INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen has led to the coronavirus disease 2019 (COVID-19) pandemic. This virus exerts multi-organ actions, after an initial respiratory infection[1]. Regarding COVID-19, while age and male gender are regarded as significant risk factors, accumulating evidence suggests a strong association with an impaired cardiometabolic profile in most severely ill patients[2]. Reports from Wuhan, China were the first to indicate a higher prevalence of hypertension and diabetes mellitus (DM) among patients with severe compared to non-severe illness[3]. From the beginning of the outbreak, cardiovascular disease (CVD), obesity, type 1 DM (T1DM), type 2 DM (T2DM) and possibly hypertension have seemed to be associated with the risk of suffering or dying from COVID-19[4-6].
DIABETES AS A PREDICTOR OF THE COURSE OF COVID-19
COVID-19 patients with T2DM and/or CVD are admitted more often to intensive care units (ICUs) compared to those without T2DM or CVD[7]. Older age and T2DM are both risk factors for COVID-19, but the observation that T2DM is a disease that is frequent in advanced age, slightly confounds this association[8].The risk of developing severe COVID-19 is higher in people with DM, especially if they have other co-morbidities, thus making patients with DM an at-risk population. The worse the glycemic control, the worse the severity of infection and the greater the risk of mortality[9]. In the initial studies of COVID-19, DM appeared to be 2.26 times (95% confidence interval [CI]: 1.47-3.49) more common in patients with more severe COVID-19 compared to those with less severe infection, while at the same time the presence of DM entailed an odds ratio of 2.85 (95%CI: 1.35-6.05) of intra-hospital mortality[2]. As already mentioned, these results were not always adjusted for age, which is a major confounding factor in the prevalence of DM. In Italy, one-third of patients who died of COVID-19 had DM (median age 80.5 years) and were predominantly male (70%)[2]. Compared with the prevalence of DM in the same population segment in Italy in 2018 (20.3%), the authors reported a relative risk of diabetes of 1.75 in patients who died from COVID-19[2]. It is therefore necessary to emphasize the advanced age of patients with severe COVID-19, as well as their multiple comorbidities, defining them as a population particularly at risk.
COVID-19 AND INFLAMMATION
COVID-19 is characterized by the excessive production of inflammatory factors, leading to an “in inflammatory storm” (a combination of pro-inflammatory immunoactive molecules, such as interleukins [ILs], interferons [IFNs], chemokines and tumor necrosis factors [TNFs]) in some patients[10]. Diffuse pulmonary alveolar damage, inflammatory cell infiltration with hyaline membranes, myocardial inflammation, lymphocyte infiltration in the liver, and pancreatitis are some of the major inflammatory findings during the course of the generalized COVID-19[11]. In sharp contrast to the above, the IFN type I response is impaired in these patients[12]. For patients with severe COVID-19, this so-called cytokine storm is a potentially life- threatening event[13].
In 317 patients with laboratory-confirmed COVID-19, inflammatory responses and higher levels of IL-6 were related to disease severity[11,14]. In patients with COVID-19, inflammatory markers such as C-reactive protein, D-dimers, ferritin, and IL-6 are increased; they have a direct effect on microvascular and macrovascular structures in patients with DM[15].
DIABETES, OBESITY, AND INFLAMMATORY SIMILARITIES WITH COVID-19
Although T1DM is not related to obesity, the majority of patients with T2DM are overweight or obese. Resembling the inflammatory processes of COVID-19, prolonged hyperglycemia, regardless of diabetes type, can also impact immune function, whereas compromised immunological status is linked to macrovascular complications of DM[11].
Inflammation begets increased oxidative stress that can damage proteins, lipids and DNA, systemically, as well as locally, both in the liver and in muscles, the predominant organs that regulate glucose output and glucose metabolism, increasing insulin resistance[16]. In T2DM, inflammation occurs in the pancreatic β cell (insulitis)[16]. Macrophages play a key role in β cell inflammation, along with IL-1β signaling (a core inflammatory process in the locally stressed β cell). Along with the local injury of the pancreatic cells, lipotoxicity further deteriorates pancreatic function. Free fatty acids can also induce the local production of IL-1β- and IL-1-dependent pro-inflammatory cytokines, which target the pancreatic islets. This process also increases nitric oxide production, lowers mitochondrial ATP, causing additional β cell dysfunction, along with the release of reactive oxygen species by hypoxia and endothelial damage[17]. TNF-α is linked to insulin resistance, obesity and islet inflammation, while IL-6 promotes islet cell apoptosis; both lead to T2DM. Obesity and DM (which often are described as “diabesity”) favor a switch from (anti-inflammatory) M2 macrophage predominance to (pro-inflammatory) M1 macrophage predominance, further contributing to exaggerated inflammation[18]. Of note, infection with respiratory syncytial virus increases the production of IFNγ, provokes natural killer (NK) cell activation and exacerbation of inflammation in muscle and adipose tissues. Moreover, NK cell activity was found to be lower in patients with DM; glycated hemoglobin A1c (A1c) levels are associated with NK cell activity[17].
T2DM is a disease that often occurs and/or is related to obesity. Insulin resistance and related progression to overt diabetes are strongly associated with hypertrophy and hyperplasia of adipose cells[18]. According to the World Obesity Federation, obesity-related conditions seem to worsen the effects of COVID-19; indeed, the Centers for Disease Control and Prevention reported that “people with heart disease and diabetes are at higher risk of COVID-19 complications” and severe obesity (body mass index of ≥ 40) entails a higher risk for severe disease or death. As previously mentioned, COVID-19 favors an inflammatory environment that may progress to a “cytokine storm” (hypersecretion of inflammatory molecules: IL-2, IL-7, granulocyte-colony stimulating factor, IFN-γ inducible protein 10, monocyte chemo-attractant protein 1 [MCP1], macrophage inflammatory protein 1-α, and TNF-α). In an analogous fashion, obesity presents a state of low-grade inflammation, as a result of the secretion of inflammatory cytokines (TNF-α, IL-1, IL-6, IL-10), transforming growth factor-β, adipokines (leptin, resistin, adiponectin), MCP-1, C-X-C motif chemokine 5, hemostatic proteins (plasminogen activator inhibitor-1), proteins affecting blood pressure (angiotensinogen) and angiogenic molecules (vascular endothelial growth factor)[13]. Hypoxia and ischemia in adipose tissue and local endothelial damage lead to the production of reactive oxygen radicals (radical oxygen species, ROS) that affect both the microenvironment and macroenvironment of blood vessels[13].
Hyperglycemia and DM affect various target organs, including the vasculature. Obesity (and its concomitant inflammation) enables another mechanism via which COVID-19 can provoke damage., which is directly related to the microvascular complications of DM[13].
COVID-19 AND GLUCOSE METABOLISM
Hyperglycemia was observed in patients with SARS in 2003, caused by another coronavirus, closely related to COVID-19, SARS-CoV-1) possibly due to potential transient impairment of pancreatic islet cell function. Two more coronaviridae (’MERS-CoV’ and ‘HCoV-EMC’, causing Middle Eastern Respiratory Syndrome and human coronavirus;) attach to cells via dipeptidyl peptidase 4 (DPP-4, an enzyme that regulates insulin secretion)[19].
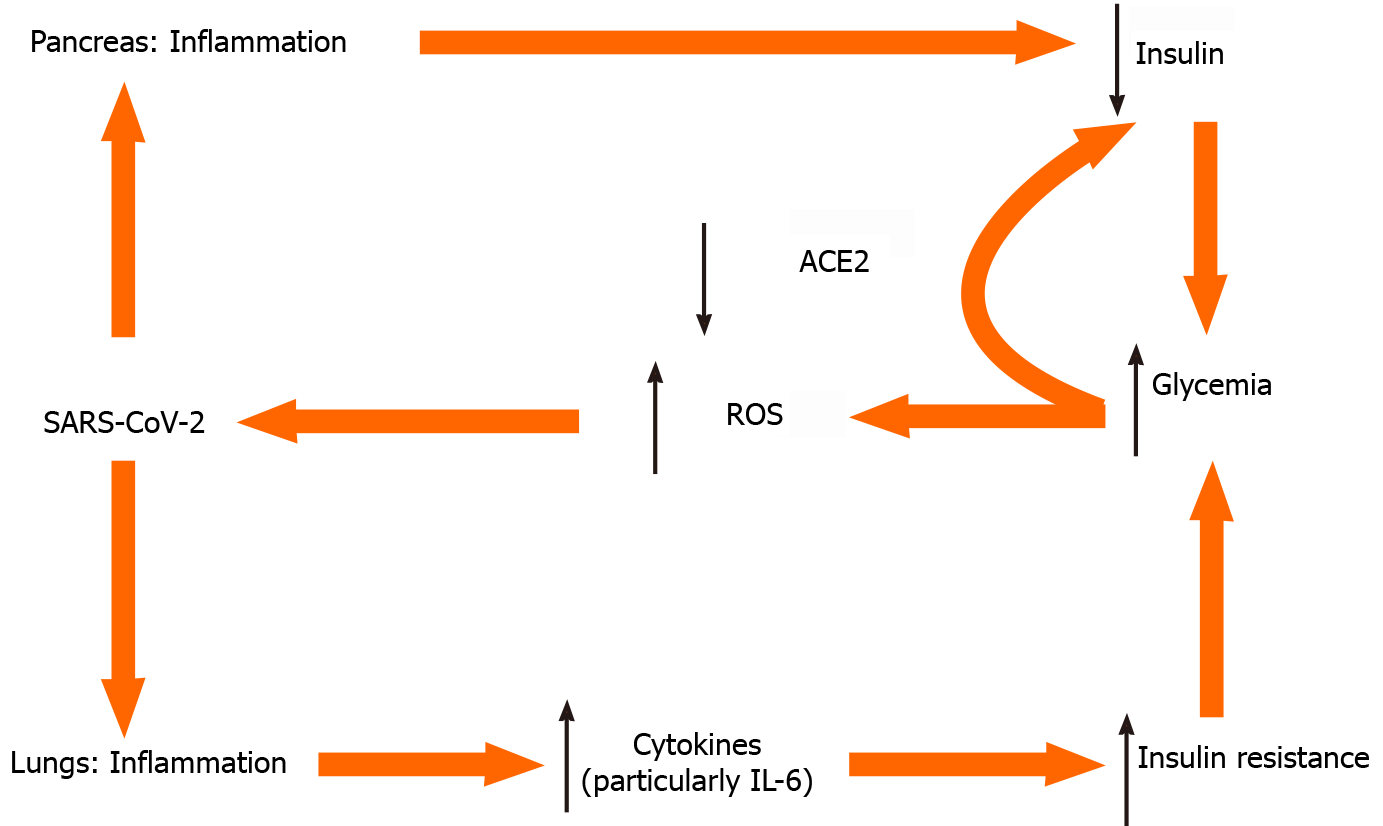
Glycemia on one hand is associated with SARS-CoV-2 replication[20]; elevated glucose levels and glycolysis increase SARS-CoV-2 replication and viral proliferation through the production of ROS (Figure 1). Notably, both T1DM and T2DM, are associated with a dysregulated immune response and increased morbidity and mortality[21]. On the other hand, in an inverse relationship, the presence of COVID-19 causes deterioration of glycemic control in already established DM. In a case series of critically ill, mostly not well-controlled patients with pre-existing T2DM (7 of 8 were on oral therapy before ICU admission), 85 to 480 units of insulin per day were needed to harness hyperglycemia[20].
Figure 1 Selected tentative pathways for hyperglycemia in severe acute respiratory syndrome coronavirus 2 infection.
ACE2: Angiotensin converting enzyme 2; IL-6: Interleukin 6; ROS: Radical oxygen species; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
The difference in diabetic ketoacidosis (DKA) rates in COVID-19 was four times higher in Black and two times higher in Hispanic patients with T1DM vs White patients with T1DM (but no statistical significance was documented)[22]. Potential explanations for these observations include the lower socioeconomic status of minority populations vis-à-vis that of the White population, the lack of appropriate nutrition and the lack of medical supervision in the use of insulin[22]. Although DKA is a major untoward event in T1DM, the majority of DKA cases with COVID-19, were observed in T2DM patients[22].
An initial diagnosis of DM was common in patients infected with SARS-COV-2, with neither any prior history of DM, nor using glucocorticoids. This new-onset hyperglycemia was an independent predictor for mortality[23] and was attributed to the binding of SARS-COV-2 to the angiotensin converting enzyme 2 (ACE2) receptor in pancreatic islets with concomitant local damage[23,24] (see also below). This ‘‘new-onset” hyperglycemia could be classified either as ‘‘stress-induced” hyperglycemia, as ‘‘new-onset DM” in previously unrecognized prediabetes, as hyperglycemia owing to the effects of SARS-CoV-2 to the pancreatic islets or as a result of ‘‘secondary DM”, following use of corticosteroids[23].
Quoting the definitions of the American Diabetes Association[25], new-onset hyperglycemia without DM is defined as fasting plasma glucose (FPG) between 5.6 mmol/L and 6.9 mmol/L (100-125 mg/dL) and/or A1c between 5.7% and 6.4%, in absence of such measurements in the past. New-onset DM is defined by either of FPG > 7.0 mmol/L (> 126 mg/dL) and/or an A1c > 6.5% and/or a random glucose > of 11.1 mmol/L (200 mg/dL)[25]. Thus, abnormal glucose measurements, in the absence of A1c > 6.5% could be expected, especially during this recent viral infection (that could not have affected the A1c levels yet). Several cases of hyperglycemia or new-onset DM in COVID-19 have been reported. As might be expected, COVID-19 has been associated with severe metabolic complications of already preexisting DM, including DKA and hyperosmolarity, necessitating high doses of insulin for glycemic control.
ACE2 is expressed in the respiratory system, in the intestines, kidneys, myocardium, vasculature and pancreatic islets. SARS-CoV-2 binds to ACE2, using it as a ligand for cell entry. Interestingly, ACE2-knockout mice are more vulnerable to β cell dysfunction[24], a fact that could explain why infection with SARS-CoV-2 can cause hyperglycemia in humans without preexisting DM. After endocytosis of the virus complex, ACE2 expression is downregulated, acting in a dual way. On one hand this impairs pancreatic islet cells’ function and causes β cell injury. On the other hand, downregulation of ACE2 Leads to unopposed angiotensin II action, which may further impair insulin secretion, by reducing blood flow and reducing insulin secretion while increasing oxidative stress in the pancreatic cell. Thus, coronaviruses might damage pancreatic islets, and give rise to hyperglycemia[24].
STUDIES REPORTING NEW-ONSET HYPERGLYCEMIA DUE TO COVID-19
Recently, a young 37-year-old patient with COVID-19 presented with all the clinical features of hyperglycemia and DKA, this being possibly the first case of new-onset DM secondary to COVID-19[26]. Another case of DKA precipitated by COVID-19 in a 54-year-old patient with newly diagnosed DM was also reported[27]. Since DKA occurs as a result of insulin deficiency, such observations give rise to questions regarding the potential effect of COVID-19 in this dangerous condition[27].
In a study by Li et al[28], among 658 hospitalized patients with confirmed COVID-19, 42 (6.4%) out of 658 patients presented with ketosis on admission with no obvious fever or diarrhea. Patients with ketosis were younger (median age 47.0 years vs 58.0 years; P = 0.003) and had a greater prevalence of fatigue (31.0% vs 10.6%; P < 0.001), DM (35.7% vs 18.5%; P = 0.007) and digestive disorders (31.0% vs 12.0%; P < 0.001). According to their data, COVID-19 infection caused ketosis or ketoacidosis, and induced DKA for patients with DM. Ketosis increased the length of hospital stay and mortality, while DM increased the length of hospital stay for patients with ketosis but had no effect on their mortality[28].
It remains to be determined whether, after resolution of COVID-19 symptoms, glucose levels are restored to normal, thus remitting the initial diagnosis of DM. To provide answers to this conundrum, a global registry of patients with COVID-19-related diabetes has been established (COVIDIAB Project)[29].
OUTCOME IN PATIENTS WITH NEW-ONSET HYPERGLYCEMIA WITHOUT DM VS NORMOGLYCEMIC COVID-19 PATIENTS
Hyperglycemia (two or more blood glucose measurements > 10 mmol/L or 180 mg/dL within any 24-h period with an A1C < 6.5%), regardless of the presence of DM, is related to an increase in COVID-19 mortality compared to normoglycemia[30]. Hyperglycemia without DM is further related to increased need for mechanical ventilation, to need for ICU hospitalization and to mortality[30]. In the same gist, complications within the first month of hospital stay were increased in hyperglycemic patients without DM[31], resulting in a higher all-cause mortality[32]. Hyperglycemia at admission (but without confirmed DM) was related to a 71% increase in mortality in 1317 patients[33].
When hyperglycemia without the presence of DM was compared to known DM (new-onset and/or preexisting DM) in COVID-19 patients, a significant increase in mortality was observed among 271 patients with new-onset hyperglycemia without DM, compared to pre-existing DM. Nevertheless, ICU admission did not seem to differ significantly[34]. Critically and non-critically ill COVID-19 patients sometimes present with higher-than-expected glycemia, even in the absence of DM. Regarding the direct association of glycemia in already admitted patients in ICU due to COVID-19 infection, hyperglycemia was noted in 20 of 36 patients. Among those, none had a prior history of DM and the incidence of hyperglycemia proved to be higher that would be expected in an ICU due to stress-induced responses[35]. In a series of 157 patients with COVID-19, a substantial number of patients with and without DM presented with hyperglycemia upon admission, while critically ill patients showed compromised insulin secretion and/or impaired sensitivity to insulin[36].
OUTCOME IN PATIENTS WITH NEW-ONSET DM VS NORMOGLYCEMIC COVID-19
Νew-onset DM (and/or DKA) has been reported to occur in 16% to 21% of COVID-19 cases[26], but the incidence of complications, need for ICU and intubation, varies among studies (n = 413), with some showing an increase and others no difference, compared to normoglycemic patients[37,38].
OUTCOME IN PATIENTS WITH NEW-ONSET DM VS PRE-EXISTING DM
The risk of all-cause death in COVID-19 patients with new-onset DM is nearly double compared to that of patients with pre-existing DM[38]. A statistically significant association of ICU admission and/or of mortality in COVID-19 patients with new-onset DM (37%), compared to patients with pre-existing DM (20%) was noted; this association persisted after adjustment for age and gender[38].
Summing up the available literature, COVID-19 patients with new-onset hyperglycemia, even without a frank diagnosis of DM due to any cause (stress-induced/COVID-19-induced/pre-existing dysglycemia), show a worse course of the disease, higher rate of complications and all-cause mortality when compared to normoglycemic or patients with DM.
TREATMENT FOR COVID-19 AND GLYCEMIA
In published reports, COVID-19 patients with hyperglycemia/secondary DM were usually treated effectively with insulin[33]. In early reports, patients were also treated with hydroxychloroquine[33]. The latter medication is known to increase endogenous insulin secretion[39]. Since the use of hydroxychloroquine for SARS-CoV-2 was—at least—controversial, and has been phased out, hyperglycemia may be seen more often in patients with SARS-CoV-2 (with or without DM). Possibly higher insulin dosage—than expected—may be needed. Hyperglycemia is also to be expected by the widespread use of dexamethasone in COVID-19 patients, per the newer treatment protocols[40-46].
CONCLUSION
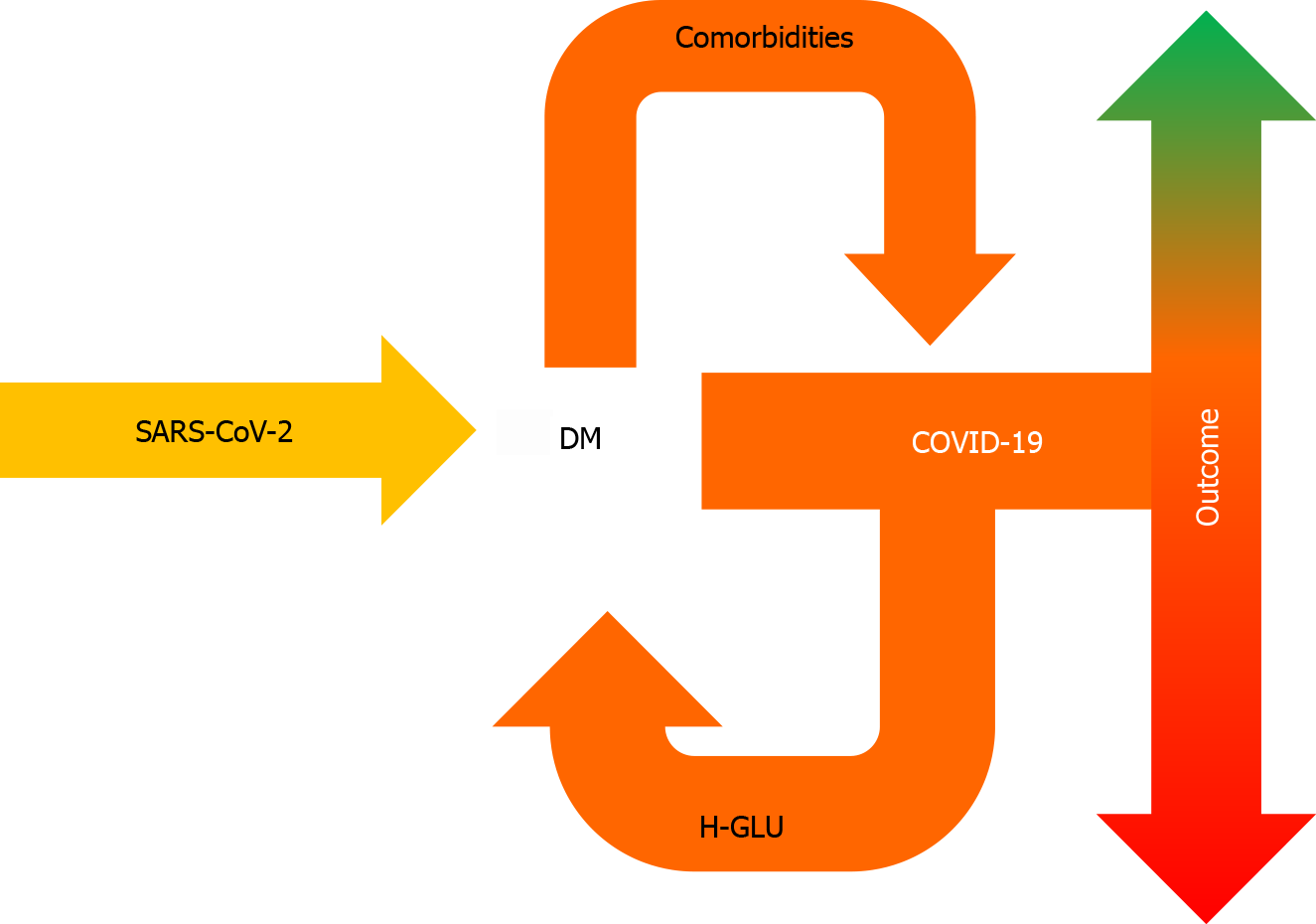
Hyperglycemia in COVID-19, irrespective of insulin resistance or history of DM, is a portent of worse prognosis (Figure 2). Further studies will help to elucidate the link between glycemia and COVID-19.
Figure 2 Pre-existing diabetes mellitus can aggravate coronavirus disease 2019, following severe acute respiratory syndrome coronavirus 2 infection, whereas coronavirus disease 2019 can lead to hyperglycemia — even in the absence of diabetes mellitus, which is associated with worse prognosis.
DM: Diabetes mellitus; COVID-19: Coronavirus disease 2019; H-GLU: Hyperglycemia; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E, Vunnam RR S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ