Published online Jun 15, 2021. doi: 10.4239/wjd.v12.i6.745
Peer-review started: February 13, 2021
First decision: March 16, 2021
Revised: March 20, 2021
Accepted: May 21, 2021
Article in press: May 21, 2021
Published online: June 15, 2021
Processing time: 111 Days and 9.9 Hours
At present, Alzheimer’s disease (AD) and type 2 diabetes mellitus (T2DM) are two highly prevalent disorders worldwide, especially among elderly individuals. T2DM appears to be associated with cognitive dysfunction, with a higher risk of developing neurocognitive disorders, including AD. These diseases have been observed to share various pathophysiological mechanisms, including alterations in insulin signaling, defects in glucose transporters (GLUTs), and mitochondrial dysfunctions in the brain. Therefore, the aim of this review is to summarize the current knowledge regarding the molecular mechanisms implicated in the association of these pathologies as well as recent therapeutic alternatives. In this context, the hyperphosphorylation of tau and the formation of neurofibrillary tangles have been associated with the dysfunction of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in the nervous tissues as well as the decrease in the expression of GLUT-1 and GLUT-3 in the different areas of the brain, increase in reactive oxygen species, and production of mitochondrial alterations that occur in T2DM. These findings have contributed to the implementation of overlapping pharmacological interventions based on the use of insulin and antidiabetic drugs, or, more recently, azeliragon, amylin, among others, which have shown possible beneficial effects in diabetic patients diagnosed with AD.
Core Tip: Alzheimer’s disease and type 2 diabetes mellitus are highly prevalent chronic diseases that have shown a significant association. Important pathways have shown to be involved in this relationship, including the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways, among others. This has led to the develop
- Citation: Rojas M, Chávez-Castillo M, Bautista J, Ortega Á, Nava M, Salazar J, Díaz-Camargo E, Medina O, Rojas-Quintero J, Bermúdez V. Alzheimer’s disease and type 2 diabetes mellitus: Pathophysiologic and pharmacotherapeutics links. World J Diabetes 2021; 12(6): 745-766
- URL: https://www.wjgnet.com/1948-9358/full/v12/i6/745.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i6.745
Alzheimer’s disease (AD) and type 2 diabetes mellitus (T2DM) are highly prevalent diseases in elderly patients[1,2]. According to the International Diabetes Federation, 1 in 11 people had DM in 2019, amounting to approximately 463 million cases worldwide, of which 90% is attributed to T2DM. These alarming numbers have quadrupled since 1990, with a prevalence of 9.3% in adults[3-5]. Moreover, numerous complications have been associated with this disorder, among which are renal disease[6], retinopathy[7], dermopathy[8], peripheral vasculopathy[9], and cognitive alterations[10]. The latter have been investigated recently, due to the prominent link between T2DM and any type of neurocognitive disorder, especially AD.
As a progressive, degenerative, and irreversible disorder, AD is characterized by neuronal loss, the formation of senile plaques composed of extracellular deposits of amyloid beta, and the presence of neurofibrillary tangles (NT) in neuronal microtubules [11]. Recent studies have demonstrated a higher incidence of AD in patients with T2DM in comparison to those without comorbidities. This epidemiologic association has spawned various hypothetical pathophysiological links between both diseases[12]. These studies focus on the role of insulin as a necessary hormone for glucose metabolism in the central nervous system (CNS), with receptors widely localized in the hippocampus, frontal area, and entorhinal cortex, which are all structures involved in memory and learning[13].
Insulin resistance (IR) is a key alteration of this hormone’s signaling and is the pathogenic cornerstone of T2DM, where it promotes a chronic state of hyperinsulinemia and hyperglycemia[14]. IR has been associated with the disruption of amyloid protein metabolism in the brain, tau phosphorylation in microtubules[15], and the formation of reactive oxygen species (ROS) in the mitochondria. Thus, these conditions may contribute to the pathogenesis and development of AD.
Therefore, the aim of this narrative review is to summarize the current knowledge on the molecular mechanisms linking T2DM with the development of AD, along with possible emerging therapeutic strategies.
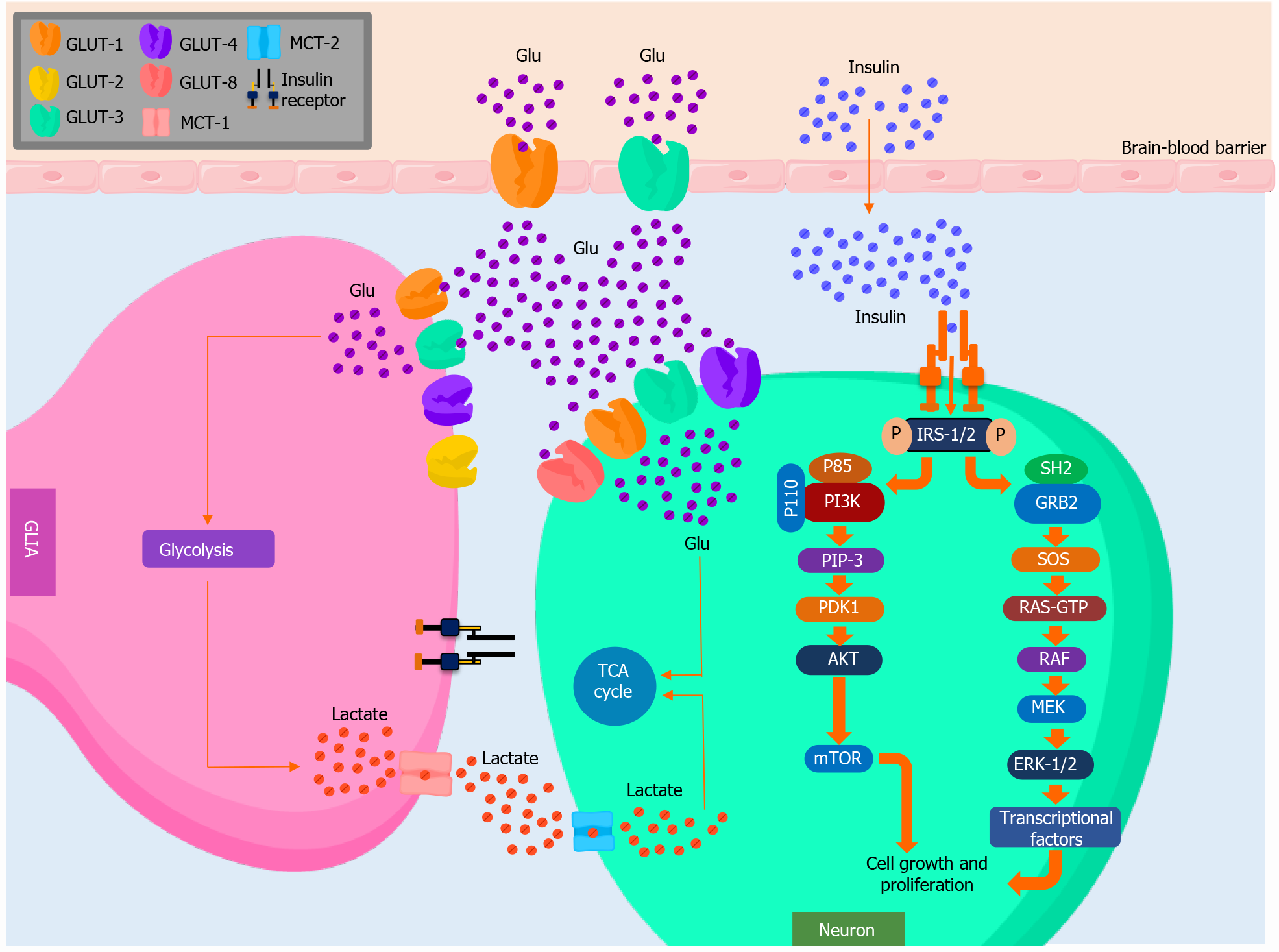
Of all organs, the brain requires the largest energetic demand[16] (Figure 1). Under physiological conditions, a constant supply of glucose, which is the main substrate, is necessary for a normal metabolic functionality. Glucose traffic to the brain is regulated in the endothelial wall of the capillaries by the blood-brain barrier (BBB), a structure composed of two semipermeable membranes, one luminal and one abluminal[17]. Both are aligned next to endothelial cells, regulating the transportation of hydrophilic metabolic substrates, such as carbohydrates and amino acids, in the CNS[18].
Glucose is a polar and hydrophilic molecule, preventing its diffusion through the blood capillaries. Thus, it needs a system of specific transporters[16]. Glucose trans
GLUT-1 and GLUT-3 isoforms display the highest affinity for glucose. Therefore, their presence in tissues that exclusively depend on glucose for their metabolic require
The GLUT-1 gene is located in the 1p35.31.3 chromosome, which is widely expressed in numerous tissues, and is considered the main GLUT of the BBB[16]. It is 3-4 times more abundant in the luminal membrane than the abluminal membrane and mediates the transport of glucose from the blood to astrocytes. GLUT-1 isoforms vary on molecular weight. 55-kD GLUT-1 is expressed in the endothelial cells of the brain blood vessels, whereas 45-kD GLUT-1 is mainly expressed in astrocytes. GLUT-1 is highly hydrophobic, and its high glucose affinity (Km = 1-2 mmol/L) allows it to transport glucose into cells at virtually any concentration[17], with selectivity for D-glucose. Therefore, it is thought to act as a basal GLUT that maintains stable glucose levels in the CNS[20].
GLUT-3 works in harmony with GLUT-1, allowing the vectorial transport of glucose to neurons[16]. It is a high-affinity GLUT (Km = 1-2 mmol/L) found primarily in the brain, although low levels of GLUT-3 have been detected in the myocardium, placenta, liver, and muscle. Regarding its kinetic properties, it appears to alternate between the intake and release of glucose[21]. Current hypotheses suggest the facilitated transport of glucose involves conformational changes in the tertiary structure of the transporter. This is triggered by the presence of glucose in its binding site on the extracellular portion of the transporter and its progressive movement to the intracellular portion of it, where another binding site is found[19].
The expression of GLUT in the nervous system varies across different cells. Astrocytes express different isoforms of the GLUT family, including GLUT-1, GLUT-2, and GLUT-4. These cells cover 99% of the BBB and it is through these GLUT isoforms that glucose is able to cross this barrier[18]. Meanwhile, neurons express GLUT-3, GLUT-4, and GLUT-8. Despite the expression of these GLUT isoforms, it has been investigated whether astrocytes, which take glucose and release lactate, are a necessary mediator between glucose and neurons or, as the conventional hypothesis proposes, neurons receive glucose directly from the interstitial fluid of the brain in aerobic conditions[21]. Through this process, glucose would enter the glycolytic pathway and the tricarboxylic acid pathway for its later oxidation, which provides the cell with the necessary energy to maintain cellular function[17]. The aforementioned glucose transportation mechanism can become the limiting step in certain situations, including the development of hypoglycemia and other conditions, such as AD, mainly limiting blood flow, BBB permeability, and changes in GLUT-1 expression[18].
Insulin is a peptide hormone necessary for maintaining glucose homeostasis[22]. In the brain, it has important neuroprotective and neuromodulatory functions, such as regulating its growth, repairing dendritic cells and neurons, and having anorexigenic effects on the hypothalamus, among other effects[23]. It is chiefly synthesized in the pancreas[24], although the presence of insulin mRNA in neurons suggests that it may be locally produced in the nervous tissues, especially in the hypothalamus, cerebral cortex, olfactory bulb, substantia nigra, and pituitary gland[16]. Insulin may also intervene in learning and memory brain functions, especially verbal memory, as evidence shows this hormone modulates the secretion of neurotransmitters, such as acetylcholine, and favors synaptic plasticity[22].
Once in the plasma, insulin can cross the BBB via active transportation mediated by its receptor, which is abundantly expressed in neurons and, in lower quantities, in glial cells[22]. After binding to its receptor, insulin promotes the autophosphorylation of tyrosine residues, triggering its intrinsic tyrosine kinase activity and phosphorylating the insulin receptor substrate (IRS) coupling protein in the tyrosine residue[24]. The majority of this response is coupled to IRS-1 and IRS-2, which are ubiquitously expressed and the main mediators of insulin-dependent mitogenesis, and the regulation of glucose metabolism in the majority of cell types[21]. Historically, IRS-1 was the first insulin substrate to be identified and represents the prototype of IRS family proteins, whereas IRS-2 is mainly involved in the regulation of brain growth. The phosphorylation of tyrosine residues on IRS activates Akt, which phosphorylates substrates, such as the mammalian target of rapamycin (mTOR) and the glycogen synthase kinase-3 (GSK3), among other targets[25]. Insulin also activates the extracellular signal receptor kinase (ERK) pathway by activating type 1 and type 2 ERKs[24]. These molecules can modify the expression of certain genes (c-fos, Elk-1) involved in cell growth and differentiation[22].
AD is a neurogenerative disorder that results in a gradual and irreversible deterioration of memory and other cognitive functions. It can also be frequently accom
Physiologically, neuronal cells release soluble amyloid beta, which is a peptide with a molecular weight of 4 kD and a length of 42-43 amino acids. The main types of amyloid (Aβ40 and Aβ42) emerge as a product of the normal secretion of the transmembrane amyloid protein precursor after a proteolytic process that requires the participation of secretases (α β γ). α-Secretase acts on the amyloid beta peptide, promoting its breakdown in two segments, which are nexin II and soluble amyloid beta peptide, which has 16 amino acids[31].
Afterward, the α-2-macroglobulin acts forming the BA-A2M complex, which will couple to a protease enzyme to reenter the neuron[32]. During this process, the secretases cleave the BA peptide from 40 to 42 amino acids. Such enzymes include the beta secretase (acting on amino acid 1) and gamma secretase (40-42 activity). The accumulation in the interstitial tissue of insoluble 1-42 beta amyloid fragments goes through various transformations in relation to its protein structure until it acquires a folded shape that is difficult to break down. Furthermore, other stable proteins are associated with this process, such as the serum amyloid P component[33]. The presence of these structures leads to the activation of the immune system, especially the phagocytic cells of the CNS (microglia), which perpetuate the lesion due to pseudoinflammation and the release of ROS. However, recent studies have suggested that the participation of amyloid beta is attributed to its deposit on the brain blood vessels, which leads to degeneration and hemorrhages, which are important events in the physiopathology of AD[34].
Tau is a protein that is highly associated with neuronal microtubules[35]. Through its isoforms and phosphorylation, tau protein interacts with tubulin to stabilize the structure of neuronal microtubules, allowing for an efficient synaptic activity[36]. The tau hypothesis indicates that an excessive or abnormal phosphorylation of this protein results in the transformation to a paired helical filament conformation (PHF-tau). This leads to its precipitation and autoaggregation, which slows the axonal transport and causes neurodegeneration due to possible apoptosis[37]. NT can be intracellular and extracellular. Intracellular NT are hyperphosphorylated and usually found in abundance in the neuritic component of the neuritic plaque. Meanwhile, extracellular NT are the result of neuronal death and the denomination of the insoluble fibrillar skeleton and is characterized by insoluble neurofibrillary components that are difficult to proteolyze. These persist even after neuronal death as remains in the extracellular medium[38].
General glucose metabolism involves various intracellular processes, including glycolysis, the Krebs cycle, and oxidative phosphorylation. Likewise, it requires extracellular factors, such as its transportation from the circulation to the intracellular environment, in which insulin has a key regulating role[12]. T2DM is associated with the progressive loss of sensitivity to this hormone in a growing IR state, which is also present in AD. This outlines a possible overlap in the pathogenesis of both condi
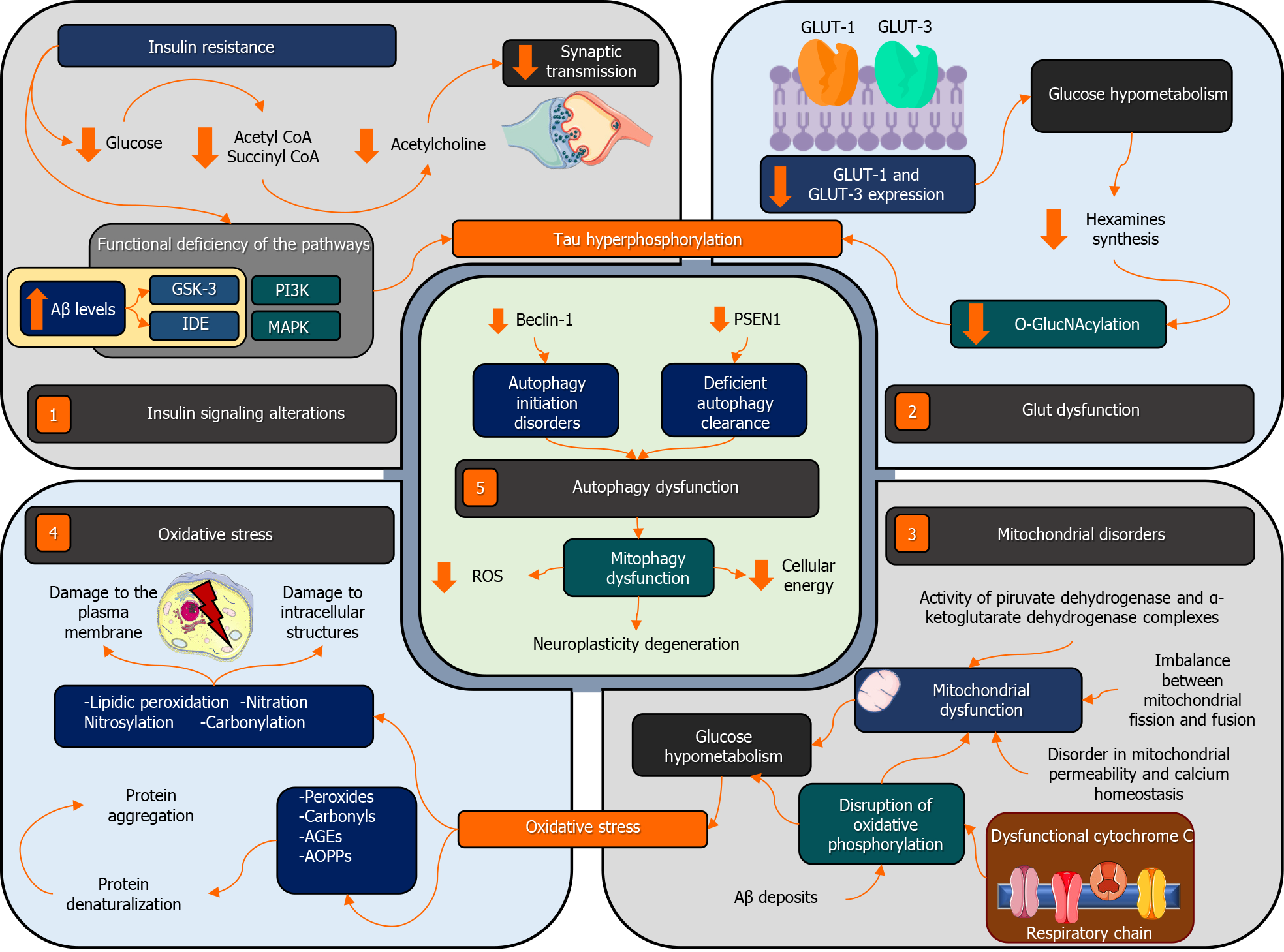
T2DM has been associated with changes in cognition and cognitive dysfunction, reporting a higher risk of developing any type of neurocognitive disorder, including AD[40]. Indeed, various clinical and preclinical studies suggest that these disorders may share multiple biochemical characteristics and signaling pathways[41] (Figure 2).
Different studies have demonstrated the association between IR and AD, even in the absence of hyperglycemia or DM. In this sense, it has been found that neurocognitive functions dependent on insulin in patients with sporadic AD could play an important role in the physiopathology of this disease, causing disruptions of insulin signaling in the brains of these individuals[42]. Similarly, a second study examining AD patients non-homozygous for the APOE-ε4 allele reported the presence of fasting insulinemia and a lower concentration of insulin in the cerebrospinal fluid (CSF), which shows decreased brain insulin uptake[43]. These findings together with those reported by other studies[44,45], suggest that insulin signaling disruption can be particularly important in the physiopathology of AD in those individuals who are not homozygous for the APOE-ε4 allele.
More recent studies point out that due to its effects on neurodegeneration, brain glucose metabolism, and cognitive performance, peripheral IR could be associated with the pathophysiology of AD in pre-diabetic[46] and non-diabetic patients[47,48]. A study that included 130 non-diabetic patients with AD reported that IR was independently associated with decreased glucose metabolism in the hippocampus and with a lower volume of gray matter[47]. Likewise, a second study reported that high serum insulin levels were significantly associated with severe AD presentations. This significance persisted when non-diabetic patients were excluded[48]. In fact, different studies have shown a decrease in AD incidence[49] and improvement of cognitive performance and brain insulin metabolism in patients with AD that have been treated with insulin or insulin-sensitizing drugs. This provides further evidence of the role of IR in the pathogenesis of AD[15,50,51].
The terms “type 3 diabetes mellitus” or “brain insulin resistance” have been coined to describe the dysfunction of insulin signaling seen in AD[52]. IR decreases the availability of glucose needed for neuronal synaptic transmissions due to the deficiency of certain metabolites produced in the glycolytic pathway, such as coenzyme A and succinyl coenzyme A. These are key precursors for the synthesis of acetylcholine, the main neurotransmitter related to cognition[53]. Similarly, it has been proposed that IR induces a series of changes in molecular mechanisms that promote the synthesis and degradation of the amyloid beta peptide and the hyperphosphorylation of the tau protein[54].
Dysfunction in insulin signaling mainly affects the efficiency of the phosphatidylinositol 3-kinase (PI3K). Studies have reported that the brains of patients suffering from AD and T2DM have decreased levels of PI3K, which leads to nervous tissue degene
In regard to GLUTs, clinical studies have revealed that part of the brain hypometabolism in individuals with AD and T2DM may be attributed to a decrease in the expression of GLUT-1 and GLUT-3 in the different areas of the brain[59,60]. Possible causes appear to be post-translational, as no changes have been found in the GLUT-1 ARNm levels in the cerebral cortex[61]. Alterations in glucose transport lead to a reduction in its metabolism, which is associated with the down-regulation of the hexosamine biosynthesis pathway. This involves a decrease in the O-glycosylation of the Ser/Thr residues of tau protein by the β-N-acetylglucosamine (or O-GlcNA cylation), which leads to its abnormal hyperphosphorylation and the formation of NT, contributing to the progression to AD[62].
Moreover, reduced neuronal levels of GLUT-3 have also been identified in patients with T2DM[63]. Likewise, this decrease in the O-GlcNAcylation and hyperphosphorylation of tau has been correlated with decreased levels of GLUT-1 and GLUT-3 in the brain tissue samples of patients with AD[64]. Similarly, other research groups have found a decreased expression of GLUT-1 and/or GLUT-3 in the brain cortex[65] and the dentate gyrus of the hippocampus[66], which were related to the formation of NT. However, the decrease in the expression of these GLUTs, rather than being the main cause of the hypometabolism observed in patients with AD, is more likely to be the result of a decrease in energy demand[64]. Further studies are needed to confirm the direct link between the alterations of GLUTs and neurodegeneration.
The brain is particularly vulnerable to oxidative damage and mitochondrial dysfunction because of the neurons’ high metabolic rate, their dependence on mitochondria to obtain energy, and their low antioxidant defenses[67,68]. Numerous findings have indicated that mitochondrial dysfunction and oxidative stress are implicated in the physiopathology of AD and T2DM[69,70].
Regarding mitochondrial disorders, a decrease in the activity of the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes has been found in the brains of patients with AD. Both complexes are involved in the Krebs cycle, which can lead to mitochondrial dysfunction, glucose hypometabolism, and neuronal oxidative stress[71,72]. Similarly, brain hypometabolism in individuals with AD is related to a disruption in mitochondrial oxidative phosphorylation[73]. Cytochrome c oxidase is the complex with greater alterations in the respiratory chain in neurons from different cortical regions[74-76]. Moreover, mitochondrial dysfunction in AD is associated with an imbalance in mitochondrial fusion and fission, alterations in mitochondrial permeability, calcium homeostasis, and the release of proapoptotic factors[77,78]. Furthermore, the accumulation of amyloid beta deposits in the mitochondria of individuals with AD alters the functions of the respiratory chain and other mitoch
On the other hand, the increase in ROS and their effects on biomolecules have been associated with the development of cell disorders related to age in AD and DM. Therefore, oxidative stress is one of the main mediators in the processes underlying both diseases[83,84]. Oxidative damage to structural components of the neuronal membrane affects enzymes, ionic channels, and receptors anchored to it. Likewise, it also affects intracellular structures, such as organelles (mitochondria) and DNA or RNA, be it through lipidic peroxidation, nitration, nitrosylation, or carbonylation[85]. Likewise, oxidative stress can lead to the formation of peroxides, carbonyls, advanced glycation end products (AGEs), and advanced oxidation protein products. Furthermore, this results in the denaturalization and aggregation of proteins[86].
The increase in ROS inhibits the cellular production of energy and decreases insulin secretion and sensitivity[87]. Similarly, oxidative damage affects a variety of signaling pathways related to the unfolded protein response and protein degradation, which could lead to IR[88]. Finally, studies have found that mitochondrial disorders related to age increase the oxidative stress in individuals with T2DM, contributing to the development and progression of AD[89-91].
Autophagy is a catabolic process which is both constitutive and inducible, wherein a cell uses the liposomal machinery to degrade components or cellular organelles that are damaged or senescent. This process can also recycle biomolecules that are then available for the ensemble of new cell structures[92]. Different studies have reported that autophagy is a crucial deleterious process in AD and T2DM[93-95]. Furthermore, evidence suggests autophagic dysfunction in β-pancreatic cells and other peripheral tissues could contribute to IR[96,97]. This can occur through the deposit of amyloid in the islets, mitochondrial dysfunction, or disorders in regulatory mechanisms, such as the AMPK pathway and the mTOR pathway[69,97-99]. All of these factors are involved in the regulation of autophagy, glucose homeostasis, and peripheral insulin sensitivity.
Moreover, autophagy also intervenes in synaptic plasticity, axonal myelinization, and inflammatory modulation by glial cells. Therefore, alterations in this process contribute to the occurrence and development of neurodegenerative disorders, such as AD[100-102]. This association has been supported by animal and human studies related to AD, reporting alterations in genes and proteins associated with autophagy [95]. Transgenic mice studies have focused on the role of defective lysosomal degradation in the pathogenesis of this condition[103,104]. Studies in patients with AD have reported that mutations in PSEN1 contribute to the defective proteolysis of autophagy substrates[105]. Furthermore, it has been suggested that the excess of autophagy vacuoles reported in dystrophic neurons in such patients is the result of a defective clearance of the autophagosome[106]. Likewise, a significant reduction in the expression of Beclin-1 has been observed in AD patients, which is a fundamental protein in the stages of initiation and maintenance of autophagy, interfering with autophagy activities[107]. Furthermore, dysfunctions in mitophagy have also been identified. This mechanism participates in the detection and elimination of damaged mitochondria. It has also been associated with AD as it induces the deterioration of neuroplasticity by increasing the levels of ROS and decreasing the levels of cellular energy[108,109]. Alterations at the beginning of autophagy, deficient clearance of autophagy substrates, and dysfunction in mitophagy constitute the physiopathological processes important in AD[110].
In synthesis, various associations between T2DM and the development of neurocognitive disorders, specifically AD, have been reported over the years. However, some authors suggest that this association may be confounded by factors, such as smoking, hypertension, APOE E ε4, or brain infarctions, which could explain the progressive emergence of cognitive deterioration and clinical diagnosis of AD in patients with T2DM[111]. At any rate, various epidemiological and postmortem studies (Table 1) have observed the high frequency of AD in patients with T2DM, which could be explained by the different common pathophysiological mechanisms explained in the previous section[112-120].
| Ref. | Methodology | Results |
| Gudala et al[112] | Meta-analysis with 28 prospective observational studies which evaluated the association between diabetes and the risk of developing AD | A 56% risk of developing AD [RR = 1.56 (95%CI: 1.41-1.73), P < 0.05] was reported in patients with diabetes |
| Profenno et al[113] | Meta-analysis of 16 cross-sectional studies evaluating the relationship between diabetes and AD | The presence of diabetes significantly and independently increased the risk of AD [OR = 1.54 (95%CI: 1.33-1.79; P < 0.001] |
| Ohara et al[114] | Prospective study that evaluated the association between glucose tolerance status and the development of neurocognitive disorders in 1017 individuals ≥ 60 yr | AD incidence was significantly higher in subjects with T2DM compared to subjects with normal tolerance to glucose [HR = 2.05 (95%CI: 1.18 to 3.57), P = 0.01] |
| Xu et al[115] | Prospective study that examined the association between diabetes and the different types of neurocognitive disorders in 1248 older adults. Diagnoses were based on the DSM-III-R criteria | Individuals with non-diagnosed diabetes had a HR of 3.29 (95%CI: 1.20-9.01) P < 0.05 for AD diagnosis |
| Xu et al[116] | Prospective study that evaluated the association between T2DM and neurocognitive disorders and AD in 1301 older adults | T2DM diagnosis was significantly associated with neurocognitive disorders [HR = 1.5 (95%CI: 1.0-2.1) P = 0.04] and AD [HR = 1.3 (95%CI: 0.9-2.1) P < 0.05] |
| Peila et al[117] | Prospective study that examines the association between T2DM and neurocognitive disorder incidence in 2574 Japanese-American men. Diagnosis of neurocognitive disorder was performed through physical exam and MRI according to the NINCDS-ADRDA and DSM-IV criteria | T2DM was significantly associated with AD diagnosis [RR = 1.8 (95%CI: 1.1-2.9) P < 0.05] |
| McIntosh et al[118] | Prospective study that examined the relationship between T2DM, biomarkers, and the risk for suffering from neurocognitive disorders in 1289 dementia-free participants. AD biomarker levels were measured from the CSF. Neurocognitive disorders were evaluated through the CDRSB | Untreated diabetic individuals had higher levels of p-tau, p-tau/Aβ1-42, and t-tau/Aβ1-42 in their CSF than normoglycemic or prediabetic individuals (P < 0.05). The untreated group did not progress to neurocognitive disorder in higher rates than normoglycemic individuals [HR = 1.602 (95%CI: 1.057-2.429); P = 0.026] |
Concerning the multiple pathophysiological links between T2DM and AD, the neuroprotective effects of lifestyle interventions, antidiabetic drugs, and other molecules have gained interest in the scientific community in the past years (Table 2).
| Ref. | Antidiabetic drug | Methodology | Results |
| Craft et al[137] | Intranasal insulin | Randomized, double-blind, placebo-controlled trial which evaluated the effects of intranasal insulin administration in 104 adults with amnestic mild cognitive impairment or mild to moderate AD | Treatment with 20 UI of insulin improved delayed memory (P ≤ 0.05). According to caretakers, a functional improvement was observed in the groups receiving 20 and 40 UI of insulin, respectively (P ≤ 0.01) |
| Alp et al[164] | Beta-glucan and gliclazide | Preclinical assay including mice with induced diabetes. These were subdivided into six groups among which two groups received treatment with beta-glucan or gliclazide. Different parameters were used to determine the level of oxidative stress, including paraoxonase-1, total antioxidative status, and malondialdehyde | Mice with induced diabetes with no treatment presented high levels of malondialdehyde with a decrease in paraoxonase-1. Groups treated with beta-glucan and gliclazide presented a return of these values to normal levels after treatment, showing a decrease in brain oxidative stress (P ≤ 0.05) |
| Mostafa et al[174] | Metformin | Preclinical study in mice in which a group received scopolamine and metformin at and the other group received scopolamine and rivastigmine. Malondialdehyde, Akt, phosphorylated Akt, phosphorylated tau, and acetylcholinesterase levels were determined | The functionality of mice receiving scopolamine and a dose of metformin of 100 mg/kg per day was better than the group that was not administered with metformin. They also presented less inflammation and oxidative stress compared with the group receiving rivastigmine. An increase in phosphorylated Akt was observed |
| Qi et al[188] | Liraglutide | Forty mice were divided into four groups. The group with amyloid beta-induced AD was administered with liraglutide for 8 weeks and their cognitive performance was evaluated using a Morris water labyrinth | A protective effect in cognitive performance was observed in mice administered with liraglutide. Likewise, less structural changes in pyramidal neurons were observed, as well as a decrease in tau phosphorylation |
| Adler et al[209] | Amylin | The amylin levels in AD patients, patients with mild cognitive dysfunctions, and the control group were determined. Likewise, pramlintide, an amylin analog, was administered in AD mice in which oxidative stress and cognition were evaluated | Lower levels of amylin in patients with AD and mild cognitive dysfunction were observed compared with the control group. Mice administered with pramlintide showed improvement in cognition and synaptic markers as well as a decrease in oxidative stress in the hippocampus |
| NCT01843075[190] | Liraglutide | Multicenter, randomized, double-blind, placebo-controlled Phase IIb study in patients with mild AD | - |
| NCT03980730[208] | Azeliragon | Multicenter, randomized, double-blind, placebo-controlled, Phase II/III studies to evaluate the safety and efficacy of azeliragon as a treatment for subjects with mild AD | - |
| NCT02462161[142] | Intranasal insulin aspart | Pilot phase I clinical trial that will examine the effects of intranasal insulin aspart on cognition, daily function, blood, and cerebral spinal fluid markers of AD | - |
| NCT02503501[143] | Intranasal insulin glulisine | A phase II, single center, randomized, double-blind, placebo-controlled study that will evaluate the safety and effectiveness of intranasal glulisine in patients with probable AD | - |
Lifestyle interventions such as nutritional counseling and physical activity are among the first indications made to diabetic and insulin-resistant patients as established by international guidelines[121]. A systematic review by Dunkley et al[122] reported the efficacy of these interventions in diabetes prevention, similar to what was observed by a second systematic review in which high-risk T2DM patients participated in lifestyle interventions, observing that there was a lower incidence of T2DM among these subjects[123].
Numerous benefits have also been observed in the context of lifestyle interventions and cognitive decline. As it has been reported, nutritional behaviors such as calorie restriction and the inclusion of an antioxidant-rich diet have shown a beneficial effect in slowing the progression of neurodegenerative illnesses[124]. In addition, dietary interventions with meals characterized by low saturated fat and low glycemic index have shown improvement not only in insulin sensitivity but also in molecular markers of AD, such as an increase of Aβ42 in the CSF, which normally shows reduced levels in patients with AD[125].
Furthermore, when examining the impact of healthy lifestyle behaviors in AD incidence, two longitudinal studies found that there is an additive effect. In consequence, those individuals who practiced two to three behaviors such as following the Mediterranean diet, performing physical activity, and having a low consumption of alcohol showed a lower incidence of AD than those performing only one or none of these behaviors[126]. Overall, lifestyle interventions for diabetic patients, patients with IR, and patients with AD show benefits at the metabolic and cognitive levels and are recommended as part of non-pharmacological treatment and preventive interventions for these conditions.
There are numerous pharmacological interventions for the treatment of T2DM and AD. The systematic administration of insulin has been widely associated with a decrease in the pathological accumulation of amyloid beta as well as with cognitive improvement[127], especially in declarative memory[128,129] and attention[127]. Nevertheless, the use of insulin has not yet proven to be a safe and effective treatment for AD. Furthermore, there are adverse effects such as hypoglycemia[130], which is one of the main issues associated with insulin therapy, related in some cases to higher cardiovascular risk and, subsequently, death[131,132]. Likewise, repeated episodes of hypoglycemia caused by insulin therapy could be involved in the worsening of the cognitive deficit observed in certain patients with DM[133,134]. Therefore, this limits the use of insulin as treatment in patients with AD and other cognitive disorders[135]. Furthermore, pharmacological preparations for insulin injections do not entirely cross the BBB, limiting its distribution in the CNS[136]. Therefore, intranasal insulin administration emerges as a highly viable alternative[137-143].
Although only a few studies have evaluated its short-term effect on healthy individuals[138,139], the chronic administration of intranasal insulin in cognitively normal patients has been associated with higher memory performance. In this context, a study performed by Reger et al[140] showed that patients with cognitive defects who were administered with intranasal insulin (20 IU, 2×/d) displayed an improvement in both memory recall and their functional state based on the observations of their caretakers. Similar findings were reported by Benedict et al[139] who administered intranasal insulin (3×/d, 40 IU/doses) for 8 wk, which led to an improvement in late words recall. Likewise, a phase II/III clinical assay conducted by Craft et al[141] included patients with AD or mild cognitive deficits who were treated with intranasal insulin through a special device (Kurve Technology). Significant score improvements were found at months 15 and 18 (-5.70 and -5.78 points, nominal P = 0.004 and 0.018).
Alternatively, leptin and ghrelin are hormones with several functions in energetic balance and have a possible role in the pathophysiology and treatment of AD[144]. Indeed, late-life weight loss and midlife obesity — both of which involve dysfunctions in leptin signaling — have been associated with a higher risk of developing AD[145]. Midlife obesity is linked to high levels of circulating leptin, which leads to the central resistance to these pathologically high levels. This has been described among obese individuals as a component in IR and T2DM. Meanwhile, late-life weight loss has been related to low circulating levels of leptin[145,146]. Low brain leptin signaling has been found to worsen hippocampus functionality and decrease the neuroprotection against various central processes in the pathogenesis of AD, such as the metabolism of amyloid beta and tau[145,147]. The administration of leptin may renew insulin sensitivity by interacting with the insulin receptor and its signaling pathway[148-150] and by decreasing the pro-inflammatory response[146]. Likewise, in mice with AD, leptin has shown a promising therapeutic effect, improving the formation of memory, synaptic plasticity, and performance in learning activities[151-153].
On the other hand, many researchers have recently focused on evaluating the influence of the ghrelin-insulin system in glucose homeostasis. Ghrelin is unable to improve insulin sensitivity as it is activated with low levels of blood glucose and acts as a counterregulatory hormone[154]. However, it activates a vast number of signaling pathways for growth factors that compensate the loss of insulin signaling[155]. Although a large long-term impact in glycogenic metabolism has not been observed, the administration of ghrelin in AD patients has emerged as a possible therapeutic target by improving the pathological markers of the disease. This could be due to an interaction with the growth hormone secretagogue receptor 1α (GHSR1α). Ghrelin/GHSR1α signaling plays an important role in the synaptic physiology of the hippocampus and memory maintenance through the regulation of the D1 dopamine receptor (DRD1)[155,156]. Current emergent evidence suggests that the disruption of GHSR1α function induces hippocampal stress and memory deficits[157]. Furthermore, animal model studies have reported that the administration of ghrelin or analogs, such as MK0677 and LY444711, can inhibit the accumulation of amyloid beta and the hyperphosphorylation of tau protein by phosphorylating GSK-3β via the AMPK and PI3K/Akt pathways[158-160]. This may also reduce oxidative stress, excitotoxicity, and neuroinflammation and improve cognition and memory[158,161,162].
Clinical evidence has shown that the use of certain oral hypoglycemic drugs is associated with a lower risk of dementia[163]. Sulfonylureas have been observed to modulate diabetes-induced oxidative stress[70]. In this context, Alp et al[164] reported that the administration of gliclazide can potentiate antioxidant mechanisms and decrease the oxidative index in the brain of diabetic rats. These findings are consistent with that of Baraka and ElGhotny[165] and Abdallah et al[166], in which the administration of glibenclamide in mice reduced the hyperphosphorylation of tau and modulation of oxidative stress. However, further research is required to demonstrate the efficacy of sulfonylureas as a possible treatment for AD. In addition, it has been demonstrated that just as insulin therapy, sulfonylureas also have severe hypogly
Evidence regarding the use of metformin in the treatment of AD has been particularly controversial recently. In theory, it could decrease tau phosphorylation[169] and the interleukin-1β-mediated activation of phosphokinases Akt and MAPK[170]. Moreover, it can inhibit complex 1 of the mitochondrial respiratory chain, inducing an increase in cyclic adenosine monophosphate (cAMP) and activating PKA and AMPK[171,172]. Likewise, a study evaluated the ability of metformin treatment for a year in mice models of AD, reporting a gender-dependent effect, wherein AMPK activation in female mice improved memory and learning, while in male mice memory and cognitive behavior worsened, possibly due to hormonal issues[173]. Furthermore, Mostafa et al[174] reported that only low doses of metformin (100 mg/kg) in rats were associated with a delay in memory loss, possibly due to the suppression of Akt. Despite this, a study assessing the risk of AD in patients treated with antidiabetics found that those with long-term metformin treatment were associated with worse cognitive performance[175]. Long-term studies, with a greater number of patients and a standardized methodology, are needed to understand the true role of metformin in AD treatment, as current evidence appears contradictory.
The use of TZD has also been a subject of study for the treatment of AD, as they cause an overexpression of PPARγ in the temporal cortex, which has been associated with a decrease in amyloid beta plaque formation, a decrease in β-secretase levels, and the expression of PPA. Likewise, it modulates calcium homeostasis in the hippocampus, in association with an improvement in cognition[176-178]. Cheng et al[179] reported that pioglitazone can improve memory and cognition in the early stages of AD. Similarly, in a pilot study performed in individuals with AD and T2DM, Sato et al[180] have found that patients treated with pioglitazone for 6 mo showed cognitive improvement compared with the placebo group. However, another study in which AD patients without diabetes were treated with TZD for 18 mo did not find any significant cognitive improvement[181]. Currently, an active clinical assay (NCT1931566) with a more extensive sample intends to determine the efficacy of pioglitazone treatment for a longer period in patients with mild cognitive impairment [182].
Since the activation of glucagon-like peptide 1 (GLP-1) receptors and glucose-dependent insulinotropic polypeptides (GIP) in the CNS has been associated with neuroprotective effects[183], the use of GLP-1 and GIP analogs in AD has emerged as a promising therapeutic option. In preclinical studies, the administration of liraglutide in mice has demonstrated to decrease tau hyperphosphorylation and the deposition of amyloid plaque. Likewise, it prevents neuronal loss and the deterioration of synaptic plasticity and promotes beneficial effects on neurogenesis and brain microcir
With regard to GIP, different analogs have been able to show improvement in neurodegenerative diseases. Gault and Hölscher[191] reported that the use of D-ala2-GIP and N-glyc-GIP analogs reversed the synaptic plasticity alterations that had been induced by amyloid. D-Ala2-GIP has also been reported to decrease the amyloid plaque load, chronic inflammation, and oxidative stress, improving memory formation and synaptic plasticity as well as normalizing neurogenesis in AD animal models[192,193]. Based on these findings, the therapeutic effect of novel dual GLP-1/GIP agonists has been evaluated, as well as more recent triple GLP-1/GIP/glucagon agonists[194]. Clear neuroprotective effects have also been observed, reducing inflammation, oxidative stress, and apoptotic signaling and protecting memory formation and synaptic activity[194-196]. On the other hand, the administration of DPP4 inhibitors, such as saxagliptin and vildagliptin, have also been associated with a decrease in amyloid beta deposition, tau phosphorylation, and improvement in memory retention[197,198]. However, these findings are limited to preclinical studies in mice.
Similarly, sodium-glucose transport protein 2 inhibitors (SGLT2i) have been proven to have neuroprotective effects in animal models[199]. This has been reported in obese and diabetic mice, in which positive results on metabolic and brain function parameters have been observed. In addition, the attenuation of physiopathological processes like mitochondrial dysfunction, IR, inflammation, oxidative stress, and apoptosis as well as improvement in cognition, neurogenesis, synaptic density, and synaptic plasticity of the hippocampus has been reported[200-202].
These findings have been recently observed in AD-T2M mice in a study performed by Hierro-Bujalance et al[203], in which it was reported that empagliflozin can reduce the density of the senile plaque and the levels of amyloid beta in the brain cortex and hippocampus.
There is a scarce number of studies providing clinical evidence on the use of SGLT2i in AD. In this sense, Wium-Andersen et al[204] performed a nested case-control study in which they established that the use of SGLT2i reduces the risk of dementia in diabetic patients (odds ratio of 0.58; 95% confidence interval: 0.42-0.81; P < 0.05). Currently, a double-blind, randomized, placebo-controlled, parallel group, 12-wk study is underway. Its goal is to investigate the effect of dapagliflozin in patients who possibly have AD[205].
Azeliragon, a novel drug, has been reported to decrease amyloid deposition and brain inflammation by antagonizing the AGE receptor[206,207]. Based on the preliminary results from a phase III 18-month clinical trial in AD patients, the use of azeliragon decreased pro-inflammatory markers, hippocampus atrophy, and cognitive deterioration. More clinical trials are needed to confirm these findings[208]. Finally, amylin, which can cross the BBB and has effects at the CNS level, has been suggested to have a role in mood disorders as well as neurodegenerative disorders[136]. In AD patients, amylin plasma levels are considerably low, and the administration of analogs, such as pramlintide, has been associated with a decrease in neuroinflammation, oxidative stress, and memory improvement in AD mice[209]. Therefore, its potential use in clinical studies in the upcoming years could be promising.
Different studies have demonstrated the existence of a marked association between T2DM and the development of AD. Significant advances in the field of neuroendocrinology have investigated the underlying molecular mechanisms involved in the link between both disorders. Although various confounding factors may intervene in this relationship, studies have found that these diseases may share pathophysiological phenomena, including several abnormalities in insulin signaling in the PI3K and MAPK pathways in the brain tissues as well as the disruption of mitochondrial function, autophagy, GLUTs 1 and 3, and oxidative stress. This overlap leads to new common therapeutic perspectives, and various antidiabetic treatments have been implemented in multiple large-scale clinical and epidemiological studies. However, future clinical trials on the efficacy of these novel therapeutic interventions are needed to better characterize the true scope of this prospect.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Venezuela
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ugo O S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | World Health Organization. Dementia. [cited 21 October 2020]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia. |
| 2. | American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S152-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 3. | International Diabetes Federation. Worldwide toll of diabetes. [cited 21 October 2020]. In: International Diabetes Federation [Internet]. Available from: https://diabetesatlas.org/en/sections/worldwide-toll-of-diabetes.html. |
| 4. | Bermudez V, Salazar J. Prevalence and Risk Factors associated with Impaired Fasting Glucose in Adults from Maracaibo City, Venezuela. J Diabetes Metab. 2016;7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | De La Cruz Vargas JA, Dos Santos F, Dyzinger W, Herzog S. Medicina del Estilo de Vida: trabajando juntos para revertir la epidemia de las enfermedades crónicas en Latinoamérica. Cienc E Innov En Salud. 2017;4:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1797] [Article Influence: 224.6] [Reference Citation Analysis (0)] |
| 7. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3524] [Cited by in RCA: 3112] [Article Influence: 239.4] [Reference Citation Analysis (3)] |
| 8. | Mirhoseini M, Saleh N, Momeni A, Deris F, Asadi-Samani M. A study on the association of diabetic dermopathy with nephropathy and retinopathy in patients with type 2 diabetes mellitus. J Nephropathol. 2016;5:139-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6:961-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 216] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (4)] |
| 10. | Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 11. | Dá Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F. Insights on the pathophysiology of Alzheimer's disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev. 2016;68:547-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Sandhir R, Gupta S. Molecular and biochemical trajectories from diabetes to Alzheimer's disease: A critical appraisal. World J Diabetes. 2015;6:1223-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 13. | Lee SH, Zabolotny JM, Huang H, Lee H, Kim YB. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol Metab. 2016;5:589-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 930] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 15. | Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer's Disease Biomarkers: A Pilot Clinical Trial. J Alzheimers Dis. 2017;57:1325-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 16. | Koepsell H. Glucose transporters in brain in health and disease. Pflugers Arch. 2020;472:1299-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 17. | Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 1050] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 18. | McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, Mayberg MR, Marroni M, Leaman S, Stanness KA, Janigro D. Mechanisms of glucose transport at the blood-brain barrier: an in vitro study. Brain Res. 2001;904:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Bermudez V, Bermudez F, Arraiz N, Leal E, Linares S, Mengual E, Valdelamar L, Seyfi H, Amell A, Carrillo M, Silva C. Biología molecular de los transportadores de glucosa: clasificación, estructura y distribución. Arch Venez Farmacol Ter. 2007;26:76-86. |
| 20. | Jurcovicova J. Glucose transport in brain - effect of inflammation. Endocr Regul. 2014;48:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Qutub AA, Hunt CA. Glucose transport to the brain: a systems model. Brain Res Brain Res Rev. 2005;49:595-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer's disease: current knowledge. Front Neurosci. 2015;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front Endocrinol (Lausanne). 2014;5:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 358] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 24. | Rojas J, Bermúdez V, Leal E, Cano R, Luti Y, Acosta L, Finol F, AparicioD, Arraiz N, Linares S, Rojas E, Canelón R, Deisiree S. Insulinorresistencia e hiperinsulinemia como factores de riesgo para enfermedad cardiovascular. Arch Venez Farmacol Ter. 2008;27:30-40. |
| 25. | Baranowska-Bik A, Bik W. Insulin and brain aging. Prz Menopauzalny. 2017;16:44-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Tascone LDS, Bottino CMC. Neurobiology of neuropsychiatric symptoms in Alzheimer's disease: A critical review with a focus on neuroimaging. Dement Neuropsychol. 2013;7:236-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Narvaez E, Pelaez J, Almeida K, Alvarez C, Mendoza C, Morales A, Godos D, Del Salto Ocaña T, Catota M. Implication of apolipoprotein e polymorphisms in the atherosclerosis and Alzheimer’s disease physiopathology. Rev Latinoam Hipertens. 2018;13:97-102. |
| 28. | Vanegas H. Buscando las bases moleculares de la enfermedad de Alzheimer. Gac Méd Caracas. 2017;125:4-11. |
| 29. | Gilbert BJ. The role of amyloid β in the pathogenesis of Alzheimer's disease. J Clin Pathol. 2013;66:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer's disease: an update. Annu Rev Neurosci. 2014;37:79-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer's disease. Med Princ Pract. 2015;24:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 32. | Varma VR, Varma S, An Y, Hohman TJ, Seddighi S, Casanova R, Beri A, Dammer EB, Seyfried NT, Pletnikova O, Moghekar A, Wilson MR, Lah JJ, O'Brien RJ, Levey AI, Troncoso JC, Albert MS, Thambisetty M. Alpha-2 macroglobulin in Alzheimer's disease: a marker of neuronal injury through the RCAN1 pathway. Mol Psychiatry. 2017;22:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Kumar A, Singh A, Ekavali. A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 1037] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 34. | Hasegawa M. Molecular Mechanisms in the Pathogenesis of Alzheimer's disease and Tauopathies-Prion-Like Seeded Aggregation and Phosphorylation. Biomolecules. 2016;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Barbier P, Zejneli O, Martinho M, Lasorsa A, Belle V, Smet-Nocca C, Tsvetkov PO, Devred F, Landrieu I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front Aging Neurosci. 2019;11:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 36. | Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013;4:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Šimić G, Babić Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L, de Silva R, Di Giovanni G, Wischik C, Hof PR. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules. 2016;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 468] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 38. | Tsartsalis S, Xekardaki A, Hof PR, Kövari E, Bouras C. Early Alzheimer-type lesions in cognitively normal subjects. Neurobiol Aging. 2018;62:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Sonne DP, Hemmingsen B. Comment on American Diabetes Association. Standards of Medical Care in Diabetes-2017. Diabetes Care 2017;40(Suppl. 1):S1-S135. Diabetes Care. 2017;40:e92-e93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 40. | Jayaraman A, Pike CJ. Alzheimer's disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr Diab Rep. 2014;14:476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 41. | Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 303] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 42. | Frölich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Türk A, Hoyer S, Zöchling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm (Vienna). 1998;105:423-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 526] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 43. | Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 44. | Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer's disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 484] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 47. | Femminella GD, Livingston NR, Raza S, van der Doef T, Frangou E, Love S, Busza G, Calsolaro V, Carver S, Holmes C, Ritchie CW, Lawrence RM, McFarlane B, Tadros G, Ridha BH, Bannister C, Walker Z, Archer H, Coulthard E, Underwood B, Prasanna A, Koranteng P, Karim S, Junaid K, McGuinness B, Passmore AP, Nilforooshan R, Macharouthu A, Donaldson A, Thacker S, Russell G, Malik N, Mate V, Knight L, Kshemendran S, Tan T, Holscher C, Harrison J, Brooks DJ, Ballard C, Edison P. Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer's subjects? Alzheimers Res Ther. 2021;13:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Thankappan S, Sen S, Subramanian S, Sinha P, Purushottam M, Bharath S. Insulin resistance in patients with Alzheimer's dementia: A controlled study from India. Asian J Psychiatr. 2018;38:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Ye F, Luo YJ, Xiao J, Yu NW, Yi G. Impact of Insulin Sensitizers on the Incidence of Dementia: A Meta-Analysis. Dement Geriatr Cogn Disord. 2016;41:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Watson KT, Wroolie TE, Tong G, Foland-Ross LC, Frangou S, Singh M, McIntyre RS, Roat-Shumway S, Myoraku A, Reiss AL, Rasgon NL. Neural correlates of liraglutide effects in persons at risk for Alzheimer's disease. Behav Brain Res. 2019;356:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 51. | Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, Detre JA, Wolk DA, Arnold SE. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis Assoc Disord. 2017;31:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 52. | Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer's disease a Type 3 Diabetes? Biochim Biophys Acta Mol Basis Dis. 2017;1863:1078-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 412] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 53. | De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement. 2014;10:S26-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 54. | Ahmad W. Overlapped metabolic and therapeutic links between Alzheimer and diabetes. Mol Neurobiol. 2013;47:399-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 459] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 56. | Ansari SA, Emerald BS. The Role of Insulin Resistance and Protein O-GlcNAcylation in Neurodegeneration. Front Neurosci. 2019;13:473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Mravec B, Horvathova L, Padova A. Brain Under Stress and Alzheimer's Disease. Cell Mol Neurobiol. 2018;38:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Simó R, Ciudin A, Simó-Servat O, Hernández C. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist's perspective. Acta Diabetol. 2017;54:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 59. | Shah K, Desilva S, Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer’s Disease. Int J Mol Sci. 2012;13:12629-12655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 60. | Chen Z, Zhong C. Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 61. | Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol Aging. 1997;18:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Liu F, Shi J, Tanimukai H, Gu J, Grundke-Iqbal I, Iqbal K, Gong CX. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer's disease. Brain. 2009;132:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 63. | Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer's disease. J Neurochem. 2009;111:242-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 64. | Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582:359-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 65. | Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann Neurol. 1994;35:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 361] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 66. | Harr SD, Simonian NA, Hyman BT. Functional alterations in Alzheimer's disease: decreased glucose transporter 3 immunoreactivity in the perforant pathway terminal zone. J Neuropathol Exp Neurol. 1995;54:38-41. [PubMed] |
| 67. | Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641-C657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 604] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 68. | Nunomura A, Honda K, Takeda A, Hirai K, Zhu X, Smith MA, Perry G. Oxidative damage to RNA in neurodegenerative diseases. J Biomed Biotechnol. 2006;2006:82323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, Oliveira CR, Moreira PI. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer's disease and diabetes interrelation. Brain Res. 2012;1441:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 70. | Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta. 2014;1842:1693-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 71. | Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 277] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 72. | Butterworth RF, Besnard AM. Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer's disease. Metab Brain Dis. 1990;5:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:14670-14675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 743] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 74. | Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 932] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 75. | Parker WD Jr, Parks JK. Cytochrome c oxidase in Alzheimer's disease brain: purification and characterization. Neurology. 1995;45:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Sims NR, Finegan JM, Blass JP, Bowen DM, Neary D. Mitochondrial function in brain tissue in primary degenerative dementia. Brain Res. 1987;436:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 123] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Bonda DJ, Wang X, Perry G, Smith MA, Zhu X. Mitochondrial dynamics in Alzheimer's disease: opportunities for future treatment strategies. Drugs Aging. 2010;27:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Lloret A, Badía MC, Mora NJ, Ortega A, Pallardó FV, Alonso MD, Atamna H, Viña J. Gender and age-dependent differences in the mitochondrial apoptogenic pathway in Alzheimer's disease. Free Radic Biol Med. 2008;44:2019-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057-9068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 637] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 80. | Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 2001;15:1439-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 784] [Cited by in RCA: 749] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 82. | Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 1028] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 83. | Di Domenico F, Perluigi M, Butterfield DA, Cornelius C, Calabrese V. Oxidative damage in rat brain during aging: interplay between energy and metabolic key target proteins. Neurochem Res. 2010;35:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Domínguez RO, Marschoff ER, González SE, Repetto MG, Serra JA. Type 2 diabetes and/or its treatment leads to less cognitive impairment in Alzheimer's disease patients. Diabetes Res Clin Pract. 2012;98:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 757] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 86. | Butterfield DA, Gu L, Di Domenico F, Robinson RA. Mass spectrometry and redox proteomics: applications in disease. Mass Spectrom Rev. 2014;33:277-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 87. | Moreira PI, Santos MS, Seiça R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J Neurol Sci. 2007;257:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 88. | Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | de la Monte SM. Insulin resistance and Alzheimer's disease. BMB Rep. 2009;42:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 90. | de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. 2014;88:548-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 91. | Orth M, Schapira AH. Mitochondria and degenerative disorders. Am J Med Genet. 2001;106:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 92. | Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 93. | Gonzalez CD, Lee MS, Marchetti P, Pietropaolo M, Towns R, Vaccaro MI, Watada H, Wiley JW. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011;7:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 94. | Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, Cedazo-Minguez A, Sita G, Morroni F, Corsi M, Carboni L. Cross-disease analysis of Alzheimer's disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci Rep. 2019;9:3965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 95. | Whyte LS, Lau AA, Hemsley KM, Hopwood JJ, Sargeant TJ. Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer's disease? J Neurochem. 2017;140:703-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 96. | Barlow AD, Thomas DC. Autophagy in diabetes: β-cell dysfunction, insulin resistance, and complications. DNA Cell Biol. 2015;34:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 97. | Lee YH, Kim J, Park K, Lee MS. β-cell autophagy: Mechanism and role in β-cell dysfunction. Mol Metab. 2019;27S:S92-S103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 98. | Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 2743] [Article Influence: 342.9] [Reference Citation Analysis (0)] |
| 99. | Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3052] [Cited by in RCA: 4444] [Article Influence: 555.5] [Reference Citation Analysis (0)] |
| 100. | Lee JA. Neuronal autophagy: a housekeeper or a fighter in neuronal cell survival? Exp Neurobiol. 2012;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 101. | Frake RA, Ricketts T, Menzies FM, Rubinsztein DC. Autophagy and neurodegeneration. J Clin Invest. 2015;125:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 102. | Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2011;8:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 103. | Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, Devidze N, Wang X, Grubb A, Gan L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer's disease. Neuron. 2008;60:247-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 104. | Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 350] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 105. | Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 923] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 106. | Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926-6937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 907] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 107. | Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 581] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 108. | Correia SC, Perry G, Moreira PI. Mitochondrial traffic jams in Alzheimer's disease - pinpointing the roadblocks. Biochim Biophys Acta. 2016;1862:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 109. | Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer's Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017;40:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 602] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 110. | Nixon RA, Yang DS. Autophagy failure in Alzheimer's disease--locating the primary defect. Neurobiol Dis. 2011;43:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 111. | Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer's disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 112. | Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 113. | Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 492] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 114. | Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, Kanba S, Kiyohara Y. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 115. | Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer's disease: a population-based cohort study. Diabetologia. 2009;52:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 116. | Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 117. | Peila R, Rodriguez BL, Launer LJ; Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 831] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 118. | McIntosh EC, Nation DA; Alzheimer’s Disease Neuroimaging Initiative. Importance of Treatment Status in Links Between Type 2 Diabetes and Alzheimer's Disease. Diabetes Care. 2019;42:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 120. | Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 121. | American Diabetes Association. . 5. Lifestyle Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S46-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 122. | Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, Khunti K. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 123. | Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 124. | Bhatti GK, Reddy AP, Reddy PH, Bhatti JS. Lifestyle Modifications and Nutritional Interventions in Aging-Associated Cognitive Decline and Alzheimer's Disease. Front Aging Neurosci. 2019;11:369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 125. | Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 126. | Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 Longitudinal studies. Neurology. 2020;95:e374-e383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 127. | Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 128. | Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 129. | Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 1996;17:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 388] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 130. | McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012;41:57-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 131. | Herman ME, O'Keefe JH, Bell DSH, Schwartz SS. Insulin Therapy Increases Cardiovascular Risk in Type 2 Diabetes. Prog Cardiovasc Dis. 2017;60:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 132. | Margolis DJ, Hoffstad O, Strom BL. Association between serious ischemic cardiac outcomes and medications used to treat diabetes. Pharmacoepidemiol Drug Saf. 2008;17:753-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 133. | Graveling AJ, Deary IJ, Frier BM. Acute hypoglycemia impairs executive cognitive function in adults with and without type 1 diabetes. Diabetes Care. 2013;36:3240-3246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 134. | Lacy ME, Gilsanz P, Eng C, Beeri MS, Karter AJ, Whitmer RA. Severe Hypoglycemia and Cognitive Function in Older Adults With Type 1 Diabetes: The Study of Longevity in Diabetes (SOLID). Diabetes Care. 2020;43:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 135. | Weinstein G, Davis-Plourde KL, Conner S, Himali JJ, Beiser AS, Lee A, Rawlings AM, Sedaghat S, Ding J, Moshier E, van Duijn CM, Beeri MS, Selvin E, Ikram MA, Launer LJ, Haan MN, Seshadri S. Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer's disease: Pooled analysis from 5 cohorts. PLoS One. 2019;14:e0212293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 136. | Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 137. | Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1018] [Cited by in RCA: 944] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 138. | Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 139. | Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 140. | Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 578] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 141. | Craft S, Raman R, Chow TW, Rafii MS, Rissman RA, Brewer JB, Donohue MC, Sun C-K, Harless K, Gessert D, Aisen PS. DT-02-03: Open label extension results from a phase ii/iii trial of intranasal insulin. Alzheimers Dement. 2019;15:P1489-P1489. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 142. | Craft S. Study of Nasal Insulin to Fight Forgetfulness - Short-Acting Insulin Aspart (SNIFF-Quick). [accessed 2020 Dec 19]. In: ClinicalTrials.gov [Internet]. Winston-Salem (CA): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02462161 ClinicalTrials.gov Identifier: NCT02462161. |
| 143. | Rosenbloom MH. Intranasal Glulisine in Amnestic Mild Cognitive Impairment and Probable Mild Alzheimer's Disease. [accessed 2020 Dec 16]. In: ClinicalTrials.gov [Internet]. Saint Paul (MN): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02503501 ClinicalTrials.gov Identifier: NCT02503501. |
| 144. | de Candia P, Matarese G. Leptin and ghrelin: Sewing metabolism onto neurodegeneration. Neuropharmacology. 2018;136:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 145. | McGuire MJ, Ishii M. Leptin Dysfunction and Alzheimer's Disease: Evidence from Cellular, Animal, and Human Studies. Cell Mol Neurobiol. 2016;36:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 146. | Salazar J, Chávez-Castillo M, Rojas J, Ortega A, Nava M, Pérez J, Rojas M, Espinoza C, Chacin M, Herazo Y, Angarita L, Rojas DM, D'Marco L, Bermudez V. Is "Leptin Resistance" Another Key Resistance to Manage Type 2 Diabetes? Curr Diabetes Rev. 2020;16:733-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 147. | Tezapsidis N, Johnston JM, Smith MA, Ashford JW, Casadesus G, Robakis NK, Wolozin B, Perry G, Zhu X, Greco SJ, Sarkar S. Leptin: a novel therapeutic strategy for Alzheimer's disease. J Alzheimers Dis. 2009;16:731-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 148. | Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 149. | Paz-Filho G, Mastronardi C, Wong ML, Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocrinol Metab. 2012;16:S549-S555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 150. | German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ Jr, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 151. | Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 152. | Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N, Casadesus G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;19:1155-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 153. | Pérez-González R, Antequera D, Vargas T, Spuch C, Bolós M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2011;24 Suppl 2:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 154. | Gómez R, Lago F, Gómez-Reino JJ, Gualillo O. Novel factors as therapeutic targets to treat diabetes. Focus on leptin and ghrelin. Expert Opin Ther Targets. 2009;13:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Seminara RS, Jeet C, Biswas S, Kanwal B, Iftikhar W, Sakibuzzaman M, Rutkofsky IH. The Neurocognitive Effects of Ghrelin-induced Signaling on the Hippocampus: A Promising Approach to Alzheimer's Disease. Cureus. 2018;10:e3285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | Kern A, Mavrikaki M, Ullrich C, Albarran-Zeckler R, Brantley AF, Smith RG. Hippocampal Dopamine/DRD1 Signaling Dependent on the Ghrelin Receptor. Cell. 2015;163:1176-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 157. | Tian J, Guo L, Sui S, Driskill C, Phensy A, Wang Q, Gauba E, Zigman JM, Swerdlow RH, Kroener S, Du H. Disrupted hippocampal growth hormone secretagogue receptor 1α interaction with dopamine receptor D1 plays a role in Alzheimer's disease. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 158. | Kunath N, van Groen T, Allison DB, Kumar A, Dozier-Sharpe M, Kadish I. Ghrelin agonist does not foster insulin resistance but improves cognition in an Alzheimer's disease mouse model. Sci Rep. 2015;5:11452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 159. | Chen Y, Cao CP, Li CR, Wang W, Zhang D, Han LL, Zhang XQ, Kim A, Kim S, Liu GL. Ghrelin modulates insulin sensitivity and tau phosphorylation in high glucose-induced hippocampal neurons. Biol Pharm Bull. 2010;33:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 160. | Dhurandhar EJ, Allison DB, van Groen T, Kadish I. Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer's disease pathology in a mouse model. PLoS One. 2013;8:e60437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 161. | Eslami M, Sadeghi B, Goshadrou F. Chronic ghrelin administration restores hippocampal long-term potentiation and ameliorates memory impairment in rat model of Alzheimer's disease. Hippocampus. 2018;28:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 162. | Santos VV, Stark R, Rial D, Silva HB, Bayliss JA, Lemus MB, Davies JS, Cunha RA, Prediger RD, Andrews ZB. Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid β (Aβ1-40) administration in mice. J Neuroendocrinol. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 163. | Hsu CC, Wahlqvist ML, Lee MS, Tsai HN. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis. 2011;24:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 164. | Alp H, Varol S, Celik MM, Altas M, Evliyaoglu O, Tokgoz O, Tanrıverdi MH, Uzar E. Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozosin. Exp Diabetes Res. 2012;2012:230342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 165. | Baraka A, ElGhotny S. Study of the effect of inhibiting galanin in Alzheimer's disease induced in rats. Eur J Pharmacol. 2010;641:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 166. | Abdallah DM, Nassar NN, Abd-El-Salam RM. Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus. Brain Res. 2011;1385:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 167. | Schloot NC, Haupt A, Schütt M, Badenhoop K, Laimer M, Nicolay C, Reaney M, Fink K, Holl RW. Risk of severe hypoglycemia in sulfonylurea-treated patients from diabetes centers in Germany/Austria: How big is the problem? Diabetes Metab Res Rev. 2016;32:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 168. | Schopman JE, Simon AC, Hoefnagel SJ, Hoekstra JB, Scholten RJ, Holleman F. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 169. | Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Köhler A, Glossmann H, Schneider R, Sutherland C, Schweiger S. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA. 2010;107:21830-21835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 170. | Li SN, Wang X, Zeng QT, Feng YB, Cheng X, Mao XB, Wang TH, Deng HP. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels. 2009;24:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 171. | Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 172. | Burcelin R. The antidiabetic gutsy role of metformin uncovered? Gut. 2014;63:706-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 173. | Ibrahim OHM, Hassan MA. The Use of Anti-Diabetic Drugs in Alzheimer’s Disease, New Therapeutic Options and Future Perspective. Pharmacol Amp Pharm. 2018;9:157-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 174. | Mostafa DK, Ismail CA, Ghareeb DA. Differential metformin dose-dependent effects on cognition in rats: role of Akt. Psychopharmacology (Berl). 2016;233:2513-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 175. | Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer's disease: a population-based case-control study. J Am Geriatr Soc. 2012;60:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 176. | Wang L, Liu W, Fan Y, Liu T, Yu C. Effect of rosiglitazone on amyloid precursor protein processing and Aβ clearance in streptozotocin-induced rat model of Alzheimer's disease. Iran J Basic Med Sci. 2017;20:474-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 177. | Pancani T, Phelps JT, Searcy JL, Kilgore MW, Chen KC, Porter NM, Thibault O. Distinct modulation of voltage-gated and ligand-gated Ca2+ currents by PPAR-gamma agonists in cultured hippocampal neurons. J Neurochem. 2009;109:1800-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 178. | Femminella GD, Bencivenga L, Petraglia L, Visaggi L, Gioia L, Grieco FV, de Lucia C, Komici K, Corbi G, Edison P, Rengo G, Ferrara N. Antidiabetic Drugs in Alzheimer's Disease: Mechanisms of Action and Future Perspectives. J Diabetes Res. 2017;2017:7420796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 179. | Cheng H, Shang Y, Jiang L, Shi TL, Wang L. The peroxisome proliferators activated receptor-gamma agonists as therapeutics for the treatment of Alzheimer's disease and mild-to-moderate Alzheimer's disease: a meta-analysis. Int J Neurosci. 2016;126:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 180. | Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 181. | Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 182. | Takeda. Biomarker Qualification for Risk of Mild Cognitive Impairment (MCI) Due to Alzheimer's Disease (AD) and Safety and Efficacy Evaluation of Pioglitazone in Delaying Its Onset (TOMMORROW). [accessed 2020 Dec 20]. In: ClinicalTrials.gov [Internet]. Zinfandel Pharmaceuticals Inc.: U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01931566 ClinicalTrials.gov Identifier: NCT01931566. |
| 183. | Hölscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2014;221:T31-T41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 184. | McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 2011;31:6587-6594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 542] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 185. | Han WN, Hölscher C, Yuan L, Yang W, Wang XH, Wu MN, Qi JS. Liraglutide protects against amyloid-β protein-induced impairment of spatial learning and memory in rats. Neurobiol Aging. 2013;34:576-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 186. | Parthsarathy V, Hölscher C. Chronic treatment with the GLP1 analogue liraglutide increases cell proliferation and differentiation into neurons in an AD mouse model. PLoS One. 2013;8:e58784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 187. | Kelly P, McClean PL, Ackermann M, Konerding MA, Hölscher C, Mitchell CA. Restoration of cerebral and systemic microvascular architecture in APP/PS1 transgenic mice following treatment with Liraglutide™. Microcirculation. 2015;22:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 188. | Qi L, Ke L, Liu X, Liao L, Ke S, Wang Y, Lin X, Zhou Y, Wu L, Chen Z, Liu L. Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced alzheimer disease mouse model. Eur J Pharmacol. 2016;783:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 189. | Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, Rodell A, Brændgaard H, Gottrup H, Schacht A, Møller N, Brock B, Rungby J. In Alzheimer's Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front Aging Neurosci. 2016;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 190. | Edison P. Evaluating Liraglutide in Alzheimer's Disease (ELAD). [accessed 2020 Dec 19]. In: ClinicalTrials.gov [Internet]. London: U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01843075 ClinicalTrials.gov Identifier: NCT01843075. |
| 191. | Gault VA, Hölscher C. Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. J Neurophysiol. 2008;99:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 192. | Duffy AM, Hölscher C. The incretin analogue D-Ala2GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer's disease. Neuroscience. 2013;228:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 193. | Faivre E, Hölscher C. Neuroprotective effects of D-Ala(2)GIP on Alzheimer's disease biomarkers in an APP/PS1 mouse model. Alzheimers Res Ther. 2013;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 194. | Hölscher C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer's and Parkinson's disease models. Neuropharmacology. 2018;136:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 195. | Li T, Jiao JJ, Hölscher C, Wu MN, Zhang J, Tong JQ, Dong XF, Qu XS, Cao Y, Cai HY, Su Q, Qi JS. A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer's disease. Hippocampus. 2018;28:358-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 196. | Camins A, Ettcheto M, Busquets O, Manzine PR, Castro-Torres RD, Beas-Zarate C, Verdaguer E, Sureda FX, Bulló M, Olloquequi J, Auladell C, Folch J. Triple GLP-1/GIP/glucagon receptor agonists, a potential novel treatment strategy in Alzheimer's disease. Expert Opin Investig Drugs. 2019;28:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 197. | Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, Madhunapantula VS, Muthureddy Nataraj SK, Basavan D. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer's disease. Neuropharmacology. 2013;72:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 198. | Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer's disease. J Pharm Pharmacol. 2013;65:1773-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 199. | Wiciński M, Wódkiewicz E, Górski K, Walczak M, Malinowski B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer's Disease and Ischemia-Related Brain Injury. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 200. | Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Jaiwongkam T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol. 2017;333:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 201. | Millar P, Pathak N, Parthsarathy V, Bjourson AJ, O'Kane M, Pathak V, Moffett RC, Flatt PR, Gault VA. Metabolic and neuroprotective effects of dapagliflozin and liraglutide in diabetic mice. J Endocrinol. 2017;234:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 202. | Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 313] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 203. | Hierro-Bujalance C, Infante-Garcia C, Del Marco A, Herrera M, Carranza-Naval MJ, Suarez J, Alves-Martinez P, Lubian-Lopez S, Garcia-Alloza M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer's disease and type 2 diabetes. Alzheimers Res Ther. 2020;12:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 204. | Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol. 2019;181:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 205. | Burns J. Dapagliflozin In Alzheimer's Disease. [accessed 2021 Mar 14]. In: ClinicalTrials.gov [Internet]. Kansas City (KS): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03801642 ClinicalTrials.gov Identifier: NCT03801642. |
| 206. | Dhananjayan K, Forbes J, Münch G. Advanced Glycation, Diabetes, and Dementia. In: Srikanth V, Arvanitakis Z. Type 2 Diabetes and Dementia. Elsevier, 2018: 169-193. |
| 207. | Burstein AH, Sabbagh M, Andrews R, Valcarce C, Dunn I, Altstiel L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer's Disease. J Prev Alzheimers Dis. 2018;5:149-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 208. | vTv Therapeutics. Study of Azeliragon in Patients With Mild Alzheimer's Disease and Impaired Glucose Tolerance (Elevage). [accessed 2020 Dec 19]. In: ClinicalTrials.gov [Internet]. Tucson (AZ): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03980730 ClinicalTrials.gov Identifier: NCT03980730. |
| 209. | Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee HG, Arnold SE, Casadesus G. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol Aging. 2014;35:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |