Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1363
Peer-review started: January 26, 2021
First decision: May 3, 2021
Revised: May 10, 2021
Accepted: August 5, 2021
Article in press: August 5, 2021
Published online: September 15, 2021
Processing time: 223 Days and 14.3 Hours
As an endocrine hormone, vitamin D plays an important role in bone health and calcium homeostasis. Over the past two decades, the non-calcemic effects of vitamin D were extensively examined. Although the effect of vitamin D on beta cell function were known for some time, the effect of vitamin D on glucose and fuel homeostasis has attracted new interest among researchers. Yet, to date, studies remain inconclusive and controversial, in part, due to a lack of understanding of the threshold effects of vitamin D. In this review, a critical examination of interventional trials of vitamin D in prevention of diabetes is provided. Like use of vitamin D for bone loss, the benefits of vitamin D supplementation in diabetes prevention were observed in vitamin D-deficient subjects with serum 25-hydroxyvitamin D < 50 nmol/L (20 ng/mL). The beneficial effect from vitamin D supplementation was not apparent in subjects with serum 25-hydroxyvitamin D > 75 nmol/L (30 ng/mL). Furthermore, no benefit was noted in subjects that achieved serum 25-hydroxyvitamin D > 100 nmol/L (40 ng/mL). Further studies are required to confirm these observations.
Core Tip: Vitamin D deficiency is a well-recognized health issue and contributes to bone loss and calcium dysregulation. Evidence suggests that excess vitamin D is not in and of itself of therapeutic benefit. Available clinical data suggests that vitamin D supplementation appears to limit the development of diabetes in vitamin D deficient subjects. However, no benefit was observed in non-vitamin D deficient subjects. Furthermore, overreplacement of vitamin D is of no beneficial effect and could possibly be harmful.
- Citation: Chang Villacreses MM, Karnchanasorn R, Panjawatanan P, Ou HY, Chiu KC. Conundrum of vitamin D on glucose and fuel homeostasis. World J Diabetes 2021; 12(9): 1363-1385
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1363.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1363
The potential role of vitamin D deficiency induced by migration of human beings has been suggested to be involved in human evolution and various modern health conditions[1]. The history prospective of vitamin D evaluation will enhance our understanding of the development in this field. The role of dietary deficiency in the pathogenesis of rickets was established by Platt[2] in 1919. Although it was thought to be caused by vitamin A deficiency initially, McCollum et al[3] identified a vitamin deficiency other than vitamin A that caused rickets in 1922. Since vitamin A, B, and C were already identified, the new molecule was named as vitamin D[4].
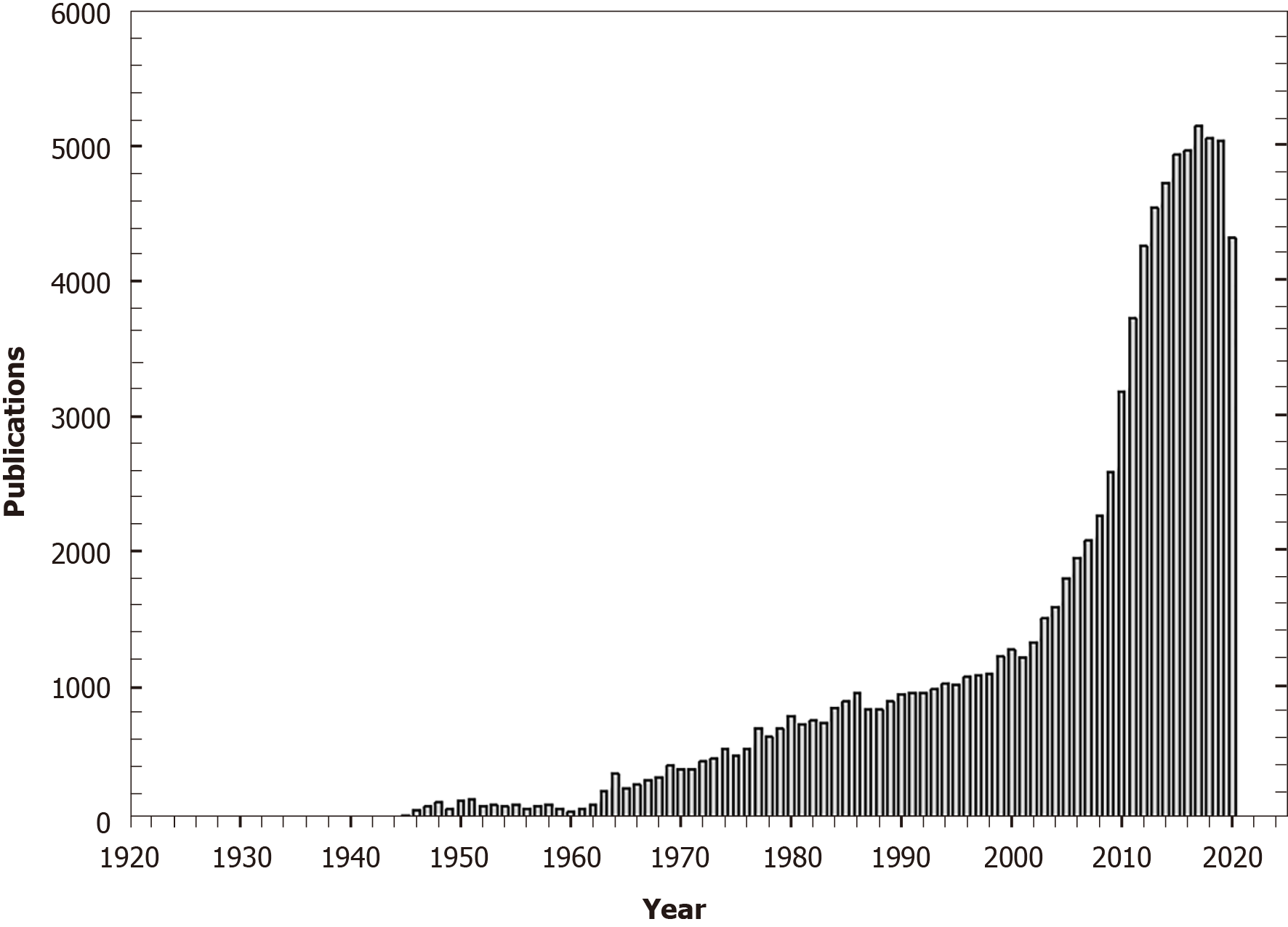
Beginning with its discovery in 1922, scientific publications focusing upon vitamin D numbered no more than some 10 per year but this increased to 35 per year by 1945 (Figure 1). As knowledge of the structure, molecular biology and function of vitamin D increased[5,6], there was a concurrent increase in vitamin D-specific publications. With the observations of the non-calcemic effects of vitamin D[7], vitamin D-focused publications peaked at 5152 in 2017. Recently, the role of vitamin D deficiency in relation to coronavirus disease 2019 (COVID-19) infection attracted attention[8].
The role of vitamin D on calcium and bone metabolism was well-summarized[9]. There is no doubt about the association between rickets and vitamin D deficiency and the reversal and prevention of rickets with vitamin D supplementation. However, controversy still surrounds the efficacy of vitamin D supplementation upon bone mineral density and fracture prevention. Multiple studies failed to demonstrate any benefit from vitamin D supplementation[10-12] and a systematic review and meta-analysis also failed to confirm any beneficial effect on bone density or fracture prevention from vitamin D supplement[13]. Nevertheless, placebo-control randomized clinical trials revealed a threshold effect of vitamin D[14,15] with no benefit observed on the subjects with baseline 25-hydroxyvitamin D level ≥ 75 nmol/L (30 ng/mL). Furthermore, possible detrimental effects on bone mineral density were observed in subjects who received a higher dose of vitamin D (250 μg or 10000 IU daily) with a mean 25-hydroxyvitamin D of 200 nmol/L or 80 ng/mL[12]. While not conclusive, these data suggest that the optimal effects of vitamin D are found at a 25-hydroxyvitamin D level of 75 nmol/L (30 ng/mL).
Vitamins are defined as micronutrients that cannot be self-synthesized and that necessary for the proper function of key enzymatic processes. Consequently, vitamins must be obtained through the diet. Vitamin D is synthesized from cholesterol to 7-dehydrocholesterol, also known as pro-vitamin D3, in the skin through the action of ultraviolet radiation[16]. In addition, the liver forms 25-hydroxyvitamin D3, also known as pre-vitamin D3. To become an active compound, further hydroxylation in the kidney is required to form 1,25-dihydroxyvitaomin D3, which is a biologically active vitamin D. Then, 1,25-dihydroxyvitamin D is released into circulation to exert its effects on the target cells and promote calcium and bone homeostasis. Thus, vitamin D is a hormone and, like the pituitary-thyroid axis, has a complex natural history in the body (Table 1).
| Pituitary-thyroid axis | Parathyroid-vitamin D axis | |
| Organ(s) | Thyroid glands | Skin/liver/kidney |
| Source compound | Iodine, tyrosine | Cholecalciferol (cholesterol), ergocalciferol |
| Prehormone | Levothyroxine, T1/2 = 6-7 d | 25-hydoxyvitamin D2/D3, T1/2 = 13-17 d |
| Active hormone | Triiodothyronine, T1/2 = 14-24 h | 1,25-dihydroxyvitamin D2/D3, T1/2 = 10-20 h |
| Transportation | Thyroxine binding globulin | Vitamin D binding protein |
| Receptor | Thyroid hormone receptor | Vitamin D receptor |
| Stimulating factor | Thyroid stimulating hormone | Parathyroid hormone |
| Effect | Energy homeostasis | Calcium homeostasis |
The half-life of thyroid hormone depends upon thyroid status[17]. The half-life for levothyroxine (T4) is 6-7 d in euthyroid subjects, 9-10 d in subjects with hypothy
In addition to the target organs, both the vitamin D receptor and 1alpha-hydroxylase (CYP27B1) are expressed in various other tissues[20], suggesting additional functions of vitamin D beyond bone metabolism and calcium homeostasis. Interestingly, the vitamin D receptor is expressed in the pancreatic islets[21], liver[22], muscle[23], and adipose tissue[24]. 1alpha-hydroxylase (CYP27B1) is expressed in pancreatic islets[25], liver[26], muscle[27], and adipose tissue[28]. Thus, it is possible that vitamin D could take part in glucose and fuel homeostasis.
In contrast to calcemic effects of vitamin D which is primary mediated by circulating 1,25-dihydroxyvitamin D produced in the kidney, the non-calcemic effects of vitamin D are mediated by circulating 25-hydroxyvitamin D through a paracrine or autocrine function[29]. Within the target cells or its vicinity, circulatory 25-hydroxyvitamin D enters cells and is converted to 1,25-dihydroxyvitamin D by the locally existing 1alpha-hydroxylase (CYP27B1). Hence, 25-hydroxyvitamin D is the key circulatory element for the non-calcemic effects of vitamin D whereas 1,25-dihydroxyvitamin D the promotes the calcemic effects.
A role for vitamin D in the pathogenesis of cancer was proposed in 1980[30] after it was observed that colon cancer rates were higher in the northern rather than the southern United States. The association of vitamin D deficiency with cancer, including breast[31], prostate[32], and colon cancer[33] was attributed to the ability of vitamin D to differentiation cells[34] and to suppress cell proliferative[35] along with other effects[36,37].
The risk of type 1 diabetes was reduced by vitamin D supplement in a birth-cohort study from Finland[38]. Furthermore, a polymorphism in the vitamin D receptor was associated with increased risk of type 1 diabetes[39]. Not unexpectedly, a role of vitamin D deficiency in the pathogenesis of type 1 diabetes was proposed[40]. In addition, the association of vitamin D deficiency with multiple sclerosis[41], systemic lupus erythematosus[42], and other autoimmune diseases[43] was attributed to the immunomodulatory and anti-inflammatory effects of vitamin D[44]. Furthermore, vitamin D plays an important role in the maintenance of B cell homeostasis[45], and vitamin D replacement may reduce B cell-mediated autoimmune disorders.
The role of vitamin D in the treatment of tuberculosis was appreciated with the observation that sun exposure altered the clinical presentation of tuberculosis[46]. Subsequently, vitamin D was administered as part of the treatment of tuberculosis[47]. Vitamin D deficiency was frequently observed in patient with untreated tuberculosis[48]. It is now known that Toll-like receptors up-regulate expression of the vitamin D receptor and the vitamin D-1-hydroxylase genes, leading to induction of the antimicrobial peptide cathelicidin and killing of intracellular Mycobacterium tuberculosis[49]. Thus, the role of vitamin D in fighting infection is established[50]. Further, vitamin D deficiency is associated with acute respiratory tract infection[51], bacterial vaginosis[52], pneumonia[53], foot infection in diabetics[54], chronic hepatitis C infection[55], and human immunodeficiency virus infection[56]. Recently, vitamin D deficiency was recognized as a risk factors for COVID-19 infection[57-61]. Thus, vitamin D could play a role in fighting infection.
An association between vitamin D receptor polymorphism and the severity of coronary artery disease was reported[62]. Deficiency was also noted to associate with an increased risk of myocardial infraction[63], hypertension[64], and stroke[65]. The mechanism proposed to account for these associations included activation of the renin-angiotensin system[66], coronary calcification[67], platelet activation and aggregation[68], increased proinflammatory cytokines[69], and vascular endothelial dysfunction[65].
In patients with vitamin D deficiency and diabetes, vitamin D supplementation improved beta cell function and glucose tolerance[70]. An association between vitamin D deficiency and glucose intolerance and beta cell dysfunction was observed in east London Asians[71]. Similarly, alternations in vitamin D metabolism in obese subjects manifesting as low 25-hydroxyvitaimin D is well-recognized[72]. This topic will be reviewed in this article.
Vitamin D deficiency was reported to be associated with depression[73], schizophrenia[74], autism[75], and Parkinson’s disease[76]. Various mechanisms have been reported to support a role of vitamin D in neuropsychiatric disorders. Vitamin D has a protective effect on dopaminergic neurons[77]. Vitamin D deficiency could result in altered synaptic plasticity through its effect on perineuronal nets leading to cognitive deficits[78]. Vitamin D deficiency alters brain protein expression in rats[79]. Furthermore, immunohistochemical study revealed the expression of vitamin D receptor and 1alpha-hydroxylase (CYP27B1) in various regions of human brain with the strong expression in the hypothalamus and in the large (presumably dopami
Vitamin D is available in two forms: ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Ergocalciferol comes from plants in the form of ergosterol (provitamin D2). Ergosterol is an important component of mushrooms. Through ultraviolet b (UVB) irradiation, which can occur within mushroom or artificially, it becomes ergocalciferol[81]. Cholecalciferol comes from animals and people through the biosynthesis of cholesterol to 7-dehydrocholesterol (Provitamin D3). Again, through UVB irradiation, this intermediate becomes cholecalciferol. Thus, dietary intake and sun exposure are the major determinants of serum 25-hydroxyvitamin D levels.
Sun, mainly UVB irradiation, plays an important role in biosynthesis of vitamin D. Since 7-dehydrocholesterol can be synthesized from cholesterol, theoretically vitamin D supplementation is not required once sun exposure is adequate. Skin color is a key determinant of vitamin D synthesis[82]. Vitamin D has been proposed to play a role in human evolution and migration away from equator by affecting skin color through the development of depigmented and tannable skin via genetic pathways under positive selection[1,83]. Sun exposure is highly effective in raising serum 25-hydroxyvitamin D concentration, while its effects diminish significantly on donning clothing and using sun screen[84]. In this regard, more body surface area exposure is more effective than longer exposure time[85]. However, the efficacy of sun exposure to increase serum 25-hydroxyvotamin D concentrations diminishes with the degree of skin tanning[86]. Thus, minimized sun exposure time for 5 min to 30 min (depending on time of day, season, latitude, and skin pigmentation) with maximize body surface exposure is recommended[9]. However, increased risk of sun-mediated skin cancer makes this approach to prevent vitamin D deficiency less optimum[87].
Vitamin D can be obtained through dietary intake. However, except for cod liver oil, vitamin D content in naturally occurring food is relatively low, even in mushrooms (Table 2). Although ergosterol is highly abundant in the membrane of mushrooms, mushroom are cultivated under shadow without UVB irradiation[81]. Thus, dietary intake of vitamin D is inadequate and vitamin D supplement is often needed to avoid deficiency.
| Food | Per serving | Percent DV | |
| IU | μg | ||
| Cod liver oil, 1 tablespoon | 1360 | 34.00 | 170 |
| Trout (rainbow), farmed, cooked, 3 ounces | 645 | 16.13 | 81 |
| Salmon (sockeye), cooked, 3 ounces | 570 | 14.25 | 71 |
| Mushrooms, white, raw, sliced, exposed to UV light, 1/2 cup | 366 | 9.15 | 46 |
| Milk, 2% milkfat, vitamin D fortified, 1 cup | 120 | 3.00 | 15 |
| Soy, almond, and oat milks, vitamin D fortified, various brands, 1 cup | 100-144 | 2.50-3.60 | 13-18 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 1 serving | 80 | 2.00 | 10 |
| Sardines (Atlantic), canned in oil, drained, 2 sardines | 46 | 1.15 | 6 |
| Egg, 1 large, scrambled (Vitamin D is in the yolk) | 44 | 1.10 | 6 |
| Liver, beef, braised, 3 ounces | 42 | 1.05 | 5 |
| Tuna fish (light), canned in water, drained, 3 ounces | 40 | 1.00 | 5 |
| Cheese, cheddar, 1 ounce | 12 | 0.30 | 2 |
| Mushrooms, portabella, raw, diced, ½ cup | 4 | 0.10 | 1 |
| Chicken breast, roasted, 3 ounces | 4 | 0.10 | 1 |
| Beef, ground, 90% lean, broiled, 3 ounces | 1.7 | 0.04 | 0 |
It is estimated that 65% of vitamin D is present as vitamin D while 35% is in the form of 25-hydroxyvitaomn D. As well, almost 75% of vitamin D is in adipose tissue, while 25-hydroxyvitamin D is distributed 20% in muscle, 30% in serum, 35% in fat, and 15% in other tissues[88]. The metabolism of vitamin D3 and vitamin D2 is summarized in Table 3. Vitamin D binding protein transports the various forms of vitamin D in circulation, including vitamin D, 25-hydroxyvtamin D, and 1,25-dihydroxyvitamin D[89]. Each vitamin D binding protein molecule has one binding site for vitamin D and/or its metabolites. The relative affinity of vitamin D binding protein to vitamin D3 is 1.14 times stronger than to vitamin D2[90]. 25-hydroxylase (CYP2R1) catalyzes 25-hydroxylation of vitamin D3 5 times more efficiently than vitamin D2[91]. Thus, after administration of a single oral dose of vitamin D3 and vitamin D2, a more sustainable and prolonged increase in serum 25-hydroxybitamin D3 concentration is observed compared to serum 25-hydroxybitamin D2 concentration[92]. 1alpha-hydroxylase (CYP27B1) coverts 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 2.4-time more efficiently than 25-hydroxyvitamin D2[93]. In receptor binding assays, 1,25-dihydroxyviramin D3 has 1.3 times more receptor affinity than 1,25-dihdroxyvitamin D3[94]. These data indicate that vitamin D3 is more biologically potent than vitamin D2.
| Ref. | Symbol | Name (chromosome location) | Function | D3/D2 |
| Haddad et al[90], 1993 | VBP | Vitamin D binding protein (4q12-q13) | Vitamin D transportation | 1.14 |
| Holmberg et al[91], 1986 | CYP2R1 | 25-hydroxylase (11p15.2) | Conversion of vitamin D to 25-hydroxy vitamin D | 5.0 |
| Zarei et al[93], 2016 | CYP27B1 | 1alpha-hydroxylase (12q13.1-q13.3) | Conversion of 25(OH)D to 1,25(OH)2D | 2.4 |
| Jones et al[94], 1980 | VDR | Vitamin D receptor (7q36) | Receptor for vitamin D | 1.3 |
Vitamin D2 and vitamin D3 were reported to have similar efficacy in raising serum 25-hydroxyviramin D concentration[95]. However, other studies demonstrated that vitamin D3 was more efficacious at raising serum 25(OH)D concentrations than vitamin D2[96-100]. This finding was confirmed by a meta-analysis of the randomized control trials[101]. Furthermore, 25-hydroxyvitamin D3 has a longer half-life compared to 25-hydroxyvitamin D2 (15.1 ± 3.1 d vs 13.9 ± 2.6 d, P = 0.001, mean ± STD)[18]. In comparison to oral vitamin D2, oral vitamin D3 achieves a higher serum concentration of 1,25-dihydroxyvitamin D[100,102] and a more effective suppression of serum parathyroid hormone concentration[97]. Physicians preferring use of vitamin D2 should be aware of its markedly lower potency and shorter duration of action when compared to vitamin D3. Thus, vitamin D3 is the preferred form of vitamin D for replacement therapy.
The primary function of vitamin D is to maintain calcium homeostasis. The minimal serum 25-hydroxyvitamin D concentration for health was defined based on the serum parathyroid hormone response to replacement therapy with ergocalciferol[103]. A serum 25-hydroxyvitamin D concentration of 50 nmol/L (20 ng/mL) was recommended since no further changes in serum parathyroid hormone levels were found in subjects with a serum 25-hydroxyvitamin D level of 50 nmol/L (≥ 20 ng/mL). In 2010, the United Sates Institute of Medicine adapted this value as a target for ensuring good bone health[104]. However, based on a larger observational study with 1569 subjects in France, serum parathyroid hormone concentration were noted to still decrease when the serum 25-hydroxyvitamin D rose to 78 mmol/L (31 ng/mL)[105]. Furthermore, a serum 25-hydroxyvitamin level of 75 nmol/L (30 ng/mL) is a recognized threshold for intestinal calcium absorption[106]. As shown in Table 4, many professional organizations and agencies have since adapted 75 nmol/L (30 ng/mL) as the minimal acceptable serum 25-hydroxyvitamin D concentration recognizing this may have beneficial effects beyond bone health, targeting beyond bone health while the Institute of Medicine define the minimal 25-hydroxyvitamin D concentration 50 nmol/L (20 ng/mL) on bone health with a public health interest.
| Organization | Daily intake | Goal | ||
| IU | μg | ng/mL | nmol/L | |
| Institute of Medicine | 600-800 | 15-20 | > 20 (20-50) | > 50 (50-125) |
| Agency of Healthcare Research and Quality, Department of Health and Human Services | > 1000 | > 25 | > 30 | > 75 |
| Office of Dietary Supplements, NIH | 600-800 | 15-20 | 20-50 | 50-125 |
| National Osteoporosis Foundation | 800-1000 | 20-25 | > 30 | > 75 |
| American Association of Clinical Endocrinologists | 1000-2000 | 25-50 | 30-60 | 75-150 |
| Endocrine Society | 1500-2000 | 37.5-50 | 30-100 | 75-250 |
The maximal allowed serum 25-hydroxyvitamin D concentration is defined by the appearance of adverse effects. Although the Institute of Medicine dose not define maximal serum 25-hydroxyvitamin D concentration[104], a warning against elevated serum 25-hydroxyvitamin D concentrations is stated. This warning is based upon the observed association of increasing mortality with serum 25-hydroxyvitamin D concentration > 125 nmol/L (50 ng/mL)[107] by limiting the maximal daily vitamin D allowance (Table 4). This notion was further supported by the finding of increased cardiovascular mortality with serum 25-hydroxyvitaminD > 125 nmol/L (50 ng/mL)[108]. In addition, a progressive decline in bone mineral density with serum 25-hydroxyvitamin D greater than 125 nmol/L (50 ng/mL) was observed in a United States population[109]. Conversely, bone mineral density improved after discontinuation of vitamin D supplementation in patients with a serum 25-hydroxyvitamin D concentration greater than 50 ng/mL[110]. Although vitamin D supplementation increased calcium absorption without a threshold effect[111], reanalysis of the data revealed a diminished response (per 1000 IU of vitamin D in Table 5) with increasing dose of vitamin D supplement suggesting a threshold effect of vitamin D on calcium absorption[112], something noted by others[106]. We reported lack of improvement in insulin sensitivity in individuals with a serum 25-hydroxyvitamin D concentration > 125 nmol/L (50 ng/mL)[113]. Although hypercalcemia from vitamin D intoxication occurs mainly when the serum 25-hydroxyvitamin D concentration is > 374 nmol/L (150 ng/mL)[114], serum 25-hydroxyvitamin D concentrations > 75 nmol/L (50 ng/mL) could be either harmful or lack beneficial effect.
| Daily vitamin D supplementation | Observed increase in calcium absorption | Estimated increase in calcium absorption per 1000 IU (25 μg) | |
| IU | μg | ||
| 800 | 20 | 3.90% | 4.88% |
| 2000 | 50 | 5.00% | 2.50% |
| 4000 | 100 | 6.70% | 1.68% |
The observation that a single oral dose of vitamin D3 2.5 mg (100000 IU) can maintain serum 25-hydroxyvitamin D above the target goal[115] provides a unique dosing strategy of vitamin D replacement therapy with greater adherence. It could even ensure 100% compliance if given by or under the direct supervision of a health care provider. Weekly[103], monthly[116], biyearly[117], and even yearly[118] schedules were reported in various trials leading to initiation of more convenient dosing schedule at less frequent intervals in clinical practice. To reduce the dosing frequency, a much higher dose of vitamin D is required which is predicted to cause a short-term spike (> 75 nmol/L or 50 ng/mL) in serum 25-hydroxyvitamin D concentration shortly after oral administration. In addition to the adverse effects as described in the above section 4.2, increased falls and fracture are observed with annual vitamin D replacement therapy. These mainly occur within the first 3 mo after oral administration of 12.5 mg vitamin D3[118]. Furthermore, the associations of high-dose vitamin D treatment with gastrointestinal complaints[119], increased bone turnover markers[120], hypercalcemia[121], hypercalciuria[122], and increased urinary magnesium loss[123] have been reported. Similar levels of serum 25-hydroxyvitamin D concentration were achieved at the end of a 56-d trial from daily (1500 IU/d), weekly (10500 IU/wk), and monthly (45000 IU/4 wk) replacement therapy. Excessive serum 25-hydroxyvitamin D concentration was not observed in those on the daily regimen but was observed in individuals on the weekly regimen and was still more common in those on monthly regimen[124]. Thus, high-dose vitamin D replacement therapy results in excessive serum 25-hydroxyvitamin D concentration.
A Lysine (K) amino acid polymorphism, in replacement of Threonine (T), at position 436 of vitamin D binding protein is associated with increased affinity of vitamin D and is associated a 416% elevation in serum 25-hydroxyvitamin D concentration if high-dose (4000 IU) vitamin D3 replacement therapy if given as opposed to low-dose (600 IU) vitamin D3 replacement therapy. Individuals carrying the TT SNP showed only a 136% increase in circulating vitamin[125]. Since the K allele is a minor allele and KK genotype accounts for less than few percent of population, the KK subjects may account for the excessive serum 25-hydroxyvitamin D-associated complications noted in certain studies. Given the above, daily vitamin D supplementation would seem to be most physiological and safest way to correct vitamin D deficiency and avoid the possible adverse effects associated with the excessive serum 25-hydroxyvitamin D concentration.
Various genetic loci are associated with serum 25-hydroxyvitamin D concentration[126] with 4 major loci identified (Table 6). These are all key proteins involved in the transportation and metabolism of vitamin D. Race and ethnicity were noted to have significant impact on serum 25-dihyrdroxyvitamin D concentration[127], again implicating a genetic influence[126] including skin color[128].
| Chromosome | SNP | Gene symbol | Protein | P value |
| 4p12 | rs2282679 | GC | Vitamin D binding protein | 1.9 × 10-109 |
| 11q12 | rs12785878 | DHCR7 | 7-dehydrocholsterol reductase | 2.1 × 10-27 |
| 11p15 | rs10741657 | CYP2R1 | 1-alpha-hydroxylase | 3.3 × 10-20 |
| 20q13 | rs6013897 | CYP24A1 | 1,25-dihydroxyvitamin D3 24-hydroxylase | 6.0 × 10-10 |
Seasonable variations in serum 25-hydroxyvitanim D concentrations related to sun exposure are well described[126]. Consistent with this, latitude has a significant impact on serum 25-dihydrocyvitamin D concentration[129]. Living closer to the equator and increasing sun exposure can improve vitamin D levels. However, the increased risk of skin cancer from sun exposure should be balanced employing maximum skin exposure area with decreased exposure time[85]. Dietary supplementation also corrects deficiency. Obesity is associated with a lower serum 25-hydroxyvitamin D concentration[72] while weight reduction with loss of adipose tissue is associated with improvement in serum 25-hydroxyvitamin D concentration[130]. These findings indicate that vitamin D status may be improved through modification of lifestyle.
As showed in Table 4, the recommended vitamin D supplement varies between organizations and agencies. The reasons for this relate to the purpose of vitamin D supplementation, visive calcemic vs non-calcemic effects. For calcemic effects, bone health is the goal of supplementation and is maximized through using a conservative daily vitamin D to achieve the minimal serum 25-hydroxyvitamin D concentration while avoiding possible adverse effects associated with overreplacement. A public health approach to this is displayed in Table 7. In contrast, a more personized approach is rationale when the target is to promote the non-calcemic effects of vitamin D.
| Vitamin D supplement | Vitamin D replacement therapy | |
| Target goal | Bone health | Beyond bone health |
| Target 25-hydroxyvitamin D level | > 20 ng/mL (50 nmol/L) | > 30 ng/mL (75 nmol/L) |
| Initial testing for 25-hydroxyvitamin D level | No | Yes |
| Concern of over-replacement | Yes | Yes |
| Follow-up testing for 25-hydroxyvitamin D level | No | Yes |
| Dose adjustment | No | Yes |
| Approach | Public health | Individualized |
We recommend using vitamin D3, instead of vitamin D2, for the rationale as discussed in the sections 3.2 and 3.3. We are in favor of daily replacement therapy and against intermittent mega dose replacement. This is supported by the recommendations of the Endocrine Society for indefinitely intermittent mega dose replacement[131]. It has been estimated that supplement with cholecalciferol 1000 IU (50 μg) daily will increase serum 25-hydroxyvitamin D concentration by 10 ng/mL[132]. Since vitamin D is a fat soluble, replacement therapy can be further enhanced by taking it with the largest meal of day[133]. We recommend vitamin D3 1000 IU daily for achievement of an initial serum 25-hydroxyvitamin D concentration between 51 nmol/L (21) ng/mL and 75 nmol/L (30 ng/mL); 2000 IU daily for between 26 nmol/L (11 ng/mL) and 50 nmol/L (20 ng/mL); and 5000 IU for equal or less than 25 nmol/L (10 ng/mL). Serum 25-hydroxyvitamin concentration should be measured within 3 mo for assessment and, if indicated, dose adjustment. We are targeting serum 25-hydroxyvitamin D concentration between 75 nmol/L (30 ng/mL) and 125 nmol/L (50 ng/mL).
To date, eight clinical trials employed vitamin D to reduce prediabetes progression to overt diabetes (Table 8). Only two studies[134,135] demonstrated positive results. Although these two studies had small sample size, they recruited true vitamin D deficient (25-hydroxyvitamin D < 50 nmol/L or 20 ng/mL) subjects and achieved final 25-hydroxyvitamin D concentration at 89-90 nmol/L, after intervention for 1 year and 6 mo, respectively. Of note, the study in India[134] was a randomized open label study demonstrating an odds ratio of 0.31 [95% confidence intervals (CI): 0.11-0.90]. The study in Iran was a randomized placebo control study[135] revealing an odds ratio of 0.06 (95%CI: 0.01-0.51). Because of relatively small sample sizes of both studies, the CI were very wide. Additional studies with similar initial and final 25-hydroxyvitamin D concentration (< 50 nmol/L and 90-100 nmol/L, respectively) and much larger sample sizes are required to confirm these data.
| Ref. | Country Race/ethnicity | Placebo control | Intervention | Dose | Frequency | Duration | Diabetes prevention | ||||
| n | 25(OH)D nmol/L | n | 25(OH)D nmol/L | ||||||||
| Initial | Final | Initial | Final | ||||||||
| Dutta et al[134], 20141 | IndiaAsian Indian | 49 | 45 | 44 | 55 | 43 | 89 | 1500 μg | Weekly X 8, monthly | 1 yr | Positive2 |
| Niroomand et al[135], 2019 | IranIranian | 83 | 32 | 40 | 83 | 31 | 90 | 1250 μg | Weekly for 3 mo, monthly | 6 mo | Positive3 |
| Wagner et al[136], 20164 | Sweden | 22 | 47 | 46 | 21 | 42 | 83 | 750 μg | weekly | 8 wk | Negative |
| Oosterwerff et al[137], 2014 | HollandNon-Western | 65 | 22 | 23 | 65 | 25 | 60 | 30 μg | daily | 16 wk | Negative |
| Barengolts et al[141], 20155 | United States African American | 86 | 35 | 50 | 87 | 37 | 120 | 1250 μg | weekly | 12 m | Negative |
| Davidson et al[139], 20136 | United States Latino and African American | 53 | 55 | 60 | 56 | 55 | 167 | 2222 μg | weekly | 12 mo | Negative |
| Jorde et al[140], 2016 | Norway | 255 | 61 | 64 | 256 | 60 | 110 | 500 μg | weekly | 5 yr | Negative |
| Pittas et al[138], 2019 | United States mixed | 1212 | 70 | 72 | 1211 | 69 | 136 | 100 μg | daily | 24 mo | Negative |
Two negative studies[136,137] were noted to have similar initial 25-hydroxyvitamin D concentrations (25-42 nmol/L). The negative results could be due to the relatively short interventions (8-16 wk) and small sample sizes. The study in Holland only achieved a final suboptimal 25-hydroxyvitamin D concentration of 60 nmol/L.
The other four studies[138-141] had a final 25-hydroxyvitamin D concentration > 100 nmol/L which might not be optimal for glucose metabolism. Among them, the study in African American[141] was the only study that recruited true vitamin D deficient subjects (initial 25-hydroxyvitamin D 37 nmol/L). Of note, ergocalciferol was used which could be less effective biologically as discussed above in 3.2 and 3.3. Enrollment of non-vitamin D deficient (25-hydroxyvitamin D < 50 nmol/L) subjects[138-140] could further reduce the chance of finding any effect. Furthermore, the study in Norway had a significant dropout rate in the interventional group with only 45% of participants completing the planned 5-year visit. The largest intervention trial[138] included more than 1000 subjects in each group. To be able to apply to the general population in the United States, this study did not target vitamin D deficient subjects and allowed the participants to take additional vitamin D up to 25 μg daily. Therefore, it had the highest initial 25-hydroxyvitamin D among these studies, 70 nmol/L in the control group and 69 nmol/L in the interventional group, which might diminish the power of this study to detect the beneficial effect of vitamin D. Regardless of the negative results in most studies, the beneficial effect of vitamin D supplementation cannot be completely excluded, especially in subjects with vitamin D deficiency (25-hydroxyvitamin D < 50 nmol/L).
Various parameters of glucose metabolism were reported in most of above-mentioned studies, except one[138]. After vitamin D intervention for 1 year, the study from India[134] observed improvement in fasting and 2-hr post-challenge glucose concentrations, insulin sensitivity by Homeostasis Model (HOMA) insulin resistance index, QUICKI, and 1/fasting insulin concentration while no impact on HbA1c and beta cell function by HOMA. Following vitamin D supplement for 6 mo, the study from Iran[135] reported the improvement in the HOMA insulin resistance index and marginal improvement in fasting insulin concentration (P = 0.05) and 2-hour post-challenge blood glucose concentration (P = 0.07) with no impact on fasting blood glucose concentration.
After an 8-wk intervention, the study from Sweden[136] assessed insulin sensitivity and beta cell function using the hyperglycemic clamp. They observed a significant improvement in deposition index based on the first phase insulin response (P = 0.005) and marginal improvement in first phase insulin response (P = 0.06), insulin sensitive index (P = 0.09), deposition index based on the second phase insulin response (P = 0.06), and A1c (P = 0.06) but no impact on the second phase insulin response and fasting and 2-hr post-challenge blood glucose concentration.
In contrast, the study from Holland[137] evaluated glucose metabolism parameters based on the 75-g glucose tolerance test following intervention for 16 wk. They reported negative results, finding no effects upon insulin area under curve, glucose area under curve, insulin sensitivity by composite insulin sensitivity index, Stumvoll index, insulin resistance index by HOMA, and beta cell function by insulinogenic index. Of note, the final 25-hydroxyvitamin D concentration was only 60 nmol/L which could be suboptimal for glucose metabolism. Similarly, after the vitamin D supplementation for 5 years, the study from Norway[140] observed no impact on fasting and 2-hr post-challenge serum glucose concentration, fasting and post challenge serum insulin concentration, fasting serum C-peptide concentration, HbA1c, and insulin sensitivity by HOMA insulin resistance index and QUICKI.
Following a 12-mo intervention, the study involving Latino and African Americans[139] observed a significant improvement in HbA1c but no effects on fasting and 2-hr post-challenge blood glucose concentration, beta cell function by the ratio of insulin and glucose area under curve, Stumvoll first and second insulin response, insulinogenic index, insulin sensitivity index by HOMA insulin resistance index and composite insulin sensitivity index, and oral disposition index. However, a significant improvement in composite insulin sensitivity index but not Matsuda index, insulinogenic index, C-peptidogenic index, and HbA1c was noted.
Excepting two studies[137,140] with negative results, favorable outcomes on parameters of glucose metabolism were reported in five studies[134-136,139,141] suggesting some benefits to supplementation under these conditions.
In vitamin D deficient (25-hydroxyvitamin D < 50 nmol/L) prediabetic subjects, vitamin D supplement appears to be effective in reduction of the development of overt diabetes. However, there appears to be no benefit in vitamin D sufficient subjects, which was noted in a study from Norway[142]. Based on pooled data from four intervention trials, in subjects without vitamin D deficiency there is no improvement in glucose metabolism with high dose vitamin D supplementation and if anything, the effect is negative[143]. This notion is consistent with the observed threshold effect of vitamin D on bone health and lack of benefit in subjects with baseline 25-hydroxyvitamin D level ≥ 75 nmol/L (30 ng/mL)[14,15].
Functional beta cell studies: The important role of vitamin D on insulin secretion has been noted in laboratory animals since 1980. Insulin secretion was reduced by about 50% in isolated perfused islets from vitamin D-deficient rats compared to controls[144]. Interestingly, 1,25-dihydroxyvitamin D3 was noted in cell nuclei in the islets of langerhans[145]. Furthermore, administration of 1,25-dihydroxyvitamin D3 to vitamin D-deficient rats improved insulin secretion significantly when compared to controls[146]. Vitamin D deficiency impaired both phases of insulin release in rats while correction of hypocalcemia failed to reverse the defect in insulin release[147]. Vitamin D, but not calcium, was essential for normal insulin secretion from the perfused rat pancreas[148]. The positive effect of single dose of 1,25-dihydroxyvitamin D3 on insulin secretion was apparent at 8 h in perfused rat pancreata, peaked at 14 h, and then decreased to pretreatment baseline values by 36 h[149]. Dietary vitamin D3 supplementation improved impaired glucose tolerance and insulin secretion in the vitamin D-deficient rats[150]. A dose-dependent effect from parenteral 1,25-dihydroxyvitamin D on insulin secretion and glucose metabolism was observed within 3 h and remained effective up to 20 h in the vitamin D-deficient rats[151]. The role of vitamin D on insulin synthesis and secretion was supported by studies in vitamin D receptor knockout mice. Insulin secretory capacity was reduced by 60% in vitamin D receptor knockout mice[152] with increased post-challenged blood glucose but normal fasting blood glucose concentration and reduced insulin mRNA levels in pancreatic islets but normal pancreatic beta cell mass, islet architecture, and islet neogenesis when compared to wild type mice. Thus, vitamin D plays an important role in pancreatic insulin synthesis and secretion in vivo.
Mechanistic studies of beta cell function: Although the essential role of vitamin D on insulin secretion has been established in vitamin D depleted laboratory animal, details of the underlying molecular mechanism remain to be defined. Employing a proteomic approach, treatment with 1,25-dihydroxyvitamin D3 resulted in 31 differentially expressed proteins in INS-1 beta-like cells[153] with 29 upregulated, some of which were implicated in insulin granule motility and insulin exocytosis as well as regulation of ions. Pretreatment of INS1E cells with 1,25-dihydroxyvitamin D or 25-hydroxyvitamin D and glucose resulted in 526 and 181 differentially expressed genes, respectively[154].
Several molecular mechanisms were proposed to account for the effects of vitamin D on beta cells, including changes in the local pancreatic islet renin-angiotensin system[155], restoration of GLUT2 expression[156], enhancement of IP3 and AMPA receptor expression[157], vitamin D-binding protein-induced beta cell dedifferentiation[158], reduction of oxidative damage[159], reduced cholinergic pancreatic effects[160], enhanced transcriptional regulation of voltage-gated calcium channels[161], and elevation of PPAR-γ expression[162]. However, further studies are required to confirm the proposed mechanisms.
Functional studies of insulin sensitivity: In contrast to beta cell function, there are fewer studies of insulin sensitivity. Dietary supplementation of vitamin D improved insulin sensitivity, hepatic steatosis, and myocardial fibrosis in Western diet fed rats[163]. In dietary-induced obese mice, vitamin D receptor activation in liver macrophages improved insulin sensitivity with reduction of hepatic inflammation and steatosis[164]. Vitamin D treatment improved insulin resistance index in a nongenetic model of type 2 diabetes[165]. However, vitamin D status were not reported in these studies.
Mechanistic studies of insulin sensitivity: Chronic central administration of 1,25-dihydroxyvitamin D3 dramatically reduced body weight, putatively by lowering food intake, in obese rodents[166]. Treatment with vitamin D increased mitochondrial function and insulin sensitivity, in part, through upregulation of perilipin 2, a perilipin protein upregulated with 1,25-dihydroxyvitamin D treatment[167]. In skeletal myocytes, vitamin D reduced insulin resistance by altering lipid partitioning and lipid droplet packaging in favor of lipid turnover[168]. FGF-23 knockout mice are hypoglycemic with profoundly increased peripheral insulin sensitivity and improved subcutaneous glucose tolerance. Ablation of vitamin D signaling in these mice normalized subcutaneous glucose tolerance tests and insulin sensitivity[169]. Caveolin-1 protein, which is necessary for vitamin D signaling, could play a role in vitamin D-induced insulin sensitivity in skeletal muscle[170]. In cultured rat osteoblasts, 1,25-dihydroxyvitamin D3 treatment increased expression of the insulin and vitamin D receptors, and elevated osteocalcin levels under high glucose exposure[171], which may in turn improve insulin sensitivity.
However, the results of vitamin D receptor knockout mice were less uniform. Skeletal muscle-specific vitamin D receptor knockout mice developed insulin resistance and glucose intolerance accompanied by increased expression and activity of FOXO1[172]. Deletion of macrophage vitamin D receptor promoted insulin resistance and monocyte cholesterol transport and accelerated atherosclerosis[173]. In contrast, deletion of the vitamin D receptor gene in endothelial cells improved glucose tolerance and insulin sensitivity in skeletal muscle and reduced expression and secretion of insulin in pancreatic islets[174]. Together these data indicate that vitamin D has positive and negative effects on insulin sensitivity that are cell and organ specific.
Due to publicity and potential non-calcemic benefits of vitamin D supplementation, the sale of vitamin D supplements increased significantly and taking vitamin D supplements is common. Thus, there are less true vitamin D deficient subjects available for inclusion in clinical trials. As well, a general lack of funding support for large trials impedes addressing the ability of researchers to address the gaps in knowledge surrounding vitamin D and its beneficial effects.
To obtain the maximal effect of vitamin D, serum 25-hydroxyvitamin D concentration should be maintained in an optimal range, namely between 75 nmol/L (30 ng/mL) and 125 nmol/L (50 ng/mL). Inadequate vitamin D replacement therapy will reduce the chance to observe the expected beneficial effect of vitamin D while adverse effects associated with excessive serum 25-hydroxyvtamin D concentration will also cloud data interpretation. Although mega doses of vitamin D given intermittently could improve compliance in a study protocol, the predicted wide swings in serum 25-hydroxyvitamin D concentrations will confound outcomes. It is important in clinical studies to use a proper daily dose to avoid these pitfalls.
The Diabetes Prevention Program demonstrated a 58% (95%CI: 48%-66%) reduction in the incidence of diabetes in the lifestyle intervention group (cumulative incidence of diabetes 14.4% in 1079 participants) and a 31% reduction in diabetes (95%CI: 17%-43%) in the metformin treated group (cumulative incidence of diabetes 21.7% in 1073 participants) when compared to the placebo (cumulative incidence of diabetes 28.9% in 1082 participants)[175]. Insulin sensitivity improved by 61.8% in the lifestyle intervention group and 28.3% in the metformin group[176]. This study can be employed to calculate a sample size sufficient for assessing the effects of vitamin D intervention.
Based on the non-linear relationship of serum 25-hydroxyvotamin D concentration and insulin sensitivity index as we reported[113], we constructed Table 9. Assuming a linear relationship between improvement in insulin sensitivity and reduction of diabetes from the Diabetes Prevention Program[175,176], we calculated the required sample size to detect the reduction of diabetes incidence after vitamin D replacement therapy in a population similar to that of the Diabetes Prevention Program[175] with a power of 0.80 to detect the proposed difference and a type I error rate, alpha, of 0.05 in a clinical trial of 3 years. Starting with a baseline serum 25-hydroxyviyamin D of 25 ng/mL (10 ng/mL), 170 subjects would be needed. Such a study cohort size is not excessive. However, if the baseline serum 25-hydroxyvitamin D is equal or greater than 50 nmol/L (20 ng/mL) the cohort size needed increases markedly. These calculations suggest that all studies to date are flawed secondary to inadequate sample size.
| Initial serum 25-hydroxy-vitamin D concentration | Estimated insulin sensitivity index(μM/min/m2/pM) | Improvement in insulin sensitivity index with postintervention Serum 25-hydroxyvitamin D concentration 40 ng/mL (100 nmol/L) | Diabetes reduction based on the Diabetes Prevention Program | Sample size | |
| ng/mL | nmol/L | ||||
| 10 | 25 | 4.1326 | 0.8664 | 0.4361 | 340 |
| 15 | 37 | 5.4144 | 0.4246 | 0.2118 | 1602 |
| 20 | 50 | 6.2812 | 0.2280 | 0.1121 | 5934 |
| 25 | 62 | 6.8674 | 0.1232 | 0.0589 | 21878 |
| 30 | 75 | 7.2638 | 0.0619 | 0.0278 | 99260 |
| 35 | 87 | 7.5319 | 0.0241 | 0.0086 | 1041162 |
It has been frustrating to confound the published negative reports while ample evidence supports the benefit of vitamin D. Accordingly, we propose these guidelines[177]. Future studies into the effects of vitamin D supplementation need to ensure the proper selection of study subjects, adequate vitamin D replacement to achieve an optimal serum 25-hydroxyvitamin D concentrations, avoidance over-placement to eliminate detrimental effects, and adequate sample size to detect the proposed effects.
Table 4 summarizes the recommended serum vitamin D concentrations from several institutions and agencies. As appreciated, studies on bone health[14,15] showed no additional benefit in the subjects with serum 25-hydroxyvitmanin D > 75 nmol/L (30 ng/mL) and this agrees with the effects upon diabetes prevention. However, increased all-cause mortality[107] and cardiovascular mortality[108] occurred prior to the 125 nmol/L (50 ng/mL) threshold, implying a much lower maximum dose for optimal serum 25-hydroxyvitamin D concentration. The question remains whether the same relationship applies to glucose homeostasis.
The detrimental effects noted in individuals with serum 25-hydroxyvitamin D concentration above a maximum threshold was observed in a cross-sectional study[109]. Further, improvement in bone density after discontinuation of vitamin D supplementation in osteoporotic patients with elevated serum 25-hydroxyvitamin D concentration was reported[110]. Elevated serum 25-hydroxyvitamin D concentrations were also associated with increased falls and fracture[118]. These reports suggest that assessment of negative effects from elevated serum 25-hydroxyvitamin D concentration may be uncovered with additional study.
Although various evidence suggests the benefit of vitamin D on glucose metabolism, published diabetes prevention trails are not convincing and suffer from improper designed and execution. To address this issue, a well-designed and well-conducted randomized, placebo-control trial to test the effects of vitamin D to limit development of diabetes is warranted, by selecting true vitamin D deficient subjects, achieving optimal but not excessive serum 25-hydroxyvitamin concentration, and enrolling adequate number of subjects. Properly monitoring serum 25-hydroxyvitamin D concentrations is required during the study.
The role of vitamin D in glucose metabolism and fuel homeostasis is supported by a number of observational studies. We reported that serum 25-hydroxyviatmin D concentration accounted for 21.2% of the variation in insulin sensitivity index in univariate analysis and 6.1% by itself among 42% with other covariates in multivariate analysis[178]. We also reported that serum 25-hydroxyviatmin D concentration accounted for 8.2% of the variation in beta cell function in univariate analysis and 4.5% by itself among 25.5% with other covariates in multivariate analysis[179]. Although the intervention studies have failed to provide concordant data for multiple reasons, laboratory studies revealed a number of molecular mechanisms that underlie the effect of vitamin D supporting the important role of the vitamin in glucose metabolism and fuel homeostasis. Since the independent contributions of vitamin D to insulin sensitivity[178] and beta cell function[179] are relatively small, vitamin D deficiency could be the last straw that breaks camel’s back in polygenetic and multifactorial diseases, such as diabetes, obesity, and hyperlipidemia.
A special acknowledgement is due to Chiu-Tien Chiu, MD, PhD for his unconditional support to KCC. We are in debt to Jeffrey Isenberg MD, MPH for critical reading and editing of the manuscript.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Endocrine Society; American Diabetes Association; American College of Endocrinology; American Association of Clinical Endocrinology; American College of Physicians
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang L S-Editor: Liu M L-Editor: A P-Editor: Guo X
| 1. | Diamond J. Evolutionary biology: geography and skin colour. Nature. 2005;435:283-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Platt BS. Sir Edward Mellanby, G.B.E., K.C.B., F.R.S. Nature. 1955;175:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | McCollum EV, Pitz W, Simmonds N, Becker JE, Shipley PG, Bunting RW. The effect of additions of fluorine to the diet of the rat on the quality of the teeth. 1925. Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. 1922. The effect of additions of fluorine to the diet of the rat on the quality of the teeth. 1925. J Biol Chem. 2002;277:E8. [PubMed] |
| 4. | Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3:479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Askew FA, Bourdillon RB, Webster TA. The production of vitamin D in a glow discharge. Biochem J. 1932;26:814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | DeLuca HF. Current concepts. Vitamin D. N Engl J Med. 1969;281:1103-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Holick MF. Noncalcemic actions of 1,25-dihydroxyvitamin D3 and clinical applications. Bone. 1995;17:107S-111S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 9. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9399] [Cited by in RCA: 9428] [Article Influence: 523.8] [Reference Citation Analysis (1)] |
| 10. | Aloia J, Fazzari M, Islam S, Mikhail M, Shieh A, Katumuluwa S, Dhaliwal R, Stolberg A, Usera G, Ragolia L. Vitamin D Supplementation in Elderly Black Women Does Not Prevent Bone Loss: A Randomized Controlled Trial. J Bone Miner Res. 2018;33:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Aspray TJ, Chadwick T, Francis RM, McColl E, Stamp E, Prentice A, von Wilamowitz-Moellendorff A, Schoenmakers I. Randomized controlled trial of vitamin D supplementation in older people to optimize bone health. Am J Clin Nutr. 2019;109:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA. 2019;322:736-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 13. | Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 433] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 14. | Reid IR, Horne AM, Mihov B, Gamble GD, Al-Abuwsi F, Singh M, Taylor L, Fenwick S, Camargo CA, Stewart AW, Scragg R. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J Intern Med. 2017;282:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25-Hydroxyvitamin D Threshold for the Effects of Vitamin D Supplements on Bone Density: Secondary Analysis of a Randomized Controlled Trial. J Bone Miner Res. 2018;33:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Glossmann HH. Origin of 7-dehydrocholesterol (provitamin D) in the skin. J Invest Dermatol. 2010;130:2139-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Nicoloff JT, Low JC, Dussault JH, Fisher DA. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest. 1972;51:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373-3381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Berry D, Hyppönen E. Determinants of vitamin D status: focus on genetic variations. Curr Opin Nephrol Hypertens. 2011;20:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1959] [Cited by in RCA: 2755] [Article Influence: 229.6] [Reference Citation Analysis (0)] |
| 21. | Lee S, Clark SA, Gill RK, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Zhang H, Shen Z, Lin Y, Zhang J, Zhang Y, Liu P, Zeng H, Yu M, Chen X, Ning L, Mao X, Cen L, Yu C, Xu C. Vitamin D receptor targets hepatocyte nuclear factor 4α and mediates protective effects of vitamin D in nonalcoholic fatty liver disease. J Biol Chem. 2020;295:3891-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 370] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Yuzbashian E, Asghari G, Hedayati M, Zarkesh M, Mirmiran P, Khalaj A. Determinants of vitamin D receptor gene expression in visceral and subcutaneous adipose tissue in non-obese, obese, and morbidly obese subjects. J Steroid Biochem Mol Biol. 2019;187:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Vuica A, Ferhatović Hamzić L, Vukojević K, Jerić M, Puljak L, Grković I, Filipović N. Aging and a long-term diabetes mellitus increase expression of 1 α-hydroxylase and vitamin D receptors in the rat liver. Exp Gerontol. 2015;72:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol. 2012;303:C396-C405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Wamberg L, Christiansen T, Paulsen SK, Fisker S, Rask P, Rejnmark L, Richelsen B, Pedersen SB. Expression of vitamin D-metabolizing enzymes in human adipose tissue -- the effect of obesity and diet-induced weight loss. Int J Obes (Lond). 2013;37:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 29. | Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 541] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 344] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 33. | Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 421] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 34. | Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990-4994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 674] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805-810. [PubMed] |
| 36. | Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutr Rev. 2008;66:S116-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1156] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 39. | Chang TJ, Lei HH, Yeh JI, Chiu KC, Lee KC, Chen MC, Tai TY, Chuang LM. Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clin Endocrinol (Oxf). 2000;52:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Zella JB, DeLuca HF. Vitamin D and autoimmune diabetes. J Cell Biochem. 2003;88:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Munger KL, Zhang SM, O'Reilly E, Hernán MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 677] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 42. | Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 43. | Maruotti N, Cantatore FP. Vitamin D and the immune system. J Rheumatol. 2010;37:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, Gangemi S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev. 2019;18:102350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 45. | Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 785] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 47. | Klip W. The tuberculostatic action of vitamin D2. Antonie Van Leeuwenhoek. 1952;18:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985;40:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2664] [Cited by in RCA: 2708] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 50. | Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 52. | Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 53. | Inamo Y, Hasegawa M, Saito K, Hayashi R, Ishikawa T, Yoshino Y, Hashimoto K, Fuchigami T. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatr Int. 2011;53:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Tiwari S, Pratyush DD, Gupta B, Dwivedi A, Chaudhary S, Rayicherla RK, Gupta SK, Singh SK. Prevalence and severity of vitamin D deficiency in patients with diabetic foot infection. Br J Nutr. 2013;109:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Terrier B, Jehan F, Munteanu M, Geri G, Saadoun D, Sène D, Poynard T, Souberbielle JC, Cacoub P. Low 25-hydroxyvitamin D serum levels correlate with the presence of extra-hepatic manifestations in chronic hepatitis C virus infection. Rheumatology (Oxford). 2012;51:2083-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Pinzone MR, Di Rosa M, Malaguarnera M, Madeddu G, Focà E, Ceccarelli G, d'Ettorre G, Vullo V, Fisichella R, Cacopardo B, Nunnari G. Vitamin D deficiency in HIV infection: an underestimated and undertreated epidemic. Eur Rev Med Pharmacol Sci. 2013;17:1218-1232. [PubMed] |
| 57. | Abrishami A, Dalili N, Mohammadi Torbati P, Asgari R, Arab-Ahmadi M, Behnam B, Sanei-Taheri M. Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: a retrospective study. Eur J Nutr. 2021;60:2249-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 58. | Panagiotou G, Tee SA, Ihsan Y, Athar W, Marchitelli G, Kelly D, Boot CS, Stock N, Macfarlane J, Martineau AR, Burns G, Quinton R. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol (Oxf). 2020;93:508-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 59. | Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, Frenkel-Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693-3702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 60. | Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, Palumbo A, Di Gioia G, Valerio VN, Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021;44:765-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 61. | Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3:e2019722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 62. | Van Schooten FJ, Hirvonen A, Maas LM, De Mol BA, Kleinjans JC, Bell DA, Durrer JD. Putative susceptibility markers of coronary artery disease: association between VDR genotype, smoking, and aromatic DNA adduct levels in human right atrial tissue. FASEB J. 1998;12:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 811] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 64. | Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 65. | Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, März W. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 66. | Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125-E132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 67. | Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 68. | Sultan M, Twito O, Tohami T, Ramati E, Neumark E, Rashid G. Vitamin D diminishes the high platelet aggregation of type 2 diabetes mellitus patients. Platelets. 2019;30:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 69. | Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 666] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 70. | Kumar S, Davies M, Zakaria Y, Mawer EB, Gordon C, Olukoga AO, Boulton AJ. Improvement in glucose tolerance and beta-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Postgrad Med J. 1994;70:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 236] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 415] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 73. | Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 74. | Zhu JL, Luo WW, Cheng X, Li Y, Zhang QZ, Peng WX. Vitamin D deficiency and Schizophrenia in Adults: A Systematic Review and Meta-analysis of Observational Studies. Psychiatry Res. 2020;288:112959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Wang T, Shan L, Du L, Feng J, Xu Z, Staal WG, Jia F. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2016;25:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 76. | Lv Z, Qi H, Wang L, Fan X, Han F, Wang H, Bi S. Vitamin D status and Parkinson's disease: a systematic review and meta-analysis. Neurol Sci. 2014;35:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Mayne PE, Burne THJ. Vitamin D in Synaptic Plasticity, Cognitive Function, and Neuropsychiatric Illness. Trends Neurosci. 2019;42:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 79. | Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, Boucraut J, Mackay-Sim A, Μgrath J, Féron F. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 80. | Eyles DW, Smith S, Kinobe R, Hewison M, Μgrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1067] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 81. | Phillips KM, Ruggio DM, Horst RL, Minor B, Simon RR, Feeney MJ, Byrdwell WC, Haytowitz DB. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J Agric Food Chem. 2011;59:7841-7853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 82. | Libon F, Cavalier E, Nikkels AF. Skin color is relevant to vitamin D synthesis. Dermatology. 2013;227:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107 Suppl 2:8962-8968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 84. | Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes. 2002;9:87-98. |
| 85. | Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87:4952-4956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Rockell JE, Skeaff CM, Williams SM, Green TJ. Association between quantitative measures of skin color and plasma 25-hydroxyvitamin D. Osteoporos Int. 2008;19:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Epstein JH. Photocarcinogenesis, skin cancer, and aging. J Am Acad Dermatol. 1983;9:487-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 130] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Heaney RP, Horst RL, Cullen DM, Armas LA. Vitamin D3 distribution and status in the body. J Am Coll Nutr. 2009;28:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 89. | Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D Binding Protein: A Historic Overview. Front Endocrinol (Lausanne). 2019;10:910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 90. | Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 91. | Holmberg I, Berlin T, Ewerth S, Björkhem I. 25-Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scand J Clin Lab Invest. 1986;46:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387-5391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 718] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 93. | Zarei A, Hulley PA, Sabokbar A, Javaid MK, Morovat A. 25-Hydroxy- and 1α,25-Dihydroxycholecalciferol Have Greater Potencies than 25-Hydroxy- and 1α,25-Dihydroxyergocalciferol in Modulating Cultured Human and Mouse Osteoblast Activities. PLoS One. 2016;11:e0165462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Jones G, Byrnes B, Palma F, Segev D, Mazur Y. Displacement potency of vitamin D2 analogs in competitive protein-binding assays for 25-hydroxyvitamin D3, 24,25-dihydroxyvitamin D3, and 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1980;50:773-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 500] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 96. | Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 412] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 97. | Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, Carnevale V, Scillitani A, Minisola S. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 98. | Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447-E452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 99. | Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA, Vasikaran SD, Taranto M, Musk AA, Fraser WD. Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone. 2009;45:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 100. | Shieh A, Chun RF, Ma C, Witzel S, Meyer B, Rafison B, Swinkels L, Huijs T, Pepkowitz S, Holmquist B, Hewison M, Adams JS. Effects of High-Dose Vitamin D2 Versus D3 on Total and Free 25-Hydroxyvitamin D and Markers of Calcium Balance. J Clin Endocrinol Metab. 2016;101:3070-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 101. | Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 102. | Cipriani C, Romagnoli E, Pepe J, Russo S, Carlucci L, Piemonte S, Nieddu L, McMahon DJ, Singh R, Minisola S. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98:2709-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 744] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 104. | Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC): National Academies Press (US), 2011. |
| 105. | Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 927] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 106. | Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80:1706S-1709S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 107. | Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 932] [Cited by in RCA: 864] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 108. | Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, Halkjær J, Lind B, Heegaard AM, Schwarz P. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J Clin Endocrinol Metab. 2015;100:2339-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 109. | Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1637] [Cited by in RCA: 1594] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 110. | Adams JS, Lee G. Gains in bone mineral density with resolution of vitamin D intoxication. Ann Intern Med. 1997;127:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Aloia JF, Dhaliwal R, Shieh A, Mikhail M, Fazzari M, Ragolia L, Abrams SA. Vitamin D supplementation increases calcium absorption without a threshold effect. Am J Clin Nutr. 2014;99:624-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 112. | Huang J, Ou HY, Karnchanasorn R, Chiu KC. Clinical implication of vitamin D threshold. Am J Clin Nutr. 2014;100:295-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 113. | Ou HY, Karnchanasorn R, Lee LZ, Chiu KC. Interaction of BMI with vitamin D and insulin sensitivity. Eur J Clin Invest. 2011;41:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Heaney RP. Nutrition and chronic disease. Mayo Clin Proc. 2006;81:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 115. | Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 116. | Ghazi AA, Hosseinpanah F, M Ardakani E, Ghazi S, Hedayati M, Azizi F. Effects of different doses of oral cholecalciferol on serum 25(OH)D, PTH, calcium and bone markers during fall and winter in schoolchildren. Eur J Clin Nutr. 2010;64:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Carnes J, Quinn S, Nelson M, Jones G, Winzenberg T. Intermittent high-dose vitamin D corrects vitamin D deficiency in adolescents: a pilot study. Eur J Clin Nutr. 2012;66:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 900] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 119. | Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 120. | Rossini M, Gatti D, Viapiana O, Fracassi E, Idolazzi L, Zanoni S, Adami S. Short-term effects on bone turnover markers of a single high dose of oral vitamin D₃. J Clin Endocrinol Metab. 2012;97:E622-E626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 121. | von Restorff C, Bischoff-Ferrari HA, Theiler R. High-dose oral vitamin D3 supplementation in rheumatology patients with severe vitamin D3 deficiency. Bone. 2009;45:747-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Tellioglu A, Basaran S, Guzel R, Seydaoglu G. Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly. Maturitas. 2012;72:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 123. | Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 124. | Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430-3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 125. | Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 126. | Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1202] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 127. | Hsu S, Hoofnagle AN, Gupta DK, Gutierrez OM, Peralta CA, Shea S, Allen NB, Burke G, Michos ED, Ix JH, Siscovick D, Psaty BM, Watson KE, Kestenbaum B, de Boer IH, Robinson-Cohen C. Race, Ancestry, and Vitamin D Metabolism: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2020;105:e4337-e4350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 128. | Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 723] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 129. | Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1091] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 130. | Gangloff A, Bergeron J, Lemieux I, Després JP. Changes in circulating vitamin D levels with loss of adipose tissue. Curr Opin Clin Nutr Metab Care. 2016;19:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6974] [Cited by in RCA: 6845] [Article Influence: 488.9] [Reference Citation Analysis (0)] |
| 132. | Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 917] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 133. | Mulligan GB, Licata A. Taking vitamin D with the largest meal improves absorption and results in higher serum levels of 25-hydroxyvitamin D. J Bone Miner Res. 2010;25:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 134. | Dutta D, Mondal SA, Choudhuri S, Maisnam I, Hasanoor Reza AH, Bhattacharya B, Chowdhury S, Mukhopadhyay S. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103:e18-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 135. | Niroomand M, Fotouhi A, Irannejad N, Hosseinpanah F. Does high-dose vitamin D supplementation impact insulin resistance and risk of development of diabetes in patients with pre-diabetes? Diabetes Res Clin Pract. 2019;148:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 136. | Wagner H, Alvarsson M, Mannheimer B, Degerblad M, Östenson CG. No Effect of High-Dose Vitamin D Treatment on β-Cell Function, Insulin Sensitivity, or Glucose Homeostasis in Subjects With Abnormal Glucose Tolerance: A Randomized Clinical Trial. Diabetes Care. 2016;39:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 137. | Oosterwerff MM, Eekhoff EM, Van Schoor NM, Boeke AJ, Nanayakkara P, Meijnen R, Knol DL, Kramer MH, Lips P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: a randomized placebo-controlled trial. Am J Clin Nutr. 2014;100:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 138. | Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O'Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M; D2d Research Group. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med. 2019;381:520-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 427] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 139. | Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 140. | Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. J Clin Endocrinol Metab. 2016;101:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 141. | Barengolts E, Manickam B, Eisenberg Y, Akbar A, Kukreja S, Ciubotaru I. Effect of high-dose vitamin D repletion on glycemic control in african-american males with prediabetes and hypovitaminosis d. Endocr Pract. 2015;21:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 142. | Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 143. | Jorde R, Strand Hutchinson M, Kjærgaard M, Sneve M, Grimnes G. Supplementation with High Doses of Vitamin D to Subjects without Vitamin D Deficiency May Have Negative Effects: Pooled Data from Four Intervention Trials in Tromsø. ISRN Endocrinol. 2013;2013:348705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 497] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 145. | Clark SA, Stumpf WE, Sar M, DeLuca HF, Tanaka Y. Target cells for 1,25 dihydroxyvitamin D3 in the pancreas. Cell Tissue Res. 1980;209:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 146. | Clark SA, Stumpf WE, Sar M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes. 1981;30:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, Deluca HF. Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology. 1983;113:1511-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 148. | Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest. 1984;73:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 149. | Kadowaki S, Norman AW. Time course study of insulin secretion after 1,25-dihydroxyvitamin D3 administration. Endocrinology. 1985;117:1765-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 150. | Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 151. | Cade C, Norman AW. Rapid normalization/stimulation by 1,25-dihydroxyvitamin D3 of insulin secretion and glucose tolerance in the vitamin D-deficient rat. Endocrinology. 1987;120:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17:509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 153. | Pepaj M, Bredahl MK, Gjerlaugsen N, Bornstedt ME, Thorsby PM. Discovery of novel vitamin D-regulated proteins in INS-1 cells: a proteomic approach. Diabetes Metab Res Rev. 2015;31:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 154. | Bornstedt ME, Gjerlaugsen N, Olstad OK, Berg JP, Bredahl MK, Thorsby PM. Vitamin D metabolites influence expression of genes concerning cellular viability and function in insulin producing β-cells (INS1E). Gene. 2020;746:144649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Cheng Q, Li YC, Boucher BJ, Leung PS. A novel role for vitamin D: modulation of expression and function of the local renin-angiotensin system in mouse pancreatic islets. Diabetologia. 2011;54:2077-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 156. | Lahbib A, Ghodbane S, Louchami K, Sener A, Sakly M, Abdelmelek H. Effects of vitamin D on insulin secretion and glucose transporter GLUT2 under static magnetic field in rat. Environ Sci Pollut Res Int. 2015;22:18011-18016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 157. | Jayanarayanan S, Anju TR, Smijin S, Paulose CS. Vitamin D3 supplementation increases insulin level by regulating altered IP3 and AMPA receptor expression in the pancreatic islets of streptozotocin-induced diabetic rat. J Nutr Biochem. 2015;26:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 158. | Kuo T, Damle M, González BJ, Egli D, Lazar MA, Accili D. Induction of α cell-restricted Gc in dedifferentiating β cells contributes to stress-induced β-cell dysfunction. JCI Insight. 2019;5:e128351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 159. | He D, Wang Y, Liu R, He A, Li S, Fu X, Zhou Z. 1,25(OH)2D3 Activates Autophagy to Protect against Oxidative Damage of INS-1 Pancreatic Beta Cells. Biol Pharm Bull. 2019;42:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 160. | Guareschi ZM, Valcanaia AC, Ceglarek VM, Hotz P, Amaral BK, de Souza DW, de Souza TA, Nardelli T, Ferreira TR, Leite NC, Lubackzeuski C, de O Emilio HR, Grassiolli S. The effect of chronic oral vitamin D supplementation on adiposity and insulin secretion in hypothalamic obese rats. Br J Nutr. 2019;121:1334-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 161. | Kjalarsdottir L, Tersey SA, Vishwanath M, Chuang JC, Posner BA, Mirmira RG, Repa JJ. 1,25-Dihydroxyvitamin D3 enhances glucose-stimulated insulin secretion in mouse and human islets: a role for transcriptional regulation of voltage-gated calcium channels by the vitamin D receptor. J Steroid Biochem Mol Biol. 2019;185:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 162. | Park S, Kim DS, Kang S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-γ expression in nonobese Type 2 diabetic rats. J Nutr Biochem. 2016;27:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 163. | Mazzone G, Morisco C, Lembo V, D'Argenio G, D'Armiento M, Rossi A, Giudice CD, Trimarco B, Caporaso N, Morisco F. Dietary supplementation of vitamin D prevents the development of western diet-induced metabolic, hepatic and cardiovascular abnormalities in rats. United European Gastroenterol J. 2018;6:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 164. | Dong B, Zhou Y, Wang W, Scott J, Kim K, Sun Z, Guo Q, Lu Y, Gonzales NM, Wu H, Hartig SM, York RB, Yang F, Moore DD. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology. 2020;71:1559-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 165. | Sadek KM, Shaheen H. Biochemical efficacy of vitamin D in ameliorating endocrine and metabolic disorders in diabetic rats. Pharm Biol. 2014;52:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 166. | Sisley SR, Arble DM, Chambers AP, Gutierrez-Aguilar R, He Y, Xu Y, Gardner D, Moore DD, Seeley RJ, Sandoval DA. Hypothalamic Vitamin D Improves Glucose Homeostasis and Reduces Weight. Diabetes. 2016;65:2732-2741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 167. | Schnell DM, Walton RG, Vekaria HJ, Sullivan PG, Bollinger LM, Peterson CA, Thomas DT. Vitamin D produces a perilipin 2-dependent increase in mitochondrial function in C2C12 myotubes. J Nutr Biochem. 2019;65:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 168. | Jefferson GE, Schnell DM, Thomas DT, Bollinger LM. Calcitriol concomitantly enhances insulin sensitivity and alters myocellular lipid partitioning in high fat-treated skeletal muscle cells. J Physiol Biochem. 2017;73:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 169. | Hesse M, Fröhlich LF, Zeitz U, Lanske B, Erben RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 170. | Boucher BJ. Does vitamin D status contribute to caveolin-1-mediated insulin sensitivity in skeletal muscle? Diabetologia. 2009;52:2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 171. | Wu YY, Yu T, Zhang XH, Liu YS, Li F, Wang YY, Gong P. 1,25(OH)2D3 inhibits the deleterious effects induced by high glucose on osteoblasts through undercarboxylated osteocalcin and insulin signaling. J Steroid Biochem Mol Biol. 2012;132:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Chen S, Villalta SA, Agrawal DK. FOXO1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J Bone Miner Res. 2016;31:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 173. | Oh J, Riek AE, Darwech I, Funai K, Shao J, Chin K, Sierra OL, Carmeliet G, Ostlund RE Jr, Bernal-Mizrachi C. Deletion of macrophage Vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015;10:1872-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 174. | Ni W, Glenn DJ, Gardner DG. Tie-2Cre mediated deletion of the vitamin D receptor gene leads to improved skeletal muscle insulin sensitivity and glucose tolerance. J Steroid Biochem Mol Biol. 2016;164:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 175. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13206] [Cited by in RCA: 12431] [Article Influence: 540.5] [Reference Citation Analysis (1)] |
| 176. | Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE; Diabetes Prevention Program Research Group. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 316] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 177. | Karnchanasorn R, Ou HY, Chiu KC. Proposed Guidelines for Future Vitamin D Studies. JAMA Intern Med. 2016;176:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 178. | Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1140] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 179. | Karnchanasorn R, Ou HY, Chiu KC. Plasma 25-hydroxyvitamin D levels are favorably associated with β-cell function. Pancreas. 2012;41:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |