Published online Jul 15, 2021. doi: 10.4239/wjd.v12.i7.1131
Peer-review started: February 8, 2021
First decision: April 20, 2021
Revised: April 28, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 15, 2021
Processing time: 153 Days and 22.2 Hours
In healthy people, the lowest daily blood glucose concentration is usually observed in the early morning, after overnight fasting. However, the clinical relevance and the prevalence of fasting biochemical hypoglycemia (FBH) are poorly understood in people who do not have diabetes, although the clinical implications of such hypoglycemia have been extensively studied in patients with diabetes. FBH can be influenced by many factors, including age, sex, body mass, smoking, alcohol drinking, exercise levels, medications, and eating behaviors, such as breakfast skipping and late-night eating.
To determine the prevalence of FBH and investigated its association with potential risk factors in a population without diabetes.
Clinical parameters and lifestyle-related factors were assessed in a cross-sectional study of 695613 people aged 40-74 years who had undergone a health check-up (390282 men and 305331 women). FBH was defined as fasting plasma glucose < 70 mg/dL (3.9 mmol/L) after overnight fasting, regardless of any symptoms. The absence of diabetes was defined as HbA1c < 6.5%, fasting plasma glucose < 126 mg/dL (7.0 mmol/L), and no pharmacotherapy for diabetes. Multivariate logistic regression analysis, with adjustment for confounding factors, was used to identify associations.
FBH was present in 1842 participants (0.26%). There were significantly more women in the FBH group (59.1%) than in the non-FBH group (43.9%). Values of most of the clinical parameters, but not age, were significantly lower in the FBH group than in the non-FBH group. Logistic regression analysis showed that a body mass index of ≤ 20.9 kg/m2 (reference: 21-22.9 kg/m2) and current smoking were significantly associated with FBH, and this was not altered by adjustment for age, sex, and pharmacotherapy for hypertension or dyslipidemia. Female sex was associated with FBH. When the data were analyzed according to sex, men in their 60s or 70s appeared more likely to experience FBH compared with their 40s, whereas men in their 50s and women aged ≥ 50 years appeared less likely to experience FBH. The relationships of FBH with other factors including alcohol drinking and pharmacotherapies for hypertension and dyslipidemia also differed between men and women.
FBH occurs even in non-diabetic people, albeit at a very low frequency. FBH is robustly associated with low body mass and smoking, and its relationship with lifestyle factors varies according to sex.
Core Tip: The clinical relevance of fasting biochemical hypoglycemia (FBH) is poorly understood in people who do not have diabetes. Therefore, we determined the prevalence of FBH and its relationships with other parameters in approximately 700000 people who did not have diabetes. FBH was identified in 0.26% of the participants and women were over-represented among these (59.1%). Low body mass and smoking were associated with FBH in both men and women. Women and men in their 60s and 70s were more likely to experience FBH, and the relationships of FBH with other factors differed between men and women.
- Citation: Tanaka K, Higuchi R, Mizusawa K, Nakamura T, Nakajima K. Fasting biochemical hypoglycemia and related-factors in non-diabetic population: Kanagawa Investigation of Total Check-up Data from National Database-8. World J Diabetes 2021; 12(7): 1131-1140
- URL: https://www.wjgnet.com/1948-9358/full/v12/i7/1131.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i7.1131
Hypoglycemia is one of the most serious complications during the treatment of diabetes, whether it be type 1, type 2, or another type[1-4]. Hypoglycemia is associated with high mortality due to cardiovascular events and impaired cognitive function. The prevalence and severity of hypoglycemia, which is particularly frequent in diabetic patients who are administering insulin[1-4], are affected by multiple factors, including medication, diet, exercise, and the presence of comorbidities. However, the clinical relevance of hypoglycemia is poorly understood in people who do not have diabetes, and its prevalence has been determined in only a few small studies[5-7]. In apparently healthy people, blood glucose concentration tends to be lowest under fasting conditions in the early morning, after overnight fasting.
We therefore aimed to determine the prevalence of fasting biochemical hypoglycemia (FBH), which was assessed using a sample of plasma instead of finger-stick blood, and to identify the associated factors from among age, sex, body mass, smoking, alcohol drinking, exercise status, and breakfast skipping in a general population of people without diabetes, using healthcare data provided by the Japanese Ministry of Health, Labour, and Welfare.
The overarching study was a composite multidisciplinary study that consisted of the secondary use of annual health check-up data collected in Japan (Kanagawa Investigation of the Total Check-up Data from the National Database) that aimed to determine the clinical factors associated with cardiometabolic diseases. Details of the study concept and design have been published elsewhere[8]. The present study was performed using data from individuals who underwent specific health check-ups and were living in Kanagawa Prefecture between April 2012 and March 2013. The study protocol was approved by the Ethics Committee of Kanagawa University of Human Services (10-43) and the Ministry of Health, Labour, and Welfare of Japan (No. 121).
We received digitally recorded anonymous data from the Ministry of Health, Labour, and Welfare of Japan in 2017, as part of its nationwide program for the provision of medical data to third parties[9]. To protect against the identification of specific individuals, their ages had been categorized as 40-44, 45-49, 50-54, 55-59, 60-64, 65-69, or 70-74 years. However, for the purposes of the present study, to evaluate age as a single numeric value, we transformed the age groups into substituted ages (st-age), corresponding to the median for each age group (42, 47, 52, 57, 62, 67, and 72 years, respectively).
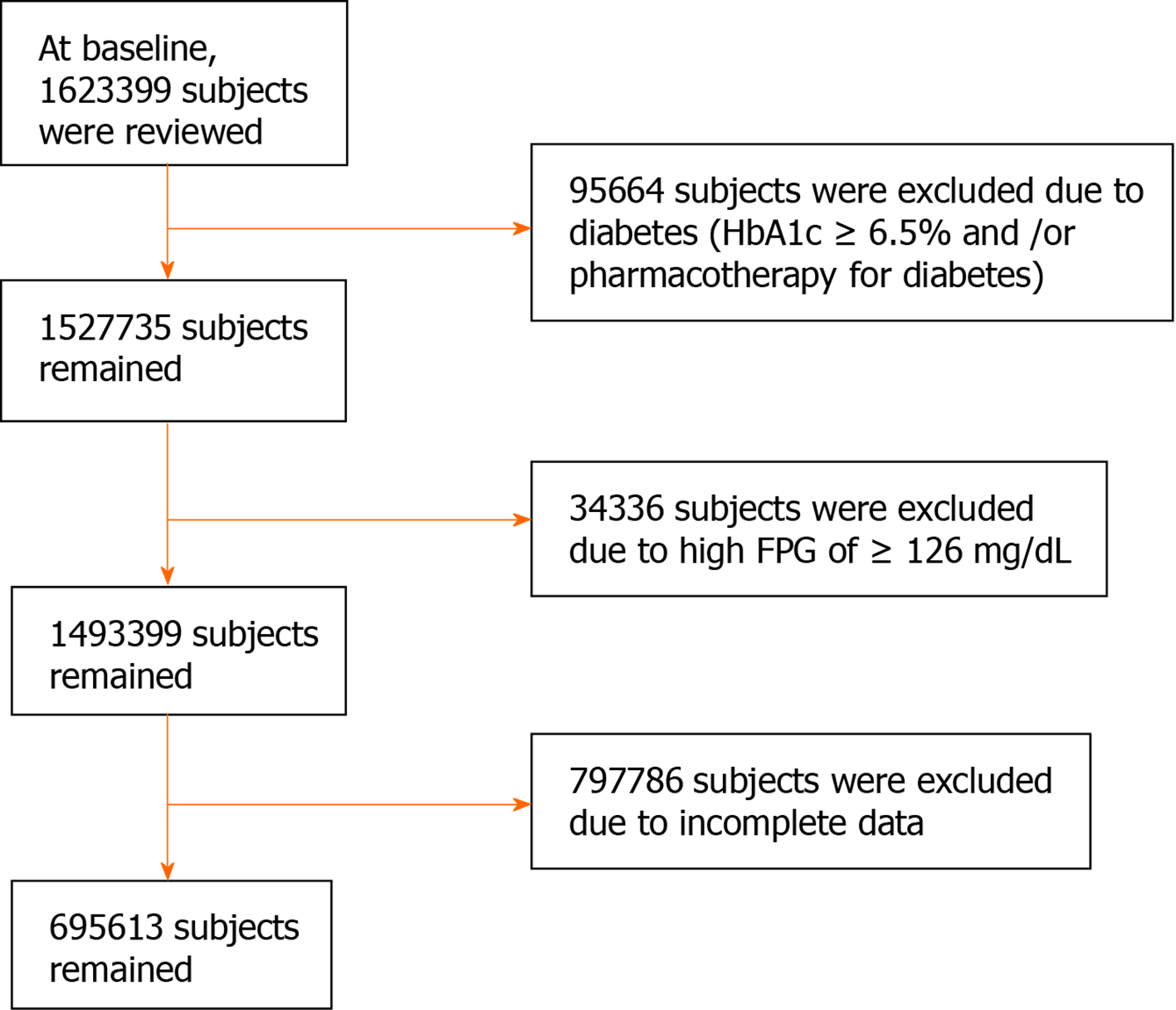
We initially reviewed data collected from 1623399 non-hospitalized people aged 40-74 years who had attended health check-ups. As shown in Figure 1, individuals who were undergoing pharmacotherapy for diabetes and/or had an HbA1c of ≥ 6.5%, regardless of their type of diabetes, were excluded (n = 95664; 5.9%). Individuals with a fasting plasma glucose (FPG) ≥ 126 mg/dL (7.0 mmol/L) were also excluded (n = 34336). After the further exclusion of those for whom incomplete clinical and lifestyle data were available (n = 797786), data from 695613 individuals remained for analysis (390282 men and 305331 women).
Anthropometric and laboratory measurements were performed in the morning following an overnight fast. Overnight fasting for > 10 h was individually confirmed and recorded by a staff member[8]. Body mass, waist circumference at the level of the navel, and height were measured by trained staff members. Body mass index (BMI) was calculated as mass (kg) divided by the square of height (m2). The participants were then placed into eight BMI groups: ≤ 16.9, 17-18.9, 19-20.9, 21-22.9, 23-24.9, 25-26.9, 27-28.9, and ≥ 29.0 kg/m2.
Fasting plasma glucose was measured (approximately 54% of samples) mainly by spectrophotometric or potentiometric (approximately 30%) method[8]. FBH was defined as an FPG < 70 mg/dL (3.9 mmol/L), regardless of the presence or absence of symptoms[4,10]. Laboratory measurements were performed using standard automated methods. Habitual breakfast skipping was defined as a positive response to the question: “Do you skip breakfast at least three times per week?”[8].
Data are expressed as mean ± SD or medians (interquartile range). Differences in continuous and categorical datasets between FBH and non-FBH groups were evaluated using Student’s t-test or the χ2 test, respectively. Trends in the prevalence of FBH with increasing alcohol consumption and age (three groups were defined: people in their 40s, 50s, and 60s + 70s) were evaluated using Cochran-Armitage tests. Because the number of participants in their 70s was relatively low, those in their 70s were grouped with those in their 60s for the analysis.
A logistic regression model was used to evaluate the relationships between FBH and plausibly related factors, with adjustment for potential confounding factors (age, sex, pharmacotherapy for hypertension or dyslipidemia, smoking status, alcohol consumption, and exercise status), yielding adjusted odds ratios (ORs) and 95%CIs. As in our previous study[11], a BMI range of 21.0-22.9 kg/m2 was used as the reference.
Statistical analyses were performed using SAS-Enterprise Guide (SAS-EG 7.1) in SAS software, version 9.4 (SAS Institute, Cary, NC, United States). P < 0.05 was considered to represent statistical significance. The statistical methods of this study were reviewed by Dr. Hiroto Narimatsu from the Kanagawa Cancer Center Research Institute, Yokohama, Japan.
The clinical characteristics of the participants, grouped according to the absence or presence of FBH, are shown in Table 1. FBH was experienced by 1842 participants (0.26% of the total). Women were significantly over-represented in the FBH group and the prevalence of FBH was significantly higher in women (0.35%, n = 1,088) than in men (0.18%, n = 754) (χ2 test, P < 0.001).
| No FBH | FBH | |
| n (% of total) | 693771 (99.7) | 1842 (0.26) |
| st-Age (yr) | 54.7 ± 10.1 | 54.7 ± 10.7 |
| 40s, n (%) | 269439 (99.7) | 758 (0.28) |
| 50s, n (%) | 185374 (99.8) | 418 (0.22) |
| 60s + 70s, n (%) | 238958 (99.7) | 666 (0.28) |
| Women, n (%) | 304243 (43.9) | 1088 (59.1) |
| BMI (kg/m2) | 22.9 ± 3.3 | 21.2 ± 3.4c |
| Systolic blood pressure (mmHg) | 122 ± 16.8 | 118 ± 17.8c |
| High-density lipoprotein-cholesterol (mg/dL) | 64.2 ± 16.8 | 70.6 ± 19.0c |
| Triglyceride, IQR (mg/dL) | 90 (64-131) | 71 (49-108)c |
| Fasting plasma glucose (mg/dL) | 94.3 ± 10.0 | 64.7 ± 5.6 |
| HbA1c (%) | 5.5 ± 0.4 | 5.4 ± 0.6c |
| Pharmacotherapy for hypertension, n (%) | 120461 (17.4) | 282 (15.3)a |
| Pharmacotherapy for dyslipidemia, n (%) | 77208 (11.1) | 213 (11.6) |
| Cardiovascular disease, n (%) | 21410 (3.1) | 71 (3.9) |
| Current smoking, n (%) | 146253 (21.1) | 492 (26.7)c |
| Habitual exercise, n (%)1 | 217322 (31.3) | 597 (32.4) |
| Breakfast skipping, n (%)3 | 102669 (14.8) | 309 (16.8)a |
| Late night dinner, n (%)4, N = 689639 | 205062 (29.8) | 482 (26.3)b |
| Alcohol consumption per day (g ethanol) | ||
| < 23 g | 379830 (54.8) | 1167 (63.4) |
| 23-45 g | 193102 (27.8) | 421 (22.9) |
| 46-68 g2 | 88021 (12.7) | 173 (9.4) |
| ≥ 69 g2 | 32818 (4.7) | 81 (4.4)c |
BMI, systolic blood pressure, serum triglyceride, and HbA1c were significantly lower in the FBH group than in the non-FBH group (P < 0.001). No difference in st-age was found, but the plasma high-density lipoprotein-cholesterol concentration was significantly higher in the FBH group (P < 0.001). The prevalence of current smoking and breakfast skipping was significantly higher in the FBH group, whereas that of pharmacotherapy for hypertension, late-night dining, and high alcohol consumption was lower in the FBH group.
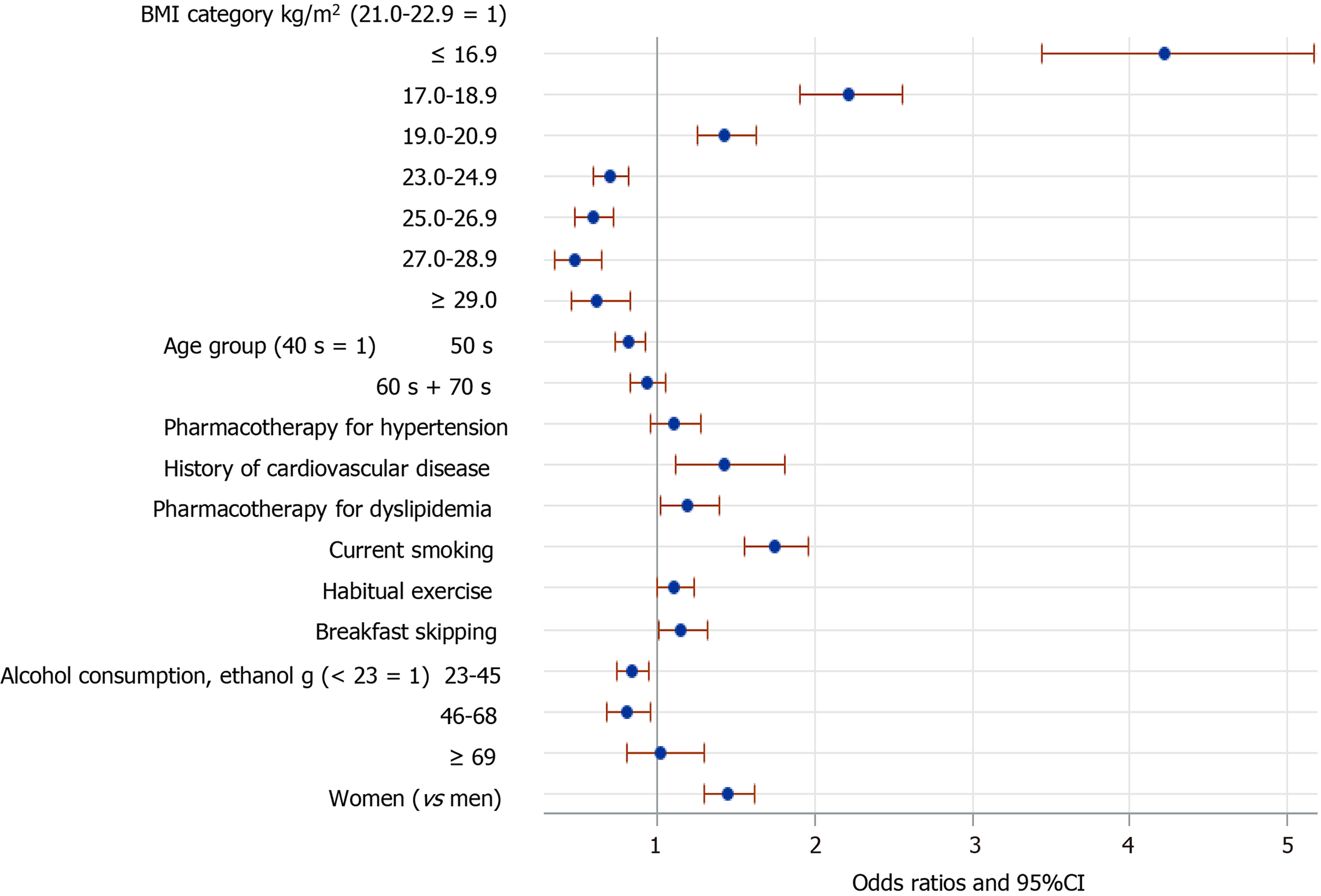
Figure 2 shows the results of logistic regression analysis, with adjustment for all the listed potential confounding factors (covariates) in the 695613 participants. Low BMI (≤ 20.9 kg/m2) was significantly associated with FBH, compared with the reference BMI category of 21-22.9 kg/m2. The OR of BMI ≤ 16.9 kg/m2 for FBH was almost four, compared with the reference BMI. By contrast, BMI ≥ 23.0 kg/m2 was inversely associated with FBH. Female sex, current smoking, a history of cardiovascular disease, pharmacotherapy for dyslipidemia, habitual exercise, and breakfast skipping were also significantly associated with FBH. Being in one’s 50s and mild-to-moderate alcohol consumption (23-68 g ethanol/day), but not heavy consumption, were inversely associated with FBH, compared with being in one’s 40s and a small amount of alcohol consumption (< 23 g ethanol/day), respectively.
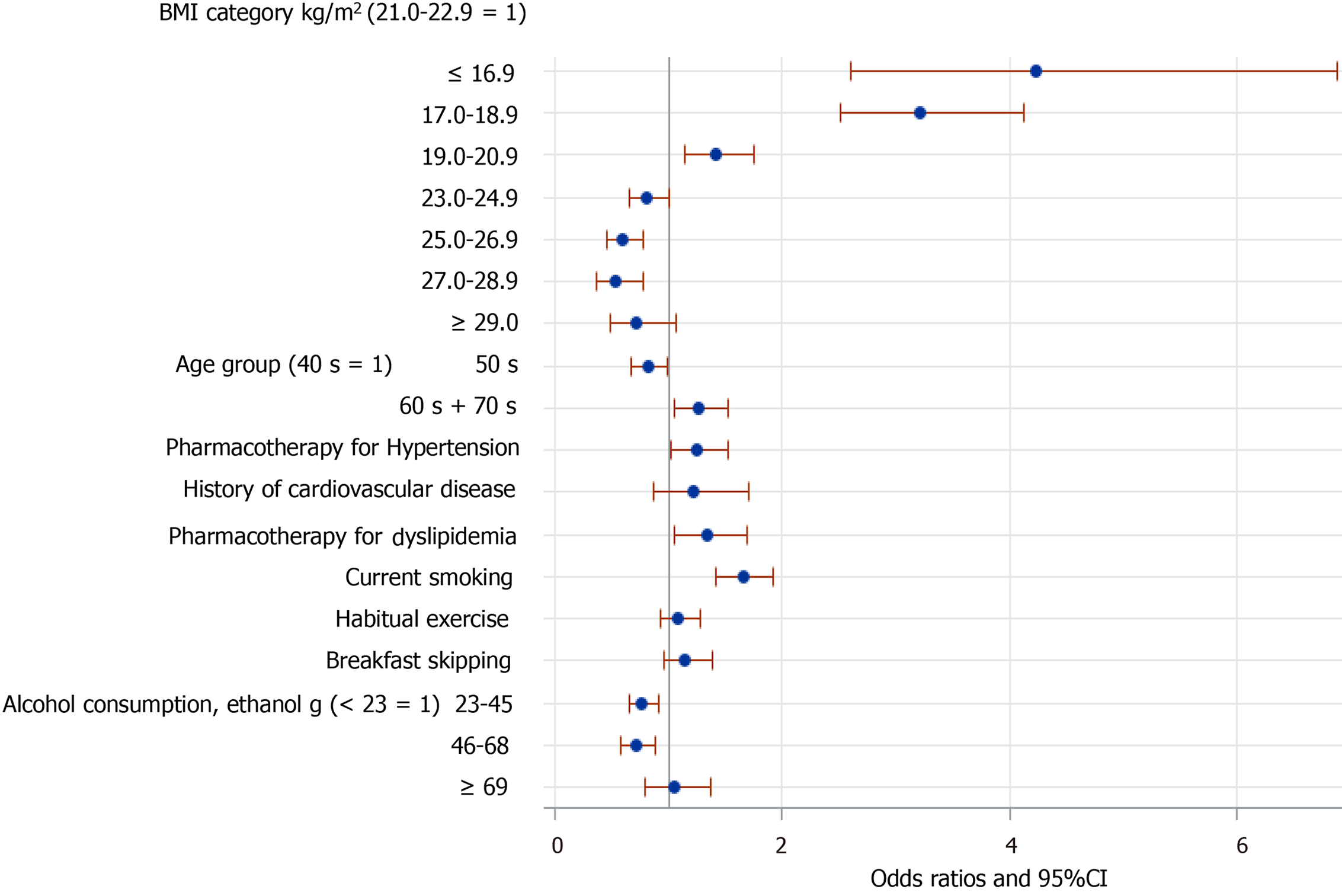
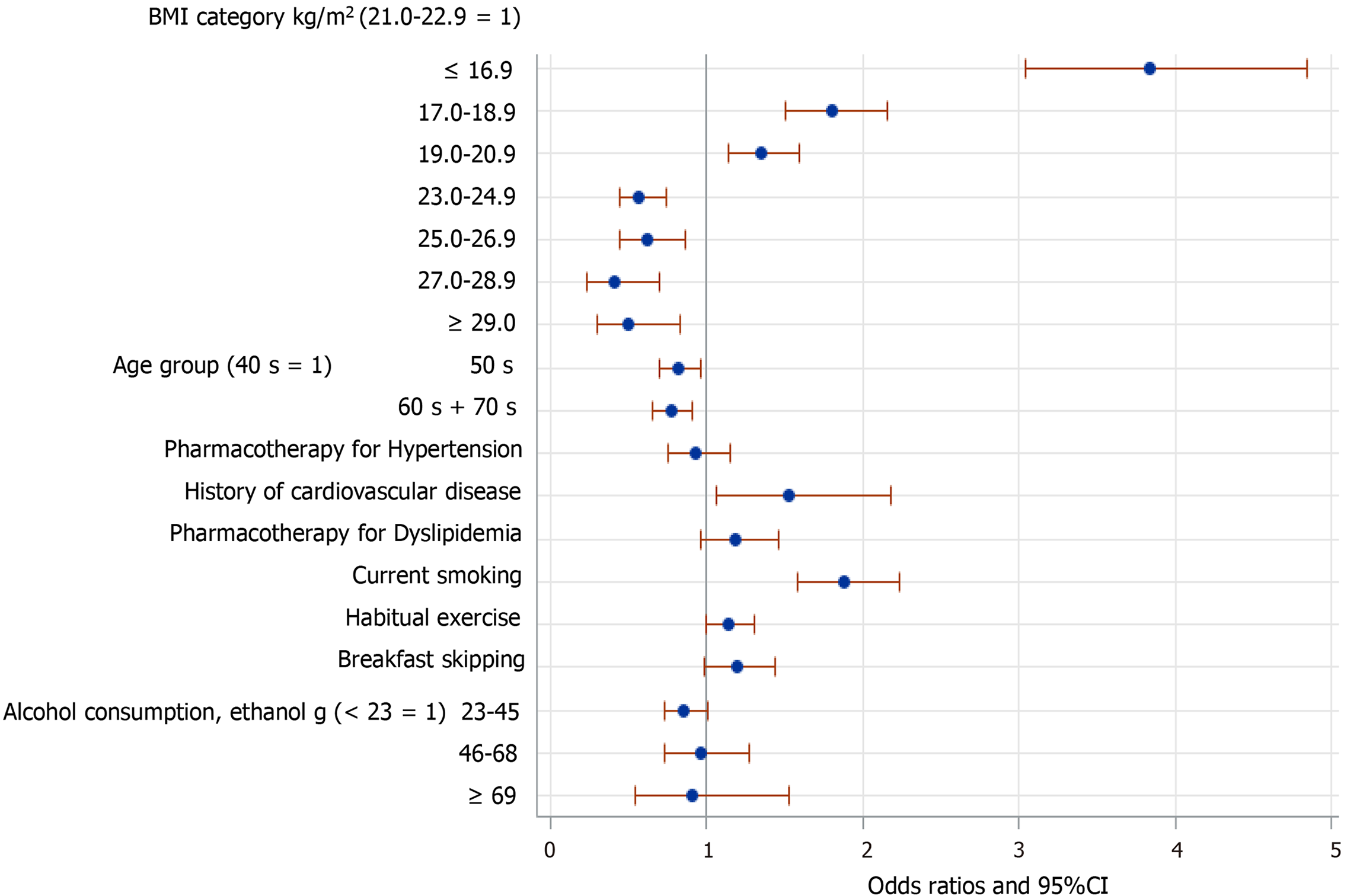
However, the relationships of FBH with several of these factors were changed when data from men and women were analyzed separately. Among the men (Figure 3), those in their 60s and 70s were more likely to have FBH. Pharmacotherapy for either hypertension or dyslipidemia was associated with FBH. Additionally, the inverse association of FBH with mild-to-moderate alcohol consumption was also present in men alone. By contrast, among women (Figure 4), those aged ≥ 50 were less likely to have FBH. A history of cardiovascular disease was significantly associated with FBH. However, mild-to-moderate alcohol consumption was not significantly associated with FBH in women. Finally, the associations of FBH with habitual exercise and breakfast skipping almost disappeared when data from men and women were analyzed separately (Figures 3 and 4), although both associations were marginally significant in women.
Hypoglycemia frequently occurs in diabetic patients undergoing pharmacotherapy and is difficult to predict[1-4]. In the present study, we have demonstrated that FBH also occurs in non-diabetic people, although the prevalence was very low (less than 1.0% of the total) in the general population studied, in contrast to that in diabetic patients[12,13]. This prevalence of FBH is not dissimilar to that of hypoglycemia (0.2%-0.5%) in non-diabetic people[3,5]. Among the potential confounding factors considered, low body mass and smoking were robustly associated with FBH in both men and women, and across the entire group, women appeared more likely to experience FBH than men. However, when data from each sex were analyzed separately, older women were found to be less likely to have FBH, whereas older men were more likely. The relationships of FBH with other factors (pharmacotherapy, a history of cardiovascular disease, and alcohol consumption) also differed between men and women. Habitual exercise and breakfast skipping were associated with FBH across the entire group, although these associations disappeared when men and women were analyzed separately, probably due to the lower statistical power.
Consistent with our findings, many previous studies have shown associations between low body mass and hypoglycemia in patients with diabetes[14-16]. Additionally, severe hypoglycemia has been shown to be prevalent in people with diabetes who are habitual smokers[17,18]. Therefore, common mechanisms may mediate the relationships between hypoglycemia, low body mass, and habitual smoking, regardless of the type of diabetes present.
FBH can occur as a reactive hypoglycemia in the non-diabetic population[19,20]. For example, reactive hypoglycemia caused by the dumping syndrome, with an incretin-driven insulin hypersecretory response occurs within a few hours of eating a meal in people who have undergone gastric or esophageal surgery[21,22]. However, because we excluded individuals from the present study who ate within the 10 h preceding blood sampling, which was established using individual questionnaires, the FBH of the individuals in the present study is unlikely to represent this type of reactive hypoglycemia, which often accompanies hyperinsulinemia[19,20]. However, hypoglycemia in the early morning may be caused by hepatic dysfunction and low gluconeogenesis, both in non-diabetic and diabetic individuals[23,24].
Many conditions and diseases are considered to contribute to the incidence of hypoglycemia in non-diabetic people[24,25]. For example, adrenal insufficiency, use of pain-relieving medication, malnutrition or low food intake, infection, low hepatic gluconeogenic capacity, and low glycogen storage have all been implicated.
The reasons why women and older men were more likely to have FBH are unknown. A plausible explanation for the former is that the insulin sensitivity of women is higher, whereas their hepatic gluconeogenesis is lower because of higher circulating 17β-estradiol concentrations in women than men[26,27]. Furthermore, older men may have a lower gluconeogenic capacity than younger men[28,29], which would result in an inadequate supply of blood glucose in the early morning before breakfast. The insulin sensitivity of individuals who habitually exercise may also be higher, possibly contributing to lower blood glucose concentrations. However, it is unknown whether such factors specifically influence the incidence of FBH.
Acute alcohol consumption increases the risk of hypoglycemia through the suppression of gluconeogenesis in the liver, which is more likely to occur in the fasting state in both diabetic and non-diabetic people[30-32]. However, the long-term effect of alcohol consumption is poorly understood[32].
In the present study, heavy alcohol consumption (≥ 69 g ethanol/day) was not associated with FBH, whereas mild-to-moderate consumption was inversely associated with FBH, particularly in men. A plausible explanation for this is that individuals who drink mild-to-moderate amounts of alcohol may concurrently consume an inadequate amount of food, predisposing toward subsequent hypoglycemia. In heavy drinkers, carbohydrate intake and glycogen storage may be inadequate, which could result in hypoglycemia several hours later, in addition to their lower renal and hepatic gluconeogenesis[31,32].
The reasons why breakfast skipping and pharmacotherapy for hypertension and dyslipidemia tended to be associated with FBH are unknown. A plausible reason for the former is that the sensitivity to hypoglycemia may be low, such that it does not elicit hunger and consequently removes the perceived need for the consumption of breakfast. In contrast, the lower prevalence of late-night dining in the FBH group (Table 1) may be less prevalent because the consumption of carbohydrates before sleep prolongs the peak blood glucose concentration until early morning.
Although we have shown that FBH occurs even in non-diabetic people, its clinical relevance in this group remains to be more fully determined. Although higher mortality in hospital[6,33], traffic accidents due to cognitive dysfunction[34], and fatal arrhythmias such as QT prolongation[35] are more prevalent in non-diabetic individuals who experience hypoglycemia, it is unknown whether these problems are also more common in non-diabetic people who experience FBH, and this deserves further study.
The main strength of the present study was that the FPG concentration was measured using a standard laboratory method, rather than using portable glucose meters, which can sometimes be inaccurate[36,37]. Furthermore, we were unable to conduct the present analysis until we had collected data from hundreds of thousands of people because of the very low prevalence of FBH. To the best of our knowledge, this study is the first to determine the prevalence of FBH and identify the associated factors in a non-diabetic population.
The present study also had several limitations. First, the assessment of hypog
We have shown that FBH occurs even in non-diabetic people, albeit very infrequently. FBH appears to be robustly associated with low body mass and smoking. Women and men in their 60s and 70s were more likely to experience FBH, and the relationships between FBH and other factors also differed between men and women.
The clinical relevance and the prevalence of fasting biochemical hypoglycemia (FBH) are poorly understood in a general population without diabetes.
FBH can be influenced by many factors, including age, sex, body mass, smoking, alcohol drinking, exercise levels, medications, and eating behaviors.
We determined the prevalence of FBH and investigated its association with potential risk factors in a population who did not have diabetes.
In a cross-sectional study of 695613 people aged 40-74 years who had undergone a health check-up, clinical parameters and lifestyle-related factors were reviewed. FBH was defined as a fasting plasma glucose < 70 mg/dL (3.9 mmol/L) after overnight fasting, regardless of any symptoms.
The prevalence of FBH was very low (0.26%) in this study. A body mass index of ≤ 20.9 kg/m2 and current smoking were significantly associated with FBH, which was not altered by adjustment for age, sex, and pharmacotherapy for hypertension or dyslipidemia. When the data were analyzed according to sex, men in their 60s or 70s appeared more likely to experience FBH compared with their 40s, whereas men in their 50s and women aged ≥ 50 years appeared less likely to experience FBH.
FBH was observed even in non-diabetic people, albeit at a very low frequency. FBH is robustly associated with low body mass and smoking, and its relationship with lifestyle factors varies according to sex.
To identify causal relationships between FBH and relevant factors (underweight, smoking, men in their 60s and so on), a prospective, large-scale study of non-diabetic people should be conducted in the future.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ng SM S-Editor: Zhang H L-Editor: A P-Editor: Wang LL
| 1. | Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Shafiee G, Mohajeri-Tehrani M, Pajouhi M, Larijani B. The importance of hypoglycemia in diabetic patients. J Diabetes Metab Disord. 2012;11:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Silbert R, Salcido-Montenegro A, Rodriguez-Gutierrez R, Katabi A, McCoy RG. Hypoglycemia Among Patients with Type 2 Diabetes: Epidemiology, Risk Factors, and Prevention Strategies. Curr Diab Rep. 2018;18:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Pratiwi C, Mokoagow MI, Made Kshanti IA, Soewondo P. The risk factors of inpatient hypoglycemia: A systematic review. Heliyon. 2020;6:e03913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Nirantharakumar K, Marshall T, Hodson J, Narendran P, Deeks J, Coleman JJ, Ferner RE. Hypoglycemia in non-diabetic in-patients: clinical or criminal? PLoS One. 2012;7:e40384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Sako A, Yasunaga H, Matsui H, Fushimi K, Hamasaki H, Katsuyama H, Tsujimoto T, Goto A, Yanai H. Hospitalization with hypoglycemia in patients without diabetes mellitus: A retrospective study using a national inpatient database in Japan, 2008-2012. Medicine (Baltimore). 2017;96:e7271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Mongraw-Chaffin M, Beavers DP, McClain DA. Hypoglycemic symptoms in the absence of diabetes: Pilot evidence of clinical hypoglycemia in young women. J Clin Transl Endocrinol. 2019;18:100202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Nakajima K, Iwane T, Higuchi R, Shibata M, Takada K, Uda J, Anan M, Sugiyama M, Nakamura T. Kanagawa Investigation of the Total Check-up Data from the National database (KITCHEN): protocol for data-driven population-based repeated cross-sectional and 6-year cohort studies. BMJ Open. 2019;9:e023323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Conceptual Diagram. Provision of Medical-Related Data to a Third Party. [cited 7 January 2021]. Available from: https://www.mhlw.go.jp/content/12401000/000402528.pdf. |
| 10. | Rozance PJ, Hay WW Jr. Describing hypoglycemia--definition or operational threshold? Early Hum Dev. 2010;86:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Nakajima K, Higuchi R, Mizusawa K. Unexpectedly High Prevalence of Breakfast Skipping in Low Body-Weight Middle-Aged Men: Results of the Kanagawa Investigation of Total Checkup Data from the National Data Base-7 (KITCHEN-7). Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 12. | Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Saito T, Ohmura H, Nojiri S, Daida H. Impact of sitagliptin combination therapy and hypoglycemia in Japanese patients with type 2 diabetes: a multi-center retrospective observational cohort study. J Pharm Health Care Sci. 2020;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Sasaki M, Mogi T, Wada Y, Hirosawa I, Koizumi A. An endemic condition of biochemical hypoglycemia among male volunteers. Ind Health. 1996;34:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Cheng PC, Hsu SR, Tu ST, Cheng YC, Liu YH. Body mass index influences the plasma glucose concentration during iatrogenic hypoglycemia in people with type 2 diabetes mellitus: a cross-sectional study. PeerJ. 2018;6:e4348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 16. | Han K, Yun JS, Park YM, Ahn YB, Cho JH, Cha SA, Ko SH. Development and validation of a risk prediction model for severe hypoglycemia in adult patients with type 2 diabetes: a nationwide population-based cohort study. Clin Epidemiol. 2018;10:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Hirai FE, Moss SE, Klein BE, Klein R. Severe hypoglycemia and smoking in a long-term type 1 diabetic population: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 2007;30:1437-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Szwarcbard N, Villani M, Earnest A, Flack J, Andrikopoulos S, Wischer N, Soldatos G, Gasevic D, Zoungas S. The association of smoking status with glycemic control, metabolic profile and diabetic complications- Results of the Australian National Diabetes Audit (ANDA). J Diabetes Complications. 2020;34:107626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Eckert-Norton M, Kirk S. Non-diabetic hypoglycemia. J Clin Endocrinol Metab. 2013;98:39A-40A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Altuntaş Y. Postprandial Reactive Hypoglycemia. Sisli Etfal Hastan Tip Bul. 2019;53:215-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 22. | van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18:68-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Frizzell RT, Campbell PJ, Cherrington AD. Gluconeogenesis and hypoglycemia. Diabetes Metab Rev. 1988;4:51-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Anno T, Kaneto H, Shigemoto R, Kawasaki F, Kawai Y, Urata N, Kawamoto H, Kaku K, Okimoto N. Hypoinsulinemic hypoglycemia triggered by liver injury in elderly subjects with low body weight: case reports. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Gosmanov AR, Gosmanova EO, Kovesdy CP. Evaluation and management of diabetic and non-diabetic hypoglycemia in end-stage renal disease. Nephrol Dial Transplant. 2016;31:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Brussaard HE, Gevers Leuven JA, Frölich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia. 1997;40:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, Han G, Newell-Fugate A, Tian Y, Majeti R, Liu W, Xu Y, Wu C, Allred K, Allred C, Sun Y, Guo S. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes. 2019;68:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 28. | Podolin DA, Gleeson TT, Mazzeo RS. Role of norepinephrine in hepatic gluconeogenesis: evidence of aging and training effects. Am J Physiol. 1994;267:E680-E686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Kim K, Cho SC, Cova A, Jang IS, Park SC. Alterations of epinephrine-induced gluconeogenesis in aging. Exp Mol Med. 2009;41:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kubota M, Virkamäki A, Yki-Järvinen H. Ethanol stimulates glycogenolysis in livers from fed rats. Proc Soc Exp Biol Med. 1992;201:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | van de Wiel A. Diabetes mellitus and alcohol. Diabetes Metab Res Rev. 2004;20:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Sumida KD, Hill JM, Matveyenko AV. Sex differences in hepatic gluconeogenic capacity after chronic alcohol consumption. Clin Med Res. 2007;5:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Mannucci E, Monami M, Mannucci M, Chiasserini V, Nicoletti P, Gabbani L, Giglioli L, Masotti G, Marchionni N. Incidence and prognostic significance of hypoglycemia in hospitalized non-diabetic elderly patients. Aging Clin Exp Res. 2006;18:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Noma Y, Komatsu M, Miya K, Shima K. Cognitive dysfunction during hypoglycemia in an elderly subject without diabetes. Diabetol Int. 2020;11:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Tsujimoto T, Yamamoto-Honda R, Kajio H, Kishimoto M, Noto H, Hachiya R, Kimura A, Kakei M, Noda M. High risk of abnormal QT prolongation in the early morning in diabetic and non-diabetic patients with severe hypoglycemia. Ann Med. 2015;47:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Klonoff DC, Parkes JL, Kovatchev BP, Kerr D, Bevier WC, Brazg RL, Christiansen M, Bailey TS, Nichols JH, Kohn MA. Investigation of the Accuracy of 18 Marketed Blood Glucose Monitors. Diabetes Care. 2018;41:1681-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | King F, Ahn D, Hsiao V, Porco T, Klonoff DC. A Review of Blood Glucose Monitor Accuracy. Diabetes Technol Ther. 2018;20:843-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |