Published online Jan 15, 2022. doi: 10.4239/wjd.v13.i1.5
Peer-review started: April 20, 2021
First decision: June 5, 2021
Revised: June 30, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 15, 2022
Processing time: 265 Days and 21.6 Hours
Polycystic ovary syndrome (PCOS) often coexists with a wide spectrum of dysglycemic conditions, ranging from impaired glucose tolerance to type 2 diabetes mellitus (T2D), which occur to a greater extent compared to healthy body mass index-matched women. This concurrence of disorders is mainly attributed to common pathogenetic pathways linking the two entities, such as insulin resistance. However, due to methodological flaws in the available studies and the multifaceted nature of the syndrome, there has been substantial controversy as to the exact association between T2D and PCOS which has not yet been elucidated. The aim of this review is to present the best available evidence regarding the epidemiology of dysglycemia in PCOS, the unique pathophysiological mechanisms underlying the progression of dysglycemia, the most appropriate methods for assessing glycemic status and the risk factors for T2D development in this population, as well as T2D risk after transition to menopause. Proposals for application of a holistic approach to enable optimal management of T2D risk in PCOS are also provided. Specifically, adoption of a healthy lifestyle with adherence to improved dietary patterns, such the Mediterranean diet, avoidance of consumption of endocrine-disrupting foods and beverages, regular exercise, and the effect of certain medications, such as metformin and glucagon-like peptide 1 receptor agonists, are discussed. Furthermore, the maintenance of a healthy weight is highlighted as a key factor in achievement of a significant reduction of T2D risk in women with PCOS.
Core Tip: Polycystic ovary syndrome (PCOS) often coexists with a wide spectrum of dysglycemic conditions, ranging from impaired glucose tolerance to type 2 diabetes mellitus (T2D), which occur to a greater extent compared to healthy body mass index-matched women. This review provides the most current knowledge on the different aspects of T2D in women with PCOS, including epidemiology, common pathophysiologic mechanisms, and methodology employed for dysglycemia assessment, as well as to scrutinize the risk factors for T2D development and to suggests the optimal management of these women in the context of T2D risk reduction.
- Citation: Livadas S, Anagnostis P, Bosdou JK, Bantouna D, Paparodis R. Polycystic ovary syndrome and type 2 diabetes mellitus: A state-of-the-art review. World J Diabetes 2022; 13(1): 5-26
- URL: https://www.wjgnet.com/1948-9358/full/v13/i1/5.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i1.5
Polycystic ovary syndrome (PCOS) constitutes the most common endocrine disorder in women of reproductive age, affecting 6%-15% of the of the global population[1]. PCOS is a multifaceted, ever-changing disease and a challenging disorder for the caring physician due to the continuous need for treatment modifications and adjustments based on the patient’s fluctuating needs and preferences throughout the course of her lifetime. Apart from oligo- or amenorrhea and/or clinical or biochemical hyperandrogenism, impaired glucose homeostasis has also been observed in patients with PCOS[1,2]. In particular, evidence from large prospective cohorts has shown progression to either prediabetes or type 2 diabetes mellitus (T2D) over time[3,4]. The emergence of T2D in PCOS can be anticipated to some extent given that the two prerequisites for T2D development, insulin resistance (IR) and ß-cell dysfunction, are frequently present in women with PCOS. Indeed, IR, which is a key player in underlying PCOS pathophysiology, has been documented in the vast majority of women suffering from the syndrome in comparison to their healthy body mass index (BMI)-matched peers. An additive effect of obesity on the degree of IR reported in these women should also be taken into account[5]. Meanwhile, the prevalence of pancreatic ß-cell dysfunction is much higher in these patients compared to their regularly ovulating, non-hyperandrogenic peers[6].
Nevertheless, there is an ongoing debate as to whether PCOS itself constitutes a risk factor for T2D or whether T2D predominantly occurs in the context of obesity in affected patients[7-9]. A recent meta-analysis of genetic studies suggests that there is no inherent T2D risk in PCOS and that T2D instead occurs as a result of either increased adiposity or hyperandrogenemia[10]. On the other hand, PCOS constitutes a polygenic trait and elegant studies have shown that clusters of genes leading to metabolic disturbances are different from those associated with overt hyperandrogenic signs in PCOS women[11]. Therefore, a genetic component of dysglycemia among PCOS women should be considered.
The presence of altered glycemic status, although a universal finding, is challenging to the clinician for several reasons. One is that the reported incidence of dysglycemia, which includes impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and T2D, varies among studies. Furthermore, agreement over a definitive recommendation regarding the optimal method for assessment of glycemic status has not been reached to date.
The aim of this narrative review was to provide the most current knowledge on the different aspects of T2D in women with PCOS, including epidemiology, common pathophysiologic mechanisms, and methodology employed for dysglycemia assessment, as well as to scrutinize the risk factors for T2D development and to suggest the optimal management of these women in the context of T2D risk reduction.
In general, the prevalence of dysglycemia is significantly higher in women with PCOS compared to their healthy BMI-matched peers. With regard to T2D, in normal women of reproductive age, the mean prevalence of T2D is 1%-3%[12], whereas in PCOS, its prevalence ranges from 1.5 to 12.4%, with a median value of 4.5%. This wide range partly depends on the age of the studied subjects, with the higher incidence (12.4%) recorded in a study evaluating mostly perimenopausal women with PCOS and a mean age of 46 years[13]. In the remaining studies, the mean age of the studied population ranged from 25 to 30 years. Another factor closely associated with T2D prevalence is ethnic variation, since a prevalence of 6.3% and 10.1% has been reported in two studies from Asia[14,15], reflecting the rising prevalence of T2D in Asia[16], a trend that has recently been corroborated in a large meta-analysis[17]. Finally, one more factor pertains to the criteria applied for PCOS diagnosis. For example, a higher degree of dysglycemia is anticipated in women diagnosed with the 1991 National Institutes of Health (NIH) criteria in comparison with the mild phenotype D of those diagnosed with the 2003 Rotterdam criteria, this due to the lower degree of IR observed in the latter group[18]. On the other hand, this logical assumption was not confirmed by a recent study evaluating more than 2000 women, which showed that T2D prevalence was similar among patients with different PCOS phenotypes[19].
Conflicting data exist regarding the prevalence of intermediate hyperglycemia, namely, IGT and IFG. The prevalence of IGT in PCOS ranges from 4%-35.4%, with an average of 16.6%; in contrast, the corresponding prevalence in the healthy peers of women with PCOS ranges from 4%-8%[12]. The reasons for this very high heterogeneity have not been fully elucidated; however, ethnic susceptibility, the various criteria applied for PCOS diagnosis, as well as age and BMI distribution in the different studied groups could partly explain this diversity. Likewise, IFG prevalence as reported in the literature ranges from 2%-21%, the average being 10.8%, higher than that of the non-PCOS population, in which it is approximately 5.9% (range 4%-8.7%)[20]. In addition to the reasons provided above, the diagnostic criteria employed to diagnose IFG also play an important role, with IFG cut-offs differing significantly between the American Diabetes Association (ADA) and the World Health Organization (WHO) clinical practice guidelines. Therefore, it is not surprising that the prevalence of IFG was higher in studies using the stricter ADA criteria[21] than in those using the corresponding WHO cut-off values[22]. The prevalence of dysglycemia as reported in different studies according to the country where the study was performed, the subjects’ age and BMI, and the diagnostic criteria to confirm the diagnosis of either PCOS or glycemia are presented in Table 1.
| Ref. | Sample size | Country | PCOS criteria | T2D criteria | Age (yr) | BMI (kg/m2)
| IFG (%) | IGT (%) | T2D (%) |
| Rajkhowa et al[142], 1996 | 90 | UK | NIH | WHO | 26 (15-39) | 31.6 (18-48) | ? | 9 | 2 |
| Legro et al[61], 1999 | 254 | USA | NIH | WHO | 14-44 | 32 ± 3 | ? | 31 | 7.5 |
| Ehrmann et al[62], 1999 | 122 | USA | NIH | ADA | 25 ± 0.7 (13-40) | 30-43 | 9 | 35 | 10 |
| Gambineri et al[3], 2004 | 121 | Italy | Rotterdam | WHO | 14-37 | 20-38 | ? | 15.7 | 2.5 |
| Legro et al[143], 2005 | 71 | USA | NIH | ADA | 30 ± 6 | 29 ± 6.4 | ? | 25 | 10 |
| Chen et al[144], 2006 | 102 | China | Rotterdam | WHO | 24.2 ± 6 | 21.7 ± 4 | ? | 20.5 | 1.9 |
| Mohlig et al[64], 2006 | 264 | Germany | NIH | WHO | 28 ± 0.4 | 30 ± 0.4 | ? | 14.3 | 1.5 |
| Vrbikova et al[145], 2007 | 244 | Czech Republic | Rotterdam | ADA | 27 ± 7.5 | 27 ± 6.9 | 12.3 | 9.4 | 1.6 |
| Gagnon et al[146], 2007 | 105 | Canada | NIH | ADA | 28.3 (14-47) | 35.5 (19-54) | ? | 23 | 5 |
| Dabadghao et al[63], 2007 | 372 | Australia | Rotterdam | ADA | 30 ± 5 (15-62) | 35 ± 8 | 3 | 15.6 | 4 |
| Espinos-Gomez et al[147], 2008 | 102 | Spain | NIH | WHO | 26 ± 6 | 30.2 ± 8 | ? | 10.7 | 7.7 |
| Cheung et al[148], 2008 | 295 | China | Rotterdam | ADA | 30 ± 6 | 25 ± 5.9 | 9.2 | 10.5 | 7.5 |
| Bhattacharya et al[149], 2009 | 264 | India | Rotterdam | WHO | 24 ± 4 | 27 ± 4.5 | ? | 14.4 | |
| Seneviratne et al[15], 2009 | 168 | Sri Lanka | Rotterdam | WHO | 29 ± 4 (20–40) | 25.92 (16-39) | ? | 23.2 | 10.1 |
| Lee et al[50], 2009 | 194 | Korea | Rotterdam | ADA | 27 ± 5 | 24 ± 4 | 17 | 1 | |
| Wei et al[51], 2009 | 356 | China | Rotterdam | WHO | 32 ± 4 (19-44) | 22 ± 4.2 | ? | 7.6 | 3,1 |
| Zhao et al[150], 2010 | 818 | China | Rotterdam | ADA | 25 ± 5 | ? | 8.5 | 35.4 | 4 |
| Stovall et al[151], 2011 | 78 | USA | NIH | ADA | 26 ± 6.4 | 29 ± 6 (18-43) | 2 | 14 | ? |
| Celik et al[66], 2013 | 252 | Turkey | Rotterdam | ADA | 24 ± 5 | 26 ± 5.7 | ? | 14.3 | 2 |
| Veltman-Verhulst et al[21], 2013 | 226 | Netherlands | Rotterdam | ADA | 29.6 ± 4 | 27 ± 6.7 | 21 | 4 | 3.5 |
| Lerchbaum et al[152], 2014 | 714 | Austria | Rotterdam | ADA | 27 (23-32) | 24.2 (21-30) | 12.8 | 1.5 | |
| Vrbikova et al[145], 2014 | 330 | Czech Republic | Rotterdam | ADA | 27.8 ± 7 | 27.6 ± 6 | 12 | 8.8 | 3 |
| Amato et al[22], 2015 | 241 | Italy | Rotterdam | WHO | 24 ± 6 (14-43) | 30 ± 6 (18-50) | 11.6 | 5.4 | 1.7 |
| Ganie et al[14], 2015 | 2014 | India | Rotterdam | ADA | 23 ± 5.4 | 25 ± 4.4 | 14.5 | 5.9 | 6.3 |
| Gracelyn et al[153], 2015 | 200 | India | Rotterdam | ADA | 16-40 | ? | ? | 14.5 | 1.5 |
| Li et al[154], 2016 | 2436 | China | Rotterdam | ADA | 27 | 21.56 | 13.5 | 19.8 | 3.9 |
| Ollila et al[127], 2017 | 265 | Finland | Rotterdam | WHO | 46 | 28.6 ± 6 | ? | ? | 12.4 |
| Pelanis et al[13], 2017 | 876 | Sweden | Rotterdam | ADA | 29 (25-34) | 28 (23-33) | 11 | 12 | 3 |
| Zhang et al[19], 2018 | 378 | China | Rotterdam | IDF | 27 ± 4.4 | 30 ± 4.3 | 31.5 | 8.7 | |
| Ortiz-Flores et al[155], 2019 | 400 | Spain | Rotterdam | WHO | 26 (14-49) | 28.6 (22-34) | 14 | 14.5 | 2.5 |
PCOS pathophysiology is characterized by a combination of androgen excess and ovulatory dysfunction. Although numerous studies have endeavored to identify the underlying pathogenetic mechanisms, this particular ‘Holy Grail’ of endocrinology has not as yet been uncovered. Irrespective of the theoretical perspective, it is widely accepted that the unusually variable phenotype in affected patients is produced by the combined effects of two separate, yet deeply intertwined, mechanisms, which are androgen over-activity (elevated androgen concentrations or hyperandrogenism) and IR[23].
IR represents a state of disrupted insulin binding to its receptor or ineffective activation of the latter by insulin, thereby forcing the pancreatic β-cells to release large amounts of insulin into the circulation in order to maintain euglycemia[24]. Such a state of chronic pancreatic stress leads to impaired glucose homeostasis, initially manifesting as IFG or IGT; however, once large numbers of islet β-cells have succumbed to stress, it leads to T2D. IR and glucose homeostasis abnormalities have been described in up to 70% of women with PCOS[25]. As early as 1980, eight obese subjects with PCOS were found to have higher serum glucose and insulin concentrations, in both a fasting state and after stimulation by an oral glucose load, compared with six obese unaffected women, despite the latter being statistically significantly more obese[26]. Even though obesity is a key risk factor for IR and T2D development in the general population, women with PCOS have higher insulin concentrations in response to an oral glucose load as compared to unaffected subjects, even in the absence of obesity[27,28]. The only clinical sign of IR is acanthosis nigricans, which correlates well with IR in either obese or lean affected individuals[29].
Of course, women with PCOS are equally exposed to the well-established association of obesity and higher degree of IR as those without PCOS. Indeed, a study comparing 198 obese and 201 non-obese women with PCOS (obesity definition: BMI > 27 Kg/m2) found that obesity was associated with lower insulin sensitivity when a variety of oral glucose tolerance test (OGTT)-derived indices was used[30]. In contrast to the latter findings, a study employing the impractical gold standard method to assess IR, namely, the hyperinsulinemic euglycemic clamp, it found more pronounced insulin secretion in lean women with PCOS compared to controls, though without significant differences in insulin sensitivity, while it confirmed the presence of higher IR compared to controls only in obese women with PCOS[31].
In addition, women with PCOS present with enhanced luteinizing hormone pulsatility, producing increased secretion of ovarian and adrenal androgens, which, along with IR, are key features of the syndrome. A meta-analysis of cross-sectional studies including 4795 women from the general population found higher testosterone concentrations in patients with T2D compared to controls[32]. In addition bioavailable testosterone correlated significantly with IR, with higher concentrations predicting the development of T2D[33]. Similar results were obtained from a systematic review and meta-analysis pooling data from the Rotterdam Study and other previously published studies, which found that subjects at the highest tertile of free androgen index had a 42% higher risk of developing T2D, in a complex multivariate analysis controlling, among others, for age, BMI, glucose, and insulin concentrations[34].
Given that PCOS is characterized by markedly higher androgen concentrations compared to those in the unaffected population, an association between the syndrome and T2D constitutes a rational hypothesis. In fact, such correlations were originally reported almost 40 years ago[35]. Ever since, multiple studies have confirmed this relationship in both lean and obese women with PCOS. A significant positive association between testosterone concentrations and IR has been described in lean women with PCOS using OGTT-derived indices[3,36] and measures of glucose disposition in hyperinsulinemic euglycemic clamps[37]. Even though PCOS is mainly characterized by hyperandrogenemia of ovarian origin, a subgroup of patients exhibits adrenal androgen hypersecretion, with the most important being dehydroepiandrosterone sulphate. In the latter subgroup, hyperandrogenemia does not seem to correlate with IR indices or metabolic abnormalities in most[38-42], but not all, studies[43,44]. It is a riddle that remains as yet unresolved, especially taking into consideration that such an association does seem to exist in other conditions of adrenal hyperandrogenism, such as premature adrenarche/pubarche[45].
Despite the similar pathophysiology of PCOS and glucose homeostasis abnor
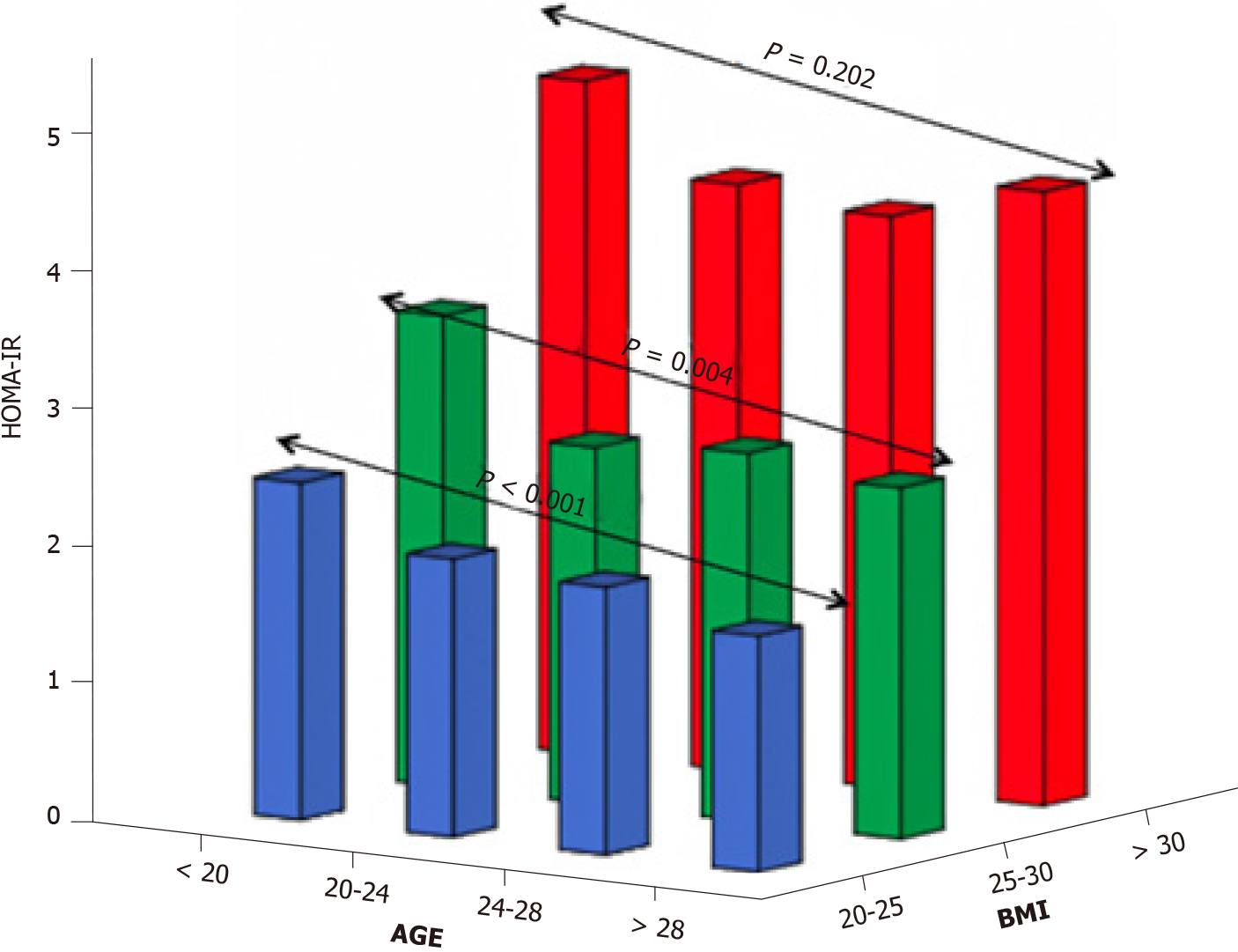
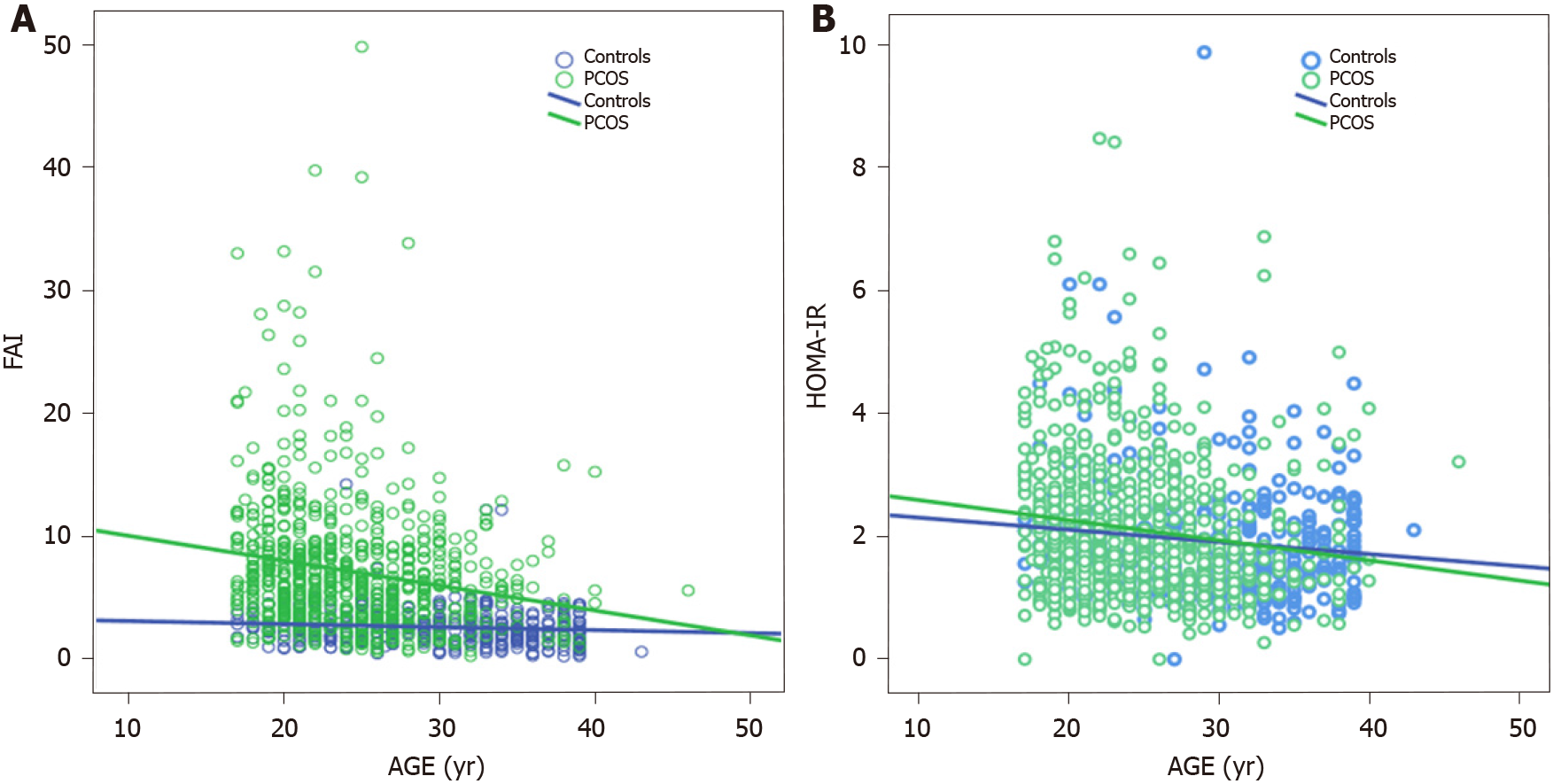
Meanwhile, the role of aging in T2D development should certainly not be underestimated. This has been demonstrated in, inter alia, a subgroup analysis of 345 Dutch women with PCOS, who were part of a large cohort study evaluating aging in women with PCOS (APOS study)[49], where the interaction of age and BMI was the most significant variable in predicting T2D in logistic regression analysis. Moreover, data assembled from several studies have also pointed to a positive association of age and BMI with T2D or intermediate hyperglycemia among women with PCOS[19,50,51]. On the other hand, a cross-sectional study conducted by our group found that aging might exert a protective effect in women with PCOS with regard to IR. In particular, obese women with PCOS demonstrated the same degree of IR through the years, although this was not the case for their lean peers in whom a gradual improvement was observed with aging (Figure 1)[52]. Furthermore, a large cross-sectional study (n = 763 normal-weight women with PCOS, according to the Rotterdam criteria; 376 controls) exhibited a parallel decrease of homeostasis insulin resistance assessment (HOMA-IR) index with free androgen index, suggesting a potential mechanism regulating this process (Figure 2)[53]. Specifically, the gradual reduction of androgen production observed in women with PCOS after their third decade of life partly explains the absence of deterioration of IR through the years which is common in the general population.
According to the Wilson and Jungner criteria, screening for a disease is essential when that condition constitutes an important health problem, an accepted treatment for patients with recognized disease exists, and facilities for diagnosis and treatment are available. Furthermore, there should be an identifiable latent or early symptomatic stage, and the natural history of the condition, including development from latent to clinical disease, must be adequately understood[54]. Furthermore, there should be an agreement upon policy as to whom to treat, taking into consideration the patients’ resources. The cost of case finding (including diagnosis) should be economically balanced in relation to possible expenditures for medical care as a whole, while case finding should be a continuing process and not a "one-off" strategy. Finally, there should be an effective screening test or examination and that test should be acceptable to the population[54]. It is obvious that PCOS covers all the prerequisites described above and, therefore, in all consensus statements by several experts, screening for T2D is recommended in women with PCOS (Table 2).
| Ref. | OGTT recommended upon diagnosis in all women with PCOS | Follow-up with OGTT |
| Joint AACE/ACE and AE-PCOS society[56] | Yes | (1) Yearly in women with IGT; and (2) Every 1–2 years based on BMI (not specified) and family history of T2D |
| Australian NHMRC[57] | Recommended if one or more criteria exist: (1) BMI > 25 kg/m2 or in Asians > 23 kg/m2; (2) History of IFG, IGT, GDM; (3) Family history of T2D; (4) Arterial hypertension; and (5) High-risk ethnicity | Every 1-3 years, based on presence of other diabetesrisk factors |
| Endocrine Society[55] | Yes | Every 3–5 years (Sooner if additional risk factors for T2D develop) |
| Royal College of Obstetricians andGynecology[59] | Recommended if one or more criteria exist: (1) BMI ≥ 25 kg/m2; (2) Age ≥ 40 years; (3) Previous GDM; and (4) Family history of T2D | Yearly in women with IGT or IFG |
| AE-PCOS Society[58] | Recommended if one or more criteria exist: (1) BMI ≥ 30 kg/m2; (2) Age ≥ 40 years; (3) Previous GDM; and (4) Family history of T2D | Every 2 years in women with risk factors (Sooner if additional risk factors for T2D develop) |
| ESHRE and ASRM[60] | Recommended if BMI ≥ 27 kg/m2 | Not specified |
However, whether glycemic status should be evaluated in every woman suffering from PCOS or in certain subgroups, as well as which is the best method for this assessment, are to date unanswered questions. With regard to which patients should be screened, there are at present two points of view. One, supported by the Endocrine Society, the Androgen Excess and Polycystic Ovary Syndrome Society, as well as by the guidelines on PCOS diagnosis and management developed in Australia, suggests universal screening in all women with PCOS[55-57]. On the other hand, a number of experts recommend screening in women with at least one risk factor, such as age >40 years, family history of either T2D or gestational diabetes mellitus, and/or obesity[58-60]. However, the latter recommendations have not been supported by solid data, since studies arguing either in favor of or against them have been published[4,61]. For example, the family history of T2D criterion has strong supportive data in studies from the USA and Australia[62,63], but not in studies originating from Italy and Germany[9,64]. Accordingly, these criteria, although reasonable, appear ultimately to be arbitrary and would not reflect the different nature of T2D development in PCOS compared to that in the unaffected population. In a similar manner, most of the studies in favor of these recommendations did not evaluate in detail the impact of age, obesity, and hyperandrogenemia on the development of dysglycemia and, thus, seem to increase controversy over this matter[4,65].
There is disagreement among experts as to whether fasting plasma glucose (FPG), OGTT, or glycated hemoglobin (HbA1c) is the best laboratory method to assess glycemic status in a patient with PCOS, despite robust data strongly pointing to OGTT as being the most accurate[66,67]. The main arguments against OGTT use are that the modality is more complex, expensive, and time-consuming than the other two screening methods. Moreover, it is characterized by high variability and its results are dependent on height[68]. However, OGTT is considered the gold standard for T2D diagnosis because it constitutes a standardized test that is easily performed and is the only method able to detect IGT, of utmost importance for women with PCOS[69]. Indeed, given that the risk of T2D development in women with IGT is considerably higher than in those with normal glucose tolerance or those with IFG[9], this at-risk population can greatly benefit from early lifestyle modification and/or pharmacological intervention[70].
In addition, the ADA and WHO are in agreement regarding the glucose concentration cut-off for the diagnosis of IGT, which is not the case for either FPG or HbA1c values. Furthermore, in several studies it has been shown that a single measurement of FPG could misclassify a substantial number of patients with either IGT or T2D, ranging from 20%-40%, as having normal glucose homeostasis[21,64,71]. This figure is certainly not negligible given that women with PCOS are at risk for T2D, even from their early reproductive years, compared to their healthy peers. Other benefits of an OGTT are that it can be applied in patients with iron deficiency, a condition commonly encountered in women of reproductive age, while the parallel measurement of insulin concentrations after a glycemic load provides the clinician with an accurate estimate of the degree of existing IR[72].
After the ADA’s recommendation for a single HbA1c measurement as an accurate index for T2D diagnosis, its use has been advocated by several research groups and international guidelines (Table 2). HbA1c cannot, however, be used for the diagnosis of dysglycemia in women suffering from PCOS for a number of reasons. First, in this group of patients, periods of oligomenorrhea are followed by periods of heavy bleeding or sychnomenorrhea and this menstrual pattern could result in major changes in hematocrit and/or ferritin levels. Since HbA1c is dependent on these parameters, iron depletion and loading might lead to significant variations in HbA1c concentrations over time, independently of the patient’s glycemic status[73]. Moreover, the specificity of HbA1c in the diagnosis of dysglycemia has been questioned in overweight and obese subjects[74], who constitute the largest group in the PCOS population. Additionally, the cut-off point for HbA1c is mainly based on the established association between HbA1c and microvascular disease in patients with established T2D. However, women with PCOS are younger and healthier overall when initially diagnosed with the syndrome, while dysglycemia does not always lead to T2D in this population compared to those evaluated in the original study of HbA1C validation[75], further calling HbA1c application into question.
Besides this, HbA1c is a costly procedure, harmonization of HbA1c assays around the globe has not yet been carried out effectively, significant variation across ethnicities has been described, and international standardization is not as yet complete[76]. The diagnostic performance of HbA1c as a marker of glucose intolerance is further compromised by the discordance between the diagnostic criteria for prediabetes proposed by the WHO [42 mmol/mol (6.0%)] and those by the ADA [39 mmol/mol (5.7%)], producing much confusion. Furthermore, available studies evaluating the ability of HbA1c to detect IGT and diabetes in PCOS have found that the test has low sensitivity when compared with OGTT for the assessment of glucose tolerance[66,67]. Finally, the diagnostic accuracy of HbA1c in detecting T2D has recently been questioned, with some investigators arguing strongly in favor of OGTT for this procedure[77].
There is, moreover, much discordance among experts regarding the frequency of glycemic status assessment, ranging from yearly to on a five-year basis depending on the coexistence of additional factors. All the latter recommendations are illustrated in Table 2. However, from a pathophysiological point of view, PCOS women with IFG constitute a different subgroup from those with IGT. In fact, data derived from a healthy population have shown that isolated IFG is usually observed in subjects with predominantly hepatic IR and normal muscle insulin sensitivity, whereas individuals with isolated IGT have normal to slightly reduced hepatic insulin sensitivity and moderate to severe muscle IR[78]. Accordingly, those with IFG may represent the general population of women prone to T2D development, whereas subjects with IGT may compose that group of patients in whom dysglycemia occurs as a consequence of hyperandrogenemia. Today, in fact, copious documentation of the detrimental effects of androgens on muscle insulin sensitivity in lean women with PCOS is underway[1].
The conversion rate from normal glucose homeostasis to IGT or from IGT to T2D in PCOS has been estimated to range from 2.5 to 3.6% annually over a period of 3–8 years[79-81]. These conversion rates are lower than those observed in individuals with IGT in the general population, who seem to develop T2D at rates of approximately 7% annually[82]. This discrepancy may be related to the fact that the underlying mechanisms of T2D development in PCOS are different from those found in healthy individuals. In fact, in non-PCOS women, the degree of insulin sensitivity pro
Several parameters which could possibly mediate the risk of developing T2D in women with PCOS have recently been evaluated. With regard to PCOS phenotypes, the presence of the most severe form of PCOS, consisting of the conglomeration of chronic anovulation and elevated androgen concentrations (former NIH criteria), was found to be one of the strongest independent predictors of glucose concentrations after a 75-g OGTT in 254 women with PCOS following adjustment for several confounders, including age, waist-to-hip ratio (WHR), and BMI[61]. However, this finding has not since been replicated[13].
Based on the well-known developmental origins of health and disease, an inverse association between birth weight and T2D risk seems highly likely. Indeed, low birth weight has been associated with PCOS diagnosis later in life, with a birth weight < 2.5 kg conferring a 76% higher likelihood of developing PCOS[84]. In a similar manner, age of menarche has been related to dysglycemia in PCOS. Indeed, PCOS women with IGT were observed to have significantly earlier menarche age (11.9 ± 1.6 years) compared to obese women with PCOS and normal glucose tolerance (12.4 ± 1.7 years) in a cross-sectional study of 121 Italian PCOS women. However, the number of subjects with T2D was too small for any correlation to be established[3]. Of note, a single-center cohort study demonstrated that a higher number of births decreased the risk of T2D in women with PCOS, corroborating a potential prophylactic effect of parity in T2D[4]. In the same context, the impact of lactation on T2D development may be hypothesized. In fact, obesity in women with PCOS is a risk factor for impaired lactogenesis and increases the risk for reduced breastfeeding initiation and duration[85]. Furthermore, since lactation is crucial for women’s post-gestational metabolic health[86], the presence of abnormal lactational function might enhance the risk for T2D in this population, even though substantial data to support this hypothesis are to date lacking. Finally, a positive link has been proposed between family history of T2D and T2D risk in PCOS; this hypothesized association is based on a defect in the first phase of insulin secretion in PCOS women with a first degree relative suffering from T2D, in contrast to BMI-matched PCOS patients without such a history[87].
Indisputably, the impact of environmental factors on T2D development is major. Endocrine disruptors constitute an emerging environmental threat, and a role for endocrine disrupting chemicals in exacerbating PCOS pathology has been proposed for over 20 years, with bisphenol A (BPA) being significantly associated with measures of IR and BMI in women with the syndrome[88]. Moreover, a positive feedback loop between BPA and hyperandrogenemia has been shown in PCOS and, therefore, BPA exposure has been particularly incriminated in PCOS pathophysiology[89]. Furthermore, the role of advanced glycation end-products (AGEs) in the patho
Three of the most common addictions have been incriminated in PCOS patho
Another risk factor contributing to impaired glucose homeostasis may be vitamin D deficiency. Regarding PCOS, lower 25-hydroxy-vitamin D [25(OH)D] concentrations have been reported in PCOS patients compared to those in controls, with vitamin D deficiency [25OHD < 20 ng/mL] being associated with higher fasting glucose and insulin concentrations, as well as IR, assessed by OGTT[98]. A meta-analysis combining data from 11 placebo-controlled randomized trials evaluating the effects of vitamin D supplementation on glucose homeostasis in 601 patients with PCOS (89% of Asian descent) found that daily supplementation with small doses of vitamin D was able to significantly lower HOMA-IR index (daily supplementation effect -0.30; P = 0.0018, low-dose supplementation effect −0.31; P = 0.0016)[99]. However, both studies failed to report data regarding the relationship between vitamin D deficiency and T2D.
Taking a more holistic approach, other emerging factors in T2D development are sleep quality and mood disorders. It is well-known that women with PCOS, even after controlling for obesity, tend to have a higher prevalence of sleep disturbances, such as reduced sleep efficiency, amount of time spent in rapid eye movement (REM) sleep, as well as non-REM sleep, and difficulty in falling asleep and maintaining sleep[100]. Moreover, the prevalence of obstructive sleep apnea is higher in women with PCOS compared to non-PCOS [odds ratio (OR) 3.83; 95%CI: 1.43-10.24][101] and is associated with higher fasting insulin levels, HOMA-IR index, HbA1c, and glucose area under the curve[102]. On the other hand, the latter findings warrant caution, since a high likelihood for selection bias is considered plausible[101]. Depression and mood disorders have been associated with IR, obesity, and T2D in multiple studies[103]. In addition, depression and mood disorders have been commonly diagnosed in women with PCOS[104,105]. Despite the theoretical possibility of an association between these two conditions, no study has evaluated such an association to date.
In recent years, the role of the gut microbiome in metabolic abnormalities, including PCOS, has been explored[106]. It was thus inevitable that an effort would be made to improve some of the features of the syndrome by intervening in the microbial population with probiotics and synbiotics. The latter intervention was shown to confer beneficial effects on body weight, fasting plasma glucose and insulin, reproductive hormones, and hirsutism, while longer duration of treatment also seemed to be more efficacious[107].
A major factor that may eliminate or reduce T2D risk in PCOS is prescription of appropriate drugs. Oral contraceptives have been the mainstay of treatment for irregular menses, hirsutism, and acne in women with PCOS with exceptional success rates. Some studies have, however, suggested an increased risk for T2DM with this strategy[4], albeit a meta-analysis of published trials identified only a minor increase in fasting insulin concentrations[108]. By contrast, given the significance of IR in the pathophysiology of the syndrome, it comes as no surprise that metformin has been the most commonly used medication to prevent or treat the metabolic abnormalities in women with PCOS[109]. Yet, despite the high expectations, metformin combined with lifestyle changes appeared to produce only a small reduction in BMI (-0.73 kg/m2). This was, namely, a decrease in subcutaneous adipose tissue volume and an improvement in menstrual cyclicity compared to lifestyle interventions alone, these as seen in a meta-analysis of published randomized controlled trials[109]. It is, however, of note that most studies were small in sample size and the risk of bias was deemed high by the authors. Patients with concurrent diabetes were excluded in most studies, not allowing for safe conclusions to be drawn in this regard. On the other hand, metformin significantly reduced the risk for gestational diabetes (pooled OR 0.20, 95%CI: 0.12-0.1, P < 0.001), early pregnancy loss (pooled OR 0.28, 95%CI: 0.10-0.75, P = 0.01), and preterm delivery (pooled OR 0.33, 95%CI: 0.18-0.60, P = 0.0003), with significant heterogeneity between studies[110]. These outcomes did not differ between patients treated prior to pregnancy and those treated throughout the duration of their pregnancy[111].
In addition to metformin, PPAR-γ agonists, such as rosiglitazone (not used cur
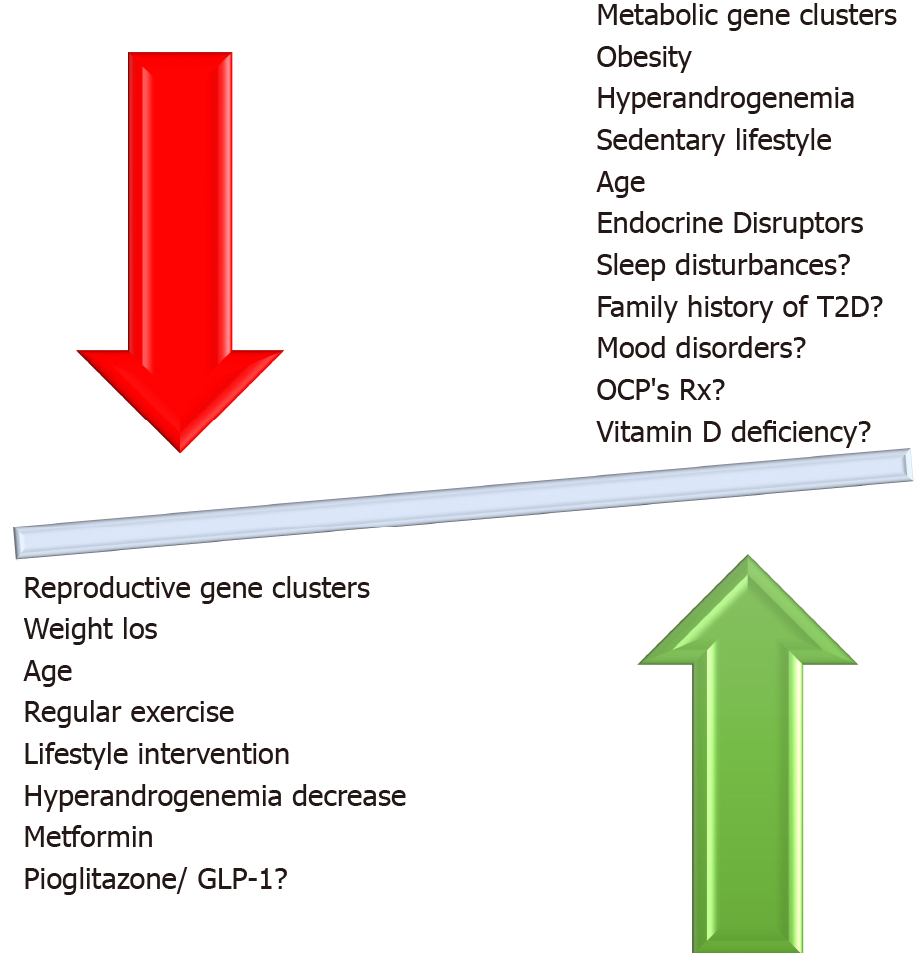
Even though supplement use has increased greatly in the past few years, supplementation with minerals, trace elements, and other supplements is, in general, still controversial. In the case of chromium supplementation in PCOS, two meta-analyses of published trials found that BMI, fasting insulin, free testosterone[119] HOMA-IR, and HOMA-β[120] seemed to improve. In addition, supplementation with omega-3 fatty acids at doses of 900-4000 mg daily appeared to improve HOMA-IR index in a meta-analysis of nine small randomized controlled trials, but no data were available on risk for T2D[121]. Myoinositol, an amino acid with potentially beneficial effects in women with PCOS, has been studied with regard to glucose metabolism, and a small positive impact on fasting insulin and HOMA-IR was found in a 2017 meta-analysis of controlled trials, without any effect on glucose concentrations or BMI[122]. The factors described above and their relationship to development of dysglycemia in PCOS are illustrated in Figure 3.
Based on the aforementioned data, a diagnosis of PCOS during the reproductive age places a woman at increased risk for T2D in later, post-reproductive life[123]—a risk which is further augmented if the issue of dysregulated glucose metabolism during transition to menopause is taken into account. The risk is even greater if the woman enters menopause before the age of 45[124]. Α recent meta-analysis of 23 cohort studies[47] assessed the long-term cardiovascular disease (CVD) risk in women with PCOS, including T2D risk. Women with a history of PCOS demonstrated a 3-fold increased risk of T2D (RR 3.00; 95%CI: 2.56-3.51) compared to non-PCOS women. However, the studies included were quite heterogeneous in terms of the participants’ age. Very few of them included postmenopausal women, either as a single or a mixed population[8,125,126]. Another shortcoming was the heterogeneity in PCOS diagnosis among studies.
A recent prospective cohort study evaluated the risk for development of T2D in 27 PCOS women (defined by the NIH criteria) after 24 years of follow-up (mean age at baseline: 29.5 ± 5.3years; mean age at the end of follow-up: 52.4 ± 5.4 years) in comparison with age-matched non-PCOS controls (n = 94). The incidence of T2D in the former group was 19% compared to 1% in the latter (P < 0.01)[8]. Interestingly, the development of T2D was independent of lifestyle factors. Although all PCOS women with T2D were obese and had a higher BMI and WHR than non-PCOS individuals, the increases in BMI and WHR per year were comparable between groups during follow-up. However, an older prospective study including 35 postmenopausal women with PCOS (mean age 70.4 ± 5 years, range 61-79), diagnosed with the Rotterdam criteria, did not find any difference in T2D incidence compared to age-matched controls after 21 years of follow-up[125]. Moreover, a retrospective cohort study published in 2000, which included 319 PCOS women (mean age 56.7 years, range 38-98; 81% postmenopausal; PCOS diagnosis based on medical records) and 1060 age-matched controls, showed a 3-fold increased risk of T2D in the PCOS group (OR 2.8; 95%CI: 1.5-5.5) after a mean follow-up time of 31 years (range 15-47). However, this risk was not significant after adjustment for BMI (OR 2.2; 95%CI: 0.9-5.2)[126].
In general, whether the increased T2D risk exists in both obese and non-obese PCOS has not as yet been fully elucidated. A recent meta-analysis by Zhu et al[10] that assessed the risk of T2D in non-obese PCOS compared to non-obese control women showed an increased risk in this PCOS subpopulation, although to a lesser extent compared to obese PCOS (five studies; OR 1.47; 95%CI: 1.11–1.93). The authors additionally reported an increased prevalence of IR, IGT, and atherogenic dyslipidemia in non-obese PCOS compared to non-obese non-PCOS women. It must be underlined that the PCOS populations in the included studies were all premenopausal[10]. Of note, a prospective cohort study of this meta-analysis assessed the incidence of T2D at the age of 46 years in a cohort of 279 women with both oligoamenorrhea and hirsutism at the age 31 years, who had been defined as “PCOS”. This cohort was compared with 1577 women, without oligoamenorrhea and hirsutism, who served as controls. Women with PCOS and BMI > 25 kg/m2 demonstrated a 2.5-fold increased risk of T2D compared to non-PCOS (OR 2.45; 95%CI: 1.28–4.67). It is notable that no such risk was identified in PCOS women of normal weight[127]. A very recent case-control study (1136 PCOS patients, aged 15 to 44 years, and 5675 controls) showed an increased risk of T2D in PCOS independently of BMI (adjusted OR in the entire cohort 2.36, 95%CI: 1.79–3.08; OR in non-obese PCOS 2.33, 95%CI: 1.71–3.18; OR in obese PCOS 2.85, 95%CI: 1.59–5.11)[128].
Aside from BMI, other factors also play an important role in the incidence of T2D in postmenopausal women with a history of PCOS. The increased ovarian androgen production and IGT observed in premenopausal women with PCOS cases seem to persist after menopause transition. On the other hand, IR and hyperinsulinemia may improve in women with PCOS during their post-reproductive years, although data are still inconsistent as to this hypothesis[129]. Furthermore, the severity of IR is also dependent on the PCOS phenotype, since hyperandrogenemia is related to a more severe metabolic dysfunction[65].
Therefore, although transition to menopause is associated with dysregulation of glucose metabolism[130], current evidence is at present too weak to support the existence of increased T2D risk in postmenopausal women with a history of PCOS compared to those without. There are currently too many confounding factors and variables, such as PCOS definition, sample size, and the precise effect of BMI and aging, to accurately determine the actual impact, if any, of a PCOS diagnosis on T2DM risk after transition to menopause.
Lifestyle intervention, including diet modification and regular exercise, still remain the mainstay of treatment in reducing T2D risk in women with PCOS, especially those who are obese or overweight. According to a recent meta-analysis of 19 randomized controlled trials (RCTs), systematic dietary intervention is superior to advice, usual diets, or no treatment with regard to HOMA-IR [mean difference (MD) -0.78, 95%CI: -0.92 to -0.65], fasting plasma insulin (FPI) (MD -4.24 mIU/L, 95%CI: -5.37 to -3.10), FPG (MD -0.11 mmol/L, 95%CI: -0.17 to -0.04 mmol/L), as well as BMI (MD -1.01 kg/m2, 95%CI: -1.38 to -0.64) and waist circumference (WC) (MD -3.25 cm, 95%CI: -5.29 to -1.22)[131]. Subgroup analysis showed that the Dietary Approaches to Stop Hypertension (DASH) diet is more effective in HOMA-IR and FPG reduction than a low-carbohydrate diet (LCD), but with comparable efficacy regarding FPI concentrations. With respect to BMI and body weight, a calorie-restricted diet is more beneficial than either DASH or LCD. All dietary patterns seem to have comparable efficacy regarding WC[131]. Moreover, data from RCTs in PCOS women have shown that LCD is quite effective in reducing BMI [standardized MD (SMD) -1.04, 95%CI: -1.38 to -0.70) and HOMA-IR (SMD -0.66, 95%CI: -1.01 to -0.30) compared to a regular diet according to a recent meta-analysis[132]. In addition, a low glycemic index diet could also be the first-line approach in PCOS patients, since it effectively reduces HOMA-IR (-0.78, 95%CI: -1.20 to -0.37), WC (-2.81 cm, 95%CI: -4.40 to -1.23), and total testosterone concentrations (-0.21 nmol/L, 95%CI: -0.32 to -0.09) compared to a high glycemic index diet[133]. Although the evidence in PCOS populations is limited, the Mediterranean diet (MedDiet) compared to the typical Western diet is also effective in reducing HOMA-IR (MD -0.42, 95%CI: −0.70 to −0.15), FPG (MD -2.98 mg/dL, 95%CI: -4.54 to -1.42), and FPI (-0.94, 95%CI: -1.72 to -0.16) compared to a usual diet. The MedDiet is also associated with a lower tendency to develop T2DM (RR 0.81; 95%CI: 0.61-1.02) and a reduction in CVD events related to metabolic syndrome[133].
The beneficial effects of structured exercise programs in metabolic syndrome, obesity, T2D, and CVD prevention and treatment are well-known[134]. Interventions consisting of lifestyle modifications in women with PCOS produce substantial improvements in glucose homeostasis and reproductive outcomes as well. These benefits are equally significant as those achieved by metformin[135]. A negative energy balance of approximately 30%, aiming to achieve an energy deficit of 500-750 kcal per day, is able to produce significant amounts of weight loss in women with PCOS. The addition of any amount of exercise, whether aerobic or anaerobic, confers additional beneficial effects on glucose homeostasis[25]. High-intensity interval training (achieving 90%-95% of the individual’s maximum heart rate) three times a week for ≥ 10 wk is effective in reducing HOMA-IR (MD -0.57, 95%CI: -0.98 to -0.16) and BMI (MD -1.90, 95%CI: -3.37 to -0.42) in women with PCOS, according to a recent meta-analysis[136]. In cases with morbid obesity, such as those with BMI > 40 kg/m2 or BMI > 35 kg/m2 with metabolic comorbidities, bariatric surgery should be considered[1]. Indeed, bariatric surgery can reduce the risk of T2D by 91% (RR 0.09, 95%CI: 0.03-0.32). The mean reduction in BMI is -14.51 kg/m2 (95%CI: -17.88 to -11.14). It also ameliorates menstrual disturbances and hirsutism in PCOS patients [RR 0.23 (95%CI: 0.13-0.43) and 0.47 (95%CI: 0.28-0.79), respectively][137].
Metformin is the most commonly used insulin sensitizer in women with PCOS, especially in those who are obese or overweight. According to the aforementioned meta-analysis, diet is superior to metformin with regard to weight loss, but comparably efficacious in improving glucose homeostasis (HOMA-IR, FPG, and FPI)[131]. In a recent meta-analysis of 12 RCTs, metformin was superior to placebo in reducing BMI [weighted MD (WMD) -1.25, 95%CI: -1.60 to -0.91] and WC (WMD -1.41, 95%CI: -2.46 to -0.37). There were no differences between groups with regard to HOMA-IR, FPG, and FPI[138].
For women who are intolerant to metformin, thiazolidinediones (TZDs) constitute another class of insulin sensitizers that have been evaluated in women with PCOS. Rosiglitazone and pioglitazone, the two commonly used TZDs, are effective in improving IR and IGT, as well as mensural cyclicity, in PCOS patients. However, weight gain, increase in transaminase levels, and potential teratogenic effects limit their use in these patients[1]. Compared to metformin, pioglitazone is superior with respect to menstrual cycle improvement and ovulation but inferior regarding hirsutism score. Both agents are equally effective in reducing HOMA-IR, FPG, and FPI, as mentioned above[112].
Glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1-RAs) have also been tested in women with PCOS. A recent meta-analysis of eight RCTs (four with exenatide 10 μg twice a day; four with liraglutide 1.2 mg/d), compared their efficacy with that of metformin in women with PCOS. The study showed that GLP-1-RAs were more effective in improving HOMA-IR (SMD -0.40, 95%CI: -0.74 to -0.06) and reducing BMI (SMD -1.02, 95%CI: -1.85 to -0.19) and WC (SMD -0.45, 95%CI: -0.89 to -0.00). No difference between GLP-1-RAs and metformin in FPG and FPI was observed either between exenatide and liraglutide[139].
Finally, in cases of post-menopausal women younger than 60 years and/or within 10 years since their last menstrual period who present with severe vasomotor symptomatology, especially those with early menopause (< 45 years of age), menopausal hormone therapy (MHT) may be of benefit, since it reduces T2D by up to 30%[140]. MHT exerts a beneficial effect on glucose homeostasis in women both with and without T2D. In cases with T2D and low CVD risk, oral estrogens may be considered[141]. In obese women with T2D or with moderate CVD risk, transdermal 17β-estradiol is the preferred treatment, along with a progestogen with minimal effects on glucose metabolism, such as progesterone, dydrogesterone, or transdermal norethisterone. However, this favorable effect on glucose homeostasis is dissipated after MHT discontinuation. In any case, MHT is not recommended for the sole purpose of T2D prevention or treatment[140,141].
It is thus clear that weight loss, preferably with LCD and a low glycemic index diet low in AGES, combined with vigorous exercise should be the first-line lifestyle intervention in overweight or obese PCOS patients due to their well-documented beneficial effects on glucose metabolism, although longitudinal data on T2D risk are thus far lacking. The MedDiet may be even more beneficial than a low glycemic index diet in CVD risk reduction, although the current evidence in PCOS patients is weak. Metformin may also be considered in cases of impaired glucose metabolism and oligo/amenorrhea. The choice of either BS, pioglitazone, or GLP-1-RA should be individualized and benefits should be weighed against costs.
The association of PCOS with increased T2D risk is relatively robust and thus should not be neglected in any woman with the syndrome. Despite the current heterogeneity of the data, the ever-changing nature of this disorder, and the uncertainty regarding the exact mechanisms regulating progression of dysglycemia in PCOS, there are several general principles that the clinician should implement in everyday practice. First, diagnosis and follow-up of dysglycemia should preferably be based on OGTT and not on FPG or HbA1c values. Second, the non-linear development of T2D in PCOS in non-obese women highlights the importance of maintaining an optimal weight in all women suffering from the syndrome. Third, menopausal women with a history of PCOS should be regularly evaluated since they may be at higher T2D risk, especially if they are obese. Fourth, a well-balanced diet coupled with regular exercise constitutes the most appropriate approach in every patient with PCOS. Metformin administration might ameliorate the biochemical and hormonal profile in PCOS and may be considered in patients in whom prior measures have failed to improve metabolic and ovulatory dysfunction. The use of pioglitazone, GLP-1-Ras, and/or MHT may be of value in selected cases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Endocrinology, No. 512990.
Specialty type: Endocrinology and Metabolism
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu QN S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R, Pfeifer M, Pignatelli D, Pugeat M, Yildiz BO; ESE PCOS Special Interest Group. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:P1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 2. | Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 996] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 3. | Gambineri A, Pelusi C, Manicardi E, Vicennati V, Cacciari M, Morselli-Labate AM, Pagotto U, Pasquali R. Glucose intolerance in a large cohort of mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes. 2004;53:2353-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and Risk Factors of Type 2 Diabetes in a Nationwide Population of Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102:3848-3857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. 2016;31:2619-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 6. | Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2021;95:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 8. | Forslund M, Landin-Wilhelmsen K, Trimpou P, Schmidt J, Brännström M, Dahlgren E. Type 2 diabetes mellitus in women with polycystic ovary syndrome during a 24-year period: importance of obesity and abdominal fat distribution. Hum Reprod Open. 2020;2020:hoz042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Gambineri A, Patton L, Altieri P, Pagotto U, Pizzi C, Manzoli L, Pasquali R. Polycystic ovary syndrome is a risk factor for type 2 diabetes: results from a long-term prospective study. Diabetes. 2012;61:2369-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Zhu T, Cui J, Goodarzi MO. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes. 2021;70:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Dapas M, Lin FTJ, Nadkarni GN, Sisk R, Legro RS, Urbanek M, Hayes MG, Dunaif A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020;17:e1003132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 12. | Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 13. | Pelanis R, Mellembakken JR, Sundström-Poromaa I, Ravn P, Morin-Papunen L, Tapanainen JS, Piltonen T, Puurunen J, Hirschberg AL, Fedorcsak P, Andersen M, Glintborg D. The prevalence of Type 2 diabetes is not increased in normal-weight women with PCOS. Hum Reprod. 2017;32:2279-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Ganie MA, Dhingra A, Nisar S, Sreenivas V, Shah ZA, Rashid A, Masoodi S, Gupta N. Oral glucose tolerance test significantly impacts the prevalence of abnormal glucose tolerance among Indian women with polycystic ovary syndrome: lessons from a large database of two tertiary care centers on the Indian subcontinent. Fertil Steril. 2016;105:194-201.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Seneviratne HR, Lankeshwara D, Wijeratne S, Somasunderam N, Athukorale D. Serum insulin patterns and the relationship between insulin sensitivity and glycaemic profile in women with polycystic ovary syndrome. BJOG. 2009;116:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, Shaw JE, Zimmet PZ. Diabetes in Asia and the Pacific: Implications for the Global Epidemic. Diabetes Care. 2016;39:472-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 17. | Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Lizneva D, Kirubakaran R, Mykhalchenko K, Suturina L, Chernukha G, Diamond MP, Azziz R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral vs unselected populations: systematic review and meta-analysis. Fertil Steril. 2016;106:1510-1520.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Zhang B, Wang J, Shen S, Liu J, Sun J, Gu T, Ye X, Zhu D, Bi Y. Association of Androgen Excess with Glucose Intolerance in Women with Polycystic Ovary Syndrome. Biomed Res Int. 2018;2018:6869705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Eades CE, France EF, Evans JM. Prevalence of impaired glucose regulation in Europe: a meta-analysis. Eur J Public Health. 2016;26:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Veltman-Verhulst SM, Goverde AJ, van Haeften TW, Fauser BC. Fasting glucose measurement as a potential first step screening for glucose metabolism abnormalities in women with anovulatory polycystic ovary syndrome. Hum Reprod. 2013;28:2228-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Amato MC, Magistro A, Gambino G, Vesco R, Giordano C. Visceral adiposity index and DHEAS are useful markers of diabetes risk in women with polycystic ovary syndrome. Eur J Endocrinol. 2015;172:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33:1602-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 619] [Cited by in RCA: 959] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 24. | Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1166] [Article Influence: 89.7] [Reference Citation Analysis (1)] |
| 25. | Pani A, Gironi I, Di Vieste G, Mion E, Bertuzzi F, Pintaudi B. From Prediabetes to Type 2 Diabetes Mellitus in Women with Polycystic Ovary Syndrome: Lifestyle and Pharmacological Management. Int J Endocrinol. 2020;2020:6276187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 554] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 426] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 351] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Dong Z, Huang J, Huang L, Chen X, Yin Q, Yang D. Associations of acanthosis nigricans with metabolic abnormalities in polycystic ovary syndrome women with normal body mass index. J Dermatol. 2013;40:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Morciano A, Romani F, Sagnella F, Scarinci E, Palla C, Moro F, Tropea A, Policola C, Della Casa S, Guido M, Lanzone A, Apa R. Assessment of insulin resistance in lean women with polycystic ovary syndrome. Fertil Steril. 2014;102:250-256.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Vrbíková J, Cibula D, Dvoráková K, Stanická S, Sindelka G, Hill M, Fanta M, Vondra K, Skrha J. Insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:2942-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1002] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 33. | Oh JY, Barrett-Connor E, Wedick NM, Wingard DL; Rancho Bernardo Study. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 34. | Muka T, Nano J, Jaspers L, Meun C, Bramer WM, Hofman A, Dehghan A, Kavousi M, Laven JS, Franco OH. Associations of Steroid Sex Hormones and Sex Hormone-Binding Globulin With the Risk of Type 2 Diabetes in Women: A Population-Based Cohort Study and Meta-analysis. Diabetes. 2017;66:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Cotrozzi G, Matteini M, Relli P, Lazzari T. Hyperinsulinism and insulin resistance in polycystic ovarian syndrome: a verification using oral glucose, I.V. Glucose and tolbutamide. Acta Diabetol Lat. 1983;20:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Takeuchi T, Tsutsumi O, Taketani Y. Abnormal response of insulin to glucose loading and assessment of insulin resistance in non-obese patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Toprak S, Yönem A, Cakir B, Güler S, Azal O, Ozata M, Corakçi A. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm Res. 2001;55:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Lerchbaum E, Schwetz V, Giuliani A, Pieber TR, Obermayer-Pietsch B. Opposing effects of dehydroepiandrosterone sulfate and free testosterone on metabolic phenotype in women with polycystic ovary syndrome. Fertil Steril. 2012;98:1318-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Moran C, Arriaga M, Arechavaleta-Velasco F, Moran S. Adrenal androgen excess and body mass index in polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Paschou SA, Palioura E, Ioannidis D, Anagnostis P, Panagiotakou A, Loi V, Karageorgos G, Goulis DG, Vryonidou A. Adrenal hyperandrogenism does not deteriorate insulin resistance and lipid profile in women with PCOS. Endocr Connect. 2017;6:601-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Patlolla S, Vaikkakara S, Sachan A, Venkatanarasu A, Bachimanchi B, Bitla A, Settipalli S, Pathiputturu S, Sugali RN, Chiri S. Heterogenous origins of hyperandrogenism in the polycystic ovary syndrome in relation to body mass index and insulin resistance. Gynecol Endocrinol. 2018;34:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Rittmaster RS, Deshwal N, Lehman L. The role of adrenal hyperandrogenism, insulin resistance, and obesity in the pathogenesis of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1993;76:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Alpañés M, Luque-Ramírez M, Martínez-García MÁ, Fernández-Durán E, Álvarez-Blasco F, Escobar-Morreale HF. Influence of adrenal hyperandrogenism on the clinical and metabolic phenotype of women with polycystic ovary syndrome. Fertil Steril. 2015;103:795-801.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Brennan K, Huang A, Azziz R. Dehydroepiandrosterone sulfate and insulin resistance in patients with polycystic ovary syndrome. Fertil Steril. 2009;91:1848-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Livadas S, Bothou C, Kanaka-Gantenbein C, Chiotis D, Angelopoulos N, Macut D, Chrousos GP. Unfavorable Hormonal and Psychologic Profile in Adult Women with a History of Premature Adrenarche and Pubarche, Compared to Women with Polycystic Ovary Syndrome. Horm Metab Res. 2020;52:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Hansen SL, Svendsen PF, Jeppesen JF, Hoeg LD, Andersen NR, Kristensen JM, Nilas L, Lundsgaard AM, Wojtaszewski JFP, Madsbad S, Kiens B. Molecular Mechanisms in Skeletal Muscle Underlying Insulin Resistance in Women Who Are Lean With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2019;104:1841-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, Laven JSE, Roeters van Lennep JE, Roseboom TJ, Hoek A. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26:942-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 48. | Anagnostis P, Paparodis R, Bosdou J, Bothou C, Goulis DG, Macut D, Dunaif A, Livadas S. The major impact of obesity on the development of type 2 diabetes in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. J Endocr Soc. 2021;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001;16:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Lee H, Oh JY, Sung YA, Chung H, Cho WY. The prevalence and risk factors for glucose intolerance in young Korean women with polycystic ovary syndrome. Endocrine. 2009;36:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Wei HJ, Young R, Kuo IL, Liaw CM, Chiang HS, Yeh CY. Prevalence of insulin resistance and determination of risk factors for glucose intolerance in polycystic ovary syndrome: a cross-sectional study of Chinese infertility patients. Fertil Steril. 2009;91:1864-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Livadas S, Kollias A, Panidis D, Diamanti-Kandarakis E. Diverse impacts of aging on insulin resistance in lean and obese women with polycystic ovary syndrome: evidence from 1345 women with the syndrome. Eur J Endocrinol. 2014;171:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Livadas S, Macut D, Bothou C, Kuliczkowska-Płaksej J, Vryonidou A, Bjekic-Macut J, Mouslech Z, Milewicz A, Panidis D. Insulin resistance, androgens, and lipids are gradually improved in an age-dependent manner in lean women with polycystic ovary syndrome: insights from a large Caucasian cohort. Hormones (Athens). 2020;19:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Wilson JMG, Jungner G; Organization WH. Principles and practice of screening for disease. World Health Organization, 1968. |
| 55. | Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1083] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 56. | Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome - Part 2. Endocr Pract. 2015;21:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 57. | Jean Hailes for women’s health on behalf of the PCOS Australian alliance. Evidence-based guidelines for the assessment and management of polycystic ovary syndrome. Melbourne: Jean Hailes Foundation for Women’s Health on behalf of the PCOS Australian Alliance, 2015: 64–65. |
| 58. | Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 660] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 59. | Royal College of Obstetricians and Gynaecologists. Polycystic Ovary Syndrome, Long-term Consequences (Green-top Guideline No. 33). Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg33/. |
| 60. | Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3363] [Cited by in RCA: 4019] [Article Influence: 191.4] [Reference Citation Analysis (0)] |
| 61. | Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 333] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 763] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 63. | Dabadghao P, Roberts BJ, Wang J, Davies MJ, Norman RJ. Glucose tolerance abnormalities in Australian women with polycystic ovary syndrome. Med J Aust. 2007;187:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Möhlig M, Flöter A, Spranger J, Weickert MO, Schill T, Schlösser HW, Brabant G, Pfeiffer AF, Selbig J, Schöfl C. Predicting impaired glucose metabolism in women with polycystic ovary syndrome by decision tree modelling. Diabetologia. 2006;49:2572-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Celik C, Abali R, Bastu E, Tasdemir N, Tasdemir UG, Gul A. Assessment of impaired glucose tolerance prevalence with hemoglobin A₁c and oral glucose tolerance test in 252 Turkish women with polycystic ovary syndrome: a prospective, controlled study. Hum Reprod. 2013;28:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Velling Magnussen L, Mumm H, Andersen M, Glintborg D. Hemoglobin A1c as a tool for the diagnosis of type 2 diabetes in 208 premenopausal women with polycystic ovary syndrome. Fertil Steril. 2011;96:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE. Differences in height explain gender differences in the response to the oral glucose tolerance test- the AusDiab study. Diabet Med. 2008;25:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Andersen M, Glintborg D. Diagnosis and follow-up of type 2 diabetes in women with PCOS: a role for OGTT? Eur J Endocrinol. 2018;179:D1-D14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 782] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 71. | Vrbikova J, Hill M, Fanta M. The utility of fasting plasma glucose to identify impaired glucose metabolism in women with polycystic ovary syndrome. Gynecol Endocrinol. 2014;30:664-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Lorenzo C, Haffner SM, Stančáková A, Kuusisto J, Laakso M. Fasting and OGTT-derived measures of insulin resistance as compared with the euglycemic-hyperinsulinemic clamp in nondiabetic Finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab. 2015;100:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Solomon A, Hussein M, Negash M, Ahmed A, Bekele F, Kahase D. Effect of iron deficiency anemia on HbA1c in diabetic patients at Tikur Anbessa specialized teaching hospital, Addis Ababa Ethiopia. BMC Hematol. 2019;19:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Chatzianagnostou K, Vigna L, Di Piazza S, Tirelli AS, Napolitano F, Tomaino L, Bamonti F, Traghella I, Vassalle C. Low concordance between HbA1c and OGTT to diagnose prediabetes and diabetes in overweight or obesity. Clin Endocrinol (Oxf). 2019;91:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968-983. [PubMed] |
| 76. | Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. PLoS One. 2017;12:e0171315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 77. | Bergman M, Abdul-Ghani M, Neves JS, Monteiro MP, Medina JL, Dorcely B, Buysschaert M. Pitfalls of HbA1c in the Diagnosis of Diabetes. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 596] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 79. | Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16:1995-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 80. | Pesant MH, Baillargeon JP. Clinically useful predictors of conversion to abnormal glucose tolerance in women with polycystic ovary syndrome. Fertil Steril. 2011;95:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep. 2006;6:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7148] [Cited by in RCA: 6609] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 83. | Celik C, Tasdemir N, Abali R, Bastu E, Yilmaz M. Progression to impaired glucose tolerance or type 2 diabetes mellitus in polycystic ovary syndrome: a controlled follow-up study. Fertil Steril. 2014;101:1123-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Sadrzadeh S, Hui EVH, Schoonmade LJ, Painter RC, Lambalk CB. Birthweight and PCOS: systematic review and meta-analysis. Hum Reprod Open. 2017;2017:hox010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Harrison CL, Teede HJ, Joham AE, Moran LJ. Breastfeeding and obesity in PCOS. Expert Rev Endocrinol Metab. 2016;11:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Gouveri E, Papanas N, Hatzitolios AI, Maltezos E. Breastfeeding and diabetes. Curr Diabetes Rev. 2011;7:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 253] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 88. | Hu Y, Wen S, Yuan D, Peng L, Zeng R, Yang Z, Liu Q, Xu L, Kang D. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol. 2018;34:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96:E480-E484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 90. | Merhi Z, Kandaraki EA, Diamanti-Kandarakis E. Implications and Future Perspectives of AGEs in PCOS Pathophysiology. Trends Endocrinol Metab. 2019;30:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 91. | Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, Panidis D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2008;69:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 92. | Tantalaki E, Piperi C, Livadas S, Kollias A, Adamopoulos C, Koulouri A, Christakou C, Diamanti-Kandarakis E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones (Athens). 2014;13:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 93. | Lee DH, Jacobs DR Jr. Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord. 2015;16:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Xirofotos D, Trakakis E, Peppa M, Chrelias C, Panagopoulos P, Christodoulaki C, Sioutis D, Kassanos D. The amount and duration of smoking is associated with aggravation of hormone and biochemical profile in women with PCOS. Gynecol Endocrinol. 2016;32:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Cupisti S, Häberle L, Dittrich R, Oppelt PG, Reissmann C, Kronawitter D, Beckmann MW, Mueller A. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010;94:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Faghfoori Z, Fazelian S, Shadnoush M, Goodarzi R. Nutritional management in women with polycystic ovary syndrome: A review study. Diabetes Metab Syndr. 2017;11 Suppl 1:S429-S432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 97. | Zhang J, Liu XF, Liu Y, Xu LZ, Zhou LL, Tang LL, Zhuang J, Li TT, Guo WQ, Hu R, Qiu DS, Han DW. Environmental risk factors for women with polycystic ovary syndrome in china: a population-based case-control study. J Biol Regul Homeost Agents. 2014;28:203-211. [PubMed] |
| 98. | He C, Lin Z, Robb SW, Ezeamama AE. Serum Vitamin D Levels and Polycystic Ovary syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2015;7:4555-4577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 99. | Łagowska K, Bajerska J, Jamka M. The Role of Vitamin D Oral Supplementation in Insulin Resistance in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 100. | Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod. 2015;30:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Kahal H, Kyrou I, Uthman OA, Brown A, Johnson S, Wall PDH, Metcalfe A, Parr DG, Tahrani AA, Randeva HS. The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep Breath. 2020;24:339-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 102. | Tasali E, Van Cauter E, Ehrmann DA. Relationships between sleep disordered breathing and glucose metabolism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, Ismail K. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36:480-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 104. | Livadas S, Chaskou S, Kandaraki AA, Skourletos G, Economou F, Christou M, Boutzios G, Karachalios A, Zerva A, Xyrafis X, Christakou C, Pighou AK, Diamanti-Kandarakis E. Anxiety is associated with hormonal and metabolic profile in women with polycystic ovarian syndrome. Clin Endocrinol (Oxf). 2011;75:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32:1075-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 106. | Guo J, Shao J, Yang Y, Niu X, Liao J, Zhao Q, Wang D, Li S, Hu J. Gut Microbiota in Patients with Polycystic Ovary Syndrome: a Systematic Review. Reprod Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 107. | Cozzolino M, Vitagliano A, Pellegrini L, Chiurazzi M, Andriasani A, Ambrosini G, Garrido N. Therapy with probiotics and synbiotics for polycystic ovarian syndrome: a systematic review and meta-analysis. Eur J Nutr. 2020;59:2841-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 108. | Amiri M, Ramezani Tehrani F, Nahidi F, Kabir A, Azizi F, Carmina E. Effects of oral contraceptives on metabolic profile in women with polycystic ovary syndrome: A meta-analysis comparing products containing cyproterone acetate with third generation progestins. Metabolism. 2017;73:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 110. | Tan X, Li S, Chang Y, Fang C, Liu H, Zhang X, Wang Y. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clin Invest Med. 2016;39:E120-E131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Bidhendi Yarandi R, Behboudi-Gandevani S, Amiri M, Ramezani Tehrani F. Metformin therapy before conception versus throughout the pregnancy and risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systemic review, meta-analysis and meta-regression. Diabetol Metab Syndr. 2019;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Xu Y, Wu Y, Huang Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS:a meta-analysis. Arch Gynecol Obstet. 2017;296:661-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, Khan AY, Kilpatrick ES, Atkin SL, Sathyapalan T. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: A randomized controlled study. Clin Endocrinol (Oxf). 2019;90:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 114. | Liu X, Zhang Y, Zheng SY, Lin R, Xie YJ, Chen H, Zheng YX, Liu E, Chen L, Yan JH, Xu W, Mai TT, Gong Y. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin Endocrinol (Oxf). 2017;87:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 115. | Tao T, Zhang Y, Zhu YC, Fu JR, Wang YY, Cai J, Ma JY, Xu Y, Gao YN, Sun Y, Fan W, Liu W. Exenatide, Metformin, or Both for Prediabetes in PCOS: A Randomized, Open-label, Parallel-group Controlled Study. J Clin Endocrinol Metab. 2021;106:e1420-e1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 116. | Tian D, Chen W, Xu Q, Li X, Lv Q. Liraglutide monotherapy and add on therapy on obese women with polycystic ovarian syndromes: a systematic review and meta-analysis. Minerva Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:2670-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 118. | Graff SK, Mario FM, Ziegelmann P, Spritzer PM. Effects of orlistat vs. metformin on weight loss-related clinical variables in women with PCOS: systematic review and meta-analysis. Int J Clin Pract. 2016;70:450-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 119. | Fazelian S, Rouhani MH, Bank SS, Amani R. Chromium supplementation and polycystic ovary syndrome: A systematic review and meta-analysis. J Trace Elem Med Biol. 2017;42:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Heshmati J, Omani-Samani R, Vesali S, Maroufizadeh S, Rezaeinejad M, Razavi M, Sepidarkish M. The Effects of Supplementation with Chromium on Insulin Resistance Indices in Women with Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Horm Metab Res. 2018;50:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Yang K, Zeng L, Bao T, Ge J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2018;16:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 122. | Zeng L, Yang K. Effectiveness of myoinositol for polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;59:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 123. | Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic ovarian syndrome (PCOS): Long-term metabolic consequences. Metabolism. 2018;86:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 124. | Anagnostis P, Christou K, Artzouchaltzi AM, Gkekas NK, Kosmidou N, Siolos P, Paschou SA, Potoupnis M, Kenanidis E, Tsiridis E, Lambrinoudaki I, Stevenson JC, Goulis DG. Early menopause and premature ovarian insufficiency are associated with increased risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Endocrinol. 2019;180:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 125. | Schmidt J, Landin-Wilhelmsen K, Brännström M, Dahlgren E. Cardiovascular disease and risk factors in PCOS women of postmenopausal age: a 21-year controlled follow-up study. J Clin Endocrinol Metab. 2011;96:3794-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 126. | Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf). 2000;52:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 127. | Ollila MM, West S, Keinänen-Kiukaanniemi S, Jokelainen J, Auvinen J, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, Franks S, Piltonen TT, Morin-Papunen LC. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective, population-based cohort study. Hum Reprod. 2017;32:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 128. | Ryu KJ, Kim MS, Kim HK, Kim YJ, Yi KW, Shin JH, Hur JY, Kim T, Park H. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: the National Health Insurance Service-National Sample Cohort Study. Fertil Steril. 2021;115:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 129. | Armeni E, Lambrinoudaki I. Cardiovascular Risk in Postmenopausal Women with Polycystic Ovary Syndrome. Curr Vasc Pharmacol. 2019;17:579-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Anagnostis P, Goulis DG. Menopause and its Cardiometabolic Consequences: Current Perspectives. Curr Vasc Pharmacol. 2019;17:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Shang Y, Zhou H, Hu M, Feng H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 132. | Zhang X, Zheng Y, Guo Y, Lai Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2019;2019:4386401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 133. | Kazemi M, Hadi A, Pierson RA, Lujan ME, Zello GA, Chilibeck PD. Effects of Dietary Glycemic Index and Glycemic Load on Cardiometabolic and Reproductive Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv Nutr. 2021;12:161-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 134. | Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1777] [Article Influence: 296.2] [Reference Citation Analysis (0)] |
| 135. | Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, Phung OJ, Wang A, Hoeger K, Pasquali R, Erwin P, Bodde A, Montori VM, Murad MH. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4655-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 136. | Santos IKD, Nunes FASS, Queiros VS, Cobucci RN, Dantas PB, Soares GM, Cabral BGAT, Maranhão TMO, Dantas PMS. Effect of high-intensity interval training on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2021;16:e0245023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 137. | Li YJ, Han Y, He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Guan Y, Wang D, Bu H, Zhao T, Wang H. The Effect of Metformin on Polycystic Ovary Syndrome in Overweight Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2020;2020:5150684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 139. | Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online. 2019;39:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 140. | Anagnostis P, Paschou SA, Katsiki N, Krikidis D, Lambrinoudaki I, Goulis DG. Menopausal Hormone Therapy and Cardiovascular Risk: Where are we Now? Curr Vasc Pharmacol. 2019;17:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 141. | Slopien R, Wender-Ozegowska E, Rogowicz-Frontczak A, Meczekalski B, Zozulinska-Ziolkiewicz D, Jaremek JD, Cano A, Chedraui P, Goulis DG, Lopes P, Mishra G, Mueck A, Rees M, Senturk LM, Simoncini T, Stevenson JC, Stute P, Tuomikoski P, Paschou SA, Anagnostis P, Lambrinoudaki I. Menopause and diabetes: EMAS clinical guide. Maturitas. 2018;117:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 142. | Rajkhowa M, Talbot JA, Jones PW, Clayton RN. Polymorphism of glycogen synthetase gene in polycystic ovary syndrome. Clin Endocrinol (Oxf). 1996;44:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 143. | Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 144. | Chen X, Yang D, Li L, Feng S, Wang L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum Reprod. 2006;21:2027-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 145. | Vrbikova J, Dvorakova K, Grimmichova T, Hill M, Stanicka S, Cibula D, Bendlova B, Starka L, Vondra K. Prevalence of insulin resistance and prediction of glucose intolerance and type 2 diabetes mellitus in women with polycystic ovary syndrome. Clin Chem Lab Med. 2007;45:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 146. | Gagnon C, Baillargeon JP. Suitability of recommended limits for fasting glucose tests in women with polycystic ovary syndrome. CMAJ. 2007;176:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 147. | Espinós-Gómez JJ, Corcoy R, Calaf J. Prevalence and predictors of abnormal glucose metabolism in Mediterranean women with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 148. | Cheung LP, Ma RC, Lam PM, Lok IH, Haines CJ, So WY, Tong PC, Cockram CS, Chow CC, Goggins WB. Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 149. | Bhattacharya SM. Polycystic ovary syndrome and abnormalities in glucose tolerance. Int J Gynaecol Obstet. 2009;105:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 150. | Zhao X, Zhong J, Mo Y, Chen X, Chen Y, Yang D. Association of biochemical hyperandrogenism with type 2 diabetes and obesity in Chinese women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2010;108:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 151. | Stovall DW, Bailey AP, Pastore LM. Assessment of insulin resistance and impaired glucose tolerance in lean women with polycystic ovary syndrome. J Womens Health (Larchmt). 2011;20:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 152. | Lerchbaum E, Schwetz V, Giuliani A, Obermayer-Pietsch B. Influence of a positive family history of both type 2 diabetes and PCOS on metabolic and endocrine parameters in a large cohort of PCOS women. Eur J Endocrinol. 2014;170:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 153. | Gracelyn LJ, Pushpagiri N. Prevalence of glucose tolerance test abnormalities in women with polycystic ovarian syndrome. Int J Reprod Contracept Obstet Gynecol. 2017;4:1739-1745. [DOI] [Full Text] |
| 154. | Li H, Li L, Gu J, Li Y, Chen X, Yang D. Should All Women with Polycystic Ovary Syndrome Be Screened for Metabolic Parameters? PLoS One. 2016;11:e0167036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 155. | Ortiz-Flores AE, Luque-Ramírez M, Fernández-Durán E, Alvarez-Blasco F, Escobar-Morreale HF. Diagnosis of disorders of glucose tolerance in women with polycystic ovary syndrome (PCOS) at a tertiary care center: fasting plasma glucose or oral glucose tolerance test? Metabolism. 2019;93:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |