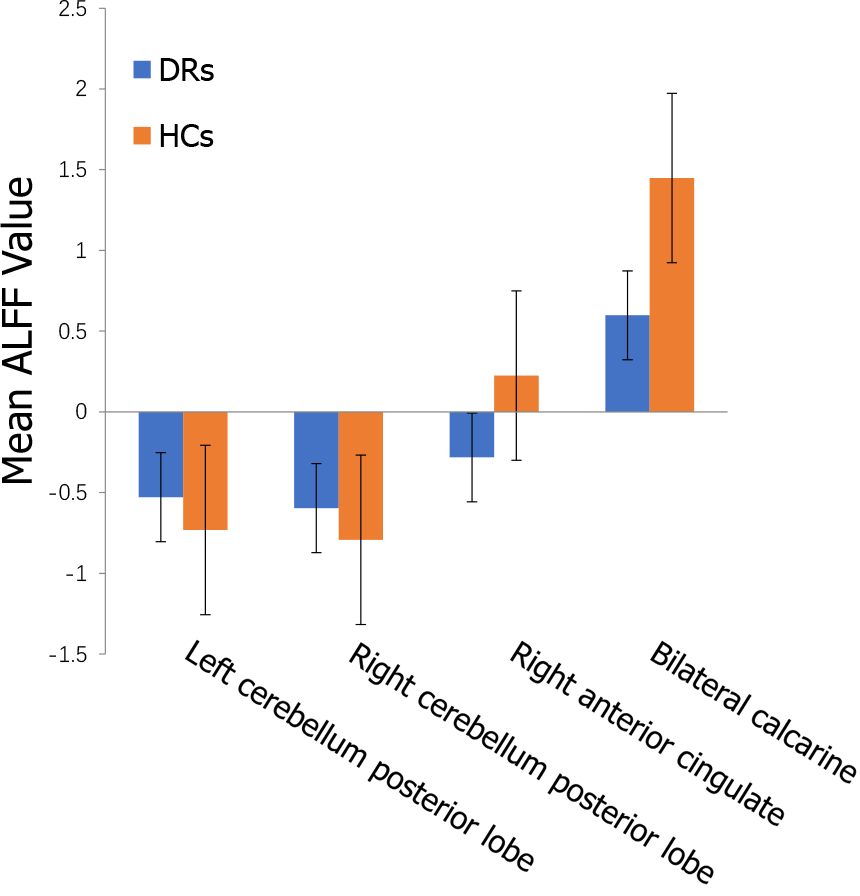
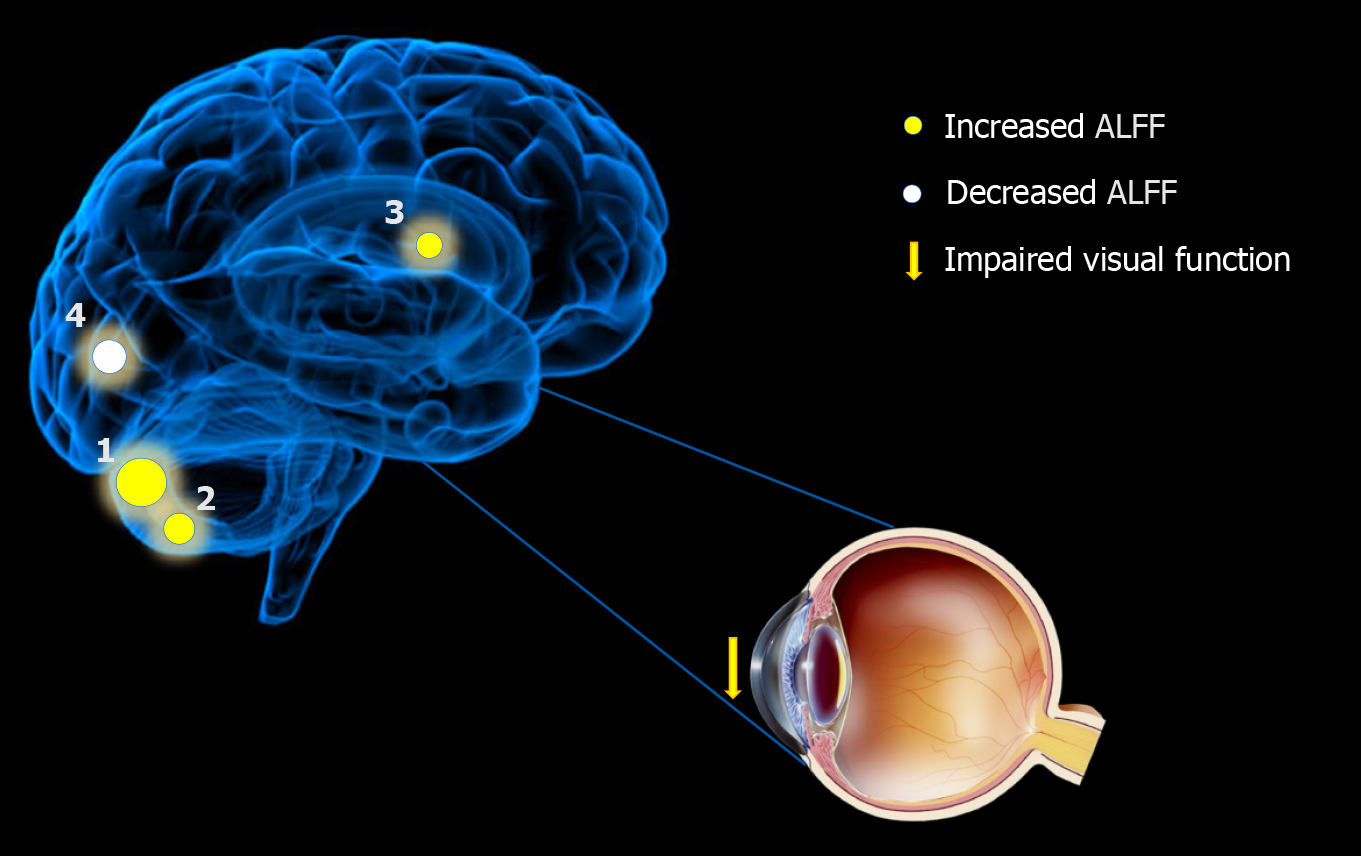
Compared with the results in the HCs, the ALFF values of the DR patients were remarkably lower in the bilateral calcarine fissures, but higher in the left and right posterior lobes of the cerebellum as well as the right anterior cingulate gyrus (Figures 2 and 3 and Table 2).
| Condition | L/R/B | Brain regions | MNI coordinates | BA | Peak voxels | t value | P value | ||
| X | Y | Z | |||||||
| DRs > HCs | |||||||||
| 1 | L | Cerebellum posterior lobe | -27 | -72 | -36 | / | 238 | 5.4338 | < 0.001 |
| 2 | R | Cerebellum posterior lobe | 33 | -57 | -42 | / | 111 | 4.6875 | < 0.001 |
| 3 | R | Anterior cingulate | 9 | 48 | 3 | 32 | 32 | -4.4176 | < 0.001 |
| DRs < HCs | |||||||||
| 1 | B | Calcarine | 3 | -66 | 21 | / | 46 | -4.3494 | < 0.001 |
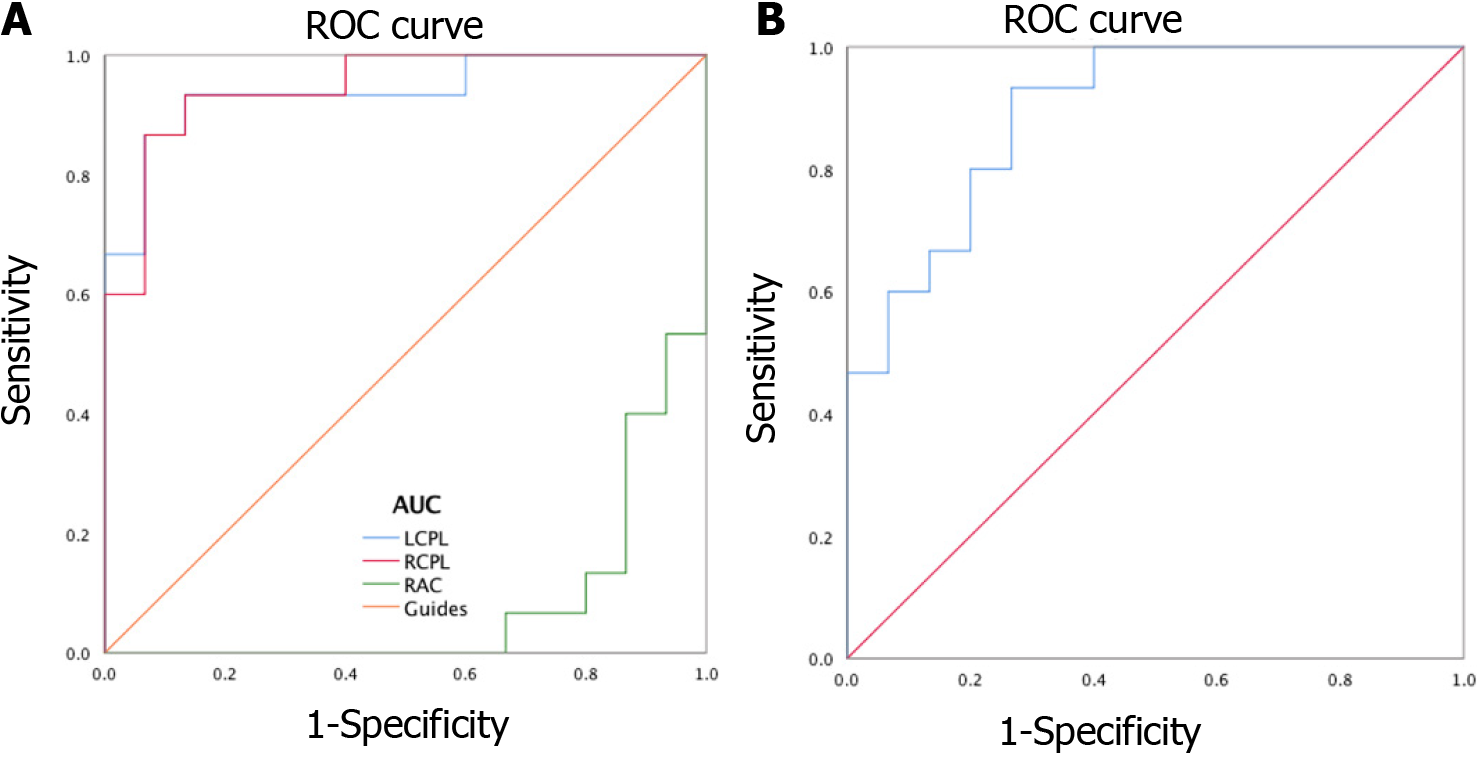
Receiver operating characteristic (ROC) curves were applied to analyze the mean ALFF values of different cerebral areas. The diagnosis rate was displayed in the area under the curve (AUC). The AUCs of ALFF values for different cerebral areas were as follows: Right anterior cingulate gyrus (0.080, P < 0.001), left posterior lobe of the cerebellum (0.938, P < 0.001), right posterior lobe of the cerebellum (0.947, P < 0.001; Figure 4A), and the bilateral calcarine fissures (0.893, P < 0.001; Figure 4B).
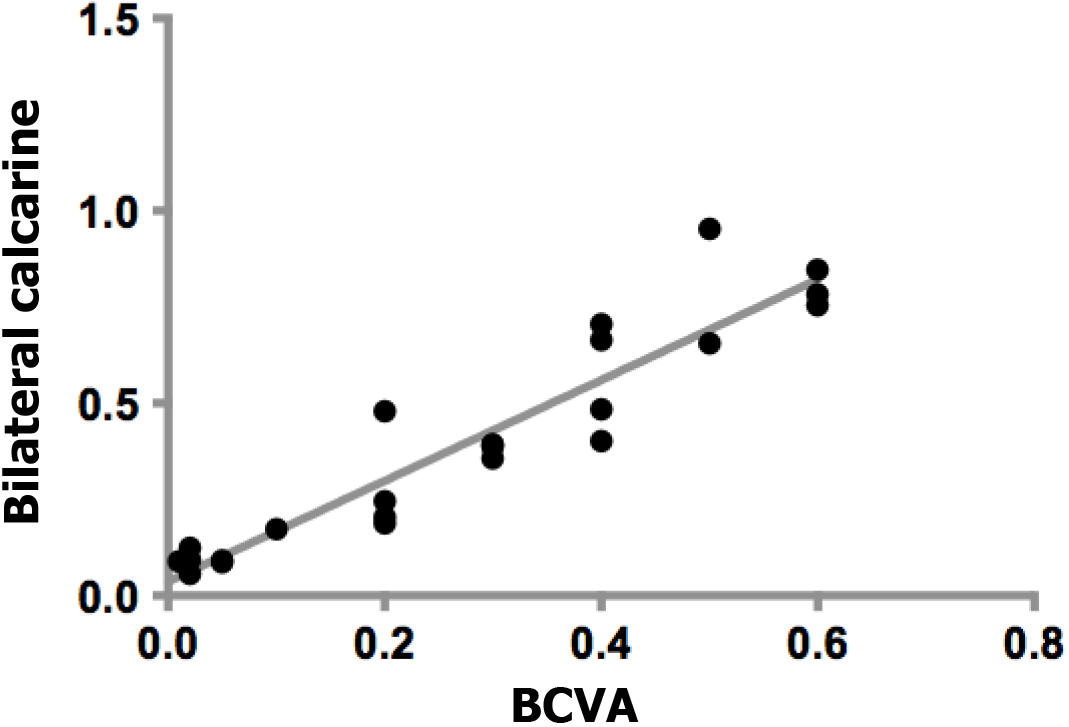
There was a positive correlation between the best-corrected visual acuity (BCVA) values of the affected eyes in the DR group and bilateral calcarine signal values (r = 0.938, P = 0.001). Figure 5 shows the specific details.
DR comprises microvessel damage, which is caused by diabetes mellitus. It is one of the most important causes of visual impairment in young adults[23]. DR is found in 33% of the diabetic patients in the world and is considered an increased risk for systemic vascular complications[24]. The retinal anatomy, physiology and embryological features are similar to cerebral small blood vessels of the brain[25]. Research has shown fundus arteriosclerosis has a positive correlation with cerebral atherosclerosis (P < 0.01)[26], and many diabetes neuroimaging markers of brain abnormalities have a relationship with microvascular disease. Previous studies have shown that the retinal pathological changes in the microcirculation (for example, the Gunn arteriovenous phenomenon, microaneurysms, soft exudate, and retinal hemorrhages) may be associated with markers of cerebral microvascular disease[27].
To our knowledge, this is the first study designed to explore spontaneous cerebral activity changes in DR patients using the ALFF method. Compared with the values in the HCs, the DR patients showed significantly lower ALFF values in the bilateral calcarine fissures, but higher values in the left and right posterior lobes of the cerebellum and the right anterior cingulate gyrus (Figure 6). The ALFF method has already been successfully applied in several eye diseases (Table 3) and it is expected that the application has good prospects for the future.
| Ref. | Disease | Brain areas | |
| PG > HG | PG < HG | ||
| Guo et al[46], 2010 | High myopia | LCN; Thalamus; Cuneus | LOL; BFL; RIPL; RPL; RMTL |
| Li et al[14], 2014 | Glaucoma | RPG | LPG; BMFG; BSFG; RP; RAG |
| Liu et al[13], 2018 | Optic neuritis | LCPL; RCPL; RITG; LPG; RFG; LFG; LCF; LIPL; LC | RCPL; RCAL; RP; RIFG; RI;LMFG; LSTG; RSG; BAC;BP;RIPL |
| Xi et al[15], 2010 | Strabismus | BT | POS |
| Liang et al[48], 2016 | Amblyopia | BC; LMOG; LPG | BPC |
| Tan et al[47], 2016 | Open-globe injury | LC; LMCC; BP | |
| Pan et al[49], 2018 | Acute eye pain | LPG; RPG; LC | LPG; RPG; LP |
The calcium troponin crack is a deep groove on the inside surface of the occipital lobe. The upper and lower parts of the sulcus are the main cortical projection areas of vision. Impulses from the upper retina are transmitted above the sulcus, and visual information from the lower retina is projected below the sulcus. Impulses from the macular area are transmitted above and below the posterior third of the sulcus. This structure is an anatomical marker or landmark.
Previous reports[28] have shown that diabetes affects the microvascular system and leads to cerebral small vascular disease (SVD; Figure 7). The underlying mechanism of visual impairment in DR may involve a compromised arterial blood supply to the visual region that results from SVD. As fMRI has shown, bilateral activation of the visual cortex and eyeball movement can be caused by stimulation of the visual system[29]. Previous studies[30] have shown that prolonged poor glycemic control manifests with basement membrane thickening, tight junction disruption, and pericyte loss, leading to dysregulation of the vascular tone and endothelial cell proliferation, which can lead to microaneurysm formation and ultimately spot and speckle hemorrhages. In this study, we found that there were decreased ALFF values bilaterally in the calcarine fissures of DR patients, which may be related to the impaired vision in these patients. We examined BCVA in both eyes of the patients and found that patients with DR had significantly lower BCVA compared to HCs (P < 0.05; Table 1). Our study observed that there was a positive correlation between the BCVA values of the eyes of the DR group and the signal values of the bilateral calcarine (r = 0.938, P = 0.001). It may be speculated that the decreased signal values of the bilateral calcarine fissures may reflect damage to visual processing in the patients.
Traditionally, the cerebellar function is considered to involve physical balance and motor coordination[31]. However, because of the development and application of neuroimaging technology in recent years, we have a better understanding of the specific effect of the cerebellum, especially its posterior lobe, in emotional processing[32].
The cerebellum’s posterior lobe is located between the primary fissure and the posterolateral fissure. In earlier studies, lesions of the posterior lobe could cause severe damage to spatial memory, emotional regulation, and executive functions[33,34]. Nitschke et al[35] showed that the cerebellum is associated with the movement of the eyeballs. Kresyun[36] found that using a transcranial magnetic to stimulate the cerebellum of DR patients helped with the recovery of the retina's ability to respond to light stress. Previous studies employing positron emission tomography found that patients with social anxiety had abnormal cerebellum signals with increasing cerebral blood flow, which were illustrative. All these studies showed that abnormal activity in the cerebellum was related to anxiety[37,38]. In this study, we found an increase in the ALFF values of the left and right posterior cerebellar lobe. While we cannot prove that DR patients have anxiety, we can propose the hypothesis that anxiety may occur in DR patients with visual and even cognitive disorders.
The anterior cingulate cortex (ACC) is a vital part of the limbic system of the brain. It has extensive and numerous fibrous connections to the cortex and subcortical structures and is involved in the regulation of emotional and motor functions[39]. In a previous study, researchers observed structural defects in the ACC in many depressed patients[40]. Yu et al[41] evaluated diabetic patients using the SF-36 Health-Related Quality of life (HRQL) and anxiety disorders, and they found that there was a statistically significant difference in impaired HRQL (SF-36 summary score) in DR patients compared to the control group (P < 0.05). Clinically, DR patients may also have serious psychosocial problems[42]. Depression is not uncommon in DR patients and it has a negative impact on their condition. However, in recent years, an increasing amount of evidence from electrophysiology[43], functional imaging[44], and behavioral studies[45] have shown that the ACC is closely related to the management of pain. The ACC can be activated by nociceptive and contextual stimuli, and it can participate in pain management, especially affective pain. The neural mechanisms of the ACC’s involvement in effective pain have not been clarified. The specific neural mechanism remains to be studied in the future.
This study demonstrated that there are some abnormal spontaneous brain activities in DR patients. The findings using these new techniques offer information that may help to explain the nerve mechanisms underlying the clinical manifestations of DR patients and contribute to improved clinical diagnoses.
Diabetic retinopathy (DR) is one of the most common complications of diabetes mellitus; however, to date, there has been little analysis of the changes in brain function in patients with DR and their relationship to the clinical manifestations in the eye. This study is the first to examine brain changes in patients with DR using the amplitude of low-frequency fluctuation (ALFF).
The current diagnosis of DR mainly involves fundus fluorescein imaging for examination, and the direct connection between eyes, other manifestations, and the brain is still unknown. In this study, we employed the ALFF technique to investigate abnormal brain activity in DR patients and its relationship with clinical characteristics. Our research may help with understanding how DR disease develops.
We investigated the underlying ALFF of local characteristics of spontaneous brain activity in DR patients and their relationship with behavioral performance. Our findings suggested possible mechanisms of clinical manifestations and behavior in DR patients.
Twenty-four DR patients and 24 healthy controls (HCs) matched for both age and sex were recruited. We measured and recorded the average ALFF values of DR patients and HCs and then classified them using receiver operating characteristic (ROC) curves.
We found that the ALFF values of both the left and right cerebellum posterior lobe and the right anterior cingulate gyrus were remarkably higher in the DR patients compared with the HCs, but DR patients also had lower values in the bilateral calcarine. ROC curve analysis of different brain regions demonstrated high accuracy of area under the curve analysis. However, there was no remarkable relationship between ALFF mean values for different regions and clinical presentations of DR patients.
We hypothesized that DR may lead to alterations in visual cortical activity. Our results showed altered spontaneous activity in three regions of the brain in patients with DR. Abnormalities in low-frequency amplitudes in the brain may be associated with alterations in contralateral best-corrected visual acuity and depression in DR patients. These findings may suggest possible mechanisms of clinical manifestations and behaviors in DR patients.
Our finding that DR may lead to multiple low-frequency amplitude frequency changes in the brain may facilitate our exploration of pathological mechanisms and disease progression in DR patients. However, the drawback is that the sample size was too small. Future studies should increase the sample size in order to ensure the validity of our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotzalidis GD S-Editor: Gao CC L-Editor: A P-Editor: Wang LYT
| 1. | Gunasekeran DV, Ting DSW, Tan GSW, Wong TY. Artificial intelligence for diabetic retinopathy screening, prediction and management. Curr Opin Ophthalmol. 2020;31:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2156] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 3. | Zhu XR, Yang FY, Lu J, Zhang HR, Sun R, Zhou JB, Yang JK. Plasma metabolomic profiling of proliferative diabetic retinopathy. Nutr Metab (Lond). 2019;16:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Su JB, Zhao LH, Zhang XL, Cai HL, Huang HY, Xu F, Chen T, Wang XQ. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc Diabetol. 2018;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Usuelli V, La Rocca E. Novel therapeutic approaches for diabetic nephropathy and retinopathy. Pharmacol Res. 2015;98:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Liu H, Wang X. Correlation of iron deposition and change of gliocyte metabolism in the basal ganglia region evaluated using magnetic resonance imaging techniques: an in vivo study. Arch Med Sci. 2016;12:163-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Meijer FJ, Goraj B. Brain MRI in Parkinson's disease. Front Biosci (Elite Ed). 2014;6:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 10. | Cakir Y. The effects of Alzheimer's disease related striatal pathologic changes on the fractional amplitude of low-frequency fluctuations. Comput Methods Biomech Biomed Engin. 2020;23:1347-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Xing XX, Zheng MX, Hua XY, Ma SJ, Ma ZZ, Xu JG. Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging. Neural Regen Res. 2021;16:388-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Rosenbaum D, Int-Veen I, Kroczek A, Hilsendegen P, Velten-Schurian K, Bihlmaier I, Fallgatter AJ, Ehlis AC. Amplitude of low frequency fluctuations (ALFF) of spontaneous and induced rumination in major depression: An fNIRS study. Sci Rep. 2020;10:21520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Jiang X, Butzkueven H, Duan Y, Huang J, Ren Z, Dong H, Shi FD, Barkhof F, Li K, Wang J. Multimodal characterization of gray matter alterations in neuromyelitis optica. Mult Scler. 2018;24:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Li T, Liu Z, Li J, Tang Z, Xie X, Yang D, Wang N, Tian J, Xian J. Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: a resting-state FMRI study. Invest Ophthalmol Vis Sci. 2014;56:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Xi S, Yao J, Zhang S, Liu R, Wu L, Ye X, Zhang P, Wen W, Zhao C. Disrupted neural signals in patients with concomitant exotropia. Ophthalmic Physiol Opt. 2020;40:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Kwak Y, Peltier SJ, Bohnen NI, Müller ML, Dayalu P, Seidler RD. L-DOPA changes spontaneous low-frequency BOLD signal oscillations in Parkinson's disease: a resting state fMRI study. Front Syst Neurosci. 2012;6:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Alzner E. International Congress of Ophthalmology — The World Meeting of Ophthalmologists, 21.–25. April 2002, Sydney — Australien. Spektrum Der Augenheilkunde. 2002;16: 189-190. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673-9678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6644] [Cited by in RCA: 6440] [Article Influence: 322.0] [Reference Citation Analysis (0)] |
| 19. | Kang HH, Shu YQ, Yang L, Zhu PW, Li D, Li QH, Min YL, Ye L, Zhou Q, Shao Y. Measuring abnormal intrinsic brain activities in patients with retinal detachment using amplitude of low-frequency fluctuation: a resting-state fMRI study. Int J Neurosci. 2019;129:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Wu YY, Yuan Q, Li B, Lin Q, Zhu PW, Min YL, Shi WQ, Shu YQ, Zhou Q, Shao Y. Altered spontaneous brain activity patterns in patients with retinal vein occlusion indicated by the amplitude of low-frequency fluctuation: A functional magnetic resonance imaging study. Exp Ther Med. 2019;18:2063-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 722] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 22. | Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 2022] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 23. | Polack S, Yorston D, López-Ramos A, Lepe-Orta S, Baia RM, Alves L, Grau-Alvidrez C, Gomez-Bastar P, Kuper H. Rapid assessment of avoidable blindness and diabetic retinopathy in Chiapas, Mexico. Ophthalmology. 2012;119:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Koch EAT, Nakhoul R, Nakhoul F, Nakhoul N. Autophagy in diabetic nephropathy: a review. Int Urol Nephrol. 2020;52:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Tso MOM, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmol. 1982;89: 1132-1145. [RCA] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 234] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Reid IR, Bolland MJ. Controversies in medicine: the role of calcium and vitamin D supplements in adults. Med J Aust. 2019;211:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 2004;3:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Szcześniak D, Rymaszewska J, Zimny A, Sąsiadek M, Połtyn-Zaradna K, Smith EE, Zatońska K, Zatoński T, Rangarajan S, Yusuf S, Szuba A. "Cerebral small vessel disease and other influential factors of cognitive impairment in the middle-aged: a long-term observational cohort PURE-MIND study in Poland". Geroscience. 2021;43:279-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Dieterich M, Bauermann T, Best C, Stoeter P, Schlindwein P. Evidence for cortical visual substitution of chronic bilateral vestibular failure (an fMRI study). Brain. 2007;130:2108-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Fang M, Selvin E. Thirty-Year Trends in Complications in U.S. Adults With Newly Diagnosed Type 2 Diabetes. Diabetes Care. 2021;44:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Ferrari C, Oldrati V, Gallucci M, Vecchi T, Cattaneo Z. The role of the cerebellum in explicit and incidental processing of facial emotional expressions: A study with transcranial magnetic stimulation. Neuroimage. 2018;169:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Adamaszek M, D'Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, Priori A, Sacchetti B, Schutter DJ, Styliadis C, Verhoeven J. Consensus Paper: Cerebellum and Emotion. Cerebellum. 2017;16:552-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 383] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 33. | Stoodley CJ, Schmahmann JD. Functional topography of the human cerebellum. Handb Clin Neurol. 2018;154:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 34. | Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 718] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 35. | Nitschke MF, Arp T, Stavrou G, Erdmann C, Heide W. The cerebellum in the cerebro-cerebellar network for the control of eye and hand movements--an fMRI study. Prog Brain Res. 2005;148:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Kresyun NV. The influence of deltalycyn and transcranial cerebellar stimulation upon recovery of retina after photo stress in patients with diabetic retinopathy. Rev Med Chir Soc Med Nat Iasi. 2014;118:1068-1073. [PubMed] |
| 37. | Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ, Nemeroff CB. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Olexová L, Štefánik P, Kršková L. Increased anxiety-like behaviour and altered GABAergic system in the amygdala and cerebellum of VPA rats - An animal model of autism. Neurosci Lett. 2016;629:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4219] [Cited by in RCA: 4267] [Article Influence: 170.7] [Reference Citation Analysis (0)] |
| 40. | Webb CA, Weber M, Mundy EA, Killgore WD. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol Med. 2014;44:2833-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Yu Y, Feng L, Shao Y, Tu P, Wu HP, Ding X, Xiao WH. Quality of life and emotional change for middle-aged and elderly patients with diabetic retinopathy. Int J Ophthalmol. 2013;6:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 42. | Crosby-Nwaobi RR, Sivaprasad S, Amiel S, Forbes A. The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care. 2013;36:3177-3186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Yu W, Wu X, Chen Y, Liang Z, Jiang J, Misrani A, Su Y, Peng Y, Chen J, Tang B, Sun M, Long C, Shen J, Yang L. Pelvic Pain Alters Functional Connectivity Between Anterior Cingulate Cortex and Hippocampus in Both Humans and a Rat Model. Front Syst Neurosci. 2021;15:642349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Ito T, Tanaka-Mizuno S, Iwashita N, Tooyama I, Shiino A, Miura K, Fukui S. Proton magnetic resonance spectroscopy assessment of metabolite status of the anterior cingulate cortex in chronic pain patients and healthy controls. J Pain Res. 2017;10:287-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Boccard SG, Pereira EA, Moir L, Van Hartevelt TJ, Kringelbach ML, FitzGerald JJ, Baker IW, Green AL, Aziz TZ. Deep brain stimulation of the anterior cingulate cortex: targeting the affective component of chronic pain. Neuroreport. 2014;25:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Guo MX, Dong HH, Zhang Y T. ALFF Changes in Brain Areas of Human with High Myopia Revealed by Resting-state Functional MRI. IEEE. 2010;1: 91-94. |
| 47. | Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, Jiang N, Zhou FQ, Shao Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12:2015-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Liang M, Xie B, Yang H, Yu L, Yin X, Wei L, Wang J. Distinct patterns of spontaneous brain activity between children and adults with anisometropic amblyopia: a resting-state fMRI study. Graefes Arch Clin Exp Ophthalmol. 2016;254:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, Zhu PW, Ye L, Ma MY, Huang X, Shao Y. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |