Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.2096
Peer-review started: September 2, 2021
First decision: October 3, 2021
Revised: October 15, 2021
Accepted: December 10, 2021
Article in press: December 10, 2021
Published online: December 15, 2021
Processing time: 104 Days and 22.6 Hours
The main pathological factor of cerebral infarction is atherosclerosis, which is the pathological process of chronic inflammatory diseases such as vascular smooth muscle hyperplasia, inflammatory cell infiltration, extracellular matrix increase, and thrombosis. At present, the focus of clinical treatment is anti-platelet aggregation and improving blood status, and current research is limited to improving symptoms only.
To observe the effect of sodium ozagrel and atorvastatin on type 2 diabetes patients with lacunar cerebral infarction.
Eighty-two patients with type 2 diabetes and lacunar cerebral infarction admitted to our hospital from January 2018 to February 2020 were equally categorized into two groups according to their treatment method. The control group was administered atorvastatin, and the observation group was administered sodium ozagrel combined with atorvastatin. The National Institutes of Health stroke scale (NIHSS) score, activities of daily living (ADL) score, blood glucose, lipid levels, inflammatory factors, high-mobility group box 1 (HMGB1) levels, paraoxonase-1 (PON-1) levels, erythrocyte sedimentation rate (ESR), and macrophage migration inhibitory factor (MIF) levels were recorded before and after treatment. The total effective rate and adverse reaction rate of the two groups were analyzed.
The total effective rate of the observation group (94.00%) was significantly higher than that of the control group (80.00%) (χ2 = 3.998; P = 0.046). The blood glucose indexes, total cholesterol levels, triglyceride levels, low-density lipoprotein cholesterol levels, high-sensitivity C-reactive protein levels, interleukin-1β levels, tumor necrosis factor-α levels, HMGB1 Levels, ESR, MIF levels, platelet aggregation rates, and plasma viscosity of the two groups decreased after treatment; however, high-density lipoprotein cholesterol and PON-1 Levels increased after treatment. After treatment, the blood glucose indexes; blood lipid indexes; inflammatory factors; HMGB1, PON-1, and MIF levels; ESR; platelet aggregation rate; and plasma viscosity of the observation group were better than those of the control group (P < 0.05). After treatment, all patients in the observation group had higher ADL scores and lower NIHSS scores than those in the control group (P < 0.05).
Sodium ozagrel with atorvastatin can reduce inflammatory reactions; regulate ESR and HMGB1, PON-1, and MIF levels; control blood glucose and lipid indexes; and alleviate nerve injury without increasing adverse effects of atorvastatin alone.
Core Tip: This study was performed to observe the effect of sodium ozagrel combined with atorvastatin on high-mobility group protein B1 and high-sensitivity C-reactive protein in patients with type 2 diabetes mellitus and lacunar infarction. The purpose was to find a treatment plan that can effectively inhibit the pathological mechanism, alleviate clinical symptoms, improve the prognosis, and guide clinical treatment.
- Citation: Yu Y, Wang L, Zhu X, Liu YF, Ma HY. Sodium ozagrel and atorvastatin for type 2 diabetes patients with lacunar cerebral infarction. World J Diabetes 2021; 12(12): 2096-2106
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/2096.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.2096
Type 2 diabetes is a common metabolic disease that is often complicated by abnormal lipid metabolism[1]. Abnormal lipid metabolism is one of the pathological causes of cerebrovascular disease. Consequently, type 2 diabetes mellitus complicated with cerebral infarction is commonly encountered in clinical settings[2]. Lacunar infarction refers to lesions and occlusion of the small perforating artery in the deep part of the cerebral hemisphere or brainstem. It results in minor harm because the perforating artery supplies only a small area. Active symptomatic treatment can help reduce the degree of disability. Statins can effectively regulate blood lipids and reduce the degree of vascular lesions[3]. However, the effect of statins alone is not ideal.
As an antiplatelet drug and thromboxane A inhibitor, sodium ozagrel is widely used to treat ischemic cerebrovascular diseases. It has been reported that sodium ozagrel combined with atorvastatin calcium is effective for treating type 2 diabetes mellitus with lacunar infarction without increasing adverse drug reactions[4]. However, research has been limited to the improvement of symptoms only, and its mechanism of action has not been investigated in depth. This study aimed to observe the effects of sodium ozagrel combined with atorvastatin on high-mobility group protein B1 (HMGB1) and high-sensitivity-C reactive protein (hs-CRP) in patients with type 2 diabetes mellitus and lacunar infarction.
Eighty-two patients with type 2 diabetes mellitus and lacunar infarction treated at our hospital from January 2018 to February 2020 were categorized into two groups according to the method of treatment (41 patients in each group). There were 24 males and 17 females in the control group. Their ages ranged from 34 to 68 years (58.69 ± 9.22 years). The mean body mass index (BMI) was 24.15 ± 2.03 kg/m2. Type 2 diabetes had been diagnosed between 1 and 10 years previously (5.69 ± 1.74 years). The mean National Institutes of Health Stroke Scale (NIHSS) score was 15.23 ± 2.33. In the observation group, there were 22 males and 19 females. Their ages ranged from 38 to 69 years (59.17 ± 10.45 years). The mean BMI was 24.09 ± 2.14 kg/m2. Type 2 diabetes had been diagnosed 1 to 10 years previously (5.61 ± 1.85 years). The mean NIHSS score was 14.96 ± 2.17. There were no significant differences in the general data of the two groups (P > 0.05).
Inclusion criteria were as follows: Type 2 diabetes meeting the criteria of the Chinese guidelines for the prevention and treatment of type 2 diabetes mellitus[5]; lacunar infarction conforming to the criteria of the European treatment guidelines for acute cerebral infarction and confirmed by cranial computed tomography and/or magnetic resonance imaging[6]; age 18 years or older and younger than 70 years; and complete clinical data.
Exclusion criteria were as follows: Mental illness or serious communication disorders; recent history of surgery or diseases with bleeding tendencies; serious diseases of the heart, liver, kidney, and other organs; malignant tumors and systemic infection; pregnancy and lactation; and allergies.
The following medications and instruments were used: atorvastatin calcium tablets (10 mg; H19990258; Beijing Jialin Co., Ltd.); sodium ozagrel (20 mg; H20093400; Guangdong Pidi Pharmaceutical Co., Ltd.); ELX800 multifunctional enzymometer (Berten Company); BS634 platelet aggregation instrument (Beijing Biochemical Instrument Factory); and HT-100B blood rheometer (Hengtuo, Zibo).
Patients in both groups were administered symptomatic treatment to control blood glucose and blood pressure, nourish brain cells, and maintain water and electrolyte balance. Patients in the control group were treated with oral atorvastatin calcium tablets 10-20 mg once per day. The observation group was administered intravenous sodium ozagrel combined with atorvastatin. Atorvastatin was administered in the same dosage as that of the control group. Sodium ozagrel 80 mg was added to intravenous 0.9% sodium chloride and 500 mL intravenous drip twice per day. All patients were treated continuously for 2 wk to evaluate the curative effect.
Changes in the NIHSS score; activities of daily living (ADL) score; blood glucose index; blood lipid index; inflammatory factors; HMGB1, paraoxonolipase-1 (PON-1), and macrophage migration inhibitor (MIF) levels; erythrocyte sedimentation rate (ESR); platelet aggregation rate; and plasma viscosity that occurred in the two groups were recorded before and after treatment. The total effective rate and adverse reaction rate of the two groups were calculated.
Fasting venous blood was obtained before treatment and 2 wk after treatment and divided into five samples. One sample was used to test the blood glucose and blood lipids using a Hitachi 7600 automatic biochemical analyzer. One sample was centrifuged at a rotational speed of 3500 rpm for 10 min, and hs-CRP, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, HMGB1, PON-1, and MIF in the serum were assayed using an enzyme-linked immunosorbent assay (American Boteng Company ELX800 multi-function enzyme label instrument; Nanjing Jiancheng Bioengineering Research Institute). One sample was tested using the Wechsler method to determine the ESR. One sample was tested using the BS634 platelet aggregation instrument (Beijing Biochemical Instrument Factory) to assess the platelet aggregation rate. One sample was tested using the Zibo Hengtuo HT-100B hemorheology instrument to assess plasma viscosity.
The NIHSS score[7,8] was used to determine the curative effect. If the NIHSS score decreased by ≥ 90% after treatment, then the condition was considered cured. If the NIHSS score decreased between 45% and < 90% after treatment, then the condition was considered to have made significant progress. If the NIHSS score decreased between 18% and < 45% after treatment, then the condition was considered to have made progress. If the NIHSS score decreased < 18% or if it increased, then no change or deterioration in the condition was considered. The total effective rate was calculated by adding the basic cure rate, significant progress rate, and progress rate.
The higher the NIHSS score, the more serious the degree of neurological impairment. NIHSS scores < 7 indicate mild defects, NIHSS scores 7-15 indicate moderate defects, and NIHSS scores > 15 indicate severe defects[9,10].
The ADL score[11,12] is determined based on the ability to independently defecate, urinate, perform basic grooming, eat, transfer from sitting to standing position, dress, climb stairs, and bathe. The total possible score is 100.
SPSS version 19.0 was used to process and analyze the data collected in this study. The NIHSS score; ADL score; blood glucose index; blood lipid index; inflammatory factors; HMGB1, PON-1, and MIF levels; ESR; and other measurements were evaluated. First, a normal distribution test was performed. Then, the measurements that were normally distributed or approximately normally distributed were evaluated using the χ2 test. An independent sample t test was used for comparisons between groups, and a paired t test was used for comparisons within groups. Sex and the incidence of adverse reactions were expressed as percentages. The χ2 test was used for comparisons between groups, and statistical significance was considered when P value was < 0.05.
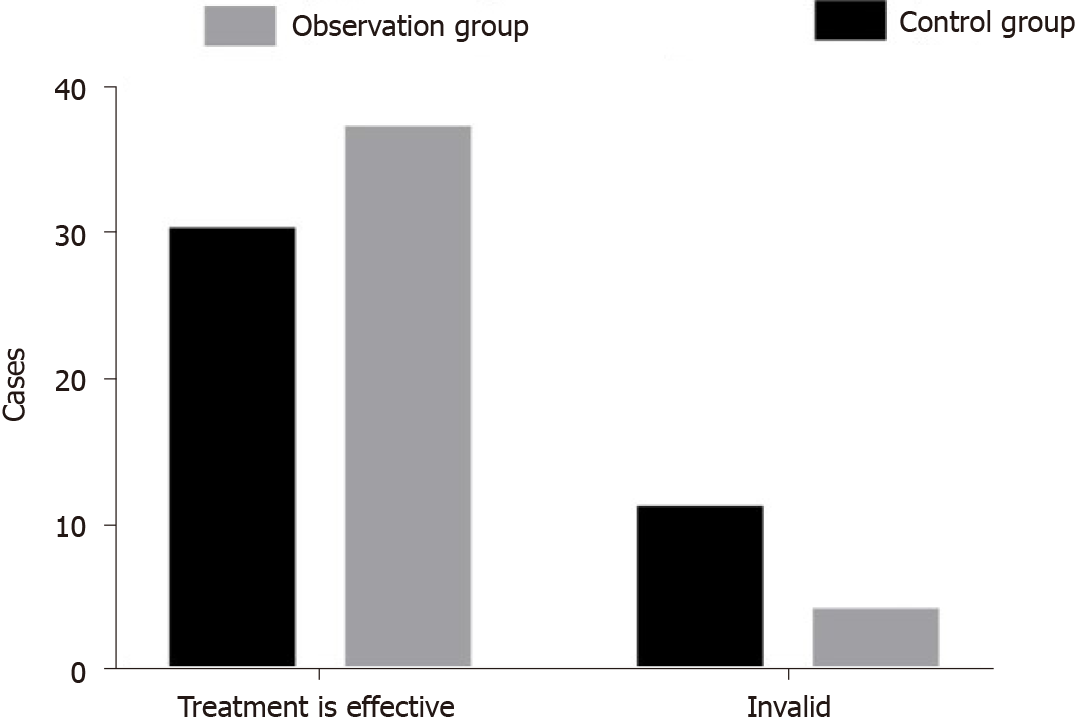
After treatment, of the 41 patients in the control group, 8 cases (19.51%), 14 cases (34.15%), 8 cases (19.51%), and 11 cases (26.83%) were considered cured, to have made significant progress, to have made progress, and to have experienced no change or deterioration, respectively. Of the 41 patients in the observation group, 13 cases (31.71%), 17 cases (41.46%), 7 cases (17.07%), and 4 cases (9.76%) were considered cured, to have made significant progress, to have made progress, and to have experienced no change or deterioration, respectively, after treatment. The total effective rate of the observation group (94.00%) was higher than that of the control group (80.00%), and this difference was statistically significant (χ2 = 3.998; P = 0.046) (Figure 1).
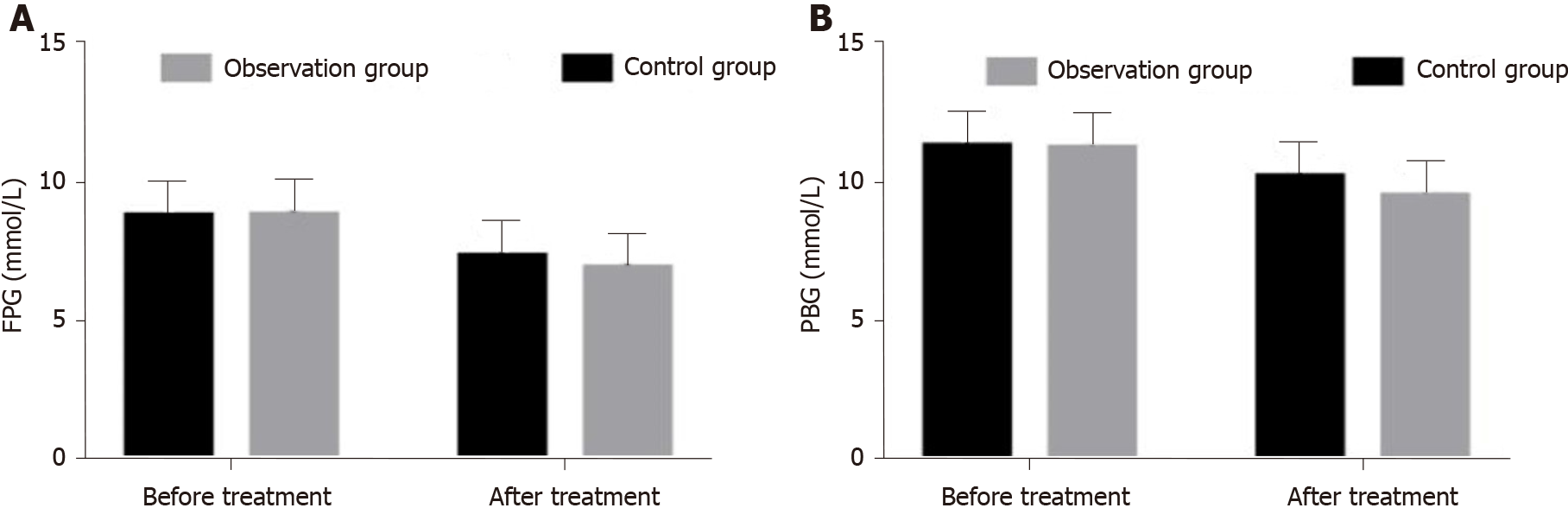
Before treatment, the mean fasting plasma glucose (FPG) and plasma blood glucose (PBG) of the observation group were 8.81 ± 1.27 mmol/L and 11.24 ± 1.27 mmol/L, respectively. The mean FPG and PBG of the control group were 8.78 ± 1.23 mmol/L and 11.32 ± 1.05 mmol/L, respectively. There was no significant difference between the groups (P > 0.05). After treatment, the mean FPG and PBG of the observation group were 6.91 ± 0.79 mmol/L and 9.53 ± 0.88 mmol/L, respectively; the mean FPG and PBG of the control group were 7.32 ± 0.96 mmol/L and 10.23 ± 1.07 mmol/L respectively. The FPG and PBG of the observation group were lower than those of the control group, and the difference was statistically significant (P < 0.05). The FPG and PBG of the two groups decreased significantly after treatment (P < 0.05) (Figure 2A and B).
The high-density cholesterol levels of the two groups increased after treatment; furthermore, that of the observation group was higher than that of the control group after treatment. Total cholesterol levels, triglyceride levels, low-density cholesterol levels, maximum platelet aggregation rates, and plasma viscosity were lower after treatment, and all these values were lower in the observation group than in the control group (P < 0.05) (Table 1).
| Group | Control group (n = 41) | Observation group (n = 41) | |
| TC (mmol/L) | Before treatment | 6.52 ± 0.47 | 6.40 ± 0.56 |
| After treatment | 5.17 ± 0.41a | 4.79 ± 0.32a,c | |
| TG (mmol/L) | Before treatment | 2.92 ± 0.41 | 2.89 ± 0.45 |
| After treatment | 2.05 ± 0.36 a | 1.68 ± 0.31a,c | |
| HDL-C (mmol/L) | Before treatment | 0.96 ± 0.31 | 0.95 ± 0.36 |
| After treatment | 1.67 ± 0.37a | 1.95 ± 0.42a,c | |
| LDL-C (mmol/L) | Before treatment | 4.25 ± 0.56 | 4.21 ± 0.53 |
| After treatment | 3.37 ± 0.45a | 2.89 ± 0.39a,c | |
| Maximum platelet aggregation rate(%) | Before treatment | 75.92 ± 9.64 | 78.40 ± 10.22 |
| After treatment | 46.48 ± 7.26a | 58.30 ± 7.74a,c | |
| Blood plasma viscosity (mPa·s) | Before treatment | 1.86 ± 0.48 | 1.94 ± 0.43 |
| After treatment | 1.40 ± 0.33a | 1.61 ± 0.35a,c | |
Before treatment, the mean NIHSS score and ADL score of the observation group were 14.96 ± 2.17 and 51.89 ± 7.54, respectively, and those of the control group were 15.23 ± 2.33 and 54.25 ± 6.36, respectively. There was no significant difference between the two groups (P > 0.05). After treatment, the NIHSS score and ADL score of the observation group were 8.79 ± 1.65 and 78.26 ± 9.22, respectively, and those of the control group were 10.23 ± 2.05 and 67.89 ± 7.98, respectively. This difference was statistically significant (P < 0.05). The ADL scores increased and NIHSS scores decreased after treatment in both groups, and the difference was statistically significant (P < 0.05).
The PON-1 Levels increased after treatment and were higher in the observation group than in the control group. Furthermore, the hs-CRP, IL-1β, TNF-α, HMGB1, and MIF levels and ESR decreased after treatment and were lower in the observation group than in the control group (P < 0.05) (Table 2).
| Group | Control group (n = 41) | Observation group (n = 41) | |
| hs-CRP (mg/L) | Before treatment | 10.63 ± 2.58 | 10.49 ± 2.44 |
| After treatment | 7.23 ± 1.96a | 5.48 ± 1.67a,c | |
| TNF-α (pg/mL) | Before treatment | 58.69 ± 6.32 | 59.02 ± 7.44 |
| After treatment | 46.58 ± 5.24a | 37.02 ± 4.63a,c | |
| HMGBl (µg/L) | Before treatment | 5.84 ± 0.56 | 5.81 ± 0.59 |
| After treatment | 3.23 ± 0.52a | 2.94 ± 0.43a,c | |
| PON-1 (U/L) | Before treatment | 135.23 ± 25.21 | 130.58 ± 22.17 |
| After treatment | 159.63 ± 25.74a | 174.25 ± 31.02a,c | |
| ESR (mm/h) | Before treatment | 32.15 ± 5.27 | 30.96 ± 6.42 |
| After treatment | 19.36 ± 3.78a | 13.02 ± 4.11a,c | |
| MIF (ng/mL) | Before treatment | 84.96 ± 5.87 | 86.02 ± 8.11 |
| After treatment | 76.36 ± 6.14a | 68.21 ± 5.47a,c | |
The rate of adverse reactions was 21.95% for the observation group and 17.07% for the control group (χ2 = 0.311; P = 0.577).
Diabetes can cause metabolic disorders that seriously affect the quality of life[13-15]. Abnormal lipid metabolism caused by diabetes leads to atherosclerotic plaque in blood vessels and may cause cerebral infarction if there is atherosclerotic plaque in cerebral vessels[16-18]. Gap infarction is the most common type of cerebral infarction in diabetic patients and is one of the main causes of death[19]. Lacunar cerebral infarction occurs in the deep part of the cerebral hemisphere or the small perforating artery of the brain stem[20]. With long-term hypertension, vascular wall lesions occur, resulting in lumen occlusion and the formation of cystic lesions 0.2-15 mm in diameter; this diameter is slightly larger than the vascular diameter, thereby causing embolism, which is common in the elderly and especially in diabetic patients. Lacunar cerebral infarction mainly occurs in the putamen, caudate nucleus, internal capsule, thalamus, and pons. Because of the limited range of arterial blood supply, occlusion of a single branch cause only a small area of ischemic necrosis of brain tissue and lacunar infarction[21]. Clinically, lacunar infarctions are more common in patients with type 2 diabetes, and the treatment effect is poor. Re-infarction or other major vascular complications are prone to occur. Therefore, early detection and prevention are particularly important[22].
Diabetes is an inflammatory disease[23]. The important pathological causes of lacunar cerebral infarction are small cerebral artery atherosclerosis and atherosclerosis, which is a chronic vascular inflammatory disease[24]. hs-CRP, IL-1β, TNF-α, and MIF are classical markers related to inflammation[25-32] and reflect the degree of inflammation in the body. When inflammation occurs in the body, serum levels of these markers increase. HMGB1 is a type of delayed inflammatory factor that can increase insulin resistance, lead to impaired glucose tolerance, promote tumor metastasis, and affect the blood-brain barrier permeability[33,34]. PON-1 is a calcium-dependent aromatic esterase that can hydrolyze lipid peroxides, protect low-density lipoprotein cholesterol from oxidative modification, and protect against cardiovascular and cerebrovascular diseases[35]. Lacunar infarctions often occur in the putamen, caudate nucleus, internal capsule, thalamus, pons, and other areas. Small perforator vessel wall lesions, stenosis, and occlusion form a small focus of infarction that only causes a small area of brain tissue damage and forms a “cavity” attributable to long-term hypertension and hyperlipidemia. Type 2 diabetes is an independent risk factor for cerebral infarction, and these two conditions often occur together. Common clinical manifestations include vertigo, limb numbness, and memory loss, resulting in a low degree of disability; however, these manifestations can occur repeatedly because the degree of neurological impairment associated with lacunar infarction is mild. During clinical treatment, attention should be focused on the regulation of lipids and improvement in microcirculation[36-39].
Actively administering symptomatic and supportive treatment is helpful for reducing the degree of disability. Statins can effectively regulate blood lipids and reduce the degree of vascular lesions[40,41]. Atorvastatin[42] selectively inhibits the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase and reduces total cholesterol and low-density lipoprotein cholesterol levels, thereby affecting the deformation and oxygen-carrying capacity of red blood cells, improving microcirculation, and promoting the recovery of neurological function. This drug has good lipid-regulating effects and pharmacological effects, such as protecting the vascular endothelium and antioxidative and anti-inflammatory properties[43-46].
Sodium ozagrel is an antiplatelet drug and thromboxane A inhibitor that can resist platelet aggregation, reduce blood viscosity, promote vasodilation to alleviate the blood hypercoagulable state, reduce thrombosis, and improve brain metabolism and microcirculation[46-49]. Ozagrel has an important role in the treatment of ischemic cerebrovascular disease and concomitant limb dyskinesia[47]. During this study, the total effective rate for patients treated with sodium ozagrel combined with atorvastatin was higher than that for patients treated with atorvastatin alone; also, the improvements in blood glucose and blood lipid indexes of patients treated with sodium ozagrel combined with atorvastatin were better than those of patients treated with atorvastatin alone[48]. Additionally, the ADL scores of patients treated with sodium ozagrel combined with atorvastatin were higher than those of patients treated with atorvastatin alone[49]. Moreover, the NIHSS scores of patients treated with sodium ozagrel combined with atorvastatin were lower than those of patients treated with atorvastatin alone. These results suggest that sodium ozagrel combined with statins may be better for controlling blood glucose and blood lipids, reducing the degree of nerve injury, and improving the self-care ability of patients with type 2 diabetes mellitus with lacunar cerebral infarction than treatment with statins alone. Ozagrel can selectively inhibit thromboxane synthase and prevent prostaglandin H2 from synthesizing thromboxane A2 to inhibit platelet aggregation and dilate blood vessels. As a result, ozagrel can increase the local perfusion in brain tissue and improve the abnormal energy metabolism caused by ischemia and hypoxia, thus reducing defects in neurological function. Blood hypercoagulability and high viscosity are risk factors for lacunar infarction. During this study, we assessed the platelet maximum aggregation rate and plasma viscosity and found that sodium ozagrel combined with atorvastatin for the treatment of type 2 diabetes mellitus with lacunar infarction can correct blood hypercoagulability and high viscosity and reduce the risk of lacunar infarction.
Inflammation is always present during the pathological process of type 2 diabetes and lacunar cerebral infarction. hs-CRP is a sensitive indicator of inflammation, and the hs-CRP serum level can reflect the degree of inflammation. Additionally, hs-CRP is associated with the severity of cardiovascular and cerebrovascular diseases. IL-1β and TNF-α are both classical proinflammatory factors that can not only directly cause tissue inflammatory damage but also expand the inflammatory response by promoting the release of other proinflammatory factors. HMGB1 is a highly conserved nuclear protein that has an important proinflammatory role in inflammation. ESR is a routine index associated with the active stage of inflammation that reflects the sedimentation rate of red blood cells[25]. MIF is a marker associated with inflammation that releases proteolytic enzymes under the actions of cytokines and growth factors, promotes atherosclerosis, and affects plaque stability. PON-1 is a calcium-dependent aromatic esterase that can hydrolyze lipid peroxides, protect low-density lipoprotein cholesterol from oxidative modification, reduce the level of oxidized low-density lipoprotein, reduce the uptake of oxidized low-density lipoprotein by macrophages, reduce the formation of foam cells, and exert protective effects on cerebrovascular vessels. The levels of hs-CRP, IL-1β, TNF-α, HMGB1, and MIF and ESR of patients treated with ozagrel combined with atorvastatin were lower than those of patients treated with atorvastatin alone. Moreover, their PON-1 Levels were lower than those of patients treated with atorvastatin alone. These results suggest that sodium ozagrel combined with statins is better for reducing inflammation and inhibiting atherosclerosis to treat type 2 diabetes mellitus with lacunar cerebral infarction than treatment with statins alone. This is because ozagrel can promote the conversion of prostaglandin H2 by endothelial cells to prostaglandin I2, regulate the balance of thromboxane A2 and prostaglandin I2, and reduce inflammation.
There was no significant difference in the rates of adverse reactions between groups. These results suggest that sodium ozagrel combined with statins does not increase the risk of adverse reactions. Type 2 diabetes mellitus with lacunar cerebral infarction is common in clinical settings. Although statins alone can alleviate the disease to a certain extent, they alone cannot achieve the ideal effect. The antiplatelet drug ozagrel was administered based on the routine treatment and lipid regulation of statins. Ozagrel is beneficial for regulating blood glucose and blood lipids, and reducing nerve injury. During this study, through the assessment of serum inflammatory indicators, it was clear that reducing inflammation and inhibiting atherosclerosis are important mechanisms for treating type 2 diabetes mellitus with lacunar cerebral infarction.
Although ozagrel has been used in combination with statins in previous clinical studies[50], most studies assessed only blood glucose or a single blood index. We assessed blood glucose, blood lipids, blood coagulation, ESR, NIHSS score, ADL score, inflammatory factors, and specific indicators, namely, HMGB1, PON-1, and MIF in a comprehensive analysis; we found that the combination of these two drugs has a good effect on blood glucose and blood lipids, reduces nerve injury, and reduces inflammation. Inhibition of atherosclerosis is an important mechanism for the treatment of type 2 diabetes mellitus complicated with lacunar infarction. Some limitations of our study should be recognized. These include the small sample size, the short follow-up time, and the lack of long-term curative effect observation. The results need to be verified with further larger scale studies and include other statins in combination with ozagrel.
Sodium ozagrel combined with atorvastatin for the treatment of type 2 diabetes mellitus with lacunar cerebral infarction can reduce inflammatory reactions and regulate the expression levels of HMGB1, PON-1, and MIF and ESR. Additionally, ozagrel can effectively control blood glucose and blood lipid indexes and reduce nerve injury, without increasing adverse reactions when compared to treatment with atorvastatin alone.
Type 2 diabetes is a common metabolic disease that is often complicated by abnormal lipid metabolism.
As an antiplatelet drug and thromboxane A inhibitor, sodium ozagrel is widely used to treat ischemic cerebrovascular diseases.
We want to observe the effects of sodium ozagrel combined with atorvastatin on high-mobility group protein B1 (HMGB1) and high-sensitivity-C reactive protein (hs-CRP) in patients with type 2 diabetes mellitus and lacunar infarction.
Eighty-two patients with type 2 diabetes mellitus and lacunar infarction treated were categorized into two groups according to the method of treatment (41 patients in each group).
After treatment, the blood glucose indexes; blood lipid indexes; inflammatory factors; HMGB1, paraoxonase-1, and macrophage migration inhibitory factor levels; erythrocyte sedimentation rate; platelet aggregation rate; and plasma viscosity of the observation group were better than those of the control group.
Sodium ozagrel with atorvastatin can reduce inflammatory reactions.
The results need to be verified with further larger scale studies and include other statins in combination with ozagrel.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Endocrinology and Metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chuang SM, Karniadakis GE, Papazafiropoulou A S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Brunton S. Pathophysiology of Type 2 Diabetes: The Evolution of Our Understanding. J Fam Pract. 2016;65. [PubMed] |
| 2. | Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14:491-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 3. | Kehler DS, Stammers AN, Susser SE, Hamm NC, Kimber DE, Hlynsky MW, Duhamel TA. Cardiovascular complications of type 2 diabetes in youth. Biochem Cell Biol. 2015;93:496-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Palitzsch D, Bührlen M. [Prevention of type 2 diabetes mellitus]. MMW Fortschr Med. 2012;154:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66:241-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 419] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 6. | Bösel J, Schönenberger S, Dohmen C, Jüttler E, Staykov D, Zweckberger K, Hacke W, Schwab S, Torbey MT, Huttner HB; stellvertretend für die Teilnehmer der „International Consensus Conference on Critical Care Management of Patients Following Large Hemispheric Infarct“ der NCS und DGNI; Neurocritical Care Society; German Society for Neurocritical and Emergency Medicine. [Intensive care therapy of space-occupying large hemispheric infarction. Summary of the NCS/DGNI guidelines]. Nervenarzt. 2015;86:1018-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Olivato S, Nizzoli S, Cavazzuti M, Casoni F, Nichelli PF, Zini A. e-NIHSS: an Expanded National Institutes of Health Stroke Scale Weighted for Anterior and Posterior Circulation Strokes. J Stroke Cerebrovasc Dis. 2016;25:2953-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Chen L, Geng L, Chen J, Yan Y, Yang L, Zhao J, Sun Q, He J, Bai L, Wang X. Effects of Urinary Kallidinogenase on NIHSS score, mRS score, and fasting glucose levels in acute ischemic stroke patients with abnormal glucose metabolism: A prospective cohort study. Medicine (Baltimore). 2019;98:e17008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Sartor EA, Albright K, Boehme AK, Morales MM, Shaban A, Grotta JC, Savitz SI, Martin-Schild S. The NIHSS Score and its Components can Predict Cortical Stroke. J Neurol Disord Stroke. 2013;2:1026. [PubMed] |
| 10. | Fischer U, Arnold M, Nedeltchev K, Brekenfeld C, Ballinari P, Remonda L, Schroth G, Mattle HP. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | van Dalen-Kok AH, Pieper MJC, de Waal MWM, van der Steen JT, Scherder EJA, Achterberg WP. The impact of pain on the course of ADL functioning in patients with dementia. Age Ageing. 2021;50:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Kato T, Kato Z, Kuratsubo I, Ota T, Orii T, Kondo N, Suzuki Y. Evaluation of ADL in patients with Hunter disease using FIM score. Brain Dev. 2007;29:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wang Z, Wang Z, Wang L, Qiu M, Wang Y, Hou X, Guo Z, Wang B. Hypertensive disorders during pregnancy and risk of type 2 diabetes in later life: a systematic review and meta-analysis. Endocrine. 2017;55:809-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Preventing type 2 diabetes. Nurs Stand. 2016;30:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Ackroyd S. Understanding type 2 diabetes. Nurs Stand. 2000;14:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z. Review: Relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol. 2018;44:347-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Huang TF, Tang ZP, Wang S, Hu MW, Zhan L, Yi Y, He YL, Cai ZY. Decrease in Serum Levels of Adiponectin and Increase in 8-OHdG: a Culprit for Cognitive Impairment in the Elderly Patients with Type 2 Diabetes. Curr Mol Med. 2019;20:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Tshikwela ML, Londa FB, Tongo SY. Stroke subtypes and factors associated with ischemic stroke in Kinshasa, Central Africa. Afr Health Sci. 2015;15:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Martins-Marques T, Catarino S, Gonçalves A, Miranda-Silva D, Gonçalves L, Antunes P, Coutinho G, Leite Moreira A, Falcão Pires I, Girão H. EHD1 Modulates Cx43 Gap Junction Remodeling Associated With Cardiac Diseases. Circ Res. 2020;126:e97-e113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Naess H, Thomassen L, Waje-Andreassen U, Glad S, Kvistad CE. High risk of early neurological worsening of lacunar infarction. Acta Neurol Scand. 2019;139:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Al-Mrabeh A. β-Cell Dysfunction, Hepatic Lipid Metabolism, and Cardiovascular Health in Type 2 Diabetes: New Directions of Research and Novel Therapeutic Strategies. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Shah MS, Brownlee M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ Res. 2016;118:1808-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 429] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 23. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 24. | Lv P, Zhao M, Liu Y, Jin H, Cui W, Fan C, Teng Y, Zheng L, Huang Y. Apolipoprotein C-III in the high-density lipoprotein proteome of cerebral lacunar infarction patients impairs its anti-inflammatory function. Int J Mol Med. 2018;41:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Stout RW. The relationship of abnormal circulating insulin levels to atherosclerosis. Atherosclerosis. 1977;27:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Ali AH, Younis N, Abdallah R, Shaer F, Dakroub A, Ayoub MA, Iratni R, Yassine HM, Zibara K, Orekhov A, El-Yazbi AF, Eid AH. Lipid-Lowering Therapies for Atherosclerosis: Statins, Fibrates, Ezetimibe and PCSK9 Monoclonal Antibodies. Curr Med Chem. 2021;28:7427-7445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Takeuchi S, Takahashi Y, Asai S. Comparison of pleiotropic effects of statins vs fibrates on laboratory parameters in patients with dyslipidemia: A retrospective cohort study. Medicine (Baltimore). 2020;99:e23427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Murphy C, Deplazes E, Cranfield CG, Garcia A. The Role of Structure and Biophysical Properties in the Pleiotropic Effects of Statins. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Rached F, Santos RD. The Role of Statins in Current Guidelines. Curr Atheroscler Rep. 2020;22:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Ferri N, Corsini A. Clinical Pharmacology of Statins: an Update. Curr Atheroscler Rep. 2020;22:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Henriksbo BD, Tamrakar AK, Phulka JS, Barra NG, Schertzer JD. Statins activate the NLRP3 inflammasome and impair insulin signaling via p38 and mTOR. Am J Physiol Endocrinol Metab. 2020;319:E110-E116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Sato K, Morofuji Y, Horie N, Izumo T, Anda T, Matsuo T. Hyperhomocysteinemia Causes Severe Intraoperative Thrombotic Tendency in Superficial Temporal Artery-middle Cerebral Artery Bypass. J Stroke Cerebrovasc Dis. 2020;29:104633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Narayan V, Shukla D, Bhat DI, Prabhuraj AR, Devi BI. Ozagrel for Postoperative Management of Aneurysmal Subarachnoid Hemorrhages. Neurol India. 2019;67:1286-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Oguro H, Mitaki S, Takayoshi H, Abe S, Onoda K, Yamaguchi S. Retrospective Analysis of Argatroban in 353 Patients with Acute Noncardioembolic Stroke. J Stroke Cerebrovasc Dis. 2018;27:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Yoshida J, Kubo Y, Yoshida K, Chida K, Kobayashi M, Ogasawara K. [Development of Intracerebral Hemorrhage and Subarachnoid Hemorrhage Shortly after Cerebral Infarction Onset in an Adult Patient with Moyamoya Disease]. No Shinkei Geka. 2017;45:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Bhatia P, Kaur G, Singh N. Ozagrel a thromboxane A2 synthase inhibitor extenuates endothelial dysfunction, oxidative stress and neuroinflammation in rat model of bilateral common carotid artery occlusion induced vascular dementia. Vascul Pharmacol. 2021;137:106827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Zhang J, Yang J, Chang X, Zhang C, Zhou H, Liu M. Ozagrel for acute ischemic stroke: a meta-analysis of data from randomized controlled trials. Neurol Res. 2012;34:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Koumura A, Hamanaka J, Kawasaki K, Tsuruma K, Shimazawa M, Hozumi I, Inuzuka T, Hara H. Fasudil and ozagrel in combination show neuroprotective effects on cerebral infarction after murine middle cerebral artery occlusion. J Pharmacol Exp Ther. 2011;338:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Park SI, Jang DK, Han YM, Sunwoo YY, Park MS, Chung YA, Maeng LS, Im R, Kim MW, Jeun SS, Jang KS. Effect of combination therapy with sodium ozagrel and panax ginseng on transient cerebral ischemia model in rats. J Biomed Biotechnol. 2010;2010:893401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Shinohara Y, Saito I, Kobayashi S, Uchiyama S. Edaravone (radical scavenger) versus sodium ozagrel (antiplatelet agent) in acute noncardioembolic ischemic stroke (EDO trial). Cerebrovasc Dis. 2009;27:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Wallemacq C. [Statins and new-onset diabetes : benefit-risk balance]. Rev Med Suisse. 2019;15:1454-1457. [PubMed] |
| 42. | Kogawa AC, Pires AEDT, Salgado HRN. Atorvastatin: A Review of Analytical Methods for Pharmaceutical Quality Control and Monitoring. J AOAC Int. 2019;102:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Taniguti EH, Ferreira YS, Stupp IJV, Fraga-Junior EB, Doneda DL, Lopes L, Rios-Santos F, Lima E, Buss ZS, Viola GG, Vandresen-Filho S. Atorvastatin prevents lipopolysaccharide-induced depressive-like behaviour in mice. Brain Res Bull. 2019;146:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 44. | Jiang R, Zhao S, Wang R, Feng H, Zhang J, Li X, Mao Y, Yuan X, Fei Z, Zhao Y, Yu X, Poon WS, Zhu X, Liu N, Kang D, Sun T, Jiao B, Liu X, Yu R, Gao G, Hao J, Su N, Yin G, Lu Y, Wei J, Hu J, Hu R, Li J, Wang D, Wei H, Tian Y, Lei P, Dong JF. Safety and Efficacy of Atorvastatin for Chronic Subdural Hematoma in Chinese Patients: A Randomized ClinicalTrial. JAMA Neurol. 2018;75:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 45. | Li S, Yu Y, Jin Z, Dai Y, Lin H, Jiao Z, Ma G, Cai W, Han B, Xiang X. Prediction of pharmacokinetic drug-drug interactions causing atorvastatin-induced rhabdomyolysis using physiologically based pharmacokinetic modelling. Biomed Pharmacother. 2019;119:109416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Kitagawa Y. [Sodium ozagrel]. Nihon Rinsho. 2006;64 Suppl 7:554-561. [PubMed] |
| 47. | Oishi M, Mochizuki Y, Hara M, Yoshihashi H, Takasu T. Effects of sodium ozagrel on hemostatic markers and cerebral blood flow in lacunar infarction. Clin Neuropharmacol. 1996;19:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Tomishima Y, Ishitsuka Y, Matsunaga N, Nagatome M, Furusho H, Irikura M, Ohdo S, Irie T. Ozagrel hydrochloride, a selective thromboxane A₂ synthase inhibitor, alleviates liver injury induced by acetaminophen overdose in mice. BMC Gastroenterol. 2013;13:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Takabatake Y, Uno E, Wakamatsu K, Yamazaki N, Hashimoto N, Tsuchiya Y. [The clinical effect of combination therapy with edaravone and sodium ozagrel for acute cerebral infarction]. No To Shinkei. 2003;55:589-593. [PubMed] |
| 50. | Ido K, Kurogi R, Kurogi A, Nishimura K, Arimura K, Nishimura A, Ren N, Kada A, Matsuo R, Onozuka D, Hagihara A, Takagishi S, Yamagami K, Takegami M, Nohara Y, Nakashima N, Kamouchi M, Date I, Kitazono T, Iihara K; J-ASPECT Study Collaborators. Effect of treatment modality and cerebral vasospasm agent on patient outcomes after aneurysmal subarachnoid hemorrhage in the elderly aged 75 years and older. PLoS One. 2020;15:e0230953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |