Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1386
Peer-review started: January 25, 2021
First decision: June 16, 2021
Revised: June 25, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: September 15, 2021
Processing time: 222 Days and 0.6 Hours
Diabetes mellitus (DM) is a noncommunicable disease reaching epidemic proportions around the world. It affects younger individuals, including women of childbearing age. Diabetes can cause diabetic retinopathy (DR), which is potentially sight threatening when severe nonproliferative DR (NPDR), proliferative DR (PDR), or sight-threatening diabetic macular oedema (STDME) develops. Pregnancy is an independent risk factor for the progression of DR. Baseline DR at the onset of pregnancy is an important indicator of progression, with up to 10% of women with baseline NPDR progressing to PDR. Progression to sight-threatening DR (STDR) during pregnancy causes distress to the patient and often necessitates ocular treatment, which may have a systemic effect. Management includes prepregnancy counselling and, when possible, conventional treatment prior to pregnancy. During pregnancy, closer follow-up is required for those with a long duration of DM, poor baseline control of blood sugar and blood pressure, and worse DR, as these are risk factors for progression to STDR. Conventional treatment with anti-vascular endothelial growth factor agents for STDME can potentially lead to foetal loss. Treatment with laser photocoagulation may be preferred, and surgery under general anaesthesia should be avoided. This review provides a management plan for STDR from the perspective of practising ophthalmologists. A review of strategies for maintaining the eyesight of diabetic women with STDR with emphasis on prepregnancy counselling and planning, monitoring and safe treatment during pregnancy, and management of complications is presented.
Core Tip: Progression of diabetic retinopathy (DR) to the sight-threatening DR (STDR) is rare during pregnancy but can cause significant ocular morbidity and distress to the mother. Good prepregnancy and intrapartum control of systemic risk factors, especially blood sugar and blood pressure, and adequate prepregnancy treatment of STDR will reduce complications during pregnancy. When STDR develops, conventional therapy for nonpregnant individuals may not be applied. This includes avoidance of anti-vascular endothelial growth factor agents conventionally for diabetic macular oedema and proliferative DR (PDR), especially during early trimesters. Panretinal photocoagulation is a safe option for PDR. Surgical treatments should be performed under local anaesthesia or preferentially deferred until postpartum.
- Citation: Choo PP, Md Din N, Azmi N, Bastion MLC. Review of the management of sight-threatening diabetic retinopathy during pregnancy. World J Diabetes 2021; 12(9): 1386-1400
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1386.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1386
Diabetes mellitus (DM) is a complex metabolic disease that involves multiple organs and may cause severe visual impairment. DM is known to affect several ocular structures, including the extraocular muscles, the intraocular lens, the optic nerve, and the retina. However, diabetic retinopathy (DR) is the most common and leading cause of blindness among working-age adults in developing countries[1]. Of 285 million people worldwide with diabetes in 2010[2], approximately one-third have signs of DR, and one-third of these patients may have vision-threatening retinopathy, defined as severe nonproliferative DR (NPDR), proliferative DR (PDR), or diabetic macular oedema (DME)[3]. In Southeast Asia alone, the total number of people with diabetes is expected to reach more than 140 million by 2040. Over 20 years, the prevalence has more than doubled among Malaysians aged 30 or more years, with a prevalence of 22.6% in 2013[4,5].
DR can be classified into several stages: (1) Mild NPDR characterized by increased vascular permeability; (2) Moderate NPDR depicted by vascular closure with less than 20 microaneurysms; (3) Severe NPDR, which is identified as any of the following clinical features: Microaneurysms in all 4 quadrants, venous beading in 2 or more quadrants, and intraretinal microvascular abnormalities in 1 or more quadrant; (4) Very severe NPDR if they have 2 or more of the criteria for severe NPDR; and (5) PDR which is characterized by the growth of new blood vessels (neovascularization) on the optic disc, retina, or on the posterior surface of the vitreous. DME is characterized by retinal thickening from leaky blood vessels that can develop at any stage of DR.
The progression of DR during pregnancy increases the frequency of perinatal follow-ups and may necessitate stressful treatments[6]. Sight-threatening DR (STDR) can cause ocular morbidity, which can lead to psychological distress in new mothers[7]. Poor vision may also lead to adverse effects on newborns through neglect and postnatal depression in the mother[7].
The incidence of DR is highly dependent on the duration and control of diabetes, and risk factors such as hyperglycaemia[8,9], hypertension[10], dyslipidaemia[11], and nephropathy[12] may accelerate DR progression in both pregnant and nonpregnant individuals.
In women with pre-existing DM, pregnancy is also known to be associated with worsening DR[13]. As the prevalence of type 1 DM (T1DM)[14] and type 2 DM (T2DM)[15] increases globally, recent studies have found that the incidence of DR in early pregnancy is approximately 63% in T1DM[16] and 14% in T2DM[13]. The adverse effects of pregnancy on retinal status occur by the end of the second trimester and regress after delivery, but some severe cases may persist into the first year postpartum[13,17-19]. Risk factors such as poor glycaemic control during pregnancy[13], longer duration of diabetes before conception[20], rapid normalization of glycated haemoglobin (HbA1c) at the beginning of pregnancy[20], hypertension[21], and preeclampsia[22] may influence the development and progression of DR during pregnancy.
The severity of DR at conception also has an impact on DR progression during pregnancy, as progression was more significant in pregnant women with moderate and severe forms of DR than in those with mild or no DR[16]. According to the Diabetes in Early Pregnancy Study, approximately 55% of pregnant women with moderate-to-severe NPDR and 21% with mild NPDR showed deterioration of DR[20]. A review by Morrison et al[23] found that when NPDR was present at baseline, 30.2% worsened, and 9.8% progressed to proliferative disease[23]. Macular oedema typically occurs alongside proteinuria or hypertension and may progress throughout pregnancy and resolve during the postpartum period; however, some cases may persist and cause long-term vision loss[24].
Screening for DR is an important aspect of diabetes management, as it aims to detect DR as early as possible to enable timely treatment and prevent vision loss[25]. Diabetic women should have a preconception retinal screening and counselling on the risk of development and progression of DR, as well as comprehensive care by a multidisciplinary team consisting of an endocrinologist, an ophthalmologist, and a perinatologist[26]. Comprehensive eye assessment, tight glycaemic control, and other assessments will be performed throughout the pregnancy period[27]. The duration of the follow-up is dependent on the stage of DR; the more severe the DR is at diagnosis during the initial check-up, the more frequent the follow-up schedule will be. Maximal control of both glucose levels and blood pressure is essential in the treatment of DR during pregnancy[16].
Currently, scatter or panretinal photocoagulation (PRP) is a preferred treatment modality for all patients, including pregnant women with DR, which involves applying laser burns on the retina while sparing the central macular area to reduce the ischaemic drive and the risk of vision loss[28]. In an unfortunate event of DR progression, pregnant women with severe NPDR and PDR at the preproliferative stage may consider either scatter or PRP, as both are effective and safe treatments with minimal side effects to the foetus[29,30]. Although the results from protocol S of DRCR.net found that both anti-vascular endothelial growth factor agents (anti-VEGF) and PRP are effective for PDR, anti-VEGF in pregnancy should be avoided whenever possible to minimize the placental transfer of drugs and risk to the foetus[30,31]. However, PRP treatment is associated with potential side effects, including worsening of macular oedema that may lead to transient or permanent vision loss, peripheral visual field defects, night vision loss, loss of contrast sensitivity, potential complications from misdirected or excessive burns, and progression of visual loss[32].
VEGF, an endothelial-cell-specific angiogenic factor[33], was suggested to be the primary mediator of diabetic retinal neovascularization, as its concentration in ocular fluid samples from patients with PDR was found to be significantly increased compared to samples from patients with NPDR[34]. Since then, clinical studies have suggested that anti-VEGF therapy is effective for PDR[30], and various anti-VEGF drugs, such as pegaptanib, ranibizumab, bevacizumab, and aflibercept, have been used. Pegaptanib (Macugen®; Pfizer Inc.) is a 28-base ribonucleic acid aptamer that specifically binds to and blocks the activity of the 165 amino acid isoform of VEGF (VEGF165)[35] and was approved by the United States Federal Drug Administration (FDA) for the treatment of neovascular age-related macular degeneration in 2004[36]; administration of a 0.3 mg (0.9 mL) dose is recommended once every six weeks by intravitreal injection. The use of pegaptanib has been shown to reduce retinal thickness and improve vision in PDR[37] and macular oedema[38]. However, its use worldwide and in Malaysia for DME and PDR in nonpregnancy diabetic patients has been largely superseded by the other 3 anti-VEGF agents.
Ranibizumab (Lucentis®; Genentech Inc.) is a humanized monoclonal antibody fragment directed at all isoforms of VEGF-A and contains only the Fab fragment of the parental anti-VEGF antibody with a weight of 48 kDa[39]. The DR Clinical Research Network’s (DRCR.net) Protocol S study found that eyes treated with ranibizumab were less likely to have vitreous haemorrhage (VH) and progress from severe NPDR to PDR than those treated with PRP[30]. The use of ranibizumab 0.3 to 0.5 mg (0.05 mL) as a monthly intravitreal injection attained FDA approval for the treatment of all forms of DR in 2017.
Bevacizumab (Avastin®; Genentech Inc.), a full-length recombinant humanized monoclonal immunoglobulin G1κ antibody weighing 149 kDa, which inactivates all VEGF isoforms[39], was FDA-approved as a treatment for colorectal carcinoma in 2004. It is used as an off-label therapy by many ophthalmologists, as trials found its side-effect profile with doses of either 1.25 mg or 2.5 mg (0.05 mL) to be similar to ranibizumab[40]. A 2-year randomized controlled trial also provided evidence supporting the use of bevacizumab for persistent centre-involving macular oedema[41].
Aflibercept (Eylea®; Regeneron Inc.) is a 115 kDa recombinant fusion protein that consists of VEGF-binding domains for human VEGF receptors 1 and 2 fused to the Fc domain of human immunoglobulin-G1 and binds to all isomers of the VEGF-A family[38]. In 2014, the FDA approved aflibercept for the treatment of macular oedema after significant improvements in the primary endpoint of mean change in best-corrected visual acuity were achieved for the aflibercept-treated group in completed phase III VIVID and VISTA[42] trials, and the 52-wk visual and anatomic superiority of the intravitreal aflibercept injection group was sustained through week 100[43]. The Panorama trial[44] was then conducted to investigate aflibercept for the improvement of moderate-severe to severe NPDR without macular oedema, and the safety data were consistent with the results of phase III VIVID and VISTA trials, and the outcome was sustained through week 100[45]; thus, it obtained FDA approval for the treatment of DR in 2019. The recommended dosage of aflibercept injection for the treatment of macular oedema and DR is 2 mg (0.05 mL) every 8 wk after five initial monthly injections.
VEGF also plays a role in the maintenance of foetal and placental vasculature[46]; thus, a reduction in VEGF expression has been linked with defective embryogenesis and foetal loss in humans[47]. Studies also found that the inhibition of VEGF activity and signalling pathways may lead to hypertension[48-50]. Despite this, the relationship between VEGF, hypertension, and preeclampsia is poorly understood. The teratogenicity of anti-VEGF drugs have been explored, categorized, and detailed by the FDA as follows[31]: Pegaptanib has been assigned to Pregnancy Category B, where no teratogenicity was found in mice when given an intravenous dose of up to 40 mg/kg/d (approximately 7000 times the recommended human dose of 0.3 mg per eye), while human studies are not yet available[51]; ranibizumab is designated Pregnancy Category C, where an embryo-foetal developmental toxicity study was performed on pregnant cynomolgus monkeys, and skeletal abnormalities were found in foetuses from monkeys treated with a dose of 1 mg/eye (approximately 13 times higher than predicted mean-steady stage Cmax levels with single eye treatment in humans); no skeletal abnormalities were observed at the lower dose of 0.125 mg/eye (equivalent to Cmax levels with single eye treatment in humans), and no adequate and well-controlled studies of the administration have been conducted in pregnant women[52]; bevacizumab has been assigned to Pregnancy Category C, as pregnant rabbits dosed with 10 mg/kg to 100 mg/kg (approximately 1 to 10 times the clinical dose of 10 mg/kg) every three days during day 6–18 of gestation showed decrease in maternal and foetal body weights, increased number of foetal resorptions, skeletal deformities, and corneal opacity in all doses, while controlled data are not yet available in human pregnancy[53]; and aflibercept is designated Pregnancy Category C, where embryo-foetal development studies on rabbits with intravenous doses of ≥ 3 mg/kg have revealed evidence of embryo-foetal toxicity such as post-implantation loss and foetal malformations including skeletal abnormalities in all doses, while no controlled data are yet available in pregnant women[54].
The pharmacokinetics of these anti-VEGF drugs have been tested in animals and humans, but not all pharmacokinetic values in humans have been obtained. Nevertheless, the pharmacokinetic characteristics of these 4 drugs appear to be similar. Following intravitreal injections, these anti-VEGF drugs leave the eye by crossing the retina and retinal pigment epithelium to the choroidal circulation, passing through the ciliary body and iris, or moving into the anterior chamber by diffusion and bulk flow before exiting through the trabecular meshwork, and none of the drugs degrades within the eye[55]. Systemic half-lives vary from hours to weeks before drug elimination via glomerular filtration or pinocytotic elimination occurs.
Pegaptanib was found to have an intravitreal half-life of 3.9 d in monkeys[56] and an estimated half-life of 7 d in humans. After entering the systemic circulation in humans, the maximum serum concentration is reached in 1–4 d, and the serum half-life is 10 d. It is metabolized by endonucleases and exonucleases, which are then excreted primarily in the urine. On the other hand, after intravitreal injection into rabbits, ranibizumab has a half-life of 2.6–2.88 d[57-59] with a maximum aqueous concentration after 3 d. Ranibizumab fully penetrates the retina one day after injection, and the concentrations in the serum are either very low (1/10000 that of the vitreous)[58] or undetectable[59]. The half-life of ranibizumab in monkeys is 3 d, and serum concentrations are 1000-fold lower than those in the vitreous[60]. Intravitreal ranibizumab is found to distribute rapidly to the monkeys’ retina within 6–24 h[60]. The half-life of intravitreal ranibizumab in humans is estimated to be 4.8–9 d, with serum concentrations approximately 90000-fold lower than intraocular concentrations[55]. The intravitreal and serum half-lives of bevacizumab in rabbits are 4.32 and 6.8 d, respectively[61,62], with a maximum serum concentration reached in 8 d. After intravitreal injections in rabbits, bevacizumab appeared in the subretinal space within 2 h[63], the inner retina and choroid within the first day, and the outer layers and choroid in subsequent days, but no drugs were found at 4 wk[64]. The half-life of intravitreal bevacizumab in a human was estimated to be 6.7–10 d depending on the use of either a one-compartment model or two-compartment model[65-68], while the half-life of bevacizumab in human serum is 17–21 d, similar to that of other full-length antibodies. Intravitreal aflibercept has a half-life of 4.7 d in rabbits[69] and an estimated 9 d in humans based on the intermediate size of the molecule (between ranibizumab and bevacizumab), while bound aflibercept in human serum has a half-life of 18 d[70]. Table 1 summarizes the structural and pharmacokinetic characteristics of the four anti-VEGF drugs. No study has been found to determine whether these drugs cross the placenta in pregnant women.
| Pegaptanib | Ranibizumab | Bevacizumab | Aflibercept | |
| Structure | Pegylated aptamer | Recombinant monoclonal antibody fragment (Fab) | Recombinant monoclonal antibody (Mab) | Fusion protein |
| Molecular weight (kDa) | 50 | 48 | 149 | 115 |
| Recommended dose (volume) | 0.3 mg (0.9 mL) | 0.5 mg (0.05 mL) | 1.25 mg (0.05 mL) | 2 mg (0.05 mL) |
| Intravitreal half-life (d) | 3.9 (monkeys) | 2.6-2.88 (rabbits) | 4.32-6.61 (rabbits) | 4.5-4.7 (rabbits) |
| 3-3.2 (monkeys) | 3.1 (monkeys) | |||
| 7.1 (humans) | 6.7-10 (humans) | |||
| Serum half-life humans (d) | 10 | 0.25 | 21 | 18 |
Several studies on the use of ranibizumab and bevacizumab in pregnant women have been reported of which some have been summarized by Polizzi and Mahajan[31]. Most of the studies in pregnant women are case reports, and initial intravitreal ranibizumab was given either 8–17 wk post last menstrual period (LMP)[70] or in the third trimester[71,72]; all reported no complications.
However, intravitreal anti-VEGF injections given as early as 5 wk postconception were associated with miscarriage within a week[73]. A total of 8 papers comprising 16 pregnancies in 15 women using intravitreal bevacizumab have been published since 2009[74-83]. The injection was given between a few days before or after the LMP and during the third trimester. There were 5 cases of abortion[76,79,82,83] and one case of pre-eclampsia[80] after the use of intravitreal bevacizumab. Petrou et al[76] described 2 women who received intravitreal bevacizumab at approximately 4 and 3 wk of gestation, respectively, followed by spontaneous miscarriage 7 and 10 d, respectively, after administration of the drug[76]. Gómez Ledesma et al[79] also reported a 41-year-old woman who received intravitreal bevacizumab a few days before or after the LMP and suffered a miscarriage approximately 7 wk after the injection[79]. Kianersi et al[82,83] reported pregnancy loss within 18 to 24 h in two patients who received intravitreal bevacizumab injection while they were between 10 and 12 wk pregnant[82,83]. Intravitreal bevacizumab given preconception and continued after 29 wk of gestation was associated with preeclampsia requiring urgent caesarean section[80].
Despite these reports of spontaneous miscarriages and preeclampsia occurring after intravitreal anti-VEGF injections given within 13 wk of gestation, other reports did not find adverse events with injections given within the same time frame[70,74,75,77,78,80,81]; thus, it is uncertain whether anti-VEGF therapy played a role in these pregnancy losses, as the rate of spontaneous miscarriage is between 15% and 20%[84] and may increase to as high as 41% if maternal age is over 35 years[85]. There were no reports on pegaptanib and aflibercept being administered in pregnant women. Hence, the use of anti-VEGF should be weighed against the possible risk of foetal developmental abnormalities or pregnancy loss and should only be administered following a thorough discussion with the patient and consultation with an obstetrician, and the potential benefit outweighs the potential risk to the foetus. Indeed, DM patients of child-bearing age should have PDR and DME treated before conceiving. This even means the need for contraception during anti-VEGF treatment.
Apart from VEGF, elevated inflammatory markers have been found in patients with DR, which suggests that inflammation may play a role in the pathogenesis of DR[86] and macular oedema[87,88]. Both animal and human studies have found increased levels of inflammatory mediators and prostaglandins (PGs) in DR in the vitreous cavity[89-91], and prostaglandin E2 levels correlate with vitreous levels of VEGF[92]. As topical nonsteroidal anti-inflammatory drugs (NSAIDs) are potent inhibitors of cyclooxygenase enzymes and reduce the synthesis of proinflammatory PGs with few documented risks, they have recently become readily available in the form of topical ophthalmic formulations[93]. New topical NSAIDs such as nepafenac (Nevanac®; Alcon Inc.) were formulated to be able to reach the posterior segment of the eye[94,95]. It rapidly penetrates the cornea and is deaminated by intraocular hydrolases in uveal tissue and retina to form the active metabolite amfenac[96].
Several small randomized case studies on the use of topical nepafenac 0.1% for the treatment of DME have been published[97-100] and revealed the effectiveness of the drug and improvement in visual acuity and retinal/foveal/macular thickness. However, a phase II, multicentre, double-masked randomized clinical trial conducted by DRCR.net found that topical nepafenac 0.1% three times a day for a year on eyes with noncentral DME does not show a beneficial effect on OCT-measured retinal thickness or visual acuity outcomes[101], which is in contrast to the results of other smaller, randomized published case reports. Small quantifiable plasma concentrations of nepafenac and amfenac have been found in subjects 2–3 h after topical administration, and the Cmax of nepafenac and amfenac in serum was approximately 0.31 and 0.42 ng/mL, respectively[102]. The elimination of orally administered nepafenac in rats was shown to be in the urine (57%) and faeces (40%) over 7 d[103]. The FDA has also categorized nepafenac under pregnancy category C, as reproduction studies performed in rabbits and rats at oral doses of up to 10 mg/kg/d have revealed maternal toxicity and no teratogenicity[104]. Animal exposure to nepafenac and amfenac was approximately 260- to 2400-fold human plasma exposure at the recommended human topical ophthalmic dose for rats and approximately 80- and 680-fold human plasma exposure for rabbits, respectively, at this dose. Dystocia increased post-implantation loss, reduced foetal weight and growth, and reduced foetal survival in maternal rats when given doses of ≥ 10 mg/kg. Although nepafenac could cross the placental barrier in rats, no adequate and well-controlled studies in pregnant women have been conducted; therefore, nepafenac should be used in pregnancy only if the potential benefit outweighs the potential risk to the foetus and should be avoided in the third trimester due to the known effects of prostaglandin biosynthesis inhibition on the foetal cardiovascular system (closure of ductus arteriosus)[105].
VH secondary to PDR is one of the most common vision-threatening complications of DR other than DME. In mild to moderate cases of VH, PRP is performed when possible to prevent further episodes of VH, and it may eventually resolve spontaneously[106]. However, approximately 5% of PDR cases develop VH even after PRP is initiated, which often requires pars plana vitrectomy (PPV)[107], a technique introduced in the 1970s[108]. Despite vision improvement reported in approximately 75% of PDR patients after PPV, major complications associated with PPV include cataract formation, elevated intraocular pressure, recurrent vitreous cavity haemorrhage (early, delayed, or persistent), iatrogenic retinal breaks, tractional and rhegmatogenous retinal detachment, and neovascular glaucoma[109]. Several studies have been conducted on the use of anti-VEGF drugs as a treatment for VH due to PDR and found that intravitreal ranibizumab[110], bevacizumab[111,112], and aflibercept[113] had good short-term safety and efficacy for new or recurrent VH in PDR eyes with and without a previously lasered approach, reducing the need for PPV. As the use of anti-VEGF drugs is associated with pregnancy loss and foetal abnormalities, PRP and PPV remain the treatment of choice for VH in pregnant patients with PDR. Surgery should be conducted under the assistance of an experienced anaesthetist to anticipate pregnancy-related anaesthetic complications[114].
Advances in PPV instrumentation have led to small-gauge vitrectomy increasing in popularity, improving the surgical experience, and allowing PPV to be performed under local anaesthesia. Nevertheless, surgical treatment of any kind is a form of stress during pregnancy. The supine position required for PPV may even prove challenging for pregnant patients due to the gravid uterus. Hence, this reiterates the need to stabilize PDR before pregnancy with a PRP laser and, if needed, PPV in diabetic patients. Although anti-VEGF has advantages, it cannot be used as a prepregnancy therapy for diabetic women with active PDR who are intending to conceive. This is due to the risk of conception loss when they subsequently conceive while treatment has to continue during pregnancy. If PDR progression occurs, surgical treatment should be delayed after delivery if this option is available.
Management of DR in pregnancy is essential, and preventing the development and progression of DR should be at all costs, as well as ensuring maternal and foetal safety. However, ophthalmic surgery during pregnancy poses additional challenges, which include the timing of the surgery, the posture during surgery, and the type of anaesthesia. Elective surgery is recommended to be postponed until 6 wk postpartum, while essential surgery should be performed in the second trimester if possible when preterm contractions and spontaneous abortions are least likely[115]. Pregnant women are susceptible to hypoxia, hypercapnia, and systemic hypotension due to altered maternal physiology, which exposes both the mother and the foetus to the risk of surgical anaesthesia, particularly general anaesthesia. Moreover, the supine position in the second and third trimesters can induce profound hypotension due to aortic and vena cava compression by the uterus. Pregnant patients should therefore be positioned with their hips, abdomen, and thighs on their left side while maintaining a normal head position for ophthalmic surgery[116].
Current anaesthetic medications, including general anaesthetics (nitrous oxide excluded), benzodiazepines, and opioids, have not been shown to have any teratogenic effects in humans when using standard concentrations at any gestational age[117,118] and have not been associated with increased rates of stillbirths or adverse pregnancy outcomes[119]. However, reports have shown an increased incidence of low birth weight and neural tube defects with exposure to general anaesthesia in the first trimester[120]; thus, general anaesthesia should be avoided whenever possible.
Local anaesthetics work by blocking sodium channels in nerve membranes, leading to absent nerve impulses and anaesthesia[121]. An extensive study on local anaesthetic use in 60000 pregnant females included benzocaine, procaine, tetracaine, and lidocaine and revealed no increased incidence of foetal complications[122] or foetal birth defects[123].
Under circumstances where general anaesthesia was necessary, an appropriate understanding of additional pregnancy-related risks should be considered, including intubation difficulties, aspiration risks, thromboprophylaxis, and foetal well-being[124]. General anaesthetics work at the level of the spinal cord and in different areas of the brain, which results in relaxation of the muscles and central nervous system depression, although the exact mechanism of action has not been ascertained[125]. Thiopentone in late pregnancy showed no significant effect on intrauterine pressure, while ketamine was found to cause a uterine contraction in early pregnancy and no effect in late pregnancy[126]. Volatile anaesthetics such as halothane, sevoflurane, desflurane, and isoflurane have been shown to inhibit uterine contractility; thus, they may be beneficial in preventing preterm contractions[127]. Nonetheless, the choice of anaesthetic technique and the selection of appropriate anaesthetic drugs should be carefully considered to preserve maternal safety, maintain the pregnancy state, and achieve the best possible foetal outcome.
According to Malaysia’s Clinical Practice Guidelines: Screening of DR, individuals with pre-existing DM who are planning for pregnancy should have their eyes examined before conception and counselled on the risk of DR development and progression[128]. Subsequent follow-up is dependent on the stage of DR found on the initial examinations: Every 3 mo for mild to no DR and referral to an ophthalmologist is necessary for moderate to severe DR. Women with gestational DM (GDM) do not require DR screening, as it carries no risk of DR unless GDM is diagnosed in the first trimester of pregnancy. GDM is a glucose intolerance state induced by pregnancy that may resolve or persist after the pregnancy period[129,130], and the prevalence of GDM in Malaysia was reported to be approximately 8.8%[131]. Women with GDM have a sevenfold increased relative risk of progressing to T2DM[132-134], and they are usually asymptomatic until macular oedema or PDR has developed.
Bastion et al[135] reported a case of a 36-year-old pregnant woman who had GDM at her previous pregnancy with an elevated post-delivery maternal glucose tolerance test. Her first-trimester fundoscopy found no DR. By the second trimester, she had developed PDR, and PRP was performed on both eyes during her pregnancy. This was followed by PPV with membrane peeling in the right eye at five months postpartum, as the right VH did not resolve spontaneously, leaving her with counting-finger vision[135]. On the other hand, Raman and Livingstone reported a case study of a 31-year-old pregnant woman with underlying T2DM who had diffuse VH on both eyes at her 22nd week of gestation, which required urgent PRP. However, she developed recurrent VH in her third trimester, and PPV was then performed at 2 wk postpartum for her right eye, as it then developed inferior combined rhegmatogenous and tractional retinal detachment (TRD). Her left eye had a nonclearing VH requiring PPV a month later[136]. Both cases reported safe delivery of the baby and good postoperative visual acuity[135,136], highlighting the rapid progression of DR and the importance of follow-up and timely surgical intervention for a good final vision outcome.
However, Helen et al[137] reported four T1DM women with PDR who had adverse maternal outcomes, including abortion in one patient, preeclampsia, and preterm delivery in one patient, renal failure requiring dialysis in one patient, neonatal death occurring in one case, and premature delivery occurring in another case. All except one woman had stable or improved visual acuity. One woman progressed to develop neovascular glaucoma[137]. Hence, prepregnancy counselling and close follow-up during pregnancy and the postpartum period are essential for diabetic women.
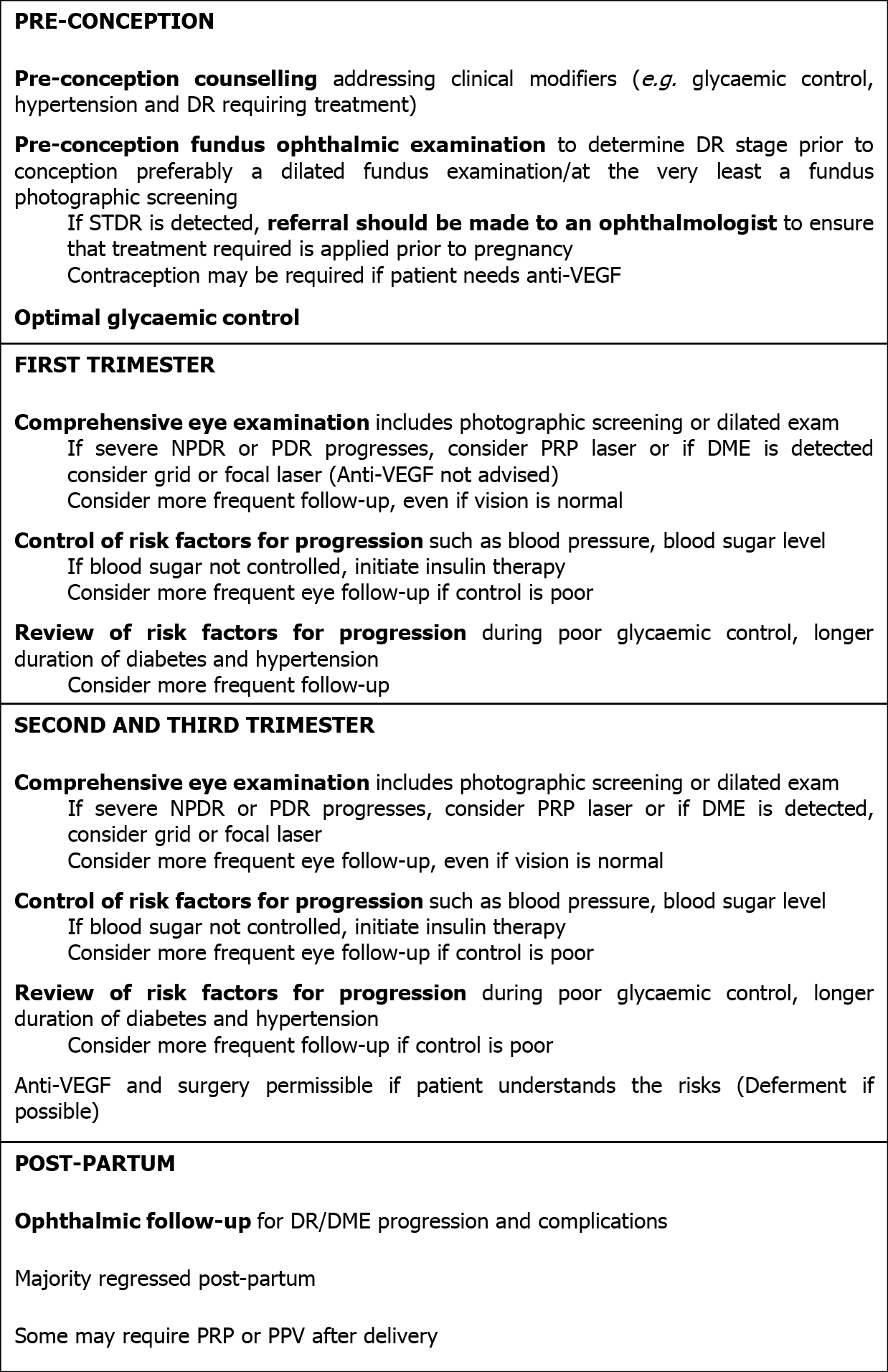
The recommended ophthalmic management of DR during pregnancy at each stage[23,26] is summarized in the following flowchart (Figure 1).
Despite the best efforts to monitor and manage DR during pregnancy, the literature suggests that compliance with treatment and follow-up is still a struggle for pregnant women with diabetes. Hampshire et al[138] looked at attendance at a prepregnancy care program for adequate retinal assessment in the subsequent pregnancy and found that 70% of women with pregestational diabetes had incomplete follow-up[138], suggesting a lack of awareness on sight-threatening complications of diabetes[139].
There is limited evidence for the management of STDR in pregnancy, with evidence mainly from case reports and series. Management of STDR in pregnancy requires prepregnancy counselling, treatment, and stabilization of DM and STDR. It involves appropriate control of systemic risk factors for DR progression, monitoring of DR with fundus imaging at least every trimester, and prompt referral to the ophthalmologist when there is DR progression during pregnancy. Treatments that are conventional for DME, such as anti-VEGF, should not be given during pregnancy in diabetic patients, particularly in the early trimester, as there have been several reports of foetal loss. PRP can be given for severe NPDR and PDR; however, surgical management for VH or TRD in pregnancy should be deferred. If at all required, surgery should be performed under local anaesthesia, at an earlier trimester, or deferred until after delivery.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tolunay HE S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J Diabetes Res. 2015;2015:582060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4374] [Article Influence: 291.6] [Reference Citation Analysis (4)] |
| 3. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3524] [Cited by in RCA: 3102] [Article Influence: 238.6] [Reference Citation Analysis (3)] |
| 4. | Wan Nazaimoon WM, Md Isa SH, Wan Mohamad WB, Khir AS, Kamaruddin NA, Kamarul IM, Mustafa N, Ismail IS, Ali O, Khalid BA. Prevalence of diabetes in Malaysia and usefulness of HbA1c as a diagnostic criterion. Diabet Med. 2013;30:825-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Hussein Z, Taher SW, Gilcharan Singh HK, Chee Siew Swee W. Diabetes Care in Malaysia: Problems, New Models, and Solutions. Ann Glob Health. 2015;81:851-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Fenwick E, Rees G, Pesudovs K, Dirani M, Kawasaki R, Wong TY, Lamoureux E. Social and emotional impact of diabetic retinopathy: a review. Clin Exp Ophthalmol. 2012;40:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Rees G, Xie J, Fenwick EK, Sturrock BA, Finger R, Rogers SL, Lim L, Lamoureux EL. Association Between Diabetes-Related Eye Complications and Symptoms of Anxiety and Depression. JAMA Ophthalmol. 2016;134:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36:1562-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Leske MC, Wu SY, Hennis A, Hyman L, Nemesure B, Yang L, Schachat AP; Barbados Eye Study Group. Hyperglycemia, blood pressure, and the 9-year incidence of diabetic retinopathy: the Barbados Eye Studies. Ophthalmology. 2005;112:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT; Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Estacio RO, McFarling E, Biggerstaff S, Jeffers BW, Johnson D, Schrier RW. Overt albuminuria predicts diabetic retinopathy in Hispanics with NIDDM. Am J Kidney Dis. 1998;31:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | You WP, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally: the role of natural selection and life expectancy at birth. BMJ Open Diabetes Res Care. 2016;4:e000161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 15. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1638] [Article Influence: 409.5] [Reference Citation Analysis (2)] |
| 16. | Vestgaard M, Ringholm L, Laugesen CS, Rasmussen KL, Damm P, Mathiesen ER. Pregnancy-induced sight-threatening diabetic retinopathy in women with Type 1 diabetes. Diabet Med. 2010;27:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Moloney JB, Drury MI. The effect of pregnancy on the natural course of diabetic retinopathy. Am J Ophthalmol. 1982;93:745-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 93] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Temple RC, Aldridge VA, Sampson MJ, Greenwood RH, Heyburn PJ, Glenn A. Impact of pregnancy on the progression of diabetic retinopathy in Type 1 diabetes. Diabet Med. 2001;18:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Schultz KL, Birnbaum AD, Goldstein DA. Ocular disease in pregnancy. Curr Opin Ophthalmol. 2005;16:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chew EY, Mills JL, Metzger BE, Remaley NA, Jovanovic-Peterson L, Knopp RH, Conley M, Rand L, Simpson JL, Holmes LB. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 179] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Rosenn B, Miodovnik M, Kranias G, Khoury J, Combs CA, Mimouni F, Siddiqi TA, Lipman MJ. Progression of diabetic retinopathy in pregnancy: association with hypertension in pregnancy. Am J Obstet Gynecol. 1992;166:1214-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 87] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Lövestam-Adrian M, Agardh CD, Aberg A, Agardh E. Pre-eclampsia is a potent risk factor for deterioration of retinopathy during pregnancy in Type 1 diabetic patients. Diabet Med. 1997;14:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Morrison JL, Hodgson LA, Lim LL, Al-Qureshi S. Diabetic retinopathy in pregnancy: a review. Clin Exp Ophthalmol. 2016;44:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Omoti AE, Waziri-Erameh JM, Okeigbemen VW. A review of the changes in the ophthalmic and visual system in pregnancy. Afr J Reprod Health. 2008;12:185-196. [PubMed] |
| 25. | Wang LZ, Cheung CY, Tapp RJ, Hamzah H, Tan G, Ting D, Lamoureux E, Wong TY. Availability and variability in guidelines on diabetic retinopathy screening in Asian countries. Br J Ophthalmol. 2017;101:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Mallika P, Tan A, S A, T A, Alwi SS, Intan G. Diabetic retinopathy and the effect of pregnancy. Malays Fam Physician. 2010;5:2-5. [PubMed] |
| 27. | Klein BE, Moss SE, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 176] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Canadian Diabetes Association Clinical Practice Guidelines Expert Committee; Cheng AY. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes. 2013;37 Suppl 1:S1-S3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 29. | American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern® Guidelines. Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology, 2014. |
| 30. | Writing Committee for the Diabetic Retinopathy Clinical Research Network. Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2015;314:2137-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 556] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 31. | Polizzi S, Mahajan VB. Intravitreal Anti-VEGF Injections in Pregnancy: Case Series and Review of Literature. J Ocul Pharmacol Ther. 2015;31:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | American Academy of Ophthalmology. Preferred practice pattern diabetic retinopathy. San Francisco, CA: American Academy of Ophthalmology, 1998. |
| 33. | Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1050] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 34. | Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2594] [Cited by in RCA: 2621] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 35. | Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 1081] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 36. | Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1663] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 37. | González VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol. 2009;93:1474-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS; Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Kubota T, Kiuchi Y, Sheridan C. Anti-vascular endothelial growth factor agents for ocular angiogenesis and vascular permeability. J Ophthalmol. 2012;2012:898207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Comparison of Age-related Macular Degeneration Treatments Trials(CATT) Research Group, Martin DF. Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 1388] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 41. | Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 289] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 42. | Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Brown DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 43. | Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, Heier JS, Terasaki H, Kaiser PK, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Korobelnik JF. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 44. | Brown DM. Intravitreal aflibercept injection (IAI) for moderately severe to severe nonproliferative diabetic retinopathy (NPDR): the phase 3 PANORAMA study. Invest Ophthalmol Vis Sci. 2018;59:1889. |
| 45. | Lim JI. Intravitreal aflibercept injection for nonproliferative diabetic retinopathy: year 2 results from the PANORAMA study. Invest Ophthalmol Vis Sci. 2020;61:1381. |
| 46. | Almawi WY, Saldanha FL, Mahmood NA, Al-Zaman I, Sater MS, Mustafa FE. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum Reprod. 2013;28:2628-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Galazios G, Papazoglou D, Tsikouras P, Kolios G. Vascular endothelial growth factor gene polymorphisms and pregnancy. J Matern Fetal Neonatal Med. 2009;22:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Granger JP. Vascular endothelial growth factor inhibitors and hypertension: a central role for the kidney and endothelial factors? Hypertension. 2009;54:465-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010;30:591-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | U.S. Food and Drug Administration. Food and Drug Administration. Highlights of prescribing information: MACUGEN® (pegaptanib sodium injection) intravitreal injection, revised: 07/2011. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2011/021756s018Lbl.pdf. |
| 52. | U.S. Food and Drug Administration. Food and Drug Administration. Highlights of prescribing information: LUCENTIS® (ranibizumab injection) for intravitreal injection, revised: 04/2017. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2017/125156s114Lbl.pdf. |
| 53. | U.S. Food and Drug Administration. Food and Drug Administration. Highlights of prescribing information: AVASTIN® (bevacizumab) injection for intravenous use, revised: 10/2020. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2020/125085s336Lbl.pdf. |
| 54. | U.S. Food and Drug Administration. S. Food and Drug Administration. Highlights of prescribing information: EYLEA® (aflibercept) injection, for intravitreal use, revised: 5/2019. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2019/125387s061 Lbl.pdf. |
| 55. | Stewart MW. Pharmacokinetics, pharmacodynamics and pre-clinical characteristics of ophthalmic drugs that bind VEGF. Expert Rev Clin Pharmacol. 2014;7:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 718] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 57. | Christoforidis JB, Williams MM, Wang J, Jiang A, Pratt C, Abdel-Rasoul M, Hinkle GH, Knopp MV. Anatomic and pharmacokinetic properties of intravitreal bevacizumab and ranibizumab after vitrectomy and lensectomy. Retina. 2013;33:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Gaudreault J, Fei D, Beyer JC, Ryan A, Rangell L, Shiu V, Damico LA. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina. 2007;27:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 60. | Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 61. | Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 62. | Christoforidis JB, Carlton MM, Knopp MV, Hinkle GH. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Invest Ophthalmol Vis Sci. 2011;52:5899-5903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Dib E, Maia M, Longo-Maugeri IM, Martins MC, Mussalem JS, Squaiella CC, Penha FM, Magalhães O Jr, Rodrigues EB, Farah ME. Subretinal bevacizumab detection after intravitreous injection in rabbits. Invest Ophthalmol Vis Sci. 2008;49:1097-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, Johnson PT, Fisher SK, Perlman I, Loewenstein A. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006;26:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 260] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 65. | Zhu Q, Ziemssen F, Henke-Fahle S, Tatar O, Szurman P, Aisenbrey S, Schneiderhan-Marra N, Xu X; Tübingen Bevacizumab Study Group; Grisanti S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750-1755, 1755.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 67. | Csaky KG, Gordiyenko N, Rabena MG, Avery RL. Pharmacokinetics of intravitreal bevacizumab in humans. Invest Ophthalmol Vis Sci. 2007;48:4936. |
| 68. | Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg vs 3.0 mg of bevacizumab in humans. Retina. 2011;31:1877-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Furfine E, Coppi A, Koehler-Stec E, Zimmer E, Tu W, Struble C. Pharmacokinetics and ocular tissue penetration of VEGF Trap after intravitreal injections in rabbits. Invest Ophthalmol Vis Sci. 2006;47:1430. |
| 70. | Fossum P, Couret C, Briend B, Weber M, Lagarce L. Safety of intravitreal injection of ranibizumab in early pregnancy: a series of three cases. Eye (Lond). 2018;32:830-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Sarhianaki A, Katsimpris A, Petropoulos IK, Livieratou A, Theoulakis PE, Katsimpris JM. Intravitreal administration of ranibizumab for idiopathic choroidal neovascularization in a pregnant woman. Klin Monbl Augenheilkd. 2012;229:451-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Jouve L, Akesbi J, Nordmann JP. Safety and efficacy of ranibizumab for pregnant women in idiopathic choroidal neovascularization. Acta Ophthalmol. 2015;93:e597-e598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Akkaya S. Early Miscarriage Occurring Six Days After Intravitreal Ranibizumab Injection. Med Hypothesis Discov Innov Ophthalmol. 2019;8:69-72. [PubMed] |
| 74. | Rosen E, Rubowitz A, Ferencz JR. Exposure to verteporfin and bevacizumab therapy for choroidal neovascularization secondary to punctate inner choroidopathy during pregnancy. Eye (Lond). 2009;23:1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Wu Z, Huang J, Sadda S. Inadvertent use of bevacizumab to treat choroidal neovascularisation during pregnancy: a case report. Ann Acad Med Singap. 2010;39:143-145. [PubMed] |
| 76. | Petrou P, Georgalas I, Giavaras G, Anastasiou E, Ntana Z, Petrou C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88:e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Introini U, Casalino G, Cardani A, Scotti F, Finardi A, Candiani M, Bandello F. Intravitreal bevacizumab for a subfoveal myopic choroidal neovascularization in the first trimester of pregnancy. J Ocul Pharmacol Ther. 2012;28:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Gómez Ledesma I, de Santiago Rodríguez MÁ, Follana Neira I, León Garrigosa F. [Neovascular membrane and pregnancy. Treatment with bevacizumab]. Arch Soc Esp Oftalmol. 2012;87:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Sullivan L, Kelly SP, Glenn A, Williams CP, McKibbin M. Intravitreal bevacizumab injection in unrecognised early pregnancy. Eye (Lond). 2014;28:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 81. | Polizzi S, Ferrara G, Restaino S, Rinaldi S, Tognetto D. Inadvertent use of bevacizumab in pregnant women with diabetes mellitus type 1. J Basic Clin Physiol Pharmacol. 2015;26:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Kianersi F, Ghanbari H, Naderi Beni Z, Naderi Beni A. Intravitreal vascular endothelial growth factor (VEGF) inhibitor injection in unrecognised early pregnancy. Invest New Drugs. 2016;34:650-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Kianersi F, Ghanbari H, Naderi Beni Z, Naderi Beni A. Intravitreal vascular endothelial growth factor (VEGF) inhibitor injection in patient during pregnancy. J Drug Assess. 2021;10:7-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Robinson GE. Pregnancy loss. Best Pract Res Clin Obstet Gynaecol. 2014;28:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 85. | Knudsen UB, Hansen V, Juul S, Secher NJ. Prognosis of a new pregnancy following previous spontaneous abortions. Eur J Obstet Gynecol Reprod Biol. 1991;39:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 242] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 87. | Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 88. | Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 345] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 89. | Johnson EI, Dunlop ME, Larkins RG. Increased vasodilatory prostaglandin production in the diabetic rat retinal vasculature. Curr Eye Res. 1999;18:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Lane LS, Jansen PD, Lahav M, Rudy C. Circulating prostacyclin and thromboxane levels in patients with diabetic retinopathy. Ophthalmology. 1982;89:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 92. | Schoenberger SD, Kim SJ, Sheng J, Rezaei KA, Lalezary M, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012;53:5906-5911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Schoenberger SD, Kim SJ. Nonsteroidal anti-inflammatory drugs for retinal disease. Int J Inflam. 2013;2013:281981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Kapin MA, Yanni JM, Brady MT, McDonough TJ, Flanagan JG, Rawji MH, Dahlin DC, Sanders ME, Gamache DA. Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation. 2003;27:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Kern TS, Miller CM, Du Y, Zheng L, Mohr S, Ball SL, Kim M, Jamison JA, Bingaman DP. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007;56:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 96. | Ke TL, Graff G, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation. 2000;24:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Hariprasad SM, Callanan D, Gainey S, He YG, Warren K. Cystoid and diabetic macular edema treated with nepafenac 0.1%. J Ocul Pharmacol Ther. 2007;23:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Callanan D, Williams P. Topical nepafenac in the treatment of diabetic macular edema. Clin Ophthalmol. 2008;2:689-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Garcia-Gonzalez JM, Emanuelli A, Berrocal MH. Topical nepafenac 0.1% for the treatment of macular edema secondary to diabetic retinopathy and retinal vascular occlusions. Invest Ophthalmol Vis Sci. 2009;50:1349. |
| 100. | Vignesh TP. Topical nepafenac in the treatment of center involving diabetic macular edema. TNOA J Ophthalmic Sci Res. 2019;57:109-112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Friedman SM, Almukhtar TH, Baker CW, Glassman AR, Elman MJ, Bressler NM, Maker MP, Jampol LM, Melia M; Diabetic Retinopathy Clinical Research Network. Topical nepafenec in eyes with noncentral diabetic macular edema. Retina. 2015;35:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 102. | Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000;24:357-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Bucci FA Jr, Waterbury LD, Amico LM. Prostaglandin E2 inhibition and aqueous concentration of ketorolac 0.4% (acular LS) and nepafenac 0.1% (nevanac) in patients undergoing phacoemulsification. Am J Ophthalmol. 2007;144:146-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | U.S. Food and Drug Administration. Food and Drug Administration. Highlights of prescribing information: NEVANAC® (nepafenac ophthalmic suspension) 0.1%, for topical ophthalmic use, revised: 11/2020. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2020/021862s017 Lbl.pdf. |
| 105. | Coceani F, Olley PM. Involvement of prostaglandins in the fetal and neonatal circulation. In: Berti F, Folco G, Velo GP (eds) Leukotrienes and Prostacyclin. NATO Advanced Science Institutes Series (Series A: Life Sciences). Boston: Springer, 1983. |
| 106. | El Annan J, Carvounis PE. Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. Int Ophthalmol Clin. 2014;54:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Flynn HW Jr, Chew EY, Simons BD, Barton FB, Remaley NA, Ferris FL 3rd. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992;99:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:813-820. [PubMed] |
| 109. | Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Diabetic Retinopathy Clinical Research Network*. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 111. | Wirkkala J, Bloigu R, Hautala NM. Intravitreal bevacizumab improves the clearance of vitreous haemorrhage and visual outcomes in patients with proliferative diabetic retinopathy. BMJ Open Ophthalmol. 2019;4:e000390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Taskintuna I, Elsayed MEAA, Taskintuna K, Ahmad K, Khandekar R, Schatz P, Kozak I. Comparison of outcomes of four different treatment modalities for diabetic vitreous haemorrhage. Sci Rep. 2020;10:3674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Mansour AM, Ashraf M, El Jawhari KM, Farah M, Souka A, Sarvaiya C, Singh SR, Banker A, Chhablani J. Intravitreal ziv-aflibercept in diabetic vitreous hemorrhage. Int J Retina Vitreous. 2020;6:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Abdelaal AM, Alqahtani AS. Mode of Delivery in the Setting of Repeated Vitreous Hemorrhages in Proliferative Diabetic Retinopathy: A Case Report and Review of the Literature. Cureus. 2020;12:e11239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Jacobson MS. Ophthalmology surgery during pregnancy. In: Nezhat C, Kavic M, Lanzafame R, Lindsay M, Polk T (eds) Non-obstetric surgery during pregnancy. Boston: Springer, 2019. |
| 116. | Kuczkowski KM. Nonobstetric surgery in the parturient: anesthetic considerations. J Clin Anesth. 2006;18:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Samples JR, Meyer SM. Use of ophthalmic medications in pregnant and nursing women. Am J Ophthalmol. 1988;106:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Schaefer C, Peters PW, Miller RK. Drugs during pregnancy and lactation: treatment options and risk assessment. London: Academic Press, 2014. |
| 119. | Reitman E, Flood P. Anaesthetic considerations for non-obstetric surgery during pregnancy. Br J Anaesth. 2011;107 Suppl 1:i72-i78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 120. | Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 303] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 121. | Hemmings HC Jr, Greengard P. Positively active: how local anesthetics work. Anesthesiology. 2010;113:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Turner MD, Singh F, Glickman RS. Dental management of the gravid patient. N Y State Dent J. 2006;72:22-27. [PubMed] |
| 123. | Hagai A, Diav-Citrin O, Shechtman S, Ornoy A. Pregnancy outcome after in utero exposure to local anesthetics as part of dental treatment: A prospective comparative cohort study. J Am Dent Assoc. 2015;146:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Upadya M, Saneesh PJ. Anaesthesia for non-obstetric surgery during pregnancy. Indian J Anaesth. 2016;60:234-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 125. | Kopp Lugli A, Yost CS, Kindler CH. Anaesthetic mechanisms: update on the challenge of unravelling the mystery of anaesthesia. Eur J Anaesthesiol. 2009;26:807-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 126. | Oats JN, Vasey DP, Waldron BA. Effects of ketamine on the pregnant uterus. Br J Anaesth. 1979;51:1163-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Yoo KY, Lee JC, Yoon MH, Shin MH, Kim SJ, Kim YH, Song TB, Lee J. The effects of volatile anesthetics on spontaneous contractility of isolated human pregnant uterine muscle: a comparison among sevoflurane, desflurane, isoflurane, and halothane. Anesth Analg. 2006;103:443-447, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Ministry of Health, Malaysia. Clinical Practice Guidelines: Screening of Diabetic Retinopathy. Putrajaya, Malaysia: Ministry of Health, 2011. |
| 129. | Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 339] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 130. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3447] [Article Influence: 313.4] [Reference Citation Analysis (16)] |
| 131. | Jeganathan R, Karalasingam SD. 4th report of national obstetric registry. Ministry of Health, Malaysia, 2013-2015. [cited 10 January 2021]. Available from: http://www.acrm.org.my/nor/reports.php.. |
| 132. | Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016;16:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 865] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 133. | Sandsæter HL, Horn J, Rich-Edwards JW, Haugdahl HS. Preeclampsia, gestational diabetes and later risk of cardiovascular disease: Women's experiences and motivation for lifestyle changes explored in focus group interviews. BMC Pregnancy Childbirth. 2019;19:448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 134. | Nguyen CL, Pham NM, Binns CW, Duong DV, Lee AH. Prevalence of Gestational Diabetes Mellitus in Eastern and Southeastern Asia: A Systematic Review and Meta-Analysis. J Diabetes Res. 2018;2018:6536974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 135. | Bastion ML, Barkeh HJ, Muhaya M. Accelerated diabetic retinopathy in pregnancy--a real and present danger. Med J Malaysia. 2005;60:502-504. [PubMed] |
| 136. | Raman P, Livingstone BI. Advanced diabetic eye disease in pregnancy. J Clin Gynecol Obstet. 2018;7:72-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 137. | Helen CC, Tajunisah I, Reddy SC. Adverse outcomes in Type I diabetic pregnant women with proliferative diabetic retinopathy. Int J Ophthalmol. 2011;4:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 138. | Hampshire R, Wharton H, Leigh R, Wright A, Dodson P. Screening for diabetic retinopathy in pregnancy using photographic review clinics. Diabet Med. 2013;30:475-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Buari NH, Dian NI. The Association of Awareness and Knowledge of Diabetic Retinopathy with Age and Residential Area In Selangor. Environ Proc J. 2017;2:125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |