Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1563
Peer-review started: April 29, 2021
First decision: June 16, 2021
Revised: June 30, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 15, 2021
Processing time: 130 Days and 16 Hours
Obesity is increasing worldwide, and this has major implications in the setting of kidney transplantation. Patients with obesity may have limited access to transplantation and increased posttransplant morbidity and mortality. Most transplant centers incorporate interventions aiming to target obesity in kidney transplant candidates, including dietary education and lifestyle modifications. For those failing nutritional restriction and medical therapy, the use of bariatric surgery may increase the transplant candidacy of patients with obesity and end-stage renal disease (ESRD) and may potentially improve the immediate and late outcomes. Bariatric surgery in ESRD patients is associated with weight loss ranging from 29.8% to 72.8% excess weight loss, with reported mortality and morbidity rates of 2% and 7%, respectively. The most commonly performed bariatric surgical procedures in patients with ESRD and in transplant patients are laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass. However, the correct timing of bariatric surgery and the ideal type of surgery have yet to be determined, although pretransplant LSG seems to be associated with an acceptable risk-benefit profile. We review the impact of obesity on kidney transplant candidates and recipients and in potential living kidney donors, exploring the potential impact of bariatric surgery in addressing obesity in these populations, thereby potentially improving posttransplant outcomes.
Core Tip: Many studies demonstrated that obese patients may have limited access to kidney transplantation and an increased rate of posttransplant complications. Diet and lifestyle modifications may have a limited impact in the treatment of obesity in these patients, while bariatric surgery has the potential to improve the candidacy of these patients and to improve perioperative outcomes. This review will evaluate the potential role of bariatric surgery in the setting of kidney transplantation.
- Citation: Veroux M, Mattone E, Cavallo M, Gioco R, Corona D, Volpicelli A, Veroux P. Obesity and bariatric surgery in kidney transplantation: A clinical review. World J Diabetes 2021; 12(9): 1563-1575
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1563.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1563
Obesity is a major public health concern, affecting more than 25% of the world population. It has been estimated that by 2030, the prevalence of the overweight population [as defined by body mass index (BMI) 25-29.9 kg/m2] will reach 38%, while 20% will be obese (BMI > 30 kg/m2)[1,2]. Obesity-related complications include but are not limited to cardiovascular disease, diabetes mellitus (DM) and cancer[3]. Kidney transplantation represents the best replacement therapy for patients with end-stage renal disease (ESRD), conferring a better quality of life than dialysis[4]. While the number of obese ESRD patients is increasing, obesity may limit access to kidney transplantation due to related comorbidities and posttransplant complications, including wound dehiscence, posttransplant DM and incisional hernia[4-6]. While class 1 obesity (BMI 30-34.9 kg/m2) is not typically a contraindication, most transplant centers consider relative and absolute average BMI cutoffs of 38 and 41 kg/m2, respectively, as contraindications to kidney transplantation[3].
Obesity may increase the risk of cardiovascular complications, DM, and metabolic syndrome, which are well-known posttransplant complications related to the chronic use of immunosuppression[4-9]. However, data on the clinical outcomes of kidney transplantation in obese patients are conflicting due to the lack of consensus on a commonly accepted definition of obesity and on a standard approach to obesity assessment[3]. Most transplant centers incorporate interventions aiming to target obesity in kidney transplant candidates, including dietary education and lifestyle modifications[3-9]. For those failing nutritional restriction and medical therapy, bariatric surgery has recently emerged as a valid therapeutic approach for improving access to kidney transplantation as well as posttransplant outcomes. However, many uncertainties remain regarding the optimal timing of bariatric surgery and the preferred surgical technique: While most transplant surgeons prefer pretransplant bariatric surgery, posttransplant surgery may theoretically reduce the impact of obesity-related complications that are magnified by immunosuppression.
In this review, we explore the impact of bariatric surgery on kidney transplant candidates and recipients and in living kidney donors, trying to address the best strategy to improve the clinical outcomes of kidney transplantation in obese individuals.
According to the 2017 Kidney Disease: Improving Global Outcomes clinical practice guidelines on the evaluation and management of candidates for kidney transplantation, obesity is usually assessed by BMI defined, according to World Health Organization, as weight in kilograms divided by height in meters squared, and by the waist-to-hip ratio, defined as the ratio of the circumference of the waist to that of the hips[10].
Obesity is defined as a BMI ≥ 30 kg/m2 and can be subdivided into classes I (BMI 30-34.9 kg/m2), II (BMI 35-39.9 kg/m2) and III (≥ 40 kg/m2), but BMI is a surrogate measure that could have significant limitations when applied to individuals, as it does not take into account fluid status, muscle mass, body shape and weight distribution[11,12]. The waist-to-hip ratio evaluates abdominal pattern obesity, and ratios > 0.85 for women and > 0.9 for men are considered obese according to the World Health Organization[10]. Recent studies suggested that in ESRD patients on dialysis and in kidney transplant recipients, a higher BMI was associated with lower mortality after adjustment for waist circumference (WC), while a higher WC was more strongly associated with higher mortality after adjustment for BMI[13,14].
Despite these limitations, BMI is currently used for the decision-making process for kidney transplantation in obese populations.
Obesity-related comorbidities, including DM, cardiovascular disease and cancer, can all prevent access to kidney transplantation[3,10,12,15]. Some authors described an “obesity paradox” among patients with ESRD on maintenance dialysis: patients with a BMI < 20 kg/m2 carry the highest relative risk (RR) of mortality, while overweight patients (BMI 25-29.9 kg/m2, RR 0.84), patients with mild obesity (BMI 30-34.9 kg/m2; RR 0.73), and patients with moderate obesity (BMI 35-39.9 kg/m2; RR 0.76) have significantly better outcomes[16]. A potential explanation of this effect is that patients with a higher BMI may have normal to high muscle mass or a favorable WC, and this could have a protective role[16-18]. However, this beneficial effect does not persist after transplantation, since obese transplant patients may have reduced graft and patient survival rates[6,19-23], particularly in patients older than 65 years[24]. As a consequence, obese patients may have limited access to waiting lists and trans
There are several reasons for the reduced access to transplantation for obese patients: in their analysis, Segev et al[25] found that obese patients who were activated on the waiting list had lower access to transplantation because they were less profitable than nonobese patients, and were more frequently bypassed; moreover, United States transplant centers may be more reluctant to transplant obese patients because they may be penalized for a higher than expected rate of patient death or allograft failure in the first posttransplant year, which could occur more frequently in obese patients[25]. Obese patients on waiting lists may therefore develop a number of comorbid conditions necessitating temporary wait-list suspension[21]. On the other hand, in the United States, many insurance payers mandate a trial of medical weight management prior to approving bariatric surgery, but centers that perform bariatric surgery could be reluctant to perform bariatric surgery in these patients due to the potential high rate of complications and death[26].
Although there is no clear consensus on the highest level of BMI to be considered a contraindication to kidney transplantation, most guidelines strongly suggest that for patients with BMI > 30 kg/m2, weight loss should be encouraged[10,25,27]. An increased risk of posttransplant death was observed in patients with a BMI of 34-36 kg/m2[25,27], suggesting that in patients with these high levels of BMI, kidney transplantation may be associated with an unacceptably high risk, and the benefit of transplant should be balanced with the risk of remaining on dialysis[10,27,28].
However, many studies failed to demonstrate a significant survival advantage for patients who lose weight during the waiting list period[18,29,30]. In the study of Molnar et al[18], among 14632 waitlisted hemodialysis patients not receiving a transplant, each 1 kg/m² increase in BMI was associated with a death hazard ratio (HR) of 0.96. However, compared with patients with minimal weight change (± 1 kg), patients who lost 3.0 to 4.9 kg and ≥ 5 kg had a RR of death of 1.3 and 1.51, respectively. More recently, Harhay et al[30] demonstrated that patients who lost ≥ 10% of their pretransplant weight had an increased risk of graft loss, mortality and longer hospitalization stay compared with those who had a < 5% weight change. Possible explanations for these adverse outcomes are the likely malnutrition status associated with weight loss and the rapid weight gain in most patients after transplantation. However, these studies did not differentiate intentional from unintentional weight loss, and only a minority of patients with higher BMI were investigated, so no conclusions about the potential benefits of intentional weight loss can be drawn.
Obese patients who lose weight may also have different access to transplantation by race and ethnicity. Ku et al[31] evaluated 10221 obese patients waitlisted for kidney transplantation to examine the association between weight changes and access to living or deceased donor transplantation by race/ethnicity. Death on the waiting list was more common among those who lost weight (15%) or gained weight (15%) than among those who maintained stable weight (13%). Overall, black people were more likely to lose weight and less likely to gain weight than whites. Overall, weight gain was associated with lower access to transplantation (HR 0.88) compared with maintenance of stable weight, but weight loss was not associated with better access to transplantation (HR 0.96) on the whole, although this correlation was different for recipients of living vs deceased donor organs. Weight loss was associated with improved access to living donor transplantation only for white recipients but not for non-Hispanic blacks or Hispanic recipients[31].
Weight loss in patients with ESRD is extremely difficult due to the restrictions of a renal diet, limited exercise tolerance due to coexisting comorbid conditions, dialysis-related fatigue, and hemodynamic instability[21]. Comprehensive weight loss programs involving regular exercise and nutrition counseling, together with pharmacotherapy, may lead to moderate weight loss among kidney transplant candidates, which could increase access to waiting lists[32]. However, medical management could have limited long-term success, while bariatric surgery could offer a reliable strategy to achieve weight loss in kidney transplant candidates[21,33], with acceptable morbidity and mortality rates[34-36]. Bariatric surgery in ESRD patients is associated with weight loss ranging from 29.8% to 72.8% excess weight loss (%EWL)[37], with reported mortality and morbidity rates of 2% and 7%, respectively[37]. Complications associated with bariatric surgery are higher in ESRD patients than in non-ESRD patients[37]: The mortality rate (2%) observed in the ESRD population is approximately 10 times higher than the mortality rate (0.18%) reported in the general population[38], while the rate of postoperative complications in ESRD patients is significantly higher than that observed in accredited hospitals for bariatric surgery (0.17%)[39]. However, many studies have consistently shown that bariatric surgery in patients with chronic kidney disease (CKD) stage 1 and 2, is associated with slower epidermal growth factor receptor (eGFR) decline and lower risk of kidney failure[40,41]. In a recent study, Kassam et al[40] evaluated the change in renal function in 164 patients with CKD stages 1 to 4 undergoing bariatric surgery. Metabolic surgery resulted in a significant reduction in the BMI in all patients, and 34.3% of patients with previous diabetes achieved complete remission. Kidney function, as measured by eGFR, significantly improved in patients with CKD stages 2, 3a, and 3b, while a similar result was not observed among patients with CKD stages 1 and 4[40], suggesting that the improvements in renal function are limited only to those patients with a mild reduction in kidney function.
The most commonly performed bariatric surgical procedures are laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (RYGB). The former is mainly a restrictive procedure with resection of the greater curve of the stomach, while the latter is a restrictive/malabsorptive procedure that entails creation of a gastric pouch and formation of a Roux-en-Y gastrojejunostomy[21,33]. Open surgery in ESRD patients may be associated with an increased mortality rate compared with the general population. In their registry analysis, Modanlou et al[34] evaluated the results of 186 ESRD patients who underwent an open bariatric surgical procedure: 72 patients underwent surgery prior to activation on the waiting list, 27 patients underwent surgery during wait-listing, and 87 patients underwent posttransplant surgery. The 30-d mortality in wait-listed and posttransplant patients was 3.5% in both groups, while the median EWL ranged between 31% and 61%[34].
Although there are few studies comparing the two surgical procedures in ESRD patients, RYGB seems to have the potential to improve access to renal transplantation and improve long-term survival compared with LSG[35]. Both LSG and RYGB achieve significant excess body weight loss (up to 80% within 24 mo) and may increase the likelihood of being listed for kidney transplantation in up to 50.3% of patients, although a recent meta-analysis reported that only 25% of patients had access to transplant at a median follow-up of 48 mo[37]. A recent analysis of the ESRD population using a probabilistic Markov model concluded that RYGB could improve access to renal transplantation and thereby increase long-term survival[42], but it could be associated with slightly higher morbidity and mortality rates[43].
In the ESRD population, LSG could be preferable and may offer significant advantages over RYGB, including an easier and faster surgical procedure and a lower incidence of surgical complications[37,44], and may increase access to the transplant waiting list and improve posttransplant outcomes[43,44-48]. Moreover, LSG does not alter immunosuppressive pharmacokinetics, avoiding under- and overimmunosuppression[37,43,48-50]. The correct timing of bariatric surgery is still a controversial issue. Although bariatric surgery in ESRD patients could be associated with an increased rate of postsurgical complications, including an increased rate of reoperation and readmission[51], most patients could benefit from pretransplant bariatric surgery to increase access to the waiting list and to reduce obesity-related complications, including diabetes and cardiovascular disease, that could worsen after transplantation. In the largest series reported in the literature, Kassam et al[48] evaluated the clinical outcomes of LSG in the ESRD population and access to the transplant waiting list. LSG reduced hypertension and the need for antihypertensive medications and reduced the incidence of diabetes (59.6% vs 32.5%, P < 0.01). Sixty-three percent of patients with ESRD who achieved a BMI of ≤ 40 kg/m2 were waitlisted and received a kidney transplant after a mean overall time from LSG to transplant of 1.9 ± 1.3 years. There was no significant difference in survival between patients who received a kidney transplant after LSG and those who remained waitlisted[48], suggesting that LSG does not increase the morbidity rate and has the potential to reduce obesity-related comorbidities, possibly improving long-term outcomes. In their retrospective study, Cohen et al[52] compared the outcome of pretransplant and posttransplant bariatric surgery: Compared to BMI-matched controls, pretransplant bariatric surgery was associated with a 1-year increased risk of acute rejection and a decreased risk of delayed graft function. Interestingly, there was no significant difference in BMI in the 5 years after bariatric surgery between the two groups, while both pretransplant and posttransplant bariatric surgery was associated with a decreased risk of allograft failure and mortality[52].
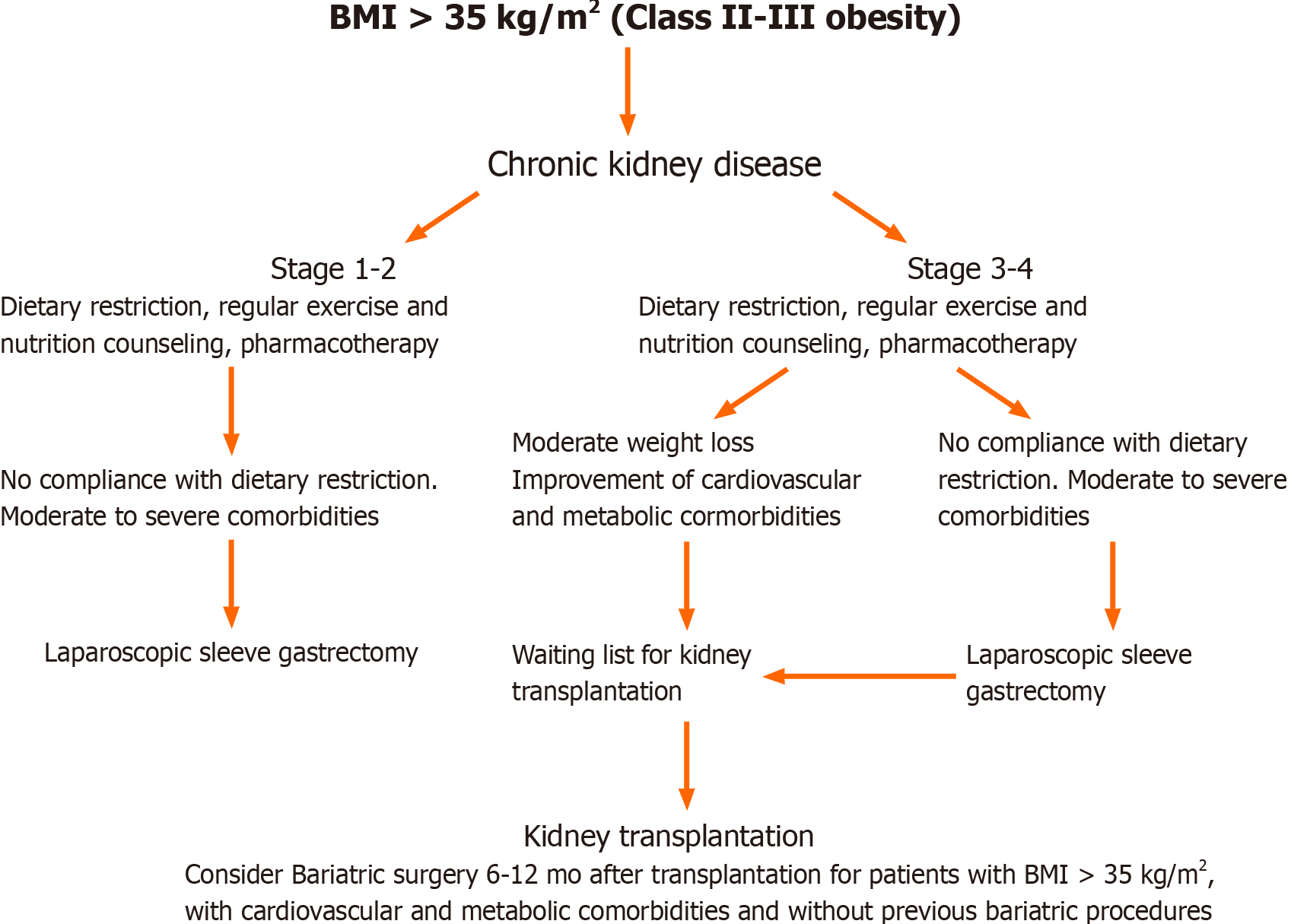
In summary, pretransplant bariatric surgery is safe and could increase access to transplantation for obese patients. LSG results in sustained weight loss and is associated with an improvement in obesity-related comorbidities (Figure 1). Although pretransplant bariatric surgery is associated with acceptable outcomes for patients undergoing kidney transplantation, the correct timing has yet to be determined.
Kidney transplantation offers significantly better patient survival and quality of life than remaining on dialysis for both obese and nonobese patients[20,22,53,54].
Obese patients may have an increased peritransplant risk of death, particularly for patients with a BMI > 30 kg/m2 receiving a graft from a marginal donor, while living donor kidney transplantation seems to offer a reduced risk[20,55]. In an analysis from the USRDS including 7521 patients, Glanton et al[56] compared the mortality rates among transplant recipients and patients on hemodialysis with class I, II and III obesity and found that kidney transplantation from both deceased (HR 0.39, 95%CI: 0.33-0.47) and living donors (HR 0.23, 95%CI: 0.16-0.34) was associated with significant lower mortality rate of those who stayed on dialysis waiting for a kidney. Interestingly, the beneficial effect of transplantation was lost in the subgroup analysis of patients with class III obesity[57]. The beneficial effect of kidney transplantation among obese patients was recently confirmed by Gill et al[20], who analyzed a large cohort from the US renal registry and reported a 66% reduction in the risk of death in all BMI groups for patients receiving a living donor kidney, whereas among the deceased donor recipients, the reduction in the risk of death was 66% in patients with class I and II obesity and 48%in patients with class III obesity[20]. The reduction in the risk of death was lower for patients receiving a graft from a marginal deceased donor, while kidney transplantation did not offer a survival benefit in African Americans with class III obesity[20]. Therefore, some authors have suggested that for patients with a higher BMI, living transplantation represents the preferred choice, while kidney transplantation from deceased donors could be associated with an unacceptable mortality risk[20,21].
The increased mortality risk observed in the peritransplant period in obese patients may be correlated with concomitant comorbid conditions that could worsen after transplantation or be the result of peritransplant complications.
Many studies have demonstrated that, similarly, graft survival in obese patients is inferior[20,21,55,58,59], and this pattern follows a U-shaped distribution, as patients with BMI values either lower or higher than the normal range (either ≤ 20 or ≥ 26 kg/m2) have worse posttransplant outcomes[58,60]. Moreover, significant post
However, the effect of BMI on posttransplant outcomes may vary by patient characteristics. In their analysis among 296807 adult kidney transplant recipients from the Scientific Registry of Transplant Recipients, Schold et al[60] demonstrated that BMI follows a “J-Shaped” risk profile with elevated risks for overall graft loss with low BMI and obesity. Moreover, the risk of graft loss associated with BMI is strictly dependent on the patients’ characteristics: Low BMI was a relatively higher risk for older recipients (> 60 years) and males but not for younger patients, while high BMI was associated with an elevated risk for Caucasians and attenuated risk among African Americans and people with type II diabetes.
Obesity may increase the surgical complexity and is significantly associated with longer operative time and risk of wound dehiscence compared to normal-weight patients[62]. In obese patients, the risk of parietal dehiscence is significantly increased for BMI > 26 kg/m2, while an increased risk of intraoperative blood loss and ureteral stenosis was observed for BMI > 32 kg/m2, and the risk of abdominal wall hematoma was increased beyond a BMI of 34 kg/m2[63]. Overall, obese patients have an incidence of wound infections and incisional hernia of 4%-40% due to the longer operative time; the concomitant use of corticosteroids, sirolimus, or everolimus; and the presence of vascular disease[26,46,56]. Moreover, obesity may also increase the risk of surgical site infection (SSI), which is a well-known cause of incisional hernia[64,65]. Wound complications are significantly associated with a BMI over 30 kg/m2, and in most cases, obesity is considered the most significant risk factor for the development of wound complications[65,66], although some authors did not find such an association[67].
Moreover, obese transplant recipients have an increased risk of delayed graft function, probably as a consequence of a longer operative time, a prolonged hospital stay, an increased rate of acute rejection, an increased rate of new-onset DM and hospital readmission[6,18,57,68-70].
Considering that some studies showed comparable outcomes between obese and nonobese patients in the absence of surgical complications, the adoption of a correct surgical procedure that could minimize the incidence of such complications is mandatory[33,71]. The adoption of a minimally invasive surgical approach, including robotic-assisted kidney transplantation (RAKT), has shown promising results compared to open KT and could increase access to kidney transplantation for obese patients[72,73]. Additionally, RAKT is associated with comparable patient and graft survival compared with open surgery[72], a significant reduction in SSI in obese recipients, and comparable graft and patient survival compared to the nonobese population[73,74].
Few studies have investigated the role of bariatric surgery after kidney transplantation in morbidly obese recipients. There are many issues related to bariatric surgery after kidney transplantation: First, surgical procedures in kidney recipients may be associated with a higher risk of complications than in the general population[75-80], and second, bariatric surgery can affect immunosuppressive therapy absorption. Bariatric surgery in kidney transplant recipients may be associated with an increased operative time, length of stay, readmission, and increased SSI but not with increased mortality[75-80]. Previous diabetes and the use of corticosteroids do not increase the risk of postoperative complications after bariatric surgery in solid organ trans
Bariatric surgery after kidney transplantation is associated with significant weight loss and a reduction in comorbidities but also with an increased risk of complications[37,52,75]. In the largest series of RYGB reported in kidney transplant recipients, Modanlou et al[34] reported a 3.5% mortality rate, with a median excess body weight loss of 31%-61%. Sleeve gastrectomy and RYGB have comparable outcomes with low postoperative complications[43,75-80]: A slight increase in mortality was observed in patients undergoing RYGB[43], but both LSG and RYGB were associated with improvements in comorbidities and graft function[43,75-80] and with a reduction in urinary protein excretion[76]. Cohen et al[52] compared the outcomes of 43 patients who underwent pretransplant bariatric surgery and 21 patients who underwent posttransplant bariatric surgery. BMI was similar between the two groups, and 5 years after bariatric surgery, there was no significant difference in BMI between the two groups (36 kg/m2 vs 32 kg/m2, P = 0.814). Compared to matched controls, post
In summary, bariatric surgery after kidney transplantation is associated with a significant and sustained weight loss, reduction in comorbidities and improvement in graft function without significant alteration of immunosuppressive therapy absorption. The potential increase in postoperative complications and mortality warrants a careful evaluation of kidney transplant recipients scheduled for bariatric surgery.
There are approximately 2700 living-donor kidney transplants performed worldwide each year, and more than 25% of these are considered obese at the time of donation[82]. Obesity may be a relevant factor influencing clinical outcomes even in living-donor kidney transplantation and may be associated with lower preoperative kidney function and longer operative time[82]. Kinoshita et al[83] compared the results of living kidney transplantations from medically complex living donors, defined by the presence of older age, obesity or DM, with standard living donors; they found that kidney recipients of medically complex living donors had a higher risk of death-censored graft loss, while no significant difference in renal function in the short term was observed between standard and medically complex living donors. When compared to donors with normal BMI, kidney transplants from donors with higher BMI (> 25 kg/m2) are associated with a higher risk of graft failure[84], and living donor obesity is associated with a 30% increased risk of long-term mortality compared with nonobese counterparts (adjusted HR: 1.32, 95%CI: 1.09-1.60, P = 0.006)[85].
Up to one-fourth of potential living kidney donors may be excluded from living donation due to obesity, which could encourage medical and surgical strategies to achieve significant weight loss. Although strongly motivated, living kidney donors are less prone to adhere to diet and lifestyle modifications for weight loss, and only 13% lose enough weight to attain a BMI < 35 kg/m2 and then undergo donation[85]. Bariatric surgery is, therefore, a potential valid weight loss strategy for potential living donors. However, many ethical issues may arise when considering the opportunity for bariatric surgery in living kidney donors. Living kidney donors should be aware that bariatric surgery is predicated only on the potential donor’s benefit and does not finalize the kidney donation or provide any future benefit to the intended recipient[86,87], and referral to an independent bariatric surgeon to assess the potential benefits and risks of surgery is recommended. Another crucial point is the timing of living donation after bariatric surgery. Montgomery et al[87] suggested that kidney donation should be performed when the potential living donor meets prespecified transplant center donation eligibility requirements, such as BMI < 30 kg/m2, and should remain stable for at least three months.
Very few studies have reported the outcomes of living kidney donation after predonation bariatric surgery. Earlier studies reported a 30%-54% decrease in BMI after bariatric surgery[88]. More recently, Nguyen et al[89] reported a series of 22 living kidney donors who underwent bariatric surgery 0.7-22 years before living donation. Interestingly, 18 donors would have been excluded from donation due to high BMI. All donors lost sufficient weight to subsequently become candidates for living kidney donation, and 17 donors reached a BMI < 35 kg/m2 after bariatric surgery. No significant differences in terms of length of stay, warm ischemic time or postoperative complications were observed when compared with 37 donors with a BMI of 35–40 kg/m2. Moreover, bariatric surgery did not significantly impact the subsequent laparoscopy for living donor nephrectomy[89]. Due to the limited cases reported in the literature, the ideal type of bariatric surgery to be performed in morbidly obese kidney donors has to be determined. RYGB has historically been the most commonly used technique since it guarantees a durable weight reduction and reversal of obesity-associated comorbidities[88,89]. However, it is associated with long-term nutritional derangements, and it has been recently supplanted by LSG as the most common bariatric surgery procedure, since it has proven comparable weight reduction with RYGB, with fewer intra- and postoperative complications, including nutritional deficiencies[88,89].
In summary, initial experience with bariatric surgery in potential living donors suggests that bariatric surgery is safe, is associated with sustained weight loss and could increase the rate of kidney donation. Sleeve gastrectomy should be preferred to RYGB due to its risk-benefit profile.
Obesity represents a major obstacle to access to kidney transplantation due to the potential increased risk of postoperative complications and mortality. However, obesity should not preclude kidney transplantation, and any efforts should be made to improve the outcomes of these patients. Bariatric surgery has been proven to be safe and helpful in reducing weight loss and obesity-related comorbidities and in increasing access to kidney transplantation. Posttransplant bariatric surgery may result in better graft survival and function but also in a high rate of postoperative complications. There is no consensus regarding the optimal timing and the ideal type of bariatric surgery, although sleeve gastrectomy seems to be associated with a reduced risk of postoperative complications. Future studies should evaluate the potential impact of bariatric surgery in the long-term reduction in cardiovascular complications and in the management of posttransplant DM in obese recipients.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mavroeidis VK S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
| 1. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2062] [Article Influence: 121.3] [Reference Citation Analysis (2)] |
| 2. | Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2156] [Article Influence: 239.6] [Reference Citation Analysis (0)] |
| 3. | Diwan TS, Lee TC, Nagai S, Benedetti E, Posselt A, Bumgardner G, Noria S, Whitson BA, Ratner L, Mason D, Friedman J, Woodside KJ, Heimbach J. Obesity, transplantation, and bariatric surgery: An evolving solution for a growing epidemic. Am J Transplant. 2020;20:2143-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Veroux M, Corona D, Veroux P. Kidney transplantation: future challenges. Minerva Chir. 2009;64:75-100. [PubMed] |
| 5. | Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 956] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 6. | Hill CJ, Courtney AE, Cardwell CR, Maxwell AP, Lucarelli G, Veroux M, Furriel F, Cannon RM, Hoogeveen EK, Doshi M, McCaughan JA. Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. 2015;30:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Veroux M, Corona D, Giuffrida G, Gagliano M, Sorbello M, Virgilio C, Tallarita T, Zerbo D, Giaquinta A, Fiamingo P, Macarone M, Li Volti G, Caglia P, Veroux P. New-onset diabetes mellitus after kidney transplantation: the role of immunosuppression. Transplant Proc. 2008;40:1885-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Veroux M, Tallarita T, Corona D, Sinagra N, Giaquinta A, Zerbo D, Guerrieri C, D'Assoro A, Cimino S, Veroux P. Conversion to sirolimus therapy in kidney transplant recipients with new onset diabetes mellitus after transplantation. Clin Dev Immunol. 2013;2013:496974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Xia M, Yang H, Tong X, Xie H, Cui F, Shuang W. Risk factors for new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. J Diabetes Investig. 2021;12:109-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Chadban SJ, Ahn C, Axelrod DA, Foster BJ, Kasiske BL, Kher V, Kumar D, Oberbauer R, Pascual J, Pilmore HL, Rodrigue JR, Segev DL, Sheerin NS, Tinckam KJ, Wong G, Knoll GA. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation. 2020;104:S11-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 360] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 11. | Camilleri B, Bridson JM, Sharma A, Halawa A. From chronic kidney disease to kidney transplantation: The impact of obesity and its treatment modalities. Transplant Rev (Orlando). 2016;30:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Glicklich D, Mustafa MR. Obesity in Kidney Transplantation: Impact on Transplant Candidates, Recipients, and Donors. Cardiol Rev. 2019;27:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Postorino M, Marino C, Tripepi G, Zoccali C; CREDIT (Calabria Registry of Dialysis and Transplantation) Working Group. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Ahmadi SF, Zahmatkesh G, Streja E, Molnar MZ, Rhee CM, Kovesdy CP, Gillen DL, Steiner S, Kalantar-Zadeh K. Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. Am J Nephrol. 2014;40:315-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Dolla C, Naso E, Mella A, Allesina A, Giraudi R, Torazza MC, Vanzino SB, Gallo E, Lavacca A, Fop F, Biancone L. Impact of type 2 diabetes mellitus on kidney transplant rates and clinical outcomes among waitlisted candidates in a single center European experience. Sci Rep. 2020;10:22000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in 'healthier' as compared with 'sicker' haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2001;16:2386-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 311] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, Kovesdy CP, Kalantar-Zadeh K. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, Krishnan M, Nissenson AR, Danovitch GM, Kalantar-Zadeh K. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11:725-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Gill JS, Hendren E, Dong J, Johnston O, Gill J. Differential association of body mass index with access to kidney transplantation in men and women. Clin J Am Soc Nephrol. 2014;9:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Gill JS, Lan J, Dong J, Rose C, Hendren E, Johnston O, Gill J. The survival benefit of kidney transplantation in obese patients. Am J Transplant. 2013;13:2083-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 21. | Lesage J, Gill JS. Management of the obese kidney transplant candidate. Transplant Rev (Orlando). 2017;31:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Poggio ED, Augustine JJ, Arrigain S, Brennan DC, Schold JD. Long-term kidney transplant graft survival-Making progress when most needed. Am J Transplant. 2021;21:2824-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 23. | Naik AS, Sakhuja A, Cibrik DM, Ojo AO, Samaniego-Picota MD, Lentine KL. The Impact of Obesity on Allograft Failure After Kidney Transplantation: A Competing Risks Analysis. Transplantation. 2016;100:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Hatamizadeh P, Molnar MZ, Streja E, Lertdumrongluk P, Krishnan M, Kovesdy CP, Kalantar-Zadeh K. Recipient-related predictors of kidney transplantation outcomes in the elderly. Clin Transplant. 2013;27:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008;19:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Orandi BJ, Purvis JW, Cannon RM, Smith AB, Lewis CE, Terrault NA, Locke JE. Bariatric surgery to achieve transplant in end-stage organ disease patients: A systematic review and meta-analysis. Am J Surg. 2020;220:566-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, Rush D, Cole E; Kidney Transplant Working Group of the Canadian Society of Transplantation. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Campbell S, Pilmore H, Gracey D, Mulley W, Russell C, McTaggart S. KHA-CARI guideline: recipient assessment for transplantation. Nephrology (Carlton). 2013;18:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Chang TI, Ngo V, Streja E, Chou JA, Tortorici AR, Kim TH, Kim TW, Soohoo M, Gillen D, Rhee CM, Kovesdy CP, Kalantar-Zadeh K. Association of body weight changes with mortality in incident hemodialysis patients. Nephrol Dial Transplant. 2017;32:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Harhay MN, Ranganna K, Boyle SM, Brown AM, Bajakian T, Levin Mizrahi LB, Xiao G, Guy S, Malat G, Segev DL, Reich D, McAdams-DeMarco M. Association Between Weight Loss Before Deceased Donor Kidney Transplantation and Posttransplantation Outcomes. Am J Kidney Dis. 2019;74:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Ku E, Whelan AM, McCulloch CE, Lee B, Niemann CU, Roll GR, Grimes BA, Johansen KL. Weighing the waitlist: Weight changes and access to kidney transplantation among obese candidates. PLoS One. 2020;15:e0242784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van Niekerk M, Macdougall IC. Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis. 2010;55:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Di Cocco P, Okoye O, Almario J, Benedetti E, Tzvetanov IG, Spaggiari M. Obesity in kidney transplantation. Transpl Int. 2020;33:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Modanlou KA, Muthyala U, Xiao H, Schnitzler MA, Salvalaggio PR, Brennan DC, Abbott KC, Graff RJ, Lentine KL. Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States renal data system and literature review. Transplantation. 2009;87:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Friedman AN, Wahed AS, Wang J, Courcoulas AP, Dakin G, Hinojosa MW, Kimmel PL, Mitchell JE, Pomp A, Pories WJ, Purnell JQ, le Roux C, Spaniolas K, Steffen KJ, Thirlby R, Wolfe B. Effect of Bariatric Surgery on CKD Risk. J Am Soc Nephrol. 2018;29:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Sheetz KH, Gerhardinger L, Dimick JB, Waits SA. Bariatric Surgery and Long-term Survival in Patients With Obesity and End-stage Kidney Disease. JAMA Surg. 2020;155:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 37. | Guggino J, Coumes S, Wion N, Reche F, Arvieux C, Borel AL. Effectiveness and Safety of Bariatric Surgery in Patients with End-Stage Chronic Kidney Disease or Kidney Transplant. Obesity (Silver Spring). 2020;28:2290-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Cardoso L, Rodrigues D, Gomes L, Carrilho F. Short- and long-term mortality after bariatric surgery: A systematic review and meta-analysis. Diabetes Obes Metab. 2017;19:1223-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 39. | De Smet J, Van Bocxlaer J, Boussery K. The influence of bypass procedures and other anatomical changes in the gastrointestinal tract on the oral bioavailability of drugs. J Clin Pharmacol. 2013;53:361-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Kassam AF, Taylor ME, Morris MC, Watkins BM, Thompson JR, Schauer DP, Smith EP, Diwan TS. The impact of sleeve gastrectomy on renal function in patients with chronic kidney disease varies with severity of renal insufficiency. Surg Obes Relat Dis. 2020;16:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Chintam K, Chang AR. Strategies to Treat Obesity in Patients With CKD. Am J Kidney Dis. 2021;77:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 42. | Choudhury RA, Hoeltzel G, Prins K, Chow E, Moore HB, Lawson PJ, Yoeli D, Pratap A, Abt PL, Dumon KR, Conzen KD, Nydam TL. Sleeve Gastrectomy Compared with Gastric Bypass for Morbidly Obese Patients with End Stage Renal Disease: a Decision Analysis. J Gastrointest Surg. 2020;24:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Dziodzio T, Biebl M, Öllinger R, Pratschke J, Denecke C. The Role of Bariatric Surgery in Abdominal Organ Transplantation-the Next Big Challenge? Obes Surg. 2017;27:2696-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Freeman CM, Woodle ES, Shi J, Alexander JW, Leggett PL, Shah SA, Paterno F, Cuffy MC, Govil A, Mogilishetty G, Alloway RR, Hanseman D, Cardi M, Diwan TS. Addressing morbid obesity as a barrier to renal transplantation with laparoscopic sleeve gastrectomy. Am J Transplant. 2015;15:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Kienzl-Wagner K, Weissenbacher A, Gehwolf P, Wykypiel H, Öfner D, Schneeberger S. Laparoscopic sleeve gastrectomy: gateway to kidney transplantation. Surg Obes Relat Dis. 2017;13:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Kim Y, Bailey AJ, Morris MC, Kassam AF, Shah SA, Diwan TS. Kidney transplantation after sleeve gastrectomy in the morbidly obese candidate: results of a 2-year experience. Surg Obes Relat Dis. 2020;16:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Kim Y, Jung AD, Dhar VK, Tadros JS, Schauer DP, Smith EP, Hanseman DJ, Cuffy MC, Alloway RR, Shields AR, Shah SA, Woodle ES, Diwan TS. Laparoscopic sleeve gastrectomy improves renal transplant candidacy and posttransplant outcomes in morbidly obese patients. Am J Transplant. 2018;18:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Kassam AF, Mirza A, Kim Y, Hanseman D, Woodle ES, Quillin RC 3rd, Johnson BL, Govil A, Cardi M, Schauer DP, Smith EP, Diwan TS. Long-term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am J Transplant. 2020;20:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 50. | Diwan TS, Lichvar AB, Leino AD, Vinks AA, Christians U, Shields AR, Cardi MA, Fukuda T, Mizuno T, Kaiser T, Woodle ES, Alloway RR. Pharmacokinetic and pharmacogenetic analysis of immunosuppressive agents after laparoscopic sleeve gastrectomy. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Cohen JB, Tewksbury CM, Torres Landa S, Williams NN, Dumon KR. National Postoperative Bariatric Surgery Outcomes in Patients with Chronic Kidney Disease and End-Stage Kidney Disease. Obes Surg. 2019;29:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 52. | Cohen JB, Lim MA, Tewksbury CM, Torres-Landa S, Trofe-Clark J, Abt PL, Williams NN, Dumon KR, Goral S. Bariatric surgery before and after kidney transplantation: long-term weight loss and allograft outcomes. Surg Obes Relat Dis. 2019;15:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Bennett WM, McEvoy KM, Henell KR, Pidikiti S, Douzdjian V, Batiuk T. Kidney transplantation in the morbidly obese: complicated but still better than dialysis. Clin Transplant. 2011;25:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Krishnan N, Higgins R, Short A, Zehnder D, Pitcher D, Hudson A, Raymond NT. Kidney Transplantation Significantly Improves Patient and Graft Survival Irrespective of BMI: A Cohort Study. Am J Transplant. 2015;15:2378-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 55. | Hoogeveen EK, Aalten J, Rothman KJ, Roodnat JI, Mallat MJ, Borm G, Weimar W, Hoitsma AJ, de Fijter JW. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation. 2011;91:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 56. | Glanton CW, Kao TC, Cruess D, Agodoa LY, Abbott KC. Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int. 2003;63:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Aziz F, Ramadorai A, Parajuli S, Garg N, Mohamed M, Mandelbrot DA, Foley DP, Garren M, Djamali A. Obesity: An Independent Predictor of Morbidity and Graft Loss after Kidney Transplantation. Am J Nephrol. 2020;51:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 394] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 59. | Grosso G, Corona D, Mistretta A, Zerbo D, Sinagra N, Giaquinta A, Caglià P, Amodeo C, Leonardi A, Gula R, Veroux P, Veroux M. The role of obesity in kidney transplantation outcome. Transplant Proc. 2012;44:1864-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Schold JD, Augustine JJ, Huml AM, Fatica R, Nurko S, Wee A, Poggio ED. Effects of body mass index on kidney transplant outcomes are significantly modified by patient characteristics. Am J Transplant. 2021;21:751-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Chang SH, McDonald SP. Post-kidney transplant weight change as marker of poor survival outcomes. Transplantation. 2008;85:1443-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Chen JH, Lee CH, Chang CM, Yin WY. Successful Management of New-Onset Diabetes Mellitus and Obesity With the Use of Laparoscopic Sleeve Gastrectomy After Kidney Transplantation-A Case Report. Transplant Proc. 2016;48:938-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Gullo-Neto S, Padoin AV, Queiroz de Carvalho JE, Wendling R, Traesel MA, Kroth L, Miranda C, Balestro AC, Siqueira R, Chao Lisot B, Lima S, Mottin CC, Saitovitch D. Metabolic surgery for the treatment of type 2 diabetes in pancreas after kidney transplant candidates. Transplant Proc. 2014;46:1741-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Valente JF, Hricik D, Weigel K, Seaman D, Knauss T, Siegel CT, Bodziak K, Schulak JA. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation. Am J Transplant. 2003;3:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Smith CT, Katz MG, Foley D, Welch B, Leverson GE, Funk LM, Greenberg JA. Incidence and risk factors of incisional hernia formation following abdominal organ transplantation. Surg Endosc. 2015;29:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Shahrestani S, Tran HM, Pleass HC, Hawthorne WJ. Optimal surgical management in kidney and pancreas transplantation to minimise wound complications: A systematic review and meta-analysis. Ann Med Surg (Lond). 2018;33:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Howard RJ, Thai VB, Patton PR, Hemming AW, Reed AI, Van der Werf WJ, Fujita S, Karlix JL, Scornik JC. Obesity does not portend a bad outcome for kidney transplant recipients. Transplantation. 2002;73:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Kwan JM, Hajjiri Z, Metwally A, Finn PW, Perkins DL. Effect of the Obesity Epidemic on Kidney Transplantation: Obesity Is Independent of Diabetes as a Risk Factor for Adverse Renal Transplant Outcomes. PLoS One. 2016;11:e0165712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 69. | Lentine KL, Delos Santos R, Axelrod D, Schnitzler MA, Brennan DC, Tuttle-Newhall JE. Obesity and kidney transplant candidates: how big is too big for transplantation? Am J Nephrol. 2012;36:575-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 70. | Erturk T, Berber I, Cakir U. Effect of Obesity on Clinical Outcomes of Kidney Transplant Patients. Transplant Proc. 2019;51:1093-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N, Englesbe MJ. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg. 2009;250:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 72. | Oberholzer J, Giulianotti P, Danielson KK, Spaggiari M, Bejarano-Pineda L, Bianco F, Tzvetanov I, Ayloo S, Jeon H, Garcia-Roca R, Thielke J, Tang I, Akkina S, Becker B, Kinzer K, Patel A, Benedetti E. Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant. 2013;13:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Tzvetanov IG, Spaggiari M, Tulla KA, Di Bella C, Okoye O, Di Cocco P, Jeon H, Oberholzer J, Cristoforo Giulianotti P, Benedetti E. Robotic kidney transplantation in the obese patient: 10-year experience from a single center. Am J Transplant. 2020;20:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 74. | Prudhomme T, Beauval JB, Lesourd M, Roumiguié M, Decaestecker K, Vignolini G, Campi R, Serni S, Territo A, Gausa L, Tugcu V, Sahin S, Alcaraz A, Musquera M, Stockle M, Janssen M, Fornara P, Mohammed N, Del Bello A, Kamar N, Sallusto F, Breda A, Doumerc N. Robotic-assisted kidney transplantation in obese recipients compared to non-obese recipients: the European experience. World J Urol. 2021;39:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Yemini R, Nesher E, Winkler J, Carmeli I, Azran C, Ben David M, Mor E, Keidar A. Bariatric surgery in solid organ transplant patients: Long-term follow-up results of outcome, safety, and effect on immunosuppression. Am J Transplant. 2018;18:2772-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 76. | Golomb I, Winkler J, Ben-Yakov A, Benitez CC, Keidar A. Laparoscopic sleeve gastrectomy as a weight reduction strategy in obese patients after kidney transplantation. Am J Transplant. 2014;14:2384-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Montgomery JR, Cohen JA, Brown CS, Sheetz KH, Chao GF, Waits SA, Telem DA. Perioperative risks of bariatric surgery among patients with and without history of solid organ transplant. Am J Transplant. 2020;20:2530-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Fagenson AM, Mazzei MM, Zhao H, Lu X, Edwards MA. Bariatric Surgery Outcomes in Patients with Prior Solid Organ Transplantation: an MBSAQIP Analysis. Obes Surg. 2020;30:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Edwards MA, Fagenson AM, Mazzei M, Zhao H. Bariatric Surgery in Prior Solid Organ Transplantation Patients: Is Race a Predictor of Adverse Outcomes? Obes Surg. 2020;30:4381-4390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Fagenson AM, Mazzei MM, Zhao H, Edwards MA. Bariatric surgery in posttransplantat patients: does diabetes influence outcomes? Surg Obes Relat Dis. 2020;16:1266-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Spaggiari M, Di Cocco P, Tulla K, Kaylan KB, Masrur MA, Hassan C, Alvarez JA, Benedetti E, Tzvetanov I. Simultaneous robotic kidney transplantation and bariatric surgery for morbidly obese patients with end-stage renal failure. Am J Transplant. 2021;21:1525-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Schussler L, Khetan P, Peacock M, Dickstein E, LaPointe-Rudow D, Palese M, Arvelakis A, Herron D, Shapiro R, Florman S, Chin EH. Is obesity a contraindication for kidney donation? Surg Endosc. 2020;34:4632-4637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Kinoshita Y, Yagisawa T, Sugihara T, Hara K, Takeshima S, Kubo T, Shinzato T, Shimizu T, Suzuki M, Maeshima A, Kamei J, Fujisaki A, Ando S, Kume H, Fujimura T. Clinical outcomes in donors and recipients of kidney transplantations involving medically complex living donors - a retrospective study. Transpl Int. 2020;33:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Naik AS, Zhong Y, Parasuraman R, Doshi M, Norman S, Lu Y, Shaban E, Shahinian V, Schaubel DE. The temporal and long-term impact of donor body mass index on recipient outcomes after kidney transplantation - a retrospective study. Transpl Int. 2020;33:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Locke JE, Reed RD, Massie AB, MacLennan PA, Sawinski D, Kumar V, Snyder JJ, Carter AJ, Shelton BA, Mustian MN, Lewis CE, Segev DL. Obesity and long-term mortality risk among living kidney donors. Surgery. 2019;166:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Sachdeva M, Sunday S, Israel E, Varghese J, Rosen L, Bhaskaran M, Molmenti EP, Mattana J. Obesity as a barrier to living kidney donation: a center-based analysis. Clin Transplant. 2013;27:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Montgomery JR, Telem DA, Waits SA. Bariatric surgery for prospective living kidney donors with obesity? Am J Transplant. 2019;19:2415-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Branco AW, Branco Filho AJ, Kondo W. Laparoscopic live donor nephrectomy in patients surgically treated for morbid obesity. Int Braz J Urol. 2007;33:377-9; discussion 379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Nguyen MJP, Carpenter D, Tadros J, Mathur A, Sandoval PR, Woodle ES, Diwan T, Ratner LE. Bariatric surgery prior to living donor nephrectomy: a solution to expand the living donor kidney pool - a retrospective study. Transpl Int. 2019;32:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |