Published online Mar 15, 2021. doi: 10.4239/wjd.v12.i3.206
Peer-review started: September 1, 2020
First decision: December 1, 2020
Revised: December 16, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: March 15, 2021
Processing time: 181 Days and 17.8 Hours
Haptoglobin (Hp) is an acidic glycoprotein, existing in the serum and other body fluids of human beings and a variety of mammals. Hp is produced in the liver, white adipose tissue, and the kidney. The genetic polymorphisms and different phenotypes of Hp have different biological functions. Hp has antibacterial, antioxidant, and angiogenic effects and is associated with multiple diseases including simple obesity, vascular complications of diabetes mellitus, nonalcoholic fatty liver disease, hypertension, blood diseases, autoimmune diseases, and malignant tumors. Hp also participates in many life activities, indicating the importance of Hp in further studies. Previously, we found that the expression of serum Hp changed after treatment of simple obesity patients in clinical trials. However, the specific mechanism of Hp in patients with simple obesity is still unclear. The purpose of this article is to introduce recent research progress on Hp, emphasizing the relationship between Hp and the development of metabolic disease, which will improve the understanding of the functions of Hp underlying metabolic diseases and discuss future research directions.
Core Tip: Although there are a large number of articles and reviews on metabolic diseases every year involving epidemiology, therapies, comparisons of treatment methods, health strategies, and comorbidities, data on different treatment methods are still limited. The current research focus is the relationship between haptoglobin (Hp) and the development of metabolic diseases, the warning effect of Hp for metabolic diseases, and the latest research progress on haptoglobin. Through this review, our ideas and future research directions will be made clearer.
- Citation: Wan BN, Zhou SG, Wang M, Zhang X, Ji G. Progress on haptoglobin and metabolic diseases. World J Diabetes 2021; 12(3): 206-214
- URL: https://www.wjgnet.com/1948-9358/full/v12/i3/206.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i3.206
Haptoglobin (Hp), a circulating tetrameric glycoprotein[1], was originally discovered in human plasma[2] and considered to be a reactive protein in the acute inflammatory phase. Hp is expressed in multiple tissues and cell types. Earlier studies suggested that Hp is an acute phase reactive protein synthesized only in internal organs. Further studies found that Hp was also expressed in other tissues including endometriosis tissue[3,4], white adipose tissue, and colorectal cancer tissues[5]. The main functions of Hp are binding with hemoglobin (Hb) and transporting Hb to macrophages to form Hp-Hb complex in a hemolyzed state, protecting Hb, and avoiding excessive Hb damage via oxidative stress. Hp has two alleles (Hp1 and Hp2) and three basic genotypes (Hp1-1, Hp1-2, and Hp2-2). Different genotypes are associated with different diseases. The Hp2 genotype is mainly related to metabolic diseases. Previous evidence demonstrated that Hp2 positively correlated with diabetic complications and can be used as a biomarker for simple obesity. Therefore, an increasing number of researchers are committed to exploring the causal relationship between Hp and metabolic diseases to better study the role of Hp and provide new therapeutic methods for the clinical treatment of metabolic syndrome. However, few reviews have focused on the metabolic functions of Hp as well as the specific mechanism of Hp in patients with simple obesity. In this review, we introduced recent research progress on Hp, emphasized the relationship between Hp and metabolic disease, and discussed further research directions of Hp.
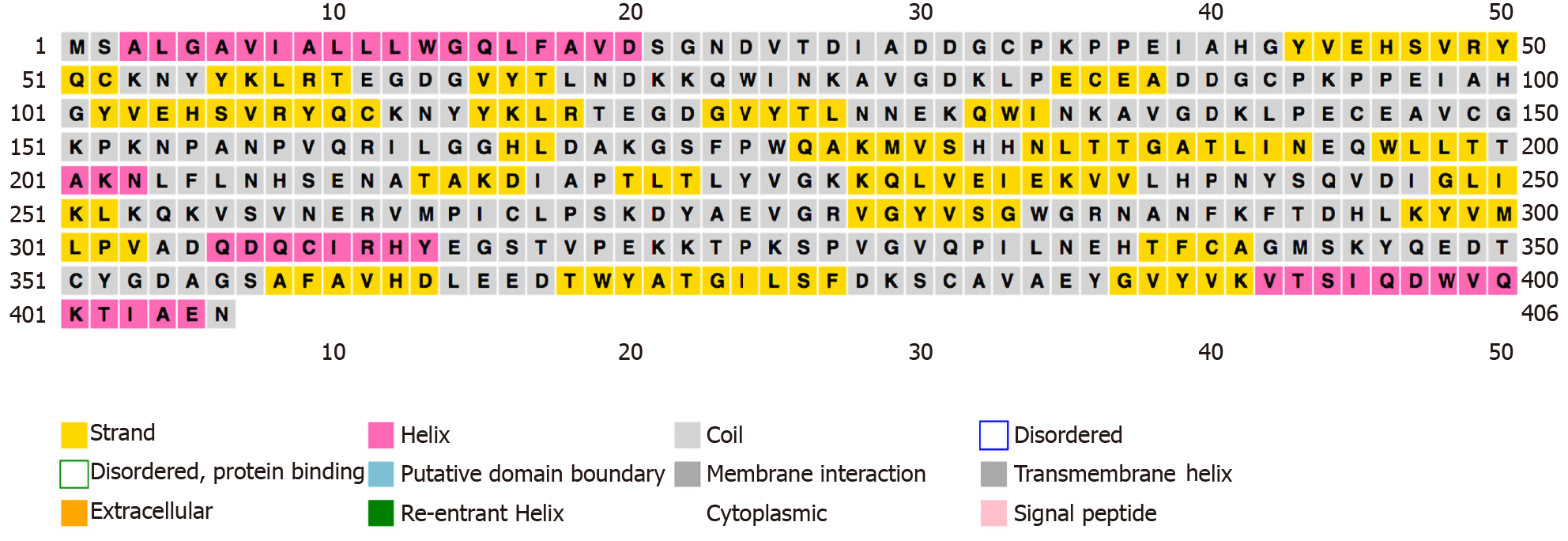
We used “haptoglobin” as a keyword to search for the Hp protein sequence information in the UniProt online database (P00738). The number of human Hp amino acids is 406, and the relative molecular weight of the protein is 45205 Dalton. The PSIPRED online analysis software was used to analyze the secondary structure of human Hp. Hp β-sheet structures were far more abundant than α-helix structures (Figure 1).
There are two major allelic forms, alpha-1 with 83 residues and alpha-2 with 142 residues, in human populations. The alleles exhibit different oligomerization properties. The sequence displayed in this protein corresponds to the alpha-2 allele. There are two pairs of peptide chains (α chain and β chain) in the molecule to form a tetramer of α2β2. The polymorphism of Hp is mainly caused by genetic variation of the α chain. The Hp gene is located at 16q22 and is codominantly inherited. These alleles determine three possible genotypes, named Hp1-1, Hp1-2, and Hp2-2, and the different genotypes are related to specific disease types.
The specific phenotype is related to the type of disease and has different functions. Hp1-1 is a susceptibility gene for infectious diseases (such as parasite infection). Hp1-1 homozygotes were more likely to have parasite infections than other types in studies related to Schistosoma parasite infections in central Sudan[6]. The Hp2-2 genotype is mainly associated with metabolic diseases and metabolic complications. In 2019, a retrospective study indicated that patients with nonalcoholic fatty liver disease had a higher Hp2-2 genotype frequency than Hp1-1 gene frequency compared with those of healthy controls; moreover, nonalcoholic fatty liver disease patients with the Hp2-2 genotype had higher body mass index (BMI), total cholesterol, liver enzyme, ferritin, and controlled attenuation parameter values than those with the non-Hp2-2 genotype[7]. Another cross-sectional study showed that in diabetes, obesity, and smoker patients, the Hp2-2 genotype is the main factor leading to increased coronary artery stenosis[8].
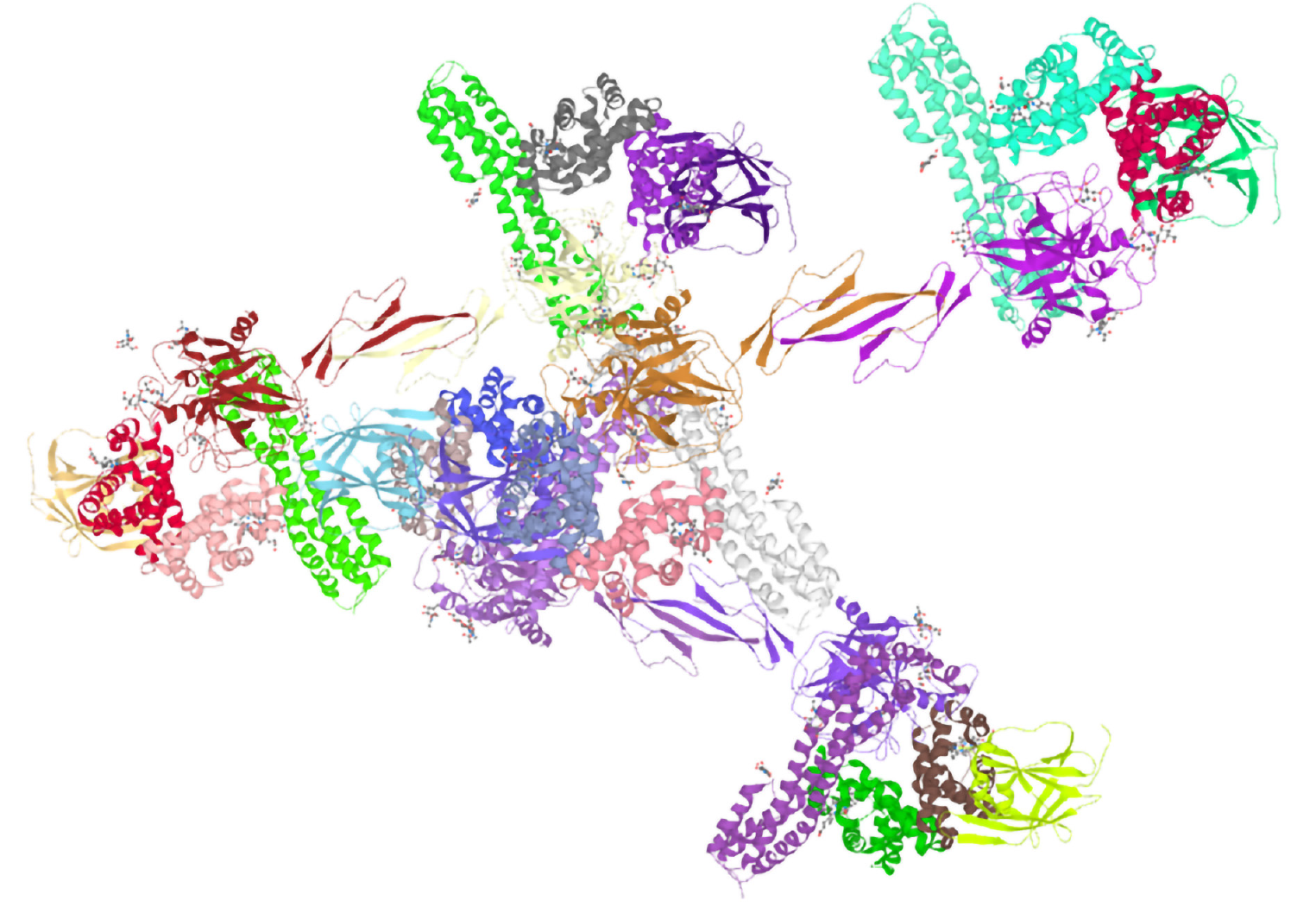
To study the function of a protein, it is necessary to grasp the high-level structural characteristics of the protein. The UniProt online database was used to predict and analyze the tertiary structure of human serum Hp (Figure 2). It is a 3-dimensional structure database cross-referencing points to data collections other than UniProtKB to show the predictive analysis of the tertiary structure of human serum Hp.
Using String online analysis software to predict and analyze the proteins interacting with human serum Hp, the results showed that there are 11 interacting proteins, and a network diagram of 11 protein interactions was predicted (Figure 3). Each node represents all the proteins produced by a single protein-coding gene locus, and the thickness of the wire indicates the strength of data support. The association between proteins is meant to be specific and meaningful, and proteins jointly contribute to a shared function. Hb subunit beta, Hb subunit alpha-1, and Hb subunit alpha-2 are mainly involved in transporting oxygen from the lung to other peripheral tissues. Apolipoprotein A-I, a cofactor for lecithin cholesterol acyltransferase, participates in the reverse transport of cholesterol from tissues to the liver for excretion by promoting cholesterol efflux from tissues. Apolipoprotein E can serve as a ligand for the low density lipoprotein receptor, and the specific apolipoprotein E receptor of hepatic tissues mediates the binding and catabolism of lipoprotein particles.
Scavenger receptor cysteine-rich type 1 protein M130 (CD163), an acute phase regulated receptor involved in clearance and endocytosis of Hb/Hp complexes by macrophages, may thereby protect tissues from free Hb-mediated oxidative damage. According to the network diagram of protein interactions, the function of Hp can be summarized, mainly including the following points.
Once the body’s antioxidant defense capacity is overwhelmed and no longer able to detoxify reactive oxygen, oxidative stress will occur. Then, oxidative damage to cell lipids, DNA, and proteins will eventually lead to cell and organ dysfunction. However, when Hp binds to Hb, it forms a stable Hp-Hb complex, which is metabolized by endocytosis mediated by CD163 to prevent free Hb from damaging the kidney. The complex formed by Hp binding to free Hb with high affinity can be rapidly cleared by reticuloendothelial cells. The complex has a large molecular weight and will not be excreted from the urine through the glomerular filter membrane, thus preventing both the loss of heme and kidney damage caused by hemolysis[9]. After Hp and free Hb binding forms a complex, it presents a new antigenic determinant, which can be recognized and bound by the Hb clearance receptor (CD163) on the surface of monocytes and macrophages and then phagocytically degraded to remove free Hb from the blood circulation[10]. Chen et al[11] used cell experiments to prove that after central nervous system hemorrhage, Hp decreased the protective response of glial cells to ferritin induction and increased the vulnerability of CD163-expressing neurons to Hb to protect neurons from Hb. In sepsis models, plasma proteins combined with cell-free Hb can improve survival.
CD163 is a member of the cysteine-rich scavenger receptor family[12] and a type I membrane protein that is a widely used marker of monocytes/macrophages and microglia. It has been demonstrated that the plasma-cleared Hp-Hb complex is a highly expressed macrophage membrane protein receptor. CD163 rapidly clears Hp-Hb complexes mainly through the endolysosomal degradation pathway. CD163-mediated (cellular) endocytosis of the Hp-Hb complex can also replace the main pathway for tissue macrophages to take up iron. One study[13] confirmed that the Hp-CD163-heme oxygenase-1 pathway is an effective capture-receptor-enzyme system, and this pathway is mediated by the acute phase regulated by interleukin 6. It generally has anti-inflammatory effects and can enhance the anti-inflammatory effects of the Hp-CD163- heme oxygenase-1 pathway. In general, the function of the CD163 and Hp connection is induced during inflammation, and CD163 is a soluble factor involved in the anti-inflammatory response and exhibits cytokine-like functions.
A recent study aimed to investigate the effect of Hb/Hp treatments on the macrophage phenotype in a lipopolysaccharide stimulated inflammatory envir-onment. It has been reported that Hb/Hp treatment can significantly reduce the expression of macrophage inflammatory factors in the environment stimulated by inflammation and lipopolysaccharide, which indicates that Hp has the greatest effect on reduction in overall inflammation and may be related to the high motility group box 1 (HMGB1) pathway. In addition, Hp treatment can also accelerate the healing of biopsy perforation wounds in diabetic mice[14]. In addition, HMGB1 is actively secreted or passively released into the extracellular environment during infection or injury, while HMGB1 is captured in the presence of Hp. The Hp and HMGB1 complex causes an anti-inflammatory response, and parallel endocytosis of the HMGB1-Hp complex acts through CD163[15].
Apolipoprotein A-I is a kind of apolipoprotein A and an activator of lecithin cholesterol acyltransferase. It can convert cholesterol into cholesterol esters, promote cholesterol transport, and regulate high density lipoprotein (HDL) metabolism and prevent the occurrence of atherosclerotic cardiovascular disease. The combination of Hp and Hb reduces the inhibitory effect of HDL in the inflammatory state of sickle cell disease, and it has been found that HDL maintained its anti-inflammatory activity in Hp knockout mice[16]. An investigation of Hp gene (HPR) polymorphism and serum Hp concentration reported a comparison of the non-HDL cholesterol, HDL cholesterol, and triglyceride concentrations of overweight subjects (25 ≤ BMI < 30) and healthy subjects (BMI < 25). Hp was positively correlated with HDL and cholesterol concentrations but not with the triglyceride concentration through linear regression analyses[17].
The expression level and gene polymorphism of Hp are also considered to relate to the occurrence and development of metabolic diseases, such as obesity, type 2 diabetes (T2DM), complications of diabetes, and so on.
The Hp protein is involved in the relationship between obesity and chronic inflammation and has many novel functions. Hp mRNA in white adipose tissue (WAT) is significantly upregulated in obese leptin receptor-deficient db/db mice compared to that in wild-type mice[18]. Hp is not only a marker of terminal differentiation of adipocytes, but also actively participates in this process. It was observed in the 3T3-L1 cell model that with the continuous differentiation of adipocytes the expression of Hp was significantly increased[19]. When the Hp gene was knocked out, the number of Hp-/- fat cells in mice was significantly reduced 30 d after birth compared with that in wild-type mice[20]. Obesity cohort studies have confirmed that circulating Hp can be used as a biomarker for simple obesity[21,22]. Recently, scientists investigated the relationship between serum Hp RS2000999 and childhood and adult obesity in two Mexican population case and control studies of 540/697 and 592/691 subjects, respectively. The results showed that Hp levels were positively correlated with childhood and adult obesity. Their data provided evidence for a causal positive association between serum Hp levels and childhood obesity in the Mexican population and proposed Hp as an important biomarker and treatment target for obesity[23].
Tumor necrosis factor receptor knockout mice were crossed with leptin-deficient ob/ob obese mice, and the resulting (double knockout) model was compared with control (ob/ob) mice. In contrast, WAT Hp expression was significantly reduced in tumor necrosis factor receptor knockout mice[24]. Obese model mice with an Hp gene knockout had significantly reduced WAT macrophage content and inflammatory factor expression. WAT inflammation characteristics were related to the onset of obesity-related complications, and these presentations improved in obese Hp-deficient mice. In the absence of Hp, obesity-associated insulin resistance and hepatosteatosis are attenuated[4].
Obese patients have chronic inflammation and insulin resistance, and chronic inflammation and insulin resistance interact with each other. It is now believed that adipose tissue is an endocrine organ, and fat cells also secrete Hp, which can be used as a biomarker[1]. Hp is an important participant in triggering WAT inflammation during the onset of obesity and is expressed in adipocytes in the initial stage of obesity. Hp induced increased expression in WAT with weight gain. Through upstream signals, including tumor necrosis factor alpha and interleukin 6, activated macrophages in adipose tissue released inflammatory factors. This caused a further increase in Hp levels through chemokine receptor 2 interaction with Hp, and circulating macrophages/ monocytes were recruited to WAT. Macrophages/ monocytes release a large amount of monocyte chemotactic protein 1 to recruit more macrophages/monocytes to infiltrate into WAT and cause the inflammation state to continue to worsen and cause a series of obesity complications[1].
Currently, increasing evidence shows that Hp is closely related to various complications of diabetes. A cohort study analyzing urine Hp to predict the risk of death in patients with T2DM indicated that Hp may be a new biomarker for the risk of death in patients with T2DM[25]. Furthermore, a recent study demonstrated that intensive glucose-lowering therapy was an effective way to prevent incident coronary heart disease and cardiovascular disease events with the Hp2-2 phenotype[26]. It has also been shown that several functional differences between Hp phenotypes seem to have a role in the occurrence of coronary artery disease (CAD) in patients with T2DM with important biological and clinical significance. Hamdy et al[27] compared the serum Hp levels and genotypes of patients with T2DM without CAD and patients with T2DM with CAD. This project further confirmed that the Hp2-2 phenotype is considered to be the main susceptibility gene for the development of T2DM with CAD. This method of disease prediction by genotype is of great convenience for predicting the development of CAD and the correct treatment and prevention of T2DM. Furthermore, the Hp phenotype was determined in 5806 non-Hispanic white Action to Control Cardiovascular Risk in Diabetes participants using a validated assay. Compared with standard therapy, intensive therapy was associated with a lower risk of incident coronary heart disease among participants with the Hp2-2 phenotype, and intensive glucose-lowering therapy was effective at preventing incident coronary heart disease and cardiovascular disease events in Action to Control Cardiovascular Risk in Diabetes study participants with the Hp2-2 phenotype[26]. Some researchers have studied 233 patients with T2DM for more than a decade and reported that serum Hp levels in T2DM patients with diabetic kidney disease (DKD) were significantly higher than those in patients without diabetic kidney disease for the first time[28]. It is fully verified that serum Hp can be used as a potential biomarker for kidney injury and provides a basis for early diagnosis and timely intervention of the disease. In diabetic nephropathy, Hp can be used as a factor to assess the prognosis risk.
In addition to metabolic diseases, Hp is also associated with many other diseases, such as respiratory diseases, cardiovascular diseases, and autoimmune diseases. As an acute phase protein, Hp levels in serum increase rapidly after infection and inflammation occur[29]. In addition, when the body is in a stress state, Hp levels in the blood are also significantly increased, such as during inflammation, trauma, infection, and other pathological conditions[30]. The use of certain hormones, such as corticosteroids, increases its serum content significantly and is related to severity and prognosis[31]. Many studies have proven that Hp is an independent factor in the incidence of these diseases, but the conclusions still need to be verified by studies with larger sample sizes. Furthermore, there are few studies about the pathways of the Hp effect on diseases, and the specific mechanisms and pathways are still unclear and have limitations. In future research, we should focus on the specific mechanism of Hp. In addition, the degree of correlation between different genotypes and diseases should be refined because Hp is genetically polymorphic.
Diabetes and obesity are serious metabolic diseases that have become widespread global diseases and pose hidden dangers to human health. According to the results of an epidemiological survey, the cause of death of approximately four million people worldwide was directly related to body mass. The number of deaths due to complications related to high BMI accounted for 7.1% of all deaths. Approximately 2.7 million (41%) of these deaths were from cardiovascular and cerebrovascular diseases. Another major reason is diabetes[32]. Therefore, exploring the prevention and treatment of metabolic diseases has become a major issue. It has been more than half a century since Hp was first discovered. Research on Hp has gradually shifted from initially determining the molecular structure and genetic variation to exploring the physio-logical function and pathological significance of Hp. Currently, Hp is used as a biomarker, and its relationship with metabolic disease has become an important way to explore the etiology and pathogenesis of diseases. After the molecular heterogeneity of Hp was discovered, many clinical studies confirmed the significance of Hp gene polymorphisms in the field of pathology. The discovery of the immunological effects of Hp provides a theoretical basis for the different effects observed in recent years.
Hp can regulate the function of lymphocytes and macrophages and may control tissue damage in the inflammatory response. Further research on the role of Hp in the immune system will provide better ideas for exploring the relationship between Hp and diseases and will eventually provide new measures for clinical treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aureliano M, Cheng TH S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Maffei M, Barone I, Scabia G, Santini F. The Multifaceted Haptoglobin in the Context of Adipose Tissue and Metabolism. Endocr Rev. 2016;37:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Polonovski M, Jayle M. Existence in the blood plasma of a substance activating the peroxidase action of hemoglobin. CR Soc Biol. 1938;129:457-460. |
| 3. | Moussa A, Rejeb J, Omezzine A, Rebhi L, Boumaiza I, Kacem S, Ben Rejeb N, Boughzala E, Ben Abdelaziz A, Bouslama A. Association between haptoglobin 2-2 genotype and coronary artery disease and its severity in a tunisian population. Biochem Genet. 2014;52:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lisi S, Gamucci O, Vottari T, Scabia G, Funicello M, Marchi M, Galli G, Arisi I, Brandi R, D'Onofrio M, Pinchera A, Santini F, Maffei M. Obesity-associated hepatosteatosis and impairment of glucose homeostasis are attenuated by haptoglobin deficiency. Diabetes. 2011;60:2496-2505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Mariño-Crespo Ó, Cuevas-Álvarez E, Harding AL, Murdoch C, Fernández-Briera A, Gil-Martín E. Haptoglobin expression in human colorectal cancer. Histol Histopathol. 2019;34:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Yousif AS, Elagib AA. Haptoglobin Phenotypes and Susceptibility to Schistosoma Parasites Infection in Central Sudan. Mediterr J Hematol Infect Dis. 2017;9:e2017042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Zhou J, Liu J, Sheng H, You N, Chen J, Mi X, Yang W, Zang S, Shi J; Chinese NAFLD Clinical Research Network (CNAFLD CRN). Haptoglobin 2-2 Genotype is Associated with More Advanced Disease in Subjects with Non-Alcoholic Steatohepatitis: A Retrospective Study. Adv Ther. 2019;36:880-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Sharpe-Timms KL, Nabli H, Zimmer RL, Birt JA, Davis JW. Inflammatory cytokines differentially up-regulate human endometrial haptoglobin production in women with endometriosis. Hum Reprod. 2010;25:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Sarpong-Kumankomah S, Gailer J. Identification of a haptoglobin-hemoglobin complex in human blood plasma. J Inorg Biochem. 2019;201:110802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Gao J, Song L, Li D, Peng L, Ding H. Clinical value of haptoglobin and soluble CD163 testing for the differential diagnosis of tuberculous and malignant pleural effusions. Medicine (Baltimore). 2019;98:e17416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Chen-Roetling J, Regan RF. Haptoglobin increases the vulnerability of CD163-expressing neurons to hemoglobin. J Neurochem. 2016;139:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Wang G, Li T, Duan SN, Dong L, Sun XG, Xue F. PPAR-γ Promotes Hematoma Clearance through Haptoglobin-Hemoglobin-CD163 in a Rat Model of Intracerebral Hemorrhage. Behav Neurol. 2018;2018:7646104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 13. | Thomsen JH, Etzerodt A, Svendsen P, Moestrup SK. The haptoglobin-CD163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxid Med Cell Longev. 2013;2013:523652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Krzyszczyk P, Kang HJ, Kumar S, Meng Y, O'Reggio MD, Patel K, Pires IS, Yarmush ML, Schloss RS, Palmer AF, Berthiaume F. Anti-inflammatory effects of haptoglobin on LPS-stimulated macrophages: Role of HMGB1 signaling and implications in chronic wound healing. Wound Repair Regen. 2020;28:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Yang H, Wang H, Levine YA, Gunasekaran MK, Wang Y, Addorisio M, Zhu S, Li W, Li J, de Kleijn DP, Olofsson PS, Warren HS, He M, Al-Abed Y, Roth J, Antoine DJ, Chavan SS, Andersson U, Tracey KJ. Identification of CD163 as an antiinflammatory receptor for HMGB1-haptoglobin complexes. JCI Insight. 2016;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Ji X, Feng Y, Tian H, Meng W, Wang W, Liu N, Zhang J, Wang L, Wang J, Gao H. The Mechanism of Proinflammatory HDL Generation in Sickle Cell Disease Is Linked to Cell-Free Hemoglobin via Haptoglobin. PLoS One. 2016;11:e0164264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Soejima M, Munkhtulga L, Furukawa K, Iwamoto S, Koda Y. Serum haptoglobin correlates positively with cholesterol and triglyceride concentrations in an obese Mongolian population. Clin Chim Acta. 2020;505:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Chiellini C, Bertacca A, Novelli SE, Görgün CZ, Ciccarone A, Giordano A, Xu H, Soukas A, Costa M, Gandini D, Dimitri R, Bottone P, Cecchetti P, Pardini E, Perego L, Navalesi R, Folli F, Benzi L, Cinti S, Friedman JM, Hotamisligil GS, Maffei M. Obesity modulates the expression of haptoglobin in the white adipose tissue via TNFalpha. J Cell Physiol. 2002;190:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Kong CS, Kim JA, Eom TK, Kim SK. Phosphorylated glucosamine inhibits adipogenesis in 3T3-L1 adipocytes. J Nutr Biochem. 2010;21:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev. 2000;16:202-210. [PubMed] |
| 21. | Engström G, Hedblad B, Janzon L, Lindgärde F. Weight gain in relation to plasma levels of complement factor 3: results from a population-based cohort study. Diabetologia. 2005;48:2525-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Doumatey AP, Lashley KS, Huang H, Zhou J, Chen G, Amoah A, Agyenim-Boateng K, Oli J, Fasanmade O, Adebamowo CA, Adeyemo AA, Rotimi CN. Relationships among obesity, inflammation, and insulin resistance in African Americans and West Africans. Obesity (Silver Spring). 2010;18:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Vázquez-Moreno M, Locia-Morales D, Perez-Herrera A, Gomez-Diaz RA, Gonzalez-Dzib R, Valdez-González AL, Flores-Alfaro E, Corona-Salazar P, Suarez-Sanchez F, Gomez-Zamudio J, Valladares-Salgado A, Wacher-Rodarte N, Cruz M, Meyre D. Causal Association of Haptoglobin With Obesity in Mexican Children: A Mendelian Randomization Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1321] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 25. | Liu JJ, Liu S, Saulnier PJ, Gand E, Choo RWM, Gurung RL, Hadjadj S, Lim SC; Singapore and SURDIAGENE Study Groups. Association of Urine Haptoglobin With Risk of All-Cause and Cause-Specific Mortality in Individuals With Type 2 Diabetes: A Transethnic Collaborative Work. Diabetes Care. 2020;43:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Carew AS, Levy AP, Ginsberg HN, Coca S, Lache O, Ransom T, Byington R, Rimm EB, Sapp J, Gardner M, Cahill LE. Haptoglobin Phenotype Modifies the Influence of Intensive Glycemic Control on Cardiovascular Outcomes. J Am Coll Cardiol. 2020;75:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Hamdy G, Hendy OM, Mahmoud H, El-sebaey A, Ali SR, Khalaf FA. Haptoglobin phenotypes as a risk factor for coronary artery disease in type 2 diabetes mellitus: an egyptian study. Egypt J Med Hum Genet. 2014;15:257-264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Huang Y, Huang Y, Zhang R, Jin L, Zhang H, Hu C. Serum haptoglobin levels are associated with renal function decline in type 2 diabetes mellitus patients in a Chinese Han population. Diabetes Res Clin Pract. 2019;156:107865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Li SC, Lee CC, Hsu CM, Huang HB, Su YC. IL-6 induces haptoglobin expression through activating STAT3 in human head and neck cancer. J Oral Pathol Med. 2020;49:49-54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Vicente J, Martinez-Guijosa J, Tvarijonaviciute A, Fernandez-de Mera IG, Gortazar C, Ceron JJ, Martinez-Subiela S. Serum haptoglobin response in red deer naturally infected with tuberculosis. Comp Immunol Microbiol Infect Dis. 2019;64:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Yang J, Zhang BL. [Value of determination of haptoglobin and α1-antitrypsin in predicting response to glucocorticoid therapy in children with primary nephrotic syndrome]. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17:227-231. [PubMed] |
| 32. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5054] [Article Influence: 631.8] [Reference Citation Analysis (2)] |