Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1463
Peer-review started: February 26, 2021
First decision: April 20, 2021
Revised: April 24, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: September 15, 2021
Processing time: 192 Days and 15.5 Hours
The gut microbiota (GM) plays a role in the development and progression of type 1 and type 2 diabetes mellitus (DM) and its complications. Gut dysbiosis contributes to the pathogenesis of DM. The GM has been shown to influence the efficacy of different antidiabetic medications. Intake of gut biotics, like prebiotics, probiotics and synbiotics, can improve the glucose control as well as the metabolic profile associated with DM. There is some preliminary evidence that it might even help with the cardiovascular, ophthalmic, nervous, and renal complications of DM and even contribute to the prevention of DM. More large-scale research studies are needed before wide spread use of gut biotics in clinical practice as an adjuvant therapy to the current management of DM.
Core Tip: The emerging role of the gut microbiome on diabetes development, progression as well as prevention has been discussed in this manuscript. The significance of gut dysbiosis in the aetiopathogenesis of diabetes mellitus and its complications has been reviewed. A bidirectional relationship exists between the antidiabetic drugs and the gut microbiome. Faecal transplantation, and bariatric surgery, typically used to treat morbid obesity, have also been shown to improve commensal gut microbiota changes. Diabetic outcomes and management can improve with better understanding of the drug-gut microbiome interactions. There is emerging evidence pointing out that gut biotics can be an add-on therapy with the antidiabetic management. To our knowledge, there is no evidence about the role of gut microbes of diabetic patients who had pancreatic cell transplantation, as well as the role of gut biotics influencing the management in this group.
- Citation: Alagiakrishnan K, Halverson T. Holistic perspective of the role of gut microbes in diabetes mellitus and its management. World J Diabetes 2021; 12(9): 1463-1478
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1463.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1463
Globally diabetes mellitus (DM) is a common medical disorder and is seen in pandemic proportions[1] with the global prevalence in adult subjects is roughly 10%[2]. The International Diabetes Federation projected by 2035, there will be 592 million cases of diabetes in the world[3]. DM type 1 is secondary to auto-immune- mediated loss of beta-cell function and is seen in 5% of the diabetic population. DM type 2 is mainly due to insulin resistance and is seen in 95% of diabetic subjects[4]. The 2016 US National Health Interview Survey data showed roughly 8.58% of the population had type 2 DM and 0.55% had type 1 DM[5].
Various research has been done in the last decade since the study of the human microbiome in 2012[6,7]. Microbes contribute to 2% of human body weight and the bacterial genomes exceeds human genes by a factor of 150[8,9]. Gut microbiota (GM) varies with age, diet, geographical location, life style, and the use of xenobiotics[10-12]. In the recent years there have been more focus on the GM in the development, progression, and distant organ complications due to DM[13]. Many studies have shown the role of the gut microbiome in DM[14-17].
The gut microbiome starts to develop with the mode of birth and it is influenced by environmental factors, diet, as well as certain medications, including antibiotics[18]. There are differences between the gut microbes seen between non-diabetic and diabetic subjects[20] (Table 1). Gut dysbiosis plays a role in numerous diseases including DM. Both altered GM and endocrine disrupters can influence the development of DM[21]. In this literature review, we analyzed the evidence for the role of GM in the development, pathogenesis, complications, management, and prevention of DM.
| Location | Change in microbiome | Ref. |
| Type 1 diabetes | ||
| Gastrointestinal tract | (1) Decreased: Prevotella; Megamona; Acidaminococcus; and (2) Increased: Bacteriodes | Elena et al[25], 2019 |
| Gastrointestinal tract | (1) Decreased: Bifidobacterium adolescentis; Bifidobacteria; and (2) Increased: Clostridium perfingens; Bacteroides | De Goffau et al[122], 2013 |
| Gastrointestinal tract | Increased: Leptotrichia goodfellowii; Bacillus cerus; Enterobacter mori LMG 25706 | Tai et al[123], 2016 |
| Gastrointestinal tract | Increased: Bacteroidetes/Firmicutes | Giongo et al[124], 2011 |
| Gastrointestinal tract | (1) Decreased: Faecalibacterium prausnitzii; and (2) Increased: Bacteroides dorei; Bacteroides vulgatus | De Goffa et al[125], 2014 |
| Gastrointestinal tract | (1) Decreased: Prevotella; Akkermansia; Bifidobacterium adolescentis; Roseburia faecis; Faecalibacterium prausnitzii; and (2) Increased: Dialister invisus; Gemella sanguinis; Difidobacterium longum | Brown et al[126], 2011 |
| Type 2 diabetes | ||
| Gastrointestinal tract | (1) Decreased: Clostridium coccoides; Clostridium leptum; and (2) Increased: Lactobacillus | Chen et al[28], 2019 |
| Gastrointestinal tract | (1) Decreased: Bifidobacterium; Bacteroides; Faecalibacterium; Akkermansia; Roseburia; and (2) Increased: Ruminococcus; Fusobacterium; Blautia | Gurung et al[30], 2020 |
| Gastrointestinal tract | (1) Decreased: Bifidobacterium; Akkermansia; and (2) Increased: Dorea | Li et al[127], 2020 |
| Gastrointestinal tract | (1) Decreased: Bifdobacterium; and (2) Increased: Lactobacillus | Sedighi et al[31], 2017 |
| Blood | (1) Decreased: Aquabacterium; Xanthomonas; Pseudonocardia; and (2) Increased: Actinotalea; Alishewanella; Seiminibacterium; Pseudoclavibacter | Qiu et al[38], 2019 |
A literature search was performed using the electronic databases MEDLINE (1966–February 2021), EMBASE and SCOPUS (1965–February 2021), and DARE (1966–February 2021). The main search items were gut bacteria, GM, intestinal flora, gut dysbiosis, type 1 DM, type 2 DM, diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, probiotics, prebiotics, synbiotics, bariatric surgery, and faecal transplantation. Non-English articles were excluded.
Studies have shown that Firmicutes/Bacteroides ratio is altered in type 1 DM[22]. In the study by Huang et al[23] (2018) negative association was seen with gut microbe Faecalibacterium and Ruminococcacea and hemoglobin A1c (HbA1c), whereas in the study by Fassatoui et al[24]. (2019) a negative association was seen between HbA1c and Akkermansia muciciniphia. A systematic review of studies done in Hispanic populations showed that patients with newly diagnosed type 1 DM have high levels of Bacteroides with a reduced proportion of Prevotella, Megamonas, and Acidaminococcus. With the initiation of insulin treatment these subjects showed an increase of Prevotella levels. Prior to the development of type 1 DM, inverse relationship of Firmicutes/ Bacteroidetes ratio has been reported[25].
The type of gut microbes and the changes seen with them influence the development of DM. The prominent GM seen in the intestine are the gram-positive Firmicutes and gram-negative Bacteroidetes and it is influenced by dietary changes[26]. A change in the ratio of Bacteroidetes to Firmicutes is associated with DM[27]. A case-control study done by Chen et al[28] (2019) in newly diagnosed type 2 DM subjects, Lactobacillus faecal count was significantly higher whereas Clostridium coccoides and Clostridium leptum was lower, and these changes in the microbes was positively correlated with glycated hemoglobin with higher Lactobacillus count subjects, and negatively correlated with decreased Clostridium count subjects when compared with healthy controls. Another study found that patients with DM showed an affiliation with the following phyla of bacteria: Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria[29]. Alterations in the gut microbe population may be related to DM, and gut microbes Ruminococcus and Fusobacterium has been shown with the development of type 2 DM, when compared to healthy adults[30]. A study by Sedighi et al[31] (2017) found that patients with type 2 DM has increased levels of Lactobacillus, while healthy controls showed increased Bifidobacterium. With respect to the Lactobacillus genus, there are various mixed results suggesting its association with type 2 DM. Certain strain such as L. acidophilus, L. gasseri, and L. salivarius have been increased where as L. amyloyorus has been decreased[30]. However, many species from this genus, such as L. plantarum, L. casei, and L. rhamnosus are often involved in probiotic preparation and have shown to be beneficial in diabetic mice models[30]. Overall, it looks that there may be a strain-specific association with DM.
Further changes in the microbiome in patients with DM are listed in Table 1. Nutrient imbalance by affecting the GM can influence the development of type 2 DM. With newly diagnosed type 2 DM different measurement parameters like age, blood lipids, body-mass index, blood pressure, and dietary nutrient intake was related to the gut microbiome composition[32].
Cani et al[33] (2008) in their animal study showed lipopolysaccharide produced by gram negative intestinal bacteria can translocate into systemic circulation through a leaky gut and can result in endotoxemia causing metabolic dysfunction and obesity. Recent evidence points out in addition to gut microbiome, the blood microbiome plays a role in DM. Blood is usually considered to be sterile, but the research suggests the presence of a microbe or microbial component in healthy humans is known as a blood microbiome. The evidence for blood human microbiome is slowly growing[34-36].
In a study by Sato et al[37] (2014) with Japanese type 2 DM subjects, blood micro
The regulation of the GI system is done by short-chain fatty acids (SCFA) derived from the metabolism of carbohydrates, and GM plays a role in this function. In addition, the gut microbes produce hormone like chemicals that can act at distant targets. Neuroactive compounds like tryptophan and neurotransmitters like serotonin, dopamine, noradrenaline, GABA, and hormones like leptin, ghrelin and glucagon-like peptide 1 (GLP-1) are indirectly regulated by SCFAs via enteroendocrine cells. Overall, the gut microbes produce several substances of a hormonal nature into the circulation which act as distant sites. Because of the GM’s ability to influence distant organs and systems as mentioned above it is considered as an endocrine organ. Overall GM functions as an autonomous endocrine organ and plays a role in bodily endocrine actions including neuroendocrine and immunoendocrine regulations[39-42].
Gut dysbiosis, is a state of increased or altered prevalence of gut bacteria which might in turn result in many disorders such as gastrointestinal, obesity, DM, immunological, and neurobehavioral diseases[43]. Shifts in the GM’s composition with more pathogenic species and phyla can contribute to the above-mentioned diseases. Hyperglycemia was associated with changes of microbiota composition, preferring the non-commensal ones, on the detriment of beneficial phyla such as Bacteroidetes, Proteobacteria, and Actinobacteria. The ratio of Firmicutes/Bacteroidetes has been found to be correlated with plasma glucose concentration. Microbiota are capable to ferment undigested carbohydrates, fiber, and other dietary and xenobiotic compounds to produce SCFAs, which through their ubiquitous receptor play an important role in host glucose metabolism[37,44,45]. The Human Microbiome plays a role in gut permeability, modification of bile acids, glucose breakdown and in the absorption of nutrients[46,47].
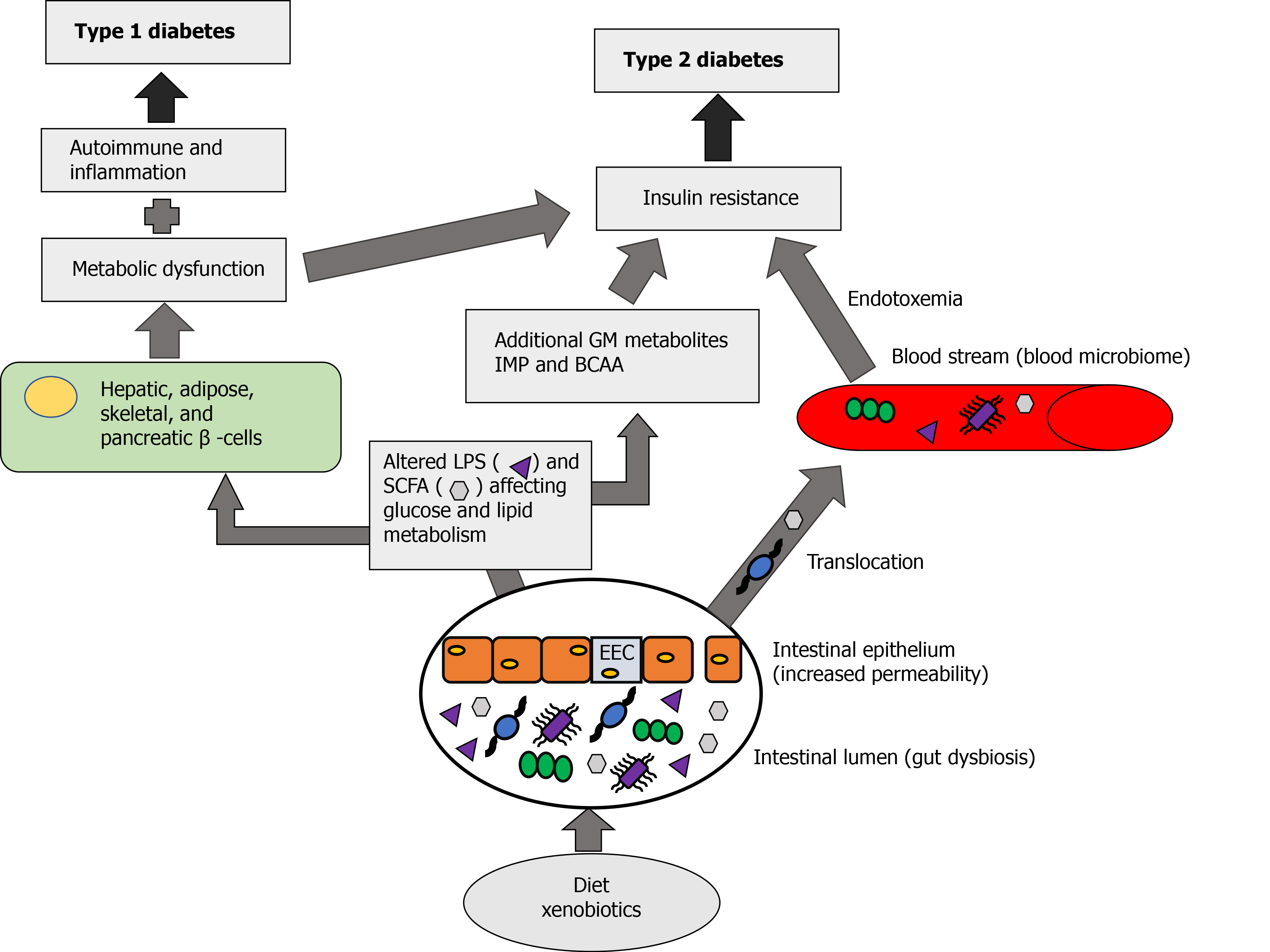
Normal commensal bacteria are helpful in maintaining the gut wall integrity, innate immunity, insulin sensitivity, metabolism, and in communication with the brain functions, as well as help to prevent the penetration of harmful microorganisms in the bowel. Bidirectional relationship exists between the GM and the brain. This chain of communication depends on the interaction of gut microbe through immune and neuroendocrine system with the central nervous system. Short-chain fatty acids, such as butyrates, acetates and propionates, produced by the GM are beneficial to different metabolic processes. The imbalance between the microbiome and host organism lead to dysbiosis. Gut microbiome dysbiosis through inflammation and metabolic dysregulation increases insulin resistance and influence the development of type 2 DM[48] (Figure 1).
Microbial dysbiosis can also be the result of nutritional imbalance which can lead to a low-grade inflammatory state, obesity, and other metabolic disorders[49]. Gut microbes affect gut permeability, glucose and lipid metabolism, energy homeostasis, and insulin sensitivity. Like any other medical conditions, gut microbes play a role in inflammation and immunity[50]. A diet rich in fat and sugar may lead to an abundance of lipopolysaccharide (LPS) release from GM and this LPS, by entering into systemic circulation, can affect β-cells, leading to decreased insulin release, and thereby altering systemic insulin sensitivity, resulting in insulin resistance, and potentially leading to DM[51].
Diets rich in carbohydrates and fat as well as xenobiotics (medications affecting the gut microbes) can cause gut dysbiosis. Normally GM produces metabolic products like SCFA, acetate, butyrate and propionate which acts locally leading to beneficial effects on different metabolic process. When there is gut dysbiosis, it can affect the enteroendocrine L-type cells in the intestinal epithelium and increase the gut permeability (leaky gut) causing these metabolic products to enter into the systemic circulation, as well as translocation of the gut microbiome into the circulation leading to the formation of the blood microbiome. This blood microbiome can cause endotoxemia and affect both metabolic dysfunction and insulin resistance. Gut dysbiosis results in excessive production of SCFA and LPS, as well as additional GM metabolites like imidazole propionate (IMP), derived from histidine, and bacteria derived amino acids. Excessive SCFA and LPS by acting on hepatic, adipose, skeletal and pancreatic cells causes metabolic dysfunction, inflammation and altered immune response. When there is a metabolic dysfunction due to gut dysbiosis combined with inflammatory and altered immune response it can cause type 1 DM, and when combined with insulin resistance due to gut dysbiosis as well as the effect of blood microbiome it can lead to the development of type 2 DM (Figure 1).
The human gut contains a wide variety of microbial communities that carry out a wide range of biochemical functions that can influence the human body through metabolite production, physiological regulation, and interacting with the host’s cellular response and immunity[52]. It has also been found that the host’s own genetics can influence the composition of their gut microbiome, making each host a unique ecosystem[53]. Dynamic changes in the gut microbiome have been seen within individuals often in various disease states, such as obesity, and DM[19,54-56]. The GM has been found to cause enhanced transcriptional changes in the intestinal cells and protein biosynthesis in the crypts within the intestine[57].
SCFAs produced by GM can serve as signaling molecules that can influence the host’s lipid and glucose levels, liver, skeletal muscle, and even immunity[52]. When there is a disruption of the gut microbiome, the altered mixture of SCFA may influence obesity, insulin sensitivity, weight gain and other comorbidities[58,59]. Obese individuals with type 2 DM have shown changes in the GM that are distinct, from non-diabetic subjects. It was found that individuals with type 2 DM showed an increase level of Proteobacteria and Bacteroides with a decreased level of Firmicutes[19].
The GM has been found to influence the host’s metabolism and show great adaptability to the changing environment within the intestines based on diet, genetics, and various physiological cues from the host[52]. The human gut microbiome can modulate absorption as well as nutrient availability within the host. This can be achieved through gene expression changes, alteration of hormones and immunity[52].
Microbial diversity and the production of SCFA as well as products such as butyrate, propionate, and acetate have been found to have a protective role against obesity and insulin resistance[60,61]. SCFAs are able to act as signaling molecule that can activate a variety of pathways that are involved in cholesterol, lipid, and glucose metabolism[58]. Modifications of the microbiome can influence metabolic parameters, in particular when there is a higher abundance of Firmicutes leading to a higher Firmicutes/Bacteroidetes ratio that may be linked to obesity[62]. This may in part be due to the fact that Firmicutes are more efficient at promoting the nutrient absorption leading to subsequent weight gain compared to Bacteroidetes[63].
A study showed the GM composition is different in obese subjects with and without type 2 DM[20]. A recent study also showed for the first time in subjects with type 2 DM the relationship between body composition and GM[64]. Faecal microbiota of obese subjects without DM had increased numbers of SCFA producing microbes, whereas obese subjects with type 2 DM had less beneficial SCFA butyrate producing microbes[65].
The progression of DM is seen as macrovascular[66] and microvascular complications like retinopathy, nephropathy, and neuropathy[67]. Gut microbes seem to play a role in the progression of DM and also shown to play a role in these complications. Diet induced diabetic animal models helps to study these complications[68]. Studies have shown that subjects with DM and eye complications have higher bacterial conjunctival flora when compared to subjects without DM[69-72]. Beli et al[73] (2018) in their animal study showed the association between the GM and diabetic retinopathy (Table 2). More research is needed to understand the mechanism how GM causes diabetic retinopathy[74].
| Intervention | Organism | Health benefit | Change in microbiome | Ref. |
| Intermittent fasting | Mice | Protection from diabetic retinopathy by increasing Tauroursodeoxycholate (a neuroprotective bile acid) producing microbes | Increased Firmicutes and decreased Bacteroidetes and Verrucomicrobia in diabetic mice undergoing intermittent fasting | Beli et al[73], 2018 |
| Antibiotic treatment (ampicillin, metronidazole, neomycin, vancomycin, or their cocktail) | Mice | Reduction in fasting glucose. Change in glucose tolerance (seen with ampicillin, vancomycin, or cocktail) | Alterations in the α- and β- diversity. An association with Akkermansia mucinipjila with decrease fasting glucose. The effect is mediated through systemic changes in glucose metabolism | Rodrigues et al[94], 2017 |
| Prebiotic: Acorn and sago | Mice | Mice fed acorn and sago derived prebiotics had an amelioration of the glucose intolerance and insulin resistance induced by a high-fat diet feeding. Intake of both novel prebiotics as well as inulin increases SCFAs levels in the mouse gut | Ahmadi et al[103], 2019 | |
| Combination of a functional fibre [PolyGlycopleX (PGX) with metformin (MET) or sitagliptin and metformin (S/MET)] | Mice | PGX + MET and PGX + S/MET showed reduced glycemia compared to controls and single treatment (P = 0.001). HbA1c was lower in PGX + S/MET compared to all other treatments (P = 0.001) | Reimer et al[93], 2014 | |
| Artificial sweetener (Neotame) | Mice | Decreased butyrate synthetic genes in Neotame group. Higher concentrations of cholesterol (P < 0.05) and fatty acids (P < 0.05) in Neotame treated mice feces | Reduction in α-diversity and altered β-diversity. Reduced Firmicutes (P < 0.01) and increased Bacteroides (P < 0.01) | Chi et al[85], 2018 |
| Combination of metformin and a prebiotic [konjac mannan-oligosaccharides (MOS)] | Mice | Combination of metformin and MOS help ameliorate insulin resistance and improved glycemic control (P < 0.05) and repair islet and hepatic histology | Metformin and MOS change the microbiome (P < 0.0001) with: Decreased: Rikenellaceae and Clostridiales; Increased: Akkermansia muciniphila and Bifidobacterium pseudolongum | Zheng et al[96], 2018 |
Diabetic neuropathy is seen as autonomic neuropathy as well as distal sensory and motor neuropathy and correlate with diabetic control, and GM also seems to play a role[75]. In a human study with early diabetic nephropathy, Barrios et al[76] (2015) showed an increase in colonic GM, whereas with end-stage renal disease patients microbes producing urease, uricase, p-cresol and indole-forming enzymes were seen[77]. The proposed mechanisms for progression of kidney disease could be due to GM imbalance, metabolic shifts, immunosuppression, inflammation, as well as accumulation of uremic toxins[78].
In DM, normal GM can be restored using diet, gut biotics, faecal transplantation, and bariatric surgery, which may help with the proper management of DM.
There is some evidence from human studies, that both faecal transplant and bariatric surgery improved the glucose and metabolic parameters by altering the GM[48]. A meta-analysis done by Magouliotis et al[79] (2017) showed some discrepancy between the human studies and the benefits witnessed from bariatric surgery. Another study looking at obese insulin resistant subjects who received allogenic faecal transplants from a lean insulin sensitive donor show improved insulin sensitivity for a short period of 6 weeks, however the benefit was not seen past 12 weeks[80] (Table 3).
| Intervention | Organism | Health benefit | Change in microbiome | Ref. |
| Probiotics | Human | Decreased fasting blood glucose and HbA1c levels. Increased HDL levels, however no significant effect on BMI and LDL levels were found | Kocsis et al[112], 2020 | |
| Artificial sweeteners (aspartame and acesulfame-K) | Human | Compared to controls, aspartame and acesulfame-K had different bacterial diversity (P < 0.01, P = 0.03 respectively), compared to controls | Frankenfeld et al[86], 2015 | |
| Probiotics, Prebiotics, or synbiotics | Human (meta-analysis) | The use of probiotics, prebiotics, or synbiotics showed a decrease in FBG (P < 0.01), total cholesterol (P = 0.02), triacylglycerols (P = 0.01) and insulinaemia (P < 0.01), as well as increased HDL-cholesterol levels (P < 0.01. Even though HbA1c reduction is seen it is not statistically significant. No effect on LDL-cholesterol was seen | Bock et al[115], 2020 | |
| Laparoscopic sleeve gastrectomy | Human | Decreased weight and BMI. Restored insulin tolerance and type 2 DM remission | Increased: Bacteroidetes/Firmicutes ratio at 1- and 3-months post surgery. Lactobacillales | Kikuchi et al[128], 2018;Li et al[129], 2013 |
| Roux-en-Y gastric bypass | Human | Type 2 DM remission and improved BMI and weight loss. Improved gastric emptying and bile acid metabolism | Decreased: Bacteroidetes/Firmicutes ratio. Improved probiotic supplementation effects due to lowered pH environment | Selber-Hnatiw et al[52], 2020; Li et al[129], 2013 |
Diet can modulate the GM and play a role in the management of DM by preventing gut dysbiosis[81] (Table 2). Fruits and vegetables contain polyphenols which can increase beneficial GM like A. muciniphila, Lactobacilli and Bifidobacteria[82]. Unbalanced dietary intake can affect the structure and abundance of GM which can play a role in the development of DM[83].
Artificial sweeteners are no-calorie sugar substitutes, may induce glucose intolerance by affecting the gut microbes. In an animal study with saccharin-fed mice showed an increase in Bacteroides and a reduction in Lactobacillus reuteri leading to GM dysbiosis and glucose intolerance[84]. Similar effects were seen in another study by Chi et al[85] (2018) using the artificial sweetener, Neotame. In a cross-sectional human study by Frankenfeld et al[86] (2015), showed sweeteners like aspartame or acesulfame-K found no effect on gut bacterial abundance. A recent randomized-blinded crossover study in healthy participants did not demonstrate measurable changes in the GM or in SCFAs after 14 d daily intake of aspartame and sucralose[87]. These preliminary observations needed to be established in future human research studies.
Antidiabetic drugs can influence the gut microbiome by affecting the drug microbiome interface, whereas the gut microbiome also influences the metabolism and play a role in the efficacy of antidiabetic drugs. The interactions of antidiabetic drugs and microbiota is getting more attention as it may play a role in the management of DM[88]. Antidiabetic agents cause alteration of the specific gut microbes. Metformin increases the population of Akkermansia muciniphila by 18-fold, enhancing the digestion of mucin and increasing SCFA[89]. Metformin, in addition to Akkermasia, causes increase in Lactobacillus and Bifidobacterium, whereas insulin increase Fusobacterium[90]. This first line antidiabetic agent in type 2 DM modifies the GM, alter the bile acid circulation and thereby a possibility that primary site of action may be gut and the GM[91].
Understanding the pharmacokinetics, pharmacodynamics and pharmacomicrobiomics of antidiabetic medications and gut microbes can help to understand drug- gut microbiome and its potential benefit with antidiabetic drugs. Overall, it may help to better manage the DM management[92].
Antidiabetic drugs have been shown to affect the different gut microbes and their metabolic effects through the medication-microbiome-metabolism axis. GM can influence the pharmacokinetics of various antidiabetic drugs such as drug absorption, drug metabolism which can affect the potency of these medications. Overall, there is a bidirectional relationship exist between antidiabetic drugs and gut microbes[88].
Different combinations of antidiabetic drugs are used to better control DM. The commonly used combination is metformin with sulphonylureas, thiazolidinediones, DPP-4 inhibitors and insulin. One animal study showed some delay in the progression of DM when sitagliptin/metformin combination given with a prebiotic fibre[93]. Currently, there is a need for more research of different combination therapies on GM.
Several animal studies have showed that gut biotics, like prebiotics and probiotics, can improve the efficacy of antidiabetic drugs. Treatment with individual or a cocktail of antibiotics reduced dysbiosis and decrease fasting glucose but did not affect body weight, as well as antibiotic treatment also changed gene expression in the ileum and liver, and shifted the alpha (α) and beta (β) diversities of GM[94]. In an animal study with mice, combining probiotics and/or prebiotics with antidiabetic medications showed an improvement in glycemic control and insulin sensitivity[95]. A study by Reimer et al[93] (2014) found that using a combination of sitagliptin and metformin with a functional fiber can delay DM progression. In an animal study usage of mannan-oligosaccharides by altering the GM increased the hypoglycemic effects of metformin[96]. Yang et al[97] (2020) found that Genistein found in soybeans and soy derived foods (prebiotic) helped to improve glucose and lipid metabolism by altering GM composition[97]. In another animal study, certain GM like Bacteroides fragilis, A. muciniphila, L. plantarum, L. casei can induce interleukin 10 (IL-10), which has been shown to improve both insulin resistance and glucose metabolism[98] (Table 2).
Many gut microbes have been shown to have antidiabetic effect in humans by different mechanisms including effect on insulin sensitivity[99]. Roseburia intestinalis can improve insulin sensitivity by increasing IL-22 production[100]. Some strains of Lactobacilli act like acarbose and have been shown to inhibit alpha glucosidase[101]. Prebiotics can feed the gut microbiome and increase the population of L-cells in the intestine and thereby increase the amount of GLP-1[102] and prevent high fat diet induced insulin resistance[103]. In the recent PREMOTE randomized control trial (RTC) study, probiotics showed antidiabetic effect by altering metabolic homeostasis[104]. Thus, GM may be useful in the management of DM[105]. Jafarnejad et al[106] (2015) and Asemi et al[107] (2014), in their two studies showed multi-probiotic supplement as well as synbiotic (L. sporogenes plus inulin) product helps to reduce glucose and other metabolic parameters. Tonucci et al[108] (2015) in their double-blind RCT study comparing fermented milk containing L. acidophilus (LA-5) plus B. animalis (Lactis BB-12) with plain fermented milk in 45 type 2 DM subjects showed decreased in HbA1c as well as low-density lipoproteins cholesterol and inflammatory cytokines. Multiple RTCs and the meta-analysis of these RCT’s with different gut microbes demonstrated antidiabetic effect as well as effect on different metabolic parameters[109-111] (Table 3).
A recent meta-analysis of 14 RCTs showed significant decrease in HbA1c in the probiotic group compared to placebo controls, weighted mean difference (WMD) is - 0.33%, 95%CI -0.53 to –0.13, P = 0.001. In this meta-analysis, probiotics significantly reduced fasting blood glucose, insulin, lipid profile and inflammatory marker in addition to blood pressure levels[112]. Another meta-analysis showed similar result with reduction in HbA1c% (WMD = - 0.24, 95%CI: - 0.44 to - 0.04, P = 0.02), fasting blood glucose (WMD = - 0.44 mmol/L, 95%CI: - 0.74 to - 0.15, P = 0.003)[113,114]. A meta-analysis study done in 2021 with probiotics, prebiotics or synbiotics on type 2 DM also showed significant improvement in glucose and other metabolic parameters[115]. Prebiotic inulin improves glycemic control in young adults with type 1 DM[116]. Certain specific species of probiotic microbes as well as certain prebiotics by altering the GM was shown to improve the auto-immune condition, which plays a major role in the pathogenesis of type 1 DM[117].
A study by Didari et al[118] (2014) looked at the safety of probiotics and synbiotics and found that certain populations, such as patients who are immunocompromised, with cardiac valvular disease, having a central venous catheter, or those with short-bowel syndrome may have an increased risk for systemic infections. Thus, caution may be warranted when using these products in diabetic patients and a risk-benefit analysis should be considered.
Some preliminary evidence in animal studies indicates altering GM may help to prevent DM[119,120]. A recent study by Gurung et al[30] (2020) showed with certain gut microbes like Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia have a negative association with DM and appears to be protective. In 42 healthy adults, GM Lactobacillus johnsonii seems to reduce the risk of type 1 DM[121].
Gut dysbiosis plays a role in the development and progression of DM. The current evidence also points out that the GM can play a role in DM related complications. Modulation of the gut bacteria or dysbiosis can be corrected by fibre, diet, antidiabetic medications, and by using gut biotics like prebiotics, probiotics, and synbiotics as well as by bariatric surgery and faecal transplantation. The interaction between gut microbes and antidiabetic agents is a promising field that may change the landscape of DM management in the future. There is some preliminary evidence to show that GM may play a role in the prevention of DM. More research is needed on a large scale to confirm these findings.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park SC S-Editor: Wang JL L-Editor: A P-Editor: Xing YX
| 1. | Toniolo A, Cassani G, Puggioni A, Rossi A, Colombo A, Onodera T, Ferrannini E. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev Med Microbiol. 2019;30:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2523] [Article Influence: 280.3] [Reference Citation Analysis (0)] |
| 3. | Ayadurai S, Hattingh HL, Tee LB, Md Said SN. A Narrative Review of Diabetes Intervention Studies to Explore Diabetes Care Opportunities for Pharmacists. J Diabetes Res. 2016;2016:5897452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2251] [Article Influence: 321.6] [Reference Citation Analysis (0)] |
| 5. | Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, Orchard TJ, Rolka DB, Imperatore G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:359-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 6. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8051] [Article Influence: 619.3] [Reference Citation Analysis (2)] |
| 7. | Chattopadhyay A, Mythili S. The journey of gut microbiome – an introduction and its influence on metabolic disorders. Front Biol. 2018;5:327-341. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Molina DK, DiMaio VJ. Normal organ weights in men: part I-the heart. Am J Forensic Med Pathol. 2012;33:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7830] [Article Influence: 522.0] [Reference Citation Analysis (4)] |
| 10. | Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 950] [Cited by in RCA: 982] [Article Influence: 89.3] [Reference Citation Analysis (1)] |
| 11. | He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, Mujagond P, Chen XJ, Rong ZH, Chen P, Lyu LY, Wang X, Wu CB, Yu N, Xu YJ, Yin J, Raes J, Knight R, Ma WJ, Zhou HW. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 589] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 12. | Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1273] [Article Influence: 181.9] [Reference Citation Analysis (0)] |
| 13. | Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 947] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 14. | Barlow GM, Yu A, Mathur R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr Clin Pract. 2015;30:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology. 2017;152:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 16. | Chen X, Devaraj S. Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr Diab Rep. 2018;18:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract. 2018;146:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Purkait D, Hameed S, Fatima Z. Chapter 15 - gut microbiome: Current development, challenges, and perspectives. In: Rastegari AA, Yadav AN, Yadav N. New and future developments in microbial biotechnology and bioengineering: Elsevier, 2020: 227-241. |
| 19. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1783] [Cited by in RCA: 2077] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 20. | Thingholm LB, Rühlemann MC, Koch M, Fuqua B, Laucke G, Boehm R, Bang C, Franzosa EA, Hübenthal M, Rahnavard A, Frost F, Lloyd-Price J, Schirmer M, Lusis AJ, Vulpe CD, Lerch MM, Homuth G, Kacprowski T, Schmidt CO, Nöthlings U, Karlsen TH, Lieb W, Laudes M, Franke A, Huttenhower C. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe. 2019;26:252-264.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 21. | Nowak K, Jabłońska E, Ratajczak-Wrona W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int. 2019;125:350-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 22. | Mrozinska S, Kapusta P, Gosiewski T, Sroka-Oleksiak A, Ludwig-Słomczyńska AH, Matejko B, Kiec-Wilk B, Bulanda M, Malecki MT, Wolkow PP, Klupa T. The Gut Microbiota Profile According to Glycemic Control in Type 1 Diabetes Patients Treated with Personal Insulin Pumps. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Huang Y, Li SC, Hu J, Ruan HB, Guo HM, Zhang HH, Wang X, Pei YF, Pan Y, Fang C. Gut microbiota profiling in Han Chinese with type 1 diabetes. Diabetes Res Clin Pract. 2018;141:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Fassatoui M, Lopez-Siles M, Díaz-Rizzolo DA, Jmel H, Naouali C, Abdessalem G, Chikhaoui A, Nadal B, Jamoussi H, Abid A, Gomis R, Abdelhak S, Martinez-Medina M, Kefi R. Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Elena RM, Gabriela GD, Arnulfo GC, Enrique CA. Studying the Gut Microbiome of Latin America and Hispanic/Latino Populations. Insight into Obesity and Diabetes: Systematic Review. Curr Diabetes Rev. 2019;15:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1347] [Cited by in RCA: 1183] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 27. | Blandino G, Inturri R, Lazzara F, Di Rosa M, Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016;42:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 28. | Chen PC, Chien YW, Yang SC. The alteration of gut microbiota in newly diagnosed type 2 diabetic patients. Nutrition. 2019;63-64:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Das T, Jayasudha R, Chakravarthy S, Prashanthi GS, Bhargava A, Tyagi M, Rani PK, Pappuru RR, Sharma S, Shivaji S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep. 2021;11:2738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 30. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 1069] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 31. | Sedighi M, Razavi S, Navab-Moghadam F, Khamseh ME, Alaei-Shahmiri F, Mehrtash A, Amirmozafari N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb Pathog. 2017;111:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 32. | Nuli R, Cai J, Kadeer A, Zhang Y, Mohemaiti P. Integrative Analysis Toward Different Glucose Tolerance-Related Gut Microbiota and Diet. Front Endocrinol (Lausanne). 2019;10:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3224] [Cited by in RCA: 3539] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 34. | Païssé S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion. 2016;56:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 35. | Olde Loohuis LM, Mangul S, Ori APS, Jospin G, Koslicki D, Yang HT, Wu T, Boks MP, Lomen-Hoerth C, Wiedau-Pazos M, Cantor RM, de Vos WM, Kahn RS, Eskin E, Ophoff RA. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl Psychiatry. 2018;8:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Whittle E, Leonard MO, Harrison R, Gant TW, Tonge DP. Multi-Method Characterization of the Human Circulating Microbiome. Front Microbiol. 2018;9:3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 37. | Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, Komiya K, Kawaguchi M, Shimizu T, Ogihara T, Tamura Y, Sakurai Y, Yamamoto R, Mita T, Fujitani Y, Fukuda H, Nomoto K, Takahashi T, Asahara T, Hirose T, Nagata S, Yamashiro Y, Watada H. Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37:2343-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 38. | Qiu J, Zhou H, Jing Y, Dong C. Association between blood microbiome and type 2 diabetes mellitus: A nested case-control study. J Clin Lab Anal. 2019;33:e22842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Evans JM, Morris LS, Marchesi JR. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37-R47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 40. | Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 374] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 41. | Hampl R, Stárka L. Endocrine disruptors and gut microbiome interactions. Physiol Res. 2020;69:S211-S223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 780] [Article Influence: 70.9] [Reference Citation Analysis (1)] |
| 43. | Rosenfeld CS. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front Cell Infect Microbiol. 2017;7:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 44. | Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol Metab. 2017;28:612-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Needell JC, Zipris D. The Role of the Intestinal Microbiome in Type 1 Diabetes Pathogenesis. Curr Diab Rep. 2016;16:89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Singer-Englar T, Barlow G, Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol. 2019;13:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 47. | Yu F, Han W, Zhan G, Li S, Jiang X, Wang L, Xiang S, Zhu B, Yang L, Luo A, Hua F, Yang C. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging (Albany NY). 2019;11:10454-10467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Halmos T, Suba I. [Physiological patterns of intestinal microbiota. The role of dysbacteriosis in obesity, insulin resistance, diabetes and metabolic syndrome]. Orv Hetil. 2016;157:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Fallucca F, Porrata C, Fallucca S, Pianesi M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab Res Rev. 2014;30 Suppl 1:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Aw W, Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig. 2018;9:5-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 51. | Acosta-Montaño P, Rodríguez-Velázquez E, Ibarra-López E, Frayde-Gómez H, Mas-Oliva J, Delgado-Coello B, Rivero IA, Alatorre-Meda M, Aguilera J, Guevara-Olaya L, García-González V. Fatty Acid and Lipopolysaccharide Effect on Beta Cells Proteostasis and its Impact on Insulin Secretion. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Selber-Hnatiw S, Sultana T, Tse W, Abdollahi N, Abdullah S, Al Rahbani J, Alazar D, Alrumhein NJ, Aprikian S, Arshad R, Azuelos JD, Bernadotte D, Beswick N, Chazbey H, Church K, Ciubotaru E, D'Amato L, Del Corpo T, Deng J, Di Giulio BL, Diveeva D, Elahie E, Frank JGM, Furze E, Garner R, Gibbs V, Goldberg-Hall R, Goldman CJ, Goltsios FF, Gorjipour K, Grant T, Greco B, Guliyev N, Habrich A, Hyland H, Ibrahim N, Iozzo T, Jawaheer-Fenaoui A, Jaworski JJ, Jhajj MK, Jones J, Joyette R, Kaudeer S, Kelley S, Kiani S, Koayes M, Kpata AJAL, Maingot S, Martin S, Mathers K, McCullogh S, McNamara K, Mendonca J, Mohammad K, Momtaz SA, Navaratnarajah T, Nguyen-Duong K, Omran M, Ortiz A, Patel A, Paul-Cole K, Plaisir PA, Porras Marroquin JA, Prevost A, Quach A, Rafal AJ, Ramsarun R, Rhnima S, Rili L, Safir N, Samson E, Sandiford RR, Secondi S, Shahid S, Shahroozi M, Sidibé F, Smith M, Sreng Flores AM, Suarez Ybarra A, Sénéchal R, Taifour T, Tang L, Trapid A, Tremblay Potvin M, Wainberg J, Wang DN, Weissenberg M, White A, Wilkinson G, Williams B, Wilson JR, Zoppi J, Zouboulakis K, Gamberi C. Metabolic networks of the human gut microbiota. Microbiology (Reading). 2020;166:96-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Gordon JI, Hooper LV, McNevin MS, Wong M, Bry L. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am J Physiol. 1997;273:G565-G570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341-3349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 55. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3078] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 56. | Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015;22:516-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 405] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 57. | El Aidy S, Hooiveld G, Tremaroli V, Bäckhed F, Kleerebezem M. The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microbes. 2013;4:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 58. | den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 3275] [Article Influence: 272.9] [Reference Citation Analysis (3)] |
| 59. | Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1486] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 60. | Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 923] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 61. | Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1572] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 62. | Rácz B, Dušková M, Stárka L, Hainer V, Kunešová M. Links between the circadian rhythm, obesity and the microbiome. Physiol Res. 2018;67:S409-S420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S, Sineok L, Lushchak O, Vaiserman A. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 670] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 64. | Hung WC, Hung WW, Tsai HJ, Chang CC, Chiu YW, Hwang SJ, Kuo MC, Chen SC, Dai CY, Tsai YC. The Association of Targeted Gut Microbiota with Body Composition in Type 2 Diabetes Mellitus. Int J Med Sci. 2021;18:511-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr Rev. 2018;39:133-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 66. | Leustean AM, Ciocoiu M, Sava A, Costea CF, Floria M, Tarniceriu CC, Tanase DM. Implications of the Intestinal Microbiota in Diagnosing the Progression of Diabetes and the Presence of Cardiovascular Complications. J Diabetes Res. 2018;2018:5205126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Zhao S, Jang S, Lee YK, Kim DG, Jin Z, Koh HJ. Genetic Basis of Tiller Dynamics of Rice Revealed by Genome-Wide Association Studies. Plants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 68. | Preguiça I, Alves A, Nunes S, Gomes P, Fernandes R, Viana SD, Reis F. Diet-Induced Rodent Models of Diabetic Peripheral Neuropathy, Retinopathy and Nephropathy. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 69. | Ham B, Hwang HB, Jung SH, Chang S, Kang KD, Kwon MJ. Distribution and Diversity of Ocular Microbial Communities in Diabetic Patients Compared with Healthy Subjects. Curr Eye Res. 2018;43:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Rowan S, Taylor A. The Role of Microbiota in Retinal Disease. Adv Exp Med Biol. 2018;1074:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 71. | Baim AD, Movahedan A, Farooq AV, Skondra D. The microbiome and ophthalmic disease. Exp Biol Med (Maywood). 2019;244:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Floyd JL, Grant MB. The Gut-Eye Axis: Lessons Learned from Murine Models. Ophthalmol Ther. 2020;9:499-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 73. | Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, Prasad R, Bhatwadekar A, White FA, Townsend SD, Chan L, Ryan CN, Morton D, Moldovan EG, Chu FI, Oudit GY, Derendorf H, Adorini L, Wang XX, Evans-Molina C, Mirmira RG, Boulton ME, Yoder MC, Li Q, Levi M, Busik JV, Grant MB. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes. 2018;67:1867-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 74. | St Leger AJ, Caspi RR. Visions of Eye Commensals: The Known and the Unknown About How the Microbiome Affects Eye Disease. Bioessays. 2018;40:e1800046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Grasset E, Burcelin R. The gut microbiota to the brain axis in the metabolic control. Rev Endocr Metab Disord. 2019;20:427-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, Pascual J, Ley RE, Spector TD, Bell JT, Menni C. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS One. 2015;10:e0134311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 77. | Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 78. | Stavropoulou E, Kantartzi K, Tsigalou C, Konstantinidis T, Romanidou G, Voidarou C, Bezirtzoglou E. Focus on the Gut-Kidney Axis in Health and Disease. Front Med (Lausanne). 2020;7:620102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 79. | Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: a Systematic Review and Meta-analysis. Obes Surg. 2017;27:1345-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 80. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2011] [Article Influence: 154.7] [Reference Citation Analysis (0)] |
| 81. | Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, Peano C, Turroni S, Rampelli S, Pozzilli P, Pianesi M, Fallucca F, Brigidi P. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 82. | Quddus MR, Lawrence WD. A Seminal Observation! Int J Surg Pathol. 2016;24:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 83. | Krezalek MA, Yeh A, Alverdy JC, Morowitz M. Influence of nutrition therapy on the intestinal microbiome. Curr Opin Clin Nutr Metab Care. 2017;20:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1358] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 85. | Chi L, Bian X, Gao B, Tu P, Lai Y, Ru H, Lu K. Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 86. | Frankenfeld CL, Sikaroodi M, Lamb E, Shoemaker S, Gillevet PM. High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann Epidemiol. 2015;25:736-42.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 87. | Ahmad SY, Friel J, Mackay D. The Effects of Non-Nutritive Artificial Sweeteners, Aspartame and Sucralose, on the Gut Microbiome in Healthy Adults: Secondary Outcomes of a Randomized Double-Blinded Crossover Clinical Trial. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 88. | Whang A, Nagpal R, Yadav H. Bi-directional drug-microbiome interactions of anti-diabetics. EBioMedicine. 2019;39:591-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 89. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1135] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 90. | Zhang F, Wang M, Yang J, Xu Q, Liang C, Chen B, Zhang J, Yang Y, Wang H, Shang Y, Wang Y, Mu X, Zhu D, Zhang C, Yao M, Zhang L. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine. 2019;66:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 91. | Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care. 2016;39:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 92. | Rizkallah MR, Saad R, Aziz RK. The human microbiome project, personalized medicine and the birth of pharmacomicrobiomics. Curr Pharmacogenomics Person Med. 2010;8:182-193. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Reimer RA, Grover GJ, Koetzner L, Gahler RJ, Lyon MR, Wood S. Combining sitagliptin/metformin with a functional fiber delays diabetes progression in Zucker rats. J Endocrinol. 2014;220:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Rodrigues RR, Greer RL, Dong X, DSouza KN, Gurung M, Wu JY, Morgun A, Shulzhenko N. Antibiotic-Induced Alterations in Gut Microbiota Are Associated with Changes in Glucose Metabolism in Healthy Mice. Front Microbiol. 2017;8:2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 95. | Stenman LK, Waget A, Garret C, Briand F, Burcelin R, Sulpice T, Lahtinen S. Probiotic B420 and prebiotic polydextrose improve efficacy of antidiabetic drugs in mice. Diabetol Metab Syndr. 2015;7:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Zheng J, Li H, Zhang X, Jiang M, Luo C, Lu Z, Xu Z, Shi J. Prebiotic Mannan-Oligosaccharides Augment the Hypoglycemic Effects of Metformin in Correlation with Modulating Gut Microbiota. J Agric Food Chem. 2018;66:5821-5831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 97. | Yang R, Jia Q, Mehmood S, Ma S, Liu X. Genistein ameliorates inflammation and insulin resistance through mediation of gut microbiota composition in type 2 diabetic mice. Eur J Nutr. 2021;60:2155-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 98. | Dagdeviren S, Jung DY, Friedline RH, Noh HL, Kim JH, Patel PR, Tsitsilianos N, Inashima K, Tran DA, Hu X, Loubato MM, Craige SM, Kwon JY, Lee KW, Kim JK. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017;31:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 99. | Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, Svendsen KD, Jakobsen M, Pedersen BK. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 100. | Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 101. | Panwar H, Calderwood D, Grant IR, Grover S, Green BD. Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur J Nutr. 2014;53:1465-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 102. | Napolitano A, Miller S, Nicholls AW, Baker D, Van Horn S, Thomas E, Rajpal D, Spivak A, Brown JR, Nunez DJ. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e100778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 103. | Ahmadi S, Nagpal R, Wang S, Gagliano J, Kitzman DW, Soleimanian-Zad S, Sheikh-Zeinoddin M, Read R, Yadav H. Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome-gut-brain axis modulation. J Nutr Biochem. 2019;67:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 104. | Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, Gu X, Xue Y, Huang S, Yang J, Chen L, Chen G, Qu S, Liang J, Qin L, Huang Q, Peng Y, Li Q, Wang X, Kong P, Hou G, Gao M, Shi Z, Li X, Qiu Y, Zou Y, Yang H, Wang J, Xu G, Lai S, Li J, Ning G, Wang W. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11:5015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 105. | Adeshirlarijaney A, Gewirtz AT. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes. 2020;11:253-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 106. | Jafarnejad S, Saremi S, Jafarnejad F, Arab A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J Nutr Metab. 2016;2016:5190846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 107. | Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2014;33:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 108. | Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 109. | Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas). 2016;52:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 110. | Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med Sci Monit. 2017;23:3044-3053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 111. | Kasińska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. 2015;125:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 112. | Kocsis T, Molnár B, Németh D, Hegyi P, Szakács Z, Bálint A, Garami A, Soós A, Márta K, Solymár M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep. 2020;10:11787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 113. | Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. Correction to: Effects of probiotics on type II diabetes mellitus: a meta-analysis. J Transl Med. 2020;18:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. Effects of probiotics on type II diabetes mellitus: a meta-analysis. J Transl Med. 2020;18:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 115. | Bock PM, Telo GH, Ramalho R, Sbaraini M, Leivas G, Martins AF, Schaan BD. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2021;64:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 116. | Ho J, Reimer RA, Doulla M, Huang C. Effect of prebiotic intake on gut microbiota, intestinal permeability and glycemic control in children with type 1 diabetes: study protocol for a randomized controlled trial. Trials. 2016;17:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 117. | Mishra SP, Wang S, Nagpal R, Miller B, Singh R, Taraphder S, Yadav H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 118. | Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. 2014;13:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 119. | Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, Yokokura T. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997;105:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Xie Y, Xiao M, Ni Y, Jiang S, Feng G, Sang S, Du G. Alpinia oxyphylla Miq. Extract Prevents Diabetes in Mice by Modulating Gut Microbiota. J Diabetes Res. 2018;2018:4230590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 121. | Marcial GE, Ford AL, Haller MJ, Gezan SA, Harrison NA, Cai D, Meyer JL, Perry DJ, Atkinson MA, Wasserfall CH, Garrett T, Gonzalez CF, Brusko TM, Dahl WJ, Lorca GL. Lactobacillus johnsonii N6.2 Modulates the Host Immune Responses: A Double-Blind, Randomized Trial in Healthy Adults. Front Immunol. 2017;8:655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 122. | de Goffau MC, Luopajärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, Vaarala O. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 123. | Tai N, Peng J, Liu F, Gulden E, Hu Y, Zhang X, Chen L, Wong FS, Wen L. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J Exp Med. 2016;213:2129-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 124. | Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 607] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 125. | de Goffau MC, Fuentes S, van den Bogert B, Honkanen H, de Vos WM, Welling GW, Hyöty H, Harmsen HJ. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 126. | Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 589] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 127. | Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:5450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 128. | Kikuchi R, Irie J, Yamada-Goto N, Kikkawa E, Seki Y, Kasama K, Itoh H. The Impact of Laparoscopic Sleeve Gastrectomy with Duodenojejunal Bypass on Intestinal Microbiota Differs from that of Laparoscopic Sleeve Gastrectomy in Japanese Patients with Obesity. Clin Drug Investig. 2018;38:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Li JF, Lai DD, Ni B, Sun KX. Comparison of laparoscopic Roux-en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Can J Surg. 2013;56:E158-E164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |