Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.524
Peer-review started: January 25, 2021
First decision: February 25, 2021
Revised: March 31, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: May 15, 2021
Processing time: 100 Days and 17 Hours
Lipid dysmetabolism is one of the main features of diabetes mellitus and manifests by dyslipidemia as well as the ectopic accumulation of lipids in various tissues and organs, including the kidney. Research suggests that impaired cholesterol metabolism, increased lipid uptake or synthesis, increased fatty acid oxidation, lipid droplet accumulation and an imbalance in biologically active sphingolipids (such as ceramide, ceramide-1-phosphate and sphingosine-1-phosphate) contribute to the development of diabetic kidney disease (DKD). Currently, the literature suggests that both quality and quantity of lipids are associated with DKD and contribute to increased reactive oxygen species production, oxidative stress, inflammation, or cell death. Therefore, control of renal lipid dysmetabolism is a very important therapeutic goal, which needs to be archived. This article will review some of the recent advances leading to a better understanding of the mechanisms of dyslipidemia and the role of particular lipids and sphingolipids in DKD.
Core Tip: The present review summarizes the recent knowledge about the role of lipids and sphingolipids in the development and progression of diabetic kidney disease (DKD). The main focus is given to the cholesterol and triglyceride metabolism abnormalities, lipid droplet accumulation and role of sphingolipids in DKD.
- Citation: Mitrofanova A, Burke G, Merscher S, Fornoni A. New insights into renal lipid dysmetabolism in diabetic kidney disease. World J Diabetes 2021; 12(5): 524-540
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/524.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.524
Lipids are essential components of a cell plasma membrane with multiple cellular functions, highlighting their importance in cell homeostasis and survival. Diabetic kidney disease (DKD) is often considered to be a consequence of hyperglycemia in a setting of diabetes mellitus. However, lipid accumulation in podocytes, which are specialized epithelial cells lining the urinary surface of the glomerular capillary tuft, has been recently reported to drive the development of DKD[1]. Lipids are also key modulators of insulin signaling in several cell types including the podocyte[2,3].
The toxicity of lipid accumulation (lipotoxicity) in the kidney was first proposed by Moorhead et al[4] in 1982 and later updated by Ruan et al[5] in 2009, suggesting that lipid dysmetabolism promotes the progression of kidney diseases, including DKD. However, the specific contribution of podocyte lipid dysmetabolism to the pathogenesis and progression of DKD has been largely unexplored. Growing evidence suggests that lipotoxicity-associated renal damage depends not only on the quantity of lipids that accumulate in the kidney but also on the lipid species[6]. In recent years, a clear role of sphingolipids and glycolipids in the pathogenesis of DKD has been also established[7-11]. Given the fact that podocytes, the terminally differentiated epithelial cells in the glomerulus, are main contributors to the proper filtration function in the kidney, changes in their number[12] and function lead to the development and progression of glomerular disease, including DKD. However, what is the cause of podocyte detachment and death in DKD remains largely unknown. We have previously published several reviews related to the role of lipids and sphingolipids in glomerular diseases with focus on insulin signaling[2], inflammation[13], and mitochondria dysfunction[14]. This review is an update on the latest knowledge with regard to the mechanisms contributing to renal lipid dysmetabolism focusing on cholesterol metabolism, fatty acid oxidation, lipid droplet accumulation and sphingolipids and how they contribute to the development and progression of DKD.
In any cell, lipid metabolism encompasses the synthesis and degradation of lipids to meet the body’s energy needs. Some lipids are being constantly oxidized, while others are being synthesized and stored. Thus, triacylglycerols are broken into free fatty acids (FFA), which undergo β-oxidation in mitochondria to produce acetyl coenzyme A (CoA), utilized in the tricarboxylic acid cycle or ketogenesis to generate energy. FFA are also involved into other biosynthetic pathways to produce membrane lipids (such as phospholipids, glycolipids, sphingolipids, or cholesterol) or signaling molecules (such as prostaglandins, leukotrienes, and thromboxanes). These metabolic pathways are tightly regulated by enzyme-catalyzed reactions and defects in any of these enzymes is associated with a wide range of health problems.
Podocytes are visceral epithelial cells of the glomerulus, which are involved in filtration and formation of primary urine. Foot processes are the most recognizable characteristic structures of podocytes and the formation of specialized junctions between foot process of neighboring podocytes, known as the slit diaphragm, and of foot processes and the glomerular basement membrane, known as the adhesion complex, are important for maintaining glomerular function[15]. The podocyte slit diaphragm is assembled in lipid rafts, which are small specialized plasma membrane domains enriched with cholesterol, sphingolipids and protein complexes with important functions in cellular signaling transduction. Cholesterol of the lipid rafts plays an important role in regulating the organization, localization and function of proteins within the slit diaphragm. Excess of cholesterol negatively affects the binding of slit diaphragm proteins to each other[16], or interferes with the ability of podocyte slit-diaphragm proteins to bind caveolin-1, an important transductor of the insulin receptor signaling in podocytes[17].
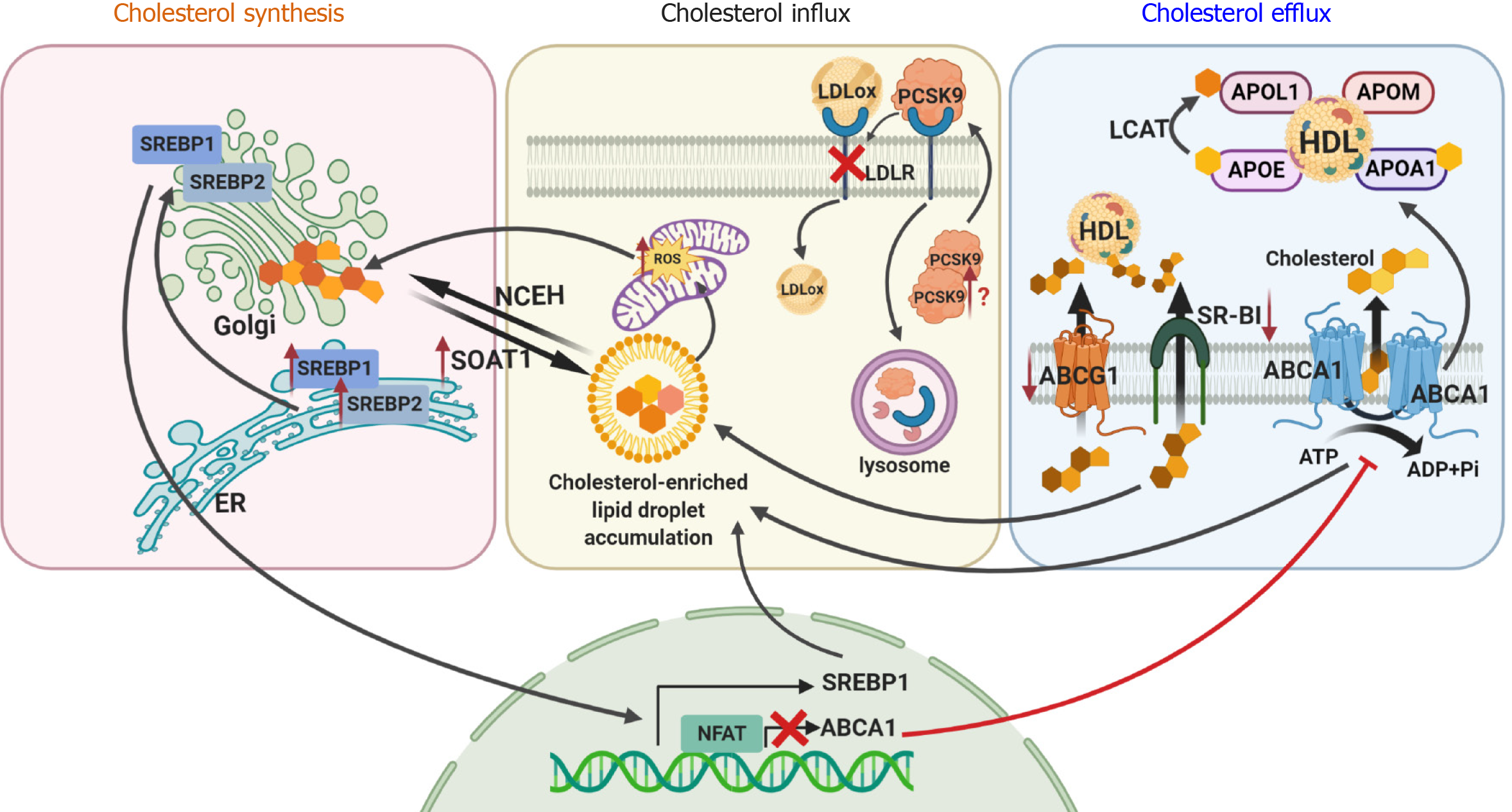
Cholesterol is synthesized starting from acetyl CoA in the de novo pathway and/or it can be imported from circulating lipoproteins by receptor-mediated endocytosis (influx). Excess cholesterol is released through several distinct pathways (efflux). Tight regulation of these three mechanisms is very important to maintain proper cholesterol metabolism within the cell, as unesterified (free) cholesterol is toxic to cells.
Intracellular cholesterol sensing is mainly regulated via sterol regulatory element-binding protein (SREBP, and its known isoforms SREBP-1a, SREBP-1c, SREBP-2), an endoplasmic reticulum resident. Increased expression of SREBP1 and SREBP2 has also been reported in glomeruli of DKD patients based on microarray data available from the Nephroseq database[18,19]. Increased expression of SREBP has been described to contribute to kidney damage in obesity-related diabetes and in mice fed on a high fat diet[20-24]. Additional studies demonstrated a role of SREBP1 in the accumulation of lipid droplet in murine models of type 1 diabetes[25]. In support, the inhibition of SREBP isoforms was found to attenuate the renal phenotype such as albuminuria or mesangial expansion in age-related renal disease and in DKD[16-20]. In contrast, a recent study reports that fatostatin treatment of 12-wk-old male mice with streptozotocin-induced diabetes, an inhibitor of SREBP-1 and SREBP-2, prevents glomerular basement membrane thickening, but does not improve albuminuria or hyperfiltration[26]. Thus, further studies are needed to determine if SREBP inhibition may be more beneficial in combination with other therapies to prevent DKD progression.
Cholesterol is transported in the circulation by two major lipoproteins, low-density lipoprotein (LDL) and high-density lipoprotein (HDL). The influx of cholesterol is primarily mediated via LDL receptors (LDLR), followed by endocytosis and the formation of LDL-containing vesicles connected to lysosomes. Free cholesterol is then transported to the endoplasmic reticulum (ER) or plasma membrane via Niemann Pick C1 or C2 transporters. In the ER, increased free cholesterol levels activate sterol-O-acyltransferase 1 (SOAT1; or acyl-CoA:cholesterol acyltransferase (ACAT1)) to form cholesterol esters for storage in lipid droplet. We recently demonstrated that genetic loss of SOAT1 in diabetic db/db mice ameliorates kidney injury by reducing cholesterol esters and lipid droplet accumulation[27]. More recently, proprotein convertase subtilisin/Kexin Type 9 (PCSK9) inhibitors, which have been developed to controlled hyperlipidemia by affecting LDL uptake and clearance in hepatocytes, have been shown to control the hyperlipidemia associated with nephrotic syndrome[28]. As PCSK9 is also expressed in the kidney[29], the contribution of PCSK9 to renal lipotoxicity remains to be explored.
Excessive cholesterol accumulation in podocytes is also associated with suppressed efflux in both experimental[22,30] and human DKD[6]. Cholesterol efflux from cells, including podocytes, occurs primarily via ATP-binding cassette transporters sub-class A (ABCA1), G (ABCG1) and scavenger receptor class B type I (SR-BI). We previously reported that normal human podocytes exposed to serum from patients with type 1 and type 2 diabetes and early stage of DKD are characterized by increased lipid droplet accumulation and reduced expression of ABCA1[3,31,32]. We also found that the expression of ABCA1 correlates with markers of DKD progression clinically and in experimental mouse models (diabetic BTBR ob/ob and db/db mice)[32]. Studies in diabetic NOD mice also demonstrated significant reduction (48%) of ABCA1 expression in kidneys[30]. While deficiency of ABCA1 is a susceptibility factor in DKD and contributes to the accumulation of lipid droplet in podocytes, it is not sufficient to cause glomerular injury itself[31,32]. Further studies demonstrated that ABCA1 overexpression reduces albuminuria in mice with podocyte-specific activation of nuclear factor of activated T cells (NFAT)[31], another susceptibility factor for cholesterol-dependent podocyte injury. Interestingly, in human glomerular cells, interleukin 1β has also been shown to inhibit cholesterol efflux possibly via suppression of ABCA1 expression[33]. By contrast, pharmacological induction of cholesterol efflux using cyclodextrin or ezetimibe, a small molecule ABCA1 inducer, resulted in amelioration of DKD progression and DKD-like glomerulosclerosis[3,32]. Exendin-4, an agonist of glucagon-like peptide 1, has also been shown to upregulate ABCA1 in glomerular endothelial cells and improve glomerular hypertrophy, basement membrane thickening and mesangial expansion[34]. Interestingly, in diabetic patients (n = 1746, all Caucasians), the ABCA1 rs9282541 (R230) polymorphism has been shown to be associated with increased risk of diabetes, while the ABCA1 rs1800977 (C69T) polymorphism was found to be associated with a significantly reduced risk of hypertriglyceridemia[35]. The rs9282541 polymorphism has also been reported to be associated with susceptibility to type 2 diabetes in patients from Mexico[36]. In contrast, studies in patients with type 2 diabetes (n = 107) from Turkey[37] and in Chinese Han population (n = 508)[38] failed to link ABCA1 rs1800977 polymorphism to lipid dysmetabolism. More recently, an association between LXR-alpha and ABCA1 gene polymorphisms was found to be associated with the risk for DKD in a Chinese population[39]. While ABCA1 mediates cholesterol transport to apolipoprotein A-I (Apo A-I) and pre-β HDL, two other transporters, ABCG1 and SR-BI, mediate cholesterol transport to mature HDL. In mouse models of DKD, significant suppression of ABCG1 and SR-BI was found in mesangial and tubular cells[40].
Taken together, these studies demonstrate that cholesterol accumulation and lipid droplet accumulation may represent a hallmark of DKD[41-43]. Based on our own studies and reports from others, we conclude that cholesterol accumulation in glomerular cells occurs independent of systemic cholesterol levels and that local lipid dysmetabolism contributes to DKD progression in patients with diabetes (Figure 1).
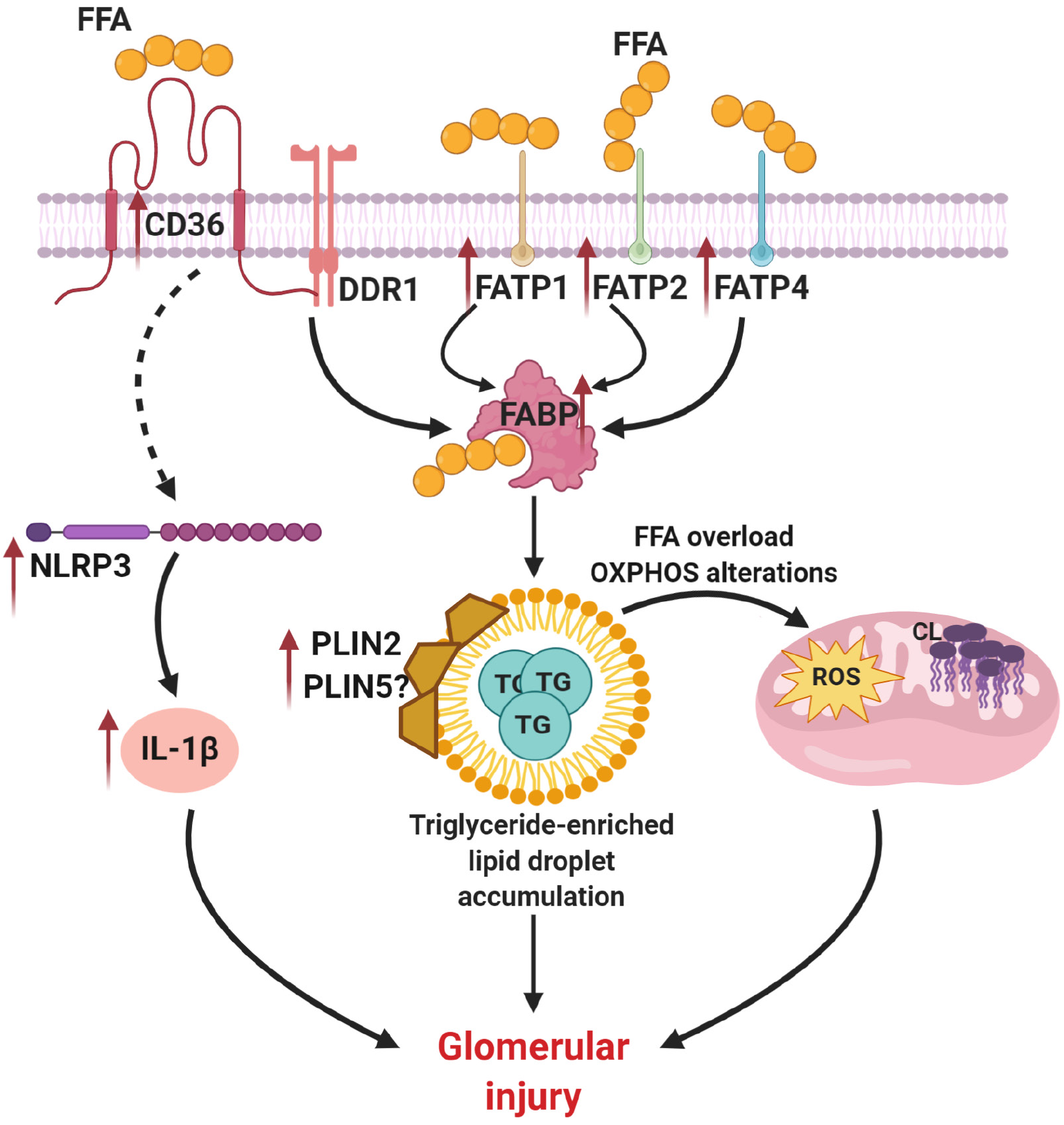
In the blood most of the circulating lipids are present as triglycerides within very low-density lipoprotein (VLDL). Triglycerides are composed of free fatty acid (FFA) and glycerol. Several fatty acid transport proteins (FATPs) control uptake of FFA into a cell. In the kidney, FATP1, FATP2, and FATP4 were shown to be mostly responsible for lipid uptake abnormalities in patients with DKD. Thus, a recent study on a population of type 2 diabetic patients (n = 268) demonstrated that expression levels of FATP1 and FATP2 in plasma are associated with progression of DKD[44]. In support, deletion of FATP2 in different mouse models of DKD (db/db and eNOS-/- diabetic mice and low dose streptozotocin-induced diabetic mice on a high fat diet) was sufficient to improve the renal outcome[45]. It has been also demonstrated that expression of FATP4 is higher in tubules of mice on a high fat diet[46], suggesting a role of FATP4 in insulin resistance and obesity. Interestingly, levels of FATP4 in db/db mice were shown to be elevated in parallel with increased renal lipid accumulation and progression of DKD, which is also associated with vascular endothelial growth factor B (VEGF-B) signaling[1]. In obese Wistar rats on high fat diet increased levels of FFA in glomerular endothelial cells were shown to be associated with microalbuminuria via VEGF-NO axis[47]. In patients with type 2 diabetes FATP4 is associated with glomerular filtration rate[48].
Other contributors to the lipid uptake abnormalities in DKD are the fatty-acid binding proteins (FABPs), which belong to a super-family of lipid-binding proteins and recognize long-chain fatty acids as substrates. Thus, urinary liver-type FABP (L-FABP) was shown to be a reliable marker of DKD development and progression in patients with diabetes[49-52]. Interestingly, in spontaneously diabetic Torii fatty rats higher levels of urinary L-FABP were shown, which was ameliorated with Liraglutide treatment[53].
Cluster of differentiation 36 (CD36), a class B scavenger receptor, is the most important transmembrane glycoprotein that mediates uptake of oxidized LDLs. CD36 is also the main uptake system of FFA in the kidney, where it is highly expressed in proximal and distal epithelial cells, podocytes and mesangial cells. Increased expression of CD36 seems to be associated with kidney damage in DKD. Earlier studies demonstrated that high glucose-mediated overexpression of CD36 induces apoptosis in renal tubular epithelial cells[54,55] and podocytes[56]. Interestingly, CD36 has also been shown to facilitate chronic inflammation, oxidation stress and fibrosis in proximal tubular cells under hyperglycemic conditions[57]. Using human podocytes, our studies suggest a novel mechanism where discoidin domain receptor 1 (DDR1), a tyrosine kinase activated by collagen I, interacts with CD36 and leads to increased CD36-dependent FFA uptake[58]. Another study demonstrated that astragaloside IV inhibits overexpression of CD36 in human glomerular mesangial cells and diabetic rats (Sprague Dawley) in response to palmitate-induced FFA accumulation and attenuates FFA uptake, oxidative stress and fibrosis[59].
In mouse podocytes treated with palmitic acid increased expression of CD36 has been shown in association with increased reactive oxygen species (ROS) production and apoptosis[60]. In mice with transgenic overexpression of CD36 in the kidney, accumulation of lipids and triglycerides in kidneys was demonstrated[61]. Additionally, CD36 is involved in the generation of other cell-specific responses via toll-like receptors (TLRs) 2, 4 and 6[62-64], CD9[65], or integrin[66] leading to the activation of pyrin domain-containing 3 (NLRP3) and nuclear factor kappa B (NF-kB) signaling pathways[67,68]. Indeed, CD36 can also recognize advanced oxidation protein products and advanced glycation end products, which are also involved in inflammatory pathway activation[69], including the kidney[57].
In patients with DKD increased expression of CD36 was reported[55,60]. Interestingly, a circulating soluble form of CD36 (sCD36), whose derivation is not entirely clear, may play a role as a cellular source of CD36 in diabetic patients and correlates with insulin resistance[70,71]. A recent study demonstrated elevated levels of sCD36 in both plasma and urine of patients with DKD[72]. However, while one study suggests that sCD36 Levels are elevated in patients with type 2 diabetes and proposes to use it as a biomarker[73], another study reports no differences in the sCD36 Levels between patients with type 1 and type 2 diabetes[74].
Thus, CD36 has an important role in the lipid homeostasis in the kidney with an important role in the crosstalk between CD36 Ligands and inflammation or apoptosis signaling pathways. Therefore, CD36 may represent a promising target for therapeutic intervention. However, further studies of the role of CD36 in DKD progression are needed to answer the questions: (1) How is sCD36 formed in patients with diabetes and what is the tissue-specific role of sCD36? (2) What are the particular mechanisms of increased FFA uptake in tubular cells vs podocytes? And (3) What are the mechanisms involved in the kidney cell-specific regulation of CD36 levels or function (tubular cells vs podocytes)? A better understanding of the mechanisms regulating the FFA uptake in rodents and its translation to humans will be a determinative factor in the development of novel peptides aimed at regulating CD36 Levels with minimum off-target effects.
Fatty acid oxidation (FAO), also called beta oxidation, is the aerobic process of fatty acid (short-, medium- or long-chain saturated fatty acyl coenzyme A, acyl-CoA) breakdown that occurs in mitochondria to provide energy from fats. During FAO, acetyl coenzyme A (acetyl-CoA), five molecules of ATP, and water are generated. Interestingly, FAO covers more than half of renal oxygen consumption. In the setting of kidney disease, genes that are associated with FAO are significantly downregulated in kidneys of mice and humans[61], which is also associated with increased fatty acid synthesis and higher intracellular lipid deposition. We have recently reported that human podocytes cultured in the presence of serum from DKD patients have significantly decreased expression of FAO genes (PPARα, ACADM, ACOX1/2), which was also observed in mouse models of DKD and in our mouse model of ABCA1 deficiency[32]. In a longitudinal study on American Indians with type 2 diabetes (n = 92), a significant reduction of FAO has been shown, which was also associated with lower abundance of C16-C20 acylcarnitines[75]. Pharmacological or genetic increase in FAO has been shown to be beneficial to improve kidney disease progression[61].
Peroxisome proliferator-activated receptors (PPARs) play a key role in the regulation of FAO in the kidney. PPARg, one of the PPARs isoforms, is highly expressed in different compartments of a nephron while decreased expression contributes to diabetes-associated kidney damage. Activation of PPARg (using thiazolidinediones) is associated with attenuation of kidney function in diabetic patients and mouse models of DKD[76-78]. Recently, a role of micro-RNA-27a (miR-27a) in the regulation of PPARg activity was demonstrated[79], suggesting miR-27a as a potential therapeutic target in DKD. In a streptozotocin-induced diabetic mouse model of DKD, activation of PPARδ ameliorates diabetes-associated renal damage [80]. Lack of PPARα, another PPAR isoform, has also been shown to accelerate DKD in a streptozotocin-induced diabetic mouse model[81]. Tesaglitazar, the PPARα/g dual agonist, markedly attenuated albuminuria and lowered collagen deposition in kidneys of db/db mice[82]. In contrast, use of a PPARα agonist, CP-900691, showed no effect on albuminuria and amelioration of DKD in the BTBR ob/ob diabetic mouse model[83]. Therefore, while activation of PPARg seems to have constitutive renoprotective effects in DKD, the role of PPARα activation in improving renal function remains questionable. A summary of the suggested mechanism of triglyceride abnormalities in DKD is shown in Figure 2.
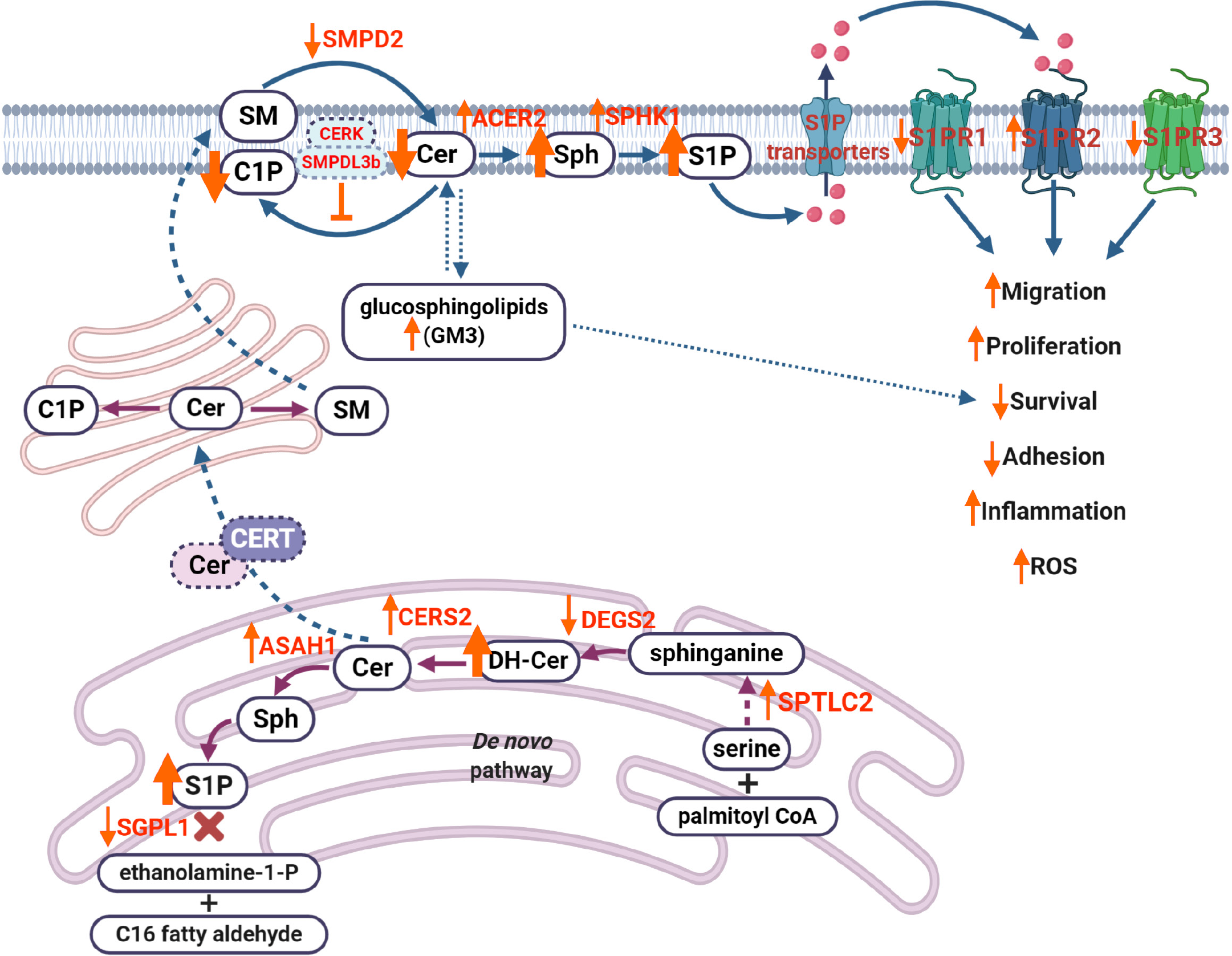
Sphingolipids are important components of cell homeostasis. Sphingolipids are a class of lipids composed of hydrophobic and hydrophilic regions with variable fatty acid composition. In recent years, sphingolipids and sphingolipid metabolites have been recognized as important regulators of cell signaling contributing to the development and progression of numerous diseases. The most studied sphingolipid metabolites are ceramide, sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P), which have been shown to regulate cell differentiation, membrane fluidity, protein anchoring, immune activation, insulin sensitivity, autophagy, and cell death. The role of S1P signaling in renal cells and in kidney diseases has been extensively reviewed[84].
In kidney cortices of diabetic db/db mice, elevated levels of long-chain ceramides (C14:0, C16:0, C18:0, C20:0) and decreased levels of very-long-chain ceramides (C24:0, C24:1) have been described[85], which is in accordance with our own studies[7]. In support of previous studies, ceramide accumulation was associated with increased reactive oxygen species production in OLEFT rats and in mice fed on a high-fat diet with DKD[86]. Elevated levels of long-chain ceramides (C16:0, C18:0 and C20:0)[87,88] and very-long-chain ceramides (C22:0, C24:0)[88] were also found in patients with early or overt DKD. Podocyte-specific deletion of the acid ceramidase main catalytic subunit (Asah1 gene) results in elevated ceramide levels in glomeruli and development of nephrotic syndrome in mice[89]. In patients with DKD enrolled into ONTARGET and TRANSCEND-randomized controlled trials rs267734 gene variant of ceramide synthase 2 (CerS2), a CerS2 isoform with high expression in the kidney, has been shown to be associated with increased albuminuria[90].
In the setting of diabetes, increased levels of S1P in plasma of rodents with type 1[91] or type 2 diabetes[92] have been reported. In mice with streptozotocin-induced diabetes increased renal levels of S1P were also reported[93,94]. Recent studies in mice and humans demonstrated that mutations in SGPL1 gene, which encodes S1P lyase 1, are associated with the development of nephrotic syndrome[9,10,95]. In rats with streptozotocin-induced DKD the use of an unselective S1P receptor agonist (FTY720) was found to have a renoprotective effect[96]. Interestingly, plasma levels of S1P in patients with type 2 diabetes negatively correlate with levels of albuminuria, while less S1P is observed in patients with macroalbuminuria[97]. A role of S1P lyase activity reduction has been demonstrated to contribute to the development of podocyte-based kidney toxicity in wildtype rodents[11]. Furthermore, S1P receptor signaling plays a significant role in glomerular injury. Five S1P receptors (S1PR1-S1PR5) exist, of which S1PR1 to S1PR4, but not S1PR5, are expressed in the kidney[98]. In mouse models of DKD, activation of S1PR1 or inhibition of S1PR2 prevented the renal injury phenotype[96]. Using a single cell RNA sequencing approach to profile glomerular cells in mouse models of DKD (streptozotocin-induced diabetic endothelial nitric oxide synthase-deficient mice), significantly lowered expression of S1P receptor 3 (S1PR3) in mesangial cells was demonstrated[99]. Previous studies also revealed a significant role of sphingosine kinase (SPHK), an enzyme that generates S1P from sphingosine, in the kidney fibrosis in STZ-induced diabetic mice and in humans with DKD[100]. In a mouse model of alloxan-induced diabetes, increased glomerular SPHK1 expression and activity were demonstrated leading to S1P accumulation[101]. In addition, SPHK1 upregulation was demonstrated in STZ-induced mouse model of DKD, where it protects from the fibrotic process[100]. A more detailed review on the role of S1P signaling in the kidney was previously published by us[84].
Even less is known about the role of C1P in the kidney. In contrast to S1P, C1P is most likely released from damaged cells[102]. Our studies demonstrated that increased sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b) in the db/db mouse model of DKD is associated with a state of C1P deficiency in podocytes[7]. SMPDL3b is a lipid-raft associated protein[103] that regulates plasma membrane fluidity[104] by blocking access of ceramide kinase, an enzyme that generates C1P from ceramide, to ceramide[8]. We also reported that elevated expression of SMPDL3b occurs in glomeruli of patients with DKD[105] and that SMPDL3b overexpression in podocytes results in the accumulation of S1P[106]. In support, podocyte-specific deficiency of Smpdl3b resulted in restoration of the renal C1P content in association with delayed DKD progression in diabetic mice[7]. To the contrary, others demonstrated that the knockout of ceramide kinase in mice is sufficient to prevent glomerular disease[107]. However, it remains to be established how bioactive sphingolipids contribute to the development of DKD and what are the best options for their use as possible biomarkers or therapeutic targets.
Dysmetabolism of other sphingolipids, such as gangliosides (mainly GM3, which is the most abundant ganglioside in the kidney), has also been reported to contribute to development of DKD[108]. Increased levels of sialic acid, a component of gangliosides, were found in patients with DKD and positively correlated with blood glucose, HbA1c, creatinine and microalbuminuria[109]. Increased GM3 species (C16:0, C18:0, C20:0, C22:0, C24:0) in kidney cortex from diabetic rats at an early stage of DKD have also been described[110]. Interestingly, GM3 was found to contribute to diabetic nephropathy via the alteration of pro-survival receptor-associated Akt signaling[111]. Another study reported that levels of glycosylated sphingolipids, such as lactosylceramide, are associated with microalbuminuria in patients with type 1 diabetes[112]. A proposed mechanism indicating how dysregulation of sphingolipid metabolism contributes to DKD is shown in Figure 3.
Lipid droplet (or lipid bodies) are lipid-rich cellular organelles that regulate storage and hydrolysis of lipids or serve as a reservoir for cholesterol and acyl-glycerol in different eukaryotic cells. Structurally, lipid droplets are composed of a neutral lipid core (triacylglycerol and cholesteryl esters) and a phospholipid monolayer. In an eukaryotic cell, lipid droplet formation may be induced by different stimuli, such as growth factors, long-chain unsaturated fatty acids, oxidative stress and inflammatory stimuli (reviewed in Ref.[113]). Once intracellular, the fatty acids can form part of the triglyceride and phospholipid components of the lipid droplet[114,115]. Increased lipid droplet accumulation is observed in patients with DKD[6] and mouse models of DKD[116,117]. We previously showed that treatment of human podocytes with serum from patients with DKD results in lipid droplet accumulation[3]. Kidneys of hyperglycemic mice (STZ-induced diabetes) are characterized by the concomitant presence of oxidative stress markers-positive (xanthine oxidoreductase and nitrotyrosine with tail-interacting protein of 47 kDa) lipid droplets in glomerular and/or tubular cells[117]. In Sprague-Dawley rats with STZ-induced diabetes, increased advanced glycation end products have been shown to cause lipid droplet accumulation[118].
While the composition of lipid droplets is not very well investigated, perilipins are the best characterized proteins of the lipid droplet coat. This family of perilipin proteins includes perilipin 1 (PLIN1), perilipin 2 (PLIN 2), perilipin 3 (PLIN 3), perilipin 4 (PLIN 4) perilipin 5 (PLIN 5). Not much data about the role of these proteins in DKD development and progression, and a recent case report suggests that mutation in PLIN1 may be associated with DKD-like kidney damage in a patient with type 4 familial partial lipodystrophy[119]. Another randomized case-control study of an Iranian population (n = 200) showed an association of the polymorphism rs4578621 in the PLIN gene with type 2 diabetes[120]. Interestingly, decreased Plin1 expression was reported in adipocytes of db/db mice, while deficiency of Plin1 in adipose tissue in wildtype mice resulted in insulin resistance and secretion of pro-inflammatory lipid metabolites, such as prostaglandins[121]. Expression of PLIN2 is significantly upregulated in kidneys from diabetic db/db mice[122] and in podocytes of patients with DKD[1], which may indicate that increased PLIN2 expression may contribute to increased lipid droplet accumulation in the diabetic kidney. Similarly, increased levels of urinary PLIN2 were reported in patients with DKD[123]. To date, no studies examining the role of other perilipin proteins in DKD have been performed. A role for PLIN5 in diabetes has recently been suggested as upregulation of PLIN5 in β-cells was shown to improve glucose tolerance in isolated islets from mice or human[124]. Because PLIN5 is also expressed in kidneys[125] under PPAR control, it would be important to investigate its role in lipid-associated kidney diseases in future investigations.
Among other factors contributing to lipid droplet accumulation in the kidney, autophagy has been shown to regulate lipid metabolism and lipid droplet formation[126-128] and to significantly contribute to renal fibrosis progression in kidney diseases. Serine/threonine protein kinase 25 (STK25), which plays an important role in skeletal muscle metabolism, is also highly expressed in human and rodent kidney[129] and was shown to aggravate renal lipid accumulation and exacerbate kidney injury in a high-fat diet mouse model of DKD[130].
The kidney is a target organ of the harmful effects of lipotoxicity in diabetes, suggesting that, similar to the liver, chronic kidney disease is a form of fatty kidney disease. In this review, we summarize new research trends and new scientific knowledge acquired within the past few years that have shed light on the role of particular lipids in diabetes-associated kidney injury. However, our knowledge with regard to the cross-talk between glucose homeostasis and lipid metabolism in health and disease remains incompletely understood and further research is needed. Similarly, more insight into the role of specific lipids in podocyte physiology is required to answer remaining questions. Which lipids are toxic to the podocytes? What factors are driving the pathophysiology of lipid accumulation in podocytes? Which lipids might be the best targets for possible therapeutic intervention in DKD? Answering these questions will help to pave the way to new diagnostic and therapeutic approaches in DKD.
We give a special thanks to the Katz family for their continuous support.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Nephrology; and American Heart Association.
Specialty type: Urology and nephrology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishibashi S, Sun X S-Editor: Gong ZM L-Editor: A P-Editor: Wang LL
| 1. | Falkevall A, Mehlem A, Palombo I, Heller Sahlgren B, Ebarasi L, He L, Ytterberg AJ, Olauson H, Axelsson J, Sundelin B, Patrakka J, Scotney P, Nash A, Eriksson U. Reducing VEGF-B Signaling Ameliorates Renal Lipotoxicity and Protects against Diabetic Kidney Disease. Cell Metab. 2017;25:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Mitrofanova A, Sosa MA, Fornoni A. Lipid mediators of insulin signaling in diabetic kidney disease. Am J Physiol Renal Physiol. 2019;317:F1241-F1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Merscher-Gomez S, Guzman J, Pedigo CE, Lehto M, Aguillon-Prada R, Mendez A, Lassenius MI, Forsblom C, Yoo T, Villarreal R, Maiguel D, Johnson K, Goldberg R, Nair V, Randolph A, Kretzler M, Nelson RG, Burke GW 3rd, Groop PH, Fornoni A; FinnDiane Study Group. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013;62:3817-3827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 538] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 468] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 7. | Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, Molina J, Varona Santos J, Ge M, Sloan A, Kim JJ, Pedigo C, Bryn J, Volosenco I, Faul C, Zeidan YH, Garcia Hernandez C, Mendez AJ, Leibiger I, Burke GW, Futerman AH, Barisoni L, Ishimoto Y, Inagi R, Merscher S, Fornoni A. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat Commun. 2019;10:2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Mallela SK, Mitrofanova A, Merscher S, Fornoni A. Regulation of the amount of ceramide-1-phosphate synthesized in differentiated human podocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:158517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, Shril S, Ashraf S, Tan W, Rao J, Airik M, Schapiro D, Braun DA, Sadowski CE, Widmeier E, Jobst-Schwan T, Schmidt JM, Girik V, Capitani G, Suh JH, Lachaussée N, Arrondel C, Patat J, Gribouval O, Furlano M, Boyer O, Schmitt A, Vuiblet V, Hashmi S, Wilcken R, Bernier FP, Innes AM, Parboosingh JS, Lamont RE, Midgley JP, Wright N, Majewski J, Zenker M, Schaefer F, Kuss N, Greil J, Giese T, Schwarz K, Catheline V, Schanze D, Franke I, Sznajer Y, Truant AS, Adams B, Désir J, Biemann R, Pei Y, Ars E, Lloberas N, Madrid A, Dharnidharka VR, Connolly AM, Willing MC, Cooper MA, Lifton RP, Simons M, Riezman H, Antignac C, Saba JD, Hildebrandt F. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest. 2017;127:912-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, Hurcombe J, Bierzynska A, Barbagelata E, Bergadá I, Cassinelli H, Das U, Krone R, Hacihamdioglu B, Sari E, Yesilkaya E, Storr HL, Clemente M, Fernandez-Cancio M, Camats N, Ram N, Achermann JC, Van Veldhoven PP, Guasti L, Braslavsky D, Guran T, Metherell LA. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J Clin Invest. 2017;127:942-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Schümann J, Grevot A, Ledieu D, Wolf A, Schubart A, Piaia A, Sutter E, Côté S, Beerli C, Pognan F, Billich A, Moulin P, Walker UJ. Reduced Activity of Sphingosine-1-Phosphate Lyase Induces Podocyte-related Glomerular Proteinuria, Skin Irritation, and Platelet Activation. Toxicol Pathol. 2015;43:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int. 2012;81:40-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Mitrofanova A, Fontanella AM, Merscher S, Fornoni A. Lipid deposition and metaflammation in diabetic kidney disease. Curr Opin Pharmacol. 2020;55:60-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Ducasa GM, Mitrofanova A, Fornoni A. Crosstalk Between Lipids and Mitochondria in Diabetic Kidney Disease. Curr Diab Rep. 2019;19:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Suleiman HY, Roth R, Jain S, Heuser JE, Shaw AS, Miner JH. Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy. JCI Insight. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Schermer B, Benzing T. Lipid-protein interactions along the slit diaphragm of podocytes. J Am Soc Nephrol. 2009;20:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Sörensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 19. | Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, Lee YS, Zhu Q, Kehata M, Li M, Jiang S, Rastaldi MP, Cohen CD, Troyanskaya OG, Kretzler M. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. 2013;23:1862-1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68:2608-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317-32325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Ishigaki N, Yamamoto T, Shimizu Y, Kobayashi K, Yatoh S, Sone H, Takahashi A, Suzuki H, Yamagata K, Yamada N, Shimano H. Involvement of glomerular SREBP-1c in diabetic nephropathy. Biochem Biophys Res Commun. 2007;364:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Sun H, Yuan Y, Sun ZL. Cholesterol Contributes to Diabetic Nephropathy through SCAP-SREBP-2 Pathway. Int J Endocrinol. 2013;2013:592576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Zhou C, Pridgen B, King N, Xu J, Breslow JL. Hyperglycemic Ins2AkitaLdlr⁻/⁻ mice show severely elevated lipid levels and increased atherosclerosis: a model of type 1 diabetic macrovascular disease. J Lipid Res. 2011;52:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Van Krieken R, Marway M, Parthasarathy P, Mehta N, Ingram AJ, Gao B, Krepinsky JC. Inhibition of SREBP With Fatostatin Does Not Attenuate Early Diabetic Nephropathy in Male Mice. Endocrinology. 2018;159:1479-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Liu X, Ducasa GM, Mallela SK, Kim JJ, Molina J, Mitrofanova A, Wilbon SS, Ge M, Fontanella A, Pedigo C, Santos JV, Nelson RG, Drexler Y, Contreras G, Al-Ali H, Merscher S, Fornoni A. Sterol-O-acyltransferase-1 has a role in kidney disease associated with diabetes and Alport syndrome. Kidney Int. 2020;98:1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Jatem E, Lima J, Montoro B, Torres-Bondia F, Segarra A. Efficacy and Safety of PCSK9 Inhibitors in Hypercholesterolemia Associated With Refractory Nephrotic Syndrome. Kidney Int Rep. 2021;6:101-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Artunc F. Kidney-derived PCSK9-a new driver of hyperlipidemia in nephrotic syndrome? Kidney Int. 2020;98:1393-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Tang C, Kanter JE, Bornfeldt KE, Leboeuf RC, Oram JF. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J Lipid Res. 2010;51:1719-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, Molina-David J, Ge M, Lassenius MI, Forsblom C, Lehto M, Groop PH, Kretzler M, Eddy S, Martini S, Reich H, Wahl P, Ghiggeri G, Faul C, Burke GW 3rd, Kretz O, Huber TB, Mendez AJ, Merscher S, Fornoni A. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest. 2016;126:3336-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Ducasa GM, Mitrofanova A, Mallela SK, Liu X, Molina J, Sloan A, Pedigo CE, Ge M, Santos JV, Hernandez Y, Kim JJ, Maugeais C, Mendez AJ, Nair V, Kretzler M, Burke GW, Nelson RG, Ishimoto Y, Inagi R, Banerjee S, Liu S, Szeto HH, Merscher S, Fontanesi F, Fornoni A. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest. 2019;129:3387-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 33. | Ruan XZ, Moorhead JF, Fernando R, Wheeler DC, Powis SH, Varghese Z. PPAR agonists protect mesangial cells from interleukin 1beta-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J Am Soc Nephrol. 2003;14:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Yin QH, Zhang R, Li L, Wang YT, Liu JP, Zhang J, Bai L, Cheng JQ, Fu P, Liu F. Exendin-4 Ameliorates Lipotoxicity-induced Glomerular Endothelial Cell Injury by Improving ABC Transporter A1-mediated Cholesterol Efflux in Diabetic apoE Knockout Mice. J Biol Chem. 2016;291:26487-26501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Yan H, Cheng L, Jia R, Yao H, Wu H, Shen Y, Zhang Y, Hao P, Zhang Z. ATP-binding cassette sub-family a member1 gene mutation improves lipid metabolic abnormalities in diabetes mellitus. Lipids Health Dis. 2019;18:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Lara-Riegos JC, Ortiz-López MG, Peña-Espinoza BI, Montúfar-Robles I, Peña-Rico MA, Sánchez-Pozos K, Granados-Silvestre MA, Menjivar M. Diabetes susceptibility in Mayas: Evidence for the involvement of polymorphisms in HHEX, HNF4α, KCNJ11, PPARγ, CDKN2A/2B, SLC30A8, CDC123/CAMK1D, TCF7L2, ABCA1 and SLC16A11 genes. Gene. 2015;565:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Ergen HA, Zeybek U, Gök O, Karaali ZE. Investigation of ABCA1 C69T polymorphism in patients with type 2 diabetes mellitus. Biochem Med (Zagreb). 2012;22:114-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Li C, Fan D. Association between the ABCA1 rs1800977 polymorphism and susceptibility to type 2 diabetes mellitus in a Chinese Han population. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Liu P, Ma L, Zhao H, Shen Z, Zhou X, Yan M, Zhao T, Zhang H, Qiu X, Li P. Association between LXR-α and ABCA1 Gene Polymorphisms and the Risk of Diabetic Kidney Disease in Patients with Type 2 Diabetes Mellitus in a Chinese Han Population. J Diabetes Res. 2020;2020:8721536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Tsun JG, Yung S, Chau MK, Shiu SW, Chan TM, Tan KC. Cellular cholesterol transport proteins in diabetic nephropathy. PLoS One. 2014;9:e105787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Russo GT, De Cosmo S, Viazzi F, Pacilli A, Ceriello A, Genovese S, Guida P, Giorda C, Cucinotta D, Pontremoli R, Fioretto P; AMD-Annals Study Group. Plasma Triglycerides and HDL-C Levels Predict the Development of Diabetic Kidney Disease in Subjects With Type 2 Diabetes: The AMD Annals Initiative. Diabetes Care. 2016;39:2278-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 42. | Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 43. | Cusick M, Chew EY, Hoogwerf B, Agrón E, Wu L, Lindley A, Ferris FL 3rd; Early Treatment Diabetic Retinopathy Study Research Group. Risk factors for renal replacement therapy in the Early Treatment Diabetic Retinopathy Study (ETDRS), Early Treatment Diabetic Retinopathy Study Report No. 26. Kidney Int. 2004;66:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Tsai IT, Wu CC, Hung WC, Lee TL, Hsuan CF, Wei CT, Lu YC, Yu TH, Chung FM, Lee YJ, Wang CP. FABP1 and FABP2 as markers of diabetic nephropathy. Int J Med Sci. 2020;17:2338-2345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Khan S, Gaivin R, Abramovich C, Boylan M, Calles J, Schelling JR. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 46. | Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 47. | Sun X, Yu Y, Han L. High FFA levels related to microalbuminuria and uncoupling of VEGF-NO axis in obese rats. Int Urol Nephrol. 2013;45:1197-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Ni X, Gu Y, Yu H, Wang S, Chen Y, Wang X, Yuan X, Jia W. Serum Adipocyte Fatty Acid-Binding Protein 4 Levels Are Independently Associated with Radioisotope Glomerular Filtration Rate in Type 2 Diabetic Patients with Early Diabetic Nephropathy. Biomed Res Int. 2018;2018:4578140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Ito H, Yamashita H, Nakashima M, Takaki A, Yukawa C, Matsumoto S, Omoto T, Shinozaki M, Nishio S, Abe M, Antoku S, Mifune M, Togane M. Current Metabolic Status Affects Urinary Liver-Type Fatty-Acid Binding Protein in Normoalbuminuric Patients With Type 2 Diabetes. J Clin Med Res. 2017;9:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Thi TND, Gia BN, Thi HLL, Thi TNC, Thanh HP. Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J Med Biochem. 2020;39:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Panduru NM, Forsblom C, Saraheimo M, Thorn L, Bierhaus A, Humpert PM, Groop PH; FinnDiane Study Group. Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2013;36:2077-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Viswanathan V, Sivakumar S, Sekar V, Umapathy D, Kumpatla S. Clinical significance of urinary liver-type fatty acid binding protein at various stages of nephropathy. Indian J Nephrol. 2015;25:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Yamada S, Tanabe J, Ogura Y, Nagai Y, Sugaya T, Ohata K, Natsuki Y, Ichikawa D, Watanabe S, Inoue K, Hoshino S, Kimura K, Shibagaki Y, Kamijo-Ikemori A. Renoprotective effect of GLP-1 receptor agonist, liraglutide, in early-phase diabetic kidney disease in spontaneously diabetic Torii fatty rats. Clin Exp Nephrol. 2021;25:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Susztak K, Ciccone E, McCue P, Sharma K, Böttinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Feng L, Gu C, Li Y, Huang J. High Glucose Promotes CD36 Expression by Upregulating Peroxisome Proliferator-Activated Receptor γ Levels to Exacerbate Lipid Deposition in Renal Tubular Cells. Biomed Res Int. 2017;2017:1414070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Yang X, Wu Y, Li Q, Zhang G, Wang M, Yang H. CD36 Promotes Podocyte Apoptosis by Activating the Pyrin Domain-Containing-3 (NLRP3) Inflammasome in Primary Nephrotic Syndrome. Med Sci Monit. 2018;24:6832-6839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WH, Xie Z, Shapiro JI, Silverstein RL. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension. 2013;61:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Kim JJ, David JM, Wilbon SS, Santos JV, Patel DM, Ahmad A, Mitrofanova A, Liu X, Mallela SK, Ducasa GM, Ge M, Sloan AJ, Al-Ali H, Boulina M, Mendez AJ, Contreras GN, Prunotto M, Sohail A, Fridman R, Miner JH, Merscher S, Fornoni A. Discoidin domain receptor 1 activation links extracellular matrix to podocyte lipotoxicity in Alport syndrome. EBioMedicine. 2021;63:103162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Su Y, Chen Q, Ma K, Ju Y, Ji T, Wang Z, Li W. Astragaloside IV inhibits palmitate-mediated oxidative stress and fibrosis in human glomerular mesangial cells via downregulation of CD36 expression. Pharmacol Rep. 2019;71:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Hua W, Huang HZ, Tan LT, Wan JM, Gui HB, Zhao L, Ruan XZ, Chen XM, Du XG. CD36 Mediated Fatty Acid-Induced Podocyte Apoptosis via Oxidative Stress. PLoS One. 2015;10:e0127507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 61. | Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1137] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 62. | Brown PM, Kennedy DJ, Morton RE, Febbraio M. CD36/SR-B2-TLR2 Dependent Pathways Enhance Porphyromonas gingivalis Mediated Atherosclerosis in the Ldlr KO Mouse Model. PLoS One. 2015;10:e0125126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol. 2013;10:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1163] [Cited by in RCA: 1179] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 65. | Huang W, Febbraio M, Silverstein RL. CD9 tetraspanin interacts with CD36 on the surface of macrophages: a possible regulatory influence on uptake of oxidized low density lipoprotein. PLoS One. 2011;6:e29092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK. αMβ₂ integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res. 2011;108:544-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 726] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 68. | Zhao J, Rui HL, Yang M, Sun LJ, Dong HR, Cheng H. CD36-Mediated Lipid Accumulation and Activation of NLRP3 Inflammasome Lead to Podocyte Injury in Obesity-Related Glomerulopathy. Mediators Inflamm. 2019;2019:3172647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Ohgami N, Nagai R, Ikemoto M, Arai H, Kuniyasu A, Horiuchi S, Nakayama H. Cd36, a member of the class b scavenger receptor family, as a receptor for advanced glycation end products. J Biol Chem. 2001;276:3195-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Kim HJ, Moon JS, Park IR, Kim JH, Yoon JS, Won KC, Lee HW. A Novel Index Using Soluble CD36 Is Associated with the Prevalence of Type 2 Diabetes Mellitus: Comparison Study with Triglyceride-Glucose Index. Endocrinol Metab (Seoul). 2017;32:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Handberg A, Lopez-Bermejo A, Bassols J, Vendrell J, Ricart W, Fernandez-Real JM. Circulating soluble CD36 is associated with glucose metabolism and interleukin-6 in glucose-intolerant men. Diab Vasc Dis Res. 2009;6:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Shiju TM, Mohan V, Balasubramanyam M, Viswanathan P. Soluble CD36 in plasma and urine: a plausible prognostic marker for diabetic nephropathy. J Diabetes Complications. 2015;29:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Ekici M, Kisa U, Arikan Durmaz S, Ugur E, Nergiz-Unal R. Fatty acid transport receptor soluble CD36 and dietary fatty acid pattern in type 2 diabetic patients: a comparative study. Br J Nutr. 2018;119:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Castelblanco E, Sanjurjo L, Falguera M, Hernández M, Fernandez-Real JM, Sarrias MR, Alonso N, Mauricio D. Circulating Soluble CD36 is Similar in Type 1 and Type 2 Diabetes Mellitus versus Non-Diabetic Subjects. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Afshinnia F, Nair V, Lin J, Rajendiran TM, Soni T, Byun J, Sharma K, Fort PE, Gardner TW, Looker HC, Nelson RG, Brosius FC, Feldman EL, Michailidis G, Kretzler M, Pennathur S. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 76. | Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes. 2005;54:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Agarwal R, Saha C, Battiwala M, Vasavada N, Curley T, Chase SD, Sachs N, Semret MH. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 2005;68:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Fujita A, Sasaki H, Doi A, Okamoto K, Matsuno S, Furuta H, Nishi M, Nakao T, Tsuno T, Taniguchi H, Nanjo K. Ferulic acid prevents pathological and functional abnormalities of the kidney in Otsuka Long-Evans Tokushima Fatty diabetic rats. Diabetes Res Clin Pract. 2008;79:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Zhou Z, Wan J, Hou X, Geng J, Li X, Bai X. MicroRNA-27a promotes podocyte injury via PPARγ-mediated β-catenin activation in diabetic nephropathy. Cell Death Dis. 2017;8:e2658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Matsushita Y, Ogawa D, Wada J, Yamamoto N, Shikata K, Sato C, Tachibana H, Toyota N, Makino H. Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice. Diabetes. 2011;60:960-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Park CW, Kim HW, Ko SH, Chung HW, Lim SW, Yang CW, Chang YS, Sugawara A, Guan Y, Breyer MD. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Cha DR, Zhang X, Zhang Y, Wu J, Su D, Han JY, Fang X, Yu B, Breyer MD, Guan Y. Peroxisome proliferator activated receptor alpha/gamma dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes. 2007;56:2036-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Askari B, Wietecha T, Hudkins KL, Fox EJ, O'Brien KD, Kim J, Nguyen TQ, Alpers CE. Effects of CP-900691, a novel peroxisome proliferator-activated receptor α, agonist on diabetic nephropathy in the BTBR ob/ob mouse. Lab Invest. 2014;94:851-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Drexler Y, Molina J, Mitrofanova A, Fornoni A, Merscher S. Sphingosine-1-Phosphate Metabolism and Signaling in Kidney Diseases. J Am Soc Nephrol. 2021;32:9-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Sas KM, Nair V, Byun J, Kayampilly P, Zhang H, Saha J, Brosius FC 3rd, Kretzler M, Pennathur S. Targeted Lipidomic and Transcriptomic Analysis Identifies Dysregulated Renal Ceramide Metabolism in a Mouse Model of Diabetic Kidney Disease. J Proteomics Bioinform. 2015;Suppl 14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Woo CY, Baek JY, Kim AR, Hong CH, Yoon JE, Kim HS, Yoo HJ, Park TS, Kc R, Lee KU, Koh EH. Inhibition of Ceramide Accumulation in Podocytes by Myriocin Prevents Diabetic Nephropathy. Diabetes Metab J. 2020;44:581-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Liu JJ, Ghosh S, Kovalik JP, Ching J, Choi HW, Tavintharan S, Ong CN, Sum CF, Summers SA, Tai ES, Lim SC. Profiling of Plasma Metabolites Suggests Altered Mitochondrial Fuel Usage and Remodeling of Sphingolipid Metabolism in Individuals With Type 2 Diabetes and Kidney Disease. Kidney Int Rep. 2017;2:470-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 88. | Morita Y, Kurano M, Sakai E, Nishikawa T, Nishikawa M, Sawabe M, Aoki J, Yatomi Y. Analysis of urinary sphingolipids using liquid chromatography-tandem mass spectrometry in diabetic nephropathy. J Diabetes Investig. 2020;11:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 89. | Li G, Kidd J, Kaspar C, Dempsey S, Bhat OM, Camus S, Ritter JK, Gehr TWB, Gulbins E, Li PL. Podocytopathy and Nephrotic Syndrome in Mice with Podocyte-Specific Deletion of the Asah1 Gene: Role of Ceramide Accumulation in Glomeruli. Am J Pathol. 2020;190:1211-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 90. | Shiffman D, Pare G, Oberbauer R, Louie JZ, Rowland CM, Devlin JJ, Mann JF, McQueen MJ. A gene variant in CERS2 is associated with rate of increase in albuminuria in patients with diabetes from ONTARGET and TRANSCEND. PLoS One. 2014;9:e106631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Fox TE, Bewley MC, Unrath KA, Pedersen MM, Anderson RE, Jung DY, Jefferson LS, Kim JK, Bronson SK, Flanagan JM, Kester M. Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res. 2011;52:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One. 2013;8:e72449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 93. | Nojiri T, Kurano M, Tokuhara Y, Ohkubo S, Hara M, Ikeda H, Tsukamoto K, Yatomi Y. Modulation of sphingosine-1-phosphate and apolipoprotein M levels in the plasma, liver and kidneys in streptozotocin-induced diabetic mice. J Diabetes Investig. 2014;5:639-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Geoffroy K, Troncy L, Wiernsperger N, Lagarde M, El Bawab S. Glomerular proliferation during early stages of diabetic nephropathy is associated with local increase of sphingosine-1-phosphate levels. FEBS Lett. 2005;579:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Linhares ND, Arantes RR, Araujo SA, Pena SDJ. Nephrotic syndrome and adrenal insufficiency caused by a variant in SGPL1. Clin Kidney J. 2018;11:462-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, Okusa MD. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 2011;79:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Bekpinar S, Yenidunya G, Gurdol F, Unlucerci Y, Aycan-Ustyol E, Dinccag N. The effect of nephropathy on plasma sphingosine 1-phosphate concentrations in patients with type 2 diabetes. Clin Biochem. 2015;48:1264-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Awad AS, Ye H, Huang L, Li L, Foss FW Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516-F1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 99. | Fu J, Akat KM, Sun Z, Zhang W, Schlondorff D, Liu Z, Tuschl T, Lee K, He JC. Single-Cell RNA Profiling of Glomerular Cells Shows Dynamic Changes in Experimental Diabetic Kidney Disease. J Am Soc Nephrol. 2019;30:533-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 100. | Ren S, Babelova A, Moreth K, Xin C, Eberhardt W, Doller A, Pavenstädt H, Schaefer L, Pfeilschifter J, Huwiler A. Transforming growth factor-beta2 upregulates sphingosine kinase-1 activity, which in turn attenuates the fibrotic response to TGF-beta2 by impeding CTGF expression. Kidney Int. 2009;76:857-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Lan T, Shen X, Liu P, Liu W, Xu S, Xie X, Jiang Q, Li W, Huang H. Berberine ameliorates renal injury in diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P signaling pathway. Arch Biochem Biophys. 2010;502:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 102. | Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26:106-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 103. | Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW 3rd. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 104. | Heinz LX, Baumann CL, Köberlin MS, Snijder B, Gawish R, Shui G, Sharif O, Aspalter IM, Müller AC, Kandasamy RK, Breitwieser FP, Pichlmair A, Bruckner M, Rebsamen M, Blüml S, Karonitsch T, Fauster A, Colinge J, Bennett KL, Knapp S, Wenk MR, Superti-Furga G. The Lipid-Modifying Enzyme SMPDL3B Negatively Regulates Innate Immunity. Cell Rep. 2015;11:1919-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 105. | Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, Mitrofanova A, Leclercq F, Faul C, Li J, Kretzler M, Nelson RG, Lehto M, Forsblom C, Groop PH, Reiser J, Burke GW, Fornoni A, Merscher S. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol. 2015;26:133-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 106. | Ahmad A, Mitrofanova A, Bielawski J, Yang Y, Marples B, Fornoni A, Zeidan YH. Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes. FASEB J. 2017;31:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 107. | Pastukhov O, Schwalm S, Römer I, Zangemeister-Wittke U, Pfeilschifter J, Huwiler A. Ceramide kinase contributes to proliferation but not to prostaglandin E2 formation in renal mesangial cells and fibroblasts. Cell Physiol Biochem. 2014;34:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993;91:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Ene CD, Penescu M, Anghel A, Neagu M, Budu V, Nicolae I. Monitoring Diabetic Nephropathy by Circulating Gangliosides. J Immunoassay Immunochem. 2016;37:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Hou B, He P, Ma P, Yang X, Xu C, Lam SM, Shui G, Zhang L, Qiang G, Du G. Comprehensive Lipidome Profiling of the Kidney in Early-Stage Diabetic Nephropathy. Front Endocrinol (Lausanne). 2020;11:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 111. | Vukovic I, Bozic J, Markotic A, Ljubicic S, Ticinovic Kurir T. The missing link - likely pathogenetic role of GM3 and other gangliosides in the development of diabetic nephropathy. Kidney Blood Press Res. 2015;40:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Lopes-Virella MF, Baker NL, Hunt KJ, Hammad SM, Arthur J, Virella G, Klein RL; DCCT/EDIC Research Group. Glycosylated sphingolipids and progression to kidney dysfunction in type 1 diabetes. J Clin Lipidol 2019; 13: 481-491. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 113. | Melo RC, D'Avila H, Wan HC, Bozza PT, Dvorak AM, Weller PF. Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J Histochem Cytochem. 2011;59:540-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 114. | Simon N, Hertig A. Alteration of Fatty Acid Oxidation in Tubular Epithelial Cells: From Acute Kidney Injury to Renal Fibrogenesis. Front Med (Lausanne). 2015;2:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 115. | Sieber J, Jehle AW. Free Fatty acids and their metabolism affect function and survival of podocytes. Front Endocrinol (Lausanne). 2014;5:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 116. | Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, Chua S, Levi M. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 117. | Kiss E, Kränzlin B, Wagenblaβ K, Bonrouhi M, Thiery J, Gröne E, Nordström V, Teupser D, Gretz N, Malle E, Gröne HJ. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors. Am J Pathol. 2013;182:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 118. | Yuan Y, Sun H, Sun Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: a pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis. 2017;16:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 119. | Chen RX, Zhang L, Ye W, Wen YB, Si N, Li H, Li MX, Li XM, Zheng K. The renal manifestations of type 4 familial partial lipodystrophy: a case report and review of literature. BMC Nephrol. 2018;19:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Saravani R, Noorzehi N, Galavi HR, Ranjbar N, Lotfian Sargazi M. Association of Perilipin and Insulin Receptor Substrate-1 Genes Polymorphism with Lipid Profiles, Central Obesity, and Type 2 Diabetes in a Sample of an Iranian Population. Iran Red Crescent Med J. 2017;19:e55100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Sohn JH, Lee YK, Han JS, Jeon YG, Kim JI, Choe SS, Kim SJ, Yoo HJ, Kim JB. Perilipin 1 (Plin1) deficiency promotes inflammatory responses in lean adipose tissue through lipid dysregulation. J Biol Chem. 2018;293:13974-13988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 122. | Jun H, Song Z, Chen W, Zanhua R, Yonghong S, Shuxia L, Huijun D. In vivo and in vitro effects of SREBP-1 on diabetic renal tubular lipid accumulation and RNAi-mediated gene silencing study. Histochem Cell Biol. 2009;131:327-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Yang W, Luo Y, Yang S, Zeng M, Zhang S, Liu J, Han Y, Liu Y, Zhu X, Wu H, Liu F, Sun L, Xiao L. Ectopic lipid accumulation: potential role in tubular injury and inflammation in diabetic kidney disease. Clin Sci (Lond). 2018;132:2407-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 124. | Trevino MB, Machida Y, Hallinger DR, Garcia E, Christensen A, Dutta S, Peake DA, Ikeda Y, Imai Y. Perilipin 5 regulates islet lipid metabolism and insulin secretion in a cAMP-dependent manner: implication of its role in the postprandial insulin secretion. Diabetes. 2015;64:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 125. | Hashani M, Witzel HR, Pawella LM, Lehmann-Koch J, Schumacher J, Mechtersheimer G, Schnölzer M, Schirmacher P, Roth W, Straub BK. Widespread expression of perilipin 5 in normal human tissues and in diseases is restricted to distinct lipid droplet subpopulations. Cell Tissue Res. 2018;374:121-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 126. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3239] [Cited by in RCA: 3110] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 127. | Takagi A, Kume S, Kondo M, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Haneda M, Chano T, Matsusaka T, Nagao K, Adachi Y, Chan L, Maegawa H, Uzu T. Mammalian autophagy is essential for hepatic and renal ketogenesis during starvation. Sci Rep. 2016;6:18944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 128. | Yan Q, Song Y, Zhang L, Chen Z, Yang C, Liu S, Yuan X, Gao H, Ding G, Wang H. Autophagy activation contributes to lipid accumulation in tubular epithelial cells during kidney fibrosis. Cell Death Discov. 2018;4:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 129. | Nerstedt A, Cansby E, Andersson CX, Laakso M, Stančáková A, Blüher M, Smith U, Mahlapuu M. Serine/threonine protein kinase 25 (STK25): a novel negative regulator of lipid and glucose metabolism in rodent and human skeletal muscle. Diabetologia. 2012;55:1797-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 130. | Cansby E, Caputo M, Gao L, Kulkarni NM, Nerstedt A, Ståhlman M, Borén J, Porosk R, Soomets U, Pedrelli M, Parini P, Marschall HU, Nyström J, Howell BW, Mahlapuu M. Depletion of protein kinase STK25 ameliorates renal lipotoxicity and protects against diabetic kidney disease. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |