Published online Jun 15, 2021. doi: 10.4239/wjd.v12.i6.794
Peer-review started: January 14, 2021
First decision: February 12, 2021
Revised: February 28, 2021
Accepted: May 27, 2021
Article in press: May 27, 2021
Published online: June 15, 2021
Processing time: 140 Days and 20.1 Hours
This article is an extensive review that provides an update on the pathophy
Core Tip: Diabetic gastroenteropathy is a common complication, poorly diagnosed in patients with long-term disease. These can present esophageal, gastric, intestinal, and even anorectal symptoms. Gastrointestinal Symptom Severity Index, Gastroparesis Cardinal Symptom Index, and Assessment of Constipation Quality of Life scores, as well as symptomatic assessment scales, contribute to the diagnosis. Apart from gastric emptying scintigraphy, currently, the use of endoscopic capsules has allowed the evaluation of abnormal transit. Jejunal fluid aspiration and culture allow assessment of bacterial overgrowth. Medical treatment, as well as adequate glycemic control, improve the symptoms, and delay the progression of the disease; in selected patients, pyloroplasty and gastric electrical stimulation are useful.
- Citation: Concepción Zavaleta MJ, Gonzáles Yovera JG, Moreno Marreros DM, Rafael Robles LDP, Palomino Taype KR, Soto Gálvez KN, Arriola Torres LF, Coronado Arroyo JC, Concepción Urteaga LA. Diabetic gastroenteropathy: An underdiagnosed complication . World J Diabetes 2021; 12(6): 794-809
- URL: https://www.wjgnet.com/1948-9358/full/v12/i6/794.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i6.794
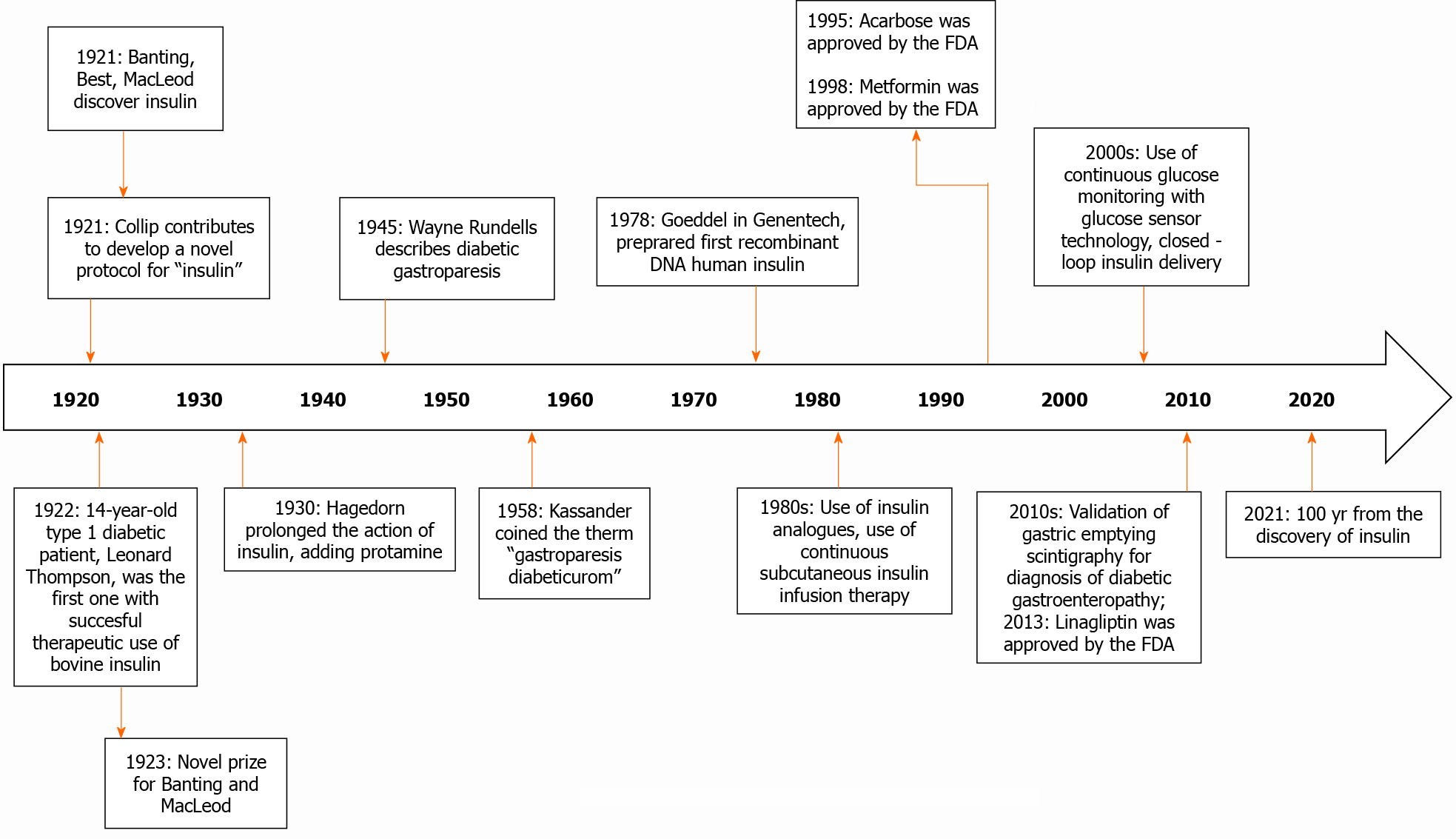
Diabetic gastroparesis was first described in 1945 by Wayne Rundells, later in 1958, Kassander coined the term gastroparesis diabeticorum[1]. This is one of the most common complications in patients with long-term type 1 and type 2 diabetes mellitus, therefore almost one quarter of this population describes gastrointestinal symptoms; manifesting itself especially in those who evolve with inadequate glycemic control or with other complications at the same time. It is mainly characterized by presenting early satiety, prolonged postprandial fullness, abdominal distention, nausea and vomiting, and abdominal pain[2]. The pathophysiology of diabetic gastroenteropathy is complex. It is postulated that the enteric nervous system neuropathy induced by hyperglycemia is one of the main causes. There is also a loss of Cajal's interstitial cells and enteric glial cells. On the other hand, oxidative stress and inflammation also affect regenerative processes and signaling[3]. As part of the treatment in some patients is used insulin, which in this 2020 are 100 years since it was discover (see Figure 1)[4,5], and around 40 years before the term gastroparesis diabeticorum was coined.
Even though, this diabetic complication has been described five decades ago, diagnosis and management still represent a challenge for the physicians. The present review focuses on the pathophysiology development, clinical, diagnosis, and brings new data about current and future treatment of this pathology. The aim of this review is an update on different treatments, including, changes in lifestyle, medical, and also surgical.
Approximately 463 million people live with diabetes worldwide, and 32 million residing in South and Central America[6].
The prevalence of diabetic gastroenteropathy has not been established. Some reports mentioned a cumulative incidence of diabetic gastroparesis at 10 years of 5.2% for patients with type 1 diabetes mellitus and 1% for patients with type 2 diabetes mellitus[7,8]. However, due to the continuous increase in the number of patients with type 2 diabetes, these diabetic patients would represent a bigger group with this complication[9].
Regarding the symptoms, the prevalence of esophageal dysmotility is 63%, reflux is 41%, 60% of patients present constipation, and 20% manifest diarrhea[10]. These differences in prevalence probably respond to the lack of recognition of the signs and symptoms of gastroenteropathy, making it necessary to learn to recognize and treat them in time.
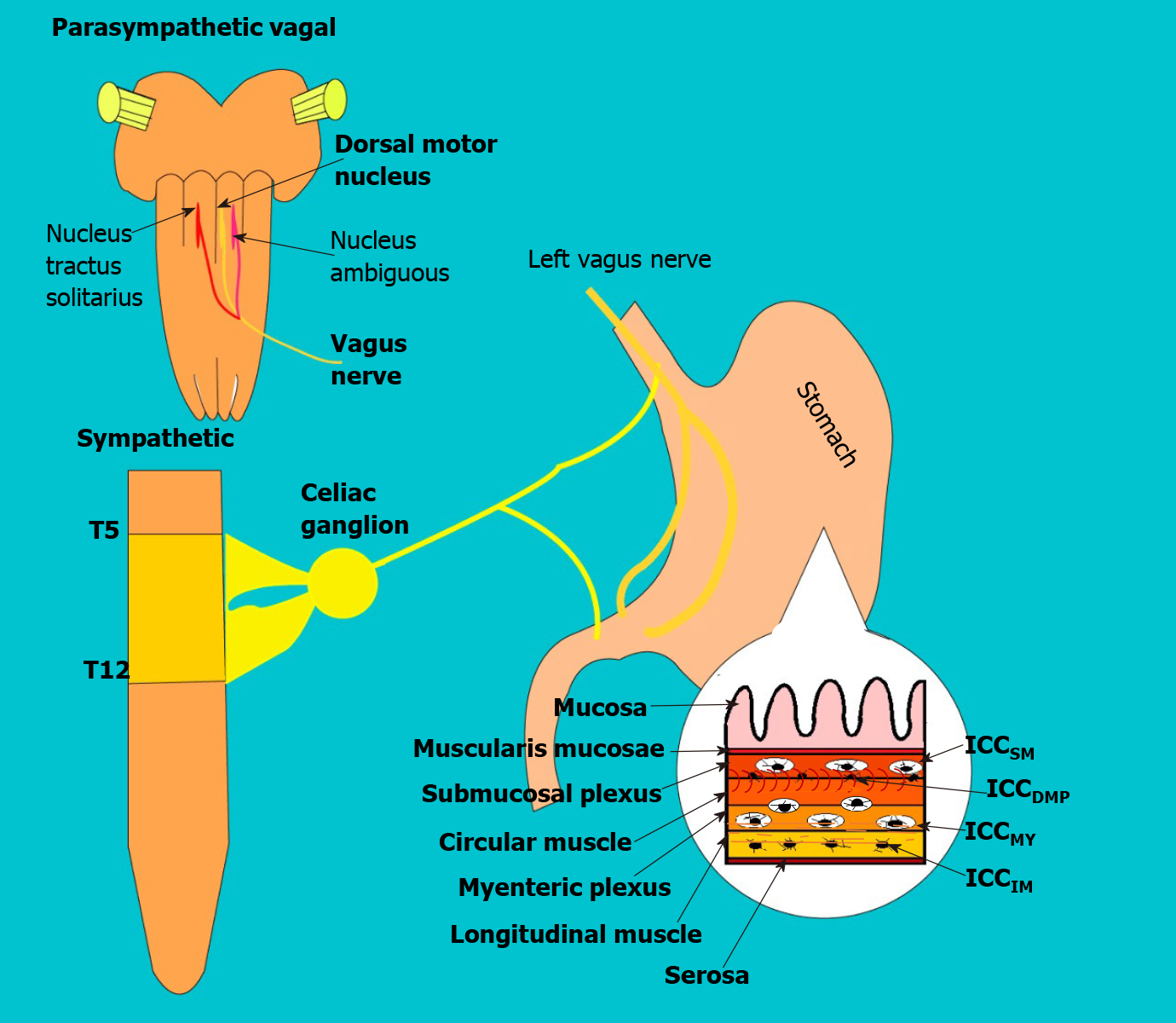
Gastric emptying is a physiological process that allows the transit of digested food to the duodenum[2]. It is a mechanical process and is regulated by a complex neurohormonal control[11,12]. In neuronal control, the parasympathetic participates through the vagus nerve which afferences fibers arrive from the enteric system to the nucleus of the solitary tract, and then pass to the vagus dorsal motor nucleus in the spinal cord, from where it exits to the myenteric plexus (see Figure 2). In the gastric wall forming two vague motor circuits: Excitatory and inhibitory, which are distributed in a heterogeneous way (allowing an integrated function in gastric biomechanics) in the body, antrum, pylorus, and into the interstitial cells of the Cajal[11]. Additional, there are several neurotransmitters, that participate in neurohumoral control at different levels (e.g., Acetylcholine, Noradrenaline, GABA, dopamine, etc.), and hormones produced in the pancreas, stomach, small intestine, and in the nervous system (Some delay gastric emptying such as cholecystokinin, GLP- 1, and leptin; and others accelerate it such as motilin and ghrelin)[11].
The speed of emptying occurs differently according to the consistency and nature of the food, thus low-calorie liquids leave the stomach very quickly[11-13]. The most solid foods stay between 2 to 3 h in the stomach (because they are transformed into smaller particles to form the acid chyme and pass into the duodenum at an average speed of 1-4 Kcal/min)[14]. However, emptying is not always complete and exists an interdigestive period in which food particles pass without been digested. Recently, with advances in non-invasive studies, such as scintigraphy, the physiology of gastric emptying has been better understood[11]. The adequate gastric emptying biomechanics is the result of adequate coordination between the proximal and distal regions of the stomach[13]. The gastric fundus serves as a reservoir of content (initially, it relaxes due to nitrogen stimuli)[2,15] and then acts as a pressure pump. In the gastric body-antrum, the food content is mixed at a speed of 3 peristaltic waves per minute[12,13,16], the pylorus acts as a gate that filters only the 1-2 mm digested food and returns the largest foods[17]. In gastric emptying, 2 phases are recognized; the digestive (whose purpose is to transport the chyme to the duodenum) and the interdigestive (which several trains of peristaltic waves have the purpose of complete the gastric emptying of the indigestible particles); the hormones that accelerate emptying participate in this latter process[11].
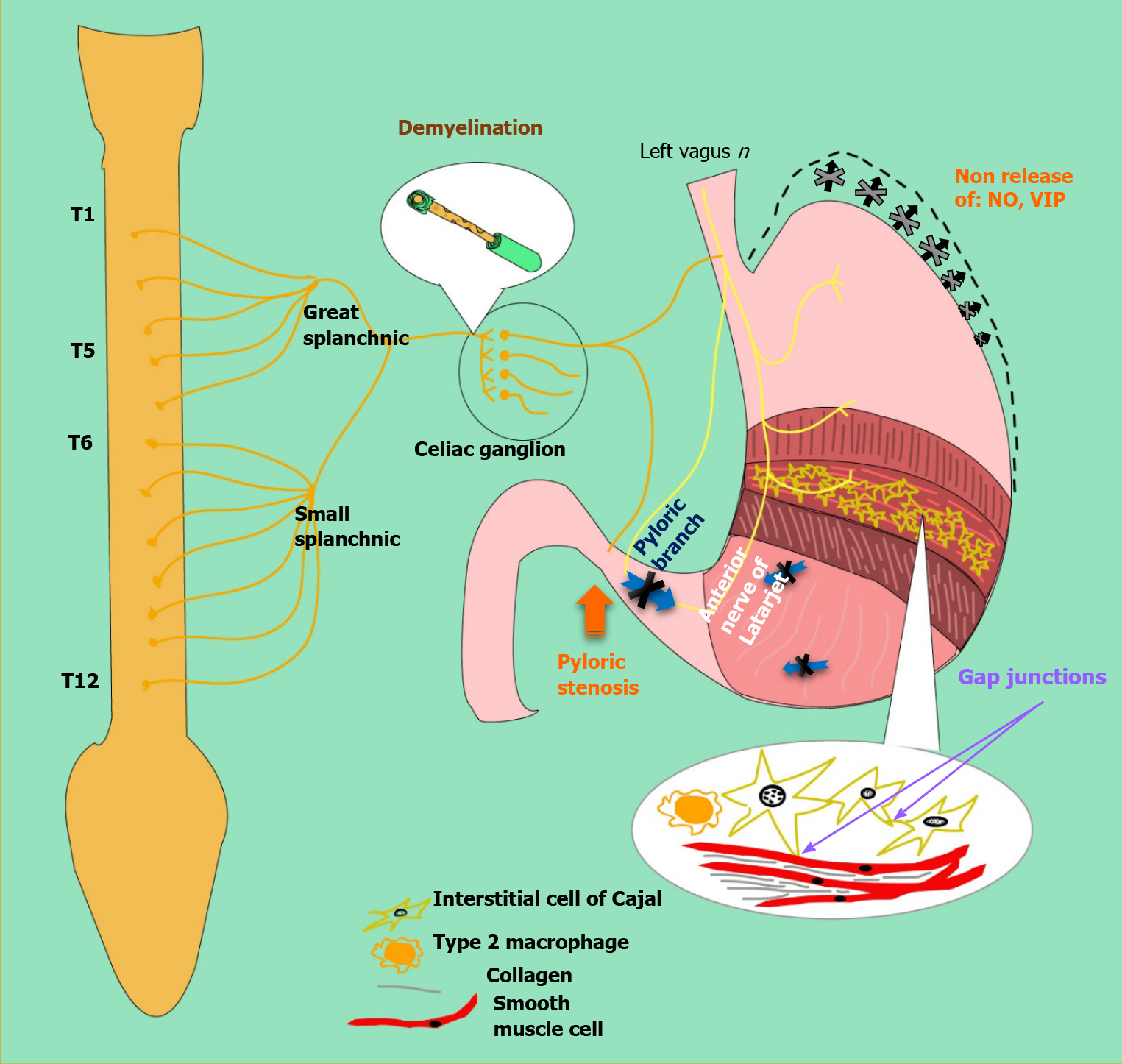
Gastroparesis is defined as delayed gastric emptying in the absence of mechanical obstruction. Although the exact mechanism of gastric dysfunction and the generation of symptoms are unknown, there are factors that promote its development, such as hyperglycemia, vagal dysfunction, loss of expression of neural nitric oxide synthase (nNOS) in the myenteric plexus, abnormalities of the Cajal interstitial cell network (ICC) and oxidative stress[17] (see Figure 3).
An acute increase twice or decrease (halve) in blood glucose can cause delayed or accelerated gastric emptying, respectively[14,18]. Additionally, the alterations in gastric emptying can produce fluctuations in glycemia, which then affects the gastric emptying rate, thus creating a vicious cycle. In the hyperglycemic state, pyloric contractions and antral hypomotility occur, leading to delayed emptying. On the other hand, hypoglycemia stimulates the vagus nerve[17].
Likewise, vagal dysfunction plays a role in diabetic gastroparesis. When food is ingested and gastric accommodation is disturbed, patients may experience symptoms such as early satiety, fullness, and discomfort. Vagal neuropathy can lead to reduced pyloric relaxation, impaired antral contraction, and impaired antropyloric coordination [19,20].
Alterations of the enteric nervous system also play an important role. The myenteric plexus contains a network of nerves found in layers between the longitudinal and circular intestinal muscular and coordinates gastric motor function. This myenteric plexus is made up of excitatory (cholinergic), inhibitory (nitrergic) motor neurons, primary afferent neurons, and interneurons[19]. Excitatory motor neurons induce muscle contractions by releasing neurotransmitters such as acetylcholine and substance P while, inhibitory neurons will relax muscle tissue by releasing nitric oxide, ATP, and vasoactive intestinal peptide[15]. Pathological changes in these pathways affect motor control and lead to delayed emptying, impaired accommodation, and gastric dysrhythmia[17].
Non-obese diabetic (NOD) mice showed a reversible loss of gastric nNOS expression, suggesting that in diabetic patients may exist negative regulation of nNOS without loss of nitrate neurons[15]. A study in rats administered streptozotocin (STZ) to induce diabetes found a reversible loss of nNOS after 4-8 wk, which progressed to irreversible loss due to apoptosis induced by oxidative stress after 12 wk. Because the active nNOS enzyme is a dimerized protein, the loss of this dimerization can cause impaired neuromuscular function, as has been reported in the antrum of STZ-induced diabetic rats[21].
Loss of ICC has been reported in animal models and diabetic patients with gastroparesis. NOD mice and STZ-induced rats show a loss of ICC in both the body and the antrum[17]. The Gastroparesis Clinical Research Consortium in America collected data from patients with gastroparesis correlating cellular changes in surgical full-thickness gastric biopsies with the patient's symptoms[22]. A decrease in ICC and the gastric emptying rate was observed. In contrast to previous data in animals and humans, nNOS expression was not significantly decreased in diabetic patients. It should be noted that the full-thickness biopsies were taken from patients undergoing gastric neurostimulator placement, and therefore may represent a subgroup that is not representative of the general population with diabetic gastroparesis[22].
It is well known that diabetes induces a state of oxidative stress and contributes to the loss of nitrogen function. Increased oxidative stress in NOD mice due to loss of macrophage heme-oxygenase-1 (HO-1), which normally protects against free radicals in the enteric nervous system, was associated with loss of ICC inducing a delayed gastric emptying. The onset of delayed gastric emptying is associated with the loss of a subset of HO-1 macrophages. Induction of HO-1 reverses the delay in gastric emptying[2].
The prevalence of gastrointestinal discomfort in diabetic patients is higher than in the general population, reaching up to 70% higher in some community studies[23].
Due to the alteration in the mechanisms of motility and secretion in the gastrointestinal tract, diabetic enteropathy could affect any portion of the gastroin
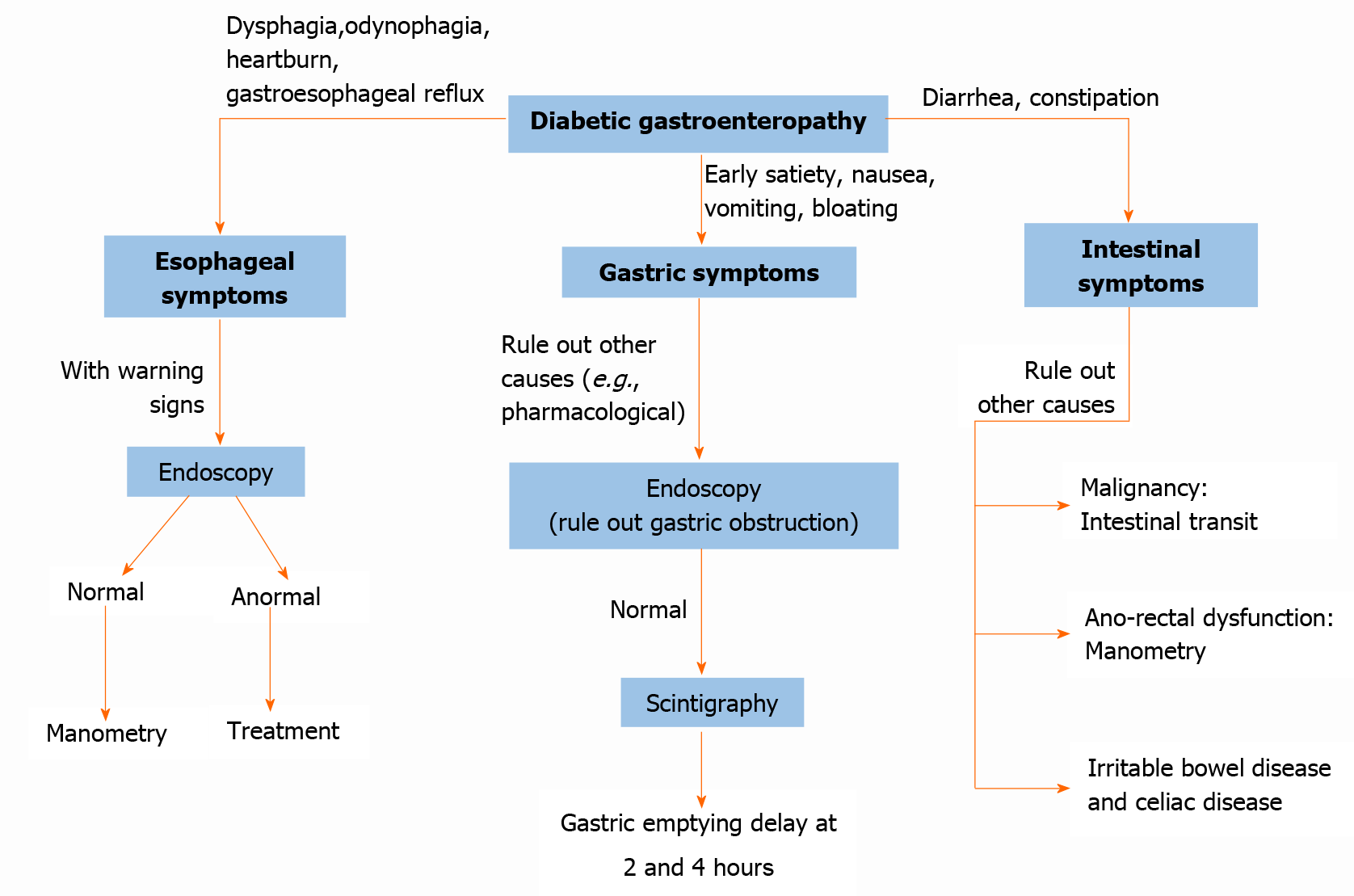
Esophageal motility disorders usually present with symptoms of gastroesophageal reflux or dysphagia (see Figure 4). However, it can also present as odynophagia, generally related to esophageal candidiasis[24].
In some cases, gastroesophageal reflux presents as a cough and worsening of respiratory parameters, delaying the diagnosis[25]. Heartburn is associated with gastroesophageal reflux in up to 41%[24].
Studies in patients with dysphagia showed that the symptoms are more frequent in women and white people. Also, about 45% of diabetic patients with dysphagia had esophageal motor abnormalities. When groups of patients with dysphagia were compared, diabetic patients had a higher percentage of smoking and body mass index than the group of non-diabetic patients with dysphagia. Manometry results indicated that patients with insulin treatment were associated with greater swallowing weakness, without significant association with glycosylated hemoglobin levels[25]. Acute hyperglycemic episodes are related to lower esophageal motility and greater dysphagia[12]. Besides, the presence of autonomic and peripheral neuropathy and retinopathy is associated with a higher frequency of erosive esophagitis compared to patients without neuropathy[26].
Clinical symptoms of gastroparesis include nausea, vomiting, early satiety, postpran
The cardinal symptoms of gastroparesis usually present in combination, not individually. Although the symptoms of idiopathic and diabetic gastroparesis are similar, vomiting and early satiety are more frequent in diabetic gastroparesis; whereas that abdominal pain is more frequent in idiopathic gastroparesis[27].
A meta-analysis of 92 studies demonstrated that delayed gastric emptying is associated with gastrointestinal symptoms such as nausea (OR = 1.6, 95%CI: 1.4-1.8), vomiting (OR = 2.0, 95%CI: 1.6-2.7), early satiety (OR = 1.8, 95%CI: 1.2-2.6) and not significant with abdominal pain (OR = 1.5, 95%CI: 1.0-2.2) with an OR = 2.0; however, the abdominal pain had a lower association[29]. In patients with documented gastroparesis, the association between symptoms and delayed gastric emptying is less clear, finding nausea as a symptom. In diabetic patients, only a strong association was found between early satiety and delayed gastric emptying. Nevertheless, the neuropathy can result in gastric motility alterations and decreased symptoms.
In patients with type 1 diabetes have been observed that women are more likely to have gastroparesis than men (5.8% vs 3.5% P < 0.001), besides, patients with gastroparesis usually were older. Patients with gastroparesis generally had a longer duration of diabetes, higher Hemoglobin A1c levels, and more frequent episodes of severe hypoglycemia[30], the influence of gender is also observed in patients with type 2 diabetes. The reason for the difference in the prevalence of diabetic gastroparesis between men and women is unknown; however, gastric motility is dependent on the neuronal synthesis of nitric oxide, uncoupling of nitric oxide synthase causes a decreased synthesis of NO, leading to a reduction in smooth muscle relaxion. These events need a cofactor that is diminished in diabetic female rats, impairs NOS activity and this mechanism is probably influenced by estrogens[28].
Among the risk factors, obesity in diabetics is associated with an increase in the risk of approximately ten times. It has been reported that 50% of patients with idiopathic gastroparesis are overweight or obese, and the symptoms differ with body mass index. Obese patients have fewer symptoms of loss of appetite or inability to finish a meal but have higher rates of gastroesophageal reflux[12].
In 25-years follow-up of diabetic patients with gastrointestinal symptoms, it has been observed that both the symptoms and gastric emptying are relatively stable over time[23].
The most common symptoms at the intestinal level are constipation, diarrhea, pain, and bloating. The frequency of chronic constipation is higher than chronic diarrhea in this group of patients (25% vs 5%)[27].
The NHANES study found that 25% of diabetics had gastrointestinal symptoms, but unlike previous studies, this one reported that only chronic diarrhea was more prevalent in diabetics than non-diabetics (11.2% vs 6.0%, P < 0.0001); However, the prevalence of chronic constipation there were no differences between diabetics and non-diabetics. Furthermore, diabetic patients with chronic diarrhea tended to use more hypoglycemic drugs, mainly metformin; and diabetics with chronic constipation had reduced kidney function. No significant relationship was found between intestinal symptoms and the presence of retinopathy, glycosylated hemoglobin levels, and duration of diabetes[31].
Diarrhea usually lasts more than 6 wk, watery, not associated with pain, not bloody, and its presentation related to the duration of diabetes is variable. It is more common in women and develops about 8 years after the diagnosis of diabetes. It occurs with a frequency interval of normal stools or even constipation, with a sudden increase in volume and frequency. Nocturnal diarrhea and fecal incontinence are two of the most distinctive findings of diabetic diarrhea[3].
Fecal incontinence occurs more frequently in diabetic patients it’s related to the duration of diabetes and the presence of microvascular complications. In diabetic patients, internal anal sphincter tone and anal contraction pressures are reduced[27].
The prevalence of fecal incontinence varies in a range of 7%-15%; however, it is often not voluntarily manifested by patients. Intestinal disorders such as diarrhea are an independent risk factor for fecal incontinence. Besides, smoking, obesity, advanced age, sedentary lifestyle, and female sex are also risk factors for fecal incontinence[8].
Due to the need to monitor gastrointestinal symptoms in patients, the use of questionnaires is preferred. These questionnaires have been changing in recent years; despite this, some studies related to treatment continue to use non-validated tools for gastrointestinal symptoms evaluation. Although the questionnaires are considered the standard for symptomatic evaluation, they are affected by recall bias, which is why these may not be optimal for monitoring changes in symptoms over time[28].
The questionnaires should specify the terms to be used, use an explicit language, evaluate all relevant symptoms, produce comparable results when evaluated in patients with stable symptoms, and detect clinically significant changes[32].
The most widely used questionnaires are the Gastrointestinal Symptom Severity Index (PAGI-SYM), Gastroparesis Cardinal Symptom Index (GCSI), Gastrointestinal Symptom Rating Scale (GSRS), Assessment of Constipation Symptom (PAC-SYM), and the Assessment of Constipation Quality of Life (PAC-QOL)[28].
PAGI-SYM assesses the severity of symptoms of the upper gastrointestinal tract. It contains 20 items and is divided into 6 sections: heartburn-regurgitation, postprandial fullness-early satiety, nausea-vomiting, abdominal distention, upper and lower abdominal pain. It's useful in the evaluation of gastroesophageal reflux, dyspepsia, and gastroparesis[24,28]. GCSI encompasses three scales that measure nausea-vomiting, postprandial fullness-early satiety, and abdominal distension. It's a 2-wk reminder, helps in assessing the severity of gastroparesis symptoms. To assess the response of gastroparesis to the treatment, the Gastroparesis Cardinal Symptom Index-Daily Diary (ANMS GCSI-DD) has been created[33].
GSRS contains 15 items combined into 5 groups: reflux, abdominal pain, indigestion, diarrhea, and constipation. It provides a broader perspective than the previous questionnaire; however, more research is still needed for an adequate correlation between the questionnaire scores and the objective measures of gastroin
PAC-SYM and PAC-QoL were developed to assess the severity of symptoms and the quality of life in patients with constipation. The first is composed of 12 items and three groups: abdominal, rectal symptoms, and stool characteristics. The M-PAC-SYM questionnaire has been developed for patients with chronic constipation and could be more useful for the evaluation of functional constipation related to diabetes[34].
The investigation of esophageal symptoms in diabetic patients is carried out in the same way as in non-diabetic patients. The typical symptoms of gastroesophageal reflux is sufficient for the diagnosis. Endoscopy and response to antisecretory therapy are not recommended as a diagnostic means. However, endoscopy shows its usefulness in the evaluation of complications in the mucosa, as well as in the detection of candidal esophagitis[23,31].
Esophageal motility disorders can be evaluated with manometry, and a video fluoroscopic swallowing exam. pH measurement studies can be useful in the evaluation of gastroesophageal reflux with or without impedance monitoring, which allows the evaluation of air and liquid transit (see Figure 4)[23].
Manometry would show delays in esophageal transit times and reduced pressure in the lower esophageal sphincter. Manometry plus pH measurement serves for a better assess esophageal motility[23,25].
Gastric emptying scintigraphy is the method of choice to evaluate gastroparesis. In this method, the patient eats a food radiolabelled with Technetium-99, after which gastric emptying is measured. It can also be used to measure the transit time of the intestine and colon. Indications for gastric emptying scintigraphy include insulin-dependent diabetes and post-prandial symptoms or diabetes with poor glycemic control, non-ulcer-associated dyspepsia, severe esophagitis caused by reflux, nausea, vomiting, weight loss, upper abdominal discomfort, new early satiety, and evaluate treatment response with prokinetic drugs. After the ingestion of radiolabeled foods, liquids diffuse rapidly through the stomach, while solids are mainly concentrated in the fundus[35]. Normal results are gastric residual less than 60% at 2 h and less than 10% at 4 h. Higher values indicate gastroparesis. Gastric retention of more than 60% at 2 h has a sensitivity of 100% and specificity of 20%. Gastric retention of more than 10% at 4 h has a sensitivity of 100% and specificity of 70% for gastroparesis[36].
In the breath test, the non-radioactive isotope C13 is bound to a digestible substance, generally octanoic acid; which is mixed with solid food, absorbed in the proximal small intestine, with subsequent metabolization in the liver towards C13-C02, which is measurable in exhalation. The breath test has a sensitivity of 89% and a specificity of 80%. Compared to scintigraphy, the test is easier to perform and does not use radiation; however, concomitant diseases such as celiac disease affect profitability[28]. This test is carried out over a period of 4 h after 8-h fasting. Breath samples are collected before meals and collect breath samples every 30 min (see Figure 4)[37].
The wireless capsule continuously measures pressure, pH, and temperature as it moves through the gastrointestinal tract. The test involves a standardized meal and subsequent ingestion of the capsule. The data from the capsule is transmitted to a receiving unit. With this method, it has been observed that around 44% of type 1 diabetic patients with some degree of sensory-motor neuropathy had abnormal transit in one or more segments, independent of the presence of gastrointestinal symptoms[38]. The change in pH across the segments may represent fermentation in the cecal region that may influence colonic transit times. The test is limited to specialized centers and has high costs. It has only 52.5% concordant results with scintigraphy, so in patients with suspected gastroparesis additional investigations will be necessary[28,37].
Other methods are the radiopaque markers (capsules containing plastic elements), which are ingested by the patient and followed through the gastrointestinal tract using plain abdominal radiographs. It is a widely available and useful test to detect significant variations in the time of intestinal transit and gastric emptying. Like the test mentioned above, a normal test does not exclude delayed gastric emptying, so additional tests are necessary[28].
The aforementioned tests should be performed when the presence of mechanical obstruction that could produce symptoms similar to gastroparesis has been ruled out[37].
Due to one of the fundamental causes of gastrointestinal symptoms is an intestinal bacterial overgrowth, his diagnostic standard is the aspiration and culture of jejunal fluid. It requires the use of endoscopy and a high probability of external contamination and false-negative results. Breath tests may help, but they do not have adequate sensitivity[23,28,37].
In constipation, we can use anorectal manometry for the evaluation of defecation disorder. The measurement of intestinal transit using the tests described above may be justified too. A thorough investigation should be conducted to rule out malignancy (see Figure 4)[25].
Medication used by patients should be considered as a possible cause of diarrhea and constipation. Furthermore, due to the higher prevalence of the celiac disease in diabetic patients than in the general population, screening using serological tests is justified[23,25].
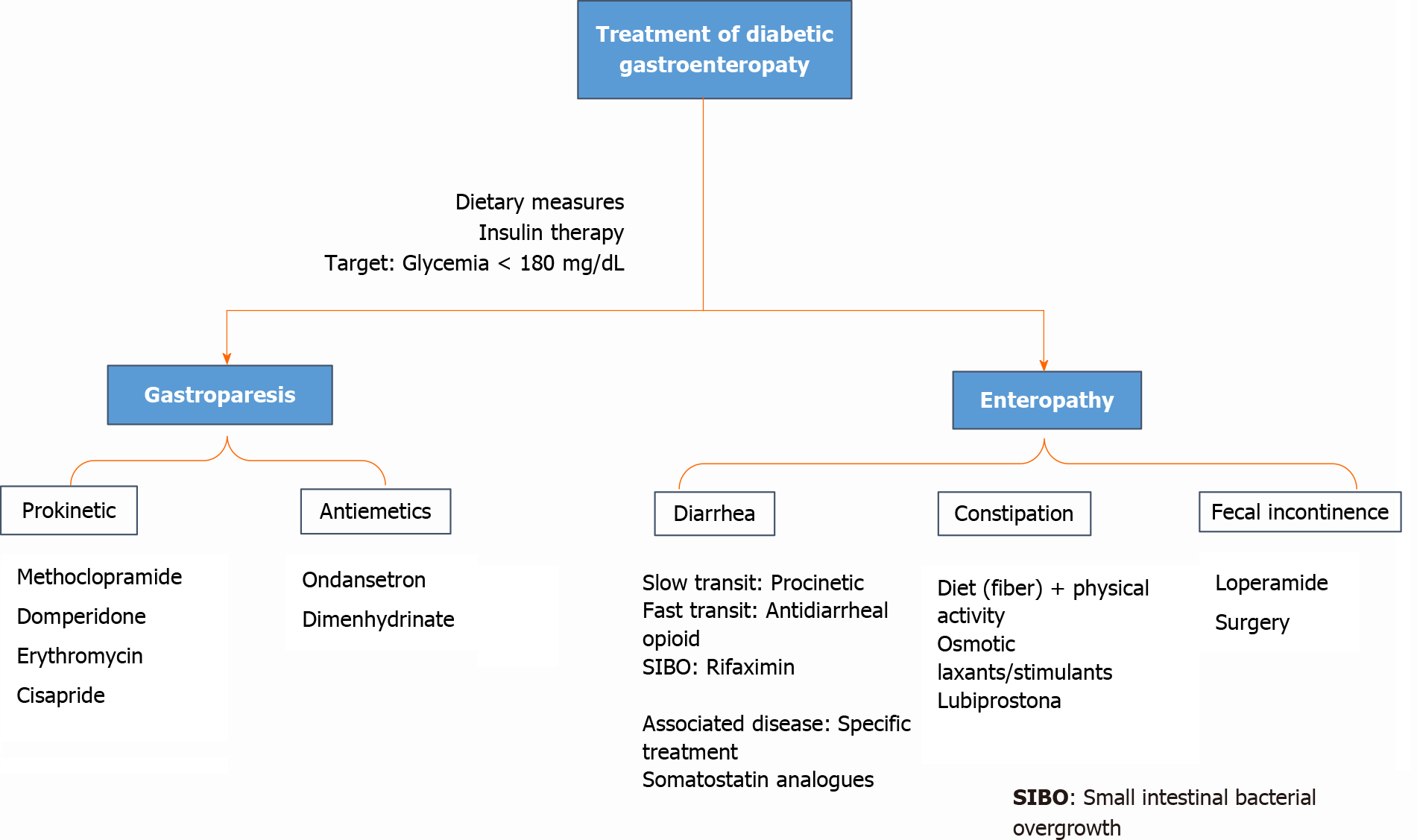
There is currently no cure for diabetic gastroenteropathy. Therefore, the goals of treatment are to delay his progression of the disease, relieve symptoms, control complications, and restore function. The management of diabetic gastroenteropathy is multidisciplinary. It requires the participation of multiple specialists such as the gastroenterologist, endocrinologist, nutritionist, psychologist, interventional radiologist, and surgeon[23].
In a didactic way, we have divided the management of gastroparesis and diabetic enteropathy, taking into account that both forms of the disease can coexist.
Most patients tend to have a mild-moderate disease and therefore respond to nutritional recommendations, dietary modifications, and adequate glycemic control, as well as prokinetic agents or antiemetic medications (with variable efficacy)[39] if there is no response to the initial measurements[3,40]. A small percentage of patients have severe disease characterized by inadequate oral tolerance, chronic malnutrition, weight loss, and frequent hospitalizations. Representing management as a therapeutic challenge[3].
On the other hand, it is also important to manage the comorbidities that usually occur in patients with diabetic gastropareses, such as gastroesophageal reflux, intestinal dysmotility, deficiency of vitamin D, and other micronutrients, bacterial and fungal infections of the gastrointestinal tract, and macrovascular-microvascular complications of Diabetes Mellitus[41,42].
Dietary modifications constitute the first-line management for gastroparesis, such as a decrease in particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis, even though their efficacy has not been clearly established[43,44].
Frequently, patients with diabetic gastroparesis tend to have a lower caloric intake than recommended, as well as significant micronutrient and macronutrient deficiencies[45]. A diet based on a low content of simple sugars and foods rich in fiber, as proposed by the American Diabetes Association (ADA) for diabetic patients[46], is not usually useful for patients with diabetic gastroparesis[3].
Measures to promote gastric emptying or do not delay it is recommended[3]. That is why the consumption of fats, fibers, and carbonated liquids, which should delay gastric emptying, should be minimized[37]. A recommended and staggered approach is to start with liquids with high nutritional value, followed by soups and shakes, and later to introduce solid foods that do not delay gastric emptying[3]. Low-fat foods should be encouraged 4-5 times per day. Patients should be instructed to drink fluids with meals and sit or walk for 1-2 h after meals.
If all these nutritional recommendations are ineffective, the patient should be suggested to consume the total calories in liquids, soups, or shakes, since fluid emptying is frequently preserved[42].
It is necessary to emphasize that all these dietary changes require education to the patients and their families. It has been found that the prevalence of nutritional consultations in diabetic patients with gastroparesis is less than 40%[45].
It is vitally important to optimize glycemic control to minimize the acute symptoms of diabetic gastroparesis and to improve gastric emptying[3,44]. Random serum to avoid inhibition of gastric myoelectric motility and control[3].
The treatment used to achieve adequate glycemic control must be individualized[3,44]. Oral antidiabetics are not recommended for patients with type 2 diabetes with clinically significant diabetic gastroparesis. The pharmacokinetics of these drugs are affected by delayed gastric emptying; therefore, agents are not ideal for effective glycemic control[3,44].
Likewise, the adverse effects of antidiabetic medications also play an important role, such as gastrointestinal intolerance frequently reported with metformin, hypoglycemia caused by sulfonylureas, diarrhea and abdominal distension due to alpha-glucosidase inhibitors, inconsistent effect inhibitors of dipeptidyl peptidase-4 on gastric emptying, and an unclear impact of SGLT-2 inhibitors. In relation to injectable therapy, GLP-1 agonists can exacerbate symptoms of delayed gastric emptying[3,44].
Patients with diabetic gastroparesis by type 1 diabetes and most of the patients with type 2 diabetes will require insulin for glycemic control[47]. It is recommended to administer prandial insulin after meals to prevent postprandial hypoglycemia if food is not fully consumed. Multiple intakes of small foods, aggressive glucose monitoring, and frequent small doses of rapid-acting insulins are recommended to prevent postprandial hyperglycemia[3]. Currently, the recommended treatment for glycemic control in patients with diabetic gastroparesis who receive insulin is continuous subcutaneous insulin infusion, based on optimizing glycemic control and reducing hospitalizations[48]; however, we must not forget the economic cost that this usually entails. The use of premixed insulins is not recommended in this group of patients[9].
The drugs most used in the treatment of diabetic gastroparesis usually include prokinetics and antiemetics[3]. Several novel targeted therapies are still under investigation (see Figure 5).
Prokinetics: Metoclopramide is a D-2 receptor antagonist with antiemetic and prokinetic effects that increase antral contractions and coordinate antral and duodenal motility. The maximum daily dose is 40 mg/d. It can be used parenterally when symptoms are severe. Among the adverse effects is an increase in serum prolactin concentration. Gynecomastia and galactorrhea can occur in adults, adolescents, and children; while, adult women can develop oligomenorrhea. Likewise, it can stimulate aldosterone synthesis and cause uncontrolled hypertension in patients with primary hyperaldosteronism, and it can also prolong the QT interval in susceptible patients[3]. It is the only drug approved by the United States Food and Drug Administration (FDA) for the management of gastroparesis; however, in February 2009, the FDA and the European Medicines Agency established black box warnings for long-term use (more than 12 wk) of metoclopramide due to the risk of irreversible tardive dyskinesia, which has limited its use[40]. Domperidone is another dopamine 2 receptor antagonist, identical to metoclopramide, with equal efficacy to the latter, but with fewer adverse effects on the central nervous system; because it does not cross the blood-brain barrier[49]. Daily doses between 10-30 mg, administered 30 min before meals and at bedtime, have been found to reduce gastrointestinal upset and hospitalizations due to gastroparesis.
Erythromycin is a macrolide with an agonist effect on motilin receptors in the gastrointestinal tract. It increases gastric emptying in a dose-dependent manner. It has been shown to stimulate gastric emptying in patients with diabetic gastroparesis. Daily dose 50-100 mg administered 3 times a day, in combination with low-volume diets can be effective for controlling gastroparesis. Cases of QT interval prolongation have also been found[11,3].
Cisapride is a potent prokinetic agent that, acting in the stomach through 5-hydroxytryptamine receptors, accelerates gastric emptying from solid foods and improves dyspeptic symptoms[3].
There are future prokinetic drugs in development, among which motilin agonists, ghrelin agonists, and new type 4 5-hydroxytryptamine receptor agonists[11,3].
Antiemetics: Both nausea and vomiting are often the most disabling symptoms in patients with diabetic gastroparesis. Antiemetic drugs can be serotonin antagonists such as ondansetron, at doses of 4-8 mg twice a day, or type 1 histamine receptor antagonists, such as dimenhydrinate at a dose of 50 mg 4 times a day. Both classes of drugs are often used alone or in combination with prokinetic agents[3].
Botulinum toxin, a potent inhibitor of neuromuscular transmission, has been postulated to improve gastric emptying and symptoms for several months; however, double-blind randomized studies have shown that improves gastric emptying, it does not improve symptoms[50].
Likewise, there has been an interest in the pylorus role in delayed gastric emptying, which is why some authors propose gastric peroral endoscopic myotomy (G-POEM) as a treatment modality in refractory patients, be it surgical or endoscopic. P. Mekaroonkamol reported 03 cases of gastroparesis refractory to conventional treatment, of varied etiology (e.g., postinfectious, postsurgical, or idiopathic) that progressed favorably after undergoing G-POEM as salvage therapy[51].
For the select group of patients with severe and refractory disease, gastric electrical stimulation may be an option. It has been shown to improve nausea, vomiting, quality of life, and nutritional status after sixth month of treatment[52,53].
A significant number of patients fail medical management and require surgical treatment. The main role of surgery is the relief of symptoms, decompression of the stomach, access to enteral nutrition, and stimulating gastric emptying[3].
The alteration of the intestine by diabetes mellitus has a variety of presentations: chronic diarrhea (involvement of the small intestine), constipation when it affects the colon, and also fecal incontinence (see Figure 4). All of them are explained by the multisystemic nature of the disease (autonomic neuropathy, infectious involvement, and autoimmune disease when in the presence of type 1 diabetes)[40,51,54].
The prevalence of chronic diarrhea in diabetic patients ranges from 3.7% to 22%[54]; compared to the general population, they have around twice the risk of having diarrhea (11% vs 6%)[33]. An important part of the management is to assess the hydration status and electrolyte imbalance that management would need. As in gastroparesis, the goal is to achieve good glycemic control and diet management. If these initial measures fail, drug therapy with opioid group antidiarrheals is an option they can be administered with caution due to their toxicity: megacolon and the potential worsening of bacterial overgrowth[12].
For small intestinal bacterial overgrowth, antibiotic therapy should be started. Rifaximin is the best research-based agent for this disorder works selectively on the gastrointestinal tract, lowers resistance, and improves symptoms in 33% to 99% of patients[55]. Somatostatin analogs like octreotide and lanreotide also improve symptoms[10]. Another common cause of diarrhea in diabetic patients is the use of drugs such as metformin, which reduces the ileal absorption of bile salts, artificial sweeteners through an osmotic mechanism (seen mainly with sorbitol intake greater than 20 g), it is split by the intestinal flora that produces hydrogen and short-chain fatty acids, which are the cause of diarrhea associated with this substance[56].
Nevertheless, there is no specific treatment for chronic diarrhea in diabetic patients. If a slow transit is targeted, the option would be prokinetics, while if the symptoms are in favor of rapid transit, the choice would be opiates. The use of somatostatin analogs that inhibit water secretion, increase absorptive capacity, and suppress the release of hormones with gastrointestinal action; is indicated in the failure of conventional therapies[56].
When constipation is the main complaint, good hydration, high-fiber meals, and routine physical activities are the first recommendations to make. Randomized placebo-controlled clinical trials are showing that the intake of natural psyllium (10 g twice a day) or flaxseed (10 g twice a day) reduces constipation symptoms and improves glycemic control in people with diabetes type 2. There are no studies specifically investigating the effects of laxatives in people with constipation as a complication due to diabetic gastroenteropathy[57,58]. Treatment focuses on its symptomatic management, with the use of a diet that promotes softening of the stool, and the use of laxatives that increase intestinal transit.
Although without much solid evidence, we can suggest starting using osmotic laxatives such as polyethylene glycol; if insufficient, stimulant laxatives such as bisacodyl or picosulfate can be added. Lubiprostone, a chloride channel activator, increases secretion from the colon, reducing colonic transit time and increasing the number of spontaneous bowel movements in people with diabetes-related consti
In the management of fecal incontinence, which is often aggravated by diarrhea, a priority is identifying an underlying cause of diarrhea and addressing it. Often improves on its own within good glycemic control[10]. Otherwise, a dietary interven
Neuromodulatory electrical stimulation of the sacral nerve is an emerging technique of treatment for fecal incontinence and possible sensitivity in the anal canal. However, it has not been specifically investigated in people with diabetic gastroenteropathy[60].
Diabetes mellitus is a chronic disease with a rising prevalence worldwide, as are its complications, including gastroenteropathy. Its pathophysiology integrates hyperglycemia, vagal dysfunction, loss of expression of neural nitric oxide synthase in the myenteric plexus, alterations in the interstitial cell network of Cajal, and oxidative stress. The clinical features are gastroesophageal reflux, gastroparesis, constipation, abdominal pain, and diarrhea. Among the diagnostic studies, manometry together with pH measurement (evaluating esophageal motility), gastric emptying scintigraphy, breath test (evaluating gastroparesis), aspiration, and jejunal culture (evaluating bacterial overgrowth) stand out. There is no definitive treatment for diabetic gastroenteropathy- The multidisciplinary approach is considered to seek to slow the progression of the disease, alleviate symptoms, and restore function. A diet low in simple sugars and high in fiber is recommended; optimize glycemic control, with a target glycemia of less than 180 mg/dL. Regarding drug therapy, prokinetics and antiemetics are included, and if bacterial overgrowth occurs, antibiotic treatment with Rifaximin. Current evidence is accumulating among the new approaches, including the use of botulinum toxin, pyroloplasty, and electrical gastric stimulation in selected patients. Although there are new techniques for diagnosis, but it does not appear a definitive cure in the near future, remaining a concern, well designed clinical trials are needed in this field.
The research team appreciates the contributions, suggestions, and corrections made by Dr. Julio Hilario Vargas, to publish this manuscript.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and Metabolism
Country/Territory of origin: Peru
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aureliano M, Zhang DM S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Kumar M, Chapman A, Javed S, Alam U, Malik RA, Azmi S. The Investigation and Treatment of Diabetic Gastroparesis. Clin Ther. 2018;40:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Borgoño CA, Zinman B. Insulins: past, present, and future. Endocrinol Metab Clin North Am. 2012;41:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Quianzon CC, Cheikh I. History of insulin. J Community Hosp Intern Med Perspect. 2012;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Shen S, Xu J, Lamm V, Vachaparambil CT, Chen H, Cai Q. Diabetic Gastroparesis and Nondiabetic Gastroparesis. Gastrointest Endosc Clin N Am. 2019;29:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Krishnasamy S, Abell TL. Diabetic Gastroparesis: Principles and Current Trends in Management. Diabetes Ther. 2018;9:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5863] [Article Influence: 977.2] [Reference Citation Analysis (8)] |
| 7. | Jung HK, Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 8. | Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Koch KL, Calles-Escandón J. Diabetic gastroparesis. Gastroenterol Clin North Am. 2015;44:39-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Kurniawan AH, Suwandi BH, Kholili U. Diabetic Gastroenteropathy: A Complication of Diabetes Mellitus. Acta Med Indones. 2019;51:263-271. [PubMed] |
| 11. | Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil. 2019;31:e13546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 12. | Sullivan A, Temperley L, Ruban A. Pathophysiology, Aetiology and Treatment of Gastroparesis. Dig Dis Sci. 2020;65:1615-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Kuo P, Rayner CK, Jones KL, Horowitz M. Pathophysiology and management of diabetic gastropathy: a guide for endocrinologists. Drugs. 2007;67:1671-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Schvarcz E, Palmér M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, Ferris CD. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Stevens JE, Jones KL, Rayner CK, Horowitz M. Pathophysiology and pharmacotherapy of gastroparesis: current and future perspectives. Expert Opin Pharmacother. 2013;14:1171-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis. Br Med Bull. 2013;105:213-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Fraser RJ, Horowitz M, Maddox AF, Harding PE, Chatterton BE, Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 341] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Meldgaard T, Keller J, Olesen AE, Olesen SS, Krogh K, Borre M, Farmer A, Brock B, Brock C, Drewes AM. Pathophysiology and management of diabetic gastroenteropathy. Therap Adv Gastroenterol. 2019;12:1756284819852047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Nguyen LA, Snape WJ Jr. Clinical presentation and pathophysiology of gastroparesis. Gastroenterol Clin North Am. 2015;44:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725-G733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Grover M, Bernard CE, Pasricha PJ, Lurken MS, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, McCallum RW, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Ünalp-Arida A, Hamilton FA, Farrugia G; NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531-539, e249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal Symptoms in Diabetes: Prevalence, Assessment, Pathogenesis, and Management. Diabetes Care. 2018;41:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Chedid V, Brandler J, Vijayvargiya P, Park SY, Szarka LA, Camilleri M. Characterization of Upper Gastrointestinal Symptoms, Gastric Motor Functions, and Associations in Patients with Diabetes at a Referral Center. Am J Gastroenterol. 2019;114:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Iyer PG, Borah BJ, Heien HC, Das A, Cooper GS, Chak A. Association of Barrett's esophagus with type II Diabetes Mellitus: results from a large population-based case-control study. Clin Gastroenterol Hepatol 2013; 11: 1108-1114. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | George NS, Rangan V, Geng Z, Khan F, Kichler A, Gabbard S, Ganocy S, Fass R. Distribution of Esophageal Motor Disorders in Diabetic Patients With Dysphagia. J Clin Gastroenterol. 2017;51:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Marathe CS, Rayner CK, Wu T, Jones KL, Horowitz M, Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP. Gastrointestinal Disorders in Diabetes 2000. [PubMed] |
| 28. | Meldgaard T, Olesen SS, Farmer AD, Krogh K, Wendel AA, Brock B, Drewes AM, Brock C. Diabetic Enteropathy: From Molecule to Mechanism-Based Treatment. J Diabetes Res. 2018;2018:3827301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Vijayvargiya P, Jameie-Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2019;68:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 30. | Aleppo G, Calhoun P, Foster NC, Maahs DM, Shah VN, Miller KM; T1D Exchange Clinic Network. Reported gastroparesis in adults with type 1 diabetes (T1D) from the T1D Exchange clinic registry. J Diabetes Complications. 2017;31:1669-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Sommers T, Mitsuhashi S, Singh P, Hirsch W, Katon J, Ballou S, Rangan V, Cheng V, Friedlander D, Iturrino J, Lembo A, Nee J. Prevalence of Chronic Constipation and Chronic Diarrhea in Diabetic Individuals in the United States. Am J Gastroenterol. 2019;114:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Mouli VP, Ahuja V. Questionnaire based gastroesophageal reflux disease (GERD) assessment scales. Indian J Gastroenterol. 2011;30:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Revicki DA, Camilleri M, Kuo B, Szarka LA, McCormack J, Parkman HP. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463, e215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Neri L, Conway PM, Basilisco G; Laxative Inadequate Relief Survey (LIRS) Group. Confirmatory factor analysis of the Patient Assessment of Constipation-Symptoms (PAC-SYM) among patients with chronic constipation. Qual Life Res. 2015;24:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Alipour Z, Khatib F, Tabib SM, Javadi H, Jafari E, Aghaghazvini L, Mahmoud-Pashazadeh A, Nabipour I, Assadi M. Assessment of the Prevalence of Diabetic Gastroparesis and Validation of Gastric Emptying Scintigraphy for Diagnosis. Mol Imaging Radionucl Ther. 2017;26:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Farrell MB. Gastric Emptying Scintigraphy. J Nucl Med Technol. 2019;47:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 38. | Farmer AD, Pedersen AG, Brock B, Jakobsen PE, Karmisholt J, Mohammed SD, Scott SM, Drewes AM, Brock C. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia. 2017;60:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Asha MZ, Khalil SFH. Pharmacological Approaches to Diabetic Gastroparesis: A systematic review of randomised clinical trials. Sultan Qaboos Univ Med J. 2019;19:e291-e304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Careyva B, Stello B. Diabetes Mellitus: Management of Gastrointestinal Complications. Am Fam Physician. 2016;94:980-986. [PubMed] |
| 41. | Kedar A, Nikitina Y, Henry OR, Abell KB, Vedanarayanan V, Griswold ME, Subramony C, Abell TL. Gastric dysmotility and low serum vitamin D levels in patients with gastroparesis. Horm Metab Res. 2013;45:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Ajumobi AB, Griffin RA. Diabetic gastroparesis: evaluation and management. Hosp Physician. 2008;44:27-35. [DOI] [Full Text] |
| 43. | Olausson EA, Störsrud S, Grundin H, Isaksson M, Attvall S, Simrén M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Jalleh R, Marathe CS, Rayner CK, Jones KL, Horowitz M. Diabetic Gastroparesis and Glycaemic Control. Curr Diab Rep. 2019;19:153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Parkman HP, Yates KP, Hasler WL, Nguyan L, Pasricha PJ, Snape WJ, Farrugia G, Calles J, Koch KL, Abell TL, McCallum RW, Petito D, Parrish CR, Duffy F, Lee L, Unalp-Arida A, Tonascia J, Hamilton F; NIDDK Gastroparesis Clinical Research Consortium. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011; 141: 486-498, 498.e1-498. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S32-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 47. | Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 48. | Sharma D, Morrison G, Joseph F, Purewal TS, Weston PJ. The role of continuous subcutaneous insulin infusion therapy in patients with diabetic gastroparesis. Diabetologia. 2011;54:2768-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Patterson D, Abell T, Rothstein R, Koch K, Barnett J. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Lacy BE, Crowell MD, Schettler-Duncan A, Mathis C, Pasricha PJ. The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care. 2004;27:2341-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Mekaroonkamol P, Li LY, Dacha S, Xu Y, Keilin SD, Willingham FF, Cai Q. Gastric peroral endoscopic pyloromyotomy (G-POEM) as a salvage therapy for refractory gastroparesis: a case series of different subtypes. Neurogastroenterol Motil. 2016;28:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 53. | Abell TL, Johnson WD, Kedar A, Runnels JM, Thompson J, Weeks ES, Minocha A, Griswold ME. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc 2011; 74: 496-503. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Lysy J, Israeli E, Goldin E. The prevalence of chronic diarrhea among diabetic patients. Am J Gastroenterol. 1999;94:2165-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Pimentel M. Review of rifaximin as treatment for SIBO and IBS. Expert Opin Investig Drugs. 2009;18:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Ebert EC. Gastrointestinal complications of diabetes mellitus. Dis Mon. 2005;51:620-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Noureddin S, Mohsen J, Payman A. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Complement Ther Med. 2018;40:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Soltanian N, Janghorbani M. A randomized trial of the effects of flaxseed to manage constipation, weight, glycemia, and lipids in constipated patients with type 2 diabetes. Nutr Metab (Lond). 2018;15:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Christie J, Shroff S, Shahnavaz N, Carter LA, Harrison MS, Dietz-Lindo KA, Hanfelt J, Srinivasan S. A Randomized, Double-Blind, Placebo-Controlled Trial to Examine the Effectiveness of Lubiprostone on Constipation Symptoms and Colon Transit Time in Diabetic Patients. Am J Gastroenterol. 2017;112:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Haas S, Brock C, Krogh K, Gram M, Lundby L, Drewes AM, Laurberg S. Does Sacral Nerve Stimulation Improve Continence Through Enhanced Sensitivity of the Anal Canal? Dis Colon Rectum. 2016;59:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |