Published online Feb 15, 2021. doi: 10.4239/wjd.v12.i2.124
Peer-review started: June 28, 2020
First decision: October 21, 2020
Revised: November 30, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: February 15, 2021
Processing time: 208 Days and 18.6 Hours
Endothelial dysfunction, a hallmark of diabetes, is a critical and initiating contributor to the pathogenesis of diabetic cardiovascular complications. However, the underlying mechanisms are still not fully understood. Ferroptosis is a newly defined regulated cell death driven by cellular metabolism and iron-dependent lipid peroxidation. Although the involvement of ferroptosis in disease pathogenesis has been shown in cancers and degenerative diseases, the participation of ferroptosis in the pathogenesis of diabetic endothelial dysfunction remains unclear.
To examine the role of ferroptosis in diabetes-induced endothelial dysfunction and the underlying mechanisms.
Human umbilical vein endothelial cells (HUVECs) were treated with high glucose (HG), interleukin-1β (IL-1β), and ferroptosis inhibitor, and then the cell viability, reactive oxygen species (ROS), and ferroptosis-related marker protein were tested. To further determine whether the p53-xCT (the substrate-specific subunit of system Xc-)-glutathione (GSH) axis is involved in HG and IL-1β induced ferroptosis, HUVECs were transiently transfected with p53 small interfering ribonucleic acid or NC small interfering ribonucleic acid and then treated with HG and IL-1β. Cell viability, ROS, and ferroptosis-related marker protein were then assessed. In addition, we detected the xCT and p53 expression in the aorta of db/db mice.
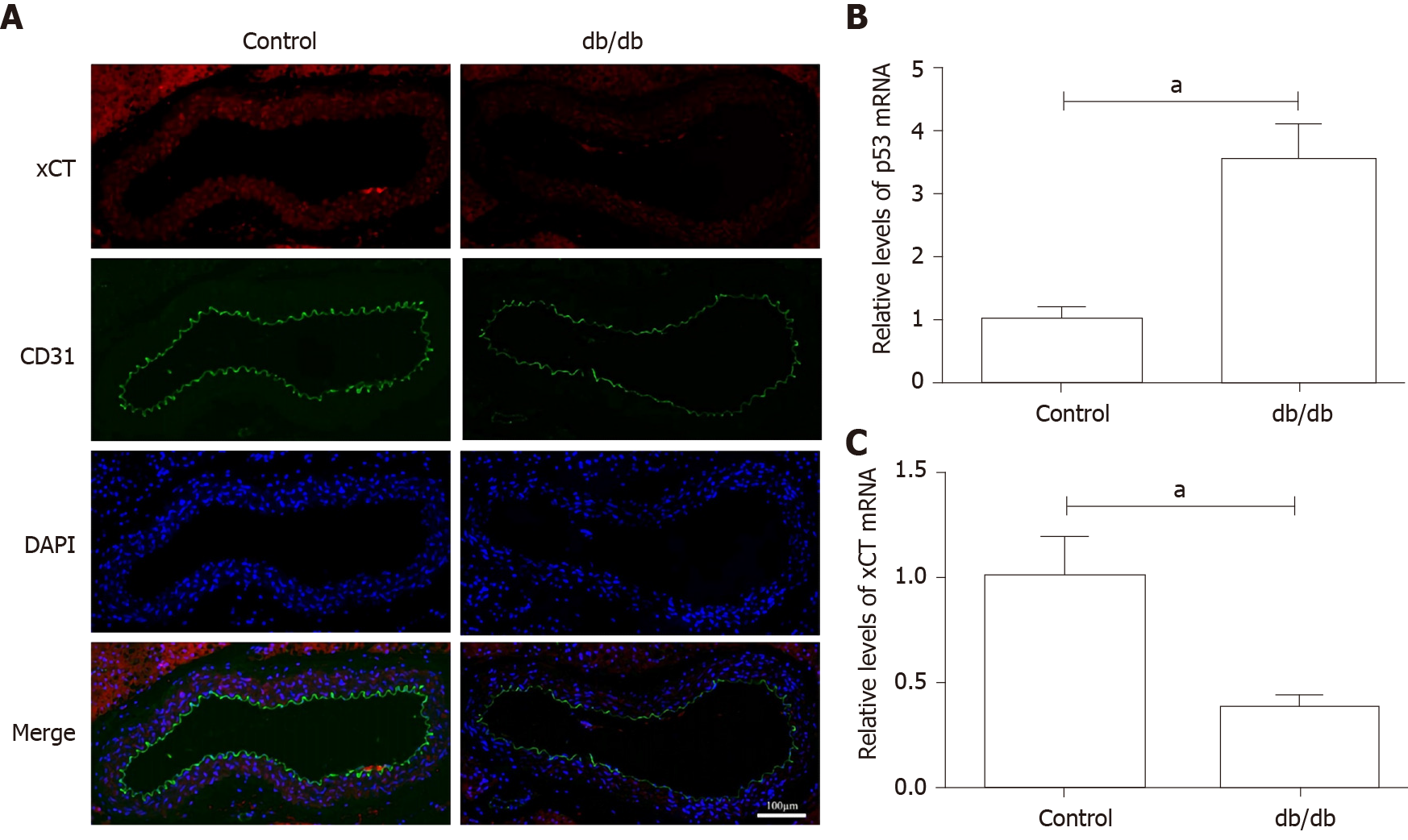
It was found that HG and IL-1β induced ferroptosis in HUVECs, as evidenced by the protective effect of the ferroptosis inhibitors, Deferoxamine and ferrostatin-1, resulting in increased lipid ROS and decreased cell viability. Mechanistically, activation of the p53-xCT-GSH axis induced by HG and IL-1β enhanced ferroptosis in HUVECs. In addition, a decrease in xCT and the presence of de-endothelialized areas were observed in the aortic endothelium of db/db mice.
Ferroptosis is involved in endothelial dysfunction and p53-xCT-GSH axis activation plays a crucial role in endothelial cell ferroptosis and endothelial dysfunction.
Core Tip: Endothelial dysfunction is a critical and initiating contributor to the pathogenesis of diabetic cardiovascular complications. Ferroptosis is characterized by the accumulation of iron-induced lipid reactive oxygen species and depletion of plasma membrane unsaturated fatty acids. Our findings demonstrated that ferroptosis is involved in endothelial dysfunction and the p53- xCT (the substrate-specific subunit of system Xc-)-glutathione axis activation plays a crucial role in endothelial cell ferroptosis and endothelial dysfunction. These results provide important insights as inhibiting activation of the p53-xCT-glutathione axis and ferroptosis could attenuate diabetes-induced endothelial dysfunction and may be a novel strategy for the treatment of vascular complications in diabetes mellitus.
- Citation: Luo EF, Li HX, Qin YH, Qiao Y, Yan GL, Yao YY, Li LQ, Hou JT, Tang CC, Wang D. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes 2021; 12(2): 124-137
- URL: https://www.wjgnet.com/1948-9358/full/v12/i2/124.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i2.124
Diabetes mellitus is a chronic, metabolic disorder characterized by insulin resistance and impaired glucose homeostasis[1]. A growing number of diabetic patients suffer from cardiovascular complications which can cause mortality and morbidity. Evidence suggests that diabetes-induced endothelial dysfunction is a critical and initiating factor in the genesis of diabetic macrovascular and microvascular complications[2], although the underlying mechanisms are still not fully understood. Extensive research has shown that endothelial dysfunction in cardiovascular complications is primarily characterized by enhanced oxidative stress, decreased release of nitric oxide (NO), increased production of inflammatory factors, impaired endothelial repair, and abnormal angiogenesis[3].
Cell death is critical in diverse aspects of mammalian development, homeostasis, and disease, and plays a vital role in diabetes-induced endothelial dysfunction. Our previous studies demonstrated that activation of NFκB signaling induced by high glucose (HG) and interleukin-1β (IL-1β) caused endothelial cell apoptosis and endothelial dysfunction[4]. Whether and how other forms of regulated cell death (RCD) are involved in diabetic endothelial dysfunction remains less clear. Ferroptosis, a recently defined, non-apoptotic, RCD process with the hallmark of the accumulation of iron-dependent lipid peroxides was named by Dixon et al[5] in 2012. Ferroptosis may occur in various physiological and pathological processes in humans and animals[6]. For example, excessive ferroptosis has been linked to neurodegeneration, kidney failure, liver fibrosis, and ischemia-reperfusion injury, whereas insufficient ferroptosis can lead to cancer[7-9]. In addition, Alzheimer’s disease, ischemic and hemorrhagic stroke, Huntington disease, and Parkinson’s disease are among the candidate neurodegenerative diseases that may involve ferroptosis[10]. Recent evidence suggests that ferroptosis plays a pathophysiological role in cardiomyopathy[11]. Bai et al[12] indicated that ferroptosis might occur during the initiation and development of atherosclerosis (AS). Inhibition of ferroptosis could protect against the aggravation of AS in thoracic aorta through attenuating lipid peroxidation and endothelial dysfunction. These studies also raise intriguing questions of whether ferroptosis is involved in diabetes-induced endothelial dysfunction. Ferroptosis is characterized by the accumulation of iron-induced lipid reactive oxygen species (ROS) and depletion of plasma membrane unsaturated fatty acids[13]. Also enhanced oxidative stress and ROS, which inactivate NO, is a dominant feature of diabetes-induced endothelial dys-function[14]. However, very little is known about the role of ferroptosis in diabetes-induced endothelial dysfunction. Therefore, this study aimed to investigate the role and regulatory mechanism of ferroptosis in diabetes-induced endothelial dysfunction.
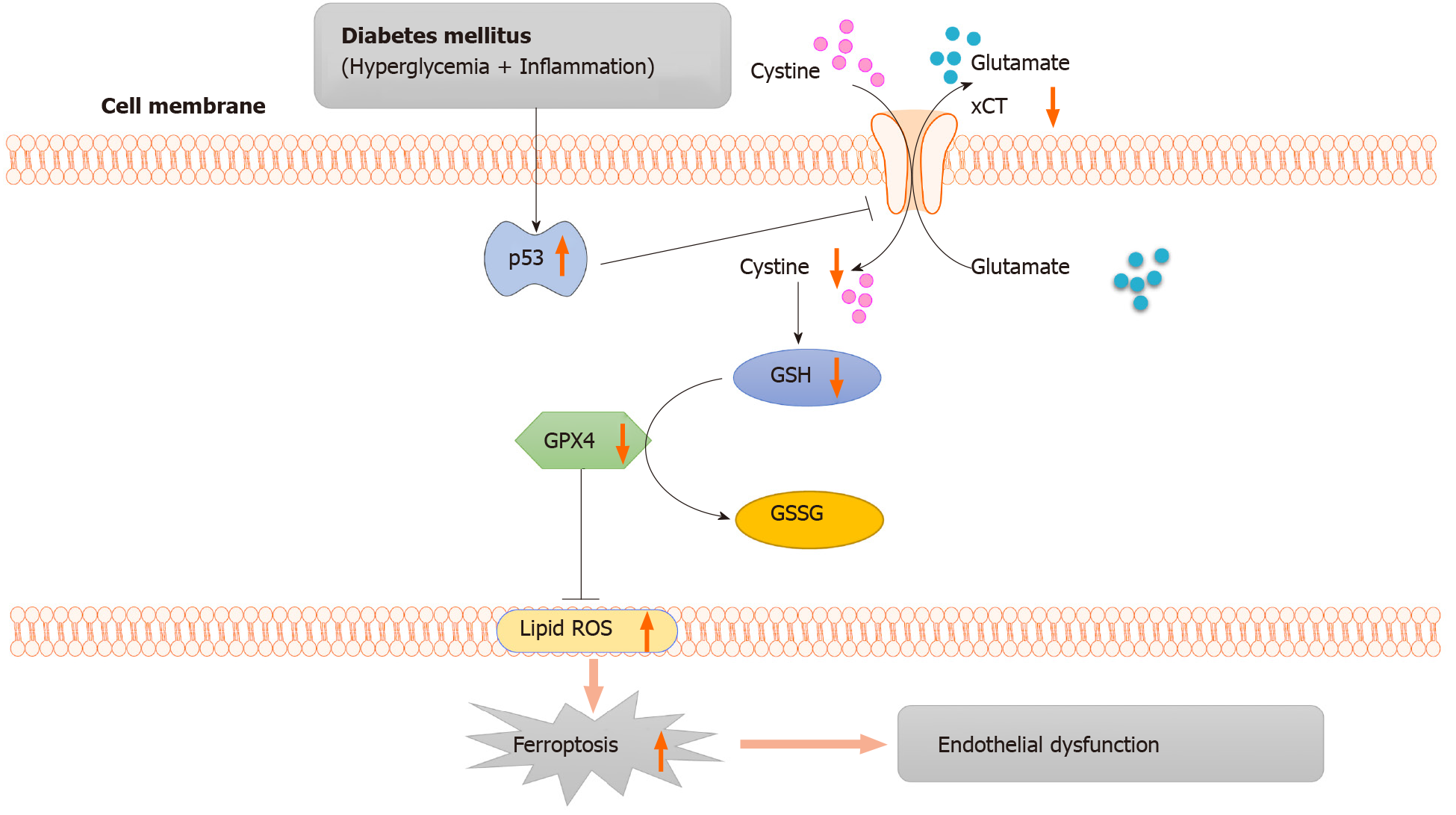
In this study, we demonstrated that HG and IL-1β induce human umbilical vein endothelial cells (HUVECs) ferroptosis and ferroptosis inhibitor attenuates the ferroptosis of HUVECs induced by HG and IL-1β. Furthermore, our findings showed that activation of the p53-xCT (the substrate-specific subunit of system Xc-)-glutathione (GSH) axis plays a vital role in HG and IL-1β induced ferroptosis. We also observed iron accumulation, down-regulation of xCT, and the presence of de-endothelialized areas in the aorta of db/db mice. These findings support our hypothesis that activation of the p53-xCT-GSH axis and increased ferroptosis have a pivotal role in diabetes-associated endothelial dysfunction.
Male C57BL/6J mice and db/db mice (C57BL/6J-Leprdb/Leprdb) were obtained from Southeast University Animal Center. 10-12 wk old mice were employed. All experiments involving animals were performed under the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (DHWE publication No. 96-01, revised in 2002) and were approved by the Care of Experimental Animals Committee of Southeast University.
Primary HUVECs were purchased from Promocell (C-12200, Heidelberg, Germany) and grown in endothelial culture medium (1001, ScienCell, United States) containing 1% endothelial cell growth supplement (1052, ScienCell), 5% fetal bovine serum (0025, ScienCell) and 1% penicillin/streptomycin solution (0503, ScienCell) at 37°C in a humidified atmosphere of 5% carbon dioxide and 95% air. All of the experiments were performed using 2-5 passages of HUVECs. HUVECs characterization is shown in Supplementary Figure 1.
Recombinant human IL-1β was purchased from Thermo Scientific (PHC0814, Waltham, MA, United States). The Cell Counting Kit-8 (CCK-8) assay kit was purchased from Dojindo (Kumamoto, Japan). Deferoxamine (DFO) and ferrostatin-1 (Fer-1) were purchased from Med Chem Express (HY-B0988 and HY-100579, Shanghai, China). Necrostatin-1s (Nec-1s) was purchased from BioVision (2263, Milpitas, United States) and Z-VAD-FMK was purchased from ApexBio (A1902, Houston, United States). The following antibodies were used: anti-glutathione peroxidase 4 [(GPX4) (ab125066, Abcam, Cambridge, MA, United States)], anti-FTH1 (ab75972, Abcam, Cambridge, MA, United States), anti-cyclooxygenase (COX)-2 (ab179800, Abcam, Cambridge, MA, United States), anti-p53 (ab1431, Abcam, Cambridge, MA, United States), anti-xCT (ab37185, Abcam, Cambridge, MA, United States), anti-CD31 (ab28364, Abcam, Cambridge, MA, United States) and anti-β-actin (ab8226, Abcam, Cambridge, MA, United States).
Cell viability was typically assessed in 96-well format by CCK-8. CCK-8 solution (10 μL) was added to HUVECs at various times, and the cells were incubated for 1-4 h at 37°C. The absorbance at 450 nm was measured on a microplate reader (SYNERGY/H4, BioTek, United States).
Approximately 1 × 106 cells/well were seeded in 6-well plates overnight followed by treatment for 48 h. Lipid peroxidation was determined by the BODIPY™ 581/591 C11 method (D3861, Invitrogen, United States). The cellular fluorescence was immediately detected using an Accuri C6 flow cytometer (BD Biosciences, United States). A total of 1 × 104 events were acquired for each sample from three independent experiments. Data were analyzed using FlowJo software (ver.10.0, FlowJo LLC, United States).
HUVECs were transfected with small interfering ribonucleic acid (siRNA) using Lipofectamine RNA interfere MAX transfection reagent (Thermo Scientific, United States) according to the manufacturer’s instructions. siRNA specific for p53 was purchased from Santa Cruz (sc-29436, United States). Control siRNA of p53 was purchased from Santa Cruz (sc-37007, United States).
Total RNA isolation was carried out with the RNAiso plus kit (9109, TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. RNA concentration and purity were confirmed with a Nanodrop 2000 (Thermo Scientific, United States). First-strand complementary deoxyribonucleic acid synthesis was then obtained using the PrimeScript™ RT Reagent Kit with gDNA Eraser (RR047A, TaKaRa, Shiga, Japan). The quantitative real-time reverse transcription-polymerase chain reaction analysis was performed with the TB Green™ Premix Ex Taq ™ II (RR820A, TaKaRa, Shiga, Japan) and Prism 7500 sodium dodecyl sulfate (Applied Biosystems; Thermo Scientific, MA, United States). The relative expression of the target genes was calculated using the 2-ΔΔCt method. β-actin was used as the internal control to normalize the data. The sequences of quantitative real-time reverse transcription-polymerase chain reaction primers are presented in Table 1.
| Sequences of the reverse transcription-polymerase chain reaction primers | |
| p53 (human) | Forward: 5′- CAGCACATGACGGAGGTTGT - 3′; Reverse: 5′- TACTCCAAATACTCCACACGC - 3′ |
| xCT (human) | Forward: 5′- TCTCCAAAGGAGGTTACCTGC - 3′; Reverse: 5′- AGACTCCCCTCAGTAAAGTGAC - 3′ |
| β-actin (human) | Forward: 5′- GGCACCCAGCACAATGAAG - 3′; Reverse: 5′- CCGATCCACACGGAGTACTTG - 3′ |
| p53 (mouse) | Forward: 5′-TGGAAGGAAATTTGTATCCCGA- 3′; Reverse: 5′-GTGGATGGTGGTATACTCAGAG- 3′ |
| xCT (mouse) | Forward: 5′-GCTGTTTTATCATCACGGGTTT - 3′; Reverse: 5′- AATCCCAAACTCATGCATAAGC- 3′ |
| β-actin (mouse) | Forward: 5′-GTGACGTTGACATCCGTAAAGA- 3′; Reverse: 5′-GCCGGACTCATCGTACTCC- 3′ |
Total cellular protein was extracted with the Tissue or Cell Total Protein Extraction Kit (C510003, Sangon Biotech, China) to evaluate the levels of GPX4, FTH1, COX-2, p53, and xCT. A BCA kit (KeyGEN, China) was used to determine the protein concentrations according to the manufacturer’s instructions. Equal amounts of proteins were electrophoresed in sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride membranes. The membranes were incubated in a blocking solution containing 5% non-fat milk at 37°C for 1 h. The blots were incubated overnight with primary antibodies against GPX4 (ab125066, Abcam), FTH1 (ab75972, Abcam), COX-2 (ab179800, Abcam), p53 (ab1431, Abcam) and xCT (ab37185, Abcam) followed by horseradish peroxidase-labelled secondary immunoglobulin G (sc-516132, Santa Cruz Biotechnology). Signals were detected using an advanced ECL system (GE Healthcare, United Kingdom). β-actin (ab8226, Abcam) was used as an internal control.
Immunofluorescence staining was performed as previously described[4]. HUVECs grown on coverslips were fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton-X100. After blocking with 10% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature, the coverslips were incubated with primary antibodies against CD31 (ab28364, Abcam) and xCT (ab37185, Abcam) at 4°C overnight. The coverslips were then incubated with secondary antibodies for 1 h at room temperature in the dark, the images were captured using a laser scanning confocal microscope (LSM 510 META, Zeiss, Germany).
Paraffin tissue sections from mice aorta were deparaffinized and rehydrated. After pre-treatment with pepsin for 30 min (1% pepsin in 0.1% HCl) for antigen retrieval, the sections were incubated overnight at 4℃ with primary CD31 and xCT antibodies diluted in Tris-buffered saline containing 1% bull serum albumin and 0.1% Tween 20. After pre-treatment, the antigen retrieval sections were washed in Tris-buffered saline and incubated with secondary antibodies. The images were captured using a laser scanning confocal microscope.
The data are shown as the mean ± SD taken from at least three independent experiments. Analyses were performed using Graphpad Prism 5 Software (Graphpad Software Inc., United States). Significance levels were determined by Student's t-test and one-way analysis of variance (ANOVA). Significance was defined as P < 0.05.
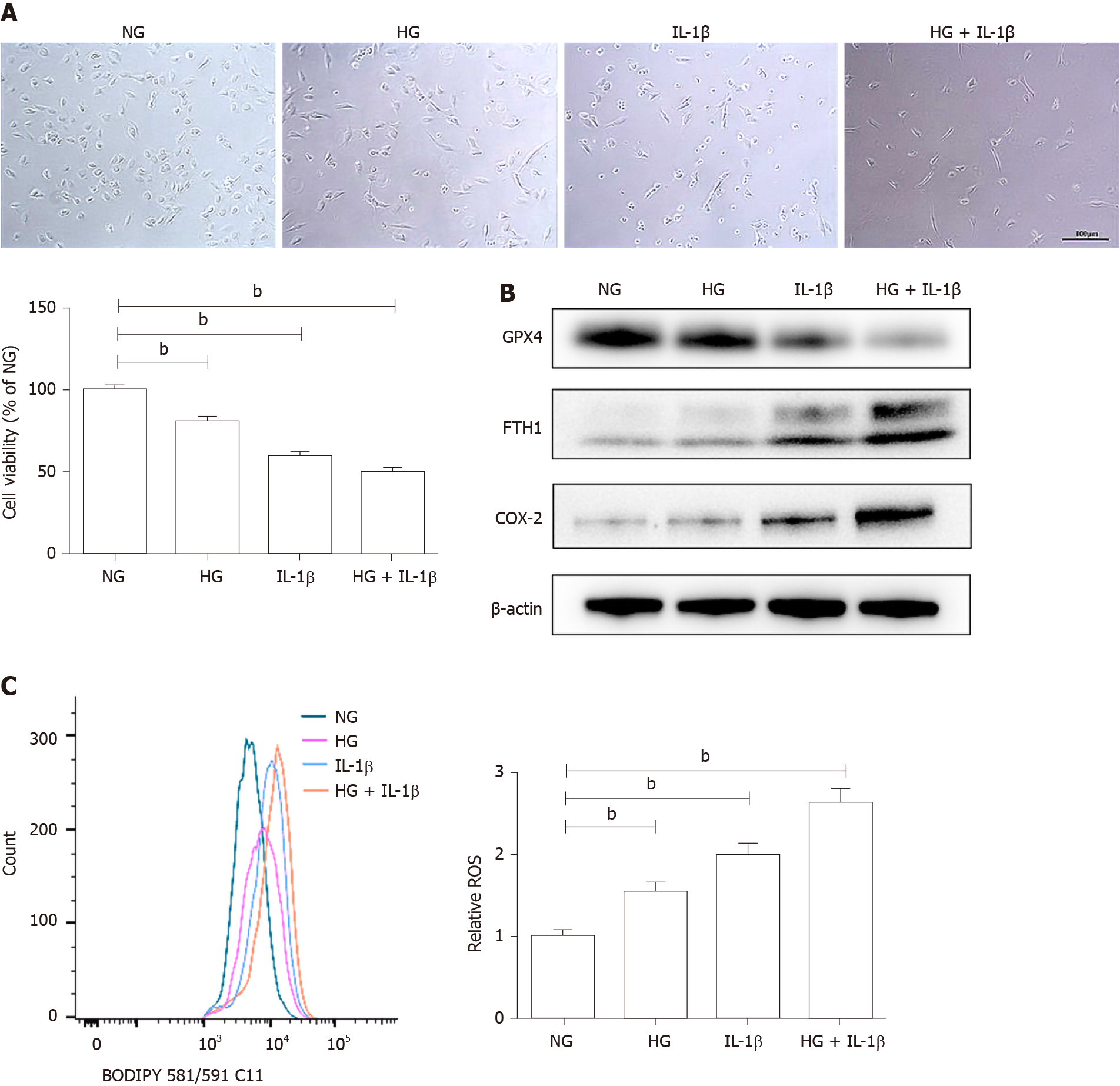
As shown in Figure 1A, HG (30 mmol/L) and IL-1β (10 ng/mL) significantly affected cell viability in HUVECs. To elucidate the involvement of ferroptosis in HG and IL-1β-mediated cell death, the expression of corresponding proteins was detected by Western blot. HG and IL-1β induced a significant reduction in GPX4 and an increase in FTH1 and COX-2 (Figure 1B). Lipid peroxidation is an essential process for conducting ferroptosis. Figure 1C shows a marked right-shift of intracellular ROS release after treatment with HG and IL-1β for 48 h.
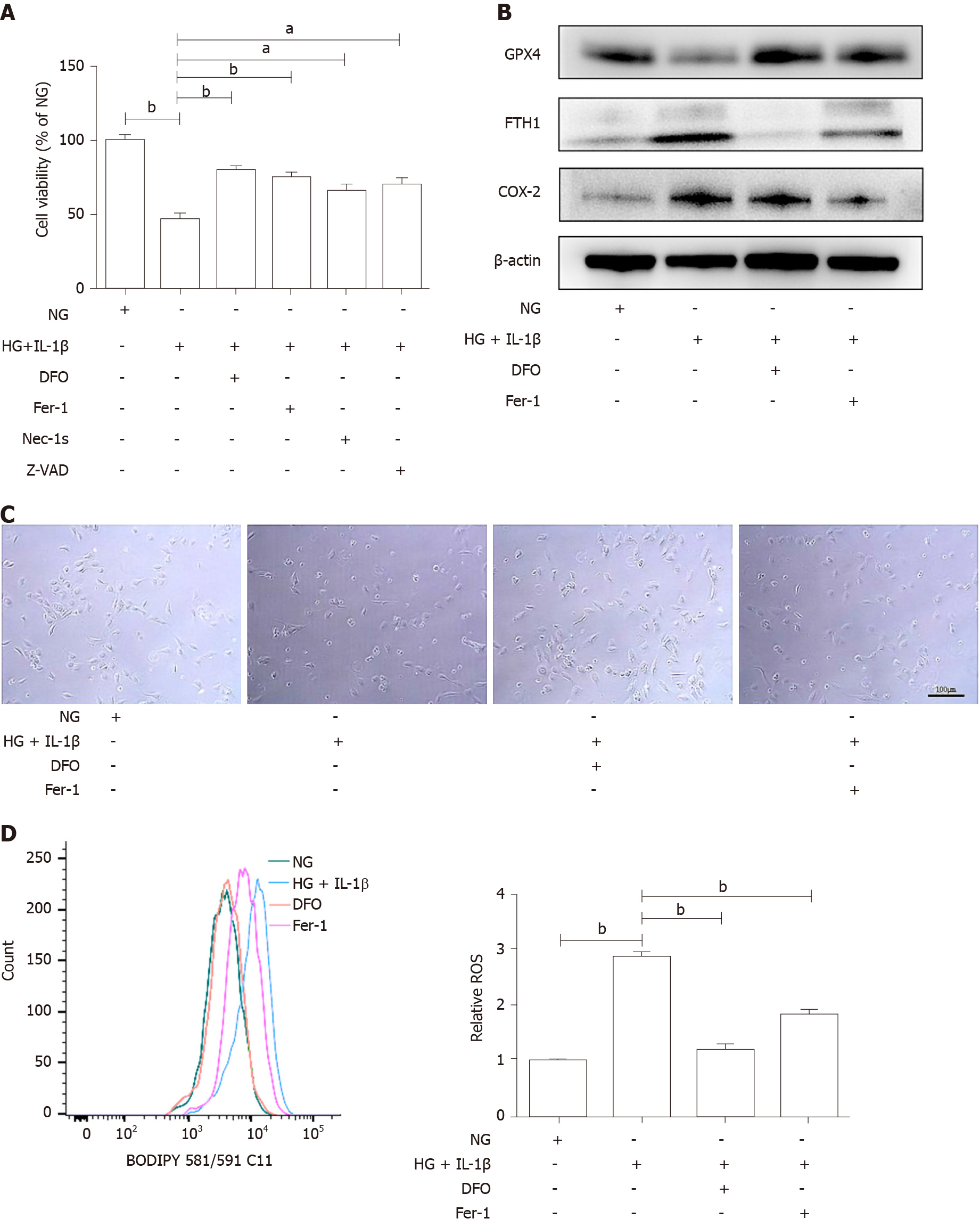
To further determine whether ferroptosis inhibitor attenuates HG and IL-1β-induced ferroptosis, HUVECs were treated with NG, HG (30 mmol/L) and IL-1β (10 ng/mL), and ferroptosis inhibitor, DFO (100 μmol/L) or Fer-1 (10 μmol/L), the necroptosis inhibitor necrostatin-1s (Nec-1s, 2 μmol/L), and the apoptosis inhibitor Z-VAD-FMK (Z-VAD, 5 μmol/L). As shown in Figure 2A, DFO, Fer-1, Nec-1s, and Z-VAD partially rescued HG and IL-1β-mediated cell death. It was demonstrated that in addition to necrosis and apoptosis, ferroptosis may play an important role in cell death induced by hyperglycemia and inflammation. Representative phase-contrast microscopy images of cell death rescued by DFO and Fer-1 are shown in Figure 2B. In addition, DFO and Fer-1 reversed the decrease in GPX4 and the increase in FTH1 and COX-2 induced by HG and IL-1β (Figure 2C). As shown in Figure 2D, after treatment with DFO and Fer-1, the level of ROS in HUVECs reduced significantly.
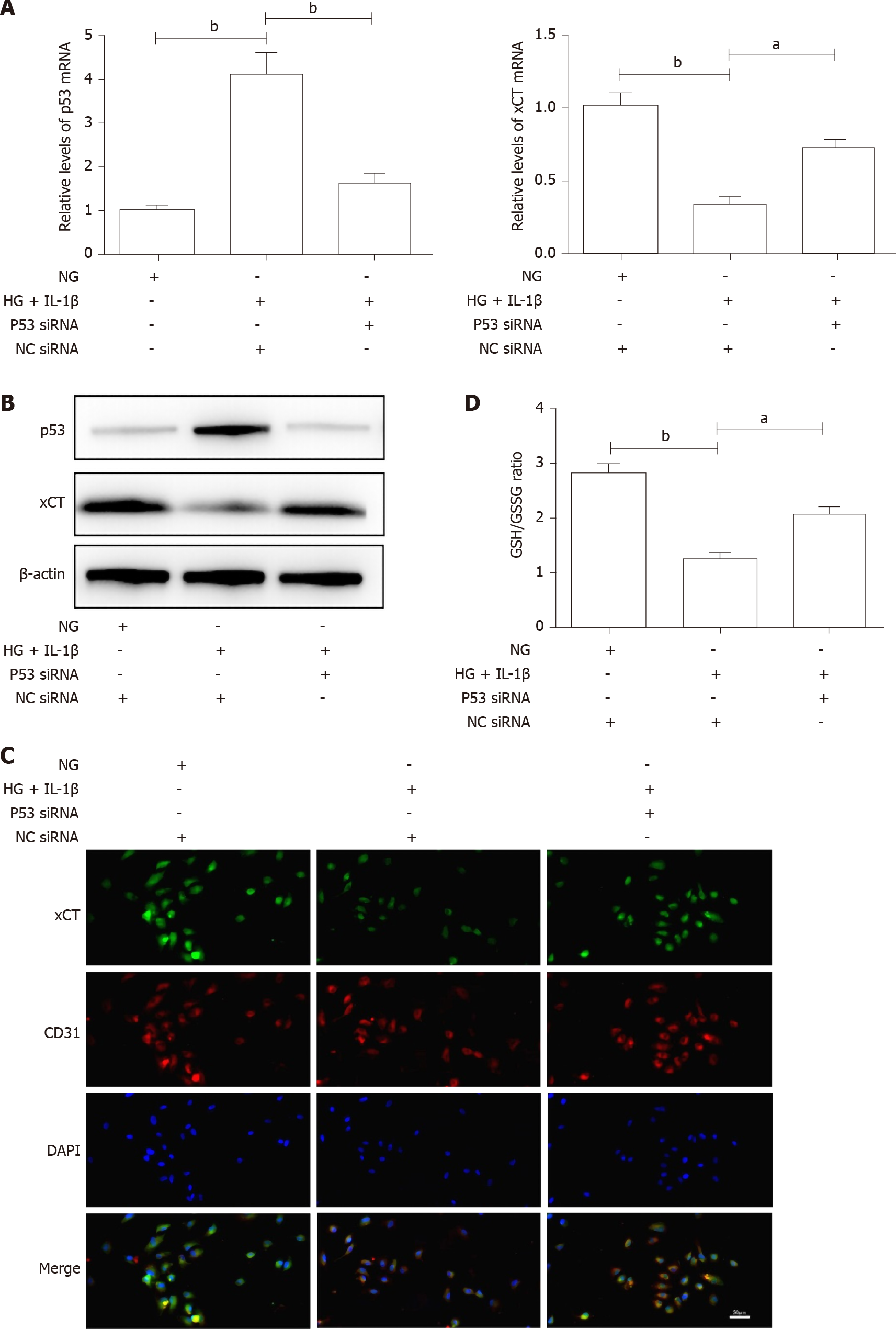
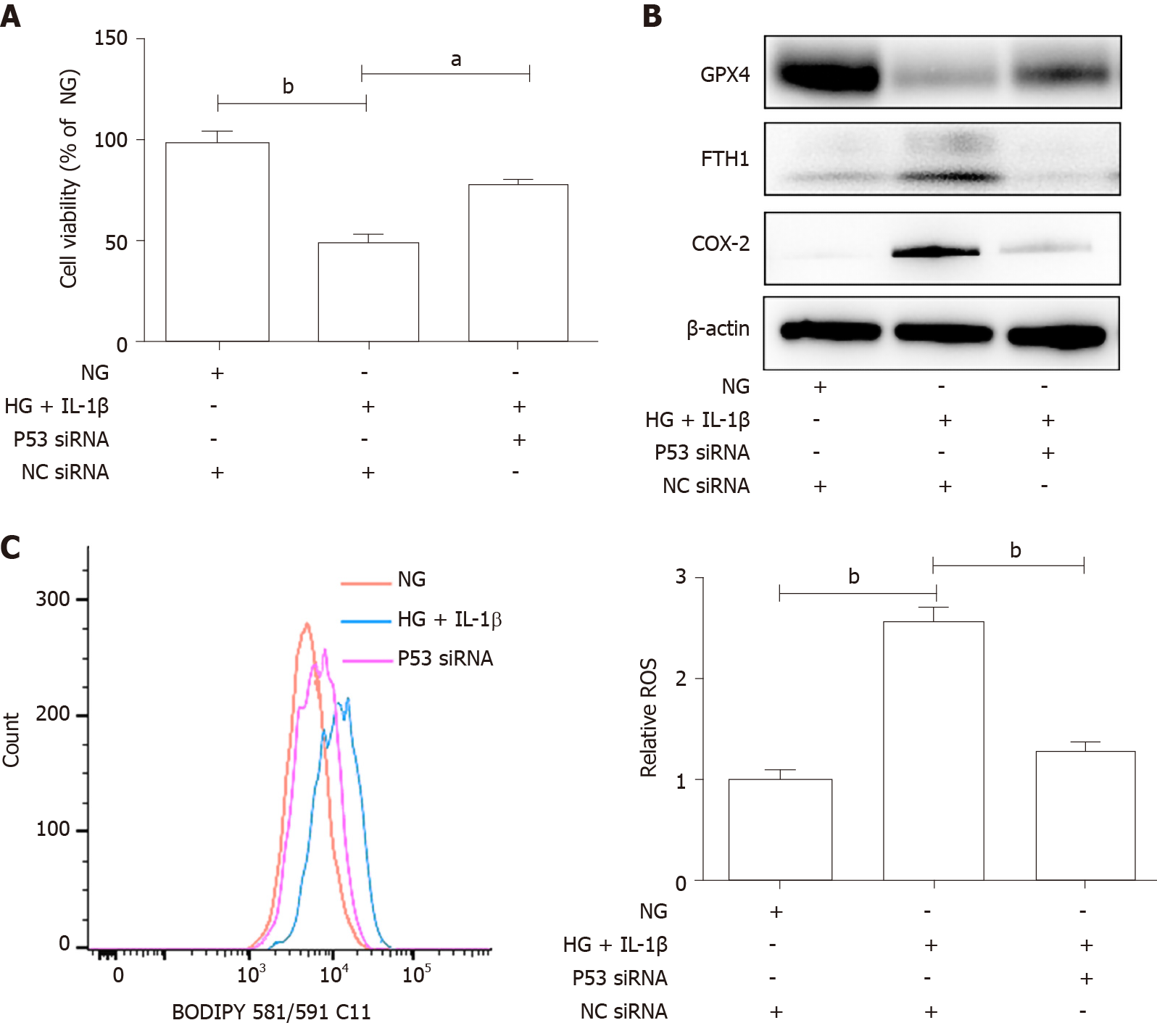
GPX4 uses GSH to protect cells from ferroptosis by eliminating phospholipid peroxides[15]. GSH is one of the major cellular non-protein antioxidants, and is a tripeptide anti-oxidant consisting of glutamate, glycine and cysteine[16]. Jiang et al[17] found that ferroptosis has p53-mediated activity during tumor suppression and p53 sensitizes cells to ferroptosis by repressing expression of xCT (SLC7A11), a key component of the cystine/glutamate antiporter, and inhibiting cystine uptake. Previous research has established that hyperglycemia promotes the activation of p53[18,19]. To further determine whether the p53-xCT-GSH axis is involved in HG and IL-1β-induced ferroptosis, HUVECs were transiently transfected with p53 siRNA or NC siRNA and then treated with NG, HG (30 mmol/L) and IL-1β (10 ng/mL). As shown in Figure 3, HG and IL-1β induced a significant increase in p53 and a reduction in xCT and GSH in HUVECs. p53 siRNA can attenuate the decrease in xCT and GSH induced by HG and IL-1β. As presented in Figure 4A, p53 siRNA significantly rescued HG and IL-1β-mediated cell death. Figure 4B shows that p53 siRNA attenuated the decrease in GPX4 and the increase in FTH1 and COX-2 induced by HG and IL-1β. As shown in Figure 4C, p53 siRNA attenuated the increase in ROS induced by HG and IL-1β.
The localization of CD31, a biomarker of endothelial cells, and xCT were detected by immunofluorescence. Figure 5A shows the decrease in xCT and the presence of de-endothelialized areas in the aortic endothelium of db/db mice. As presented in Figure 5B and C, p53 mRNA was up-regulated, and xCT mRNA was down-regulated in the aorta of db/db mice.
Previous research has established that endothelial dysfunction is among the most important factors for vascular complications in diabetic patients[20]. The endothelium secretes various factors, regulating blood vessel development, cell adhesion, inflammation, metabolism, angiogenesis, vascular permeability, and vessel integrity[21]. Endothelial dysfunction is an imbalance in the production of vaso-constricting factors, e.g., prostaglandin, angiotensin II, endothelin-1, and vasodilator factors, e.g., prostacyclin, NO, and endothelial-derived hyperpolarizing factor. Disruption of this balance results in vasoconstriction, vascular inflammation, leukocyte adherence, platelet activation, impaired coagulation, thrombosis, and AS[22].
Existing research recognizes the critical role played by hyperglycemia-induced oxidative stress and increased production of ROS in the development of endothelial dysfunction[23]. Ferroptosis, a newly defined, iron-dependent form of the RCD process that is induced by excessive lipid peroxidation, is morphologically and mechanistically distinct from apoptosis[5,7,24]. And the current study found that HG and IL-1β induced ferroptosis in HUVECs, as evidenced by the protective effect of DFO and Fer-1, resulting in an increase in lipid ROS and a decrease in cell viability. In addition, activation of the p53-xCT-GSH axis enhanced ferroptosis in HUVECs. Interestingly, p53 siRNA attenuated the decrease in xCT and GSH, the increase in ROS and ferroptosis induced by HG and IL-1β. Our findings were in accordance with the study by Yokoyama et al[25], which showed that, in the diabetic state, endothelial p53 expression was markedly up-regulated and endothelium-dependent vasodilatation was significantly impaired. Wu et al[18] verified the relationship between HG-induced p53 signaling activation and suppression of antioxidant systems in the vascular endothelium, which was consistent with our findings. Moreover, Ortega-Camarillo et al[26] reported that p53 is part of a death signaling pathway in rat insulin-producing cells under hyperglycemic conditions. Gu et al[27] indicated that the inhibition of p53 attenuated diabetes-induced cell senescence, and improved both glycolytic and angiogenic defects. These results showed that endothelial p53 plays a significant role in the development of diabetic vascular complications and inhibition of endothelial p53 could be a novel therapeutic target in diabetics. xCT, a key component of the cystine-glutamate antiporter, is important for the exchange of glutamate and cystine at a ratio of 1:1 across the plasma membrane[28]. Our findings showed that, in diabetes, p53 could transcriptionally suppress xCT and the downregulation of xCT inhibited cystine uptake and reduced GSH synthesis. These results were also supported by Jiang, who found that retinal cells treated with HG for four days showed a decrease in xCT. Furthermore, a decrease of xCT in the retina of diabetic rats was also found[29]. Our results were further supported by recent studies which indicated that xCT expression and glutathione levels were reduced whereas ROS were increased in diabetic rats[30]. Lutchmansingh et al[31] showed that patients with type 2 diabetes have GSH deficiency, especially if they have microvascular complications.
In short, the results of this study indicated that the p53-xCT-GSH axis induced by HG and IL-1β can repress the expression of xCT and inhibit cystine uptake, ultimately causing increased endothelial cell ferroptosis and endothelial dysfunction. From the perspective of ferroptosis and GSH synthesis disorders, we describe a new mechanism of endothelial vascular injury in diabetes.
As illustrated in Figure 6, in diabetes, p53 signaling is activated, and p53 can transcriptionally suppress xCT, in endothelial cells. The downregulation of xCT inhibits cystine uptake and reduces glutathione synthesis, which is involved in triggering ferroptosis in endothelial cells and ultimately leads to endothelial dysfunction. In conclusion, the current study demonstrated that ferroptosis is involved in endothelial dysfunction for the first time, and p53-xCT-GSH axis activation plays a crucial role in endothelial cell ferroptosis and endothelial dysfunction. These facts support the hypothesis that inhibiting activation of the p53-xCT-GSH axis and ferroptosis could attenuate diabetes-induced endothelial dysfunction.
Endothelial dysfunction, a hallmark of diabetes, is a critical and initiating contributor to the pathogenesis of diabetic cardiovascular complications. However, the underlying mechanisms are still not fully understood. Ferroptosis is a newly defined regulated cell death driven by cellular metabolism and iron-dependent lipid peroxidation. Although the involvement of ferroptosis in disease pathogenesis has been shown in cancers and degenerative diseases, the participation of ferroptosis in the pathogenesis of diabetic endothelial dysfunction remains unclear.
We tried to provide new insight into the mechanism of diabetic endothelial dysfunction.
The objective of this study is to investigate the role and regulatory mechanism of ferroptosis in diabetes-induced endothelial dysfunction.
Human umbilical vein endothelial cells (HUVECs) were treated with high glucose (HG), interleukin-1β (IL-1β) and ferroptosis inhibitor, and then cell viability, reactive oxygen species (ROS) and ferroptosis-related marker protein were assessed. To further determine whether the p53-xCT (the substrate-specific subunit of system Xc-)-glutathione (GSH) axis is involved in HG and IL-1β-induced ferroptosis, HUVECs were transiently transfected with p53 small interfering ribonucleic acid or NC small interfering ribonucleic acid and then treated with HG and IL-1β. Cell viability, ROS and ferroptosis-related marker protein were then tested. In addition, we detected xCT and p53 expression in the aorta of db/db mice.
It was found that HG and IL-1β induced ferroptosis in HUVECs, as evidenced by the protective effect of the ferroptosis inhibitors, Deferoxamine and Ferrostatin-1, resulting in increased lipid ROS and decreased cell viability. Mechanistically, activation of the p53-xCT-GSH axis induced by HG and IL-1β enhanced ferroptosis in HUVECs. In addition, the decrease in xCT and de-endothelialized areas were observed in the aortic endothelium of db/db mice.
The current study demonstrated that ferroptosis is involved in endothelial dysfunction and p53-xCT-GSH axis activation plays a crucial role in endothelial cell ferroptosis and endothelial dysfunction.
These results provide important insights as inhibiting activation of the p53-xCT-GSH axis and ferroptosis could attenuate diabetes-induced endothelial dysfunction and may be a novel strategy for the treatment of vascular complications in diabetes mellitus.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng JT, Li W S-Editor: Zhang L L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Rother KI. Diabetes treatment--bridging the divide. N Engl J Med. 2007;356:1499-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1183] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 3. | Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9:434-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 4. | Luo E, Wang D, Yan G, Qiao Y, Zhu B, Liu B, Hou J, Tang C. The NF-κB/miR-425-5p/MCT4 axis: A novel insight into diabetes-induced endothelial dysfunction. Mol Cell Endocrinol. 2020;500:110641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11652] [Article Influence: 896.3] [Reference Citation Analysis (1)] |
| 6. | Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020;30:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 776] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 7. | Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4608] [Cited by in RCA: 4945] [Article Influence: 618.1] [Reference Citation Analysis (0)] |
| 8. | Feng H, Stockwell BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 563] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 9. | Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 407] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 10. | Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol. 2019;56:4880-4893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 360] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 11. | Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, Gao F, Yu Y, Song Z, Wu Q, An P, Huang S, Pan J, Chen HZ, Chen J, Linkermann A, Min J, Wang F. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ Res. 2020;127:486-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 12. | Bai T, Li M, Liu Y, Qiao Z, Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med. 2020;160:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 13. | Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195-2209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1001] [Cited by in RCA: 1117] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 14. | Yang C, Kelaini S, Caines R, Margariti A. RBPs Play Important Roles in Vascular Endothelial Dysfunction under Diabetic Conditions. Front Physiol. 2018;9:1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 5163] [Article Influence: 469.4] [Reference Citation Analysis (0)] |
| 16. | Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 887] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 17. | Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 2393] [Article Influence: 239.3] [Reference Citation Analysis (0)] |
| 18. | Wu Y, Lee S, Bobadilla S, Duan SZ, Liu X. High glucose-induced p53 phosphorylation contributes to impairment of endothelial antioxidant system. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2355-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Chen YW, Chenier I, Chang SY, Tran S, Ingelfinger JR, Zhang SL. High glucose promotes nascent nephron apoptosis via NF-kappaB and p53 pathways. Am J Physiol Renal Physiol. 2011;300:F147-F156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Dhananjayan R, Koundinya KS, Malati T, Kutala VK. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J Clin Biochem. 2016;31:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 564] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 22. | Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 365] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Gao M, Jiang X. To eat or not to eat-the metabolic flavor of ferroptosis. Curr Opin Cell Biol. 2018;51:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Yokoyama M, Shimizu I, Nagasawa A, Yoshida Y, Katsuumi G, Wakasugi T, Hayashi Y, Ikegami R, Suda M, Ota Y, Okada S, Fruttiger M, Kobayashi Y, Tsuchida M, Kubota Y, Minamino T. p53 plays a crucial role in endothelial dysfunction associated with hyperglycemia and ischemia. J Mol Cell Cardiol. 2019;129:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Ortega-Camarillo C, Guzmán-Grenfell AM, García-Macedo R, Rosales-Torres AM, Avalos-Rodríguez A, Durán-Reyes G, Medina-Navarro R, Cruz M, Díaz-Flores M, Kumate J. Hyperglycemia induces apoptosis and p53 mobilization to mitochondria in RINm5F cells. Mol Cell Biochem. 2006;281:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Gu J, Wang S, Guo H, Tan Y, Liang Y, Feng A, Liu Q, Damodaran C, Zhang Z, Keller BB, Zhang C, Cai L. Inhibition of p53 prevents diabetic cardiomyopathy by preventing early-stage apoptosis and cell senescence, reduced glycolysis, and impaired angiogenesis. Cell Death Dis. 2018;9:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond). 2018;38:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 565] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 29. | Carpi-Santos R, Ferreira MJ, Pereira Netto AD, Giestal-de-Araujo E, Ventura AL, Cossenza M, Calaza KC. Early changes in system [Formula: see text] and glutathione in the retina of diabetic rats. Exp Eye Res. 2016;146:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Carpi-Santos R, Calaza KC. Alterations in System xc- Expression in the Retina of Type 1 Diabetic Rats and the Role of Nrf2. Mol Neurobiol. 2018;55:7941-7948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Lutchmansingh FK, Hsu JW, Bennett FI, Badaloo AV, McFarlane-Anderson N, Gordon-Strachan GM, Wright-Pascoe RA, Jahoor F, Boyne MS. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS One. 2018;13:e0198626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |