Published online Nov 15, 2022. doi: 10.4239/wjd.v13.i11.926
Peer-review started: August 5, 2022
First decision: August 20, 2022
Revised: September 9, 2022
Accepted: November 2, 2022
Article in press: November 2, 2022
Published online: November 15, 2022
Processing time: 98 Days and 6.8 Hours
With the high incidence of diabetes around the world, ischemic complications cause a serious influence on people’s production and living. Neovascularization plays a significant role in its development. Therefore, neovascularization after diabetic ischemia has aroused attention and has become a hot spot in recent years. Neovascularization is divided into angiogenesis represented by atherosclerosis and arteriogenesis characterized by coronary collateral circulation. When mononuclear macrophages successively migrate to the ischemia anoxic zone after ischemia or hypoxia, they induce the secretion of cytokines, such as vascular endothelial growth factor and hypoxia-inducible factor, activate signaling pathways such as classic Wnt and phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) pathways, trigger oxidative stress response, activate endothelial progenitor cells or enter the glycolysis or lactic acid process and promote the formation of new blood vessels, remodeling them into mature blood vessels and restoring blood supply. However, the hypoglycemic condition has different impacts on neovascularization. Consequently, this review aimed to introduce the mechanisms of neovascularization after diabetic ischemia, increase our un-derstanding of diabetic ischemic complications and their therapies and provide more treatment options for clinical practice and effectively relieve patients’ pain. It is believed that in the near future, neovascularization will bring more benefits and hope to patients with diabetes.
Core Tip: This review aimed to give an overview of neovascularization in patients with diabetes. First, we introduced the basic concepts and influencing factors of neovascularization, including angiogenesis and arteriogenesis. Second, the mechanisms regarding cytokines, classical and novel signaling pathways, glycolysis and lactic acid process and so on described in detail. Then, the neovascularization after diabetic ischemia was further described in combination with the complications of diabetes, such as diabetic atherosclerosis, diabetic retinopathy, diabetic nephropathy and diabetic foot ulcer. Last but not least, the treatment plans listed, with advantages and disadvantages, that may offer more treatment options.
- Citation: Cai Y, Zang GY, Huang Y, Sun Z, Zhang LL, Qian YJ, Yuan W, Wang ZQ. Advances in neovascularization after diabetic ischemia. World J Diabetes 2022; 13(11): 926-939
- URL: https://www.wjgnet.com/1948-9358/full/v13/i11/926.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i11.926
Diabetes mellitus is a complex, heterogeneous, whole-body chronic metabolic disease; it is predicted that by 2045, the number of patients with diabetes, aged 20-79 years, will increase to 783 million[1]. Diabetes-related complications include microvascular plaque formation, ischemia and hypoxia caused by atherosclerosis. They are characterized by severe arterial ischemia, increased risk of amputation of peripheral artery disease and so on. Promoting neovascularization to restore the blood flow of the ischemic area is conducive to disease outcomes while avoiding risk. Hence, it is an urgent issue for scientific researchers.
Neovascularization is caused by angiogenesis and arteriogenesis. Angiogenesis refers to the budding of new blood vessels in the vascular bed in an original way to form new vasculature, mainly in the capillaries[2]. Arteriogenesis is the formation of new arteries by expanding lumen diameter and remodeling tube walls to restore blood flow in the ischemic area. The new route gradually disappears when the previously blocked artery is recanalized[3].
Physiological angiogenesis mainly occurs in embryo development, endometrial thickening and wound healing; the process depends on the ratio of proangiogenic factors to antiangiogenic factors. Pathological angiogenesis is often triggered in disease states, such as atherosclerosis, tumors, systemic lupus erythematosus, etc[4], to form abnormal blood vessels with thinner walls and higher permeability. Angiogenesis includes the following steps: (1) Endothelial cells sprout under the action of angiogenic factors[5]; (2) Pericytes aggregate if their absence leads to increased lumen permeability[6] and vascular instability; and (3) The basement membrane is reconstructed to develop mature and stable blood vessels.
Atherosclerosis can lead to severe complications such as myocardial infarction (MI), resulting in heart failure. Endothelial cells, which sprout under the action of angiogenic factors, significantly improve the patient’s recovery, which is the focus of current research.
After MI, blood congestion, thromboembolism and compression of the surrounding tissue, proinflammatory factors are released, leading to impaired endothelial integrity, loss of myocardial cells, endothelial cell damage, increased capillary permeability and secretion of proinflammatory cytokines to activate white blood cells and endothelial cells. This results in the release of a large number of inflammatory factors into the infarcted myocardium, thus promoting myocardial inflammation[7]. The formation of neovascularization in the ischemic infarct area to provide nutrients and oxygen is the key to post-MI repair. Neovascularization in the surrounding area of infarction increases vascular density and extends to the core area of infarction[8], and hypoxia plays an essential role in this process.
The most studied factor is hypoxia-inducible factor-1α (HIF-1α), whose reduced protein expression under high glucose conditions leads to increased MI[9]. In anoxic environments, the HIF-1α/vascular endothelial growth factor (VEGF) pathway acts by releasing angiogenic factors. After MI, reactive oxygen species (ROS) in cardiac fibroblasts increase by about 50%, resulting in mutations in the HIF phenotype marked by scar contraction and dysfunction[10]. HIF activation induces VEGF release, which activates endothelial cells (ECs) through the paracrine mechanism, and can be expressed in ECs to participate in angiogenesis. In addition, angiopoietin-like protein 4 stabilizes VEGF receptor 2/Ca2+-dependent cell adhesion molecule 5 complex to maintain endothelial structural integrity and promote macrophage transformation into a repair phenotype to enhance boundary region angiogenesis.
Arteriogenesis refers to the growth of new arteries or the derived collateral vessels, mainly involved in the active proliferation of ECs and smooth muscle cells, resulting in lumen enlargement and wall remodeling. It comprises primarily two stages[3]. In the early stage, the diameter of lateral branches and tube walls increases under the influence of fluid shear stress. Subsequently, monocytes promote lumen remodeling by secreting metalloproteinases and cytokines. In addition, M1-type macrophages promote the progression of myocardial inflammation[11]. The release of inflammatory factors under ischemia and hypoxia promotes the progression of inflammation and the occurrence of glycolysis. Under this action, fibroblasts are transformed into ECs, triggering epigenetic modification. ECs and fibroblasts contribute to the formation of arteries[12-14].
Human coronary circulation has an extensive anastomotic network. Even one-third of ordinary people have collateral circulation to cope with MI caused by transient vascular occlusion[15]. Further, 20%-25% of patients with coronary artery disease can prevent MI, improve survival and reduce mortality by promoting normal blood flow through collateral circulation during coronary artery occlusion. Approximately 1 in 5 patients cannot tolerate percutaneous coronary intervention or coronary artery bypass grafting. Therefore, collateral growth promotion is a promising therapeutic strategy targeting arteriogenesis. In the absence of coronary artery disease, these arteries are only 100-200 µm in diameter, and the lumen is impassable. When coronary artery disease causes a major artery occlusion, the collateral arteries are remodeled and the lumen is expanded to 100-800 µm in diameter to serve as part of the major artery[16], which is in line with normal routes and has one to two layers of smooth muscle cells. At the same time, the expansion of the diameter of the tube is accompanied by a decrease in the number of collateral arteries. The myocardial protection of large blood flow is more significant in the collateral arteries than in the new capillaries surrounding the infarct area[17].
Although collateral maturation is essential for preserving cardiac function, the related markers are still lacking[18]. Although the influence of coronary artery collateral formation and prognosis has been controversial at present[19], some studies showed[20] that patients with MI having coronary collateral circulation have more severe stenosis and worse cardiac function. Therefore, from a macro point of view, it is believed that collateral circulation benefits at least one-fifth of patients who cannot undergo percutaneous coronary intervention and coronary artery bypass grafting. Physiological or pathological vascular reconstruction and blood flow redistribution can prevent excessive MI and reduce injury to the body. This therapeutic strategy has broad research prospects and is worth further exploration to benefit patients.
As mentioned earlier, neovascularization is affected by various proangiogenic/antiangiogenic factors. The mechanisms and roles of proangiogenic factors, such as HIF, macrophages, VEGF family, noncoding RNA and hepatocyte growth factor, and antiangiogenic factors, such as thrombospondin-1 and interleukin 12 (IL-12), summarized in this study (Tables 1 and 2)[7,21-44].
| Trigger factors | Mechanism | Function | Ref. |
| HIF | When hypoxia occurs, HIF-α dimerizes with HIF-β, binding to hypoxia response elements in the nucleus, transcribing thousands of genes and promoting angiogenesis | Promote angiogenesis and increase vascular density; stimulate collateral vessel compensatory formation; regulate EPO and other downstream factors; mobilize endothelial progenitor cells | [21-24] |
| Macrophages | Macrophages are divided into M1 type, which is proinflammatory and phagocytic, and M2 type, which is anti-inflammatory and promotes angiogenesis | Transform into perivascular cells to control vascular permeability; remodel extracellular matrix to provide conduit for apical cells and promote blood vessel germination; endothelial cells and trim abnormal blood vessels | [25-30] |
| Monocytes | Angiogenesis is directly dependent on the number of circulating monocytes | Induce HIF-mediated release of chemokines and growth factors to stimulate angiogenesis; express angiogenin receptor Tie-2 and exacerbate inflammation | [7,31] |
| VEGF family | VEGF-A regulates angiogenesis, vascular permeability and inflammation. VEGF-B regulates angiogenesis and apoptosis. VEGF-C and VEGF-D regulate lymphangiogenesis, apoptosis and fiber formation | VEGF activates a variety of downstream signaling pathways and promotes the proliferation, migration and vascular remodeling of ECs; activate ERK1/2 and promote angiogenesis | [32-34] |
| Noncoding RNAs | Many noncoding RNAs regulate complex processes of angiogenesis | MiR-25-3p enhances endothelial permeability and angiogenesis. MiR-590-5p subtype NF90 has angiogenic effects | [35,36] |
| HGF | Stimulates angiogenesis by inducing endothelial cell proliferation, migration and tubular blood vessel formation | HGF significantly increases the expression of VEGF, decreases the activation of NF-κB and vascular leakage, promotes angiogenesis, is anti-inflammatory, is anti-oxidative and reduces vascular permeability | [37] |
| Angiotensin II | Angiogenesis is induced by activation of angiotensin 1 receptor and nicotinamide adenine dinucleotide phosphate oxidase | Induction of angiotensin II synthesis can lead to a proangiogenic state | [38] |
| asTF | asTF is widely expressed in macrophages and neovascularization in AS plaques | asTF affects all key stages of angiogenesis, including proliferation, migration and differentiation and induces increased levels of HIF and VEGF to promote angiogenesis | [39] |
| Classical Wnt pathway | Under the influence of Wnt factor, β-catenin isolates and enters the nucleus, binding to TCF/LEF and initiating transcription of downstream genes | The Wnt pathway promotes angiogenesis by regulating endothelial cell proliferation | [40,41] |
| Inhibitory factor | Mechanism | Function | Ref. |
| Platelet reactive protein-1 (TSP-1) | TSP-1 levels increase under hypoxia | TSP-1 inhibits angiogenesis by stimulating endothelial cell apoptosis and inhibiting endothelial cell migration and proliferation as well as inhibiting VEGF and eNOS | [42,43] |
| IL-12 | IL-12 stimulates the expression of proinflammatory and antiangiogenic genes in monocytes | Neutralization of IL-12 can enhance angiogenesis in ischemic areas and reduce body dysfunction | [7,44] |
| Noncoding RNAs | Noncoding RNA has the dual role of promoting and inhibiting angiogenesis | Upregulation of MiR-15a and MiR-16 reduce Tie2 protein levels and inhibit angiogenesis | [35,36] |
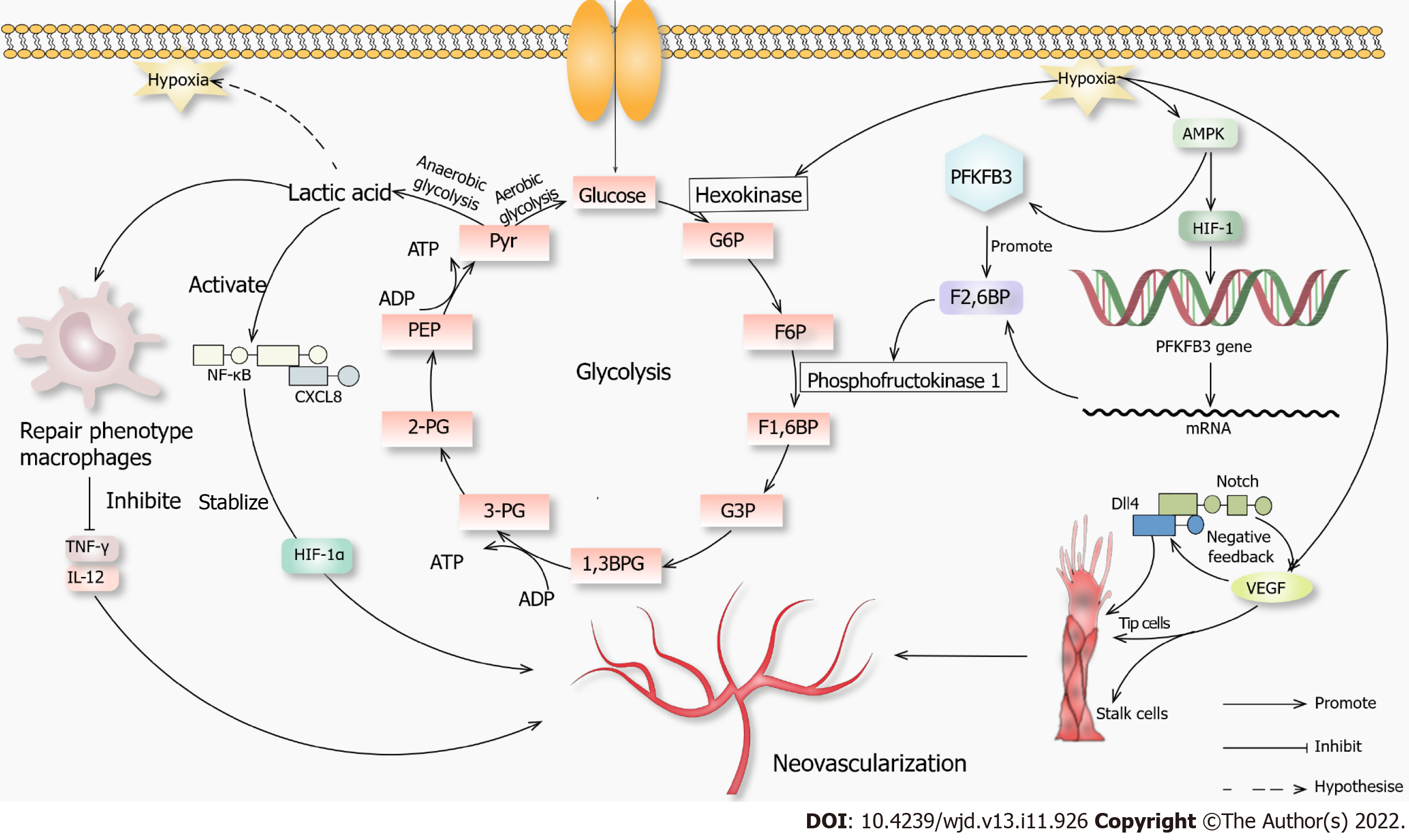
Studies have shown that 80% of ATP in ECs is produced by glycolysis[45], mainly due to the limited number of mitochondria. The energy generated needs to be supplied to the distal tissues. In addition, the ATP generation by glycolysis is faster than that by oxidative phosphorylation. Therefore, glycolysis is the primary energy supply for ECs in normal and hypoxic states. Hypoxia or lack of nutrition can promote the production of VEGF, fibroblast growth factor and other angiogenic factors[46], the concentration and the activity of hexokinase and membrane expression of glucose transporter protein 1. The ECs differentiate into specific cells under high VEGF levels, forming platelet pseudopodia and filiform pseudopodia, promoting migration and stem cell proliferation. Stable glycolysis produces lactic acid[47], promoting VEGF expression and inducing angiogenesis under multiple effects.
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) transcription through HIF-1α is induced by hypoxia, which is an essential regulator of glycolysis and can be phosphorylated by activating kinases such as mitogen-activated protein kinase[48]. PFKFB3 can promote the synthesis of fructose-2 6-diphosphate, activate phosphofructokinase 1 and promote glycolysis. The Notch-Delta-like ligand 4 signaling pathway can promote stem cell extension, tip cell growth and blood vessel germination[49].
Some intermediate products of glycolysis enter the pentose phosphate pathway. The NADPH produced during this process is necessary for nitric oxide (NO) biosynthesis, promoting angiogenesis. When macrophage metabolism shifts to glycolysis, M1 proinflammatory macrophages facilitate glycolysis, interrupting the tricarboxylic acid cycle and producing lactic acid rather than metabolizing pyruvate to acetyl-CoA. Anti-inflammatory M2 macrophages inhibit the pentose phosphate pathway.
Lactic acid has long been considered as a metabolic waste. Recent studies have shown that lactic acid can be expressed as a signaling molecule in wound healing and angiogenesis[50]. Lactic acid can modify histones to regulate macrophage polarity and expression of tissue repair genes such as arginase-1[51]. In inflammatory diseases such as atherosclerosis, macrophages secrete proinflammatory cytokines such as tumor necrosis factor γ and interleukin 12 to cause extensive damage to surrounding tissue, and anaerobic glycolysis causes lactic acid accumulation[51]. The macrophage polarity transition is a hallmark of the disease, and lactic acid converts macrophages from a proinflammatory phenotype into a repairing phenotype, removes cell debris and promotes wound healing. Inflammatory macrophages undergo modifications, which promote the repair characteristics of macrophages in response to inflammatory damage[52]. In addition, lactic acid can covalently couple with various histone lysine residues during histone acetylation to promote the transcription of homeostasis-related genes. In the late stage of lactic acid and histone lactate modification and accumulation, the cells switch to a steady-state phenotype, in which inflammatory genes are difficult to induce.
Studies have shown that lactic acid can promote the secretion of VEGF, activate the nuclear factor kappa-B/C-X-C motif chemokine 8 pathway and stabilize HIF-1α, playing a role in promoting angiogenesis signaling molecules. The overall function of lactic acid is to transform the inflammatory phenotype of macrophages into a repair phenotype. We hypothesized that lactic acid was associated with angiogenesis. However, the integration mechanism of lactic acid and hypoxia, such as HIF, chromatin remodeling and other processes extending to angiogenesis, is still unclear and needs further research (Figure 1).
When the body is subjected to various diabetic stimuli, the mitochondria is stimulated to produce superoxide, leading to the formation of the powerful oxidant nitrite, which damages DNA and depletes intracellular NAD (+)[53], resulting in a pathological state. Two common mechanisms[54] contribute to increased oxidative stress[55] in diabetes: One is an increase in free radical production and the other is a decrease in the levels of protective endogenous antioxidants. Also, natural antioxidants include dandelion[56], saffron[57,58], hawthorn[59], vitamin C and vitamin E[60]. However, rhizoma polygonate in traditional Chinese medicine can dephosphorylate DNA to damage DNA[61]. In addition, hyperglycemia activates nuclear factor kappa-B, which can lead to changes in the inflammatory response, upregulation of COX-2, inducible NO synthase (NOS)[62], tumor necrosis factor α and interleukin 1, promotion of cell proliferation and inhibition of cell death. The increased expression of inducible NOS catalyzes the production of large amounts of NO[63]. The inhibition of TLR2/4 signaling can avoid nuclear factor kappa-B translocation, ultimately reducing cell apoptosis[64]. The hyperglycemic environment can stimulate the mitochondrial respiratory chain to produce a large number of oxygen free radicals, activate protein kinases C[65] and promote the NADPH-related processes of oxidative stress, leading to endothelial cell apoptosis. A small number of ROS can maintain normal physiological function[66]; however, an excess of ROS causes oxidative stress[67], which can activate multiple stress kinases and related proteases and affect their activities[68], aggravate cytotoxicity and attack cells, leading to endothelial progenitor cell senescence, apoptosis and inhibition of migration and proliferation. Superoxide anions and H2O2 in the ROS family play a major role in this process. In addition, the activity of endodermal NOS is reduced, the metabolism of tetrahydrobiopurine (BH4) is abnormal, and dihydrobiopterin (BH2) cannot be recovered in diabetes, resulting in a lower level of BH4[69]. NOS induces the formation of many superoxide anions instead of NO, aggravating oxidative stress. Advanced glycation end products lead to an imbalance in ROS production and clearance and increased endothelial permeability[70]. Oxidative stress impairs angiogenesis through multiple mechanisms.
Endothelial progenitor cells (EPCs) are fusiform cells with limited proliferative capacity in the early stage and cells with high proliferative capacity in the late stage[71]. During tissue ischemia, EPCs can be mobilized from bone marrow to damaged blood vessels for vascular repair or angiogenesis, or the number of circulating EPCs increases via various factors, including VEGF, stromal cell-derived factor-1 or stem cell factor. EPCs can proliferate, migrate, adhere and differentiate into ECs, repair damaged ECs and secrete angiogenic factors such as VEGF to promote angiogenesis in ischemic tissues[72].
In diabetes, endothelial dysfunction and delayed angiogenesis promote the occurrence and development of diabetic vascular complications. In the high glucose environment, the number of EPCs is reduced and their functions are impaired[73]. In addition, EPCs are less responsive to ischemia, VEGF, stromal cell-derived factor-1 and other stimuli, and the mobilization mechanism is damaged. EPCs may also secrete antiangiogenic factors. The high glucose environment leads to the excessive production of ROS, excessive activation of NADPH oxidase and a significant decrease in the levels of manganese-containing superoxide dismutase and other antioxidant enzymes, resulting in EPC dysfunction[74]. The excessive production of ROS significantly increases the levels of oxLDL, inhibits Akt phosphorylation and endothelial NOS (eNOS) expression, decreases NO activity, inhibits the PI3K/Akt/eNOS signaling pathway and induces the apoptosis of EPCs, migration of EPCs and formation of functional defects in the lumen[75]. In addition, the severe inflammatory environment of diabetes causes impaired adhesion and proliferation of EPCs as well as neovascularization.
However, when patients with diabetes suffer from vascular complications, the number and function of EPCs in different parts (microvessels and large vessels) are different. For example, when such patients suffer from peripheral artery disease, the number of EPCs decreases. However, the proliferative capacity of EPCs is increased in patients with proliferative retinopathy.
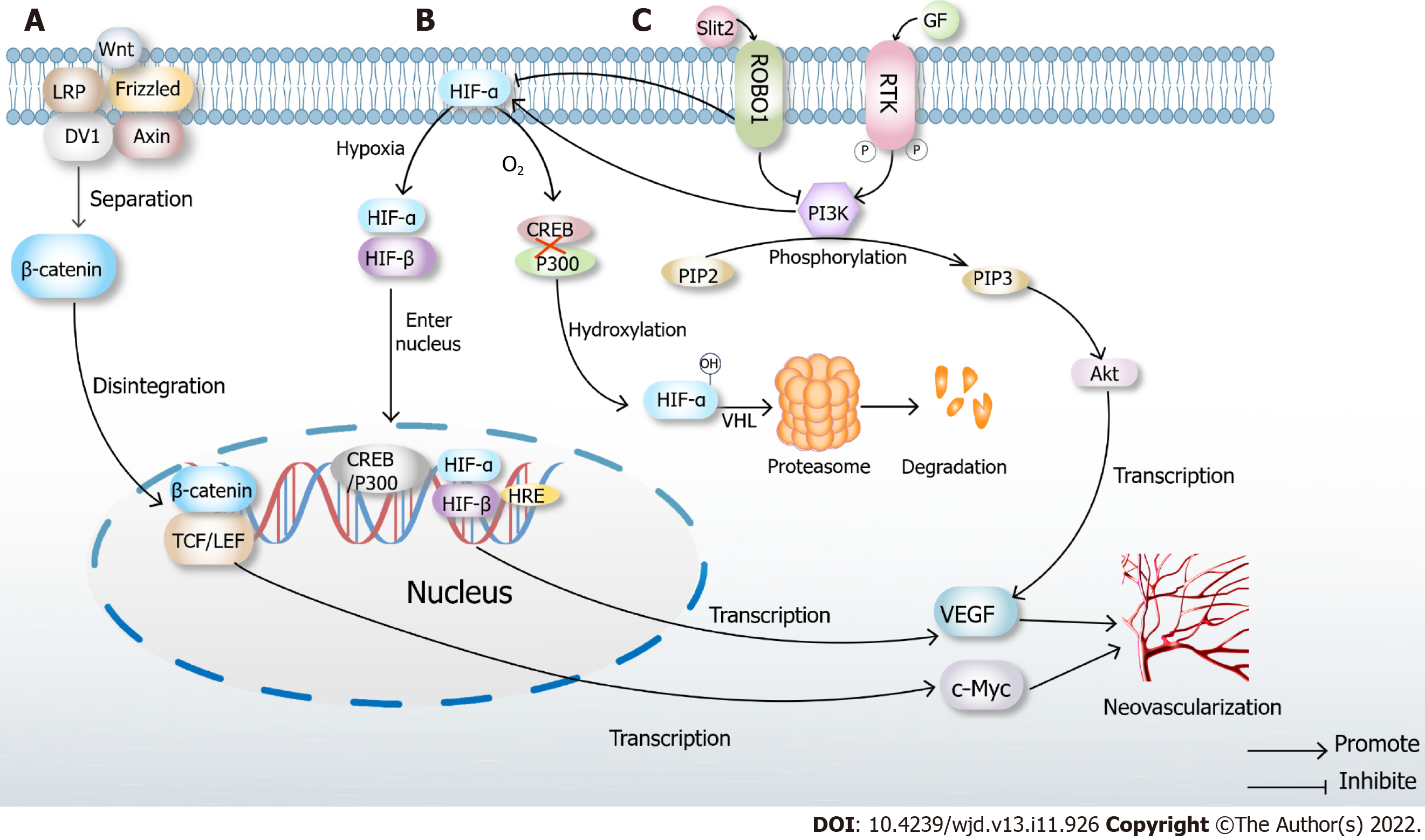
Slit2/ Roundabout 1/PI3K/Akt/VEGF signaling pathways
Endothelial cell-derived Slit2 plays a proangiogenic role in EC migration and lumen formation through its Roundabout 1 (Robo1) receptor. High glucose levels directly induce Slit2 production or Slit2/Robo1 binding. Robo1 inhibits the activation of the PI3K/Akt pathways and HIF-1α/VEGF signaling pathways and inhibits angiogenesis. PI3K inhibitors also inhibit the HIF-1α/VEGF signaling pathway. Hence, Robo1 may be a potential therapeutic target in diabetic ischemic complications with abnormal angiogenesis, such as diabetic nephropathy and diabetic retinal disease[76]. The interference of this signaling pathway can inhibit angiogenesis (Figure 2).
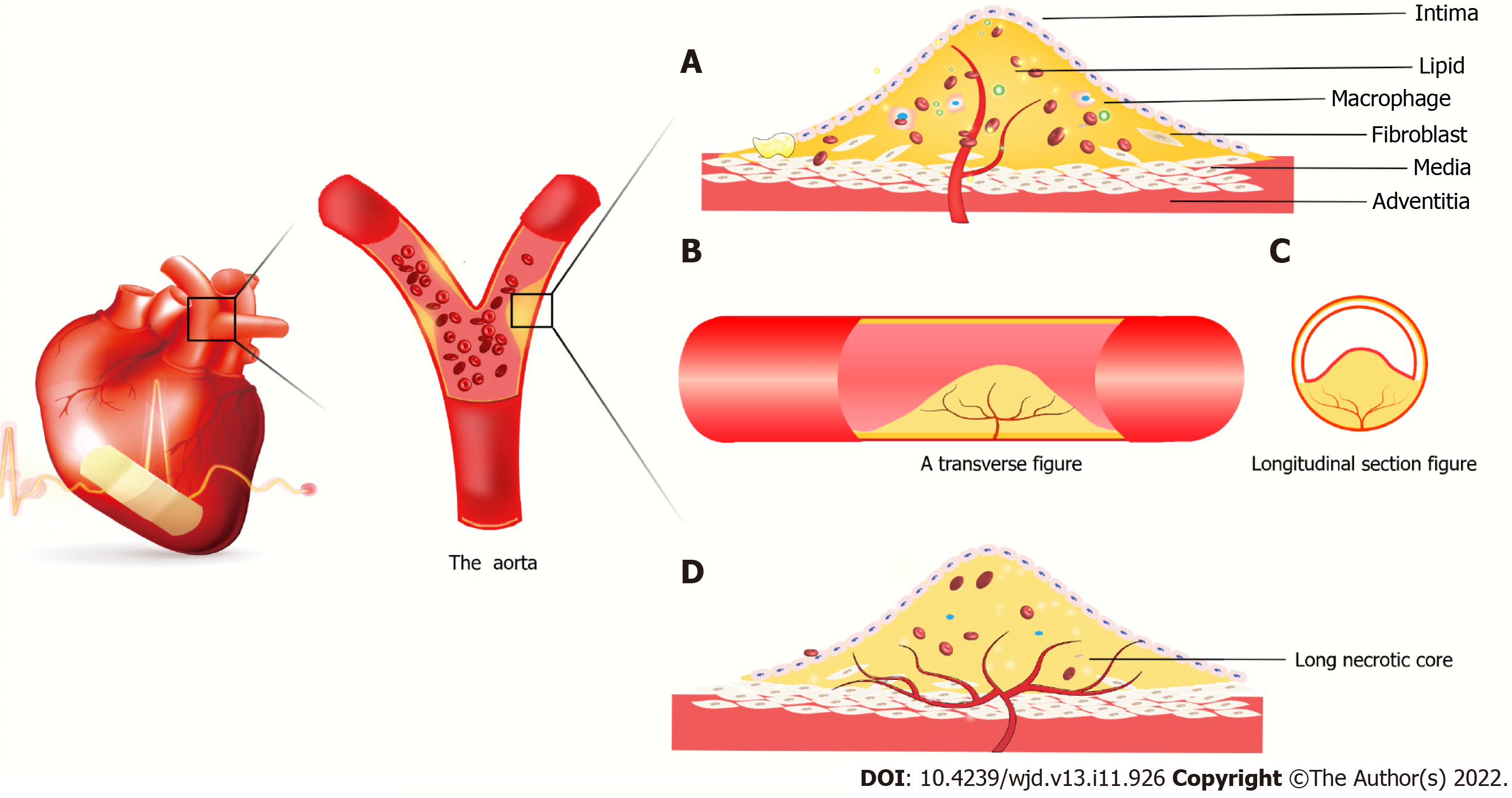
Atherosclerotic plaques lead to local hypoxia and trigger angiogenesis of the outer membrane of the vessel. The newly formed vessels are immature and leaky due to the lack of a tight connection between the pericellular cells and ECs. Lipids, inflammatory factors and red blood cells invade the plaques, leading to vessel rupture within the plaques. Proteolytic enzymes released by neovascularization promote inflammatory cell infiltration, and inflammatory factors trigger angiogenesis[77]. At the same time, intraplaque bleeding leads to a rapid increase in plaque volume, the fibrous cap changes to a contraction phenotype, and the fibrous cap becomes thinner[78], increasing the risk of plaque rupture[79]. However, Brezinski et al[80,81] put forward an opposite view that plaque rupture is caused by insufficient angiogenesis. The axial extension of neovascularization allows the healing of most plaques, while insufficient angiogenesis cannot sustain the growth of ECs in long necrotic plaques, resulting in plaque rupture and acute coronary syndrome.
Under high glucose conditions, the senescence and death of EPCs are accelerated, the PI3K/Akt/eNOS signaling pathway is inhibited, advanced glycation end products are increased, and fibrin formation is accelerated[82,83]. This triggers endoplasmic reticulum stress and oxidative stress, resulting in insulin resistance and accelerated atheromatous plaque formation. The endothelial cell differentiation is blocked, and angiogenesis is damaged (Figure 3).
Diabetic retinopathy (DR) can be divided into early nonproliferative DR and late proliferative DR in terms of progression, leading to pathological retinal angiogenesis[84]. Neovascularization extends along the surface of the retina into the vitreous cavity. Still, such vessels are fragile and easily broken, easily leading to vitreous hemorrhage, retinal detachment or macular nonperfusion and related photoreceptor dysfunction[85]. The earliest change is the thickening of the vascular basement membrane. In a high glucose environment, the basement membrane hardens and changes the elasticity of blood vessels, affecting the retinal blood flow and the dynamic balance between the inside and outside of blood vessels[86].
After the blood glucose level increases, the blood-retinal barrier degrades briefly in a few days or weeks, and then Muller cells are activated. Pericytes are lost in about 2 mo, followed by ECs, leading to vascular degeneration 6 mo after diabetes[85]. Vascular degeneration is caused by the loss of pericytes that control vascular patency, resulting in decreased vascular patency, vascular blockage, endothelial cell fusion/degeneration and finally basal membrane dissolution, vascular degeneration and formation of capillaries without ECs. However, under physiological conditions, ECs of retinal capillaries express a high level of tight connections, limiting the circulation of nutrients, soluble factors and cells into tissues[87], and vascular degeneration destroys this structural function.
Consistent with numerous pathological processes, in a hypoxic and ischemic environment, angiogenesis-related factors are promoted, retinal neovascularization occurs, and newly formed blood capillaries migrate to other capillaries and merge to form new blood capillaries[87]. However, the high glucose environment can promote the development of diabetic retinal neovascularization. Proliferative retinopathy is accompanied by tractional retinal detachment and vision loss. Therefore, the treatment aims to inhibit neovascularization. The standard treatment methods include laser therapy and anti-VEGF therapy. The intravitreal injection of VEGF inhibitors has excellent short-term safety. However, whether long-term anti-VEGF may have long-term adverse effects on the function of retinal neurons is unclear.
In the early stage of diabetic nephropathy, hypoxia induces HIF generation, and endothelial growth factor or angiopoietin maintains renal vascular density, resulting in increased abnormal angiogenesis, vascular immaturity, plasma protein leakage, increased proteinuria and significantly increased glomerular filtration rate. In the late stage, glomerular capillaries are sparse, and the production of nephrogenic erythropoietin is increased, which aggravates renal hypoxia, and glomerular cells lose vitality[88]. In this process, eNOS activity declines, the utilization rate of NO decreases, oxidative stress abates HIF activation, and VEGF expression is significantly lowered. Also, the levels of antiangiogenetic factors such as platelet response protein 1 and endothelial inhibition significantly increase, the levels of inflammatory cytokines increase, and VEGF expression is inhibited. Further, EPC function is impaired, the inflammatory response is enhanced, and the expression of adherence factors is upregulated, which is accompanied by impaired capillary ECs, endothelial barrier dysfunction and reduced angiogenesis, resulting in abnormal angiogenesis and vascular leakage. Podocytes are an important source of growth factors that regulate endothelial cell proliferation and angiogenesis. Their number increases in the early stage of diabetes and decreases in the late stage. The number of mesangial cells increases, the capillary basement membrane thickens, and the capillary number and area increase, directly or indirectly resulting in glomerular hyperplasia and mesangial expansion.
At present, no precise treatment is available for diabetic nephropathy to inhibit angiogenesis. VEGFA inhibitors can be used, but their levels need to be maintained at an appropriate level in vivo[89]. A deviation from moderate levels can cause damage.
Diabetic foot ulcer (DFU) is characterized by neuropathy caused by hyperglycemic levels, arterial stenosis caused by lipid deposition, and ischemic lesions of lower extremities. The DFU healing process can be divided into three overlapping phases: early steady-state and inflammation, arteriogenesis and matrix deposition; mid-late reshaping; and epithelial cell remodeling[90]. Neutrophil granulocyte and macrophages produce cytokines and promote cell proliferation; fibroblasts are rich in collagen fibers and induce angiogenesis and vascularization[91]. Neurological and ischemic lesions lead to impaired healing.
DFU arteriogenesis is reduced in diabetes. Hypoxia and ROS decrease transcriptional activities of HIF, VEGF, angiopoietin 2 and fibroblast growth factor and inhibit collateral development and arteriogenesis after limb ischemia[92]. Hyperglycemic levels result in impaired growth factor production and macrophage function, collagen accumulation inhibition and poor migration and proliferation of keratinocytes and fibroblasts, leading to impaired angiogenesis[91]. At the same time, wound infection reduces the active matrix metalloproteinase 8 (MMP-8) level, increases the active MMP-9 level, inhibits laminin, enhances keratinocyte migration, reduces arteriogenesis and slows wound healing[93].
For the treatment of DFU, the current strategy is to increase arteriogenesis, eliminate oxidative stress and ulcer infection[94]. The best strategy for DFU treatment is to inhibit MMP-9 without affecting MMP-8, which can reduce inflammation and increase arteriogenesis. Applying prolyl hydroxylase domain inhibitors with clinical potential can stabilize HIF and increase its activity to promote arteriogenesis. Mesenchymal stem cells produce growth hormones that drive arteriogenesis and re-epithelialization.
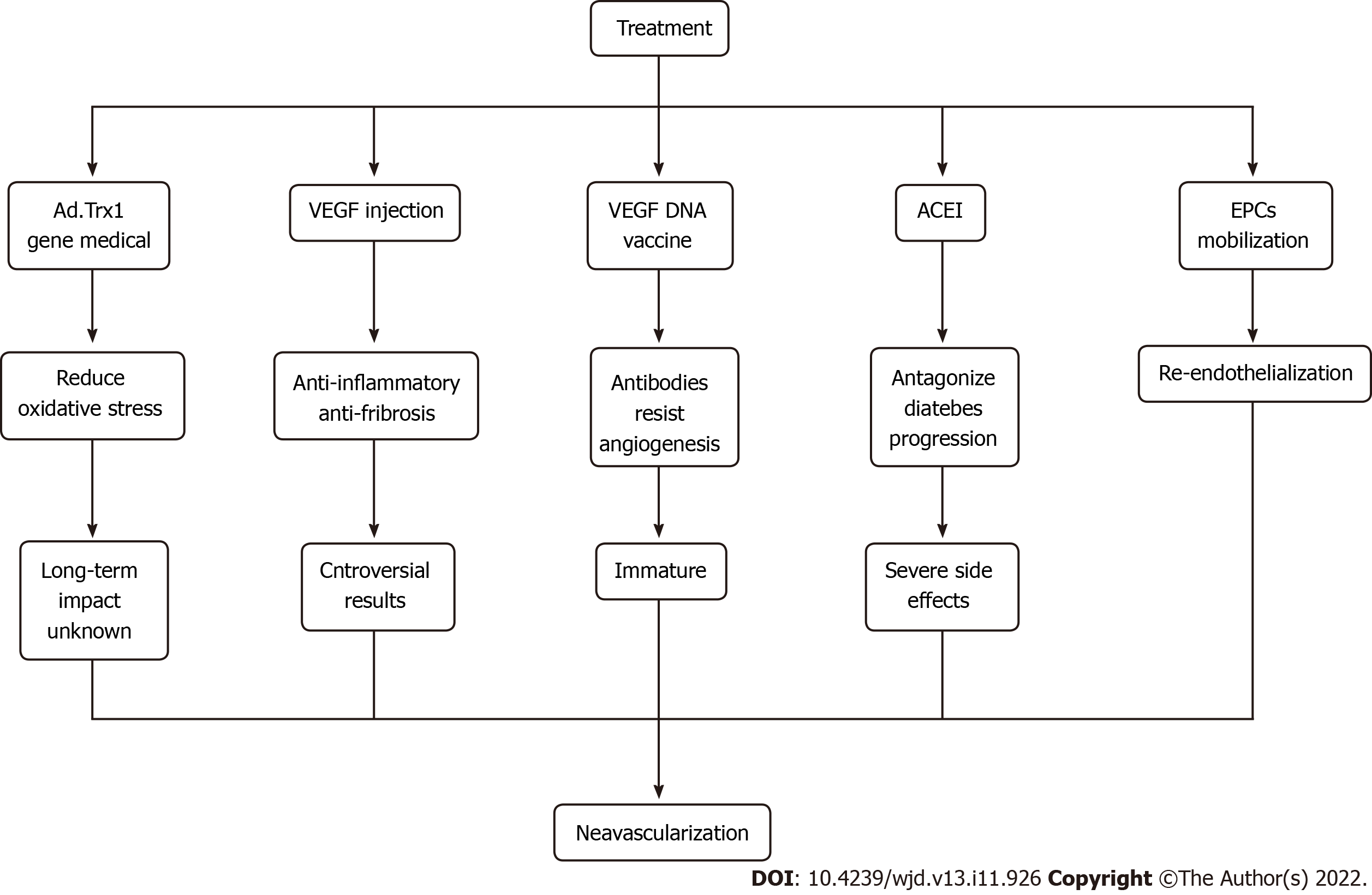
For treating diabetic ischemic neovascularization, the most crucial way is to control blood glucose levels. On this basis, various therapeutic methods, including gene therapy and vaccine research, have been proposed, which have broad prospects. For example, Ad.trx1 gene therapy can stabilize the microenvironment in the myocardium, reduce oxidative stress and cell death and induce neovascularization and maturation. This is a novel treatment that may improve disease progression and patient recovery[95]. Injection of VEGF and hepatocyte growth factor can promote neovascularization and exert anti-inflammatory and anti-fibrotic effects, but gene therapy is still controversial due to its insignificant effect and needs further study.
Antiangiogenesis antibodies can be generated by the intramuscular injection of the VEGF DNA vaccine; however, the technology is still immature, and the efficacy and long-term impact are not apparent. Hence, further clinical research is needed. Also, angiotensin-converting enzyme inhibitors can antagonize inflammation, increase the number of EPCs and improve the mobilization ability in patients with diabetes. However, this treatment causes an irritating cough, bilateral renal artery stenosis and other adverse side effects[96]. In addition, EPC mobilization promotes re-endothelialization, repairs damaged ECs, promotes angiogenesis and restores blood flow[97,98]. It is believed that with further research, more treatments can be developed to benefit mankind (Figure 4).
The advantages and disadvantages of neovascularization to the body are based on different environments. For , MI, peripheral arterial disease, coronary collateral circulation and DFU, neovascularization needs to be promoted to restore the perfusion of the ischemic area and reduce body damage. Neovascularization should be inhibited for DR and tumors to reduce the risk of retinal stripping or tumor metastasis. Although the involvement of neovascularization in disease pathogenesis is still not specific, it has huge prospects for treating ischemia in diabetes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amin A, United Arab Emirates; Hettiarachchi P, Sri Lanka; Jovandaric MZ, Serbia S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | International Diabetes Federation. IDF diabetes atlas[EB/OL].. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Zhu L, Lama S, Tu L, Dusting GJ, Wang JH, Liu GS. TAK1 signaling is a potential therapeutic target for pathological angiogenesis. Angiogenesis. 2021;24:453-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Annex BH, Cooke JP. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ Res. 2021;128:1944-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 4. | Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 5. | Dalton AC, Shlamkovitch T, Papo N, Barton WA. Constitutive Association of Tie1 and Tie2 with Endothelial Integrins is Functionally Modulated by Angiopoietin-1 and Fibronectin. PLoS One. 2016;11:e0163732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Xiong YY, Gong ZT, Tang RJ, Yang YJ. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11:1046-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 7. | Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC. Angiogenesis after acute myocardial infarction. Cardiovasc Res. 2021;117:1257-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 8. | Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Janbandhu V, Tallapragada V, Patrick R, Li Y, Abeygunawardena D, Humphreys DT, Martin EMMA, Ward AO, Contreras O, Farbehi N, Yao E, Du J, Dunwoodie SL, Bursac N, Harvey RP. Hif-1a suppresses ROS-induced proliferation of cardiac fibroblasts following myocardial infarction. Cell Stem Cell. 2022;29:281-297.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 10. | Hutchings G, Kruszyna Ł, Nawrocki MJ, Strauss E, Bryl R, Spaczyńska J, Perek B, Jemielity M, Mozdziak P, Kempisty B, Nowicki M, Krasiński Z. Molecular Mechanisms Associated with ROS-Dependent Angiogenesis in Lower Extremity Artery Disease. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Meng S, Zhou G, Gu Q, Chanda PK, Ospino F, Cooke JP. Transdifferentiation Requires iNOS Activation: Role of RING1A S-Nitrosylation. Circ Res. 2016;119:e129-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Chanda PK, Meng S, Lee J, Leung HE, Chen K, Cooke JP. Nuclear S-Nitrosylation Defines an Optimal Zone for Inducing Pluripotency. Circulation. 2019;140:1081-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Lai L, Reineke E, Hamilton DJ, Cooke JP. Glycolytic Switch Is Required for Transdifferentiation to Endothelial Lineage. Circulation. 2019;139:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Möbius-Winkler S, Uhlemann M, Adams V, Sandri M, Erbs S, Lenk K, Mangner N, Mueller U, Adam J, Grunze M, Brunner S, Hilberg T, Mende M, Linke AP, Schuler G. Coronary Collateral Growth Induced by Physical Exercise: Results of the Impact of Intensive Exercise Training on Coronary Collateral Circulation in Patients With Stable Coronary Artery Disease (EXCITE) Trial. Circulation. 2016;133:1438-48; discussion 1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Liu R, Zhao H, Wu S, Li H. Incomplete protective effect of coronary collateral circulation for acute myocardial infarction patients. Medicine (Baltimore). 2020;99:e22750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34:2674-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Chu AA, Li W, Zhu YQ, Meng XX, Liu GY. Effect of coronary collateral circulation on the prognosis of elderly patients with acute ST-segment elevation myocardial infarction treated with underwent primary percutaneous coronary intervention. Medicine (Baltimore). 2019;98:e16502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Nathoe HM, Koerselman J, Buskens E, van Dijk D, Stella PR, Plokker TH, Doevendans PA, Grobbee DE, de Jaegere PP; Octopus Study Group. Determinants and prognostic significance of collaterals in patients undergoing coronary revascularization. Am J Cardiol. 2006;98:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Fei Y, Hou J, Xuan W, Zhang C, Meng X. The relationship of plasma miR-503 and coronary collateral circulation in patients with coronary artery disease. Life Sci. 2018;207:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Elias J, Hoebers LPC, van Dongen IM, Claessen BEPM, Henriques JPS. Impact of Collateral Circulation on Survival in ST-Segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention With a Concomitant Chronic Total Occlusion. JACC Cardiovasc Interv. 2017;10:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Lucero García Rojas EY, Villanueva C, Bond RA. Hypoxia Inducible Factors as Central Players in the Pathogenesis and Pathophysiology of Cardiovascular Diseases. Front Cardiovasc Med. 2021;8:709509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Ullah K, Wu R. Hypoxia-Inducible Factor Regulates Endothelial Metabolism in Cardiovascular Disease. Front Physiol. 2021;12:670653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Pan Z, Ma G, Kong L, Du G. Hypoxia-inducible factor-1: Regulatory mechanisms and drug development in stroke. Pharmacol Res. 2021;170:105742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Matuszewska K, Pereira M, Petrik D, Lawler J, Petrik J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Karger A, Nandigama R, Stenzinger A, Grimminger F, Pullamsetti SS, Seeger W, Savai R. Hidden Treasures: Macrophage Long Non-Coding RNAs in Lung Cancer Progression. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Zheng K, Niu W, Lei B, Boccaccini AR. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021;133:168-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 27. | Alvarez-Argote S, O'Meara CC. The Evolving Roles of Cardiac Macrophages in Homeostasis, Regeneration, and Repair. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Martin P, Gurevich DB. Macrophage regulation of angiogenesis in health and disease. Semin Cell Dev Biol. 2021;119:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 2021;21:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 309] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 30. | Hong H, Tian XY. The Role of Macrophages in Vascular Repair and Regeneration after Ischemic Injury. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 31. | Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 32. | Zhou Y, Zhu X, Cui H, Shi J, Yuan G, Shi S, Hu Y. The Role of the VEGF Family in Coronary Heart Disease. Front Cardiovasc Med. 2021;8:738325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 33. | Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 1781] [Article Influence: 296.8] [Reference Citation Analysis (0)] |
| 34. | Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59:455-467. [PubMed] |
| 35. | Razavi ZS, Asgarpour K, Mahjoubin-Tehran M, Rasouli S, Khan H, Shahrzad MK, Hamblin MR, Mirzaei H. Angiogenesis-related non-coding RNAs and gastrointestinal cancer. Mol Ther Oncolytics. 2021;21:220-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Ghafouri-Fard S, Shoorei H, Mohaqiq M, Taheri M. Non-coding RNAs regulate angiogenic processes. Vascul Pharmacol. 2020;133-134:106778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Shimamura M, Nakagami H, Sanada F, Morishita R. Progress of Gene Therapy in Cardiovascular Disease. Hypertension. 2020;76:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Xie S, Wang Y, Huang Y, Yang B. Mechanisms of the antiangiogenic effects of aspirin in cancer. Eur J Pharmacol. 2021;898:173989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Giannarelli C, Alique M, Rodriguez DT, Yang DK, Jeong D, Calcagno C, Hutter R, Millon A, Kovacic JC, Weber T, Faries PL, Soff GA, Fayad ZA, Hajjar RJ, Fuster V, Badimon JJ. Alternatively spliced tissue factor promotes plaque angiogenesis through the activation of hypoxia-inducible factor-1α and vascular endothelial growth factor signaling. Circulation. 2014;130:1274-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 3112] [Article Influence: 389.0] [Reference Citation Analysis (0)] |
| 41. | Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT Signaling in Cardiac and Vascular Disease. Pharmacol Rev. 2018;70:68-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 42. | Dou M, Chen Y, Hu J, Ma D, Xing Y. Recent Advancements in CD47 Signal Transduction Pathways Involved in Vascular Diseases. Biomed Res Int. 2020;2020:4749135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Bazzazi H, Zhang Y, Jafarnejad M, Isenberg JS, Annex BH, Popel AS. Computer Simulation of TSP1 Inhibition of VEGF-Akt-eNOS: An Angiogenesis Triple Threat. Front Physiol. 2018;9:644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Xu Y, Yan Y, Geng T, Wang C, Xu Y, Yang P, Yan J. CD137-CD137L Signaling Affects Angiogenesis by Mediating Phenotypic Conversion of Macrophages. J Cardiovasc Pharmacol. 2020;75:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Zhang W, Zhang X, Huang S, Chen J, Ding P, Wang Q, Li L, Lv X, Zhang P, Zhou D, Wen W, Wang Y, Lei QY, Wu J, Hu W. FOXM1D potentiates PKM2-mediated tumor glycolysis and angiogenesis. Mol Oncol. 2021;15:1466-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 46. | De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1141] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 47. | Russo S, Kwiatkowski M, Govorukhina N, Bischoff R, Melgert BN. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front Immunol. 2021;12:746151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 48. | Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci U S A. 2020;117:30628-30638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 49. | Yuan C, Wu C, Xue R, Jin C, Zheng C. Suppression of human colon tumor by EERAC through regulating Notch/DLL4/Hes pathway inhibiting angiogenesis in vivo. J Cancer. 2021;12:5914-5922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 50. | Dichtl S, Lindenthal L, Zeitler L, Behnke K, Schlösser D, Strobl B, Scheller J, El Kasmi KC, Murray PJ. Lactate and IL6 define separable paths of inflammatory metabolic adaptation. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 51. | Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, Geng Z, Johnson C, Young B, Henke C, Gourley GR, Zhang J. Lactate Promotes Synthetic Phenotype in Vascular Smooth Muscle Cells. Circ Res. 2017;121:1251-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 52. | Alvarez R, Mandal D, Chittiboina P. Canonical and Non-Canonical Roles of PFKFB3 in Brain Tumors. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 53. | Al-Shamsi M, Amin A, Adeghate E. Effect of vitamin C on liver and kidney functions in normal and diabetic rats. Ann N Y Acad Sci. 2006;1084:371-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Al Shamsi MS, Amin A, Adeghate E. Beneficial effect of vitamin E on the metabolic parameters of diabetic rats. Mol Cell Biochem. 2004;261:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Amin A, Lotfy M, Mahmoud-Ghoneim D, Adeghate E, Mohamed A. Al-Akhras, Al-Saadi M, Al-Rahmoun S, Hameed R. Pancreas-protective effects of chlorella in STZ-induced diabetic animal model: insights into the mechanism. J Diabetes Mellitus. 2011;1:36-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Hamza AA, Mohamed MG, Lashin FM, Amin A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. JoBAZ. 2020;81:43. [DOI] [Full Text] |
| 57. | Nelson DR, Hrout AA, Alzahmi AS, Chaiboonchoe A, Amin A, Salehi-Ashtiani K. Molecular Mechanisms behind Safranal's Toxicity to HepG2 Cells from Dual Omics. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 58. | Abdalla Y, Abdalla A, Hamza AA, Amin A. Safranal Prevents Liver Cancer Through Inhibiting Oxidative Stress and Alleviating Inflammation. Front Pharmacol. 2021;12:777500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 59. | Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn Herbal Preparation from Crataegus oxyacantha Attenuates In Vivo Carbon Tetrachloride -Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 60. | Al-Shamsi M, Amin A, Adeghate E. Vitamin E ameliorates some biochemical parameters in normal and diabetic rats. Ann N Y Acad Sci. 2006;1084:411-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Benassi E, Fan HY, Sun QL, Dukenbayev K, Wang Q, Shaimoldina A, Tassanbiyeva A, Nurtay L, Nurkesh A, Kutzhanova A, Mu CL, Dautov A, Razbekova M, Kabylda A, Yang Q, Li ZY, Amin A, Li XG, Xie YQ. Generation of particle assemblies mimicking enzymatic activity by processing of herbal food: the case of rhizoma polygonati and other natural ingredients in traditional Chinese medicine. Nanoscale Adv. 2021;3:2222-2235. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 62. | Hamza AA, Fikry EM, Abdallah W, Amin A. Mechanistic insights into the augmented effect of bone marrow mesenchymal stem cells and thiazolidinediones in streptozotocin-nicotinamide induced diabetic rats. Sci Rep. 2018;8:9827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Hamza AA, Hassanin SO, Hamza S, Abdalla A, Amin A. Polyphenolic-enriched olive leaf extract attenuated doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress and inflammation. JoBAZ. 2021;82:54. [DOI] [Full Text] |
| 64. | Abdel-Latif R, Heeba GH, Hassanin SO, Waz S, Amin A. TLRs-JNK/ NF-κB Pathway Underlies the Protective Effect of the Sulfide Salt Against Liver Toxicity. Front Pharmacol. 2022;13:850066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 65. | El-Dakhly SM, Salama AAA, Hassanin SOM, Yassen NN, Hamza AA, Amin A. Aescin and diosmin each alone or in low dose- combination ameliorate liver damage induced by carbon tetrachloride in rats. BMC Res Notes. 2020;13:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 66. | Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 487] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 67. | Amin A, Hamza AA, Daoud S, Hamza W. Spirulina protects against cadmium-induced hepatotoxicity in rats. Am J Pharmacol Toxicol. 2006;1:21-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Nurtay L, Sun QL, Mu CL, Cao ZS, Wang Q, Liang ZS, Ma CP, Li XG, Amin A, Xie YQ. Rhizoma polygonati from Mount Tai: nutritional value and usefulness as a traditional Chinese medicine, source of herbzyme, and potential remediating agent for COVID-19 and chronic and hidden hunger. Acupuncture Herb Med. 2021;1:31-38. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 497] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 70. | Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 573] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 71. | Chopra H, Hung MK, Kwong DL, Zhang CF, Pow EHN. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018;2018:9847015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 72. | Lopes-Coelho F, Silva F, Gouveia-Fernandes S, Martins C, Lopes N, Domingues G, Brito C, Almeida AM, Pereira SA, Serpa J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res. 2010;106:854-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 74. | Kaushik K, Das A. Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 2019;21:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 75. | Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, Yan C, Xie X, Lin Z, Panayi AC, Mi B, Liu G. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 76. | Liu J, Hou W, Guan T, Tang L, Zhu X, Li Y, Hou S, Zhang J, Chen H, Huang Y. Slit2/Robo1 signaling is involved in angiogenesis of glomerular endothelial cells exposed to a diabetic-like environment. Angiogenesis. 2018;21:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front Immunol. 2018;9:706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 78. | Ma Q, Reiter RJ, Chen Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis. 2020;23:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 79. | Parma L, Baganha F, Quax PHA, de Vries MR. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur J Pharmacol. 2017;816:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 80. | Brezinski ME. Comparing the Risk Factors of Plaque Rupture and Failed Plaque Healing in Acute Coronary Syndrome. JAMA Cardiol. 2019;4:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Brezinski M, Willard F, Rupnick M. Inadequate Intimal Angiogenesis as a Source of Coronary Plaque Instability: Implications for Healing. Circulation. 2019;140:1857-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Wang Y, Jiang C, Shang Z, Qiu G, Yuan G, Xu K, Hou Q, He Y, Liu Y. AGEs/RAGE Promote Osteogenic Differentiation in Rat Bone Marrow-Derived Endothelial Progenitor Cells via MAPK Signaling. J Diabetes Res. 2022;2022:4067812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Gryszczyńska B, Budzyń M, Begier-Krasińska B, Osińska A, Boruczkowski M, Kaczmarek M, Bukowska A, Iskra M, Kasprzak MP. Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Ji L, Tian H, Webster KA, Li W. Neurovascular regulation in diabetic retinopathy and emerging therapies. Cell Mol Life Sci. 2021;78:5977-5985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 85. | Hammes HP. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 243] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 86. | Roy S, Kim D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2021;82:100903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 87. | Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021;24:213-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 88. | Li T, Shen K, Li J, Leung SWS, Zhu T, Shi Y. Glomerular Endothelial Cells Are the Coordinator in the Development of Diabetic Nephropathy. Front Med (Lausanne). 2021;8:655639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 89. | Zhang A, Fang H, Chen J, He L, Chen Y. Role of VEGF-A and LRG1 in Abnormal Angiogenesis Associated With Diabetic Nephropathy. Front Physiol. 2020;11:1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: Is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2016;42:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 91. | Gorecka J, Kostiuk V, Fereydooni A, Gonzalez L, Luo J, Dash B, Isaji T, Ono S, Liu S, Lee SR, Xu J, Liu J, Taniguchi R, Yastula B, Hsia HC, Qyang Y, Dardik A. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther. 2019;10:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 92. | Catrina SB, Zheng X. Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab Res Rev. 2016;32 Suppl 1:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 93. | Chang M, Nguyen TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54:1080-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 363] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 94. | An T, Chen Y, Tu Y, Lin P. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in the Treatment of Diabetic Foot Ulcers: Application and Challenges. Stem Cell Rev Rep. 2021;17:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 95. | Samuel SM, Thirunavukkarasu M, Penumathsa SV, Koneru S, Zhan L, Maulik G, Sudhakaran PR, Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 96. | Yang JX, Pan YY, Wang XX, Qiu YG, Mao W. Endothelial progenitor cells in age-related vascular remodeling. Cell Transplant. 2018;27:786-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 97. | Kshitiz, Ellison DD, Suhail Y, Afzal J, Woo L, Kilic O, Spees J, Levchenko A. Dynamic secretome of bone marrow-derived stromal cells reveals a cardioprotective biochemical cocktail. Proc Natl Acad Sci US A. 2019;116:14374-14383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |