Published online Dec 15, 2022. doi: 10.4239/wjd.v13.i12.1154
Peer-review started: August 10, 2022
First decision: October 5, 2022
Revised: October 18, 2022
Accepted: December 1, 2022
Article in press: December 1, 2022
Published online: December 15, 2022
Processing time: 119 Days and 20.1 Hours
Diabetes mellitus is considered a leading contributor to severe coronavirus disease 2019 (COVID-19).
To characterize differences between hospitalized diabetic patients with vs without COVID-19, and parameters associated with COVID-19 severity for prediction.
This case-control study included 209 patients with type 2 diabetic mellitus hospitalized at the Galilee Medical Center (Nahariya, Israel) and recruited between September 2020 and May 2021, 65 patients with COVID-19 infection in dedicated wards and 144 COVID-19-negative patients in internal medicine wards hospitalized due to other reasons. Clinical parameters - including age, type of antiglycemic medications, presence of retinopathy, smoking history, body mass index (BMI), glycosylated hemoglobin, maximum neutrophil:lymphocyte ratio (NLRmax), C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), and albumin (blood and urine) - were compared between the two primary patient groups, and then between COVID-19-negative patients hospitalized due to infectious vs non-infectious disease. Finally, we explored which parameters were associated with severe COVID-19 pneumonia.
COVID-19-negative patients were older (63.9 ± 9.9 vs 59.8 ± 9.2, P = 0.005), and had longer duration of diabetes (P = 0.031), lower eGFR (P = 0.033), higher albumin (P = 0.026), lower CRP (P < 0.001), greater smoking prevalence (P < 0.001), and more baseline albuminuria (54.9% vs 30.8%, P = 0.005) at admission; 70% of COVID-19 patients with albuminuria had moderate-range albuminuria (albumin:creatinine 30-300 mg/g). Most of the patients with albuminuria had chronic kidney disease stage II (CKD II). Oral antiglycemic therapies were not significantly different between the two groups. Multivariable logistic regression showed that higher BMI was significantly associated with severe COVID-19 (OR 1.24, 95%CI: 1.01-1.53, P = 0.04), as was higher NLRmax (OR 1.2, 95%CI: 1.06-1.37, P = 0.005). Surprisingly, pre-hospitalization albuminuria, mostly moderate-range, was associated with reduced risk (OR 0.09, 95%CI: 0.01-0.62, P = 0.015). Moderate-range albuminuria was not associated with bacterial infections.
Moderate-range albuminuria in COVID-19-positive diabetic patients with CKD II is associated with less severe COVID-19. Further studies should explore this potential biomarker for risk of COVID-19-related deterioration and early interventions.
Core Tip: Type 2 diabetes mellitus and its risk factors are considered to be contributors to severe coronavirus disease 2019 (COVID-19). In this study, we analyzed our single-center clinical data of adults with type 2 diabetes between September 2020 and May 2021 to determine the impact of risk factors on severity of COVID-19 pneumonia. Surprisingly, we found that moderate-range pre-hospitalization albuminuria was associated with reduced risk of severe COVID-19 pneumonia. Further studies are needed to explore this association and pathogenesis relating to immunomodulation, which may indicate a biomarker for patients at reduced risk for COVID-19-related deterioration that may translate to therapeutic interventions.
- Citation: Bashkin A, Shehadeh M, Shbita L, Namoura K, Haiek R, Kuyantseva E, Boulos Y, Yakir O, Kruzel-Davila E. Baseline moderate-range albuminuria is associated with protection against severe COVID-19 pneumonia. World J Diabetes 2022; 13(12): 1154-1167
- URL: https://www.wjgnet.com/1948-9358/full/v13/i12/1154.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i12.1154
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the causative pathogen for coronavirus disease 2019 (COVID-19) pneumonia[1]. COVID-19 infection can cause various symptoms of varying severity, starting from mild disease with upper respiratory tract infection and continuing to moderate and severe pneumonia with a systemic inflammatory response syndrome, acute respiratory distress syndrome (ARDS), multi-organ involvement, and shock[2].
Several risk factors for severe COVID-19 disease have been described, and include advanced age, male sex, smoking history, and underlying chronic diseases such as cardiovascular disease (CVD), diabetes mellitus, obesity, underweight, and chronic kidney disease (CKD) [defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m²], as well as socioeconomic deprivation[3,4]. The presence of diabetes and the individual degree of hyperglycemia appear to be independently associated with COVID-19 severity and increased mortality[5].
Given the compromised immune function in patients with diabetes, especially innate immunity with impaired natural killer (NK) cells[5-7], impaired T cell responses with increased Th1 and Th17 cells, reduced T regulatory cells, and altered cytokine response[8], diabetes is considered to be a risk factor for severe COVID-19 pneumonia[5,9]. A recent retrospective study demonstrated elevated cytokines, imbalance of Th1/Th2 secreted cytokines and reduced levels of CD8+ T cells and NK cells in patients with diabetes suffering from COVID-19 pneumonia compared to patients without diabetes, with reduced level of CD8+ T cells and NK cells being more pronounced in non-survivors[10]. In addition, other risk factors for severe COVID-19 pneumonia are associated with diabetes, e.g., obesity, hyperglycemia, CVD, and CKD[1-3].
In this case-control study, we aimed to characterize the differences between patients with diabetes hospitalized in internal medicine departments and patients with diabetes suffering from COVID-19 pneumonia in designated wards at the Galilee Medical Center. Among the patients with COVID-19 infection, we explored clinical parameters that were associated with severe COVID-19 pneumonia for predictive value.
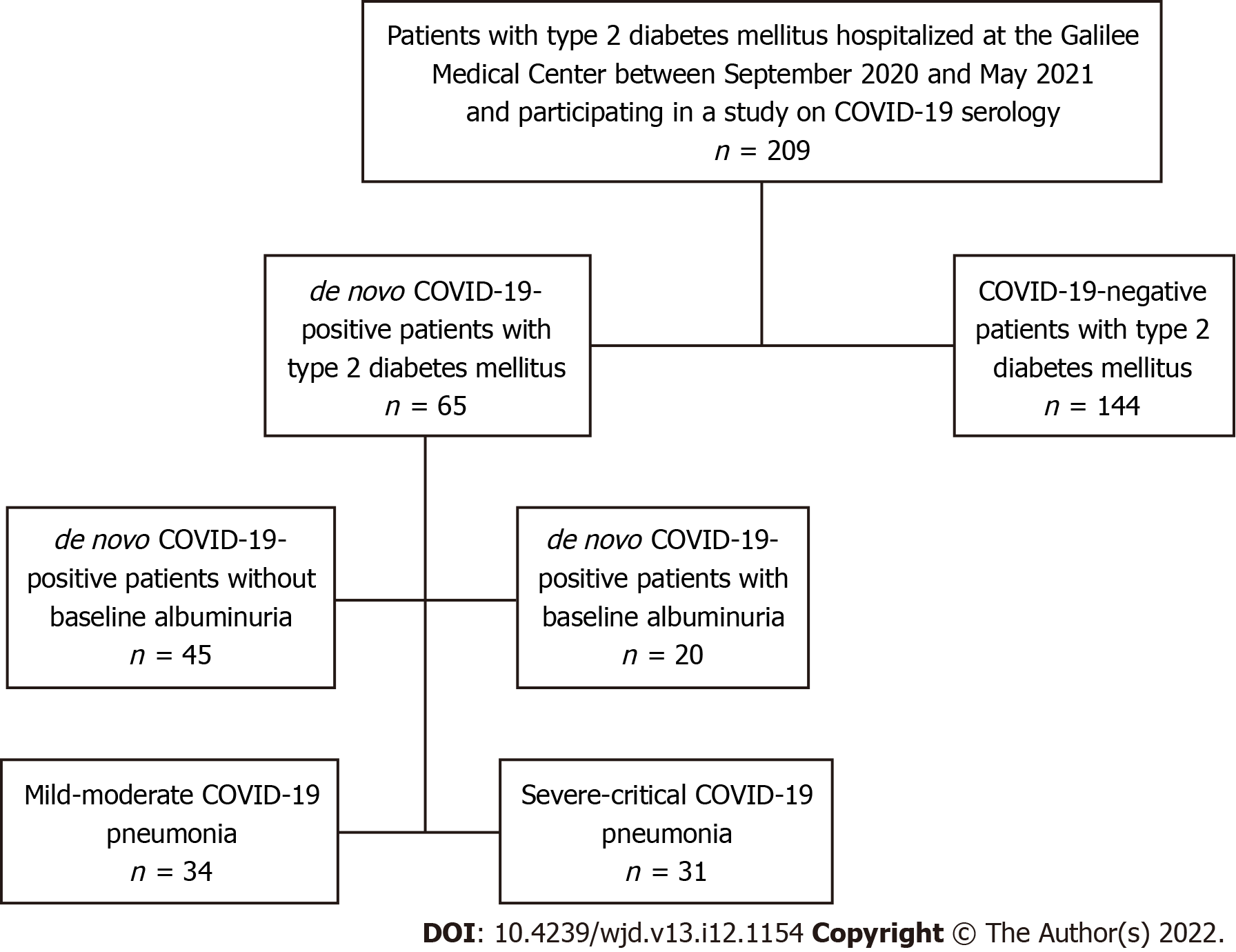
Data were analyzed from 209 type 2 diabetic patients hospitalized at the Galilee Medical Center between September 2020 and May 2021 and participating in a study evaluating the prevalence of and related factors for de novo positive COVID19 serology (ethical committee approval number 0073-21-NHR, dated 05-Jul-2021). Sixty-five patients suffering from COVID-19 infection were hospitalized in the COVID-19 wards and 144 patients with other diseases were hospitalized in the internal medicine wards, the latter recruited concurrently for unbiased comparison (Figure 1). Diabetes was defined by glycosylated hemoglobin (HbA1c) ≥ 6.5% and by medical history of type 2 diabetes diagnosis in the past. Patients hospitalized in the internal medicine wards were recruited during their hospitalization period, while patients suffering from COVID-19 pneumonia were recruited after their discharge from the hospital. All participants signed the informed consent form as approved by the Galilee Medical Center Helsinki Committee (investigational review board). The total number of participants was reached per enrollment criteria of patients hospitalized during the selected time period.
This case-control study included the following two parts: (1) First we compared the clinical parameters between the two primary patient groups, followed by a comparison of the clinical characteristics between patients hospitalized due to infectious vs non-infectious disease in the internal medicine wards; and (2) Second, we explored which clinical parameters were associated with severe COVID-19 pneumonia.
Demographic, clinical, and laboratory parameters were collected from electronic hospital and community records using Chameleon and Ofek software, respectively. The following baseline parameters were recorded: Age, sex, religion, type of antiglycemic medications [metformin, dipeptidylpeptidase-4 (DPP-4) inhibitors, sulfonylurea, sodium glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and insulin], presence of retinopathy, smoking history, body mass index (BMI), and HbA1c. The following parameters were collected during the hospitalization period: maximum neutrophil:lymphocyte ratio (NLRmax) and C-reactive protein (CRP) (plus values at admission), eGFR, and albumin. COVID-19 infection was defined as a positive SARS-CoV-2 polymerase chain reaction.
Baseline moderate-range albuminuria was defined as an albumin-to-creatinine ratio between > 30 and < 300 mg/g in two urine analyses performed during the 18 mo prior to hospitalization; macroalbuminuria was defined as > 300 mg/g. eGFR was calculated by using the CKD Epidemiology Collaboration creatinine equation[11]. Baseline HbA1c and eGFR were calculated as the average of up to two last values of the respective tests during the 12 mo prior to hospitalization. Retinopathy was defined according to fundoscopic examination conducted during the 18 mo prior to hospitalization (it included background retinopathy, proliferative retinopathy, or macular edema).
Quantitative variables other than albuminuria were not divided into subgroups. Albuminuria was divided dichotomously for patients with and without, based on known categorization by urine-albumin-to-creatinine ratio (< 30 mg/g and ≥ 30 mg/g); and for those with, further divided into three groups according to albuminuria severity (albuminuria < 30 mg/g, 30-300 mg/g, and > 300 mg/g).
The severity of COVID-19 infection was determined in accordance with the following Israel Ministry of Health criteria published July 12, 2020 (according to the United States National Institutes of Health): (1) Mild illness when there are symptoms of mild viral upper respiratory tract infection; (2) Moderate illness per imaging and oxygen saturation ≥ 94% on room air; (3) Severe illness when one of the following criteria were met: respiratory rate > 30 breaths/minute, oxygen saturation < 94% on room air, PaO2/FiO2 < 300 mmHg, or lung infiltration > 50%; and (4) Critical illness per hemodynamic instability, need for mechanical ventilation, and/or multiorgan failure.
Quantitative variables were analyzed for mean (SD), median, and interquartile range (IQR). Categorical variables were analyzed with frequencies and percentages.
Differences between groups for continuous variables were compared using independent sample t-test or Mann-Whitney test. We chose independent sample t-test when the compared variables did not deviate significantly from the normal distribution. Differences between groups for categorical variables were compared with Chi-square or Fisher’s exact tests (if expectancy < 5).
Correlations between continuous variables were examined with Spearman’s correlation coefficient test, which was chosen over Pearson’s correlation coefficient test according to the variable’s distribution shape. Multivariable logistic regression modelling was used to determine the risk factors for severe or critical COVID-19 and separately for infectious compared to non-infectious disease in patients without COVID-19 infection. In the multivariable analysis, the severity of COVID-19 pneumonia and presence of infectious disease were the dependent variables, while the following baseline parameters were independent variables: age, sex, BMI, last eGFR measured before hospitalization, HbA1c, NLRmax, and albuminuria before hospitalization. These risk factors were chosen according to the univariable analysis results and theoretical considerations. We defined the following three models according to variables: Model 1 included sex, age, and BMI; Model 2 included Model 1 variables plus HbA1c, eGFR, and NLRmax; and Model 3 included Model 2 variables plus the presence of albuminuria. Model 3 presents an adjustment of background and clinical measures (e.g., age, eGFR, NLRmax, etc.) for the albuminuria variable.
Odds ratios (OR) and 95% confidence intervals (CI) for OR are provided as estimates of risk for each variable.
Analyses were performed with IBM SPSS Statistics software version 27.0 (Chicago, IL, United States). A P value of < 0.05 was considered statistically significant. Two-sided P values are presented unless otherwise specified.
Between September 2020 and May 2021, 65 patients with diabetes suffering from COVID-19 infection and 144 diabetic COVID-19-negative patients hospitalized due to other reasons were enrolled in this study.
Clinical characteristics of patients with diabetes, with COVID-19 infection compared to diabetic patients without COVID-19 infection, are presented in Table 1. Patients without COVID-19 were older than patients with COVID-19 (63.9 ± 9.9 years compared to 59.8 ± 9.2 years, respectively, P = 0.005), had lower prevalence of smoking (6.2% compared to 33.3%, P < 0.001), longer duration of diabetes (P = 0.031), lower eGFR (P = 0.033), higher albumin (P = 0.026), and lower CRP at admission, as well as lower maximum value of CRP (CRPmax) during hospitalization (P < 0.001). Interestingly, baseline albuminuria was more common in patients without COVID-19 infection (54.9% compared to 30.8%, P = 0.005). There was a trend toward a higher percentage of insulin therapy in patients without COVID-19 (P = 0.047 and P = 0.064 for prandial and basal insulin, respectively). Use of other antiglycemic therapies were not significantly different between the two groups.
| Patients without COVID-19 infection, n = 144 | Patients with COVID-19 infection, n = 65 | P value | |
| Age (yr), mean (SD) | 63.9 (9.9) | 59.8 (9.2) | 0.0051 |
| Median (IQR) | 65 (57-71) | 61 (53-66) | |
| Sex, female, n (%) | 50 (34.7) | 32 (49.2) | 0.0662 |
| Population group, n (%) | 0.0672 | ||
| Jews | 63 (44.7) | 20 (30.8) | |
| Arabs | 78 (55.3) | 45 (69.2) | |
| BMI (kg/m2), mean (SD) | 30.7 (5.9) | 32.1 (4.6) | 0.091 |
| Median (IQR) | 30.3 (27.4-33.8) | 31.6 (29.1-34.7) | |
| Diabetes duration (yr), median (IQR) | 13 (8-17.8) | 10 (5.5-14.5) | 0.0313 |
| eGFR (mL/min/1.73 m2 body surface area) at baseline, median (IQR) | 85.4 (62.2-97.6) | 91.9 (75.3-101.0) | 0.0333 |
| HbA1c (%), median (IQR) | 7.6 (6.5-9.1) | 7.4 (6.6-9.1) | 1.003 |
| Metformin, n (%) | 110 (76.9) | 56 (86.2) | 0.142 |
| DPP-4 inhibitors, n (%) | 29 (20.1) | 18 (27.7) | 0.282 |
| Sulfonylurea, n (%) | 8 (5.6) | 7 (10.8) | 0.254 |
| SGLT2 inhibitors, n (%) | 36 (25.0) | 18 (27.7) | 0.732 |
| GLP-1 agonists, n (%) | 25 (17.4) | 12 (18.5) | 1.002 |
| Basal insulin, n (%) | 60 (41.7) | 18 (27.7) | 0.0642 |
| Prandial insulin, n (%) | 30 (21.0) | 6 (9.2) | 0.0472 |
| Current smoking, n (%) | 48 (33.3) | 4 (6.2) | < 0.0012 |
| No albuminuria, n (%) | 65 (45.1) | 45 (69.2) | 0.0022 |
| Albuminuria < 30 mg/g, n (%) | 79 (54.9) | 20 (30.8) | 0.0053 |
| Albuminuria 30-300 mg/g, n (%) | 65 (45.1) | 14 (21.5) | |
| Albuminuria > 300 mg/g, n (%) | 14 (9.7) | 6 (9.2) | |
| Retinopathy, n (%) | 21 (21.4) | 7 (15.2) | 0.502 |
| NLRmax at hospitalization, median (IQR) | 4.0 (2.5-7.8) | 6.5 (2.6-10.0) | 0.123 |
| CRP (mg/L) at admission, median (IQR) | 9.7 (4.8-45.4) | 71.8 (12.2-145.9) | < 0.0013 |
| CRPmax (mg/L) at hospitalization, median (IQR) | 11.3 (5.2-68.1) | 86.6 (12.2-167.6) | < 0.0013 |
| eGFR (mL/min/1.73 m2 body surface area) at hospitalization, median (IQR) | 86.0(62.2-96.0) | 92.1(76.2-100.8) | 0.0303 |
| Albumin, median (IQR) | 3.8 (3.4-4.0) | 3.6(3.2-3.9) | 0.0261 |
Among the 65 patients suffering from COVID-19 infection in this cohort, 20 had documented albuminuria prior to hospitalization, whereas 45 did not. Most of the patients with albuminuria had CKD II and moderately increased albuminuria (A2) (Table 2). Basal insulin therapy was more common in patients with albuminuria (50% compared to 17.8%, P = 0.015). As expected, patients with albuminuria had significantly higher values of HbA1c (median 8.9% and IQR25-75 7.3%-10.4%, compared to 7.2% and 6.6%-8.4%, respectively, P = 0.02). Other parameters were not significantly different between the two groups (Table 2).
| Diabetic patients without baseline albuminuria, n = 45 | Diabetic patients with baseline albuminuria, n = 20 | P value | |
| Age (yr), mean (SD) | 58.7 (9.2) | 62.2 (9.0) | 0.161 |
| Median (IQR) | 60.0 (50.5-65.0) | 63.0 (56.5-68.8) | |
| Sex, female, n (%) | 21 (46.7) | 11 (55.0) | 0.602 |
| Population group, n (%) | 0.772 | ||
| Jews | 13 (28.9) | 7 (35.0) | |
| Arabs | 32 (71.1) | 13 (65.0) | |
| BMI (kg/m2), mean (SD) | 31.6 (4.5) | 33.2 (4.6) | 0.191 |
| Median (IQR) | 30.9 (28.6-33.7) | 32.3 (29.7-35.9) | |
| Diabetes duration (yr), median (IQR) | 10.0 (5.5-14.0) | 11.0 (5.3-17.0) | 0.251 |
| eGFR (mL/min/1.73 m2 body surface area) at baseline, median (IQR) | 90.5 (77.8-99.5) | 95.3 (69.6-101.1) | 0.903 |
| HbA1c (%), median (IQR) | 7.2 (6.6-8.4) | 8.9 (7.3-10.4) | 0.023 |
| Metformin, n (%) | 41 (91.1) | 15 (75) | 0.124 |
| DPP-4 inhibitors, n (%) | 15 (33.3) | 3 (15) | 0.152 |
| Sulfonylurea, n (%) | 5 (11.1) | 2 (10) | 1.004 |
| SGLT2 inhibitors, n (%) | 15 (33.3) | 3 (15) | 0.152 |
| GLP-1 agonists, n (%) | 8 (17.8) | 4 (20) | 1.004 |
| Basal insulin, n (%) | 8 (17.8) | 10 (50) | 0.0152 |
| Prandial insulin, n (%) | 2 (4.4) | 4 (20) | 0.0674 |
| Current smoking, n (%) | 2 (4.4) | 2 (10) | 0.584 |
| Retinopathy, n (%) | 4 (12.9) | 3 (20) | 0.674 |
| NLRmax, median (IQR) | 6.2 (2.5-9.4) | 6.7 (2.6-18.0) | 0.483 |
| CRP (mg/L) at admission, median (IQR) | 90.4 (10.9-153.6) | 43.9 (12.5-106.8) | 0.333 |
| CRPmax (mg/L) at hospitalization, median (IQR) | 92.8 (11.4-171.3) | 61.0 (12.5-157.3) | 0.603 |
| eGFR (mL/min/1.73 m2 body surface area) at hospitalization, median (IQR) | 90.3 (77.0-98.4) | 94.3 (67.8-101) | 0.763 |
| Albumin, median (IQR) | 3.6 (3.3-4.0) | 3.6 (3.1-3.9) | 0.251 |
Univariable analysis demonstrated increased risk for severe COVID-19 pneumonia with higher inflammatory markers NLRmax and CRP (P < 0.001) and lower albumin level (P < 0.001). Oral antiglycemic therapies were not significantly different between patients with moderate or severe pneumonia (Table 3).
| Mild-moderate COVID-19 pneumonia, n = 34 | Severe-critical COVID-19 pneumonia, n = 31 | P value | |
| Age (yr), mean (SD) | 58.6 (10.0) | 61.2 (8.1) | 0.261 |
| Median (IQR) | 59.5 (50.8-65.3) | 63 (57.0-67.0) | |
| Sex, female, n (%) | 20 (58.8) | 12 (38.7) | 0.142 |
| Population group, n (%) | 1.002 | ||
| Jews | 10 (29.4) | 10 (32.3) | |
| Arabs | 24 (70.6) | 21 (67.7) | |
| BMI (kg/m2), mean (SD) | 31.4 (3.8) | 32.8 (5.2) | 0.221 |
| Median (IQR) | 31.2 (28.9-33.6) | 31.7 (29.3-36.1) | |
| Diabetes duration (yr), median (IQR) | 9.5 (4.8-12.5) | 11 (7-17) | 0.133 |
| eGFR (mL/min/1.73 m2 body surface area) at baseline, median | 90.4 (74.9-105.5) | 93.3 (76.0-100.3) | 0.783 |
| HbA1C (%), median (IQR) | 7.4 (6.6-9.1) | 7.4 (6.6-9.4) | 0.933 |
| Metformin, n (%) | 30 (88.2) | 26 (83.9) | 0.734 |
| DPP-4, n (%) | 10 (29.4) | 8 (25.8) | 0.792 |
| Sulfonylurea, n (%) | 5 (14.7) | 2 (6.5) | 0.434 |
| SGLT2 inhibitors, n (%) | 11 (32.4) | 7 (22.6) | 0.422 |
| GLP-1 agonists, n (%) | 5 (14.7) | 7 (22.6) | 0.532 |
| Basal insulin, n (%) | 8 (23.5) | 10 (32.3) | 0.582 |
| Prandial insulin, n (%) | 3 (8.8) | 3 (9.7) | 1.004 |
| Current smoking, n (%) | 4 (11.8) | 0 (0) | 0.124 |
| No albuminuria, n (%) | 21 (61.8) | 24 (77.4) | 0.192 |
| 2-sided | |||
| 0.142 | |||
| 1-sided | |||
| Albuminuria < 30 mg/g, n (%) | 13 (38.2) | 7 (22.6) | 0.1453 |
| 2-sided | |||
| Albuminuria 30-300 mg/g, n (%) | 8 (23.5) | 6 (19.4) | 0.0733 |
| Albuminuria > 300 mg/g, n (%) | 5 (14.7) | 1 (3.2) | 1-sided |
| Retinopathy, n (%) | 4 (16.7) | 3 (13.6) | 1.004 |
| NLRmax median (IQR) | 2.7 (1.7-8.5) | 9.2 (5.7-15.4) | < 0.0013 |
| CRP (mg/L) at admission, median (IQR) | 15.4 (5.1-80.9) | 122.1 (70.8-177.2) | < 0.0013 |
| CRPmax (mg/L) at hospitalization, median (IQR) | 15.4 (5.1-86.2) | 142.3 (87.1-205.1) | < 0.0013 |
| eGFR (mL/min/1.73 m2 body surface area) at hospitalization, median (IQR) | 91.2 (76.0-105.0) | 93.3 (76.5-100.3) | 0.903 |
| Albumin (g/dL), mean (SD) | 3.8 (0.4) | 3.3 (0.4) | < 0.0011 |
| Median (IQR) | 3.9 (3.6-4.1) | 3.3 (3.1-3.5) |
The following variables were considered to be confounders due to putative correlation with albuminuria and COVID-19 severity: age, BMI, eGFR, HbA1c, and NLRmax. We therefore examined the correlation between each of these variables and albuminuria (see Table 2) and COVID-19 severity (see Table 3). According to the univariable analysis (Tables 2 and 3), HbA1c was found to be significant correlated with albuminuria (P = 0.02), but was not found to be significant in the COVID-19 severity univariable analysis (P = 0.93); NLRmax was not found to be correlated with albuminuria (P = 0.48), but was found to be correlated with COVID-19 severity (P < 0.001); age and BMI were found only to trend toward correlations with albuminuria (P = 0.16 and P = 0.19 respectively) and COVID-19 severity (P = 0.26, P = 0.22), possibly due to the small sample size; eGFR was not found to correlate with either albuminuria or COVID-19 severity (P = 0.90 and P = 0.78, respectively).
Because of the theoretical consideration and the above findings, we decided to include those variables in the multivariable analysis. Given the association between sex and COVID-19 severity reported in the literature, we included this variable in the univariable analysis, wherein male sex showed only a trend toward correlation with COVID-19 severity (P = 0.14), with no correlation for albuminuria (P = 0.60).
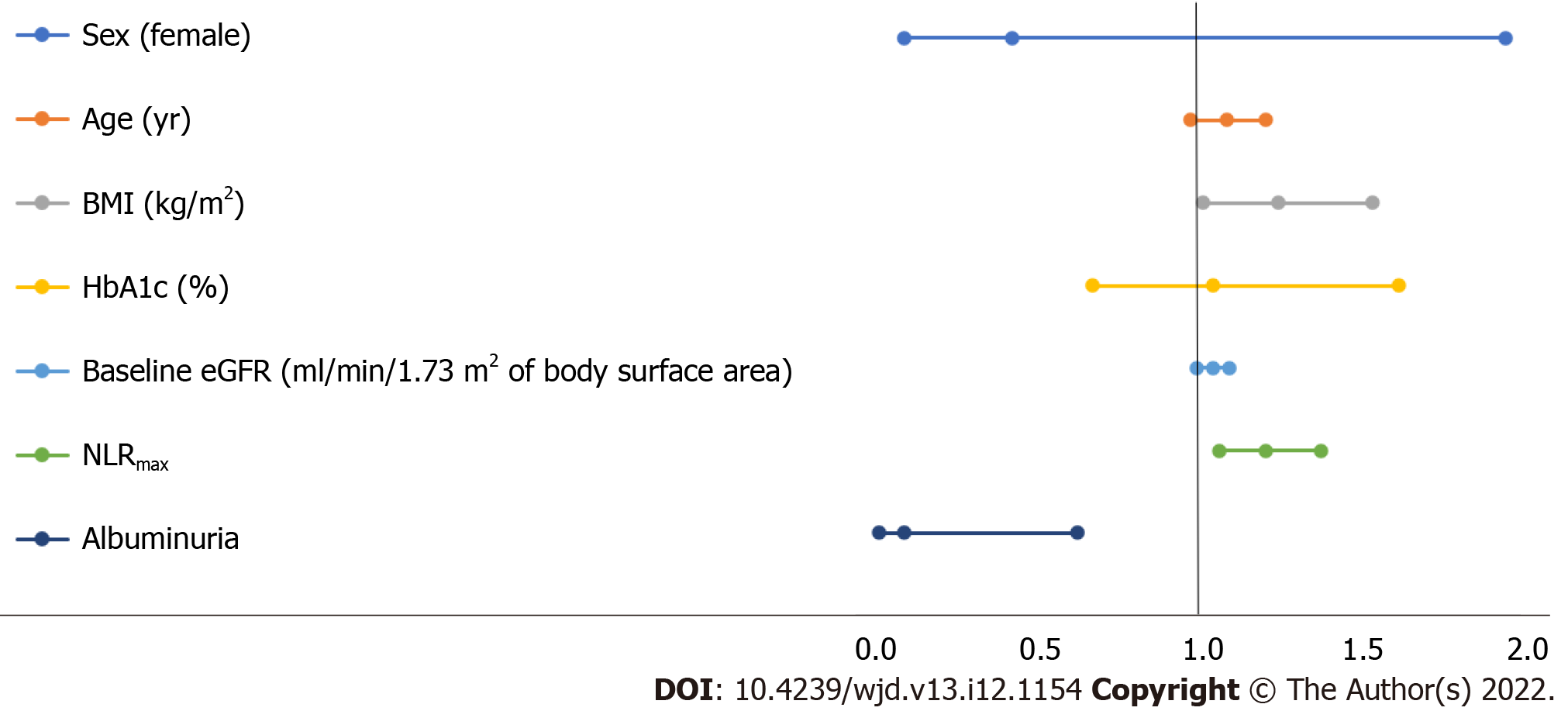
Variables associated with severe COVID-19 pneumonia in the multivariable logistic analysis according to the Models 1-3 are presented in Table 4, and the multivariable regression in Model 3 is depicted in Figure 2. The dependent variable is the severity of COVID-19 pneumonia, while the following parameters are independent variables: age, sex, BMI, last eGFR measured before hospitalization, HbA1c, NLRmax, and albuminuria before hospitalization. In the final model, as expected, a higher BMI was significantly associated with severe COVID-19 pneumonia (OR 1.24, 95%CI: 1.01-1.53, P = 0.04), as was higher NLRmax (OR 1.20, 95%CI: 1.06-1.37, P = 0.005). Surprisingly, the presence of moderate-range albuminuria before hospitalization was associated with reduced risk (OR 0.09, 95%CI: 0.01-0.62, P = 0.015). Of note, 70% of COVID-19 patients with proteinuria had moderate-range albuminuria.
| OR | 95%CI | P value | ||
| Model 1 | Sex (female vs male) | 0.30 | 0.10-0.93 | 0.038 |
| Baseline characteristics | Age (yr) | 1.03 | 0.98-1.09 | 0.28 |
| R-square: 14.4% | BMI (kg/m2) | 1.12 | 0.99-1.28 | 0.073 |
| Model 2 | Sex (female vs male) | 0.57 | 0.15-2.16 | 0.41 |
| Baseline characteristics | Age (yr) | 1.02 | 0.94-1.11 | 0.62 |
| R-square: 34.4% | BMI (kg/m2) | 1.14 | 0.96-1.35 | 0.13 |
| HbA1c (%) | 0.85 | 0.59-1.23 | 0.39 | |
| Baseline eGFR (mL/min/ 1.73 m2 of body surface area) | 1.02 | 0.98-1.07 | 0.28 | |
| NLRmax | 1.18 | 1.05-1.33 | 0.007 | |
| Model 3 | Sex (female vs male) | 0.42 | 0.09-1.94 | 0.27 |
| Baseline characteristics | Age (yr) | 1.08 | 0.97-1.20 | 0.18 |
| R-square = 46.9% | BMI (kg/m2) | 1.24 | 1.01-1.53 | 0.040 |
| HbA1c (%) | 1.04 | 0.67-1.61 | 0.89 | |
| Baseline eGFR (mL/min/1.73 m2 of body surface area) | 1.04 | 0.99-1.09 | 0.12 | |
| NLRmax | 1.20 | 1.06-1.37 | 0.005 | |
| Albuminuria | 0.09 | 0.01-0.62 | 0.015 |
As expected, moderate correlation strength was found between age and diabetes duration in the COVID-19 group (Spearman’s correlation coefficient test r = 0.58, P < 0.001). Given this correlation, only age was included in the multivariable regression model. Similarly, moderate-to-strong correlation strength was found between NLRmax and CRPmax in the COVID-19-positive patients (Spearman’s correlation coefficient test r = 0.59, P < 0.001 for the COVID-negative patients, and r = 0.73, P < 0.001 for the COVID-19-positive patients). Given the significant correlation between CRP and NLRmax, only NLRmax was included in the multivariable regression model.
Given the surprising protective association between moderate-range albuminuria and severe COVID-19 infection, we wanted to explore whether this association is similarly observed in bacterial infections. We hypothesized that this protective association is specific for viral infections such as COVID-19 and not to bacterial infections. For this, similar multivariable logistic regression models were conducted in COVID-19-negative patients without bacterial infections vs patients with bacterial infections to characterize which variables are associated with the latter. Variables associated with the absence vs presence of bacterial infection in the multivariable analysis according to the Models 1-3 are presented (Table 5). In the final model, NLRmax was significantly associated with bacterial infection. The protective effect of albuminuria was not observed with regard to bacterial infection. The apparent protective effect of moderate-range albuminuria in patients with CKD II was specific to COVID-19 infection in this cohort, but may be relevant to other viral infections as well.
| OR | 95%CI | P value | ||
| Model 1 | Sex (female vs male) | 1.88 | 0.70-5.05 | 0.21 |
| Baseline characteristics | Age (yr) | 0.98 | 0.93-1.02 | 0.34 |
| R-square: 2.8% | BMI (kg/m2) | 0.95 | 0.88-1.04 | 0.27 |
| Model 2 | Sex (female vs male) | 7.23 | 1.57-33.23 | 0.011 |
| Baseline characteristics | Age (yr) | 1.00 | 0.92-1.09 | 0.97 |
| R-square: 54.0% | BMI (kg/m2) | 0.94 | 0.84-1.05 | 0.26 |
| HbA1c (%) | 1.19 | 0.88-1.60 | 0.25 | |
| Baseline eGFR (mL/min/1.73 m2 of body surface area) | 1.05 | 1.01-1.08 | 0.013 | |
| NLRmax | 1.36 | 1.21-1.54 | < 0.001 | |
| Model 3 | Sex (female vs male) | 7.26 | 1.58-33.44 | 0.011 |
| Baseline characteristics | BMI (kg/m2) | 0.94 | 0.84-1.05 | 0.26 |
| R-square: 54.0% | Age (yr) | 1.00 | 0.92-1.09 | 0.98 |
| HbA1c (%) | 1.19 | 0.87-1.61 | 0.28 | |
| Baseline eGFR (mL/min/1.73 m2 of body surface area) | 1.05 | 1.01-1.08 | 0.012 | |
| NLRmax | 1.37 | 1.21-1.55 | < 0.001 | |
| Albuminuria | 1.12 | 0.32-3.96 | 0.86 |
Diabetes mellitus has been associated with severe COVID-19 pneumonia[3-5,8]. Hyperglycemia increases SARS-CoV-2 replication in human monocytes, and glycolysis sustains SARS-CoV-2 replication via the production of mitochondrial reactive oxygen species and activation of hypoxia-inducible factor 1α[12]. Further, individuals suffering from diabetes are thought to have chronic low-grade inflammation, which might facilitate the cytokine storm that can lead to clinical deterioration of COVID-19 patients[13]. In addition, patients with diabetes have impaired NK cell activity and altered T cell subpopulations that may increase the susceptibility to severe COVID-19 pneumonia[5,6,9,13,14]. Moreover, other risk factors for severe COVID-19 pneumonia are associated with diabetes, e.g., obesity, CVD, and CKD[5,8,10]. Therefore, in the current study, we focused on patients with type 2 diabetes who were hospitalized at the Galilee Medical Center due to COVID-19 infection or other acute diseases for comparison.
Interestingly, baseline albuminuria was more common in patients without COVID-19 infection (54.9% compared to 30.8%, P = 0.005). The observed low rate of albuminuria in the COVID-19 group led us to explore the associations of several baseline clinical variables, including baseline albuminuria and antiglycemic therapies, as predictors of severe COVID-19 pneumonia. Similarly to previous publications, we identified factors that were significantly associated with increased severity of COVID-19 pneumonia[3-5], including higher BMI (OR 1.24, 95%CI: 1.01-1.53, P = 0.04) and NLRmax (OR 1.2, 95%CI: 1.06-1.37, P = 0.005), the latter reflecting the immune modulation caused by COVID-19 viremia. Surprisingly, the presence of moderate-range albuminuria before hospitalization was associated with reduced risk for severe COVID-19 pneumonia (OR 0.09, 95%CI: 0.01-0.62, P = 0.015).
Several oral antiglycemic therapies may yield protective anti-COVID-19 properties due to their pleotropic effects. Given that DPP-4 is thought to be one of the COVID-19 receptors and DPP-4 inhibitors have anti-inflammatory activity[15], these medications were indeed associated with a better clinical outcome in COVID-19 patients[8,16]. GLP-1 receptor agonists have several immune modulation activities, including inhibition of nuclear factor-κB[5,17]. SGLT2 inhibition decreases the mRNA expression levels of some cytokines and chemokines, such as tumor necrosis factor, interleukin-6, and monocyte chemoattractant protein 1[14]. Metformin treatment reduces the circulating levels of inflammatory biomarkers in diabetics, and was associated with significantly lower in-hospital mortality in a retrospective study of COVID-19 patients[5,18,19]. Despite the above properties, in the current study we did not detect a protective effect of any class of antiglycemic medication. It is possible that the current cohort was underpowered to detect such differences.
The protective association of baseline moderate-range albuminuria with severe COVID-19 pneumonia was unexpected and counterintuitive, given the well-established role of CKD in enhancing severity of the disease. This association was not observed in patients suffering from bacterial infection, suggesting a specific protective effect against COVID-19, as we cannot draw any conclusions about other viral pathogens in the current cohort. To our knowledge, baseline moderate-range albuminuria with eGFR > 60 mL/min has not been described as conferring such an advantage as demonstrated here, and we cannot draw any conclusions about other viral pathogens in the current cohort.
Post-mortem findings in the lungs of people with fatal COVID-19 demonstrated diffuse alveolar damage and inflammatory cell infiltration[20]. The inflammatory response in patients with severe COVID-19 pneumonia is impaired. Specifically, it has been demonstrated that interferon (IFN) type I response is disrupted with low IFNα activity in the blood, indicating high blood viral load and an impaired inflammatory response[21]. Despite IFN's protective role in COVID-19 infection, IFN can lead to proteinuria. The role of activation of type I IFN signaling in mediating proteinuria is well-known. Podocytes respond to toll-like receptor ligand-like polyinosinic:polycytidylic acid [poly (I:C)] that simulates viral infection, by releasing pro-inflammatory cytokines and activation of type I IFN signaling. IFN signaling enhances podocyte B7-1 expression and actin remodeling in vitro and leads to transient proteinuria in vivo. Interestingly, mice treated with a type I IFN receptor-blocking antibody were protected from lipopolysaccharide-induced proteinuria[22]. Therefore, we hypothesize that the presence of moderate-range albuminuria may represent intact type 1 IFN signaling, and in the case of COVID-19 infection, such intact IFN signaling can confer protection from severe COVID-19 pneumonia. We emphasize that moderate-range albuminuria has a protective association in the context of patients with mild CKD (eGFR > 60 mL/min), who constitute the majority of participants in our cohort. Notwithstanding, it was recently demonstrated that patients with eGFR < 60 mL/min or severe albuminuria are at risk for severe COVID-19 infection[23], probably due to advanced CKD contributing to impaired immune function.
A similarly counterintuitive association was recently demonstrated in a cohort of inflammatory bowel disease (IBD) patients. Severe sequelae of COVID-19 were lower in IBD patients compared to matched non-IBD controls, suggesting that baseline immune activity may modulate the progression of COVID-19 pneumonia[24]. In addition, the unexpected finding with regard to moderate-range albuminuria and protection from severe COVID-19 pneumonia might be the result of confounding by as yet unidentified factors or collider bias.
As with all observational studies, our study has limitations. We did not have information regarding other co-morbidities such as liver disease, respiratory disease, alcohol abuse, cognitive impairment, etc., which can potentially serve as confounding factors. Residual confounding might also have resulted from the use of only several measurements to identify baseline characteristics. We did not have data about urine albumin:creatinine during hospitalization; therefore, the contribution of this variable was not included in the final model. In addition, we did not have baseline clinical data for patients who did not survive COVID-19 infection, as described in the methods relating to patient enrollment. Of note, the sample size was small, but was adequate to demonstrate statistical significance of the protective association between moderate-range albuminuria and severe COVID-19 pneumonia.
The presence of moderate-range albuminuria in patients with diabetes suffering from COVID-19 pneumonia may represent an intact IFN type 1 response that may translate to protection against severe disease. Further studies are needed to explore whether this association is also relevant to other viral infections and characterize the pathogenesis relating to immunomodulation, which may indicate a biomarker for patients at reduced risk for COVID-19-related deterioration that may translate to therapeutic interventions.
Several risk factors for severe coronavirus disease 2019 (COVID-19) disease have been described, including: Advanced age, male sex, smoking history, and underlying chronic diseases such as cardiovascular disease, diabetes mellitus, obesity, underweight, and chronic kidney disease (CKD). This case-control study was conducted to identify risk factors for severe COVID-19 pneumonia in patients with type 2 diabetic mellitus hospitalized at the Galilee Medical Center (Nahariya, Israel).
We aimed to characterize differences between hospitalized diabetic patients with vs patients without COVID-19, and parameters associated with COVID-19 severity for prediction.
Similar to previous reports, multivariable logistic regression showed higher body mass index (BMI) and neutrophil:lymphocyte ratio (NLR) were significantly associated with severe COVID-19. Surprisingly, pre-hospitalization albuminuria, mostly moderate-range (albumin:creatinine 30-300 mg/g), was associated with reduced risk for severe COVID-19 pneumonia. The counterintuitive protective association in patients with stage II CKD was not described before. Given the causative association between type I interferon (IFN) signaling and proteinuria, we hypothesize that the presence of moderate-range albuminuria may represent an intact type I IFN signaling, which confers protection from severe COVID-19 pneumonia and its complications.
This case-control study included 209 patients with type 2 diabetic mellitus hospitalized at the Galilee Medical Center (Nahariya, Israel) and recruited between September 2020 and May 2021, 65 patients with COVID-19 infection in dedicated wards and 144 COVID-19-negative patients in internal medicine wards hospitalized due to other reasons. Clinical parameters – including age, type of antiglycemic medications, presence of retinopathy, smoking history, BMI, glycosylated hemoglobin, maximum NLR (NLRmax), C-reactive protein (CRPmax), estimated glomerular filtration rate (eGFR), and albumin (blood and urine) - were compared between the two primary patient groups, and then between COVID-19-negative patients hospitalized due to infectious vs non-infectious disease. Finally, we explored which parameters were associated with severe COVID-19 pneumonia.
COVID-19-negative patients were older and had longer duration of diabetes, lower eGFR, higher albumin, lower CRP, greater smoking history, and more baseline albuminuria at admission. 70% of COVID-19 patients with albuminuria had moderate-range albuminuria. Most of the patients with albuminuria had CKD II. Oral antiglycemic therapies were not significantly different between the two groups. As previously reported, multivariable logistic regression showed higher BMI and higher NLR were significantly associated with severe COVID-19. Surprisingly, pre-hospitalization albuminuria, mostly moderate-range, was associated with reduced risk for severe COVID-19 pneumonia. This protective association was specific to COVID-19 infection and was not observed in bacterial infections.
Moderate-range albuminuria in COVID-19-positive diabetic patients with CKD II is associated with less severe COVID-19. We hypothesize that this counterintuitive association may represent intact IFN signaling that on the one hand can lead to harmful proteinuria via podocyte injury, and on the other hand can serve as a protective cytokine with the potential to mitigate COVID-19 infection and complications. Given the importance of intact type I IFN response in controlling COVID-19, we suggest that moderate-range albuminuria in diabetic patients with mild CKD may serve as a biomarker for intact IFN signaling and therefore is associated with reduced risk for severe COVID-19 pneumonia.
Further studies should explore the potential role of albuminuria in the presence of mild CKD as a biomarker for reduced risk of COVID-19-related deterioration that may translate to therapeutic interventions.
Ossie Sharon contributed to the editing of this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diaconu CT, Romania; Elfaki I, Saudi Arabia S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17649] [Article Influence: 3529.8] [Reference Citation Analysis (0)] |
| 2. | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2083] [Cited by in RCA: 3174] [Article Influence: 793.5] [Reference Citation Analysis (0)] |
| 3. | Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, Sattar N, Young B, Valabhji J. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 612] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 4. | Sandoval M, Nguyen DT, Vahidy FS, Graviss EA. Risk factors for severity of COVID-19 in hospital patients age 18-29 years. PLoS One. 2021;16:e0255544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 591] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 6. | Kim JH, Park K, Lee SB, Kang S, Park JS, Ahn CW, Nam JS. Relationship between natural killer cell activity and glucose control in patients with type 2 diabetes and prediabetes. J Diabetes Investig. 2019;10:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 778] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 8. | Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33:479-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 9. | Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 10. | Han M, Ma K, Wang X, Yan W, Wang H, You J, Wang Q, Chen H, Guo W, Chen T, Ning Q, Luo X. Immunological Characteristics in Type 2 Diabetes Mellitus Among COVID-19 Patients. Front Endocrinol (Lausanne). 2021;12:596518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH, Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2354] [Article Influence: 588.5] [Reference Citation Analysis (0)] |
| 12. | Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo Dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:498-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 13. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6752] [Article Influence: 1350.4] [Reference Citation Analysis (0)] |
| 14. | Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 15. | Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P, Lu J. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. iScience. 2020;23:101400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Rhee SY. Effects of a DPP-4 Inhibitor and RAS Blockade on Clinical Outcomes of Patients with Diabetes and COVID-19 (Diabetes Metab J 2021;45:251-9). Diabetes Metab J. 2021;45:619-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Cameron AR, Morrison VL, Levin D, Mohan M, Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Wong AK, Viollet B, Sakamoto K, Fagerholm SC, Foretz M, Lang CC, Rena G. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119:652-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 499] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 19. | Luo P, Qiu L, Liu Y, Liu XL, Zheng JL, Xue HY, Liu WH, Liu D, Li J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am J Trop Med Hyg. 2020;103:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 20. | Eketunde AO, Mellacheruvu SP, Oreoluwa P. A Review of Postmortem Findings in Patients With COVID-19. Cureus. 2020;12:e9438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2291] [Cited by in RCA: 2171] [Article Influence: 434.2] [Reference Citation Analysis (0)] |
| 22. | Gurkan S, Cabinian A, Lopez V, Bhaumik M, Chang JM, Rabson AB, Mundel P. Inhibition of type I interferon signalling prevents TLR ligand-mediated proteinuria. J Pathol. 2013;231:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA, Hutchinson S, Caparrotta TM, Mellor J, Jeyam A, O'Reilly JE, Wild SH, Hatam S, Höhn A, Colombo M, Robertson C, Lone N, Murray J, Butterly E, Petrie J, Kennon B, McCrimmon R, Lindsay R, Pearson E, Sattar N, McKnight J, Philip S, Collier A, McMenamin J, Smith-Palmer A, Goldberg D, McKeigue PM, Colhoun HM; Public Health Scotland COVID-19 Health Protection Study Group; Scottish Diabetes Research Network Epidemiology Group. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 24. | Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS; Jill Roberts Center Study Group Study Group; Weill Cornell Medicine-Gastrointestinal Study Group. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients With Inflammatory Bowel Disease. Gastroenterology. 2020;159:1541-1544.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |