Published online Feb 15, 2021. doi: 10.4239/wjd.v12.i2.158
Peer-review started: October 8, 2020
First decision: November 18, 2020
Revised: November 26, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: February 15, 2021
Processing time: 106 Days and 16.5 Hours
In rare instances, primary liver cancer can be associated with intraocular metastasis (IOM).
To investigate the correlation between a diverse range of clinical characteristics and IOM in diabetic patients with primary liver cancer, and to determine potential risk factors in predicting IOM.
We recruited a total of 722 diabetic patients with primary liver cancer. The differences between the IOM and non-intraocular metastasis (NIOM) groups in these patients were assessed using the chi-squared test and Student’s t-test. Binary logistic regression analysis was subsequently used to determine risk factors. Finally, the diagnostic value of IOM in this cohort with primary liver cancer was analyzed by receiver operating characteristic (ROC) curve analysis.
In all, 13 patients had IOM. There were no remarkable intergroup differences with respect to age, sex, histopathological sub-types, or blood biochemical parameters. However, the IOM group had significantly higher alpha-fetoprotein (AFP) and cancer antigen 125 (CA125) values than the NIOM group. Binary logistic regression identified AFP and CA125 to be significant risk factors for IOM in diabetic patients with primary liver cancer. ROC curve analysis showed that the area under the curve values for AFP and CA125 were 0.727 and 0.796, with the cut-off values of 994.20 ng/mL and 120.23 U/mL, respectively. The sensitivity and specificity for AFP were 92.3% and 59.9%, while those for CA125 were 84.6% and 70.1%, respectively.
Elevated AFP and CA125 represent significant risk factors for IOM in diabetic patients with primary liver cancer.
Core Tip: This is a retrospective study designed to evaluate the risk factors for ocular metastasis in patients with diabetic primary liver cancer. Elevated alpha-fetoprotein (AFP) and cancer antigen 125 (CA125) represent significant risk factors for intraocular metastasis (IOM) in diabetic patients with primary liver cancer. Notably, the combination of AFP and CA125 is more reliable for differentiation between IOM and non-intraocular metastasis in diabetic patients with primary liver cancer.
- Citation: Yu K, Tang J, Wu JL, Li B, Wu SN, Zhang MY, Li QY, Zhang LJ, Pan YC, Ge QM, Shu HY, Shao Y. Risk factors for intraocular metastasis of primary liver cancer in diabetic patients: Alpha-fetoprotein and cancer antigen 125. World J Diabetes 2021; 12(2): 158-169
- URL: https://www.wjgnet.com/1948-9358/full/v12/i2/158.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i2.158
Primary liver carcinoma is among the most frequent causes of cancer-related deaths worldwide and shows increasing rates of incidence and mortality. In China, primary liver carcinoma is the fourth most common malignant tumor and the third major cause of tumor-associated deaths, and therefore a significant public health threat. This disease has a relatively high incidence among the middle aged (45-64 years old) and elderly population (> 65 years old). Of the various types of primary liver cancers, hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) are the most common, accounting for approximately 70% and 15% of all diagnosed cases, respectively[1]. The main risk factors of HCC involve hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, alcoholic liver diseases, and nonalcoholic fatty liver disease. Chronic HBV and HCV infection regulate the pathogeny of HCC in low- and middle-incomes countries; furthermore, aflatoxin exposure leads to HCC progression in Africa and East Asia[2]. The most reliable approach to minimize HCC risk is to prevent the potential hepatopathy by means such as hepatitis B vaccination. Less common causes of HCC involve hereditary hemochromatosis, alpha1-antitrypsin deficiency, autoimmune hepatitis, some types of porphyria, and Wilson’s disease[3]. In recent years, it was found that diabetes and cancer are related diseases that may co-exist in the same individual[4]. An increasing body of significant evidence suggests that patients with diabetes have a greater risk of developing certain cancers, especially primary hepatic cancer[5].
Our previous study showed that carbohydrate antigen 125, calcium, and hemoglobin can be predictive clinical indicators for ocular metastasis in male liver cancer patients[6]. However, things may be different when it comes to diabetic patients with primary liver cancer.
The most common mode of ICC dissemination is intrahepatic, involving the venous system[7]. A variety of signaling pathways dominate the expression of metastasis-associated genes in primary liver carcinoma[8]. Overexpression of vascular endothelial cell growth factor and epidermal growth factor receptor are associated with intrahepatic metastases and perineural, lymph-vascular aggression, respectively, in ICC[7].
Hepatocellular carcinoma, the major form of primary liver cancer, is specifically widespread in Southeast Asia involving areas of China. Extrahepatic metastases (EHM) of HCC are commonly observed in sites such as the lung, lymph nodes, bone and adrenal gland[9].
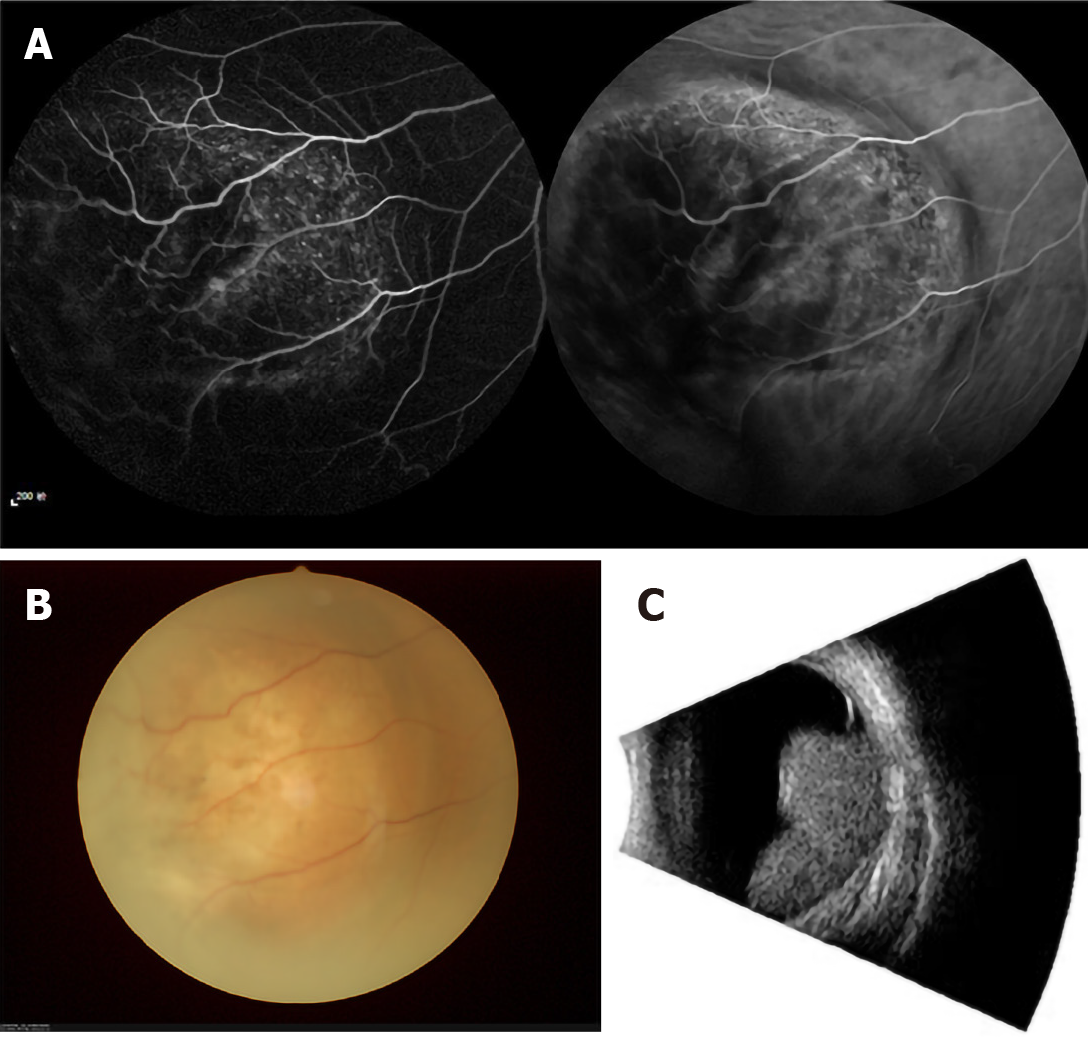
Although primary liver carcinoma metastasis to the eye is rare, intraocular metastasis (IOM) is closely associated with primary liver carcinoma. The detection, diagnosis, and management of IOM in the early stages are extremely important for good outcomes. IOM is more common in middle-aged patients; the average age of subjects in different series being around 60 years[10]. The overall prognosis of IOM is poor, with proptosis and diplopia being the most frequent presenting features, per a previous report[10]. The treatment for IOM aims at reducing the chance of visual impairment and improving the quality of life of patients. Radiotherapy is the main choice of treatment, as it can effectively control regional progression of the cancer and preserve vision. Metastasis to the choroid of HCC can be treated with focal plaque brachytherapy, with conservation of vision[11]. It is noteworthy that the clinical significance and underlying laboratorial value of diagnosing IOM has increased in the past few years owing to the expanding role of chemotherapy and radiation in the treatment of metastatic disease[12]. Because the eye provides a distinct opportunity for the imagery and identification of even a micro focus of neoplasm, treatment can be designed without delay and the effect observed immediately.
Imaging plays a crucial role in the diagnosis of primary liver carcinoma. The diagnostic criteria for HCC are mainly based on the visualization of neoan-giogenesis[13]. The prompt detection of IOM may significantly influence the choice of treatment for primary liver carcinoma. Consequently, there is an urgent need to identify the potential risk factors of IOM in patients with primary liver carcinoma and determine clinically meaningful predictors.
Therefore, the aim of this retrospective research was to detect the potential risk factors for IOM by investigating a range of clinicopathological parameters and biomarkers in diabetic patients with primary liver carcinoma.
This retrospective study was performed between January 1998 and May 2015, and involved a series of 722 consecutive middle-age and elderly diabetic patients diagnosed with primary liver cancer. All the patients were treated at the Endocrinology Department of the First Affiliated Hospital of Nanchang University. The diagnosis in all cases with primary liver cancer was confirmed by histo-pathological analysis of specimens obtained through needle biopsy or radical nephrectomy. The diagnosis of IOM was confirmed using computed tomography or magnetic resonance imaging. Patients with primary intraocular carcinoma and those with secondary liver carcinoma were excluded from the study. The experimental design was explained to each patient and written informed consent was obtained.
For each diabetic participant, we retrospectively recorded a range of demographic and clinical characteristics, including sex, age at the time of diagnosis of the primary tumor, histopathological tumor sub-type, sites of metastases, and treatments received. We also retrospectively recorded the plasma levels of several tumor biomarkers including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), cancer antigen 199 (CA199), cancer antigen 153 (CA153), cancer antigen 724 (CA724), ferritin (FER), and alkaline phosphatase (ALP), and the serum levels of total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), lipoprotein A [Lp(a)], calcium and hemoglobin (Hb). We subsequently analyzed the incidence of IOM, and investigated data for the potential correlation between clinical parameters and IOM.
The chi-squared test and Student’s t-test were applied to determine differences in clinical characteristics between the IOM and non-intraocular metastasis (NIOM) groups. Subsequently, binary logistic regression analysis was conducted to confirm potential risk factors of IOM. In addition, receiver operating characteristic curves were plotted, and area under the curve (AUC) values were used to evaluate the accuracy in predicting IOM. P values less than 0.05 were considered statistically significant. SPSS17.0 software (SPSS; IBM Corp, United States) or Excel 2016 software (Excel; Microsoft Corp, United States) were used for all statistical analyses in this study.
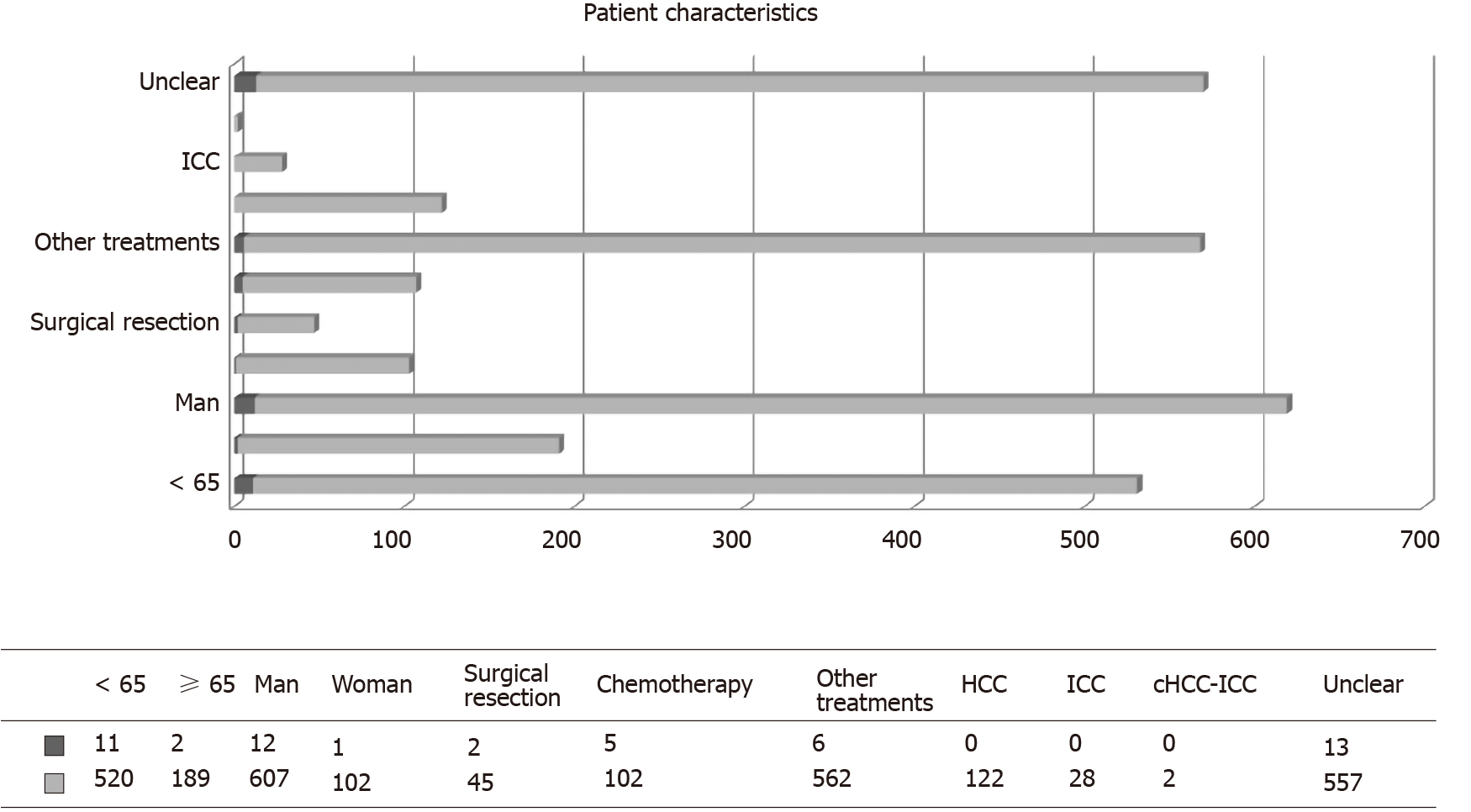
Overall, 722 diabetic patients with primary liver cancer were recruited into this study. The demographic data are shown in Table 1 and Figure 1. The majority of patients were male (n = 619, 85.7%). Of the 722 patients, 13 were diagnosed with IOM. The mean age of patients in the IOM and NIOM groups was 53.62 ± 8.61 years and 58.66 ± 9.66 years, respectively. There were no significant intergroup differences in terms of age, sex, and histopathological sub-type (P > 0.05). However, treatment received was significantly different between the two groups (P < 0.05).
| Patient characteristics | IOM group (%) | NIOM group (%) | Total numbers of patients (%) | P value |
| Age (yr)1 | 53.62 ± 8.61 | 58.66 ± 9.66 | 0.062 | |
| < 65 | 11 (84.6) | 520 (73.3) | 531 (73.5) | |
| ≥ 65 | 2 (15.4) | 189 (26.7) | 191 (26.5) | |
| Gender2 | 0.777 | |||
| Men | 12 (92.3) | 607 (85.6) | 619 (85.7) | |
| Women | 1 (7.7) | 102 (14.4) | 103 (14.3) | |
| Treatment2 | 0.015 | |||
| Surgical resection | 2 (15.4) | 45 (6.3) | 47 (6.5) | |
| Chemotherapy | 5 (38.5) | 102 (14.4) | 107 (14.8) | |
| Other treatments | 6 (46.2) | 562 (79.3) | 568 (78.7) | |
| Histopathological type2 | 0.317 | |||
| HCC | 0 | 122 (17.2) | 122 (16.9) | |
| ICC | 0 | 28 (3.9) | 28 (3.9) | |
| cHCC-ICC | 0 | 2 (0.3) | 2 (0.3) | |
| Unclear | 13 (100) | 557 (78.6) | 570 (78.9) | |
| HbA1c | 6.04 ± 0.54 | 6.23 ± 0.67 | - | 0.892 |
Our analysis showed that the levels of AFP and levels of CA125 were significantly higher (P < 0.05) in the IOM group than in the NIOM group. There were no significant intergroup differences in the levels of CEA, CA199, CA153, CA724, FER, ALP, TC, TG, HDL, LDL, ApoA1, ApoB, Lp(a), calcium or Hb (Table 2). Furthermore, binary logistic regression analysis showed that AFP and CA125 levels were independent risk factors for IOM. The value of AFP was 1.001 (1.000-1.001) and that of CA125 was 1.001 (1.000-1.002), both of which were statistically significant (P < 0.05) (Table 3).
| Clinical features | IOM group | NIOM group | t | P value |
| AFP | 1030.37 ± 342.38 | 526.37 ± 587.16 | -5.17 | < 0.001 |
| CEA | 6.54 ± 14.75 | 389.37 ± 10012.62 | 0.138 | 0.89 |
| CA125 | 419.78 ± 356.43 | 148.03 ± 305.88 | -3.165 | 0.002 |
| CA199 | 200.35 ± 329.82 | 314.22 ± 4482.59 | 0.092 | 0.927 |
| CA153 | 19.81 ± 15.06 | 20.32 ± 23.28 | 0.078 | 0.938 |
| CA724 | 6.68 ± 8.44 | 6.91 ± 12.32 | 0.066 | 0.947 |
| FER | 198.23 ± 52.50 | 259.43 ± 224.57 | 0.981 | 0.327 |
| ALP | 192.54 ± 94.07 | 174.15 ± 171.39 | -0.386 | 0.7 |
| TC | 4.75 ± 1.20 | 5.14 ± 8.62 | 0.161 | 0.872 |
| TG | 1.44 ± 0.81 | 1.37 ± 1.03 | -0.257 | 0.797 |
| HDL | 1.30 ± 0.64 | 1.50 ± 1.17 | 0.602 | 0.548 |
| LDL | 2.52 ± 1.16 | 2.58 ± 1.43 | 0.144 | 0.885 |
| ApoA1 | 1.65 ± 0.45 | 1.57 ± 0.87 | -0.331 | 0.741 |
| ApoB | 0.77 ± 0.32 | 1.10 ± 0.82 | 1.426 | 0.154 |
| Lp(a) | 181.92 ± 219.72 | 221.66 ± 240.54 | 0.591 | 0.555 |
| Ca | 14.56 ± 44.90 | 2.14 ± 0.27 | -0.997 | 0.338 |
| Hb | 114.69 ± 43.54 | 117.19 ± 22.31 | 0.207 | 0.84 |
| Factors | B | OR | OR (95%CI) | P value |
| AFP | 0.001 | 1.001 | 1.000-1.001 | 0.01 |
| CA125 | 0.001 | 1.001 | 1.000-1.002 | 0.036 |
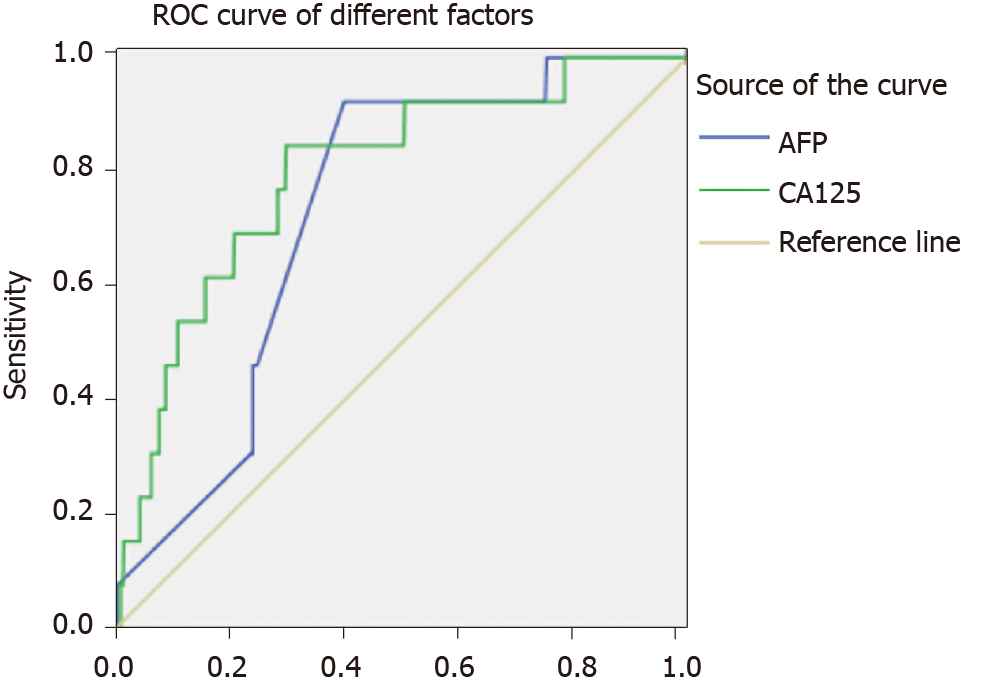
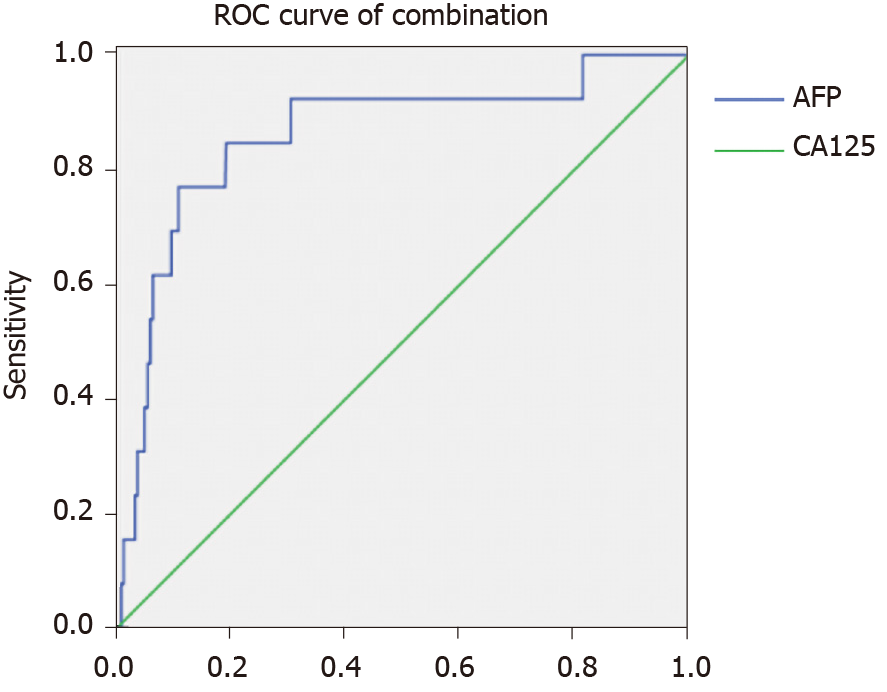
The AUC value for AFP was 0.727, and the associated sensitivity and specificity values for predicting IOM were 92.3% and 59.9%, respectively. The AUC value for CA125 was 0.796, and the associated sensitivity and specificity values for predicting IOM were 84.6% and 70.1%, respectively. These data are based on cut-off values of 994.20 ng/mL and 120.23 U/mL for AFP and CA125, respectively. We also found that the combination of AFP and CA125 data exhibited higher AUC (0.860) and specificity (89.3%) values. All results were statistically significant (P < 0.05) (Figures 2 -4, Table 4).
| Cut-off value | Sensitivity (%) | Specificity (%) | AUC | 95%CI | P value | |
| AFP | 994.20 | 0.923 | 0.599 | 0.727 | 0.627-0.827 | 0.005 |
| CA125 | 120.23 | 0.846 | 0.701 | 0.796 | 0.678-0.915 | < 0.001 |
| AFP + CA125 | 0.769 | 0.893 | 0.860 | 0.745-0.975 | < 0.001 |
Primary liver cancer is a major cause of tumor-associated mortality worldwide with increasing incidence. Although several treatment options such as ablation, surgical resection, and liver transplantation are available for primary liver cancer, the treatment is still complicated, because considerations involve not only the cancer pathology and liver anatomy but also the hepatic function and the general condition of patients. Moreover, distant metastasis is the main characteristic of malignancy. In China, many patients suffer from primary liver cancer, with some patients, especially in the middle and elderly age groups, having already developed regional or distant metastases at diagnosis. Hence, providing optimal treatment is challenging. While IOM of primary liver cancer is relatively uncommon, it is a predictor of unfavorable prognosis. Therefore, confirmation of cancer with IOM in the early stage is critical.
To date, carcinoma-related IOM has been reported in patients with rectal cancer[14], lung cancer[15], breast cancer[16], esophageal carcinoma[17], thyroid cancer[18], gastric carcinoma[19], renal cancer[20], and choriocarcinoma[21] (Table 5).
| Ref. | Diseases with IOM |
| Tei et al[14], 2014 | Rectal cancer |
| Shah et al[15], 2014 | Lung cancer |
| Levison et al[16], 2018 | Breast cancer |
| Chang et al[17], 2018 | Esophageal carcinoma |
| Fountas et al[18], 2017 | Thyroid cancer |
| Wu et al[19], 2019 | Gastric carcinoma |
| Essadi et al[20], 2017 | Renal cancer |
| Hazan et al[21], 2014 | Choriocarcinoma |
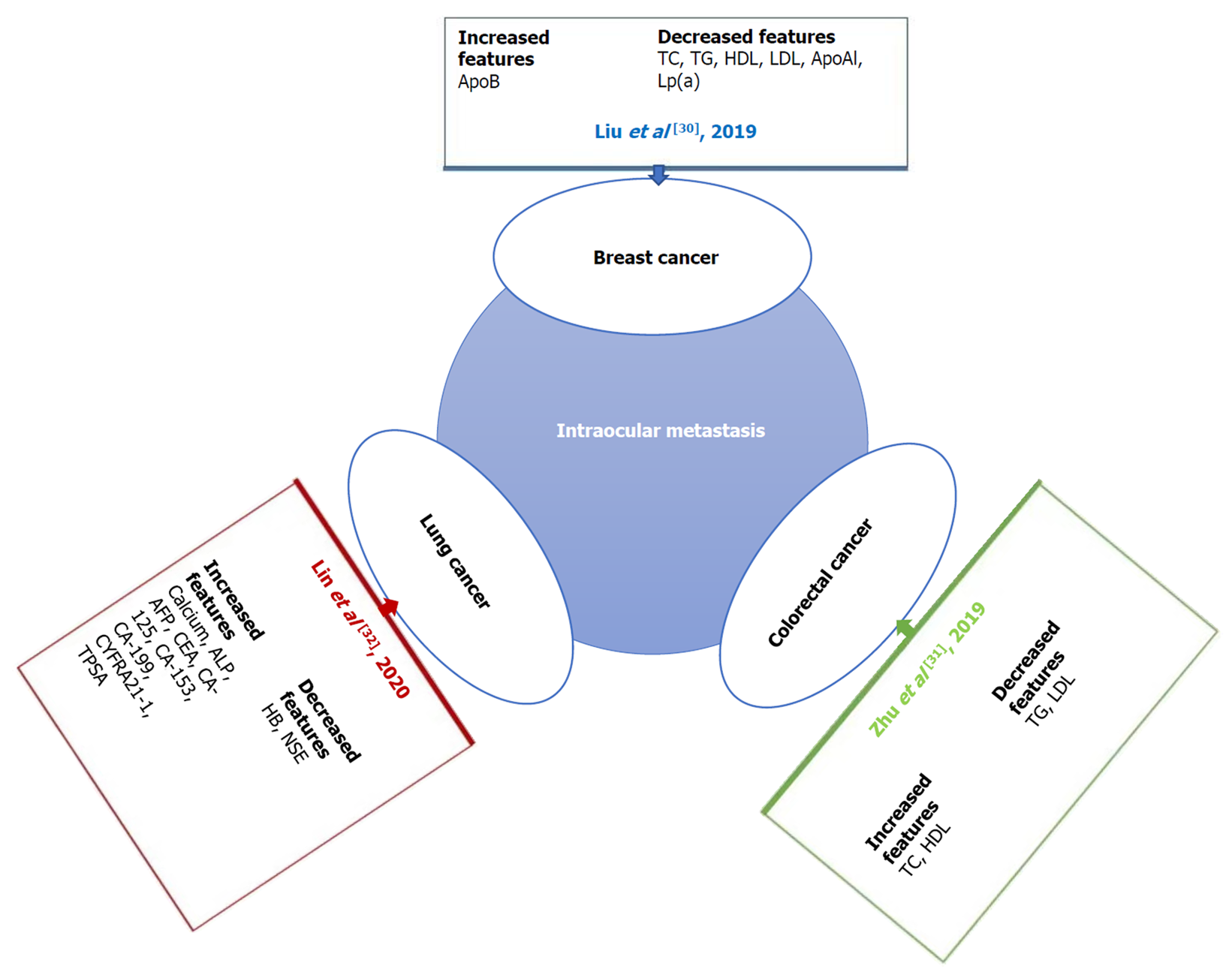
Previous studies[22-29] on the risk factors for local or distant metastases of primary liver cancer are shown in Table 6. The potential of tumor markers to predict metastases of carcinoma and address the limitations of imaging examinations is indicated (Figure 5)[30-32]. The content of serological tumor markers also changed when different tumors had IOM. Serological detection and imaging detection have different advantages, and the combination of the two can more accurately determine the occurrence and stage of tumors (Table 6).
| Ref. | Histopathological type | Metastatic sites | Risk factor |
| Lin et al[22], 2003 | HCC | Lung | Tumor size |
| Ogawa et al[23], 2004 | HCC | Distant metastasis | CD44v3 |
| Xiang et al[24], 2009 | HCC | Bone | CXCR4 |
| Lin et al[25], 2010 | HCC | Vessel | Aurora B |
| Kwak et al[26], 2012 | HCC | Peritoneum | HCC rupture |
| Morimoto et al[27], 2014 | HCC | EHM | Platelet, DCP |
| Chen et al[28], 2015 | Small HCC | IHM and EHM | VEGF |
| Lee et al[29], 2019 | HCC | EHM | AFP |
Tumor markers can be detected in blood, cerebrospinal fluid, or serous fluid, and tumor-marker evaluation has been used in tumor screening as well as for prognostic analysis. As one of the earliest discovered protein tumor markers, AFP has found considerable experimental and clinical acceptance and application. AFP is a 67-kDa glycoprotein produced in the early fetal stage by the liver, or later by various carcinomas such as HCC and hepatoblastoma and ovarian and testicular non-spermatogonial germ cells[33]. In the sera of normal healthy individuals, the concentrations of AFP are < 20 ng/mL. In patients with HCC, increased AFP levels could be prognostic indicators in the case of large tumors, advanced cancer stage, EHM, portal vein thrombosis, and recurrence after liver transplantation resulting in poor prognosis. Although its application in HCC screening and diagnosis is debatable, AFP levels have been used to guide therapeutic decision-making in HCC and manage the overall treatment in recent years[34]. Additionally, AFP is now considered a significant marker of postoperative HCC relapse and metastasis. Increased AFP level plays a crucial role in accelerating tumor growth and distant metastasis of HCC cells by increasing the expression of metastasis-associated proteins. The molecular mechanism by which AFP accelerates metastasis of HCC cells is by activating the phosphatidylinositol 3-kinase/protein kinase B signaling pathway to stimulate expression of metastasis-related factors such as CXC motif chemokine receptor 4. Liu et al[35] found that AFP levels ≥ 20000 ng/mL confirm HCC with portal vein tumor thrombosis (PVTT), thereby providing significant guidance for clinical practice. Our results indicated that elevated AFP is a risk factor for IOM in diabetic patients with primary liver cancer, because patients with AFP levels > 994.20 ng/mL were prone to IOM.
CA125, also known as mucin 16, is a large transmembrane glycoprotein, the largest of the class of membrane-associated mucins to which it belongs[36]. In addition, a previous study indicated that CA125 was overexpressed in 80% of cases with epithelial ovarian cancer[37]. CA125 is a useful tumor marker for early diagnosis and monitoring of the reaction to chemotherapy in epithelial ovarian cancer, and routine testing of CA125 levels after initial therapy can help detect cancer recurrence several months before laboratorial evidence or clinical signs of the disease. Since it was introduced in 1983, CA125 has been used worldwide in the management of patients with ovarian cancer. Yuan et al[38] reported that CA125 promotes ovarian cancer cell invasion through the Wnt signaling pathway, and presented a cut-off value of 82.9 U/mL, which was likely to indicate metastasis of ovarian cancer. Furthermore, CA125 has also been tested in the sera of patients with primary liver cancer. Li et al[39] analyzed the medical records of 60 patients (30 patients with HCC and 30 with ICC) and found that joint detection of CA125, AFP, CA199, and CEA was of great importance in the diagnosis of ICC. In another study, Liu et al[35] reported that AFP levels > 32.91 ng/mL and CA125 levels > 113.65 U/mL can detect HCC with PVTT. Our results demonstrate that CA125 is a risk factor for IOM in primary liver cancer, with a cut-off value of 120.23 U/mL. In addition, diabetes is often accompanied by various secondary diseases. Although cancer is generally considered a genetically controlled disease. Related studies have shown that the metabolic characteristics of various cancers are consistent with the metabolic changes in diabetic patients[40]. Furthermore, data analysis has also shown that the probability of diabetic patients suffering from HCC is 2-3 times that of patients without diabetes[41]. To date, there is no direct evidence that diabetic patients are more likely to develop eye metastases from liver cancer. However, some studies have shown that diabetes is significantly related to eye diseases such as retina diseases and liver diseases such as cirrhosis[42,43]. Combined with the data from our research, we speculate that diabetic patients are relatively more likely to develop liver cancer ocular metastasis. However, this requires further clinical data to confirm. Thus, diabetic patients with primary liver cancer whose CA125 levels were > 120.23 U/mL were more prone to IOM.
A combination of AFP and CA125 levels showed higher specificity than either level alone in predicting IOM in diabetic patients with primary liver cancer. In addition, AUC results showed higher accuracy of combined AFP and CA125 than either level alone in differentiating between patients with IOM and NIOM. Overall, combined AFP and CA125 levels appeared to be more useful in predicting IOM than either level alone.
Our study has some limitations. First, it was a retrospective study with insufficient data in the IOM group, which may have affected the overall analysis. Second, owing to the long period of time, some patient data, such as for prognosis, were either unknown or missing. Third, the number of cases with IOM was very low, so it is necessary to further expand the sample size in future studies to obtain more accurate results. In addition, all patients were from the same hospital, which may have led to inherent confounding factors. Furthermore, we only found altered serum concentrations of AFP and CA125 in diabetic patients with IOM of primary liver cancer. Therefore, how these two factors change remains unclear. Finally, in the basic clinical characteristics of diabetic patients, statistics on body mass index (BMI), smoking history and other clinical complications are not performed, which may cause errors in the statistical results. Pairing the patient’s BMI with diabetes, smoking history, and other clinical complications will make the results more accurate and more convincing. We will further study their potential relationships in future experiments.
In conclusion, based on this retrospective study of 722 diabetic patients with primary liver cancer, serum concentrations of AFP and CA125 were independent risk factors for IOM. Moreover, the combined levels of AFP with CA125 most likely have higher accuracy than either level alone in predicting IOM among diabetic patients with primary liver cancer.
The number of patients with primary liver cancer is increasing, and the development of metastasis is closely related to the clinical prognosis of patients.
Intraocular metastases (IOM) are rare in primary liver cancer, but once they occur, they often predict a poor prognosis.
To investigate the correlation between a diverse range of clinical characteristics and IOM in diabetic patients with primary liver cancer, and to determine potential risk factors in predicting IOM.
A total of 722 diabetic patients with primary liver cancer were evaluated for IOM. The general information and biochemical indices between the IOM and non-IOM groups were then statistically analyzed.
There was no significant difference in general information between the two groups, but the contents of alpha-fetoprotein (AFP) and cancer antigen 125 (CA125) in the IOM group were significantly higher than those in the non-IOM group.
Elevated levels of AFP and CA125 are risk factors for IOM of primary liver cancer in diabetic patients.
We used a retrospective study to evaluate the risk factors for ocular metastasis in patients with diabetic primary liver cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakajima K, Papazafiropoulou A S-Editor: Chen XF L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 433] [Article Influence: 54.1] [Reference Citation Analysis (1)] |
| 2. | Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 4. | Petrick JL, Freedman ND, Demuth J, Yang B, Van Den Eeden SK, Engel LS, McGlynn KA. Obesity, diabetes, serum glucose, and risk of primary liver cancer by birth cohort, race/ethnicity, and sex: Multiphasic health checkup study. Cancer Epidemiol. 2016;42:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Su Q, Sun F, Li J, Zhang H, Wang M, Zhou H, Qiao L. The correlation analysis of primary liver cancer with Type 2 diabetes. Indian J Cancer. 2015;52 Suppl 3:E148-E152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Xu QH, Zhu PW, Li B, Shi WQ, Lin Q, Min YL, Ge QM, Yuan Q, Shao Y. Carbohydrate antigen-125, calcium, and hemoglobin as predictive clinical indicator for ocular metastasis in male liver cancer patients. Biosci Rep. 2020;40:BSR20194405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 8. | Lee JH, Cho HS, Lee JJ, Jun SY, Ahn JH, Min JS, Yoon JY, Choi MH, Jeon SJ, Lim JH, Jung CR, Kim DS, Kim HT, Factor VM, Lee YH, Thorgeirsson SS, Kim CH, Kim NS. Plasma glutamate carboxypeptidase is a negative regulator in liver cancer metastasis. Oncotarget. 2016;7:79774-79786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Gupta R, Honavar SG, Vemuganti GK. Orbital metastasis from hepatocellular carcinoma. Surv Ophthalmol. 2005;50:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Malaviya L, Shields CL, Turaka K, Ehya H, Shields JA. Choroidal metastasis from hepatocellular carcinoma, diagnosed by fine needle aspiration biopsy and treated by iodine-125 brachytherapy. Graefes Arch Clin Exp Ophthalmol. 2011;249:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Taake WH, Allen RA, Straatsma BR. Metastasis of a hepatoma to the choroid. Am J Ophthalmol. 1963;56:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Ronot M, Purcell Y, Vilgrain V. Hepatocellular Carcinoma: Current Imaging Modalities for Diagnosis and Prognosis. Dig Dis Sci. 2019;64:934-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Tei M, Wakasugi M, Akamatsu H. Choroidal metastasis from early rectal cancer: Case report and literature review. Int J Surg Case Rep. 2014;5:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Shah SU, Mashayekhi A, Shields CL, Walia HS, Hubbard GB 3rd, Zhang J, Shields JA. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Levison AL, Erenler F, Zhao Y, Martin DF, Lowder C, Budd GT, Singh AD. Late-onset choroidal metastasis from breast cancer. Retin Cases Brief Rep. 2018;12:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Chang SY, Tsai SH, Chen LJ, Chan WC, Tsao YP. Choroidal metastasis from esophageal squamous cell carcinoma. Taiwan J Ophthalmol. 2018;8:104-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Fountas A, Tigas S, Giotaki Z, Tsatsoulis A, Kalogeropoulos CD. Choroidal metastasis from papillary thyroid cancer: An unusual feature of a common disease. Ann Endocrinol. 78:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Wu SQ, Li QS, Zhang Y, Zhu LW. Spontaneous rupture of the eyeball due to choroidal metastasis of gastric carcinoma: A case report. Medicine (Baltimore). 2019;98:e17441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Essadi I, Lalya I, Kriet M, El Omrani A, Belbaraka R, Khouchani M. Successful management of retinal metastasis from renal cancer with everolimus in a monophthalmic patient: a case report. J Med Case Rep. 2017;11:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Hazan A, Katz MS, Leder H, Blace N, Szlechter M. Choroidal metastases of choriocarcinoma. Retin Cases Brief Rep. 2014;8:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Lin SC, Shih SC, Kao CR, Chou SY. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol. 2003;9:1208-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Ogawa M, Yamamoto T, Kubo S, Uenishi T, Tanaka H, Shuto T, Tanaka S, Hirohashi K. Clinicopathologic analysis of risk factors for distant metastasis of hepatocellular carcinoma. Hepatol Res. 2004;29:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Xiang ZL, Zeng ZC, Tang ZY, Fan J, Zhuang PY, Liang Y, Tan YS, He J. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Lin ZZ, Jeng YM, Hu FC, Pan HW, Tsao HW, Lai PL, Lee PH, Cheng AL, Hsu HC. Significance of Aurora B overexpression in hepatocellular carcinoma. Aurora B Overexpression in HCC. BMC Cancer. 2010;10:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Kwak MS, Lee JH, Yoon JH, Yu SJ, Cho EJ, Jang ES, Kim YJ, Lee HS. Risk factors, clinical features, and prognosis of the hepatocellular carcinoma with peritoneal metastasis. Dig Dis Sci. 2012;57:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Morimoto Y, Nouso K, Wada N, Takeuchi Y, Kinugasa H, Miyahara K, Yasunaka T, Kuwaki K, Onishi H, Ikeda F, Miyake Y, Nakamura S, Shiraha H, Takaki A, Yamamoto K. Involvement of platelets in extrahepatic metastasis of hepatocellular carcinoma. Hepatol Res. 2014;44:E353-E359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Gao SG, Chen JM, Wang GP, Wang ZF, Zhou B, Jin CH, Yang YT, Feng XS. Risk factors for the Long-Term Efficacy, Recurrence, and Metastasis in Small Hepatocellular Carcinomas. Cell Biochem Biophys. 2015;72:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Lee CH, Chang CJ, Lin YJ, Yen CL, Shen CH, Cheng YT, Lin CC, Hsieh SY. Nomogram predicting extrahepatic metastasis of hepatocellular carcinoma based on commonly available clinical data. JGH Open. 2019;3:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Liu JX, Yuan Q, Min YL, He Y, Xu QH, Li B, Shi WQ, Lin Q, Li QH, Zhu PW, Shao Y. Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag Res. 2019;11:2881-2888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Zhu PW, Gong YX, Min YL, Lin Q, Li B, Shi WQ, Yuan Q, Ye L, Shao Y. The predictive value of high-density lipoprotein for ocular metastases in colorectal cancer patients. Cancer Manag Res. 2019;11:3511-3519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Lin Q, Chen XY, Liu WF, Zhu PW, Shi WQ, Li B, Yuan Q, Min YL, Liu JM, Shao Y. Diagnostic value of CA-153 and CYFRA 21-1 in predicting intraocular metastasis in patients with metastatic lung cancer. Cancer Med. 2020;9:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Charrière B, Maulat C, Suc B, Muscari F. Contribution of alpha-fetoprotein in liver transplantation for hepatocellular carcinoma. World J Hepatol. 2016;8:881-890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A, Houessinon A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016;463:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 35. | Liu Y, Wang X, Jiang K, Zhang W, Dong J. The diagnostic value of tumor biomarkers for detecting hepatocellular carcinoma accompanied by portal vein tumor thrombosis. Cell Biochem Biophys. 2014;69:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Bottoni P, Scatena R. The Role of CA 125 as Tumor Marker: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:229-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Yang WL, Lu Z, Bast RC Jr. The role of biomarkers in the management of epithelial ovarian cancer. Expert Rev Mol Diagn. 2017;17:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Yuan Q, Song J, Yang W, Wang H, Huo Q, Yang J, Yu X, Liu Y, Xu C, Bao H. The effect of CA125 on metastasis of ovarian cancer: old marker new function. Oncotarget. 2017;8:50015-50022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Li Y, Li DJ, Chen J, Liu W, Li JW, Jiang P, Zhao X, Guo F, Li XW, Wang SG. Application of Joint Detection of AFP, CA19-9, CA125 and CEA in Identification and Diagnosis of Cholangiocarcinoma. Asian Pac J Cancer Prev. 2015;16:3451-3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Gillies RJ, Pilot C, Marunaka Y, Fais S. Targeting acidity in cancer and diabetes. Biochim Biophys Acta Rev Cancer. 2019;1871:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Li X, Wang X, Gao P. Diabetes Mellitus and Risk of Hepatocellular Carcinoma. Biomed Res Int. 2017;2017:5202684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in Diabetic Retinopathy. Rev Diabet Stud. 2015;12:159-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 43. | Li X, Jiao Y, Xing Y, Gao P. Diabetes Mellitus and Risk of Hepatic Fibrosis/Cirrhosis. Biomed Res Int. 2019;2019:5308308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |