Published online Dec 15, 2022. doi: 10.4239/wjd.v13.i12.1066
Peer-review started: September 29, 2022
First decision: October 21, 2022
Revised: November 4, 2022
Accepted: November 23, 2022
Article in press: November 23, 2022
Published online: December 15, 2022
Processing time: 77 Days and 9.4 Hours
Chronic wound healing has long been an unmet medical need in the field of wound repair, with diabetes being one of the major etiologies. Diabetic chronic wounds (DCWs), especially diabetic foot ulcers, are one of the most threatening chronic complications of diabetes. Although the treatment strategies, drugs, and dressings for DCWs have made great progress, they remain ineffective in some patients with refractory wounds. Stem cell-based therapies have achieved specific efficacy in various fields, with mesenchymal stem cells (MSCs) being the most widely used. Although MSCs have achieved good feedback in preclinical studies and clinical trials in the treatment of cutaneous wounds or other situations, the potential safety concerns associated with allogeneic/autologous stem cells and unknown long-term health effects need further attention and supervision. Recent studies have reported that stem cells mainly exert their trauma repair effects through paracrine secretion, and exosomes play an important role in intercellular communication as their main bioactive component. MSC-derived exosomes (MSC-Exos) inherit the powerful inflammation and immune modulation, angiogenesis, cell proliferation and migration promotion, oxidative stress alleviation, collagen remodeling imbalances regulation of their parental cells, and can avoid the potential risks of direct stem cell transplantation to a large extent, thus demonstrating promising performance as novel "cell-free" therapies in chronic wounds. This review aimed to elucidate the potential mechanism and update the progress of MSC-Exos in DCW healing, thereby providing new therapeutic directions for DCWs that are difficult to be cured using conventional therapy.
Core Tip: Diabetic chronic wounds (DCWs) are one of the most serious chronic complications of diabetes, and the efficacy of stem cell therapies for refractory chronic wounds has been studied previously. Stem cell-derived exosomes are one of the important active components of stem cell paracrine secretion, which inherit the wound repair capacity of parental cells as parts of novel cell-free therapies in addition to cell-bases ones. Herein we discuss the mechanism and latest progress of mesenchymal stem cell-derived exosomes in promoting DCW healing.
- Citation: Wu J, Chen LH, Sun SY, Li Y, Ran XW. Mesenchymal stem cell-derived exosomes: The dawn of diabetic wound healing. World J Diabetes 2022; 13(12): 1066-1095
- URL: https://www.wjgnet.com/1948-9358/full/v13/i12/1066.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i12.1066
Wound healing after skin tissue injury relies on a dynamic chain of physiological reactions including hemostasis, inflammation, cell proliferation, and tissue remodeling[1]. Any step out of balance, such as excessive inflammation, impaired fibroblast migration and proliferation, abnormal collagen formation and deposition, and hindered re-epithelialization, ultimately leads to delayed wound healing and formation of chronic wounds. Chronic wounds are those that have failed to proceed through an orderly and timely reparative process to produce anatomical and functional integrity of the injured site[2]. They refer to wounds caused by multiple factors that have not healed or have not demonstrated a tendency to heal after a certain period clinically, with a chronic duration ranging from 4 to 12 wk[3,4]. Various pathological states result in chronic wound development, including diabetes, pressure injuries, infections, and arterial/venous insufficiency of which reports are similar in China and developed Western countries[4-6], which have the most complicated pathogenesis and therapeutic strategies being diabetic chronic wounds (DCWs).
Diabetes mellitus (DM) is a metabolic disease characterized by elevated blood glucose levels, of which DCWs are among the most threatening complications. The combination of a high-glucose environment and several biological factors, including ischemia and hypoxia, abnormal inflammatory response, excessive oxidative stress, and peripheral neuropathy, contributes to wound formation[7-9]. Such wounds have problems of protracted healing, long treatment time, difficulties in management, high cost, repeated attacks, and high disability/mortality rates, resulting in heavy physical, psychological and economic burdens[10,11]. The intervention of DCWs cannot be underestimated based on what is mentioned above. Hence, solving persistent inflammation, impaired cell proliferation and migration, decreased angiogenesis, and remodeling of the extracellular matrix (ECM) is important. Innovative wound repair methods, such as local negative pressure, growth factors, and autologous platelet-rich gels, have remarkable effects on healing DCWs[12-15]. However, more specific treatment options are required for refractory and contraindicated wounds.
With the rapid development of tissue engineering, cell therapies have gradually become widely used in various disciplines. Stem cells can be used in regenerative medicine and play an indispensable role in wound repair[16], of which mesenchymal stem cells (MSCs) are the most commonly used. MSCs have self-renewal abilities and multi-directional differentiation potential, participating in damage repair through intercellular communication and bioactive factor secretion, finally achieving the effect of promoting wound healing[17]. Clinical trials of MSCs for treating various types of cutaneous wounds are currently in full swing, and their efficacy and safety in promoting wound regeneration have been initially demonstrated. As clinical trials continue to progress, further attention and supervision need to be paid to their potential safety issues of proliferative lesion formation, abnormal organ reaction and unknown long-term health effects after transplantation[18-20].
Studies have revealed stem cells promote repair and regeneration mainly through paracrine signaling, whereas exosomes are one of their important paracrine active components[21]. MSC-derived exosomes (MSC-Exos) carry genetic information, functional RNAs, and proteins from parental cells, demonstrating wound healing effects via intercellular communication after these biologically active substances are acquired by recipient cells[22-24]. Thus, MSC-Exos have broad application prospects in diabetic wound repair[25]; however, they have not yet been carried out in clinical practice. The important role of MSC-Exos in all stages of diabetic wound healing and the preclinical application are highlighted in this review, to pave the way for their use as an effective tool in the management of these harmful diabetic complications.
DM is a metabolic disease characterized by elevated blood glucose levels, which poses a serious threat to human health. The continuous progression of hyperglycemic toxicity without effective control will affect macrovascular, microvascular, and peripheral nerves throughout the body and involve various organs such as the brain, eyes, heart, kidney, and skin, resulting in various diabetic chronic complications[26]. DCWs are one of the most common and threatening chronic complications, often accompanied by infection or deep-tissue destruction[27]. Protracted wounds are the most common cause of non-traumatic amputations. Diabetic foot ulcers (DFUs) are characterized by wounds on the feet, which are the most typical, and patients with DFUs have a 2.5 times higher risk of 5-year mortality than those with none[28]. The overall mortality of DFUs within 5 years is nearly 50%[29], and approximately 20% of moderate-to-severe DFUs will lead to amputation; the 5-year mortality rate after amputation exceeds 70%[30].
Impaired wound healing processes caused by hyperglycemia-induced disturbances in wound-linked cellular behaviors contribute to diabetic wound healing difficulties[7, 31]. Hyperglycemia, oxidative stress, and insulin resistance affect the function of vascular smooth muscle cells, endothelial cells, and platelets, which in turn may lead to abnormal coagulation processes and affect platelets of triggering for subsequent inflammatory and proliferative phases[32]. The hyperglycemic microenvironment can lead to dysfunction of immune and inflammatory cells and dysregulation of inflammatory factors. Perpetuated inflammatory states induced by increased mast cell degranulation[33], excessive extracellular traps produced by neutrophils[34], dysregulated and persistent M1 (pro-inflammatory) macrophage polarization[35], pro-inflammatory factors (IL-1β, TNF-α, and IL-6) overexpression, and anti-inflammatory factors (IL-10 and TGF-β) deficiency finally hinder wound healing[7]. The proliferative phase of diabetic wound healing is characterized by disturbed physiological functions of keratinocytes[36], fibroblasts[37], and endothelial cells[38], then the impaired re-epithelialization, granulation tissue formation, matrix deposition, and angiogenesis affect wound healing. Various factors also affect the function and activity of these cells during this phase, including decreased chemokines with pro-angiogenesis produced by macrophages, hemoglobin glycation, vascular stenosis, increased oxygen consumption affecting oxygen-dependent cellular behaviors, and impaired nerve fiber regeneration[7,31,39,40]. Remodeling of the ECM spans the entire injury response, and fibroblasts are the major cell type responsible for this phase[31]. Sequential changes in the ECM require a balance between collagen degradation and synthesis, achieved through temporal regulation of the dynamic changes in the ratio of matrix metalloproteinases (MMPs) to tissue inhibitors of metalloproteinases (TIMPs)[41,42]. Such changes in DCWs are unbalanced and lead to difficult wound healing and excessive scarring[41,43]. However, no clear demarcation exists between the various stages of wound healing, and functionally impaired cells can interact, eventually leading to poor diabetic wound healing, progressing to local infection, gangrene, and even amputation. Therefore, the most important aspect of effectively treating DCWs is to identify an appropriate approach that can comprehensively improve abnormalities in all phases of wound healing.
Traditional strategies for DCWs management include glycemic control, conventional dressings (e.g., hydrocolloids, alginates, and silver ions, etc.), thorough debridement (e.g., surgical, mechanical, ultrasonic waterjet, collagenase, and maggot, etc.), wound off-loading, autologous skin and skin substitute grafting, infection control, and revascularization, etc. These strategies are used to create the wound bed microenvironment suitable for repair through moisture balance maintenance, necrotic or inactivated tissues removal, systemic and local infections control, and local blood flow improvement[13,44-46]. Negative pressure wound therapy can also be used to achieve its role in improving wound exudate drainage, enhancing local perfusion, removing bacterial products, promoting granulation tissue growth, and facilitating wound healing[47]. However, these conventional treatments are often ineffective in many patients because of impaired cell function around the wound sites caused by underlying microenvironmental alterations[48]. Several innovative wound adjuvant therapies, including exogenous supplementation of growth factors[49], platelet-rich plasma[50], autologous platelet-rich gels[15,51], and hyperbaric oxygen therapy[52] have been developed to promote the activity and function of damaged cells and offer the possibility of treating unselected refractory wounds. However, an updated systematic review has revealed that some measures had positive effects on accelerating wound healing, while others had limited impacts on diabetic ulcer healing[53]. However, the overall efficacy of various treatment modalities for DCWs remains unsatisfactory, and effective therapeutic strategies need to be continued.
Stem cells have the potential for self-renewal and multidirectional differentiation with great research and application value in life sciences, clinical trials and disease research. Stem cell-based therapies are now approved by several countries, and have been widely used in various disciplines. MSCs are currently the main experimental cell sources and have shown their excellent therapeutic potential and value in clinical trials in the field of regenerative medicine[16,54].
MSCs provide assistance in all phases of wound healing by exerting their functions of regulating skin homeostasis and wound healing through migration into the skin damage site and interaction with skin cells and can influence the function of these cells by paracrine secretion of bioactive factors and differentiation into them[55,56]. As MSCs have exhibited wound healing in many preclinical studies as powerful tools for regulating inflammation, promoting cell proliferation and migration, angiogenesis, and collagen synthesis[57-60], the application of MSCs for DCWs contributes to progress toward clinical trials. Twenty-five clinical trials of MSCs for diabetic ulcers have been conducted or are recruiting subjects, which are recorded in the ClinicalTrials.gov database (clinicaltrials.gov).
Previous clinical studies have demonstrated that MSC transplantation in patients with DFUs is safe and feasible with the properties of improving microcirculation, wound healing, ulcer recurrence, and amputation[61-63]. However, stem cell therapies are still in their early clinical stage, further attention and supervision are required of declined performance during production and application as cellular senescence and loss of multipotency during ex vivo expansion and from variable donors[64,65], decreased survival rate caused by advanced glycosylation end products[66], potential safety issues as proliferative lesion formation and abnormal organ reaction[20], and unknown long-term health effects after transplantation. Basic and clinical researches related to allogeneic/autologous stem cells are subject to the International Society for Stem Cell Research Guidelines for Clinical Translation of Stem Cells and national ethical guidelines and related guidelines/regulations[20,67].
MSCs exert their repair and regenerative effects mainly through paracrine signaling, and exosomes are one of the important active components[21] that provide a more stable entity that minimizes the potential safety concerns for cell transplantation. MSC-Exos play an important role in intercellular communication by carrying various important functional substances of parental cells, being used of promoting wound healing[68,69]. Compared to direct cell transplantation, MSC-Exos avoid the immune rejection because of low immunogenicity; allow to cross various biological barriers and avoid the risk of embolism from intravenous injection based on their smaller sizes[70]; the dose and fraction can be adjusted artificially and genetic modifications are easier and safer[71]; avoid the problem of malignant transformation; and allow to repair diabetic complications through multiple actions[72]. They can also be used as ideal carriers for carrying and delivering therapeutic drugs, genes, enzymes, or RNAs[73], and their efficiency and targeted transport capacity can be tuned through pretreatment or engineering transformation[74], demonstrating their promising applications in the field of repair and regeneration.
The concept of “exosomes” was first proposed in 1981 by Trams et al[75], using to collectively refer to extracellular vesicles (EVs) that originated from the exudation of various cell line cultures. The currently defined exosomes were first discovered in sheep reticulocytes and considered cellular waste[76-78]. Of note, “EVs” is the preferred term by the International Society for Extracellular Vesicles (ISEV) to describe all nanoparticles with lipid bilayer structures released by cells[79].
Exosomes, the biological nanoscale spherical lipid bilayer vesicles[80], can be secreted by almost all cell types and are widely present in cell culture supernatants and many body fluids[81]. Their diameters range from 10 to 200 nm. In addition to exosomes, EVs also include microvesicles that are also called ectosomes with a diameter of 100-1000 nm, and apoptotic bodies larger than 1000 nm according to different sizes and biogenesis[82,83]. The types and functions of the bioactive substances carried by exosomes differ according to their cellular origins and adjacent cellular components[84]. The major substances include genetic information, RNA species (mRNA, tRNA, rRNA, miRNA, lncRNA, circRNA, etc.), proteins, lipids, cytokines, and growth factors[85,86]. Exosomal proteins include intrinsic components involved in exosome biogenesis, such as fusion-related proteins (GTPases, annexins, flotillin, and Rab proteins), heat shock proteins (HSP70 and HSP90), tetraspanins (CD63, CD81, CD82, and CD9), ESCRT complex, and specific functional proteins originating from parental cells[87]. Apart from serving as a medium for cellular communication, some proteins are also involved in the membrane composition and biosynthesis as identified biomarker proteins and can provide stability and permeability in concert with phospholipid bilayers.
Exosomes originate from endosomes during generation, circulation, degradation, and liberation[88]. Extracellular substances fuse with early sorting endosomes through plasma membrane invagination and endocytosis, and begin to accumulate bioactive substances. Eventually, they mature into late sorting endosomes, which invaginate to form intraluminal vesicles that can then generate multivesicular bodies (MVBs)[68,88]. MVBs can be absorbed by lysosomes comprising a degradative pathway, or they can undergo a specific exocytotic process whereby they fuse with the plasma membrane to release exosomes into the extracellular space[89]. After release, they act as mediators of intercellular and intra-organ communication to transfer the contained bioactive substances to recipient cells through direct fusion, endocytosis, and receptor-ligand binding to affect their functions[90,91], participating in the body's physiological and pathological state adjustment[92].
The extraction of exosomes is primarily based on their physicochemical properties. This process is difficult because of the heterogeneity of exosomes derived from different cell origins, the possible existence of subpopulations of exosomes with different functions and phenotypes even when extracted from a single cell line, and multiple EV subtypes with similar biophysical properties[93]. Therefore, different isolation methods should be targeted for different purposes[87]. Differential ultracentrifugation is the most widely used separation technique and is also known as the gold standard for isolation, while the main principle is to harvest the desired components based on size and density differences[94]. Polymer precipitation uses polyethylene glycol to harvest exosomes under centrifugal conditions by reducing their solubility[95]. Size-exclusion chromatography[96] and ultrafiltration[97] are both based on size differences between exosomes and other components, although they may adulterate other particles of similar size. Immunoaffinity capture is based on the specific binding of antibodies and ligands to isolate exosomes from a heterogeneous mixture[98]. Current isolation and purification techniques have varying effects and many problems such as low purity and recovery, structural damage, and time and cost consumption, making achieving efficient enrichment difficult, which has become a bottleneck of the translational applications of exosomes[87]. Hence, continuously exploring new isolation and purification techniques or combining multiple techniques is necessary to improve the isolation efficiency and thus obtain ideal exosomes.
Exosomes are mainly characterized by external characteristics (morphology and size detection) and the identification of surface markers[87]. As mentioned above, some protein components of exosomes serve as surface protein markers for identification. The ISEV has proposed the need to identify two types of proteins as follows: one is the biomarker proteins shared by exosomes to determine whether the extracted components are exosomes, and the other is cell-type-specific exosomal proteins that need to be identified to determine cellular origin[79]. Therefore, exosomes can be characterized by detecting their morphology using transmission electron microscopy, their size and concentration by dynamic light scattering, and nanoparticle tracking analysis technology, and their marker proteins by western blot, enzyme-linked immunoassay, and flow cytometry[87].
Stem cells have self-renewal abilities and multi-directional differentiation potential, while MSCs are one of the most frequently used and promising adult stem cells that can be derived from most adult tissues such as the bone marrow, adipose tissue, and umbilical cord[99,100]. Bone marrow-derived MSC-Exos (BMSC-Exos) are biologically stable, have low immunogenicity, and exhibit good proliferation and viability after transplantation. They are most commonly used in clinical trials and can play a prominent role in various disorders, especially bone-related diseases[101]. Umbilical cord-derived MSC-Exos (UCMSC-Exos) can be isolated non-invasively, with low immunogenicity and strong self-renewal and proliferation ability, although it has limitations in maintaining bioactive and clinical therapeutic transport[102]. Adipose-derived MSC-Exos (AMSC-Exos) have relatively abundant sources that can be easily obtained by painless minimally invasive surgery; they are also pluripotent, plastic, easy to store, and stable in blood or body fluids[103]. Exosomes of different origins share most of their bioactive factors and are generally similar in their biological functions; however, their specific biological properties depend on the molecules that are specifically expressed[104].
MSC-Exos are involved in intercellular communication through the transfer of proteins, RNA, DNA, and bioactive lipids that can be delivered to target cells to regulate their activities and functions[68]. They are generally involved in the regulation of cell survival and differentiation, the immune system, and inflammation modulation, and are also capable of promoting angiogenesis and tissue remodeling[73]. Considering these multiple biological functions, several studies have also reported that the MSC-Exos play a therapeutic role in autoimmune diseases[105], ischemic injuries[106], and metabolic diseases[107], and are also related to dynamically modulating tumor biological functions[108], promoting repair and regeneration of damaged osteochondral, neural, and tendon tissues, and facilitating wound healing[109-112]. Current studies also discovered that they can improve COVID-19-related cytokine storms and the deterioration of lung function due to severe pneumonia[113].
MSC-Exos play an important role in each phase of wound healing[81]. They can regulate diverse cell types related to wound repair by enhancing or suppressing certain bioactivities, achieving hemostasis, inflammatory regulation, cell migration to the wound site, cell proliferation, and differentiation to form granulation tissue, angiogenesis, and ECM reorganization[69]. They can also be expected to be therapeutic agents for different types of diabetes by alleviating autoimmune damages[114], attenuating insulin resistance, and improving β-cell exhaustion[115]. Additionally, they can be used to prevent and treat DM-related complications. Based on these potentials, MSC-Exos may be of considerable importance in DCW treatment.
Tissue factor (TF) is an initiator of coagulation activation and was identified in the plasma membrane of exosomes[116]. TF can transfer to the platelets and initiate the extrinsic coagulation cascade, leading to the conversion of prothrombin to thrombin and fibrin clot formation[117]. Induced coagulation and stimulated thrombogenicity were observed using EVs carrying TF from the pericardial blood of patients who received cardiac surgery[116]. Rat BMSC-Exos were applied to the bleeding site in the hemorrhage liver model, which exhibited an inhibited amount of bleeding and shortened bleeding time, demonstrating their excellent hemostatic properties. However, no studies related to exosomes' promotion of coagulation in cutaneous wound healing have been conducted. Further studies are needed to demonstrate the potential role of exosomes in the hemostasis phase of wound healing.
Excessive inflammation is a major cause of persistent diabetic wounds. Abnormal macrophage polarization and cytokine overexpression lead to an uncontrolled and persistent inflammatory state and can cause secondary tissue damage[7]. MSCs-Exos can inhibit the differentiation, activation, and proliferation of T cells as well as reduce IFN-γ release[118]. They can reduce the concentration of the inflammatory cytokines, TNF-α, iNOS, IL-1β, and IL-6[119] and upregulate the expression of the anti-inflammatory cytokine IL-10[120,121]. MSCs-Exos can also induce M2 polarization of macrophages to promote wound healing by delivering exosome-derived miR-223 to target regulating the expression of pknox1 protein[122].
Such abilities can also be observed in diabetic wounds. Topical application of native AMSC-Exos to diabetic mice dorsal full-thickness skin wounds also downregulated inflammatory cytokines (IL-6, TNF-α, CD14, CD19, and CD68) expression and promoted wound healing[123]. Similar alleviated inflammatory effects achieved by regulating inflammatory factors could also be observed in the combination of intraperitoneal Nrf2 pharmaceutical activator and BMSC-Exos subcutaneous injection, demonstrating decreased inflammatory cytokines TNF-α and IL-1β and increased anti-inflammatory cytokines IL-4 and IL-10[124]. Intradermal injection of MSC-Exos derived from human menstrual blood could induce macrophage polarization from the M1 to M2 phenotype, while this capacity is better than that of menstrual blood-derived MSCs[125]. Significantly lower M1 polarized macrophages and higher M2 polarized macrophages were also observed in the diabetic mouse air pouch model and diabetic rat full-thickness skin wound model using BMSC-Exos, while melatonin-stimulated BMSC-Exos (MT-Exos) had stronger effects[121]. Immunomodulatory capacity was enhanced after preconditioning. Moreover, MT-Exos could improve wound healing by activating the PTEN/PI3K/AKT signaling pathway to promote macrophage M2 polarization, angiogenesis, and collagen synthesis; promote the resolution of persistent inflammation; and drive the transition from inflammation to proliferation[121]. HUCMSC-Exos pretreated with lipopolysaccharides have better regulatory properties for macrophage polarization and resolution of chronic inflammation by transferring miR-let7b, while the TLR4/NF-κB/STAT3/AKT pathway is important in regulating this mechanism to promote wound healing[126]. The use of engineered TNF-α/hypoxia-pretreated HUVMSC-Exos in infected DCWs also decreased proinflammatory cytokines (TNF-α, IL-1β, and IL-6), induced M2 macrophage polarization, reduced bacterial burden, and bacterial colonization at the wound sites. Reduced levels of oxidative biomarkers and increased levels of antioxidant mediators also demonstrated the ability of oxidative stress suppression[127]. The combination of BMSC-Exos and carboxyethyl chitosan-dialdehyde carboxymethyl cellulose hydrogel revealed skewed macrophage functional polarity from M1 toward an anti-inflammatory M2 phenotype, as well as enhanced antibacterial effects by significantly inhibiting bacterial growth[128].
Fibroblasts, keratinocytes, and endothelial cells participate in the proliferative phase. Unlike the dual regulatory effects on the tumor, MSC-Exos directly affect the proliferative phase of wound healing by stimulating the proliferation and differentiation of these cells, as well as promoting angiogenesis at injury sites[104]. Enhanced migratory and proliferative capacity and inhibited apoptosis of keratinocytes by activating the AKT/HIF-1α and Wnt/β-catenin pathways were observed with AMSC-Exos[129,130]. BMSC-Exos demonstrated the ability to promote fibroblast proliferation, migration, and secretion of growth factors and can induce tube formation in human umbilical vein cells (HUVECs)[131]. AMSC-Exos induced angiogenesis in both in vivo and in vitro experiments, and the promotion of angiogenesis in endothelial cells was achieved by transferring miR-125a to inhibit DLL4 expression, accompanied by the downregulation of pro-angiogenic genes (Ang1 and Flk1), and upregulation of anti-angiogenic genes (Vash1 and TSP1)[132]. In addition to its pro-proliferative ability in vitro, the pro-healing effect of MSC-Exos has also been observed in acute non-diabetic wounds. MSC-Exos from human umbilical cord Wharton’s jelly could regulate HaCaT cell function by suppressing AIF nucleus translocation and PARP-1 hyperactivation, thus attenuating full-thickness skin wounds by enhancing re-epithelialization and angiogenesis[133]. Fetal dermal-derived MSC-Exos accelerated wound closure in a mouse full-thickness skin wound model by activating the Notch signaling pathway to promote the motility and secretory capacity of fibroblasts[134].
Similarly, exosomes from MSCs improve proliferation and angiogenesis in diabetic wounds. AMSC-Exos accelerated cutaneous wound healing in diabetic mice with full-thickness skin wounds model by enhancing cell proliferation, inhibiting apoptosis, and promoting angiogenesis. They also repaired skin barrier functions, and produced large amounts, regular arrangement, and dense distribution of new collagen[123]. Shabbir et al[131] have also reported that these cells significantly increased their proliferation when treated with MSC-derived exosomes. Enhanced angiogenesis and fibroblasts proliferation, migration, and differentiation abilities were observed in diabetic wounds treated with human decidua derived MSC-Exos, as well as an improved fibroblast senescent state, reduced scar width, and larger and better-organized collagen deposition[135].
Various methods have been used to modify MSC-Exos to enhance fibroblast proliferation and angiogenesis. Co-culture of lncRNA H19-transfected BMSC-Exos with fibroblasts extracted from foot tissue of patients with DFUs revealed that overexpressed exosomes regulated the PTEN-mediated PI3K/AKT signaling pathway by competitively binding miR-152-3p to enhance proliferation and migration of fibroblasts and inhibit apoptosis and inflammation[136]. Injecting such exosomes into the peri-wound tissue of diabetic mice revealed the same changes in expression and accelerated wound healing[136]. Atorvastatin-pretreated BMSC-Exos promoted proliferation, migration of HUVECs, and vascular endothelial growth factor (VEGF) expression and accelerated wound healing in diabetic full-thickness skin injury rat models[137]. Pioglitazone-pretreated BMSC-Exos-treated full-thickness wounds in diabetic rats achieved faster-wound closure, with more adequate re-epithelialization and extensive collagen deposition, significantly enhanced wound perfusion, and had significantly upregulated levels of VEGF and CD31[138]. Subcutaneous injection of mmu_circ_0000250-modified AMSC-Exos via miR-128-3p/SIRT1-mediated autophagy promoted wound healing in diabetic mice, and increased capillary and granulation tissue production was detected owing to promoted proliferation and migration and reduced apoptosis of endothelial cells[139].
Biological scaffolds can improve the survival of exosomes in the inflammatory environment of diabetic wounds and maintain their sustained release. UCMSC-Exos combined with the Pluronic F127 hydrogel revealed promoted chronic wound healing in diabetic mice. The elevated number of blood vessels and microvascular density, enhanced regeneration of granulation tissue, and cell proliferation were also observed, with the significant formation of new hair follicles in the center of the wounds, sufficient subepidermal collagen deposition, and orderly arrangement of collagen fibers[140]. Similar changes were observed in the wounds of diabetic mice using engineered bioactive self-healing antimicrobial exosome hydrogels (FHE@exo), and the elevated number of dermal appendages and differentiation and re-epithelialization of the epidermis were also observed[141]. The combination of human gingival tissue-derived MSC-Exos (GMSC-Exos) and a chitosan/silk hydrogel sponge promoted re-epithelialization, angiogenesis, and collagen deposition, while the increased nerve fiber density also reflected enhanced neuronal ingrowth in the proliferative stage[142].
In the final stage of wound healing, the production and remodeling of the ECM are key factors in determining the time of wound healing and degree of scarring. Recently, some studies have reported on the effects of exosomes on matrix remodeling. BMSC-Exos have been demonstrated to restore normal skin morphology in rats with full-thickness skin injury[143], while these capacities relied on the downregulation of TGF-β1 and upregulation of TGF-β3 by inhibiting the TGF-β/Smad signaling pathway. UCMSC-Exos had large amounts of miR-21, miR-23a, miR-125b, and miR-145, while it inhibited the differentiation and excessive aggregation of myofibroblasts and exerted an anti-scarring effect via the TGF-β2/Smad2 pathway in vivo[144]. UCMSC-Exos can also promote the phosphorylation of YAP, a key site of the Hippo pathway, to negatively regulate the Wnt4/β-catenin pathway to balance tissue regeneration and repair, with excessive cell proliferation and collagen deposition in the remodeling stage[145]. It was noted that intravenous injection of ADSC-Exos could increase the ratio of type III collagen to type I and TGF-β3 to TGF-β1, prevent fibroblast-to-myofibroblast differentiation, and reduce scarring at incisions in the full-thickness skin injury models[146]. They could also induce the ERK/MAPK pathway in fibroblasts to increase the expression of MMP3, thereby increasing MMP3/TIMP1 to regulate ECM remodeling[146].
In contrast to the promoted cell proliferation and abundant granulation tissue in the early stage of healing, proliferative activities were reduced during the late repair stage to prohibit tissue hyperplasia when using FHE@exo, suggesting entry into the remodeling phase that prevents excessive tissue proliferation to promote wound healing[141]. The application of GMSC-Exos with chitosan/silk hydrogel sponge on the wounds of diabetic rats revealed more collagen deposition and thick wavy collagen fibers that were arranged in an orderly fashion, which is similar to that in normal skin, implying enhanced ECM remodeling[142]. These were also observed in the local transplantation of HUCMSC-Exos with polyvinyl alcohol/alginate nano hydrogel and of miR-126-3p overexpressed synovial-derived MSC-Exos with hydroxyapatite/chitosan composite hydrogel[147,148]. Altogether, these studies indicate that MSC-Exos play a pivotal role in the ECM remodeling phase of wound healing.
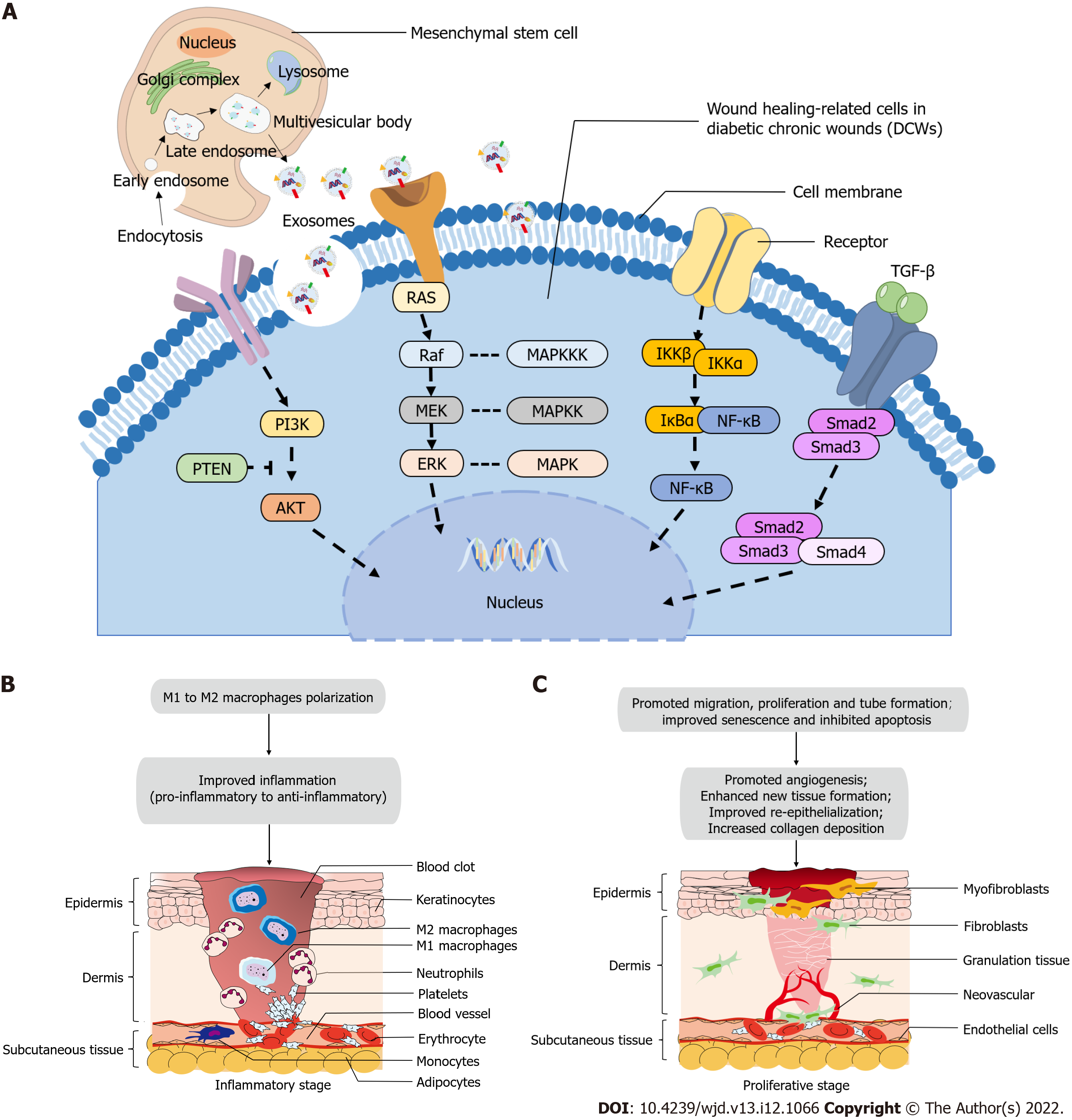
The various stages of wound healing are closely interwoven. MSC-Exos inherit the genetic information of their parental cells and can transfer the therapeutic bioactive substances to target cells to participate in intercellular communication, resulting in the regulation of target cell function and promotion of wound healing[81,149]. We analyzed the current preclinical application of MSC-Exos in diabetic wound models, and the cell source, administration method, dose, frequency, animal type, wound diameter, efficacy, and possible molecular mechanisms are summarized in Table 1[104,121,123-128,147,148,135-142,150-158]. Additionally, MSC-Exos were not only responsible for a specific stage but also promote microenvironment changes in the wounds at each stage to exert a pro-healing effect. Although the biological functions of promoting diabetic wound healing are generally similar, certain differences exist in the regulated signaling pathways of different cell-derived exosomes or receiving different preconditioning, according to previous studies. The regulatory mechanisms most frequently studied in diabetic wound models and may potentially confirmed in DCWs, as well as the microenvironmental changes in inflammatory and proliferative stages of wound healing after using MSC-Exos, are depicted in Figure 1.
| No. | Ref. | Institution(Nation) | Exosomes source | Intervention, administration, dose and time | Control | Model species | Wound diameter | Therapeutic effect | Molecular mechanism | |
| 1 | Yang et al[140], 2020 | The Third Affiliated Hospital of Southern Medical University(China) | Human umbilical cord | 1 HUCMSC-Exos + PF-127 hydrogel; injected topically; 100 µg in 100 µL PF-127 (24%); at Day 0 | PBS (100 µL) | Rats (Sprague-Dawley) | 10 mm × 2 (1.5 cm apart) | 1 Accelerated wound closure rate | — | |
| 2 New hair follicle formation, fibroblasts proliferation, sufficient and order collagen deposition | ||||||||||
| 2 HUCMSC-Exos + PF-127 hydrogel; injected topically; 100 µg in 100 µL PBS; at Day 0 | ||||||||||
| 3 Reduced inflammatory cell infiltration | ||||||||||
| 4 Higher microvessel densities and higher number of blood vessels (CD31, MVD) | ||||||||||
| 3 PF-127 hydrogel; injected topically; 100 µL PF-127 (24%); at Day 0 | ||||||||||
| 5 Promoted cell proliferation (Ki67) and enhanced regeneration of granulation tissue | ||||||||||
| 6 Upregulated expression of VEGF and TGF-β | ||||||||||
| 7 Hydrogel supported exosome survival and biological activity | ||||||||||
| 2 | Wang et al[141], 2019 | The Affiliated Hospital of Wenzhou Medical University; Xi'an Jiaotong University(China) | Mouse adipose tissue | 1 AMSC-Exos + F127/OHA-EPL hydrogel; covered the wound; 10 μg; at Day 0 | Saline | Mice (ICR) | 8 mm × 2 mm | 1 Accelerated wound closure rates | — | |
| 2 Promoted cell proliferation and abundant granulation tissue in early stage of healing; reduced proliferative activities during the late repair stage to prohibit tissue hyperplasia | ||||||||||
| 2 AMSC-Exos; covered the wound; 10 μg; at Day 0 | ||||||||||
| 3 Abundant and well-organized collagen fibers, more collagen deposition (Col I, Col III) | ||||||||||
| 3 F127/OHA-EPL hydrogel; covered the wound; 10 μg; at Day 0 | ||||||||||
| 4 Faster re-epithelization (cytokeratin) and epithelial cell differentiation | ||||||||||
| 5 Promoted angiogenesis (α-SMA) and blood vessels formation | ||||||||||
| 6 Complete skin regeneration: skin appendages and less scar tissue appeared | ||||||||||
| 3 | Liu et al[121], 2020 | Second Military Medical University; Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University(China) | Human bone marrow | 1 Melatonin-pretreated BMSC-Exos (MT-Exo); injected subcutaneously at least six sites per wound; dose not mentioned; at Day 0 | PBS | Rats (Sprague-Dawley) | 20 mm | 1 Accelerated diabetic wound healing | PTEN/AKT signaling pathway | |
| 2 Anti-inflammatory effect on macrophages by promoting M2 and inhibiting M1 polarization | ||||||||||
| 3 Enhanced re-epithelialization (increased neoepithelium length) | ||||||||||
| 4 Improved angiogenesis (α-SMA, CD31, Microfli perfusion) and collagen synthesis (Col I and III) | ||||||||||
| 5 Activated the PTEN/AKT signaling pathway | ||||||||||
| 2 BMSC-Exos; injected subcutaneously at least six sites per wound; dose not mentioned; at Day 0 | ||||||||||
| 4 | Pomatto et al[104], 2021 | University of Turin(Italy) | Human bone marrow | BMSC-EVs + carboxymethylcellulose; applied on the wound; 1 × 109 in 25 µL of vehicle; at Day 0, 3, 7 and 10 | carboxymethylcellulose high viscosity 10 mg/mL (25 µL) | Mice (NSG) | 6 mm × 8 mm | Not effective and did not reduce the wound closure rate | — | |
| Human adipose tissue | AMSC-EVs + carboxymethylcellulose; applied on the wound; 1 × 109 in 25 µL of vehicle; at Day 0, 3, 7, 10 and 14 | 1 Accelerated cutaneous wound healing | ||||||||
| 2 Reduced size of the scar | ||||||||||
| 3 Increased epithelial thickness and re-epithelization | ||||||||||
| 4 Promoted angiogenesis (the number of vessels) | ||||||||||
| 5 | Shi et al[139], 2020 | Affiliated Hospital of Nantong university(China) | Human adipose tissue | 1 mmu_circ_0000250-modified AMSC-Exos;injected subcutaneously at four sites around the wound;200 μg in 100 μL PBS;at Day 0 | PBS (100 μL) | Mice (C57BL) | 4 mm | 1 Accelerated cutaneous wound healing | mmu_circ_0000250/miR-128-3p/SIRT1-mediated autophagy | |
| 2 Reduced scar areas | ||||||||||
| 3 Enhanced angiogenesis (CD31, vessel density) | ||||||||||
| 4 Suppressed apoptosis of skin tissue | ||||||||||
| 5 Suppressed expression of miR-128-3p but promoted SIRT1 expression | ||||||||||
| 2 AMSC-Exos; injected subcutaneously at four sites around the wound; 200 μg in 100 μL PBS; at Day 0 | ||||||||||
| 6 Increased expression of autophagy-related gene (LC3) | ||||||||||
| 6 | Hu et al[138], 2021 | Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology(China) | Rat bone marrow | 1 Pioglitazone-treated BMSC-Exos (PGZ-Exos); injected subcutaneously(at least six sites per wound); 100 μg in 100 μL PBS; at Day 0 | PBS (100 μL) | Rats (Sprague-Dawley) | 15 mm | 1 Accelerated cutaneous wound healing | PTEN/PI3K/AKT/eNOS pathway | |
| 2 Enhanced re-epithelization | ||||||||||
| 3 Promoted collagen synthesis (Col I, Col III) and collagen deposition, indicating more superior ECM remodeling ability | ||||||||||
| 4 Enhanced angiogenesis (VEGF, CD31) and blood flow of the wound | ||||||||||
| 2 BMSC-Exos; injected subcutaneously (at least six sites per wound); 100 μg in 100 μL PBS; at Day 0 | ||||||||||
| 7 | Yu et al[137], 2020 | Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University; Second Military Medical University(China) | Human bone marrow | 1 Atorvastatin-pretreated BMSC-Exos (ATV-Exos); injected subcutaneously (six points); dose not mentioned; at Day 0 | PBS | Rats (Sprague-Dawley) | 20 mm | 1 Accelerated cutaneous wound healing | miR-221-3p /PTEN/AKT/eNOS pathway | |
| 2 Increased re-epithelization (more epithelial structures and longer neuroepithelium) | ||||||||||
| 2 BMSC-Exos; injected subcutaneously (six points); dose not mentioned; at Day 0 | ||||||||||
| 3 Promoted collagen synthesis and deposition, indicating more superior ECM remodeling ability (thicker wavy collagen fibers and more extensive collagen deposition arranged neatly) | ||||||||||
| 4 Superior biosafety of the therapy of exosomes | ||||||||||
| 5 Enhanced angiogenesis (CD31, α-SMA and Microfil perfusion) | ||||||||||
| 8 | Zhao et al[123], 2021 | Tongji University(China) | Human adipose tissue | 1. AMSC-Exos; smeared at the wound; 200 μg in 200 μL PBS; 3 times/day, 2 wk | PBS;Untreated | Mice (db/db) | 15 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Exosomes entered the dermis of wounds after smearing | ||||||||||
| 2 Recombinant human epidermal growth factor (rhEGF); smeared at the wound;3 times/day, 2 wk | ||||||||||
| 3 Mild hyperkeratosis and typical fibrous structures with new glands and hair follicles, implying enhanced tissue remodeling | ||||||||||
| 3 AMSC-CM; smeared at the wound; 3 times/day, 2 wk | ||||||||||
| 4 Enhanced collagen synthesis (Col I, Col III), deposition and remodeling (large amounts, large area, regular arrangement and dense distribution of new collagen) | ||||||||||
| 5 Enhanced cell proliferation and inhibited apoptosis | ||||||||||
| 6 Increased blood vessel intensity and promoted angiogenesis (CD31, VEGF) | ||||||||||
| 7 Repaired skin barrier functions (elevated expression levels Filaggrin, Loricrin, and AQP3) | ||||||||||
| 8 Suppressed expression of inflammatory cytokines (IL-6, TNF-α, CD14, CD19 and CD68) | ||||||||||
| 9 Negatively regulated MMP1 and MMP3 expression in promoting collagen synthesis | ||||||||||
| 9 | Tao et al[150], 2017 | Shanghai Jiao Tong University Affiliated Sixth People’s Hospital(China) | Human synovial membrane | 1 miR-126-3p overexpressed SMSC-Exos + chitosan wound dressings; placed on the wound bed with pressure dressing; at Day 0 | Untreated | Rats (Sprague-Dawley) | 18 mm | 1 Accelerated cutaneous wound healing | PI3K/AKT and MAPK/ERK signaling pathways | |
| 2 Enhanced angiogenesis (microcomputed tomography, CD31, α-SMA) | ||||||||||
| 3 Promoted re-epithelialization, granulation tissue formation, collagen alignment and deposition, implying enhanced ECM remodeling | ||||||||||
| 2 Chitosan wound dressings; placed on the wound bed with pressure dressing; at Day 0 | ||||||||||
| 4 Accelerated development of hair follicles and sebaceous glands | ||||||||||
| 10 | Ti et al[126], 2015 | Chinese PLA General Hospital(China) | Human umbilical cord | 1 LPS-pretreated HUCMSC-Exos; injected dispersively into the wound edge; 60 μg in 0.5 mL PBS; at Day 0 | Untreated | Rats | 10 mm | 1 Accelerated cutaneous wound healing | let-7b/TLR4/NF-κB/STAT3/AKT pathway | |
| 2 Decreased inflammatory cell infiltration | ||||||||||
| 3 Regulate macrophage polarization to M2 macrophages | ||||||||||
| 2 HUCMSC-Exos; injected dispersively into the wound edge; 60 μg in 0.5 mL PBS; at Day 0 | ||||||||||
| 4 Promoted the appearance of new small capillaries | ||||||||||
| 11 | Li et al[136], 2020 | The Fourth Affiliated Hospital of Harbin Medical University(China) | Mouse bone marrow | 1 lncRNA H19 overexpressed BMSC-Exos; injected into the skin around the wound; at Day 0 | Untreated | Mice (C57BL/6) | 10 mm | 1 Accelerated cutaneous wound healing. | lncRNA H19/miR-152-3p/PTEN/ PI3K/AKT signaling pathway | |
| 2 Ameliorated inflammation of the wound (IL-10 ↑, IL-1β↓, TNF-α↓ and fewer inflammatory cells around the wound) | ||||||||||
| 2 BMSC-Exos; injected into the skin around the wound; at Day 0 | ||||||||||
| 3 Promoted granulation tissue formation | ||||||||||
| 4 Enhanced angiogenesis (Increased expression of VEGF, TGF-β1, α-SMA, and Col I) | ||||||||||
| 5 Suppressed cell apoptosis | ||||||||||
| 6 Interacted with miR-152-3p via PTEN-mediated PI3K/AKT signaling pathway (diminished miR-152-3p expression, elevated PTEN expression and decreased expression of PI3K, AKT and p-AKT) | ||||||||||
| 12 | Shi et al.(2017)[142] | Chinese PLA General Hospital(China) | Human gingival tissue | 1 GMSC-Exos+ chitosan/silk hydrogel sponge; covered the wound with restraining bandage; 150 μg in 100 μl PBS; at Day 0, changed every 3 d | 1. PBS (100 μL);2. gauze (13 mm× 13 mm) covered the wound | Rats (Sprague-Dawley) | 10 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Promoted re-epithelialization, deposition and remodeling of ECM (more collagen deposition and thick wavy collagen fibers, the collagen fibers arranged in an orderly fashion similar to that of normal skin) | ||||||||||
| 2 Chitosan/silk hydrogel sponge; covered the wound with restraining bandage; in 100 μL PBS; at Day 0, changed every 3 d | ||||||||||
| 3 Enhanced angiogenesis (CD34, microvessel density) | ||||||||||
| 4 Enhanced neuronal ingrowth (nerve fiber density) | ||||||||||
| 13 | Xiao et al[151], 2021 | Nan Fang Hospital of Southern Medical University(China) | Human adipose tissue | 1 AMSC-Exos + human acellular amniotic membrane (hAAM) scaffold; covered on the wound; 100 μg in 100 μL PBS; at Day 0, every other day, 3 times in total | PBS (100 μL) | Mice (BALB/c) | 10 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Suppressed wound inflammatory responses (fewer inflammatory cells around the wound and higher recruitment of M2 macrophages to the wound sites) | ||||||||||
| 2 AMSC-Exos; covered on the wound;100 μg in 100 μL PBS; at Day 0, every other day, 3 times in total | ||||||||||
| 3 Enhanced angiogenesis (CD31) | ||||||||||
| 4 Enhanced extracellular matrix (ECM) deposition (Col III) | ||||||||||
| 5 Promoted re-epithelialization (completed epithelial and dermal regenerated) | ||||||||||
| 3 hAAM patch; covered on the wound; at Day 0, every other day, 3 times in total | ||||||||||
| 6 Failed regenerated hair follicle and sebaceous glands | ||||||||||
| 14 | Yan et al[152], 2022 | Union Hospital, Tongji Medical College, Huazhong University of Science and Technology(China) | Human umbilical cord | 1 HUCMSC-Exos injected locally to the wound site; 100 μL, 50 μg/ml; at days 0, 3, 5, 7, 9, and 11 | PBS (100 μL) | Mice (C57BL/6J) | 10 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Reduced oxidative stress (ROS) | ||||||||||
| 3 Promoted granulation tissue formation | ||||||||||
| 2 HUCMSC-Exos injected locally to the wound site; 100 μL, 100 μg/mL; at days 0, 3, 5, 7, 9, and 11 | ||||||||||
| 4 Enhanced angiogenesis (CD31, mean perfusion unit ratio) | ||||||||||
| 15 | Geng et al[128], 2022 | Jinzhou Medical University(China) | Rat bone marrow | 1 BMSC-Exos + carboxyethyl chitosan-dialdehyde carboxymethyl cellulose hydrogel; covered the wound; twice a day, two weeks | Untreated | Rats (Sprague-Dawley) | 20 mm | 1 Accelerated cutaneous wound healing | VEGF-mediated PI3K/AKT signaling pathways | |
| 2 Promoted collagen deposition and remodeling, and fibrin regeneration | ||||||||||
| 2 Carboxyethyl chitosan-dialdehyde carboxymethyl cellulose hydrogel; covered the wound; twice a day, two weeks | 3 Enhanced antibacterial effects by significantly inhibiting bacterial growth | |||||||||
| 4 Skew macrophage functional polarity from M1 (iNOS) towards an anti-inflammatory M2 phenotype (CD206) | ||||||||||
| 5 Decreased inflammatory factors (IL-1β, TNF-α) | ||||||||||
| 6 Promoted proliferation of blood vessels and angiogenesis (CD31) | ||||||||||
| 16 | Gondaliya et al[153], 2022 | National Institute of Pharmaceutical Educationand Research(India) | Bone marrow | 1 BMSC-Exos loaded with miR-155 inhibitor; injected subcutaneously; 0.1 μg/μL; 1 d after wound induction | Untreated | Mice (C57BL/6) | 4 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Declined miR-155 levels with a concomitant increase in FGF-7 | ||||||||||
| 2 BMSC-Exos; injected subcutaneously; 0.1 μg/μL; 1 d after wound induction | ||||||||||
| 3 Downregulated expression of MMP-2 and MMP-9 | ||||||||||
| 4 Declined expression of pro-inflammatory cytokines (TIMP-2, lymphotactin, sTNF RI, sTNF RII, and LIX); declined regulated upon activation, normal T cell expressed and secreted (RANTES) chemokine; downregulated pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and TGF-β1 | ||||||||||
| 3 BMSC-Exos loaded with negative control sequences; injected subcutaneously; 0.1 μg/μL; 1 d after wound induction | ||||||||||
| 5 Promoted re-epithelialization, collagen synthesis and deposition, angiogenesis (α-SMA) and vascularization (CAM) | ||||||||||
| 17 | Dalirfardouei et al[125], 2019 | Mashhad University of Medical Sciences(Iran) | Human menstrual blood | 1 MenSC-Exos; injected intradermally; 10 μg in 100 μL of PBS; at Day 0 | PBS (100 μL) | Mice (C57BL/6) | 8 mm | 1 Accelerated cutaneous wound healing | NF-κB signaling pathway (possible) | |
| 2 Promoted re-epithelialization | ||||||||||
| 2 MenSCs; injected intradermally; 1 × 106 cells in 100 μL of PBS; at Day 0 | ||||||||||
| 3 Induced macrophage polarization from M1 (iNOS) to M2 (Arg) phenotype | ||||||||||
| 4 Enhanced angiogenesis (VEGF, microvessel density) | ||||||||||
| 5 Improved collagen deposition (upregulated Col I/Col III ratio at Day 7, downregulated at Day 14) | ||||||||||
| 6 Decreased size of scar tissues | ||||||||||
| 7 Decreased cellularity in the granulation tissue | ||||||||||
| 8 Decreased Rela gene expression at Day 4, enhanced at Day 7. | ||||||||||
| 18 | Wang et al[124], 2022 | Affiliated Hospital of Nantong University(China) | Rat bone marrow | 1 BMSC-Exos + 50 mg/kg intraperitoneal tertbutylhydroquinone (tBHQ); injected subcutaneously of 4 sites at the base and edge of the wound; 100 μg/mL, 200 μL; at Day 0 and 7 | PBS | Rats (Sprague-Dawley) | 15 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Promoted re-epithelialization and collagen deposition | ||||||||||
| 3 Enhanced angiogenesis (CD31) | ||||||||||
| 4 Reduced inflammation (decreased inflammatory cytokines TNF-α, IL-1β and increased anti-inflammatory cytokines IL-4, IL-10). | ||||||||||
| 2 BMSC-Exos + 200 μL intravenous Lenti-sh-NC; injected subcutaneously of 4 sites at the base and edge of the wound; 100 μg/mL, 200 μL; at Day 0 and 7 | ||||||||||
| 3 BMSC-Exos; injected subcutaneously of 4 sites at the base and edge of the wound; 100 μg/mL, 200 μL; at Day 0 and 7 | ||||||||||
| 4 BMSC-Exos + 200 μL intravenous Lenti-sh-Nrf2; injected subcutaneously of 4 sites at the base and edge of the wound; 100 μg/mL, 200 μL; at Day 0 and 7 | ||||||||||
| 19 | Sun et al[127], 2022 | Nanjing Normal University; Nanjing University; Nanjing medical University; Nanjing Tech University(China) | Human umbilical vein | 1 Engineering TNF-α/hypoxia-pretreated HUVMSC-Exos +PCOF; each subsequent day later, total 21 d | PBS | Mice (C57BL/6) | 15 mm (S.aureus-infected chronic wounds) | 1 Accelerated cutaneous wound healing | miR-126/ SPRED1/RAS/ERK pathway (possible) | |
| 2 Reduced bacterial burden and suppressed bacterial colonization in the wound sites | ||||||||||
| 2 Engineering TNF-α/hypoxia-pretreated HUVMSC-Exos; each subsequent day later, total 21 d | ||||||||||
| 3 Reduced the inflammatory response (immune cells counting); decreased proinflammatory cytokines (TNF-α, IL-1β, IL-6); induced M2 (CD206) macrophages polarization | ||||||||||
| 3 Vancomycin; each subsequent day later, total 21 d | ||||||||||
| 4 PCOF; each subsequent day later, total 21 d | ||||||||||
| 4 Promoted collagen deposition and remodeling, granulation formation, re-epithelialization and enhanced proliferation of fibroblasts | ||||||||||
| 5 Enhanced cell proliferation (Ki67) | ||||||||||
| 6 Suppressed oxidative stress induced by bacteria and peroxide substrates (reduced the content of oxidative biomarkers and (MDA) increased the antioxidant mediators (GSH-Px, SOD) | ||||||||||
| 7 Promoted angiogenesis (upregulated miR-126, HIF-1α, VEGF, CD31 and α-SMA; increased neovascularization) | ||||||||||
| 8 In vivo biosafety (blood system, heart, liver, kidney and other organs) | ||||||||||
| 20 | Li et al[147], 2016 | Shanghai Normal University; Shanghai Jiao Tong University Affiliated Sixth People's Hospital(China) | Human synovial tissue | 1 miR-126-3p overexpressed SMSC-Exos + hydroxyapatite/chitosan composite hydrogel; placed on the wound bed with pressure dressing | Untreated | Rats (Sprague-Dawley) | 18 mm | 1 Accelerated cutaneous wound healing | Activated MAPK/ERK and PI3K/AKT pathways | |
| 2 Enhanced angiogenesis (μCT), formation and maturation of new vessels (CD31, α-SMA) | ||||||||||
| 3 Promoted re-epithelialization, granulation tissue maturation, collagen alignment and deposition that indicated improved ECM remodeling | ||||||||||
| 2 Hydroxyapatite/chitosan composite hydrogel; placed on the wound bed with pressure dressing | ||||||||||
| 4 Accelerated growth of follicles and sebaceous glands | ||||||||||
| 21 | Zhang et al[148], 2021 | Jinzhou Medical University(China) | Human umbilical cord | 1 HUCMSC-Exos + polyvinyl alcohol (PVA)/alginate (Alg) nanohydrogel; locally transplanted; 300 μL; once a day | Untreated | Rats (Sprague-Dawley) | 15 mm × 2 mm | 1 Accelerated cutaneous wound healing | ERK1/2 pathway | |
| 2 Enhanced re-epithelialization and hair follicles formation | ||||||||||
| 3 Promoted collagen deposition and remodeling (increased and orderly arranged collagen fibers) | ||||||||||
| 2 HUCMSC-Exos; locally transplanted; 300 μL; once a day | ||||||||||
| 3 PVA/Alg nanohydrogel; locally transplanted; 300 μL; once a day | ||||||||||
| 4 Promoted angiogenesis (CD31, α-SMA, SR-B1, VEGF) | ||||||||||
| 22 | Han et al[154], 2022 | The First Affiliated Hospital of Zhengzhou University(China) | Human bone marrow | 1 lncRNA KLF3-AS1 overexpressed BMSC-Exos; injected via tail vein; 100 µL; at Day 0 | Untreated | Mice (BALB/c) | Not mentioned | 1 Accelerated cutaneous wound healing | lncRNA KLF3-AS1/miR-383/VEGFA signaling pathway | |
| 2 Minimized weight loss. | ||||||||||
| 2 Negative control silenced BMSC-Exos;injected via tail vein;100 µL;at Day 0 | 3 Reduced inflammation (decreased IL-6 and IL-1β) | |||||||||
| 4 Promoted angiogenesis (CD31), collagen deposition and follicle regeneration | ||||||||||
| 3 Negative control overexpressed BMSC-Exos; injected via tail vein; 100 µL; at Day 0 | ||||||||||
| 5 Decreased expression of miR-383 and increased VEGFA | ||||||||||
| 4 lncRNA KLF3-AS1 silenced BMSC-Exos; injected via tail vein; 100 µL; at Day 0 | ||||||||||
| 23 | Ding et al[155], 2019 | Shanghai Jiao Tong University Affiliated Sixth People's Hospital(China) | Human bone marrow | 1 Deferoxamine-preconditioned BMSC-Exos (DFO-Exos); injected subcutaneously around the wounds at four sites; 100 μg in 100 μL PBS; at Day 0 | PBS (100 μL) | Rats (Sprague-Dawley) | 20 mm × 2 mm | 1 Accelerated cutaneous wound healing | miR-126/PTEN/PI3K/AKT pathway | |
| 2 Enhanced re-epithelialization and lower scar formation | ||||||||||
| 3 Promoted collagen deposition (increased wavy collagen fibers) | ||||||||||
| 2 BMSC-Exos; injected subcutaneously around the wounds at four sites; 100μg in 100μL PBS; at Day 0 | ||||||||||
| 4 Promoted angiogenesis (vessel density by micro-CT, CD31, α-SMA) | ||||||||||
| 24 | Bian et al[135], 2020 | Chinese PLA General Hospital(China) | Human decidua | dMSC-sEVs; injected around the wounds at 4 sites (25 μL per site); 100 μL, 5.22 × 1011 particles/mL; at Day 7, 14, 21and 28 | PBS (100 μL) | Mice (BKS-db) | 16 mm | 1 Accelerated cutaneous wound healing | RAGE/RAS; Smad pathways | |
| 2 Reduced scar width | ||||||||||
| 3 Accelerated collagen deposition (larger and better-organized collagen deposition) | ||||||||||
| 4 Enhanced fibroblast proliferation (PCNA), migration (CXCR4), and differentiation abilities of fibroblast | ||||||||||
| 5 Promoted angiogenesis (α-SMA) | ||||||||||
| 6 Improved fibroblast senescent state (p21) | ||||||||||
| 25 | Zhang et al[156], 2022 | Xijing Hospital of Fourth Military Medical University(China) | Human adipose tissue | AMSC-Exos; injected subcutaneously; 200 μg; 3 d after wound induction, for three consecutive days | PBS (100 μL) | Mice (db/db) | 10 mm | 1 Accelerated cutaneous wound healing | SIRT3/SOD2 pathway | |
| 2 Enhanced re-epithelialization | ||||||||||
| 3 Promoted angiogenesis (CD34, VEGF) | ||||||||||
| 4 Improved oxidative stress (MDA, T-AOC, SOD) | ||||||||||
| 5 Reduced inflammatory cytokines (IL-1β, IL-6, TNF-α, MCP-1) | ||||||||||
| 26 | Born et al[157], 2021 | University of Maryland; Johns Hopkins University School of Medicine(USA) | Human bone marrow | 1 HOX transcript antisense RNA (HOTAIR) overexpressed BMSC-EVs; injected around the wound in a cross pattern of four sites; 50 μg in 50 μL PBS; at Day 3, four times | PBS (50 μL) | Mice (db/db) | 8 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Promoted angiogenesis (CD31, VEGFA) | ||||||||||
| 2 BMSC-EVs; injected around the wound in a cross pattern of four sites; 50 μg in 50 μL PBS; at Day 3, four times | ||||||||||
| 27 | Teng et al[158], 2022 | Jiangnan University (China) | Human umbilical cord | HUCMSC-Exos; injected subcutaneously around the wounds at four sites; 100 μL (100 μg/mL); at Day 0 | PBS (100 μL) | Rats (Sprague-Dawley) | 10 mm | 1 Accelerated cutaneous wound healing | — | |
| 2 Inhibited chronic inflammation: (decreased number of inflammatory cells); inhibited pro-inflammatory cytokines (TNF-α); induced M2 (CD206) macrophages polarization | ||||||||||
| 3 Enhanced re-epithelialization | ||||||||||
| 4 Promoted angiogenesis (increased new blood vessels, CD31, VEGF) | ||||||||||
| 5 Promoted collagen synthesis and skin regeneration | ||||||||||
Preclinical studies have demonstrated the ability of MSC-Exos to promote diabetic wound healing. No evident pathological abnormalities in the heart, liver, spleen, lung, and kidneys sampled after exosome treatment were observed, and biomarkers reflecting liver and kidney function blood biochemistry were also within normal limits[127]. Meanwhile, no erythema, edema, or irritation was observed in the wound area after exosome treatment[137], confirming the superior biosafety of exosome therapy.
We also searched for applications of exosomes secreted by stem cells from other sources in diabetic wounds and summarized them in Supplementary Table 1. Noteworthy, the types of animals used for modeling were limited to mice and rats. Most of the studies involved acute diabetic wounds, that is, exosomes were administered immediately after successful modeling of full-thickness skin wounds. Only one study introduced Staphylococcus aureus to establish infected chronic wounds after the establishment of full-thickness cutaneous wounds and confirmed that exosomes were effective in treating infectious DCWs[127]. The efficacy and safety of MSC-Exos need to be further confirmed in larger animal models and DCW models. Because the islet morphology, structure and function, blood biochemical indices, and skin structure of minipigs are more similar to those of the human body, they are ideal animal models for studying diabetic wounds[159]. Our team has established a chronic skin ulcer model in diabetic miniature pigs in the early stage[160] and is researching on exosome products to explore the optimal administration methods and dosages and to verify their therapeutic effects.
According to the search results in ClinicalTrials.gov, no clinical trials of MSC-Exos and exosomes from other sources for diabetic cutaneous wound healing have been registered. Therefore, we expanded the scope of clinical trials to search for exosomes derived from any sources and exosome-enriched stem cell-conditioned medium in various wound types (Table 2). None of the included four registered clinical trials had related results published, while they were all non-randomized one-arm pilot studies. Thus, more high-quality randomized controlled trials are required to further confirm these research results. Of note, the application of cell-free therapies in clinical patients requires special attention to security, although no adverse reactions of exosomes have been reported in preclinical studies. Moreover, ADSC-Exos has been confirmed to not induce any irritation or toxicity in skin sensitization, irritation, or oral toxicity tests[161]; therefore, they can be considered in clinical practice to promote wound healing in combination with basic wound care measures. Nevertheless, toxicological analysis of different tissue-derived MSCs-Exos and more evidence of short and long-term health safety assessments are required to confirm their safety.
| Start year | Institution (Nation) | Type of wounds | Intervention | Autologous/Allogeneic | Administration, frequency | Patients number | Follow-up period | Outcome measures | Phase | Study design | ClinicalTrials.gov identifier | Status |
| 2022 | Shanghai Ninth People's Hospital Affiliated to Shanghai Jiao Tong University (China) | Full-layer skin wounds | Adipose tissue derived exosomes(200-300 mL of the subject adipose tissue) | Autologous | Applied directly to the wound (mixed with sterile hydrogel), twice a week | 5 | 4 wk | Primary: Percentage of wound healing | Not Applicable | Non-randomized, single group assignment, open label | NCT05475418 | Not yet recruiting |
| 2015 | Kumamoto University (Japan) | Intractable cutaneous ulcers (e.g., rheumatic disease, peripheral arterial disease, chronic venous insufficiency, decubitus or burns) | Plasma-derived exosomes (Plasma samples will be filtered through 0.45 μm and 0.20 μm filters. The samples will be filtered through 0.02 μm filter to trap exosomes with the filter. Saline solution will be loaded from the other side of the 0.02 μm filter to obtain exosome rich buffer.) | Autologous | Applied to the ulcer, daily | 5 | 28 d | Primary: Ulcer size (length, width, depth) | Early Phase 1 | Non-randomized, single group assignment, open label | NCT02565264 | Unknown |
| Secondary: Pain of cutaneous wounds (VAS) | ||||||||||||
| 2023 | Aegle Therapeutics (USA) | Dystrophic Epidermolysis Bullosa (DEB); chronic wounds (< 20% closure of wound during observation period); 10-50 cm2 | Bone marrow mesenchymal stem cells derived extracellular vesicle (AGLE-102) | Allogeneic | Multiple administrations of 2 ascending dose levels of AGLE-102; (up to 6 administrations); (each administration will occur 14 ± 7 d but no less than 7 d apart); (each administration no more than 3 mo); (wound closes prior to 6 administrations, no additional doses will be given) | 10 | 8 mo; if the wound closes before receiving all 6 doses, for 4 mo after the wound closes | Primary: Dose limiting toxicity | Phase 1/2 | Non-randomized, multicenter, ascending dose, single group assignment, open label | NCT04173650 | Not yet recruiting |
| Secondary: Wound size | ||||||||||||
| 2019 | Mayapada Hospital (Indonesia) | Chronic wounds | Human Wharton's Jelly mesenchymal stem cells conditioned medium (WJ-MSC-CM) | Allogeneic | Applied to the wound (the conditioned medium gel), every week | 38 | 2 wk | Primary: Success rate of chronic ulcer healing | Phase 1 | Non-randomized, single group assignment, open label | NCT04134676 | Completed |
Exosome research is still in its infancy, and the realization of the transformation from preclinical research to clinical application still has great exploration value. The problems of optimal preparation, extraction, isolation, and storage of exosomes on a large scale and their production efficiency have not yet been determined; preparation and identification of components due to different source cells and the high heterogeneity of exosome components have not yet been solved; specific regulatory mechanisms in DCWs have not yet been fully elucidated; efficacy and safety of different cell sources and/or administrations have not been proven, and reasonable and effective methods of fusing exosomes with other biomaterials have not yet been implemented, all these issues are barriers that limit the clinical application of exosomes.
Thus, efficient, stable, safe, and mass-producible stem cells and related products for the treatment of diabetic wounds are yet to be explored and developed. More research is required in future clinical trials and routine practice to determine the most effective cell sources for diabetic wounds; to establish optimal large-scale culture conditions of MSCs; to solve the preparation problem of huge heterogeneity of exosome components; to explore standardized isolation, quality control, purification, and characterization techniques of MSC-Exos; and to determine the best approach for long-term storage[162]. Researchers also need to fully understand the abilities, loss, distribution, diffusion efficiency, and clearance efficiency of exosomes after transporting them to target areas. Physical, chemical, or biological methods for preconditioning, genetic engineering, and transfection are used to specifically enhance a certain therapeutic potential to achieve relatively better wound healing than native exosomes, thus becoming new treatment directions[163]. Additionally, combining exosomes with biomaterials is possible to create bioactive dressings to enhance or combine repair ability, provide local microenvironment stability, and achieve sustained release of exosomes[74]. Additionally, starting clinical trials as soon as possible is necessary to verify the optimal dosages, administration methods, and efficacy evaluation of MSC-Exos in clinical patients, looking forward to its broad application prospects in promoting DCW healing in clinical practice[162].
DCWs, which are one of the most common chronic refractory wounds, pose a heavy burden to patients, families, and society. Current studies have suggested that MSC-Exos can play an important role in various aspects of wound healing and hold sufficient promise for promoting diabetic wound healing. However, recent clinical applications of MSC-Exos in DCW repair are still limited. Moreover, clinical translational issues, such as exosome production, isolation, purification, and storage processes, the most effective route of administration and dose, and efficacy evaluation remain. Accurate and efficient exosome products need to be established, and experiments in animals that have a greater resemblance to human skin tissues and clinical trials need to be initiated as soon as possible to validate the optimal dosage and administration, and efficacy evaluation for using MSC-Exos to provide safety assurance for further clinical applications. Modification of MSC-Exos and integration with biomaterials to improve their efficacy and reduce their elimination rate may be a promising direction. We look forward to the clinical application of MSC-Exos for diabetic wound healing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roncalli J, France; Shalaby MN, Egypt S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S-34S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 922] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 2. | Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994;2:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 274] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117:35S-41S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 397] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 4. | Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4:560-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1534] [Cited by in RCA: 1439] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 5. | Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, Schmidtchen A, Car J, Järbrink K. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 374] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 6. | Cheng B, Jiang Y, Fu X, Hao D, Liu H, Liu Y, Huang Z, Tan Q, Wang L, Hu D, Yang Y, Han C, Cheng Z, Ran X, Li Y. Epidemiological characteristics and clinical analyses of chronic cutaneous wounds of inpatients in China: Prevention and control. Wound Repair Regen. 2020;28:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 8. | Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 9. | Chen L, Gao Y, Li Y, Wang C, Chen D, Ran X. Severe Intermittent Hypoxia Modulates the Macrophage Phenotype and Impairs Wound Healing Through Downregulation of HIF-2α. Nat Sci Sleep. 2022;14:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Bowling FL, Rashid ST, Boulton AJ. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol. 2015;11:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med. 2014;31:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Bowers S, Franco E. Chronic Wounds: Evaluation and Management. Am Fam Physician. 2020;101:159-166. [PubMed] |
| 13. | Jones RE, Foster DS, Longaker MT. Management of Chronic Wounds-2018. JAMA. 2018;320:1481-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 14. | Zarei F, Negahdari B, Eatemadi A. Diabetic ulcer regeneration: stem cells, biomaterials, growth factors. Artif Cells Nanomed Biotechnol. 2018;46:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Li Y, Gao Y, Chen D, Wang C, Liu G, Yang X, Ran X. Autologous platelet-rich gel treatment for diabetic chronic cutaneous ulcers: A meta-analysis of randomized controlled trials. J Diabetes. 2019;11:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Kanji S, Das H. Advances of Stem Cell Therapeutics in Cutaneous Wound Healing and Regeneration. Mediators Inflamm. 2017;2017:5217967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Lopes L, Setia O, Aurshina A, Liu S, Hu H, Isaji T, Liu H, Wang T, Ono S, Guo X, Yatsula B, Guo J, Gu Y, Navarro T, Dardik A. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther. 2018;9:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Hyun I, Lindvall O, Ahrlund-Richter L, Cattaneo E, Cavazzana-Calvo M, Cossu G, De Luca M, Fox IJ, Gerstle C, Goldstein RA, Hermerén G, High KA, Kim HO, Lee HP, Levy-Lahad E, Li L, Lo B, Marshak DR, McNab A, Munsie M, Nakauchi H, Rao M, Rooke HM, Valles CS, Srivastava A, Sugarman J, Taylor PL, Veiga A, Wong AL, Zoloth L, Daley GQ. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Lovell-Badge R, Anthony E, Barker RA, Bubela T, Brivanlou AH, Carpenter M, Charo RA, Clark A, Clayton E, Cong Y, Daley GQ, Fu J, Fujita M, Greenfield A, Goldman SA, Hill L, Hyun I, Isasi R, Kahn J, Kato K, Kim JS, Kimmelman J, Knoblich JA, Mathews D, Montserrat N, Mosher J, Munsie M, Nakauchi H, Naldini L, Naughton G, Niakan K, Ogbogu U, Pedersen R, Rivron N, Rooke H, Rossant J, Round J, Saitou M, Sipp D, Steffann J, Sugarman J, Surani A, Takahashi J, Tang F, Turner L, Zettler PJ, Zhai X. ISSCR Guidelines for Stem Cell Research and Clinical Translation: The 2021 update. Stem Cell Reports. 2021;16:1398-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 20. | Marks PW, Witten CM, Califf RM. Clarifying Stem-Cell Therapy's Benefits and Risks. N Engl J Med. 2017;376:1007-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 21. | Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 22. | Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum Gene Ther. 2015;26:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Deng H, Sun C, Sun Y, Li H, Yang L, Wu D, Gao Q, Jiang X. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell Reprogram. 2018;20:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 25. | An T, Chen Y, Tu Y, Lin P. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev Rep. 2021;17:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Demir S, Nawroth PP, Herzig S, Ekim Üstünel B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv Sci (Weinh). 2021;8:e2100275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 27. | Grennan D. Diabetic Foot Ulcers. JAMA. 2019;321:114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5938] [Article Influence: 989.7] [Reference Citation Analysis (8)] |
| 29. | Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: A systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (1)] |
| 30. | Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 31. | Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 848] [Article Influence: 169.6] [Reference Citation Analysis (0)] |
| 32. | Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 33. | Dong J, Chen L, Zhang Y, Jayaswal N, Mezghani I, Zhang W, Veves A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv Ther. 2020;37:4519-4537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 874] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 35. | Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Transl Res. 2021;236:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 369] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 36. | Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: Focusing on diabetic wound healing. J Dermatol Sci. 2016;84:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 37. | Liu Y, Liu Y, He W, Mu X, Wu X, Deng J, Nie X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front Immunol. 2022;13:918223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (2)] |
| 38. | Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 627] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 39. | Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1170] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 40. | Papachristou S, Pafili K, Papanas N. Skin AGEs and diabetic neuropathy. BMC Endocr Disord. 2021;21:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr Diab Rep. 2018;18:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 42. | Li L, Chen D, Wang C, Liu G, Ran X. The Effect of Autologous Platelet-Rich Gel on the Dynamic Changes of the Matrix Metalloproteinase-2 and Tissue Inhibitor of Metalloproteinase-2 Expression in the Diabetic Chronic Refractory Cutaneous Ulcers. J Diabetes Res. 2015;2015:954701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Chang M. Restructuring of the extracellular matrix in diabetic wounds and healing: A perspective. Pharmacol Res. 2016;107:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 511] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 45. | Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: Chronic wound care and management. J Am Acad Dermatol. 2016;74:607-25; quiz 625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 418] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 46. | Braun L, Kim PJ, Margolis D, Peters EJ, Lavery LA; Wound Healing Society. What's new in the literature: an update of new research since the original WHS diabetic foot ulcer guidelines in 2006. Wound Repair Regen. 2014;22:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Ji S, Liu X, Huang J, Bao J, Chen Z, Han C, Hao D, Hong J, Hu D, Jiang Y, Ju S, Li H, Li Z, Liang G, Liu Y, Luo G, Lv G, Ran X, Shi Z, Tang J, Wang A, Wang G, Wang J, Wang X, Wen B, Wu J, Xu H, Xu M, Ye X, Yuan L, Zhang Y, Xiao S, Xia Z. Consensus on the application of negative pressure wound therapy of diabetic foot wounds. Burns Trauma. 2021;9:tkab018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Pop MA, Almquist BD. Biomaterials: A potential pathway to healing chronic wounds? Exp Dermatol. 2017;26:760-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 50. | Mastrogiacomo M, Nardini M, Collina MC, Di Campli C, Filaci G, Cancedda R, Odorisio T. Innovative Cell and Platelet Rich Plasma Therapies for Diabetic Foot Ulcer Treatment: The Allogeneic Approach. Front Bioeng Biotechnol. 2022;10:869408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Li L, Chen D, Wang C, Yuan N, Wang Y, He L, Yang Y, Chen L, Liu G, Li X, Ran X. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015;23:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Zhang Z, Zhang W, Xu Y, Liu D. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis. Asian J Surg. 2022;45:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Vas P, Rayman G, Dhatariya K, Driver V, Hartemann A, Londahl M, Piaggesi A, Apelqvist J, Attinger C, Game F. Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, Al-Gazally ME, Mustafa YF, Siahmansouri H. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 2022;13:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 204] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 55. | Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 56. | Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 57. | Yu Q, Qiao GH, Wang M, Yu L, Sun Y, Shi H, Ma TL. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front Cell Dev Biol. 2022;10:812262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 58. | Maranda EL, Rodriguez-Menocal L, Badiavas EV. Role of Mesenchymal Stem Cells in Dermal Repair in Burns and Diabetic Wounds. Curr Stem Cell Res Ther. 2017;12:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Shi R, Lian W, Jin Y, Cao C, Han S, Yang X, Zhao S, Li M, Zhao H. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim Biophys Sin (Shanghai). 2020;52:620-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | De Gregorio C, Contador D, Díaz D, Cárcamo C, Santapau D, Lobos-Gonzalez L, Acosta C, Campero M, Carpio D, Gabriele C, Gaspari M, Aliaga-Tobar V, Maracaja-Coutinho V, Ezquer M, Ezquer F. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 61. | Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D, Gastens MH, Quast T, Negrean M, Stirban OA, Nandrean SG, Götting C, Minartz P, Kleesiek K, Tschoepe D. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract. 2012;66:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 62. | Lu D, Jiang Y, Deng W, Zhang Y, Liang Z, Wu Q, Jiang X, Zhang L, Gao F, Cao Y, Chen B, Xue Y. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant. 2019;28:645-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Moon KC, Suh HS, Kim KB, Han SK, Young KW, Lee JW, Kim MH. Potential of Allogeneic Adipose-Derived Stem Cell-Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes. 2019;68:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 64. | Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 422] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 65. | Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 66. | Wang Z, Li H, Zhang D, Liu X, Zhao F, Pang X, Wang Q. Effect of advanced glycosylation end products on apoptosis in human adipose tissue-derived stem cells in vitro. Cell Biosci. 2015;5:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Jin J. Stem Cell Treatments. JAMA. 2017;317:330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6572] [Article Influence: 1314.4] [Reference Citation Analysis (0)] |
| 69. | Than UTT, Guanzon D, Leavesley D, Parker T. Association of Extracellular Membrane Vesicles with Cutaneous Wound Healing. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Nair S, Salomon C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol. 2018;40:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 71. | Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 72. | Newton WC, Kim JW, Luo JZQ, Luo L. Stem cell-derived exosomes: a novel vector for tissue repair and diabetic therapy. J Mol Endocrinol. 2017;59:R155-R165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100-3110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 74. | Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 75. | Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 797] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 76. | Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420. [PubMed] |
| 77. | Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1470] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 78. | Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 794] [Cited by in RCA: 941] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 79. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7702] [Article Influence: 1100.3] [Reference Citation Analysis (1)] |
| 80. | Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V, Daglia M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 81. | Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112:384-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 82. | Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4900] [Cited by in RCA: 6160] [Article Influence: 513.3] [Reference Citation Analysis (0)] |
| 83. | Lai P, Weng J, Guo L, Chen X, Du X. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 84. | Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 85. | Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 798] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 86. | Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H, Hamblin MR. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 87. | Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020;15:6917-6934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 830] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 88. | Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS, Khan MK. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 89. | Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1505] [Cited by in RCA: 1413] [Article Influence: 235.5] [Reference Citation Analysis (0)] |
| 90. | Rai AK, Johnson PJ. Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc Natl Acad Sci U S A. 2019;116:21354-21360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 91. | Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release. 2017;266:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 92. | Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33:1744-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 464] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 93. | Zabeo D, Cvjetkovic A, Lässer C, Schorb M, Lötvall J, Höög JL. Exosomes purified from a single cell type have diverse morphology. J Extracell Vesicles. 2017;6:1329476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 94. | Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 477] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 95. | Rider MA, Hurwitz SN, Meckes DG Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci Rep. 2016;6:23978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 510] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 96. | Böing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 855] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 97. | Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, Gevaert K, Miinalainen I, Cocquyt V, Braems G, Van den Broecke R, Denys H, De Wever O, Hendrix A. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep. 2017;7:2704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 98. | Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, Muhhina J, Fondelli C, Gavrilova J, Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 99. | Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6:288-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (16)] |
| 100. | Álvarez-Viejo M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J Stem Cells. 2020;12:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 101. | Wang Z, Wu Y, Zhao Z, Liu C, Zhang L. Study on Transorgan Regulation of Intervertebral Disc and Extra-Skeletal Organs Through Exosomes Derived From Bone Marrow Mesenchymal Stem Cells. Front Cell Dev Biol. 2021;9:741183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 103. | Schreml S, Babilas P, Fruth S, Orsó E, Schmitz G, Mueller MB, Nerlich M, Prantl L. Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 104. | Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini F, Lopatina T, Brizzi MF, Camussi G. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 105. | Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front Immunol. 2021;12:749192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 106. | Babaei M, Rezaie J. Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations. J Transl Med. 2021;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 107. | Akbar N, Azzimato V, Choudhury RP, Aouadi M. Extracellular vesicles in metabolic disease. Diabetologia. 2019;62:2179-2187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 108. | Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1418] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 109. | Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano. 2019;13:10015-10028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 110. | Behera J, Tyagi N. Exosomes: mediators of bone diseases, protection, and therapeutics potential. Oncoscience. 2018;5:181-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 111. | Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, Yang P, Duan X, Zhang J, Fu X, Hu X, Ao Y. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 112. | Guillamat-Prats R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 227] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 113. | Askenase PW. COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? J Extracell Vesicles. 2020;10:e12004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 114. | Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119:9433-9443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 115. | Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B, Wu P, Shi Y, Mao F, Yan Y, Xu W, Qian H. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano. 2018;12:7613-7628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 116. | Biró E, Sturk-Maquelin KN, Vogel GM, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 117. | Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 759] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 118. | Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol. 2014;5:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 119. | Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 306] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 120. | Wang J, Xia J, Huang R, Hu Y, Fan J, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res Ther. 2020;11:424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 121. | Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 338] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 122. | He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 123. | Zhao B, Zhang X, Zhang Y, Lu Y, Zhang W, Lu S, Fu Y, Zhou Y, Zhang J. Human Exosomes Accelerate Cutaneous Wound Healing by Promoting Collagen Synthesis in a Diabetic Mouse Model. Stem Cells Dev. 2021;30:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 124. | Wang L, Cai Y, Zhang Q, Zhang Y. Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells. Int J Stem Cells. 2022;15:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 125. | Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 126. | Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 522] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 127. | Sun B, Wu F, Wang X, Song Q, Ye Z, Mohammadniaei M, Zhang M, Chu X, Xi S, Zhou N, Wang W, Yao C, Shen J. An Optimally Designed Engineering Exosome-Reductive COF Integrated Nanoagent for Synergistically Enhanced Diabetic Fester Wound Healing. Small. 2022;18:e2200895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 128. | Geng X, Qi Y, Liu X, Shi Y, Li H, Zhao L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv. 2022;133:112613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 129. | Zhang Y, Han F, Gu L, Ji P, Yang X, Liu M, Tao K, Hu D. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. J Mol Histol. 2020;51:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 130. | Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120:10847-10854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 131. | Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015;24:1635-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 506] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 132. | Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 433] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 133. | Zhao G, Liu F, Liu Z, Zuo K, Wang B, Zhang Y, Han X, Lian A, Wang Y, Liu M, Zou F, Li P, Liu X, Jin M, Liu JY. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res Ther. 2020;11:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 134. | Wang X, Jiao Y, Pan Y, Zhang L, Gong H, Qi Y, Wang M, Shao M, Wang X, Jiang D. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019;2019:2402916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 135. | Bian X, Li B, Yang J, Ma K, Sun M, Zhang C, Fu X. Regenerative and protective effects of dMSC-sEVs on high-glucose-induced senescent fibroblasts by suppressing RAGE pathway and activating Smad pathway. Stem Cell Res Ther. 2020;11:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 136. | Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z, Fu Y, Zhai A, Bi C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 137. | Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 138. | Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, Yan C, Xie X, Lin Z, Panayi AC, Mi B, Liu G. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 139. | Shi R, Jin Y, Hu W, Lian W, Cao C, Han S, Zhao S, Yuan H, Yang X, Shi J, Zhao H. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318:C848-C856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 140. | Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int J Nanomedicine. 2020;15:5911-5926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 316] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 141. | Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 667] [Cited by in RCA: 593] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 142. | Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front Physiol. 2017;8:904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 143. | Jiang T, Wang Z, Sun J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res Ther. 2020;11:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 144. | Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl Med. 2016;5:1425-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 145. | Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H, Fu H, Yan Y, Zhang X, Wang M, Zhu W, Qian H, Xu W. HucMSC Exosome-Delivered 14-3-3ζ Orchestrates Self-Control of the Wnt Response via Modulation of YAP During Cutaneous Regeneration. Stem Cells. 2016;34:2485-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 146. | Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HMA, Hu B, Song J, Chen L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:13321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 147. | Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B. 2016;4:6830-6841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 148. | Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021;120:111671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 149. | Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 459] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 150. | Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl Med. 2017;6:736-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 151. | Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, Hu Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 152. | Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, Hu L, Chen L, Cao F, Zhou W, Zhang P, Liu G. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front Bioeng Biotechnol. 2022;10:829868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 153. | Gondaliya P, Sayyed AA, Bhat P, Mali M, Arya N, Khairnar A, Kalia K. Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-155 Inhibitor Ameliorate Diabetic Wound Healing. Mol Pharm. 2022;19:1294-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 154. | Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX, Xu HL. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract. 2022;183:109126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 155. | Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed Res Int. 2019;2019:9742765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 156. | Zhang Y, Bai X, Shen K, Luo L, Zhao M, Xu C, Jia Y, Xiao D, Li Y, Gao X, Tian C, Wang Y, Hu D. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 157. | Born LJ, Chang KH, Shoureshi P, Lay F, Bengali S, Hsu ATW, Abadchi SN, Harmon JW, Jay SM. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv Healthc Mater. 2022;11:e2002070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 158. | Teng L, Maqsood M, Zhu M, Zhou Y, Kang M, Zhou J, Chen J. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 159. | Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Müller TD, Tschöp MH. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14:140-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 585] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 160. | Li Y, Liang Y, Gao Y, Chen D, Ran X. Dynamic changes of wound-related miRNAs after application of autologous platelet-rich gel in diabetic wounds. Chin Med J. 2022;Epub ahead of print. [DOI] [Full Text] |
| 161. | Ha DH, Kim SD, Lee J, Kwon HH, Park GH, Yang SH, Jung JY, Lee JH, Park SR, Youn J, Lee SH, Kim JE, Lim J, Lee HK, Cho BS, Yi YW. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul Toxicol Pharmacol. 2020;115:104686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 162. | Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979-5997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 163. | Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |