Published online Mar 15, 2021. doi: 10.4239/wjd.v12.i3.215
Peer-review started: November 6, 2020
First decision: December 11, 2020
Revised: December 20, 2020
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: March 15, 2021
Processing time: 115 Days and 12.9 Hours
Coronavirus disease 2019 (COVID-19) is a global pandemic where several comorbidities have been shown to have a significant effect on mortality. Patients with diabetes mellitus (DM) have a higher mortality rate than non-DM patients if they get COVID-19. Recent studies have indicated that patients with a history of diabetes can increase the risk of severe acute respiratory syndrome coronavirus 2 infection. Additionally, patients without any history of diabetes can acquire new-onset DM when infected with COVID-19. Thus, there is a need to explore the bidirectional link between these two conditions, confirming the vicious loop between “DM/COVID-19”. This narrative review presents (1) the bidirectional association between the DM and COVID-19, (2) the manifestations of the DM/COVID-19 loop leading to cardiovascular disease, (3) an understanding of primary and secondary factors that influence mortality due to the DM/COVID-19 loop, (4) the role of vitamin-D in DM patients during COVID-19, and finally, (5) the monitoring tools for tracking atherosclerosis burden in DM patients during COVID-19 and “COVID-triggered DM” patients. We conclude that the bidirectional nature of DM/COVID-19 causes acceleration towards cardiovascular events. Due to this alarming condition, early monitoring of atherosclerotic burden is required in “Diabetes patients during COVID-19” or “new-onset Diabetes triggered by COVID-19 in Non-Diabetes patients”.
Core Tip: This narrative review hypothesizes that there is a bidirectional link between diabetes mellitus (DM) and coronavirus disease 2019 (COVID-19). The first bidirectional link is from COVID-19 to DM due to pancreatic damage or renin-angiotensin-aldosterone system dysregulation or cytokine storm. This is caused by the endocytosis of severe acute respiratory syndrome coronavirus 2. The second bidirectional link is from DM to COVID-19 and is due to drug-induced or impaired immunity or raised furin levels in DM. The review furthers explores the five pathways leading to cardiovascular diseases.
- Citation: Viswanathan V, Puvvula A, Jamthikar AD, Saba L, Johri AM, Kotsis V, Khanna NN, Dhanjil SK, Majhail M, Misra DP, Agarwal V, Kitas GD, Sharma AM, Kolluri R, Naidu S, Suri JS. Bidirectional link between diabetes mellitus and coronavirus disease 2019 leading to cardiovascular disease: A narrative review. World J Diabetes 2021; 12(3): 215-237
- URL: https://www.wjgnet.com/1948-9358/full/v12/i3/215.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i3.215
Coronavirus disease 2019 (COVID-19) is a global pandemic and an ongoing international public health emergency caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. Our understanding of the COVID-19 epidemic is limited. However, the information gleaned from previous viral outbreaks may shed light on new approaches to prevent and cure this pandemic. As of November 5, 2020, there are over 48.5 million laboratory-confirmed cases of COVID-19 in around 200 countries, with nearly 1.2 million deaths, mostly in comorbid and high-risk groups[1].
Diabetes mellitus (DM) is a highly prevalent metabolic disorder, affecting more than 400 million people globally[2-4]. It is now also considered an independent risk factor of COVID-19[5-9]. Long-standing DM leads to macrovascular and microvascular complications that ultimately affect patients’ quality of life[10]. DM has a long history of being associated with several other infections[11], such as the 2008 epidemic SARS-CoV-1[12], the 2009 pandemic influenza A (H1N1)[13], and the 2010 epidemic Middle East respiratory syndrome-related coronavirus (MERS-CoV)[14]. Similarly, it has been shown that DM is associated with the current COVID-19 pandemic[5,15-18]. A study of 20982 COVID-19 patients by the Chinese Centre for Disease Control and Prevention showed a 5% prevalence of DM. Further, in an Italian study, Onder et al[6] reported that out of 355 COVID-19 patients, 36% had DM. Similarly, another study in the United States by Bhatraju et al[8] reported that in 24 patients, 58% were diabetic. There is, therefore, an extensive range (5.3%-58%) of reported prevalence of DM in COVID-19 patients[5], which could be attributed to the fact that the studies were conducted in different countries (or geographical locations), assessed significantly different sample sizes, and had different objectives.
The majority of people suffering from COVID-19 escape major complications, but a significant minority develops severe illness leading to death. Several factors have been implicated in the development of severe illness, including (1) older age, (2) comorbidities, (3) professional risk of exposure to SARS-CoV-2 at work, and (4) socioeconomic and household conditions. People of any age can get the COVID-19 infection, including children, but typically, COVID-19 related serious complications are present in those over 60 years[19,20]. A supporting study from the Centers for Disease Control and Prevention, United States[21], consisting of 4226 people, reported that 80% of deaths were in people aged ≥ 60 years that required intensive care unit admission and long-term care. People of any age with a history of serious past chronic health problems are more vulnerable to COVID-19, possibly because of a weak immune system. Such comorbidities include long-standing heart and brain problems[22,23],
Obesity is a major risk factor for many diseases, and an increasing number of reports show obesity as a risk factor for COVID-19[25,26], similar to what had been seen with previous coronavirus infections such as SARS-CoV-1 and MERS[28]. Our observations point to the reason for comorbidity (that includes diabetes) as one of the highly probable causes of COVID-19 mortality. The top seven countries in diabetes prevalence (India, United States, Pakistan, Bangladesh, Indonesia, Mexico, and Brazil) are listed in the top 8% of countries contributing COVID-19 deaths globally. This suggests that DM and its comorbid conditions may be a major contributor to COVID-19-related mortality[1]. Front-line workers, including drivers, sanitation handlers, police, security guards, doctors, and paramedics, come into contact with the public more frequently and may have a higher chance of COVID infection[29,30]. In addition, poor living conditions, discrimination, lifestyle, and low socioeconomic status are associated with a higher risk of severe COVID-19 related infection, complications, and death[31,32].
Recent studies have also indicated a plausible reverse association between DM and COVID-19, which means that COVID-19 patients without a history of DM could experience a new onset of diabetes[33-35]. To investigate this association, an international group of diabetes researchers from the CoviDIAB Project (covidiab.e-dendrite.com) has set up a global registry of patients with COVID-19–associated diabetes. Although there is a high prevalence of DM in COVID-19, a possible bidirectional association and the link between these two conditions are still unclear. Therefore, to prevent long term cardiovascular events, it is essential to collect more evidence from different peer-reviewed studies and validate this bidirectional mechanism between DM and COVID-19.
We analyzed this possible bidirectional hypothesis by splitting it into two unidirectional flows. We hope that this will lead to a better understanding of these two global health emergencies. In section 2, we address the question “How does diabetes increase the viral entry of SARS-CoV-2?”, while in section 3, we address the question “How can COVID-19 infections lead to new-onset diabetes or the worsening of pre-existing diabetes?” Table 1 provides an evidence-based summary of studies supporting sections 2 and 3. Section 4 addresses the question “How can the interplay between DM and COVID-19 increase the risk of cardiovascular disease (CVD)?”. Further, it also discusses the role of vitamin D (Vit D) during the COVID-19 pandemic. Finally, section 5 presents the role of atherosclerosis imaging for diabetes patients during the COVID-19 pandemic and long term follow-up of survivors[36,37].
| SN | Ref. | Country | Pathway validation | Type of relation shown | Conclusion and overview derived from the study | Place added at the manuscript |
| 1 | Abbas et al[59] (2020) | Egypt | Pathway I, IV | Bidirectional | The authors had concluded that there exists a bidirectional relationship between COVID-19 and DM. Furthermore, the authors also discussed the SARS-CoV-2 cellular entry using overexpression of ACE2 receptors in DM patients. Hence, DM is a risk factor for COVID-19. SARS-CoV-2 also uses additional ACE2, which is also observed on pancreatic beta cells. This leads to beta cell destruction that results in triggering of new-onset diabetes and worsening of pre-existing DM | Section 2, page 9, and section 3, page 11 |
| 2 | Muniangi-Muhitu et al[60] (2020) | The United Kingdom and Singapore | Pathway I-b, II, V, VI-a | Bidirectional | Authors had concluded that DM worsens the COVID-19, and this is due to weakened immunity. Weak immunity support SARS-CoV2 to infect primarily monocytes and dendritic cells in DM patients. They showed that the use of common medications in DM and HTN can increase the expression of ACE2 levels, which favors SARS-CoV-2 viral binding. Additionally, the authors also mentioned that COVID-19 can trigger a cytokine storm, which results in insulin resistance and causes worsening in glycemic levels | Section 2, page 9 and section 3, page 11 |
| 3 | Mota et al[58] (2020) | Romania | Pathway I, II, III, IV | Bidirectional | Drugs frequently used by patients with diabetes, like GLP-1 receptor agonists, thiazolidinediones, antihypertensives such as ACE inhibitors, and statins, up-regulate ACE2. Increased cellular furin in poor glycemic control can result in cellular viral entry | Section 2, page 9 and section 3, page 11 |
| 4 | Kalra et al[68] (2020) | United Kingdom, India, and Kazakhstan | Pathway II | Bidirectional | The authors clearly mentioned that diabetes is known to be characterized by an impaired immune response, especially in those with uncontrolled glucose. This may increase the susceptibility to COVID-19 infection | Section 2, page 10 |
| 5 | Rubino et al[35] (2020) | Australia, United Kingdom, Germany | Pathway IV | Bidirectional | In this study, the authors clearly mentioned that there is the possibility of glycemic alteration with the SARS-CoV-2 virus because it can directly affect the pancreatic beta cells, which results in new-onset DM or worsening pre-existing DM | Section 3, page 10 |
| 6 | Balasubramanyam et al[88] (2020) | Chennai (India) | Pathway IV | Unidirectional | COVID-19 infection in diabetic patients aggravates morbidity and may be linked to increased mortality. The biological explanations for this could be virus exploitation of multiple organs | Section 3, page 11 |
| 7 | Baracchini et al[66] (2020) | Germany | Pathway II, IV | Unidirectional | This study is clear evidence showing people with DM have a severe risk of SARS-CoV-2 because of the defective immune response. Secondly, this study also supported an overexpression of ACE2 receptors in DM patients facilitating cellular entry. At last, this study showed an increase in blood glucose levels due to the SARC-CoV-2-driven infection to the pancreatic beta cells | Section 3, page 11 |
| 8 | Fang et al[17] (2020) | Greece | Pathway I | Unidirectional | In this study, the authors suggested that patients with cardiac diseases, hypertension, or diabetes, who are treated with ACE2 increasing drugs, are at higher risk for severe COVID-19 infection. Therefore, such patients should be monitored for ACE2-modulating medications, such as ACE inhibitors or ARBs | Section 1, page 6 |
| 9 | Muniyappa et al[64] (2020) | Maryland | Pathway II | Unidirectional | DM inhibits neutrophil chemotaxis, phagocytosis, and intracellular killing of microbes. Impairments in adaptive immunity characterized by an initial delay in the activation of Th1 cell-mediated immunity and a late hyperinflammatory response is often observed in patients with diabetes | Section 2, page 9 |
| 10 | Chen et al[65] (2020) | China and Sweden | Pathway II | Unidirectional | Authors concluded that patients associated with DM have over-expression of ACE2, which will worsen the prognosis during a COVID-19 infection | Section 2, page 9 |
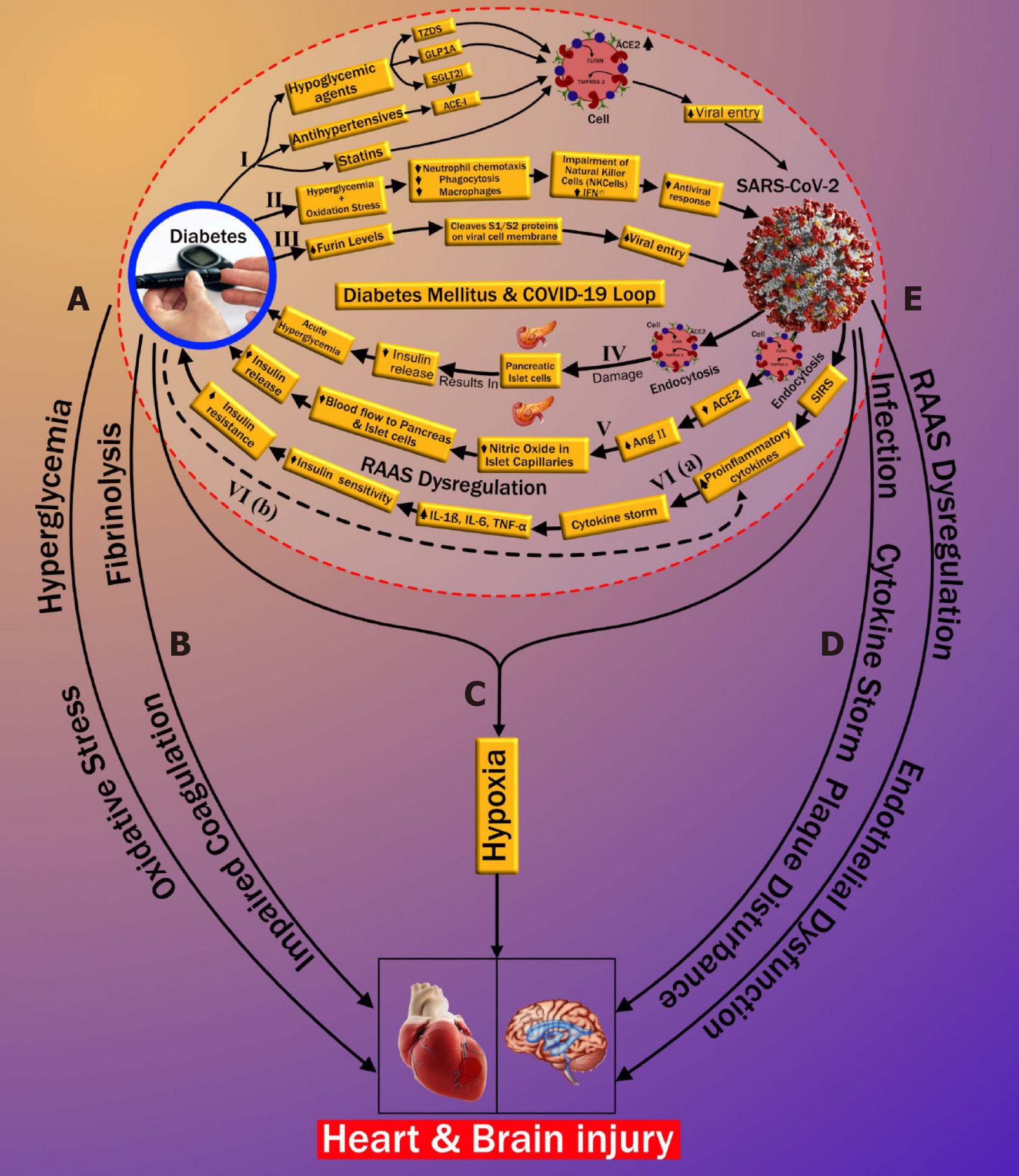
Three possible pathways that might increase COVID-19 susceptibility in patients with DM are depicted in Figure 1. Increased susceptibility to COVID-19 infection may occur through (1) medication-induced angiotensin-converting enzyme 2 (ACE2) expression (pathway-I), (2) impaired immunity (pathway-II), and (3) increased furin levels (pathway-III). In the first pathway, patients with DM exhibit a high prevalence of increased blood pressure and CVD[38]. Hence, along with hypoglycemic agents, these patients are mostly treated with antihypertensive medications like angiotensin-converting enzyme inhibitors (ACE-I), angiotensin-II type I receptor blockers (ARB)[39], and lipid-lowering drugs such as statins[40]. Hypoglycemic agents, such as (1) glucagon-like peptide-1 agonists (e.g., liraglutide)[41] and (2) thiazolidinediones (e.g., pioglitazone)[42,43], facilitate viral entry following overexpression of ACE2. A new hypoglycemic agent, sodium-glucose cotransporter 2 inhibitors (SGLT2i), has been used for treating type 2 diabetes[44]. SGLT2i may also promote cellular viral entry by increasing ACE2 levels indirectly, mainly when used alongside ACE-I. Moreover, using SGLT2i in patients with COVID-19 may cause serious complications such as dehydration and could increase the risk of diabetic ketoacidosis (DKA)[45-47]. Antihypertensive medications such as ACE-I and ARBs are also associated with an increase in ACE2 expression[48-51]. Increased expression of ACE2 receptors by the epithelial cells of the lung [alveolar type 2 (AT2) cells][52,53], intestine (enterocytes)[54], kidneys (proximal tubule cells)[52], and heart (myocardial cells)[52] facilitates the human cell entry of SARS-CoV-2[41-43,45,48-51]. Additionally, Hodgson et al[55] showed that patients with DM and hypertension treated with ACE-I and ARB are more susceptible to SARS-CoV-2 infection due to higher expression of ACE receptors. Furthermore, experimental animal studies demonstrated increased expression of ACE2 receptors by using statins[56,57]. In an experimental rabbit model, atorvastatin use resulted in overexpression of ACE2 receptors in both the heart and the kidney[56]. A similar study in diabetic rats treated with fluvastatin resulted in overexpression of ACE2 receptors in the heart and blood vessels[57]. Recently Mota et al[57] reported in their study that frequently used drugs in patients with DM, like glucagon-like peptide-1 receptor agonist, thiazolidinediones, anti-hypertensives such as ACE-I, and lipid-lowering drugs such as statins, hike ACE2 expression, increasing the risk of COVID-19. On the other hand, it was shown that people who were on insulin had less ACE2 expression (Table 1)[58]. Furthermore, Abbas et al[59] and Muniangi-Muhitu et al[60] also validated that “Drug-induced” causes an increase in ACE2 expression in DM, which further increases the possibility of SARS-CoV-2 viral entry. These studies suggest that a medication-induced ACE2 overexpression may play a role in the pathophysiology of COVID-19, as shown in pathway-I (Table 1).
The second pathway (marked as II in Figure 1) is related to the impaired immune response in DM patients. Patients with DM have an increased susceptibility to infections[61]. The presence of hyperglycemia and oxidative stress in DM inhibits (1) neutrophil chemotaxis, (2) phagocytosis, and (3) macrophage activity[55,58,62-66]. Furthermore, in DM, impairment of natural killer cells and interferons (IFN-γ) has been observed. Additionally, the SARS-CoV-2 virus primarily infects monocytes and dendritic cells that results in a weakened immune system (Table 1)[60,67]. All these play a vital role in increasing susceptibility to viral proliferation in COVID-19 patients, especially those with poor blood glucose control[55,61-63]. Hence, with the above explanation, we can conclude that there is a possible association between impaired immune response and an increased risk of COVID-19 infection in diabetes patients. Recently, Kalra et al[68] also supported the same pathway II in their newly published article.
In the third pathway (marked as III in Figure 1), DM increases the presence of furin, which is a type-1 membrane-bound protease belonging to the “proprotein convertase subtilisin/Kexin” receptor family[55]. Interestingly, recent studies showed an increased furin level in DM patients facilitating the cellular entry of SARS-CoV-2 (Table 1)[58,69,70]. Furthermore, furin is associated with the cleavage and priming of the spike protein of SARS-CoV-2 (S1 and S2 proteins), thereby mediating viral entry in the host cell[71,72]. The presence of furin is likely associated with the replication of SARS-CoV-2 in patients with DM[16,69]. Although pathway-III is well seen and better understood compared with pathway-I and pathway-II, the cumulative effect of the three pathways validates the possible link that indicates the increased cellular entry of SARS-CoV-2 in patients with pre-existing DM.
This section illustrates the reasons causing “new-onset DM” or “worsening of pre-existing DM” in post-COVID-19 cases. It has been observed that patients that were not having a prior history of diabetes, but when infected by COVID-19, lead to severe complications such as DKA[33-35]. It has been shown that DKA occurs mainly due to total or subtotal insulinogenic (reduced insulin levels) and the overproduction of counter regulators, which favors the production of ketones[34,73]. Further, DKA is most commonly observed in patients with type 1 DM but may also occur in type 2 diabetes[74,75]. In an observational study, Li et al[33] reported that COVID-19 infection also induces DKA in patients with diabetes. Henceforth, we hypothesized the three plausible series of pathways of new-onset DM or worsening of pre-existing diabetes after COVID-19 infection, which is depicted in Figure 1 (pathways-IV, V, and VI). A zoomed version of Figure 1 indicating the bidirectional association between DM and COVID-19 is provided in Supplementary Figure 1.
Pathway IV explains the effect of COVID-19 causing insulin-dependent DM. It is well known that viral infections are associated with the development of pancreatic autoantibodies leading to insulin-dependent DM or type 1 DM. These respiratory viruses were identified as one of the potential causative pathogens in “the environmental determinants of diabetes in the young” (TEDDY) study[76,77]. SARS-CoV-2 uses the ACE2 receptor as an entry gate[78] into the pancreas. An interesting study by Thaweerat et al[79] showed that the ACE2 receptors are more densely populated in the endocrine area when compared with the exocrine area of the pancreas through ACE2 immunostaining of pancreatic tissue[79]. The main function of the exocrine area of the pancreas is to facilitate blood glucose regulation[80]. SARS-CoV-2 enters into the pancreas thereby triggering autoimmunity and resulting in pancreatic cell destruction; this is particularly prevalent in severe COVID-19 cases[81-84]. This pathway, activated due to viral infection, may lead to the production of cross-reactive antibodies against pancreatic cells (molecular mimicry hypothesis)[85,86]. Thus, the hypothesis of the bidirectional involvement of DM-COVID-19 holds, which states that the SARS-CoV-2 infection results in direct damage to pancreatic islet cells, leading to the impairment of insulin levels[79,87] and potentially triggering DM (shown in pathway-IV in Figure 1). Recently Abbas et al[59] also validated the existence of this pathway (Table 1). Additionally, Baracchini et al[66] and Mota et al[58] also mentioned this pathway in their recent work on COVID-19 (Table 1). Balasubramanyam et al[88] and Rubino et al[35] further asserted their views on this pathway, establishing its validation.
Pathway V suggests that endocytosis of SARS-CoV-2 decreases ACE2 levels that causes the increase of angiotensin II (AngII) levels, which is a potent vasoconstrictor. Constriction of vessel lumen may be due to inhibition of nitric oxide in the endothelium of islet capillaries[89]. This results in a decrease in blood supply to the pancreas. Islet cells receive 15% of the total blood supply to the pancreas, even though they constitute only 1%-2% of pancreatic volume[90]. Hence, a decrease in the blood flow to the pancreatic islets due to vasoconstriction may impair insulin secretion in the pancreas[91] (see pathway V of Figure 1). This further confirms the bidirectional association between COVID-19 and DM, also listed in Table 1.
In pathway VI, increased proinflammatory cytokines due to COVID-19 are higher in patients with DM in comparison with patients without DM. This likely contributes to a poorer prognosis when both diseases coexist[92]. The severe illness that accompanies COVID-19 causes a systemic inflammatory response. This can be seen even with mild COVID-19 infection, resulting in an increase of proinflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor alpha (TNF-α)[93]. Increased proinflammatory cytokines result in decreased insulin sensitivity, which then leads to hyperglycemia. Further obesity, a significant coexisting condition associated with type 2 diabetes, is linked to the development of insulin resistance (IR). Obesity and type 2 diabetes further aggravate the proinflammatory cytokine response, which worsens the IR[93] (see pathway VI in Figure 1). Wang et al[70] reported that SARS-CoV-2 infection in patients with diabetes results in increased levels of stress hormones such as glucocorticoids that can lead to a hyperglycemic state. An acute rise in glycemic levels may result in life-threatening complications like ketoacidosis. Finally, the systemic inflammatory state associated with COVID-19 may plausibly worsen the pre-existing IR state in such individuals, manifesting as overt DM[94]. The long-term sequelae of this process are currently unknown, and clinical studies are needed to validate the hypothesis further. However, our hypothesis is supported by some recent studies that also indicate a similar thought that “the association between COVID-19 and hyperglycemia is because of metabolic inflammation and exaggerated cytokine release” (Table 1)[60,95]. This thought emerged because of the potential role of SARS-COV-2 in the impairment of insulin secretion, leading to hyperglycemia. Reddy et al[96] presented a case study of two patients positive with COVID-19 and no personal history of diabetes. The authors indicated precipitation of DKA, which can occur in newly diagnosed diabetes patients. Pal et al[97] also provided an overview of this bidirectional interaction between DM and COVID-19, where COVID-19 may lead to diabetes and, in turn, further increases the severity of COVID-19. These studies validate our thought process and indicate a need for a global study to investigate this bidirectional hypothesis. The recent announcement of the CoviDIAB project will shed light on this possible hypothesis of the bidirectional association between both of these global healthcare emergencies[35,98].
Throughout the world, DM is one of the leading causes of mortality and morbidity due to its association with several microvascular and macrovascular complications, which include CVD. Since there is a positive correlation between DM and COVID-19, it is imperative to understand its implication on CVD risk. Several studies have found that patients with COVID-19 and DM are at increased risk of vascular complications[99,100]. Poor glycemic control with the presence of IR plays a vital role in the worsening of CVD risk in DM patients[101]. As supporting evidence, the study by Madjid et al[102] highlighted the identification of heart damage through high levels of troponin in the blood due to COVID-19 leading to mortality of the patient. Further, it was shown that there is a role for inflammation of the heart due to COVID-19, e.g., myocarditis, vascular inflammation, and cardiac arrhythmias. Other supporting evidence by Javanmardi et al[103] is a meta-analysis showing the prevalence of pre-existing diseases in COVID-19 patients. The data in this study were pooled from 10 articles having 76993 patients and showed a prevalence of 7.87% [95% confidence interval (CI) 6.57-9.28] diabetes, 16.37% (95%CI: 10.15-23.65) hypertension, 12.11% (95%CI 4.40-22.75) CVD, and 7.63% (95%CI 3.83-12.43) smoking history, respectively, in patients infected with SARS-CoV-2. Further, Azar et al[104] have shown that the presence of pre-existing diseases such as DM, hypertension, and CVD are more likely to be associated with an increased risk of mortality in COVID-19 patients. Azar et al[104] focused on the cytokine storm concept that showed the connection between DM and COVID-19. Further, they showed that the higher basal levels of proinflammatory cytokines were seen in diabetic patients, which resulted in a cytokine storm with an increase in viral infection. They demonstrated the link between high levels of IL-6 and the AMP-activated protein kinase (AMPK)/mechanistic target of rapamycin signaling pathway and their role in exacerbating diabetes-related complications and IR. Both statements in the article support pathway VI of our article, which shows the possibility of aggravating preexisting diabetes or new-onset diabetes in COVID-19 due to cytokine storm. Additionally, they highlighted the role of the ACE2 receptor during viral binding to the host cell, thereby causing an increased risk of viral uptake in diabetes patients.
The work of Azar et al[104] is one of the bases of our hypothesis, where we discuss the two-way relationship between DM and COVID-19, i.e. triggering of COVID-19 on the new onset of DM and worsening glycemic levels of DM. Further, our study demonstrated the importance of early imaging to prevent CVD among all patients with COVID-19. On the contrary, the studies by Madjid et al[102] and Javanmardi et al[103] did not directly support the concept of bidirectional relationship. Additionally, Sattar et al[105] showed that there was worsening of cardiac events in COVID-19 patients with preexisting cardiac conditions, such as coronary artery disease, hypertension, and DM[105]. Furthermore, six different studies across various hospitals in China reported the prevalence of comorbid conditions in COVID-19 patients. Out of 1527 COVID-19 admissions, 9.7% had diabetes, leading to increased CVD prevalence by 16.4%[7,106-110].
In the previous two sections, we have explained the possible bidirectional link between DM and COVID-19. Current data from many countries such as China, Italy, and the United States have shown that COVID-19 can lead to mild symptoms in most individuals. However, a minority of individuals suffer from severe complications due to underlying chronic complications (explained in detail in section I, page 3)[111]. Possible reasons for increased CVD risk in known DM patients infected with COVID-19 are explained in five subsections (labeled as pathways A to E) of Figure 1. The first two subsections discuss the possible connection between DM and CVD, which includes (1) oxidative stress due to chronic hyperglycemia (subsection A) and (2) increased coagulation activity (subsection B). Subsection C shows how COVID-19 and DM jointly affect CVD due to hypoxia. In the last two subsections (D and E), we show the possible pathways between COVID-19 and CVD. This includes the role of (1) cytokine storm (subsection D) and (2) renin-angiotensin-aldosterone system (RAAS) dysregulation along with endothelial dysfunction (subsection E).
Oxidative stress is defined as the pathology of hyper-production of “reactive oxygen species” (ROS) and the counterbalancing part of the endogenous antioxidant defensive system[106]. Chronic hyperglycemia and IR in DM result in the production of proinflammatory cytokines and an increase of “advanced glycation end products” (AGEs)[61,107]. High levels of AGEs increase CVD risk two-fold when compared with low AGE levels[112]. A further increase in AGE levels results in ROS production, which accelerates AGE production, producing a cyclic effect[113]. Increased ROS results in oxidative stress as a systemic manifestation that plays a vital role in DM[108]. These further result in endothelial dysfunction due to (1) nitric oxide inhibition in the endothelial cells of the blood vessels and (2) increased inflammation and fibrosis[109], eventually leading to increased risk of atherosclerotic CVD (marked as subsection A in Figure 1). Interestingly, many studies found that AMPK has a protective role in cardiac injury by acting against oxidative stress and turns into a potential therapeutic target in patients with diabetes and COVID-19[110,114]. Besides this link between oxidative stress, DM, and CVD, some recent studies have also pointed out a possible role of oxidative stress in the pathogenesis of COVID-19 related infections[112,113,115,116]; however, this is beyond the scope of this review.
Increase in coagulation activity occurs due to the loss of fibrinolytic activity associated with DM. In general, the fibrinolytic process helps to degrade clots and remove them from blood vessels. It counters clot formation and risk occlusion in blood vessels by eliminating fibrin from the vasculature. In patients with DM, there is an increase in clotting factors and a relative reduction of the fibrinolytic system[117]. The impaired coagulation in DM is associated with alterations of the fibrin network and increased antifibrinolytic proteins[118]. Hence the reduced coagulation activity in patients with DM causes endothelial dysfunction by triggering platelet activation and aggregation, which further favors atherosclerotic plaque formation[119], increasing cardiovascular risk (marked as subsection B in Figure 1).
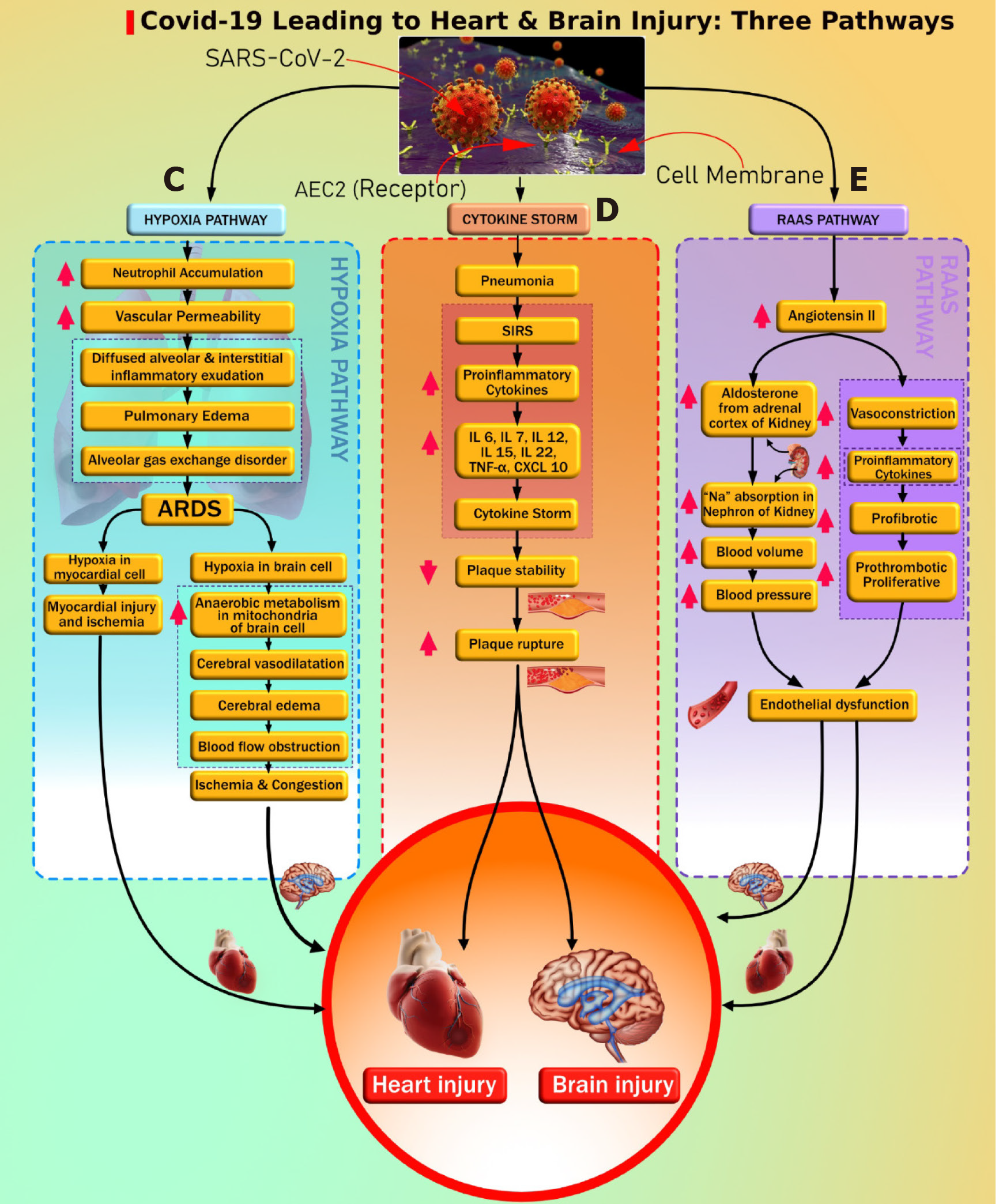
There is clear evidence that SARS-CoV-2 causes pulmonary as well as extrapulmonary complications like CVD[120]. Primary SARS-CoV-2 enters through the respiratory route and anchors to AT2 cells in the alveolar pulmonary epithelium[121]. This fusion is occurring due to the presence of ACE2 receptors on the surface of AT2 cells and the resulting development of respiratory symptoms as the most common clinical presentation of COVID-19 patients[122]. Infected AT2 cells further initiate the immune response by producing inflammatory mediators shown with SARS-CoV-1 and stimulate the production of proinflammatory cytokines and chemokines[123]. Hyperproduction of chemokines and cytokines results in endothelial dysfunction, causing vasodilation and an increase in sub-endothelial space's vascular permeability. This further leads to diffused alveolar interstitial exudate[124] and causes pulmonary edema resulting in an alveolar gas exchange disorder known as “acute respiratory distress syndrome” (ARDS)[125]. Additionally, DM patients can have reduced lung function indicated by decreased levels of “forced vital capacity” (FVC) and “forced expiratory volume in one second” (FEV1). Generally, FVC and FEV1 are vital parameters for accessing lung function[126-128]. Impending lung function is associated with chronic hyperglycemia resulting in an increased risk of ARDS[126-128]. Decreased lung function in DM can increase the risk of ARDS if infected with COVID-19. This was further supported by Huang et al[129], where the authors showed that roughly 30% of diabetes patients with COVID-19 developed impairment in lung function, shown as a decline in FEV1/FVC ratio. Hence, ARDS risk is increased with the coexistence of DM and COVID-19 and can further lead to depletion in the oxygen levels in the blood[130-132].
Ongoing hypoxia in myocardial cells results in myocardial ischemia and heart injury[132], and ongoing hypoxia in brain cells results in brain injury[133]. Nan et al[134] showed that COVID-19 patients with comorbidity had an acute cardiac injury and needed invasive mechanical ventilation, while Kwenandar et al[135] showed that cardiovascular manifestations in COVID-19 patients like myocardial injury, arrhythmias, sudden cardiac arrest, heart failure, and coagulation abnormality occur in up to 33% of patients[135]. Zunyou, Wu et al[136], and Clerkin et al[137] submitted a summary of the report to the Chinese center for disease control and prevention indicating 1023 deaths in 44672 confirmed cases with COVID-19, i.e. a case-fatality rate (CFR) of 2.3, and stating that patients with underlying CVD or hypertension had a higher CFR compared with people without comorbidities[136,137].
Guo et al[92] reported a higher risk of pneumonia in COVID-19 patients with DM when compared with patients without a history of DM. Patients with DM experience an advanced stage of illness that causes multiple organ dysfunction, triggering an exaggerated inflammatory response compared with non DM. This results in the production of proinflammatory cytokines that include IL-6, IL-7, IL-12, IL-15, IL-22, C-reactive protein, and TNF-α, leading to cytokine storm[138-141]. Another study by Guo et al[142] showed that cardiac injury in patients with COVID-19 had elevated troponin and C-reactive protein, suggestive of increased morbidity and mortality[122,142]. Zheng et al[122] and Wu et al[143] exemplified that cardiac injury may happen due to cytokine storms caused by the inflammatory response of T helper cells. Huang et al[129] also supported that imbalance in T helper cells results in triggering cytokine storm leading to destabilization of carotid plaque and micro thrombosis. Supporting evidence by Kang et al[144] showed that significant risk of cardiac complications, such as arrhythmia, heart failure, and myocardial infarction, in COVID-19 was due to a combination of (1) hyper inflammation with cytokine release, (2) plaque instability, (3) myocardial inflammation, (4) hypercoagulable state, and (5) direct myocardial injury. Vinayagam et al[145] concluded that chronic inflammation through cytokines and chemokines promotes hypercoagulability, causing multiorgan dysfunction leading to heart and brain injury.
RAAS plays an important role in maintaining cardiovascular health and electrolyte balance[146] and has been well-described before. In COVID-19 patients, the SARS-CoV-2 gains entry into the cells by attaching to the ACE2 receptor of the cell. The anchoring ability of the virus is due to its spike protein, which is present on its surface[71,147,148]. The dysregulation of RAAS occurs due to the loss of a counter-balance between Ang II levels and ACE2 levels after SARS-CoV-2 infection[149]. ACE2 levels degrade Ang II and produce Ang (1-7), which opposes the negative impact of Ang II[132,150]. ACE2 and Ang (1-7) are recognized as a cardio-cerebral protective factor[151]. The reduced levels of ACE2 receptors and increased levels of Ang II after a SARS-CoV-2 infection can lead to atherosclerotic CVD in two possible ways. First, an increased Ang II level causes stimulation of the adrenal gland, triggering the production of the mineralocorticoid hormone aldosterone[146], which causes Na (sodium) and water retention in the collecting duct of the kidney[152]. This increases blood volume and blood pressure[153], causing endothelial dysfunction that progresses to atherosclerotic CVD. Second, excessive Ang II levels result in vasoconstriction, proinflammation, prothrombotic, and proliferative effects[132,150]. This has a detrimental effect on the blood vessels, thereby leading to endothelial cell damage and subsequent atherosclerotic cardiovascular events. Additionally, DM patients using ACE inhibitors and ARBs have increased ACE2 expression, which is beneficial to vascular health by reducing profibrotic and proinflammatory function. But, increased ACE2 levels promote the entry of SARS-CoV-2 infection that potentially results in a loss of ACE2 in blood vessels in diabetes patients causing vascular complications like CVD[154]. Recently, Suri et al[23] showed that COVID-19 is an independent risk factor for developing CVD due to hypoxia, cytokine storm, and RAAS dysregulation.
In Table 2, we briefly illustrated the difference in COVID-19 severity between patients with DM and non-DM. We have shown that DM has an added risk in patients with COVID-19. There are many reasons to explain why COVID carries a worse prognosis in DM patients. They include age, culture, comorbidities like hypertension and pre-existing CVD, higher body mass index, and proinflammatory and pro-coagulable state, all of which may contribute to the risk of worse outcomes[155].
| Pathophysiology of CVD | Non-diabetes | Diabetes | Reason |
| Hypoxia | ↑ | ↑ ↑ | In COVID-19 patients compared with non-DM cases, DM reduced pulmonary function by reduced levels of FVC and FEV1 this condition further deteriorated in COVID-19 causing hypoxia[126-128,134] ongoing ischemia results in hypoxia causing CVD |
| Cytokine storm | ↑ | ↑ ↑ | In COVID-19 patients compared with non-DM cases, DM increases the severity of the cytokine storm is due to exaggerated inflammatory response[138-141]. Thus, it increases the endothelial dysfunction causing a decrease in plaque stability, and an increase in plaque rupture results in CVD |
| RAAS Dysregulation | ↑ | ↑ ↑ | DM patients using ACE inhibitors and ARBs have increased ACE2 expression is beneficial to vascular health by reducing profibrotic and proinflammatory function. But increased ACE2 levels increase the entry of SARS-CoV-2 infection, which potentially results in loss of ACE2 in blood vessels in diabetes patients causing vascular complications like CVD (see Obukhov et al[154]) |
A meta-analysis by Santoso et al[156] showed a total of 2389 patients taken from 13 studies that had a cardiac injury and were associated with a higher risk of mortality when affected by COVID-19. Further, the authors stated that these patients required intensive care unit admission during the COVID-19 period[156]. In another meta-analysis by the same group (see Huang et al[157]), which included 6452 patients with DM from 30 studies, DM associated with the worst outcome and mortality when affected by COVID-19. Additionally, Pranata et al[158] showed in a total of 4448 patients from 16 studies that 77% of people had poor health outcomes due to cerebrovascular disease, and 60% of people had poor health outcomes due to CVD.
Vit D has many beneficial roles in the maintenance of musculoskeletal health, and its deficiency causes calcium malabsorption resulting in fractures[159]. This can be prevented by a daily intake requirement of 800-2000 IU of Vit D, co-administrated with calcium, thereby reducing the risk of fracture by 15%-30%. This range of doses is recommended by major organizations during pre-COVID times[160]. Interestingly in recent publications on COVID-19 by Ilie et al[161] and Rhodes et al[162], the authors showed that low Vit D levels are associated with higher mortality rates in SARS-CoV-2 infections. Further ecological studies have shown that major risk factors of low Vit D levels are older age, higher latitudes, winter season, less sunlight exposure, and dietary habits. Vit D is responsible for the modulation of innate and adaptive immunity via Vit D receptor (VDR) and CYP27B1 (enzyme converting it to active metabolite calcitriol), and both are expressed in immune cells[163,164].
Many studies showed that the major role of Vit D in COVID-19 is that it lessens the cytokine production after SARS-CoV-2 infection including IL6, TNF-α, and IFN-β[165]. Other anti-viral properties include modulation of macrophage chemotactic protein1, IL 8, type 1 IFN, TNF-α, and lowering of ROS[166]. Ongoing clinical trials on pharma-ceutical interventions of 2019 Novel Coronavirus Research Compendium[167] and primary registry trials of World Health Organization[168] include trials of Vit D supplementation in COVID-19 infection.
Figure 2 shows the cytokine storm leading to atherosclerosis and pathway “E” leading to endothelial dysfunction and atherosclerosis formation. Both DM and COVID-19 are associated with vascular wall damage[169] (such as plaque erosion or atherosclerotic plaque vulnerability), and this elevates the risk of atherosclerotic CVD events[170-172]. Vinciguerra et al[173] reported that atherosclerosis may be an ideal pathogenetic substrate for high viral replication ability, leading to adverse cardiovascular outcomes. Atherosclerosis is generally initiated by damage to the endothelial cells[174]. Recent histological findings suggest the involvement of COVID-19 viral elements in endothelial cell damage and the accumulation of inflammatory cells leading to endothelial cell death[175]. The damage to endothelial cells and the hyperdynamic circulation due to COVID-19 may lead to atherosclerotic plaque instability and rupture[176].
Similarly, the formation of atherosclerotic thrombus in the arteries has been reported in recent studies[177]. Lapergue et al[177] reported COVID-19 patients that showed the development of large thrombus in the cervical carotid artery with underlying mild non-stenosing atheroma. Indes et al[178] also reported an elevation of risk for acute arterial thromboembolic complications in patients with COVID-19 infection. Esenwa et al[179] presented a radiology-pathology case series of three COVID-19 patients and reported that a disproportionately high intra-luminal thrombus in the carotid artery showing mild-to-moderate atherosclerotic disease with intimal thickening and plaque calcification. Mohamud et al[180] presented a case study of six COVID-19 patients that had atherosclerotic plaque vulnerability and the development of thrombotic events. This was the result of the inflammatory response and cytokine storm, which has also been considered as an important phenomenon for cardiac events in our review (Figure 2). Similarly, Alkhaibary et al[181] reported complete occlusion of the left common carotid artery and left middle cerebral artery of the COVID-19 patients.
These studies have also indicated the presence of vascular risk factors such as hypertension and DM that exaggerate the risk of cardiovascular and stroke events in COVID-19 patients. Since the COVID-19 driven cytokine storm is associated with atherosclerotic plaque instability, it is essential to screen such patients for the presence of arterial plaque burden. Imaging techniques have shown promising results in screening, diagnosis, and patient management during this pandemic. COVID-19 symptoms of the patients and seriousness have helped to decide which imaging technique is most appropriate: Portable and non-portable[23]. In COVID-19 patients, non-invasive carotid ultrasound may be adopted for low-risk patients to investigate the presence of carotid atherosclerotic plaque[182,183], which is also considered as a surrogate marker CVD[184-186] and also used for CVD risk assessment in diabetic patients[187-197]. Similarly, magnetic resonance imaging and X-rays can be useful for screening of medium risk patients[198-200]. Most of the studies prefer the use of these non-portable imaging techniques. For critical cases, intravascular ultrasound imaging, computed tomography angiography, and ventriculography are generally followed for arterial imaging[201-203]. Among all these techniques, ultrasound is a portable, less expensive, and radiation-free imaging technique and, therefore, can be adopted for the screening of atherosclerotic plaque in patients with COVID-19[204]. Several studies used ultrasound to detect the rupture-prone atherosclerotic plaque and the tissue characterization of such plaque for the prevention of future cardiac events[205-207]. Furthermore, studies have used ultrasound-based imaging techniques for CVD risk assessment in patients with diabetes[187-189,191,193,195,196,208-212] and, thus, could also be adopted for diabetic patients with COVID-19. Several other imaging-based studies have shown the interaction of plaque measurements in diabetes patients[196,211,212]. In the imaging category, plaque area was recently measured and correlated with hemoglobin A1c in diabetes patients[213]. These further assert the role of atherosclerotic imaging and phenotype measurements in post-COVID-19 patients. Image-based risk calculators can be adapted for 10-year risk assessment on diabetes patients, which can be adapted for post-COVID-19 follow-up[211,214].
In the current pandemic, it is also equally important for radiologists and medical practitioners to follow the guidelines while conducting an imaging-based screening of COVID patients. These include isolating the imaging equipment, taking the images through the isolation room glasses, and using disposable sterile protection to imaging probes[23].
We showed clearly the six pathways between DM and COVID-19, establishing the DM-COVID-19 loop. Three pathways were unidirectional from DM to COVID-19 and vice-versa establishing the bidirectional flow. Further, we demonstrated the effect of this DM-COVID-19 loop on CVDs, causing the acceleration towards cardiovascular and cerebrovascular events. The mini-review also shows why only a minority group of people develop severe complications, unlike the majority group of people who escape. The review also sheds light on the role of Vit D during the COVID-19 pandemic. Finally, the review presents the role of vascular imaging for tracking atherosclerotic burden during COVID-19 and on long term follow-up patients. We conclude that (a) DM/COVID-19 loop is detrimental to the patient’s heart and brain and (b) early monitoring of atherosclerotic burden is required in “Diabetes patients during COVID-19” or “new-onset Diabetes triggered by COVID-19 in Non-Diabetes patients”.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li T S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Worldometer. COVID-19 Coronavirus Pandemic. Available from: https://www.worldometers.info/coronavirus/. |
| 2. | Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab. 2014;18:868-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Organization WH. World Health Organization: Diabetes - Key Facts. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. |
| 4. | Blind E, Janssen H, Dunder K, de Graeff PA. The European Medicines Agency's approval of new medicines for type 2 diabetes. Diabetes Obes Metab. 2018;20:2059-2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 479] [Article Influence: 95.8] [Reference Citation Analysis (1)] |
| 6. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1653] [Cited by in RCA: 2123] [Article Influence: 424.6] [Reference Citation Analysis (0)] |
| 7. | Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liuxingbing Xue Zazhi. 2020;2:145-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1049] [Article Influence: 209.8] [Reference Citation Analysis (0)] |
| 8. | Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020;382:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1819] [Cited by in RCA: 1861] [Article Influence: 372.2] [Reference Citation Analysis (0)] |
| 9. | Klonoff DC, Umpierrez GE. Letter to the Editor: COVID-19 in patients with diabetes: Risk factors that increase morbidity. Metabolism. 2020;108:154224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 507] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 11. | Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, Sun GZ, Yang GR, Zhang XL, Wang L, Xu X, Xu XP, Chan JC. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 497] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 13. | Schoen K, Horvat N, Guerreiro NFC, de Castro I, de Giassi KS. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19:964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Banik GR, Alqahtani AS, Booy R, Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: Analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31:81-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 423] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 16. | Gupta R, Hussain A, Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74:864-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 17. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:E21. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1951] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 18. | Pititto BA, Ferreira SRG. Diabetes and covid-19: more than the sum of two morbidities. Rev Saude Publica. 2020;54:54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020;. [DOI] [Full Text] |
| 20. | Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell LF, Chernyak Y, Tobin K, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020;. [DOI] [Full Text] |
| 21. | CDC. Coronavirus Disease 2019 (COVID-19)-Global COVID-19 World Map. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/world-map.html. |
| 22. | Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020;382:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 719] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 23. | Suri JS, Puvvula A, Biswas M, Majhail M, Saba L, Faa G, Singh IM, Oberleitner R, Turk M, Chadha PS, Johri AM, Sanches JM, Khanna NN, Viskovic K, Mavrogeni S, Laird JR, Pareek G, Miner M, Sobel DW, Balestrieri A, Sfikakis PP, Tsoulfas G, Protogerou A, Misra DP, Agarwal V, Kitas GD, Ahluwalia P, Kolluri R, Teji J, Maini MA, Agbakoba A, Dhanjil SK, Sockalingam M, Saxena A, Nicolaides A, Sharma A, Rathore V, Ajuluchukwu JNA, Fatemi M, Alizad A, Viswanathan V, Krishnan PR, Naidu S. COVID-19 pathways for brain and heart injury in comorbidity patients: A role of medical imaging and artificial intelligence-based COVID severity classification: A review. Comput Biol Med. 2020;124:103960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. medRxiv. 2020;. [DOI] [Full Text] |
| 25. | Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, Southern WN, Mantzoros CS. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 586] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 26. | Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M; LICORN and the Lille COVID-19 and Obesity study group. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020;28:1195-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1263] [Cited by in RCA: 1576] [Article Influence: 315.2] [Reference Citation Analysis (0)] |
| 27. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP; Becker LB; Chelico JD; Cohen SL; Cookingham J; Coppa K; Diefenbach MA; Dominello AJ; Duer-Hefele J; Falzon L; Gitlin J; Hajizadeh N; Harvin TG; Hirschwerk DA; Kim EJ; Kozel ZM; Marrast LM; Mogavero JN; Osorio GA; Qiu M; Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6515] [Article Influence: 1303.0] [Reference Citation Analysis (0)] |
| 28. | Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health. 2016;16:1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Desborough J, Hall SL, de Toca L, Davis S, Roberts L, Kelaher C, Kidd M. Australia's national COVID-19 primary care response. Med J Aust. 2020;1. |
| 30. | Farkas KJ, Romaniuk JR. Social work, ethics and vulnerable groups in the time of Coronavirus and COVID-19. Society Register. 2020;4:67-82. [DOI] [Full Text] |
| 31. | White C, Nafilyan V. Coronavirus (COVID-19) related deaths by ethnic group, England and Wales: 2 March 2020 to 10 April 2020. Office for National Statistics 2020. |
| 32. | Chowkwanyun M, Reed AL Jr. Racial Health Disparities and Covid-19 - Caution and Context. N Engl J Med. 2020;383:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 455] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 33. | Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 34. | Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 35. | Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z, Del Prato S, Ji L, Hopkins D, Herman WH, Khunti K, Mbanya JC, Renard E. New-Onset Diabetes in Covid-19. N Engl J Med. 2020;383:789-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 550] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 36. | Kanc K, Komel J, Kos M, Wagner J. H(ome)bA1c testing and telemedicine: High satisfaction of people with diabetes for diabetes management during COVID-19 lockdown. Diabetes Res Clin Pract. 2020;166:108285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Anjana RM, Pradeepa R, Deepa M, Jebarani S, Venkatesan U, Parvathi SJ, Balasubramanyam M, Radha V, Poongothai S, Sudha V, Shanthi Rani CS, Ranjani H, Amutha A, Manickam N, Unnikrishnan R, Mohan V. Acceptability and Utilization of Newer Technologies and Effects on Glycemic Control in Type 2 Diabetes: Lessons Learned from Lockdown. Diabetes Technol Ther. 2020;22:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Solini A, Zoppini G, Orsi E, Fondelli C, Trevisan R, Vedovato M, Cavalot F, Lamacchia O, Arosio M, Baroni MG, Penno G, Pugliese G; Renal Insufficiency And Cardiovascular Events (RIACE) Study Group. Resistant hypertension in patients with type 2 diabetes: clinical correlates and association with complications. J Hypertens. 2014;32:2401-10; discussion 2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Saglimbene V, Palmer SC, Ruospo M, Natale P, Maione A, Nicolucci A, Vecchio M, Tognoni G, Craig JC, Pellegrini F, Lucisano G, Hegbrant J, Ariano R, Lamacchia O, Sasso A, Morano S, Filardi T, De Cosmo S, Pugliese G, Procaccini DA, Gesualdo L, Palasciano G, Johnson DW, Tonelli M, Strippoli GFM; Long-Term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) Investigators. The Long-Term Impact of Renin-Angiotensin System (RAS) Inhibition on Cardiorenal Outcomes (LIRICO): A Randomized, Controlled Trial. J Am Soc Nephrol. 2018;29:2890-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Rosenblit PD. Common medications used by patients with type 2 diabetes mellitus: what are their effects on the lipid profile? Cardiovasc Diabetol. 2016;15:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, Santisteban P, González-Matías LC, Vigo E, Mallo F. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology. 2015;156:3559-3569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 42. | Zhang W, Li C, Liu B, Wu R, Zou N, Xu Y-Z, Yang Y-Y, Zhang F, Zhou H-M, Wan K-Q. Pioglitazone upregulates hepatic angiotensin converting enzyme 2 expression in rats with steatohepatitis. Ann Hepatol. 2013;12:892-900. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Ali RM, Al-Shorbagy MY, Helmy MW, El-Abhar HS. Role of Wnt4/β-catenin, Ang II/TGFβ, ACE2, NF-κB, and IL-18 in attenuating renal ischemia/reperfusion-induced injury in rats treated with Vit D and pioglitazone. Eur J Pharmacol. 2018;831:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 45. | Filippatos TD, Liontos A, Papakitsou I, Elisaf MS. SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses. Postgrad Med. 2019;131:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546-550. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 562] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 47. | Hampp C, Swain RS, Horgan C, Dee E, Qiang Y, Dutcher SK, Petrone A, Chen Tilney R, Maro JC, Panozzo CA. Use of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Type 1 Diabetes and Rates of Diabetic Ketoacidosis. Diabetes Care. 2020;43:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2912] [Article Influence: 582.4] [Reference Citation Analysis (0)] |
| 49. | Cüre E, Cumhur Cüre M. Comment on 'Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract. 2020;162:108132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 51. | Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 52. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1528] [Article Influence: 305.6] [Reference Citation Analysis (0)] |
| 53. | Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 54. | Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv. 2020;. [DOI] [Full Text] |
| 55. | Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 56. | Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 57. | Shin YH, Min JJ, Lee JH, Kim EH, Kim GE, Kim MH, Lee JJ, Ahn HJ. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Vessels. 2017;32:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Mota M, Stefan A. Covid-19 and Diabetes-A Bidirectional Relationship? Rom J Diabetes Nutr Metab Dis. 2020;27:77-79. |
| 59. | Abbas AM. Bidirectional Relationship between COVID-19 and Diabetes. Am J Biomed Sci Res. 2020;9:424-426. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Muniangi-Muhitu H, Akalestou E, Salem V, Misra S, Oliver NS, Rutter GA. Covid-19 and Diabetes: A Complex Bidirectional Relationship. Front Endocrinol (Lausanne). 2020;11:582936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 61. | Knapp S. Diabetes and infection: is there a link? Gerontology. 2013;59:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 62. | Moutschen M, Scheen A, Lefebvre P. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18:187. |
| 63. | Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 777] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 64. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 65. | Chen X, Hu W, Ling J, Mo P, Zhang Y, Jiang Q, Ma Z, Cao Q, Deng L, Song S. Hypertension and diabetes delay the viral clearance in COVID-19 patients. medRxiv. 2020;. [DOI] [Full Text] |
| 66. | Baracchini C, Pieroni A, Kneihsl M, Azevedo E, Diomedi M, Pascazio L, Wojczal J, Lucas C, Bartels E, Bornstein NM, Csiba L, Valdueza J, Tsivgoulis G, Malojcic B. Practice recommendations for neurovascular ultrasound investigations of acute stroke patients in the setting of the COVID-19 pandemic: an expert consensus from the European Society of Neurosonology and Cerebral Hemodynamics. Eur J Neurol. 2020;27:1776-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1373] [Article Influence: 274.6] [Reference Citation Analysis (0)] |
| 68. | Kalra S, Kalhan A, Akanov ZA. COVID-19 and Endocrinology - A Bidirectional Relationship. 2020. Available from: https://www.touchendocrinology.com/insight/covid-19-and-endocrinology-a-bidirectional-relationship/. |
| 69. | Fernandez C, Rysä J, Almgren P, Nilsson J, Engström G, Orho-Melander M, Ruskoaho H, Melander O. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284:377-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 70. | Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 71. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14253] [Article Influence: 2850.6] [Reference Citation Analysis (0)] |
| 72. | Walls AC, Park Y, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. BioRxiv. 2020;. [DOI] [Full Text] |
| 73. | Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism. 2016;65:507-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 74. | Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 75. | Misra S, Oliver NS. Diabetic ketoacidosis in adults. BMJ. 2015;351:h5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Caruso P, Longo M, Esposito K, Maiorino MI. Type 1 diabetes triggered by covid-19 pandemic: A potential outbreak? Diabetes Res Clin Pract. 2020;164:108219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Lönnrot M, Lynch KF, Elding Larsson H, Lernmark Å, Rewers MJ, Törn C, Burkhardt BR, Briese T, Hagopian WA, She JX, Simell OG, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Hyöty H; TEDDY Study Group. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 78. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4601] [Article Influence: 209.1] [Reference Citation Analysis (0)] |
| 79. | Thaweerat W. Current evidence on pancreatic involvement in SARS-CoV-2 infection. Pancreatology. 2020;20:1013-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 549] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 81. | Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol 2020; 18: 2128-2130. e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 473] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 82. | Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, Holl RW. Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA. 2020;324:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 83. | Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol. 2020;57:1265-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 84. | Oriot P, Hermans MP. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg. 2020: 1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 85. | Chowdhury S, Goswami S. COVID-19 and type 1 diabetes: dealing with the difficult duo. Int J Diabetes Dev Ctries. 2020: 1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus--why the β cells? Nat Rev Endocrinol. 2016;12:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 87. | Liu F, Long X, Zou W, Fang M, Wu W, Li W, Zhang B, Zhang W, Chen X, Zhang Z. Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection. medRxiv. 2020;. [DOI] [Full Text] |
| 88. | Balasubramanyam M. Does COVID-19 Warn Us to Revisit Virus-Induced Diabetes? Explor Res Hypothesis Med. 2020;5:129-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, and hypertension. Semin Nephrol. 2004;24:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Jansson L, Hellerström C. Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia. 1983;25:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 91. | Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020: e3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 884] [Cited by in RCA: 899] [Article Influence: 179.8] [Reference Citation Analysis (1)] |
| 93. | Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21:e13034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 94. | Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 649] [Cited by in RCA: 768] [Article Influence: 76.8] [Reference Citation Analysis (10)] |
| 95. | American Diabetes Association. How COVID-19 Impacts People with Diabetes. Available from: https://www.diabetes.org/coronavirus-covid-19/how-coronavirus-impacts-people-with-diabetes. |
| 96. | Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: A report of two cases and review of literature. Diabetes Metab Syndr. 2020;14:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 97. | Pal R, Bhadada SK. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14:513-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 98. | COVIDIAB registry. A joint initiative of King's College London and Monash university, Australia. Available from: http://covidiab.e-dendrite.com/. |
| 99. | Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 100. | Fox CS, Coady S, Sorlie PD, D'Agostino RB Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 101. | Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3494] [Cited by in RCA: 3615] [Article Influence: 180.8] [Reference Citation Analysis (0)] |
| 102. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1249] [Article Influence: 249.8] [Reference Citation Analysis (2)] |
| 103. | Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID-19: A systematic review and meta-analysis. PLoS One. 2020;15:e0241265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 104. | Azar WS, Njeim R, Fares AH, Azar NS, Azar ST, El Sayed M, Eid AA. COVID-19 and diabetes mellitus: how one pandemic worsens the other. Rev Endocr Metab Disord. 2020;21:451-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 105. | Sattar Y, Ullah W, Rauf H, Virk HUH, Yadav S, Chowdhury M, Connerney M, Mamtani S, Pahuja M, Patel RD, Mir T, Almas T, Moussa Pacha H, Chadi Alraies M. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. Int J Cardiol Heart Vasc. 2020;29:100589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21:425-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 107. | Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34:575-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1034] [Article Influence: 147.7] [Reference Citation Analysis (1)] |
| 108. | Savoia C, Sada L, Zezza L, Pucci L, Lauri FM, Befani A, Alonzo A, Volpe M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int J Hypertens. 2011;2011:281240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 109. | Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183-198. [PubMed] |
| 110. | Li T, Jiang S, Yang Z, Ma Z, Yi W, Wang D, Yang Y. Targeting the energy guardian AMPK: another avenue for treating cardiomyopathy? Cell Mol Life Sci. 2017;74:1413-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 111. | Shaw ML. Mortality, Risk Factors of Patients With Cardiac Injury and COVID-19. Available from: https://www.ajmc.com/view/mortality-risk-factors-of-patients-with-cardiac-injury-and-covid19. |
| 112. | Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease-a systematic review. J Infect Dis Epidemiol. 2020;6:121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Delgado-Roche L, Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51:384-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 406] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 114. | Li T, Mu N, Yin Y, Yu L, Ma H. Targeting AMP-Activated Protein Kinase in Aging-Related Cardiovascular Diseases. Aging Dis 2020; 11: 967-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 115. | Ntyonga-Pono M. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35:12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 116. | Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 117. | Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 514] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 118. | Kearney K, Tomlinson D, Smith K, Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol. 2017;16:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 119. | Dunn EJ, Grant PJ. Type 2 diabetes: an atherothrombotic syndrome. Curr Mol Med. 2005;5:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Geng YJ, Wei ZY, Qian HY, Huang J, Lodato R, Castriotta RJ. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc Pathol. 2020;47:107228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 121. | Filardi T, Morano S. COVID-19: is there a link between the course of infection and pharmacological agents in diabetes? J Endocrinol Invest. 2020;43:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 122. | Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2333] [Cited by in RCA: 2076] [Article Influence: 415.2] [Reference Citation Analysis (0)] |
| 123. | Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, Trilling M, Lu M, Dittmer U, Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 124. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 125. | Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 126. | McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;161:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 127. | Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, Selvin E, Brancati FL. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2008;31:741-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 128. | Shah SH, Sonawane P, Nahar P, Vaidya S, Salvi S. Pulmonary function tests in type 2 diabetes mellitus and their association with glycemic control and duration of the disease. Lung India. 2013;30:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 129. | Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, Zhou X, Liu X, Huang X, Yuan S, Chen C, Gao F, Huang J, Shan H, Liu J. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 352] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 130. | Wang L, Zhou X, Yin Y, Mai Y, Wang D, Zhang X. Hyperglycemia Induces Neutrophil Extracellular Traps Formation Through an NADPH Oxidase-Dependent Pathway in Diabetic Retinopathy. Front Immunol. 2018;9:3076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 131. | Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1102] [Article Influence: 220.4] [Reference Citation Analysis (0)] |
| 132. | Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 133. | Abdennour L, Zeghal C, Dème M, Puybasset L. [Interaction brain-lungs]. Ann Fr Anesth Reanim. 2012;31:e101-e107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 134. | Nan J, Jin Y-B, Myo Y, Zhang G. Hypoxia in acute cardiac injury of coronavirus disease 2019: lesson learned from pathological studies. J Geriatr Cardiol. 2020;17:221. |
| 135. | Kwenandar F, Japar KV, Damay V, Hariyanto TI, Tanaka M, Lugito NPH, Kurniawan A. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 136. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11504] [Article Influence: 2300.8] [Reference Citation Analysis (0)] |
| 137. | Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1220] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 138. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6746] [Article Influence: 1349.2] [Reference Citation Analysis (0)] |
| 139. | Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1169] [Cited by in RCA: 1119] [Article Influence: 223.8] [Reference Citation Analysis (0)] |
| 140. | Yin C, Wang C, Tang Z, Wen Y, Zhang S, Wang B. Clinical analysis of multiple organ dysfunction syndrome in patients suffering from SARS. Zhongguo Weizhongbing Jijiu Yixue. 2004;16:646-650. |
| 141. | Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 340] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 142. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2840] [Article Influence: 568.0] [Reference Citation Analysis (0)] |
| 143. | Wu L, O'Kane AM, Peng H, Bi Y, Motriuk-Smith D, Ren J. SARS-CoV-2 and cardiovascular complications: From molecular mechanisms to pharmaceutical management. Biochem Pharmacol. 2020;178:114114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 144. | Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 145. | Vinayagam S, Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260:118431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 146. | Leenen FHH, Blaustein MP, Hamlyn JM. Update on angiotensin II: new endocrine connections between the brain, adrenal glands and the cardiovascular system. Endocr Connect. 2017;6:R131-R145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 147. | de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2157] [Cited by in RCA: 2280] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 148. | Wu K, Peng G, Wilken M, Geraghty RJ, Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J Biol Chem. 2012;287:8904-8911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 149. | Augoustides JGT. The Renin-Angiotensin-Aldosterone System in Coronavirus Infection-Current Considerations During the Pandemic. J Cardiothorac Vasc Anesth. 2020;34:1717-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 151. | Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 621] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 153. | Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 154. | Obukhov AG, Stevens BR, Prasad R, Li Calzi S, Boulton ME, Raizada MK, Oudit GY, Grant MB. SARS-CoV-2 Infections and ACE2: Clinical Outcomes Linked With Increased Morbidity and Mortality in Individuals With Diabetes. Diabetes. 2020;69:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 155. | Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 603] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 156. | Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 157. | Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 158. | Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 159. | Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr Rev. 2019;40:1109-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 668] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 160. | Chakhtoura M, Chamoun N, Rahme M, Fuleihan GE. Impact of vitamin D supplementation on falls and fractures-A critical appraisal of the quality of the evidence and an overview of the available guidelines. Bone. 2020;131:115112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 161. | Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 467] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 162. | Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 163. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2913] [Article Influence: 582.6] [Reference Citation Analysis (0)] |
| 164. | Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1018] [Cited by in RCA: 1095] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 165. | Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 166. | Calton EK, Keane KN, Newsholme P, Soares MJ. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS One. 2015;10:e0141770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 167. | Johns Hopkins Bloomberg School of Public Health. 2019 Novel Coronavirus Research Compendium (NCRC). Available from: https://ncrc.jhsph.edu/. |
| 168. | World Health Organization. Important information related to the COVID-19 outbreak. Available from: https://www.who.int/ictrp/en/. |
| 169. | Saba L, Gerosa C, Wintermark M, Hedin U, Fanni D, Suri JS, Balestrieri A, Faa G. Can COVID19 trigger the plaque vulnerability-a Kounis syndrome warning for "asymptomatic subjects". Cardiovasc Diagn Ther. 2020;10:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 170. | Colling ME, Kanthi Y. COVID-19-associated coagulopathy: An exploration of mechanisms. Vasc Med. 2020;25:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 171. | Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 172. | Bandyopadhyay D, Akhtar T, Hajra A, Gupta M, Das A, Chakraborty S, Pal I, Patel N, Amgai B, Ghosh RK, Fonarow GC, Lavie CJ, Naidu SS. COVID-19 Pandemic: Cardiovascular Complications and Future Implications. Am J Cardiovasc Drugs. 2020;20:311-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 173. | Vinciguerra M, Romiti S, Fattouch K, De Bellis A, Greco E. Atherosclerosis as Pathogenetic Substrate for Sars-Cov2 Cytokine Storm. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 174. | Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2783] [Article Influence: 198.8] [Reference Citation Analysis (0)] |
| 175. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4576] [Article Influence: 915.2] [Reference Citation Analysis (0)] |
| 176. | Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020;5:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 177. | Lapergue B, Lyoubi A, Meseguer E, Avram I, Denier C, Venditti L, Consoli A, Guedon A, Houdart E, Weisenburger-Lile D, Piotin M, Maier B, Obadia M, Broucker TDE. Large vessel stroke in six patients following SARS-CoV-2 infection: a retrospective case study series of acute thrombotic complications on stable underlying atherosclerotic disease. Eur J Neurol. 2020;27:2308-2311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 178. | Indes JE, Koleilat I, Hatch AN, Choinski K, Jones DB, Aldailami H, Billett H, Denesopolis JM, Lipsitz E. Early experience with arterial thromboembolic complications in patents with COVID-19. J Vasc Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 179. | Esenwa C, Cheng NT, Lipsitz E, Hsu K, Zampolin R, Gersten A, Antoniello D, Soetanto A, Kirchoff K, Liberman A, Mabie P, Nisar T, Rahimian D, Brook A, Lee SK, Haranhalli N, Altschul D, Labovitz D. COVID-19-Associated Carotid Atherothrombosis and Stroke. AJNR Am J Neuroradiol. 2020;41:1993-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 180. | Mohamud AY, Griffith B, Rehman M, Miller D, Chebl A, Patel SC, Howell B, Kole M, Marin H. Intraluminal Carotid Artery Thrombus in COVID-19: Another Danger of Cytokine Storm? AJNR Am J Neuroradiol. 2020;41:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 181. | Alkhaibary A, Abbas M, Ahmed ME, Khatri IA, Alkhani A. Common Carotid Artery Occlusion in a Young Patient: Can Large-Vessel Stroke Be the Initial Clinical Manifestation of Coronavirus Disease 2019? World Neurosurg. 2020;144:140-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 182. | Singh TM, Lee A, Ali SF, Cheon BM, Josephson M, Boulom V. Urgent Carotid Endarterectomy in a COVID-19 Patient: Standard Approach With Some Adjustments. Vasc Dis Manag. 2020;17:E104-109. |
| 183. | Piscaglia F, Stefanini F, Cantisani V, Sidhu PS, Barr R, Berzigotti A, Chammas MC, Correas JM, Dietrich CF, Feinstein S, Huang P, Jenssen C, Kono Y, Kudo M, Liang P, Lyshchik A, Nolsøe C, Xie X, Tovoli F. Benefits, Open questions and Challenges of the use of Ultrasound in the COVID-19 pandemic era. The views of a panel of worldwide international experts. Ultraschall Med. 2020;41:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 184. | Gudmundsson EF, Bjornsdottir G, Sigurdsson S, Eiriksdottir G, Thorsson B, Aspelund T, Gudnason V. Carotid Plaque is a Strong Surrogate Marker for CAC and Subclinical CHD in the General Population. Atheroscler Suppl. 2018;32:14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 185. | Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020;12:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 186. | Morassi M, Bigni B, Cobelli M, Giudice L, Bnà C, Vogrig A. Bilateral carotid artery dissection in a SARS-CoV-2 infected patient: causality or coincidence? J Neurol. 2020;267:2812-2814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 187. | Jamthikar A, Gupta D, Saba L, Khanna NN, Araki T, Viskovic K, Mavrogeni S, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Viswanathan V, Sharma A, Nicolaides A, Kitas GD, Suri JS. Cardiovascular/stroke risk predictive calculators: a comparison between statistical and machine learning models. Cardiovasc Diagn Ther. 2020;10:919-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 188. | Viswanathan V, Jamthikar AD, Gupta D, Shanu N, Puvvula A, Khanna NN, Saba L, Omerzum T, Viskovic K, Mavrogeni S, Turk M, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Kitas GD, S C, Joshi S, Fiscian H, Folson AA, Wu DH, Ruzsa Z, Nicolaides A, Sharma A, Bhatt DL, Suri JS. Low-cost preventive screening using carotid ultrasound in patients with diabetes. Front Biosci (Landmark Ed). 2020;25:1132-1171. [PubMed] |
| 189. | Jamthikar A, Gupta D, Cuadrado-Godia E, Puvvula A, Khanna NN, Saba L, Viskovic K, Mavrogeni S, Turk M, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Kitas GD, Shankar C, Nicolaides A, Viswanathan V, Sharma A, Suri JS. Ultrasound-based stroke/cardiovascular risk stratification using Framingham Risk Score and ASCVD Risk Score based on "Integrated Vascular Age" instead of "Chronological Age": a multi-ethnic study of Asian Indian, Caucasian, and Japanese cohorts. Cardiovasc Diagn Ther. 2020;10:939-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 190. | Viswanathan V, Jamthikar AD, Gupta D, Puvvula A, Khanna NN, Saba L, Viskovic K, Mavrogeni S, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Sharma A, Kancharana P, Misra DP, Agarwal V, Kitas GD, Nicolaides A, Suri JS. Does the Carotid Bulb Offer a Better 10-Year CVD/Stroke Risk Assessment Compared to the Common Carotid Artery? Angiology. 2020;71:920-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 191. | Jamthikar A, Gupta D, Khanna NN, Saba L, Laird JR, Suri JS. Cardiovascular/stroke risk prevention: A new machine learning framework integrating carotid ultrasound image-based phenotypes and its harmonics with conventional risk factors. Indian Heart J. 2020;72:258-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 192. | Khanna NN, Jamthikar AD, Gupta D, Nicolaides A, Araki T, Saba L, Cuadrado-Godia E, Sharma A, Omerzu T, Suri HS, Gupta A, Mavrogeni S, Turk M, Laird JR, Protogerou A, Sfikakis PP, Kitas GD, Viswanathan V, Suri JS. Performance evaluation of 10-year ultrasound image-based stroke/cardiovascular (CV) risk calculator by comparing against ten conventional CV risk calculators: A diabetic study. Comput Biol Med. 2019;105:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 193. | Jamthikar A, Gupta D, Khanna NN, Araki T, Saba L, Nicolaides A, Sharma A, Omerzu T, Suri HS, Gupta A, Mavrogeni S, Turk M, Laird JR, Protogerou A, Sfikakis PP, Kitas GD, Viswanathan V, Pareek G, Miner M, Suri JS. A Special Report on Changing Trends in Preventive Stroke/Cardiovascular Risk Assessment Via B-Mode Ultrasonography. Curr Atheroscler Rep. 2019;21:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 194. | Jamthikar A, Gupta D, Khanna NN, Saba L, Araki T, Viskovic K, Suri HS, Gupta A, Mavrogeni S, Turk M, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Kitas GD, Viswanathan V, Nicolaides A, Bhatt DL, Suri JS. A low-cost machine learning-based cardiovascular/stroke risk assessment system: integration of conventional factors with image phenotypes. Cardiovasc Diagn Ther. 2019;9:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 195. | Saba L, Jamthikar A, Gupta D, Khanna NN, Viskovic K, Suri HS, Gupta A, Mavrogeni S, Turk M, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Kitas GD, Viswanathan V, Nicolaides A, Bhatt DL, Suri JS. Global perspective on carotid intima-media thickness and plaque: should the current measurement guidelines be revisited? Int Angiol. 2019;38:451-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 196. | Viswanathan V, Jamthikar AD, Gupta D, Puvvula A, Khanna NN, Saba L, Viskovic K, Mavrogeni S, Turk M, Laird JR, Pareek G, Miner M, Ajuluchukwu J, Sfikakis PP, Protogerou A, Kitas GD, Nicolaides A, Sharma A, Suri JS. Integration of estimated glomerular filtration rate biomarker in image-based cardiovascular disease/stroke risk calculator: a south Asian-Indian diabetes cohort with moderate chronic kidney disease. Int Angiol. 2020;39:290-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 197. | Saba L, Biswas M, Suri HS, Viskovic K, Laird JR, Cuadrado-Godia E, Nicolaides A, Khanna NN, Viswanathan V, Suri JS. Ultrasound-based carotid stenosis measurement and risk stratification in diabetic cohort: a deep learning paradigm. Cardiovasc Diagn Ther. 2019;9:439-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 198. | Jacobi A, Chung M, Bernheim A, Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin Imaging. 2020;64:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 199. | Vasilev Y, Sergunova K, Bazhin A, Masri A, Vasileva Y, Suleumanov E, Semenov D, Kudryavtsev N, Panina O, Khoruzhaya A. MRI of the lungs in patients with COVID-19: clinical case. medRxiv. 2020;. |
| 200. | Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C, Rieke GJ, Zachoval C, Heine A, Velten M, Duerr GD. Diffuse Myocardial Inflammation in COVID-19 Associated Myocarditis Detected by Multiparametric Cardiac Magnetic Resonance Imaging. Circ Cardiovasc Imaging. 2020;13:e010897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 201. | Lo STH, Yong AS, Sinhal A, Shetty S, McCann A, Clark D, Galligan L, El-Jack S, Sader M, Tan R, Hallani H, Barlis P, Sechi R, Dictado E, Walton A, Starmer G, Bhagwandeen R, Leung DY, Juergens CP, Bhindi R, Muller DWM, Rajaratnum R, French JK, Kritharides L; Interventional council of CSANZ and COVID-19 Interventional cardiology working group. Consensus guidelines for interventional cardiology services delivery during covid-19 pandemic in Australia and new Zealand. Heart Lung Circ. 2020;29:e69-e77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 202. | Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1827] [Cited by in RCA: 1694] [Article Influence: 338.8] [Reference Citation Analysis (0)] |
| 203. | Behzad S, Velez E, Najafi MH, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) pneumonia incidentally detected on coronary CT angiogram: a do-not-miss diagnosis. Emerg Radiol. 2020;27:721-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 204. | Olusanya O. Ultrasound in Times of COVID-19. Available from: https://healthmanagement.org/c/icu/issuearticle/ultrasound-in-times-of-covid-19#:~:text=Cardiac%2C%20abdominal%20and%20vascular%20ultrasound, support%20tool%20in%20COVID%2D19. |
| 205. | Skandha S, Gupta S, Saba L, Koppula V, Suri JS. Ultrasound-based Carotid Plaque Tissue Risk Stratification using 3-D Optimized Artificial Intelligence Paradigm: a Cardiovascular/Stroke Application: Atheromatic 2.0. Comput Biol Med. 2020;In Press. |
| 206. | Acharya UR, S VS, Molinari F, Saba L, Nicolaides A, Shafique S, Suri JS. Carotid ultrasound symptomatology using atherosclerotic plaque characterization: a class of Atheromatic systems. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:3199-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 207. | Acharya UR, Faust O, Sree SV, Alvin AP, Krishnamurthi G, Seabra JC, Sanches J, Suri JS. Atheromatic™: symptomatic vs. asymptomatic classification of carotid ultrasound plaque using a combination of HOS, DWT & texture. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:4489-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 208. | Puvvula A, Jamthikar AD, Gupta D, Khanna NN, Porcu M, Saba L, Viskovic K, Ajuluchukwu JNA, Gupta A, Mavrogeni S, Turk M, Laird JR, Pareek G, Miner M, Sfikakis PP, Protogerou A, Kitas GD, Nicolaides A, Viswanathan V, Suri JS. Morphological Carotid Plaque Area Is Associated With Glomerular Filtration Rate: A Study of South Asian Indian Patients With Diabetes and Chronic Kidney Disease. Angiology. 2020;71:520-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 209. | Cuadrado-Godia E, Jamthikar AD, Gupta D, Khanna NN, Araki T, Maniruzzaman M, Saba L, Nicolaides A, Sharma A, Omerzu T, Suri HS, Gupta A, Mavrogeni S, Turk M, Laird JR, Protogerou A, Sfikakis P, Kitas GD, Viswanathan V, Suri JS. Ranking of stroke and cardiovascular risk factors for an optimal risk calculator design: Logistic regression approach. Comput Biol Med. 2019;108:182-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 210. | Khanna NN, Jamthikar AD, Gupta D, Araki T, Piga M, Saba L, Carcassi C, Nicolaides A, Laird JR, Suri HS, Gupta A, Mavrogeni S, Protogerou A, Sfikakis P, Kitas GD, Suri JS. Effect of carotid image-based phenotypes on cardiovascular risk calculator: AECRS1.0. Med Biol Eng Comput. 2019;57:1553-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 211. | Kotsis V, Jamthikar AD, Araki T, Gupta D, Laird JR, Giannopoulos AA, Saba L, Suri HS, Mavrogeni S, Kitas GD, Viskovic K, Khanna NN, Gupta A, Nicolaides A, Suri JS. Echolucency-based phenotype in carotid atherosclerosis disease for risk stratification of diabetes patients. Diabetes Res Clin Pract. 2018;143:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 212. | Porcu M, Mannelli L, Melis M, Suri JS, Gerosa C, Cerrone G, Defazio G, Faa G, Saba L. Carotid plaque imaging profiling in subjects with risk factors (diabetes and hypertension). Cardiovasc Diagn Ther. 2020;10:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 213. | Cuadrado-Godia E, Maniruzzaman M, Araki T, Puvvula A, Jahanur Rahman M, Saba L, Suri HS, Gupta A, Banchhor SK, Teji JS, Omerzu T, Khanna NN, Laird JR, Nicolaides A, Mavrogeni S, Kitas GD, Suri JS; Fellow AIMBE. Morphologic TPA (mTPA) and composite risk score for moderate carotid atherosclerotic plaque is strongly associated with HbA1c in diabetes cohort. Comput Biol Med. 2018;101:128-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 214. | Khanna NN, Jamthikar AD, Araki T, Gupta D, Piga M, Saba L, Carcassi C, Nicolaides A, Laird JR, Suri HS, Gupta A, Mavrogeni S, Kitas GD, Suri JS. Nonlinear model for the carotid artery disease 10-year risk prediction by fusing conventional cardiovascular factors to carotid ultrasound image phenotypes: A Japanese diabetes cohort study. Echocardiography. 2019;36:345-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |