Published online Aug 15, 2021. doi: 10.4239/wjd.v12.i8.1282
Peer-review started: January 28, 2021
First decision: April 6, 2021
Revised: May 24, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 15, 2021
Processing time: 192 Days and 17.6 Hours
Diabetic macrovascular complications (DMCs) are the most common complications encountered during the course of diabetes mellitus (DM) with extremely high mortality rates. Therefore, there is an urgent need to identify specific and sensitive biomarkers for the early diagnosis of DMCs.
To investigate the expression and significance of serum miR-129-5p in patients with DM and macrovascular complications.
Serum samples were collected from 36 healthy controls, 58 patients with DM presenting no macrovascular complications, and 62 patients with DMCs. The expression of miR-129-5p was detected using quantitative real-time polymerase chain reaction. Pearson’s correlation assay was performed to analyze the correlation between serum miR-129-5p levels and clinical indicators. Receiver operator characteristic (ROC) analysis was conducted to analyze the diagnostic value of serum miR-129-5p in patients with DM or DMCs.
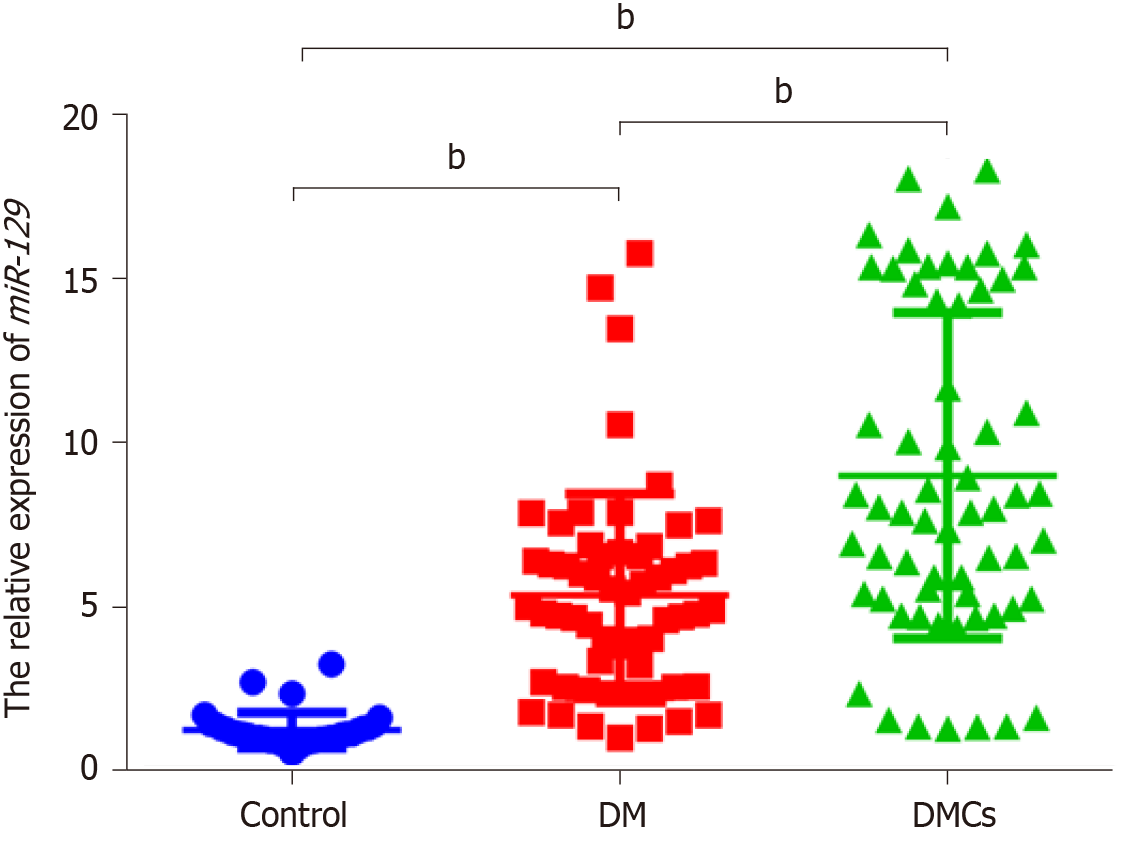
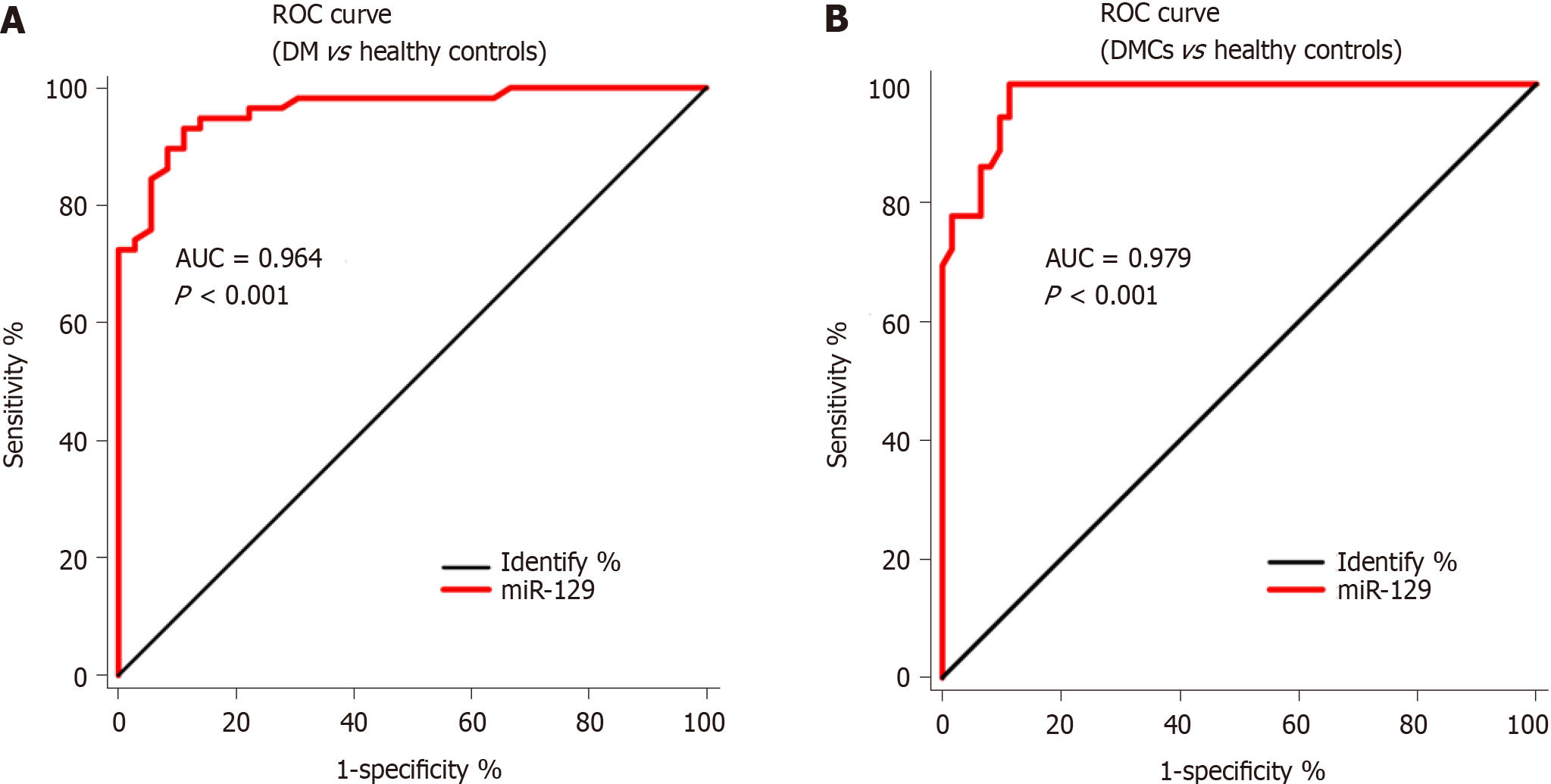
There was a 4.378-fold and 7.369-fold increase in serum miR-129-5p expression in the DM (5.346 ± 0.405) and DMCs (8.998 ± 0.631) groups, respectively (P < 0.001), compared with the control group (1.221±0.090). In addition, the expression of serum miR-129-5p in patients with DMCs was higher than that in patients with DM, revealing a 1.683-fold increase (P < 0.001). Additionally, serum miR-129-5p expression significantly correlated with smoking history, disease duration, and glycated hemoglobin (HbA1c) in patients with DMCs (P < 0.001). The area under the ROC curve (AUC) of miR-129-5p as a serum marker was 0.964 (95% confidence interval [CI]: 0.930-0.997, P < 0.001) in distinguishing between patients with DM and healthy controls, whereas the AUC of miR-129-5p as a serum marker was 0.979 (95%CI: 0.959-0.999, P < 0.001) in distinguishing between patients with DMCs and healthy controls.
Elevated serum miR-129-5p expression levels correlate with the development of DMCs and can be utilized as a novel early diagnostic biomarker for DM combined with macrovascular complications.
Core Tip: Diabetic macrovascular complications (DMCs) are the most common complications encountered during the course of diabetes mellitus (DM) with extremely high mortality rates. Therefore, there is an urgent need to identify specific and sensitive biomarkers for the early diagnosis of DMCs. Here, we revealed that the high serum miR-129-5p expression is related to the development of DMCs and can be employed as a novel early diagnostic biomarker for DM combined with macro
- Citation: He XY, Ou CL. Clinical significance of serum miR-129-5p in patients with diabetes mellitus presenting macrovascular complications. World J Diabetes 2021; 12(8): 1282-1291
- URL: https://www.wjgnet.com/1948-9358/full/v12/i8/1282.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i8.1282
Diabetes mellitus (DM) is a metabolic disease characterized by chronic hyperglycemia and is considered one of the most common human diseases worldwide[1,2]. Long-term hyperglycemia may cause systemic vascular damage following the occurrence and development of DM, thereby inducing diabetic macrovascular complications (DMCs)[3-5]. DMCs are among the most critical factors related to DM-associated deaths, accounting for approximately 70% of mortalities[6,7]. Therefore, there is an urgent need to identify effective biomarkers for early diagnosis, monitoring progression, and targeted therapy of DMCs[8].
MicroRNAs (miRNAs) are a class of non-coding RNAs (ncRNAs) with a length of approximately 22 nucleotides, which are known to inhibit the expression of crucial molecules by binding to the untranslated region of target mRNA[2,9], thereby affecting the process of human metabolic diseases, including DM[10]. Moreover, miRNAs have been detected in tissues as well as in various body fluids, including blood, urine, saliva, and milk, especially in serum. Recently, accumulating evidence has revealed that serum miRNAs are closely associated with DM as well as DM-associated complications, such as DMCs. For example, Barutta et al[11] have demonstrated that serum miR-126 levels can be associated with vascular complications in DM. However, the patterns and role of a large number of serum miRNAs remain unclear.
In the present study, we aimed to detect changes in the serum expression of miR-129-5p in DM combined with DMCs and analyze the relationship between miR-129-5p expression and the clinical characteristics of DMCs, thus evaluating its potential as a novel biomarker for predicting the prognosis of DMCs.
In total, 156 serum samples, including samples from 36 healthy controls, 58 patients with DM without DMCs, and 62 patients with DM presenting DMCs, were collected between March 2017 and January 2019 at the Affiliated Hospital of Guilin Medical University (Guilin, China). We used the most common clinical non-invasive examination, B-mode ultrasound, to diagnose patients with DM presenting macro
Fasting venous blood samples (5 mL) were collected from all participants. After standing for 2 h, samples were centrifuged at 3500 rpm for 20 min at 4 °C; the obtained serum samples were transferred to freshly sterilized tubes and immediately stored at -80 °C.
Total RNA was extracted from serum specimens using a miRNA Serum/Plasma Isolation Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Reverse transcription was performed using Mir-XTM miRNA First-Strand Synthesis Kit (Clontech, United States), and quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed as described previously[12]. The relative expression of miR-129-5p was normalized to the expression of U6 and calculated using the comparative threshold cycle (Ct) method using the formula 2−ΔΔCt.
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, United States) and SPSS 18.0 (IBM Corp., Chicago, IL, United States). Student’s t-test and one-way analysis of variance (ANOVA) were employed to evaluate the differential expression of serum miR-129-5p between patient groups and the healthy control group. Pearson’s correlation coefficient was utilized to analyze the correlation between two indicators. The relationship between clinical factors and miR-129-5p expression was estimated using the Chi-square test. Receiver-operating characteristic (ROC) curve analysis was performed using the SigmaPlot suite 13.0 software. Results with P < 0.05, P < 0.01, or P < 0.001 were deemed statistically significant.
Overall, no significant differences in age, sex, BMI, TCHO, or HDL-c were observed among the three groups. Data values for systolic pressure, diastolic pressure, OGTT, HbA1c, TG, and LDL-c in the DM and DMC groups were significantly higher than those in the control group (P < 0.05). Moreover, there was a 1.964-fold (P < 0.001) increase in the disease duration in the DMC group (10.560 ± 1.230 years), compared to that in the control group (5.376 ± 0.716 years); this finding was consistent with a previous study indicating that a DM duration of more than 5 years might induce DMCs more often[13].
Next, we used qRT-PCR to detect the differential expression of serum miR-129-5p in patients with DM presenting no DMCs, patients with DMCs, and healthy controls. There was a 4.378-fold and 7.369-fold increase in serum miR-129-5p expression in the DM (5.346 ± 0.405) and DMC (8.998 ± 0.631) groups, respectively, compared to the control group (1.221 ± 0.090; P < 0.001; Figure 1). Further analysis showed that serum miR-129-5p expression level in patients with DMCs was significantly higher than that in patients with DM, demonstrated as a 1.683-fold increase (P < 0.001, Figure 1).
Pearson’s correlation coefficient was employed to analyze the correlation between the expression of serum miR-129-5p and clinical variables in DM and DMCs. The results demonstrated that the expression of serum miR-129-5p positively correlated with OGTT (glucose 2 h after dinner) in patients with DM (P < 0.05, Table 1); however, in patients with DMCs, serum miR-129-5p expression positively correlated with the disease duration, smoking history, DM family history, fasting glucose (OGTT), and HbA1c (P < 0.05, Table 2). Our findings revealed that the serum expression of miR-129-5p was more closely related to the clinical factors of DMCs than those of DM, suggesting that serum miR-129-5p can serve as a potential molecular marker for detecting the development of DMCs.
| Parameter | Control (n = 36) | DM (n = 58) | DMC (n = 62) |
| Age (yr) | 53.310 ± 1.535 | 55.860 ± 1.400 | 57.050 ± 1.190 |
| Gender (male/female) | (13/23) | (32/26) | (33/29) |
| BMI (kg/m2) | 24.150 ± 0.306 | 24.660 ± 0.388 | 25.050 ± 0.305 |
| Duration of disease (yr) | - | 5.376 ± 0.716 | 10.560 ± 1.230e |
| Systolic pressure (mmHg) | 123.510 ± 2.039 | 134.703 ± 2.456b | 137.712 ± 2.497c |
| Diastolic pressure (mmHg) | 73.081 ± 1.704 | 82.261 ± 1.471c | 81.732 ± 1.349c |
| OGTT (mmol/L) | |||
| Fasting glucose | 5.397 ± 0.051 | 11.511 ± 0.597c | 11.341 ± 0.709c |
| 1-h glucose | 8.835 ± 0.094 | 13.763 ± 0.596c | 13.113 ± 0.339c |
| 2-h glucose | 6.568 ± 0.108 | 14.061 ± 0.622c | 13.650 ± 0.483c |
| HbA1c, (%) | 5.589 ± 0.111 | 10.64 ± 0.323c | 10.290 ± 0.287c |
| Comprehensive metabolic indicators | |||
| TG (mmol/L) | 1.335 ± 0.121 | 2.265 ± 0.2483b | 1.867 ± 0.154a |
| TCHO (mmol/L) | 4.792 ± 0.208 | 4.986 ± 0.153 | 5.047 ± 0.205 |
| HDL-c (mmol/L) | 1.410 ± 0.060 | 1.312 ± 0.049 | 1.303 ± 0.043 |
| LDL-c (mmol/L) | 2.831 ± 0.1643 | 3.566 ± 0.188b | 3.735 ± 0.227b |
| Parameter | DM | DMV | ||
| r | P value | r | P value | |
| Age (yr) | 0.205 | 0.122 | 0.24 | 0.06 |
| Gender | -0.025 | 0.85 | -0.178 | 0.167 |
| BMI (kg/m2) | -0.193 | 0.146 | -0.069 | 0.597 |
| Duration of disease (yr) | 0.122 | 0.361 | 0.335 | 0.008 |
| Smoking history | -0.135 | 0.312 | 0.346 | 0.006 |
| DM family history | -0.06 | 0.656 | 0.299 | 0.018 |
| Systolic pressure, mmHg | -0.133 | 0.318 | -0.136 | 0.29 |
| Diastolic pressure, mmHg | -0.19 | 0.153 | -0.191 | 0.137 |
| OGTT (mmol/L) | ||||
| Fasting glucose | 0.111 | 0.405 | 0.268 | 0.036 |
| 1-h glucose | 0.258 | 0.05 | 0.071 | 0.582 |
| 2-h glucose | 0.283 | 0.031 | 0.183 | 0.154 |
| HbA1c, % | 0.149 | 0.263 | 0.255 | 0.045 |
| TG | -0.149 | 0.263 | -0.176 | 0.172 |
| CHOL | -0.057 | 0.673 | 0.138 | 0.285 |
| HDL-c | -0.052 | 0.696 | -0.105 | 0.418 |
| LDL-c | -0.115 | 0.389 | -0.105 | 0.417 |
Based on the median serum level of miR-129-5p, patients with DM presenting DMCs were divided into high- and low-level groups. The relationship between the expression levels of serum miR-129-5p and clinical factors in DMCs was analyzed using the Chi-square test. As shown in Table 3, the expression levels of serum miR-129-5p showed no significant correlation with patient gender, age, family history of DM, high blood pressure, or fasting blood glucose (P > 0.05). However, serum miR-129-5p levels significantly correlated with smoking history, disease duration, and HbA1c in patients with DMCs (P < 0.05).
| Parameter | No. of cases (n = 62) | miR-129 level | χ2 | P value | |
| High (n = 24) | Low (n = 38) | ||||
| Gender | |||||
| Male | 33 | 15 | 18 | 1.353 | 0.245 |
| Female | 29 | 9 | 20 | ||
| Age (yr) | |||||
| ≥ 60 | 31 | 15 | 16 | 2.447 | 0.118 |
| < 60 | 31 | 9 | 22 | ||
| BMI (kg/m2) | |||||
| ≥ 24 | 42 | 13 | 29 | 3.302 | 0.069 |
| 0 < 24 | 20 | 11 | 9 | ||
| Smoking history | |||||
| Yes | 16 | 10 | 6 | 5.144 | 0.023 |
| No | 46 | 14 | 32 | ||
| DM family history | |||||
| Yes | 25 | 13 | 12 | 3.119 | 0.077 |
| No | 37 | 11 | 26 | ||
| Duration of disease (yr) | |||||
| ≥ 10 | 29 | 16 | 13 | 6.224 | 0.013 |
| < 10 | 33 | 8 | 25 | ||
| HBP | |||||
| Yes | 28 | 12 | 16 | 0.37 | 0.543 |
| No | 34 | 12 | 22 | ||
| HbA1c (%) | |||||
| ≥ 10 .28 | 33 | 18 | 15 | 7.457 | 0.006 |
| < 10.28 | 29 | 6 | 23 | ||
| FBG (mmol/L) | |||||
| ≥ 10 .34 | 21 | 10 | 11 | 1.062 | 0.303 |
| < 10.34 | 41 | 14 | 27 | ||
| PBS 1 h (mmol/L) | |||||
| ≥ 13.11 | 36 | 15 | 21 | 0.316 | 0.574 |
| < 13.11 | 26 | 9 | 17 | ||
| PBS 2 h (mmol/L) | |||||
| ≥ 13.65 | 33 | 14 | 19 | 0.41 | 0.522 |
| < 13.65 | 29 | 10 | 19 | ||
| TG (mmol/L) | |||||
| ≥ 1.867 | 20 | 5 | 15 | 2.339 | 0.126 |
| < 1.867 | 42 | 19 | 23 | ||
| TCHO (mmol/L) | |||||
| ≥ 5.047 | 24 | 9 | 15 | 0.024 | 0.876 |
| < 5.047 | 38 | 15 | 23 | ||
| HDL-c (mmol/L) | |||||
| ≥ 1.303 | 30 | 10 | 20 | 0.708 | 0.4 |
| < 1.303 | 32 | 14 | 18 | ||
| LDL-c (mmol/L) | |||||
| ≥ 3.735 | 27 | 11 | 16 | 0.083 | 0.773 |
| < 3.735 | 35 | 13 | 22 | ||
Furthermore, we performed ROC curve analysis to evaluate the diagnostic value of serum miR-129-5p as a serum marker in patients with DM lacking DMCs and patients with DMCs. The results revealed that the area under the ROC curve (AUC) of miR-129-5p as a serum marker was 0.964 (95%CI: 0.930-0.997, P < 0.001, Figure 2A) in distinguishing between patients with DM and healthy controls; the AUC of miR-129-5p as a marker was 0.979 (95%CI: 0.959-0.999, P < 0.001, Figure 2B) in distinguishing between patients with DMC and healthy controls. This result implied that the diagnostic value of serum miR-129-5p in patients with DMCs was higher than that in patients with DM.
DMCs are the most common complications associated with DM and are typically accompanied by pathological changes in endothelial cells, cardiomyocytes, vascular cells, and stem cells. The occurrence and development of DMCs involve various underlying mechanisms, including autophagy[14], oxidative stress[15], inflammatory response, and immune response[16]. In the present study, we revealed that the disease duration in the DMC group was significantly prolonged when compared with the DM group (P < 0.001), indicating that vascular complications are important factors in DM-related deaths. Therefore, there is considerable urgency in the search for early diagnostic markers and drug targets for DMCs.
With the development of RNA-seq and next-generation sequencing technologies[17,18], an increasing number of miRNAs have been discovered and identified to be strongly associated with the development of DM[19-21]. miRNAs are present in human plasma in a stable form, showing a higher specificity and sensitivity of detection than serum proteins. Therefore, serum miRNAs have been widely employed as novel diagnostic biomarkers for DM. To date, numerous serum miRNAs are found to be aberrantly expressed during DMCs, including miR-148[22], miR-342[23], and miR-133a[24], with a few recent studies elaborating the relationship between serum miRNAs and DMCs.
miR-129-5p is a member of the miRNA family and is closely related to the development and progression of human cancers[25,26]. Recently, miR-129-5p was found to regulate diabetic wound healing[27], DM-associated inflammatory response[28], and revascularization[29]. However, the patterns and role of high serum miR-129-5p expression in DMCs remain unclear. In the present study, we observed that serum expression of miR-129-5p was significantly higher in patients with DM and patients with DMCs than in the control group. Furthermore, the expression of serum miR-129-5p was significantly higher in patients with DMCs than that in patients with DM (P < 0.05). Further evaluation revealed that the expression of serum miR-129-5p correlated significantly with smoking history, disease duration, and HbA1c in patients with DMCs (all P < 0.001). Additionally, the AUC of miR-129-5p as a serum marker was 0.964 (95%CI: 0.930-0.997, P < 0.001) in distinguishing between patients with DM and healthy controls, whereas the AUC of miR-129-5p as a serum marker was 0.979 (95%CI: 0.959-0.999, P < 0.001) in distinguishing between patients with DMCs and healthy controls.
Collectively, our findings provide convincing evidence demonstrating the upregulation of serum miR-129-5p, which can serve as a novel molecular marker for the early diagnosis of DMCs. In addition, the expression of serum miR-129-5p significantly correlates with smoking history, disease duration, and HbA1c in patients with DMCs.
Diabetic macrovascular complications (DMCs) are the most common complications encountered during the course of diabetes mellitus (DM) with extremely high mortality rates.
There is an urgent need to identify specific and sensitive biomarkers for the early diagnosis of DMCs.
To investigate the expression and significance of serum miR-129-5p in patients with DM and macrovascular complications.
Serum samples were collected from 36 healthy controls, 58 patients with DM presenting no macrovascular complications, and 62 patients with DMCs. The expression of miR-129-5p was detected using quantitative real-time polymerase chain reaction. Pearson’s correlation assay was performed to analyze the correlation between serum miR-129-5p levels and clinical indicators. Receiver operator characteristic analysis was conducted to analyze the diagnostic value of serum miR-129-5p in patients with DM or DMCs.
These finding demonstrated that serum expression of miR-129-5p was significantly higher in patients with DM and patients with DMCs than in the control group. Furthermore, the expression of serum miR-129-5p was significantly higher in patients with DMCs than in patients with DM (P < 0.05). Further evaluation revealed that the expression of serum miR-129-5p correlated significantly with smoking history, disease duration, and HbA1c in patients with DMCs (P < 0.001). Additionally, the AUC of miR-129-5p as a serum marker was 0.964 (95%CI: 0.930-0.997, P < 0.001) in distinguishing between patients with DM and healthy controls, whereas the AUC of miR-129-5p as a serum marker was 0.979 (95%CI: 0.959-0.999, P < 0.001) in distinguishing between patients with DMCs and healthy controls.
Elevated serum miR-129-5p expression levels correlate with the development of DMCs and can be utilized as a novel early diagnostic biomarker for DM combined with macrovascular complications.
The high serum miR-129-5p expression is related to the development of DMCs and can be employed as a novel early diagnostic biomarker for DM combined with macrovascular complications.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aureliano M, Hussein A, Spartalis M S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Alegre-Díaz J, Herrington W, López-Cervantes M, Gnatiuc L, Ramirez R, Hill M, Baigent C, McCarthy MI, Lewington S, Collins R, Whitlock G, Tapia-Conyer R, Peto R, Kuri-Morales P, Emberson JR. Diabetes and Cause-Specific Mortality in Mexico City. N Engl J Med. 2016;375:1961-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 2. | Liu B, Wang Y, Zhang Y, Yan B. Mechanisms of Protective Effects of SGLT2 Inhibitors in Cardiovascular Disease and Renal Dysfunction. Curr Top Med Chem. 2019;19:1818-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 4. | Yehualashet AS. Toll-like Receptors as a Potential Drug Target for Diabetes Mellitus and Diabetes-associated Complications. Diabetes Metab Syndr Obes. 2020;13:4763-4777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus - A comprehensive review. J Diabetes Complications. 2020;34:107613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 6. | Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14-E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 804] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 7. | Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2020] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 8. | Lysy PA, Corritore E, Sokal EM. New Insights into Diabetes Cell Therapy. Curr Diab Rep. 2016;16:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Ou C, Sun Z, Li X, Ren W, Qin Z, Zhang X, Yuan W, Wang J, Yu W, Zhang S, Peng Q, Yan Q, Xiong W, Li G, Ma J. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Rajkumar KV, Lakshmanan G, Sekar D. Identification of miR-802-5p and its involvement in type 2 diabetes mellitus. World J Diabetes. 2020;11:567-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Barutta F, Bruno G, Matullo G, Chaturvedi N, Grimaldi S, Schalkwijk C, Stehouwer CD, Fuller JH, Gruden G. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol. 2017;54:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Ou C, Sun Z, He X, Li X, Fan S, Zheng X, Peng Q, Li G, Ma J. Targeting YAP1/LINC00152/FSCN1 Signaling Axis Prevents the Progression of Colorectal Cancer. Adv Sci (Weinh). 2020;7:1901380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 13. | Annani-Akollor ME, Addai-Mensah O, Fondjo LA, Sallah L, Owiredu EW, Acheampong E, Akamugri S. Predominant Complications of Type 2 Diabetes in Kumasi: A 4-Year Retrospective Cross-Sectional Study at a Teaching Hospital in Ghana. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Xu J, Kitada M, Ogura Y, Koya D. Relationship Between Autophagy and Metabolic Syndrome Characteristics in the Pathogenesis of Atherosclerosis. Front Cell Dev Biol. 2021;9:641852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Byrne NJ, Rajasekaran NS, Abel ED, Bugger H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med. 2021;169:317-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 16. | Navas-Madroñal M, Castelblanco E, Camacho M, Consegal M, Ramirez-Morros A, Sarrias MR, Perez P, Alonso N, Galán M, Mauricio D. Role of the Scavenger Receptor CD36 in Accelerated Diabetic Atherosclerosis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Armingol E, Officer A, Harismendy O, Lewis NE. Deciphering cell-cell interactions and communication from gene expression. Nat Rev Genet. 2021;22:71-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 719] [Article Influence: 179.8] [Reference Citation Analysis (0)] |
| 18. | Zheng T, Ellinghaus D, Juzenas S, Cossais F, Burmeister G, Mayr G, Jørgensen IF, Teder-Laving M, Skogholt AH, Chen S, Strege PR, Ito G, Banasik K, Becker T, Bokelmann F, Brunak S, Buch S, Clausnitzer H, Datz C; DBDS Consortium; Degenhardt F, Doniec M, Erikstrup C, Esko T, Forster M, Frey N, Fritsche LG, Gabrielsen ME, Gräßle T, Gsur A, Gross J, Hampe J, Hendricks A, Hinz S, Hveem K, Jongen J, Junker R, Karlsen TH, Hemmrich-Stanisak G, Kruis W, Kupcinskas J, Laubert T, Rosenstiel PC, Röcken C, Laudes M, Leendertz FH, Lieb W, Limperger V, Margetis N, Mätz-Rensing K, Németh CG, Ness-Jensen E, Nowak-Göttl U, Pandit A, Pedersen OB, Peleikis HG, Peuker K, Rodriguez CL, Rühlemann MC, Schniewind B, Schulzky M, Skieceviciene J, Tepel J, Thomas L, Uellendahl-Werth F, Ullum H, Vogel I, Volzke H, von Fersen L, von Schönfels W, Vanderwerff B, Wilking J, Wittig M, Zeissig S, Zobel M, Zawistowski M, Vacic V, Sazonova O, Noblin ES; 23andMe Research Team; Farrugia G, Beyder A, Wedel T, Kahlke V, Schafmayer C, D'Amato M, Franke A. Genome-wide analysis of 944 133 individuals provides insights into the etiology of haemorrhoidal disease. Gut. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Price NL, Goedeke L, Suárez Y, Fernández-Hernando C. miR-33 in cardiometabolic diseases: lessons learned from novel animal models and approaches. EMBO Mol Med. 2021;13:e12606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Bali KK, Gandla J, Rangel DR, Castaldi L, Mouritzen P, Agarwal N, Schmelz M, Heppenstall P, Kuner R. A genome-wide screen reveals microRNAs in peripheral sensory neurons driving painful diabetic neuropathy. Pain. 2021;162:1334-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Hua F. New insights into diabetes mellitus and its complications: a narrative review. Ann Transl Med. 2020;8:1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Grieco GE, Cataldo D, Ceccarelli E, Nigi L, Catalano G, Brusco N, Mancarella F, Ventriglia G, Fondelli C, Guarino E, Crisci I, Sebastiani G, Dotta F. Serum Levels of miR-148a and miR-21-5p Are Increased in Type 1 Diabetic Patients and Correlated with Markers of Bone Strength and Metabolism. Noncoding RNA. 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Eissa S, Matboli M, Bekhet MM. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed Pharmacother. 2016;83:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | de Gonzalo-Calvo D, van der Meer RW, Rijzewijk LJ, Smit JW, Revuelta-Lopez E, Nasarre L, Escola-Gil JC, Lamb HJ, Llorente-Cortes V. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci Rep. 2017;7:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Cao J, Shen Y, Zhu L, Xu Y, Zhou Y, Wu Z, Li Y, Yan X, Zhu X. miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat Cell Biol. 2012;14:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Chen X, Ruan A, Wang X, Han W, Wang R, Lou N, Ruan H, Qiu B, Yang H, Zhang X. miR-129-3p, as a diagnostic and prognostic biomarker for renal cell carcinoma, attenuates cell migration and invasion via downregulating multiple metastasis-related genes. J Cancer Res Clin Oncol. 2014;140:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Wang W, Yang C, Wang XY, Zhou LY, Lao GJ, Liu D, Wang C, Hu MD, Zeng TT, Yan L, Ren M. MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes. 2018;67:1627-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Umehara T, Mori R, Mace KA, Murase T, Abe Y, Yamamoto T, Ikematsu K. Identification of Specific miRNAs in Neutrophils of Type 2 Diabetic Mice: Overexpression of miRNA-129-2-3p Accelerates Diabetic Wound Healing. Diabetes. 2019;68:617-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Ma XL, Li SY, Shang F. Effect of microRNA-129-5p targeting HMGB1-RAGE signaling pathway on revascularization in a collagenase-induced intracerebral hemorrhage rat model. Biomed Pharmacother. 2017;93:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |