Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.556
Peer-review started: February 16, 2021
First decision: March 16, 2021
Revised: March 27, 2021
Accepted: April 23, 2021
Article in press: April 23, 2021
Published online: May 15, 2021
Processing time: 78 Days and 22.8 Hours
Renal gluconeogenesis is one of the major pathways for endogenous glucose production. Impairment in this process may contribute to hyperglycemia in cases with insulin resistance and diabetes. We reviewed pertinent studies to elucidate the role of renal gluconeogenesis regulation in insulin resistance and diabetes. A consensus on the suppressive effect of insulin on kidney gluconeogenesis has started to build up. Insulin-resistant models exhibit reduced insulin receptor (IR) expression and/or post-receptor signaling in their kidney tissue. Reduced IR expression or post-receptor signaling can cause impairment in insulin’s action on kidneys, which may increase renal gluconeogenesis in the state of insulin resistance. It is now established that the kidney contributes up to 20% of all glucose production via gluconeogenesis in the post-absorptive phase. However, the rate of renal glucose release excessively increases in diabetes. The rise in renal glucose release in diabetes may contribute to fasting hyperglycemia and increased postprandial glucose levels. Enhanced glucose release by the kidneys and renal expression of the gluconeogenic-enzyme in diabetic rodents and humans further point towards the significance of renal gluconeogenesis. Overall, the available literature suggests that impairment in renal gluconeogenesis in an insulin-resistant state may contribute to hyperglycemia in type 2 diabetes.
Core Tip: Recently, investigators have begun elucidating the role of renal gluconeogenesis in physiology and pathology. Recent evidence suggests a significant role of the kidney in glucose metabolism under pathological conditions, such as insulin resistance and diabetes. This review summarizes the findings from the literature that have enhanced our knowledge related to the significance of renal gluconeogenesis in normal and pathological states.
- Citation: Sharma R, Tiwari S. Renal gluconeogenesis in insulin resistance: A culprit for hyperglycemia in diabetes. World J Diabetes 2021; 12(5): 556-568
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/556.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.556
Gluconeogenesis is the process of glucose production by non-carbohydrate carbon substrates. During the process, glucose-6-phosphate is produced from precursors, like lactate, glycerol, and amino acids, with subsequent hydrolysis by glucose-6-phosphatase (G6Pase) to glucose. Previously, kidney was not considered to significantly contribute to the overall glucose release[1], however, re-evaluation using the net balance techniques suggested up to 20% contribution to overall glucose production[2]. The rate of renal gluconeogenesis varies in response to physiological activities, such as fasting, postprandial, exercise, stress, and pathological stimuli, like diabetes and insulin sensitivity[3-5].
The liver, kidney, and intestine are the three tissues that express the key gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase (FBPase), and G6Pase. G6Pase helps in the final release of glucose into the circulation by dephosphorylating glucose-6-phosphate. PEPCK is involved in the phosphorylation of oxaloacetic acid and FBPase dephosphorylates fructose-1,6 bisphosphate to fructose-6-phosphate.The activity of these enzymes is regulated by insulin. Besides, insulin also regulates the other rate-limiting step, like the availability of gluconeogenesis substrates[6-8]. Renal gluconeogenesis is more sensitive to insulin activity than hepatic gluconeogenesis[3]. Impaired insulin action due to inefficient receptor expression/signaling may blunt insulin’s suppressive effect on gluconeogenesis. It could contribute to hyperglycemia as seen in insulin-resistant and diabetic rat models and humans[9-15]. Patients with type-2 diabetes mellitus exhibit an increase of about 300% in glucose production[16,17]. Glucose-induced glucose release by the kidneys may potentially contribute to postprandial hyperglycemia in diabetic patients[3].Renal gluconeogenesis contributes to normal glucose levels in the post-absorptive state and plays a key role in postprandial hyperglycemia in diabetic patients[5].
The kidneys’ substantial contribution to systemic glucose levels via gluconeogenesis has now been recognized[18-20]. The first evidence of glucose release by the kidneys emerged in 1938 when Bergman et al[21] reported doubled glucose utilization in the hepatectomized animals along with nephrectomy. Several studies confirmed that renal cortex can produce glucose from non-carbohydrate precursors[9,22-25]. The primary sources for renal glucose production involve lactate from cellular respiration, glutamine from protein, and glycerol from triglyceride breakdown[26]. Other than the in vitro studies, incorporating these precursors into glucose by the human kidney has also been quantitated[27,28]. Studies using the isotopic approach in human subjects suggested lactate to be the most important renal gluconeogenic substrate, followed by glutamine and glycerol[3,28,29]. Several studies have suggested kidney's role in maintaining glucose homeostasis through gluconeogenesis[18,19,26]. Early human studies using a combination of net renal glucose balance and isotopic measurements have demonstrated that the kidney releases significant amount of glucose in post-absorptive state[30]. The kidney was once thought to contribute mainly to whole-body glucose production only during acidosis or prolonged starvation[6,18,26]. The role and contribution of the glucose production by the kidney in other physiological and pathological conditions have emerged[18,31]. The kidney accounts for 10% systemic gluconeogenesis in the absorptive phase; the rate rises to as much as 25% in the post-absorptive phase[32]. Moreover, in the case of prolonged fasting, the kidney prevents and reverses hypoglycemia by a counter-regulatory process of increased gluconeogenesis and inhibition of glucose uptake[33]. Besides such adaptive changes, impaired renal insulin signaling/sensitivity affects renal gluconeogenesis[15]. Improving renal insulin sensitivity may reduce systemic glucose levels via gluconeogenesis inhibition [34]. In the postprandial state, the renal glucose release accounts for approximately 50% of the endogenous glucose release for several hours. These observations suggested that increased renal glucose release may play an important role in facilitating efficient liver glycogen repletion by permitting substantial suppression of hepatic glucose release. Hormones (notably insulin and catecholamines), substrates, enzymes, and glucose transporters are some of the other factors which affect glucose production by the kidney[31,35-39].
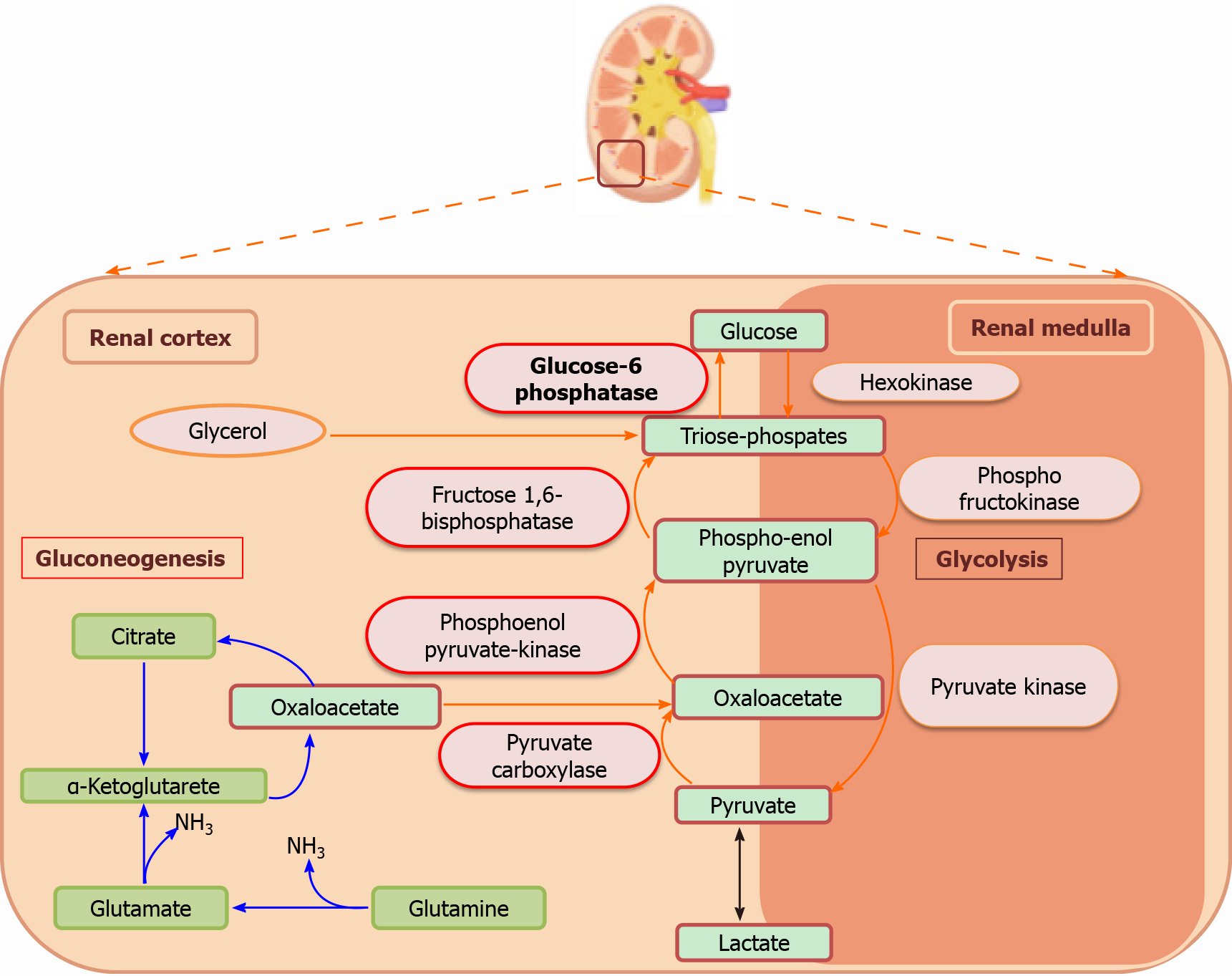
The kidney differentially regulates glucose levels in the medulla and the cortex, with glucose utilization in the renal medulla and glucose production in the kidney cortex[19]. The separation of these processes is based on the differences in the distribution of various enzymes. The nephrons present in the renal medulla have glucose-phosphorylating and glycolytic enzymes; thus, they are involved in the phosphorylation and accumulation of glycogen. However, these cells lack gluconeogenic enzymes, and therefore, cannot synthesize or release free glucose into the circulation. On the other hand, renal cortex cells, more precisely the proximal tubule cells, possess gluconeogenic enzymes, and can produce and release glucose[26,40]. Therefore, the net equilibrium of glucose in the kidney is represented by the difference between renal glucose release by the cortex and renal glucose uptake by the medulla (Figure 1).
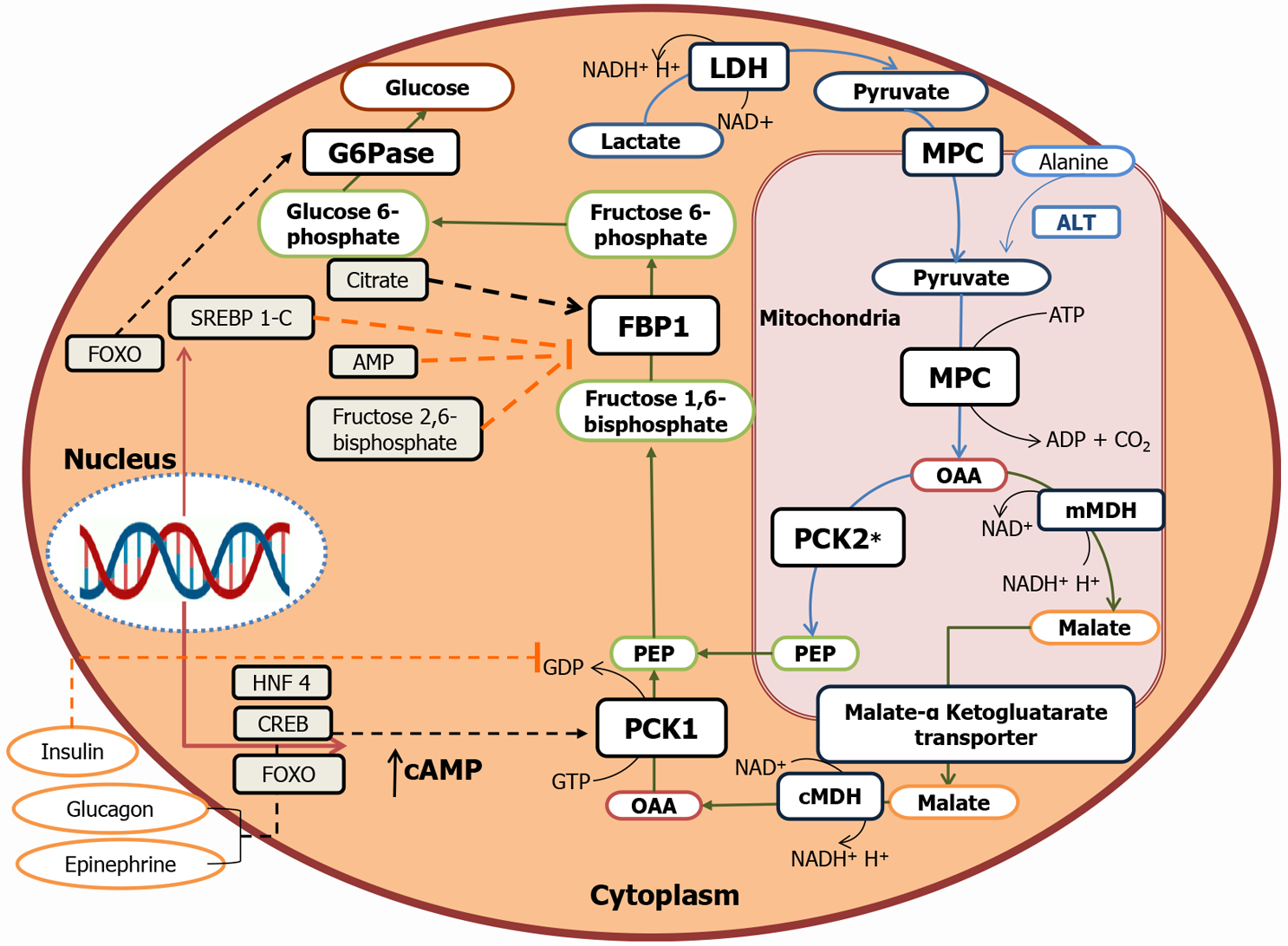
PEPCK, FBPase, G6Pase, and pyruvate carboxylase catalyze the irreversible steps in gluconeogenesis. All these key enzymes are exclusively expressed in the S1–S3 segments of the proximal tubule[41-43]. PEPCK enzymes exist in two isoforms: cytosolic and mitochondrial. These enzymes are encoded by the two nuclear genes. According to human data, 60% of PEPCK is confined to mitochondria, while 40% to cytosol[44]. The cytoplasmic form is regulated at the transcriptional level by nutritional and hormonal stimuli, whereas the expression of mitochondrial form remains constitutive[45] (Figure 2). These three key enzymes are rate-limiting and, under metabolic alterations, PEPCK has been most extensively reported to be regulated. For example, in acidotic conditions, the expression and the activity of renal PEPCK have been found to be upregulated, while G6Pase and FBPase were marginally regulated[15,23,46]. Similarly, under insulin resistance conditions, PEPCK expression increased significantly compared to the levels of FBPase and G6Pase[12,15]. Further, the PEPCK/PCK1 activity in the kidney and the liver of diabetic patients correlates with the levels of PCK1 mRNA, with PEPCK and G6P being regulated at the post-transcriptional level, while FBP being regulated at the pre-or the post-translational level[8,47,48]. PEPCK and G6Pase have been shown to be transcriptionally regulated by a complex network of transcription factors and cofactors, including CREB, HNF-4α, and FOXO1[49].
As discussed in the above sections, kidneys contribute significantly towards the total endogenous glucose production in normal physiological conditions, including fasting and postprandial states[26,50]. After an overnight fast, 75% of glucose entering the circulation is released by the liver, and the remaining 25% is released by the kidney[19,32,51].After a prolonged fast of 48 h, liver glycogen stores are depleted, and renal gluconeogenesis becomes the major source of glucose that is released into the circulation[51,52].Thus, as the duration of fasting increases, the overall proportion of glucose released via renal gluconeogenesis increases[53]. A few studies based on glucose release and glucose uptake by metabolic tissues suggest that the postprandial phase is also important in regulating glucose homeostasis. For example, a 61% decrease in overall glucose release via hepatic glycogenolysis was reported previously in a human study, virtually ceasing in 4 to 6 h[54]. This finding was attributed to the need for replenishing the liver glycogen stores and to limit postprandial hypergly
Insulin has been demonstrated to attenuate enhanced renal gluconeogenesis in rodent models of type 1 diabetes[59,60-66]. Insulin is a known suppressor of gluconeogenesis in both, liver and kidney; however, kidneys are more sensitive to the suppressive effects of insulin[67]. Using the combined isotopic and net balance approach, insulin was shown to suppress renal glucose release and stimulated renal glucose uptake by 75% in conscious dogs[28]. A human study also showed that administration of insulin inhibitor increased renal glucose production in type 1 diabetic patients[19]. At molecular levels, insulin has been demonstrated to reduce the mRNA expressions of PCK1 and G6P[59]. This inhibitory effect is mediated through phosphorylation of FOXO1 via the IRS/Pi3k/Akt/FOXO1 pathway[59,68].Insulin inhibits the availability of gluconeogenic substrates or redirect the substrates to the oxidative pathways [6,26,28]. Moreover, it indirectly affects glucose release via reduction of free fatty acid uptake[6,69,70]. A few reports have documented an inhibitory effect of insulin on renal gluconeogenesis through the substrates glycerol and glutamine in the post-absorptive state in humans[6,28]. However, regulation of renal gluconeogenesis by insulin, glucagon, and epinephrine is not widely studied in humans[6,71,72].
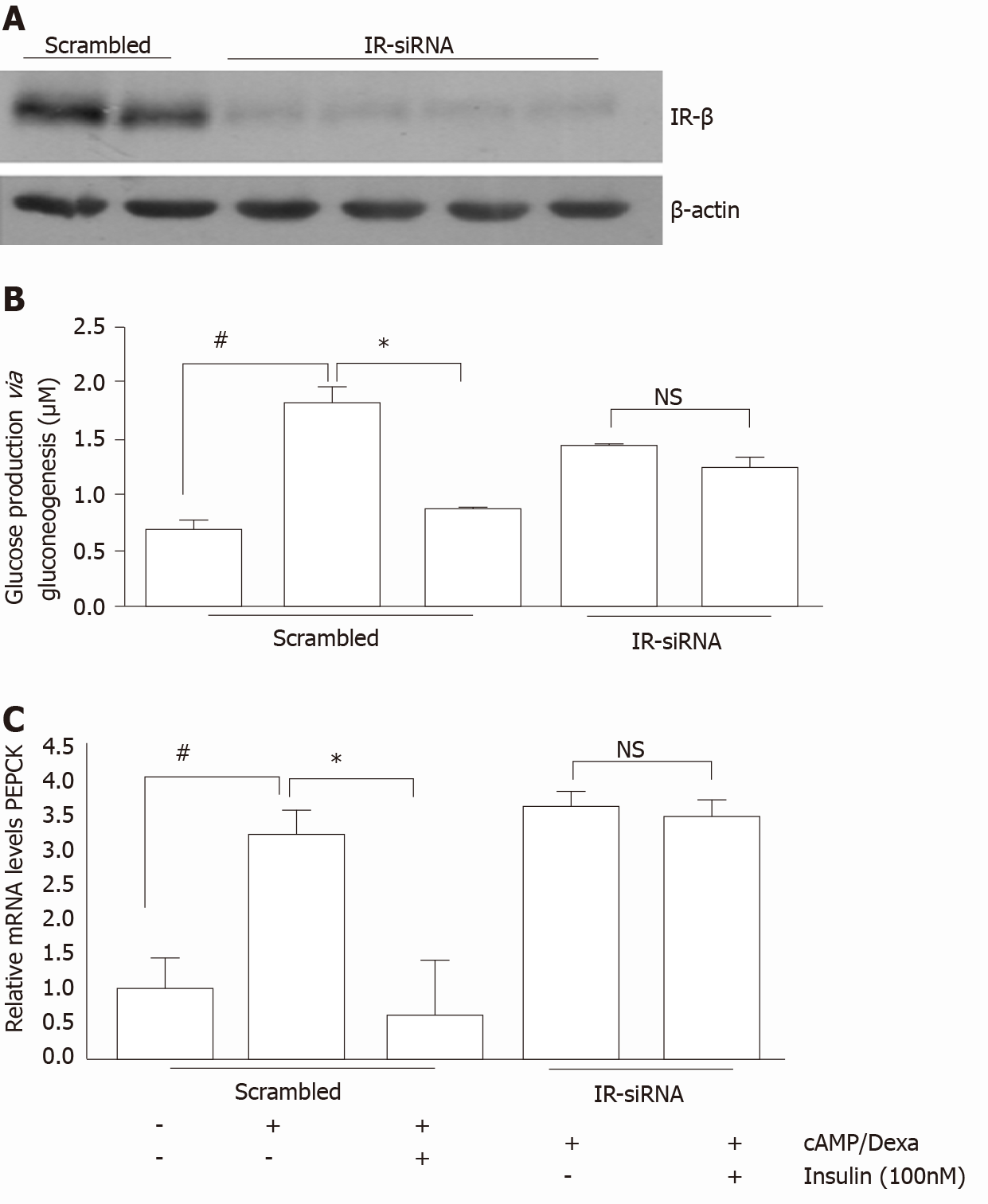
In the liver, the role of insulin or insulin receptor (IR) signaling in transcriptional regulation of gluconeogenic genes, that is, PCK1 and G6PC, is well known[73,74]. However, only a handful of studies have investigated the role of insulin via IR signaling in renal gluconeogenesis regulation. DeFronzoet al[75] reported the inhibitory effect of insulin on renal gluconeogenesis. Previously, we demonstrated high blood glucose and renal gluconeogenic-enzyme upregulation in mice with targeted deletion of IRs from the proximal tubule[13,59]. These IR knock-out (IRKO) mice exhibited normal insulin sensitivity, throughout their bodies. Additionally, increased activity and elevated mRNA expression of G6Pase observed in the IRKO mice indicates the role of the IR in regulating renal gluconeogenesis. In another study, reduced IR expression with a concomitant increase in PEPCK levels were reported in the kidney cortex of mice with high-fat-induced insulin resistance[76].In addition, in vitro studies in primary human proximal tubule (PT)cells also revealed insulin’s inhibitory action on cAMP/DEXA-induced gluconeogenesis, while silencing of the IR attenuated this inhibitory effect[65] (Figure 3). Further down the signaling mechanism, Nakamura et al[77] demonstrated that, unlike the liver, insulin-induced inhibition of proximal tubule gluconeogenesis inhibition might be mediated via the IRS1/ Akt2/mTORC1/2 pathway. In another study, IRS2 (IRS2–/–) knockdown has been shown to result in elevated blood glucose levels in mice[78].However, the post-receptor signaling mechanism for insulin-induced inhibition of renal gluconeogenesis is not yet clear. Nevertheless, these studies indicate the significance of IR signaling in renal gluconeogenesis and suggest that defect in IR signaling to the kidneys may contribute to hyperglycemia in insulin resistance state[9-13,79].
Insulin resistance refers to inefficient sensitivity of primary metabolic tissues towards insulin and is characterized by a reduced insulin action despite hyperinsulinemia [80-82]. Like the other metabolic tissues, kidneys also lose their insulin sensitivity during insulin resistance[14,61,83].The mechanism of insulin resistance is different among different organs and even cells of the same organ. For example, in case of insulin resistance, IRS2 signaling is impaired in liver too. However, in the renal proximal tubules, insulin signaling via IRS1 is impaired; however, the signaling via IRS2 is preserved[84-87].
Insulin resistance has frequently been associated with renal abnormalities, such as impaired glucose metabolism[12,79,88]. These studies suggest that impairment of the expression or post-receptor signaling of the IR can enhance renal gluconeogenesis in the diabetic patients. A wide distribution of IR throughout the nephron segments and their reduced expression in renal epithelial cells in insulin resistance models have been reported[14].We and others have demonstrated reduced expression of IR and its phosphorylated form in the kidney cortex of diabetic rodents and humans [14,61,65,89]. In a previous study, newly diagnosed cases of type-2 diabetes were reported to exhibit impaired insulin-induced suppression of gluconeogenesis [9,11,79]. Our recent study also suggested impairment in meal-induced inhibition of renal PEPCK in individuals with reduced insulin sensitivity[15].Thus, insulin resistance might be responsible for high levels of gluconeogenic enzymes found in renal biopsies from T2D human and rodent models[61,65,90].
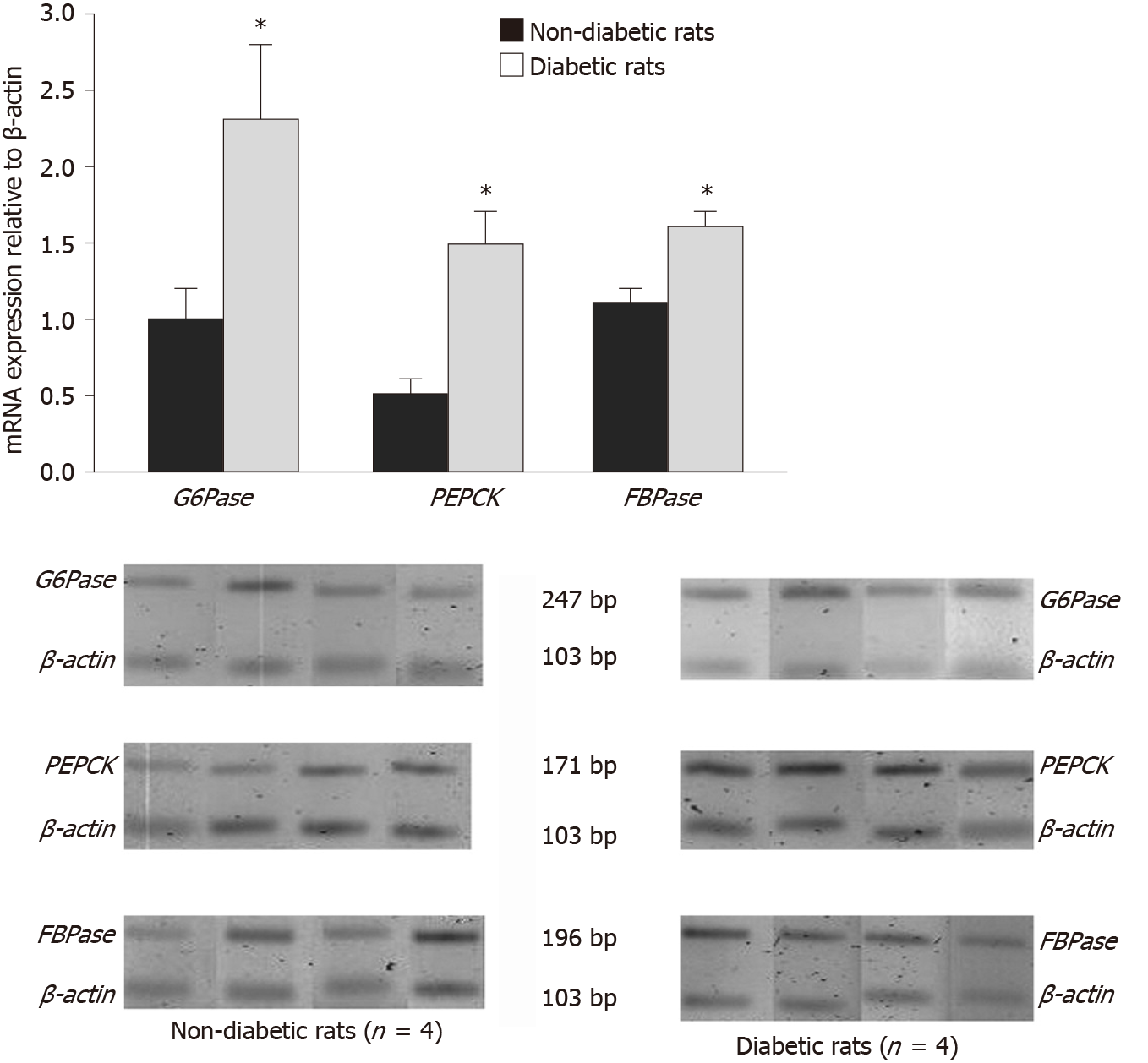
Nevertheless, impaired IR signaling to the kidneys also affects kidneys’ vital functions, including the endogenous glucose production by the kidneys[13,91-93]. We previously reported altered systemic glucose metabolism in IRKO mice, which further strengthens this proposition[13].Thus, similar to the liver, insulin resistance could impair renal gluconeogenesis in diabetes patients[14,61]. Previous studies on diabetic animal models have reported increased renal gluconeogenic enzyme activity and glucose release[48,94-98]. In 1999, Meyer reported significantly higher systemic glucose levels in diabetic patients compared to normal subjects, of which 40% of glucose content was contributed by renal glucose release[16]. Another in vitro study conducted by Eid et al[12], for the very first time, reported increased gluconeogenesis in the proximal tubules of obese Zucker rats. Another in vivo study reported an intrinsic increase in renal gluconeogenesis and increased PEPCK mRNA levels in type 2 diabetic model[12,61,83,99].The other key enzymes, FBPase and G6Pase, were, however, marginally regulated[12] (Figure 4). Moreover, recent rodent model studies conducted by us and others also indicated the significant role of renal gluconeogenesis in fasting hyperglycemia[13,15,59,65]. Furthermore, increased renal gluconeogenesis contributed to increased level of fasting glucose in T2DM patients and raised postprandial glucose. Furthermore, many human studies also reported an increase in the release of glucose by the kidney in the fasting state in T2DM patients[100-104], which might be attributed to gluconeogenesis[105].Additionally, abnormal postpran
Insulin resistance is a known risk factor for developing pre-diabetes, and eventually, type-2 diabetes. Insulin resistance at the kidney level could further contribute to hyperglycemia by enhancing renal gluconeogenesis. Thus, improving insulin sensitivity via lifestyle modifications, such as dieting and physical activity, could be a preventive strategy for pre-diabetes and improving glycemic levels in diabetes patients. Two classes of drugs, biguanides and thiazolidinediones, are available commercially for improving insulin sensitivity. In clinical practice, both these agents are in common use for glucose-lowering in patients with type-2 diabetes[26,109,110]. By enhancing renal insulin sensitivity, these agents exhibit great potential in regulation of renal function in T2DM patients[111,112]. Apart from the known insulin sensitizers, SGLT2 inhibitors are emerging as another promising anti-hyperglycemic agent. They induce glucosuria by inhibiting glucose reabsorption in the renal proximal tubules[113]. Inhibition of renal glucose reabsorption and induction of glucosuria by these agents are considered to be effective and safe in patients with T2DM. Moreover, their insulin-independent action lowers hypoglycemia risk commonly associated with other anti-diabetic drugs[26].
Interestingly, SGLT2 inhibitors have been postulated to act by modulating insulin sensitivity and/or renoprotective actions in T2DM patients[114]. Dapagliflozin, an SGLT2 inhibitor, has been shown to improve renal function and renal insulin signaling in an animal model of diet-induced obesity[115]. Dapagliflozin, either as monotherapy or add-on therapy to insulin or metformin, was found to reduce glucose and HbA1c levels in T2DM in clinical trials[116]. Also, dapagliflozin or empagliflozin, along with insulin therapy, imparts clinical benefits in patients with type-1 diabetes[117,118]. However, more studies are warranted to confirm their therapeutic potential as an adjunct therapy.
Renal gluconeogenesis plays a key role in normal physiology, where its impairment contributes adversely with pathological implications. Overall, this review suggested enhancement or insulin-mediated impairment of renal gluconeogenesis in cases of insulin resistance. Such impairment may further contribute to hyperglycemia in type-2 diabetes. However, more research is warranted in this area to further elucidate the associated mechanism.
We would like to thank Mr. Shashank Mathur for helping with the Figures 1 and 2. We would also like to thank Dr. Maurice B Fluitt (Assistant Professor, Endocrinology and Metabolism, Department of Medicine, Howard University College of Medicine, Washington, DC, United States) and Dr. Kath Clark (Lecturer, Biological Sciences, Department of Molecular and Cell Biology, University of Leicester, University Road, Leicester, LE1 7RH, United Kingdom) for proofreading the manuscript.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Physiological Society, No. 00058535; and American Society of Nephrology, No. 579089.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo GH, Primadhi RA S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ
| 1. | Björkman O, Felig P, Wahren J. The contrasting responses of splanchnic and renal glucose output to gluconeogenic substrates and to hypoglucagonemia in 60-h-fasted humans. Diabetes. 1980;29:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab. 2002;282:E428-E434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 3. | Cersosimo E, Garlick P, Ferretti J. Renal substrate metabolism and gluconeogenesis during hypoglycemia in humans. Diabetes. 2000;49:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 4. | Cersosimo E, Garlick P, Ferretti J. Renal glucose production during insulin-induced hypoglycemia in humans. Diabetes. 1999;48:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Meyer C, Dostou JM, Gerich JE. Role of the human kidney in glucose counterregulation. Diabetes. 1999;48:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Meyer C, Dostou J, Nadkarni V, Gerich J. Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am J Physiol. 1998;275:F915-F921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Miyake K, Ogawa W, Matsumoto M, Nakamura T, Sakaue H, Kasuga M. Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J Clin Invest. 2002;110:1483-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:423-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Basu R, Barosa C, Jones J, Dube S, Carter R, Basu A, Rizza RA. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J Clin Endocrinol Metab. 2013;98:E409-E417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Chung ST, Courville AB, Onuzuruike AU, Galvan-De La Cruz M, Mabundo LS, DuBose CW, Kasturi K, Cai H, Gharib AM, Walter PJ, Garraffo HM, Chacko S, Haymond MW, Sumner AE. Gluconeogenesis and risk for fasting hyperglycemia in Black and White women. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Chung ST, Hsia DS, Chacko SK, Rodriguez LM, Haymond MW. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia. 2015;58:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Eid A, Bodin S, Ferrier B, Delage H, Boghossian M, Martin M, Baverel G, Conjard A. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J Am Soc Nephrol. 2006;17:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Tiwari S, Singh RS, Li L, Tsukerman S, Godbole M, Pandey G, Ecelbarger CM. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J Am Soc Nephrol. 2013;24:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol. 2007;18:2661-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Sharma R, Kumari M, Prakash P, Gupta S, Tiwari S. Phosphoenolpyruvate carboxykinase in urine exosomes reflect impairment in renal gluconeogenesis in early insulin resistance and diabetes. Am J Physiol Renal Physiol. 2020;318:F720-F731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Meyer C, Woerle HJ, Dostou JM, Welle SL, Gerich JE. Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E1049-E1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, Gerich J. Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol. 1998;274:E817-E826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Cano N. Bench-to-bedside review: glucose production from the kidney. Crit Care. 2002;6:317-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE. Renal glucose production and utilization: new aspects in humans. Diabetologia. 1997;40:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 158] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Perriello G, Nurjhan N, Stumvoll M, Bucci A, Welle S, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Regulation of gluconeogenesis by glutamine in normal postabsorptive humans. Am J Physiol. 1997;272:E437-E445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 21. | Bergman H, Drury D. The relationship of kidney function to the glucose utilization of the extra abdominal tissues. American Journal of Physiology-Legacy Content. 1938;124:279-284. |
| 22. | Stumvoll M, Meyer C, Mitrakou A, Gerich JE. Important role of the kidney in human carbohydrate metabolism. Med Hypotheses. 1999;52:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Alleyne GA, Scullard GH. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969;48:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Ambühl PM, Amemiya M, Danczkay M, Lötscher M, Kaissling B, Moe OW, Preisig PA, Alpern RJ. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol. 1996;271:F917-F925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA. Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis. 2004;43:636-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 27. | Gerich JE. Control of glycaemia. Baillieres Clin Endocrinol Metab. 1993;7:551-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Cersosimo E, Garlick P, Ferretti J. Insulin regulation of renal glucose metabolism in humans. Am J Physiol. 1999;276:E78-E84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Conjard A, Martin M, Guitton J, Baverel G, Ferrier B. Gluconeogenesis from glutamine and lactate in the isolated human renal proximal tubule: longitudinal heterogeneity and lack of response to adrenaline. Biochem J. 2001;360:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Buczkowska EO. [The role of the human kidney for glucose homeostasis]. WiadLek. 2004;57:158-160. [PubMed] |
| 31. | Mutel E, Gautier-Stein A, Abdul-Wahed A, Amigó-Correig M, Zitoun C, Stefanutti A, Houberdon I, Tourette JA, Mithieux G, Rajas F. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes. 2011;60:3121-3131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Gerich JE. Lilly lecture 1988.Glucose counterregulation and its impact on diabetes mellitus. Diabetes. 1988;37:1608-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Derlacz RA, Hyc K, Usarek M, Jagielski AK, Drozak J, Jarzyna R. PPAR-gamma-independent inhibitory effect of rosiglitazone on glucose synthesis in primary cultured rabbit kidney-cortex tubules. Biochem Cell Biol. 2008;86:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 35. | Kurokawa K, Massry SG. Evidence for stimulation of renal gluconeogenesis by catecholamines. J Clin Invest. 1973;52:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Lupiáñez JA, Dileepan KN, Wagle SR. Interrelationship of somatostatin, insulin, and calcium in the control of gluconeogenesis in kidney cortex slices. Biochem Biophys Res Commun. 1979;90:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Swe MT, Pongchaidecha A, Chatsudthipong V, Chattipakorn N, Lungkaphin A. Molecular signaling mechanisms of renal gluconeogenesis in nondiabetic and diabetic conditions. J Cell Physiol. 2019;234:8134-8151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavaré JM, Mathieson PW, Saleem MA. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Vallon V. Glucose transporters in the kidney in health and disease. Pflugers Arch. 2020;472:1345-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 40. | Legouis D, Faivre A, Cippà PE, de Seigneux S. Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol Dial Transplant. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 41. | Schmid H, Scholz M, Mall A, Schmidt U, Guder WG, Dubach UC. Carbohydrate metabolism in rat kidney: heterogeneous distribution of glycolytic and gluconeogenic key enzymes. Curr Probl Clin Biochem. 1977;8:282-289. [PubMed] |
| 42. | Guder WG, Schmidt U. The localization of gluconeogenesis in rat nephron.Determination of phosphoenolpyruvate carboxykinase in microdissected tubules. Hoppe Seylers Z Physiol Chem. 1974;355:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Vandewalle A, Wirthensohn G, Heidrich HG, Guder WG. Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol. 1981;240:F492-F500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Hanson RW, Garber AJ. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am J ClinNutr. 1972;25:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Quintas A, Freire AP, Halpern MJ. Bioquímica, Organização Molecular da Vida.Lisboa: Lidel 2008. |
| 46. | Iynedjian PB, Ballard FJ, Hanson RW. The regulation of phosphoenolpyruvate carboxykinase (GTP) synthesis in rat kidney cortex.The role of acid-base balance and glucocorticoids. J Biol Chem. 1975;250:5596-5603. [PubMed] |
| 47. | Bertinat R, Pontigo JP, Pérez M, Concha II, San Martín R, Guinovart JJ, Slebe JC, Yáñez AJ. Nuclear accumulation of fructose 1,6-bisphosphatase is impaired in diabetic rat liver. J Cell Biochem. 2012;113:848-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Mithieux G, Vidal H, Zitoun C, Bruni N, Daniele N, Minassian C. Glucose-6-phosphatase mRNA and activity are increased to the same extent in kidney and liver of diabetic rats. Diabetes. 1996;45:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Kim HJ, Jee HJ, Yun J. DNA damage induces down-regulation of PEPCK and G6P gene expression through degradation of PGC-1alpha. Acta Biochim Biophys Sin (Shanghai). 2011;43:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Benoy MP, Elliott KA. The metabolism of lactic and pyruvic acids in normal and tumour tissues: Synthesis of carbohydrate. Biochem J. 1937;31:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 338] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Consoli A, Kennedy F, Miles J, Gerich J. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987;80:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 53. | Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med. 1997;102:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Meyer C, Dostou JM, Welle SL, Gerich JE. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;282:E419-E427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. ExpMol Med. 2016;48:e219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 549] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 56. | Geary N. Postprandial Suppression of Glucagon Secretion: A Puzzlement. Diabetes. 2017;66:1123-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Joseph SE, Heaton N, Potter D, Pernet A, Umpleby MA, Amiel SA. Renal glucose production compensates for the liver during the anhepatic phase of liver transplantation. Diabetes. 2000;49:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Gerich JE. Hepatorenal glucose reciprocity in physiologic and pathologic conditions. Diabetes Nutr Metab. 2002;15:298-302; discussion 302. [PubMed] |
| 59. | Sasaki M, Sasako T, Kubota N, Sakurai Y, Takamoto I, Kubota T, Inagi R, Seki G, Goto M, Ueki K, Nangaku M, Jomori T, Kadowaki T. Dual Regulation of Gluconeogenesis by Insulin and Glucose in the Proximal Tubules of the Kidney. Diabetes. 2017;66:2339-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Patel SN, Parikh M, Lau-Cam CA. Impact of light ethanol intake and of taurine, separately and together, on pathways of glucose metabolism in the kidney of diabetic rats. Adv Exp Med Biol. 2015;803:279-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Gatica R, Bertinat R, Silva P, Carpio D, Ramírez MJ, Slebe JC, San Martín R, Nualart F, Campistol JM, Caelles C, Yáñez AJ. Altered expression and localization of insulin receptor in proximal tubule cells from human and rat diabetic kidney. J Cell Biochem. 2013;114:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Tojo A, Hatakeyama S, Kinugasa S, Nangaku M. Angiotensin receptor blocker telmisartan suppresses renal gluconeogenesis during starvation. Diabetes Metab Syndr Obes. 2015;8:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Tojo A, Hatakeyama S, Nangaku M, Ishimitsu T. H+-ATPase blockade reduced renal gluconeogenesis and plasma glucose in a diabetic rat model. Med Mol Morphol. 2018;51:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 64. | von Morze C, Chang GY, Larson PE, Shang H, Allu PK, Bok RA, Crane JC, Olson MP, Tan CT, Marco-Rius I, Nelson SJ, Kurhanewicz J, Pearce D, Vigneron DB. Detection of localized changes in the metabolism of hyperpolarized gluconeogenic precursors 13 C-lactate and 13 C-pyruvate in kidney and liver. Magn Reson Med. 2017;77:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Pandey G, Shankar K, Makhija E, Gaikwad A, Ecelbarger C, Mandhani A, Srivastava A, Tiwari S. Reduced Insulin Receptor Expression Enhances Proximal Tubule Gluconeogenesis. J Cell Biochem. 2017;118:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Winiarska K, Jarzyna R, Dzik JM, Jagielski AK, Grabowski M, Nowosielska A, Focht D, Sierakowski B. ERK1/2 pathway is involved in renal gluconeogenesis inhibition under conditions of lowered NADPH oxidase activity. Free Radic Biol Med. 2015;81:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Cersosimo E, Garlick P, Ferretti J. Regulation of splanchnic and renal substrate supply by insulin in humans. Metabolism. 2000;49:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1081] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 69. | Ader M, Bergman RN. Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am J Physiol. 1990;258:E1020-E1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Lewis GF, Vranic M, Harley P, Giacca A. Fatty acids mediate the acute extrahepatic effects of insulin on hepatic glucose production in humans. Diabetes. 1997;46:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Stumvoll M, Meyer C, Kreider M, Perriello G, Gerich J. Effects of glucagon on renal and hepatic glutamine gluconeogenesis in normal postabsorptive humans. Metabolism. 1998;47:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest. 1995;96:2528-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285:E685-E692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 74. | Valenti L, Rametta R, Dongiovanni P, Maggioni M, Fracanzani AL, Zappa M, Lattuada E, Roviaro G, Fargion S. Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis. Diabetes. 2008;57:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 75. | DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 357] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 76. | Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 Suppl 3:S215-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 769] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 77. | Nakamura M, Tsukada H, Seki G, Satoh N, Mizuno T, Fujii W, Horita S, Moriya K, Sato Y, Kume H, Nangaku M, Suzuki M. Insulin promotes sodium transport but suppresses gluconeogenesis via distinct cellular pathways in human and rat renal proximal tubules. Kidney Int. 2020;97:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Hashimoto S, Maoka T, Kawata T, Mochizuki T, Koike T, Shigematsu T. Roles of Insulin Receptor Substrates (IRS) in renal function and renal hemodynamics. PLoS One. 2020;15:e0242332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | DeFronzo RA, Ferrannini E. Insulin resistance.A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2964] [Cited by in RCA: 2851] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 81. | Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1011] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 82. | Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12:721-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 293] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 83. | Liu Q, Zhang L, Zhang W, Hao Q, Qiu W, Wen Y, Wang H, Li X. Inhibition of NF-κB Reduces Renal Inflammation and Expression of PEPCK in Type 2 Diabetic Mice. Inflammation. 2018;41:2018-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 85. | Mima A, Ohshiro Y, Kitada M, Matsumoto M, Geraldes P, Li C, Li Q, White GS, Cahill C, Rask-Madsen C, King GL. Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 2011;79:883-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 86. | Horita S, Nakamura M, Suzuki M, Satoh N, Suzuki A, Seki G. Selective Insulin Resistance in the Kidney. Biomed Res Int. 2016;2016:5825170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 87. | Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 742] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 88. | Martyn JA, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 89. | Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Yañez AJ, Ludwig HC, Bertinat R, Spichiger C, Gatica R, Berlien G, Leon O, Brito M, Concha II, Slebe JC. Different involvement for aldolase isoenzymes in kidney glucose metabolism: aldolase B but not aldolase A colocalizes and forms a complex with FBPase. J Cell Physiol. 2005;202:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Kumari M, Sharma R, Pandey G, Ecelbarger CM, Mishra P, Tiwari S. Deletion of insulin receptor in the proximal tubule and fasting augment albumin excretion. J Cell Biochem. 2019;120:10688-10696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VR, Ranjan S, Wolter J, Kohli S, Shahzad K, Heidel F, Krueger M, Schwenger V, Moeller MJ, Kalinski T, Reiser J, Chavakis T, Isermann B. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun. 2015;6:6496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 93. | Pandey G, Makhija E, George N, Chakravarti B, Godbole MM, Ecelbarger CM, Tiwari S. Insulin regulates nitric oxide production in the kidney collecting duct cells. J Biol Chem. 2015;290:5582-5591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Weber G, Lea MA, Convery HJ, Stamm NB. Regulation of gluconeogenesis and glycolysis: studies of mechanisms controlling enzyme activity. Adv Enzyme Regul. 1967;5:257-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | BEARN AG, BILLING BH, SHERLOCK S. Hepatic glucose output and hepatic insulin sensitivity in diabetes mellitus. Lancet. 1951;2:698-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Carlsten A, Hallgren B, Jagenburg R, Svanborg A, Werkö L. Arterio-hepatic venous differences of free fatty acids and amino acids. Studies in patients with diabetes or essential hypercholesterolemia, and in healthy individuals. Acta Med Scand. 1967;181:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Felig P, Wahren J, Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes. 1978;27:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Waldhäusl W, Bratusch-Marrain P, Gasić S, Korn A, Nowotny P. Insulin production rate, hepatic insulin retention and splanchnic carbohydrate metabolism after oral glucose ingestion in hyperinsulinaemic Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1982;23:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Rosella LC, Lebenbaum M, Fitzpatrick T, Zuk A, Booth GL. Prevalence of Prediabetes and Undiagnosed Diabetes in Canada (2007-2011) According to Fasting Plasma Glucose and HbA1c Screening Criteria. Diabetes Care. 2015;38:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 100. | TENG CT. Studies on carbohydrate metabolism in rat kidney slices. II. Effect of alloxan diabetes and insulin administration on glucose uptake and glucose formation. Arch Biochem Biophys. 1954;48:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | LANDAU BR. Gluconeogenesis and pyruvate metabolism in rat kidney, in vitro. Endocrinology. 1960;67:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | FLINN RB, LEBOEUF B, CAHILL GF Jr. Metabolism of C14-labeled substrates in kidney cortical slices from normal and alloxan-diabetic rats. Am J Physiol. 1961;200:508-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Joseph PK, Subrahmanyam K. Effect of growth hormone, insulin, thyroxine and cortisone on renal gluconeogenesis. Arch Biochem Biophys. 1968;127:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Chang AY, Schneider DI. Rate of gluconeogenesis and levels of gluconeogenic enzymes in liver and kidney of diabetic and normal Chinese hamsters. Biochim Biophys Acta. 1970;222:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J. Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. J Clin Invest. 1998;102:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 187] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 106. | Lemieux G, Aranda MR, Fournel P, Lemieux C. Renal enzymes during experimental diabetes mellitus in the rat. Role of insulin, carbohydrate metabolism, and ketoacidosis. Can J Physiol Pharmacol. 1984;62:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Krebs HA, Speake RN, Hems R. Acceleration of renal gluconeogenesis by ketone bodies and fatty acids. Biochem J. 1965;94:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 108. | Williamson JR. Mechanism for the stimulation in vivo of hepatic gluconeogenesis by glucagon. Biochem J. 1966;101:11C-14C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis. 2010;55:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 110. | Lachin JM, Viberti G, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Holman RR, Kahn SE; ADOPT Study Group. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol. 2011;6:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Fujii M, Takemura R, Yamaguchi M, Hasegawa G, Shigeta H, Nakano K, Kondo M. Troglitazone (CS-045) ameliorates albuminuria in streptozotocin-induced diabetic rats. Metabolism. 1997;46:981-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1540] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 113. | Ferrannini E. Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell Metab. 2017;26:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 232] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 114. | Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, Wanner C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 115. | Jaikumkao K, Pongchaidecha A, Chueakula N, Thongnak L, Wanchai K, Chatsudthipong V, Chattipakorn N, Lungkaphin A. Renal outcomes with sodium glucose cotransporter 2 (SGLT2) inhibitor, dapagliflozin, in obese insulin-resistant model. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 339] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 117. | Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, Bogle A, Iqbal N, List J, Griffen SC. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 118. | Perkins BA, Cherney DZ, Partridge H, Soleymanlou N, Tschirhart H, Zinman B, Fagan NM, Kaspers S, Woerle HJ, Broedl UC, Johansen OE. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |