Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.2087
Peer-review started: February 3, 2021
First decision: August 19, 2021
Revised: September 1, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: December 15, 2021
Processing time: 316 Days and 1.3 Hours
Omarigliptin is one of several once-weekly dipeptidyl peptidase-4 inhibitors (DPP-4is). Despite the high frequency of switching from various daily DPP-4is to omarigliptin in actual clinical practice, data regarding its efficacy in patients with type 2 diabetes (T2D) after switching are limited.
To analyze the efficacy of omarigliptin in Japanese patients with T2D who had previously received treatment with other glucose-lowering agents.
Forty-nine T2D patients treated for the first time with omarigliptin were recruited retrospectively and divided into four groups defined as either add-on or switched from daily DPP-4is: switched from linagliptin, switched from sitagliptin, and switched from vildagliptin. During a 3-mo follow-up, the clinical parameters among these groups were assessed and compared, with the impact of the switch on glycemic variability as measured by continuous glucose monitoring also being evaluated in the switched groups.
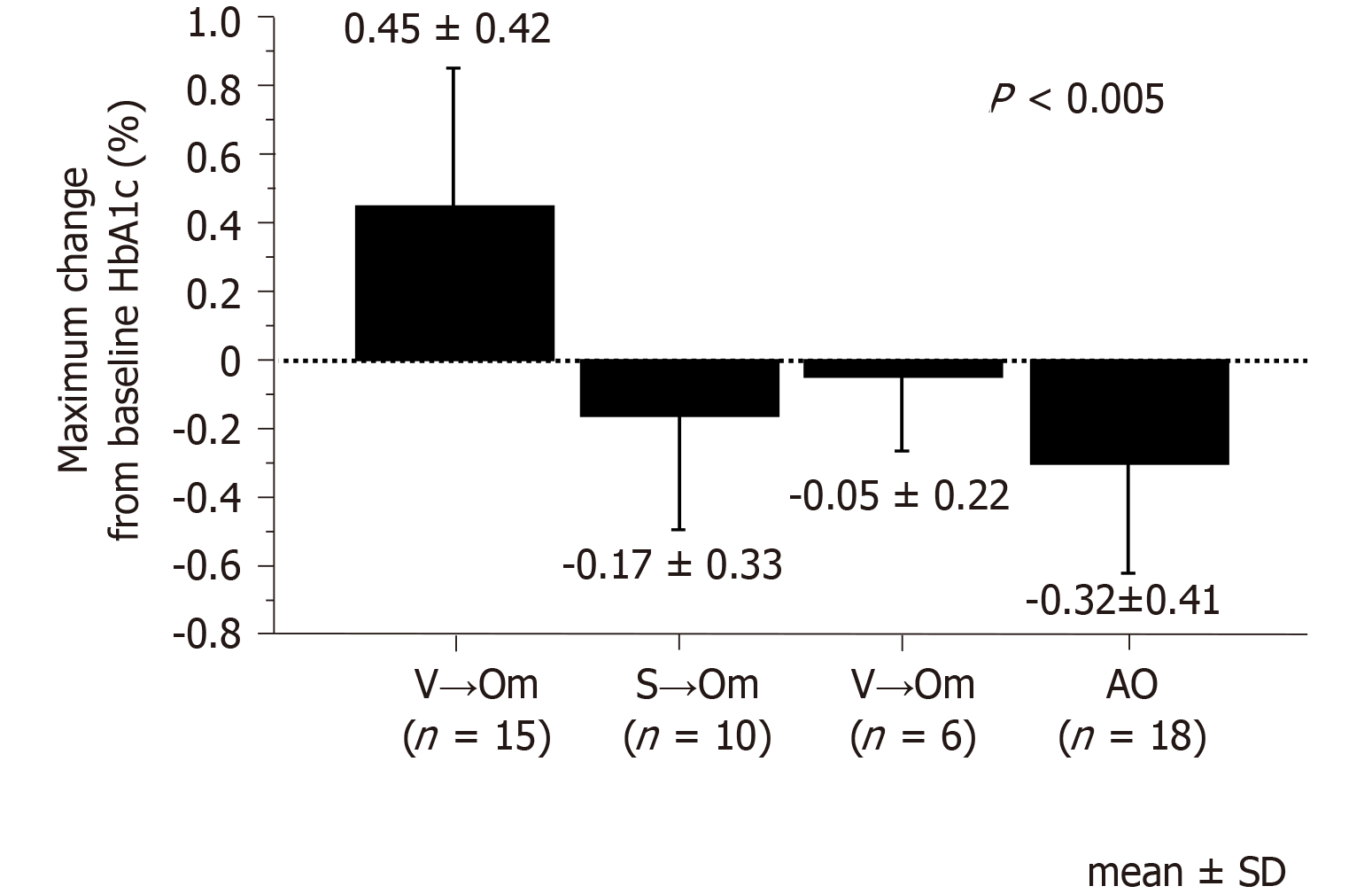
Hemoglobin A1c levels saw a significant decrease of -0.32% ± 0.41% in the add-on group (P = 0.002). However, the other groups’ variables depended on the pre-switch daily DPP-4i: switched from linagliptin, -0.05% ± 0.22%; switched from sitagliptin, -0.17% ± 0.33%; and switched from vildagliptin, 0.45% ± 0.42%, which saw significant worsening (P = 0.0007). Multivariate logistic regression analysis revealed that switching from vildagliptin to omarigliptin was independently associated with worsening glycemic control (P = 0.0013). The mean and standard deviation of sensor glucose value, the mean amplitude of glycemic excursions, and the mean of daily difference significantly improved when switching the patient from either linagliptin or sitagliptin to omarigliptin. However, in patients switched from vildagliptin, not only did the glucose variability indices see no improvements, the mean of daily difference even underwent significant worsening.
Administering omarigliptin as add-on therapy or switching to it from sitagliptin and linagliptin, but not vildagliptin, improves glycemic control and thus should help in decision making when selecting DPP-4is for T2D patients.
Core Tip: This paper reported on the efficacy of omarigliptin in Japanese patients with type 2 diabetes who had previously received treatment with other glucose-lowering agents. The present study demonstrated that administering omarigliptin as add-on therapy or switching to it from sitagliptin and linagliptin, but not vildagliptin, provides more effective glycemic control. Ultimately, these findings should help decision-making in the actual clinical setting when selecting and using dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes.
- Citation: Kawasaki E, Nakano Y, Fukuyama T, Uchida A, Sagara Y, Tamai H, Tojikubo M, Hiromatsu Y, Koga N. Efficacy of omarigliptin, once-weekly dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes. World J Diabetes 2021; 12(12): 2087-2095
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/2087.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.2087
Type 2 diabetes (T2D) is a chronic metabolic disease characterized by hyperglycemia, predominantly associated with varying degrees of β-cell dysfunction, and insulin resistance. Although medical nutrition therapy and exercise are central to managing T2D, many patients require pharmacological treatment to achieve their glycemic targets. Over the past decade, we have witnessed a rapid development in antidiabetic agents, including, but not limited to, once-weekly medications. Among a range of currently available oral hypoglycemic agents, the most frequently prescribed in Japan belong to the dipeptidyl peptidase-4 inhibitors (DPP-4is) class of medication, with more than 70% of patients with oral hypoglycemic agents receiving DPP-4is[1].
Recently, several once-weekly DPP-4is, which may improve patient adherence to a medication regimen due to a lower medication burden, received approval in Japan. Omarigliptin is one such once-weekly DPP-4i, whose non-inferiority in efficacy and safety as add-on therapy to glucose-lowering agents has been demonstrated in comparison with daily DPP-4is[2]. However, despite the high frequency of switching from daily DPP-4is to omarigliptin in actual clinical practice, data regarding its efficacy in patients with T2D after switching are limited. Therefore, we carried out the present retrospective study in real-world practice to explore the efficacy of omarigliptin as add-on therapy to other oral hypoglycemic agents or in switching to omarigliptin from daily DPP-4is in patients with T2D. Additionally, we examined the impact of the switch from daily DPP-4is to omarigliptin on glycemic variability using a continuous glucose monitoring (CGM) devise.
Forty-nine T2D patients who received treatment with omarigliptin for the first time, either with or without other hypoglycemic agents for a course lasting at least 3 mo, were retrospectively recruited. The study consisted of 35 males and 14 females, with the mean age at examination and mean duration of diabetes being 68.2 ± 11.9 and 12.4 ± 7.8 years, respectively. All patients received omarigliptin 25 mg on an outpatient basis and had no changes made to their diabetes treatment (e.g., medical nutrition therapy, exercise, medication) for at least 3 mo after being administered omarigliptin. Patients were divided into four groups according to add-on to other glucose-lowering agents or switched from daily DPP-4is: add-on (AO, n = 18), switched from linagliptin 5 mg (L→Om, n = 6), switched from sitagliptin 50 mg (S→Om, n = 10), and switched from vildagliptin 100 mg (V→Om, n = 15). The clinical characteristics of these groups are described in Table 1.
| AO (n = 18) | L→Om (n = 6) | S→Om (n = 10) | V→Om (n = 15) | |
| Male : Female | 13 : 5 | 4 : 2 | 5 : 5 | 13 : 2 |
| Age (yr) | 66.8 ± 12.1 | 75.2 ± 9.0 | 63.6 ± 15.7 | 70.1 ± 8.7 |
| Duration (yr) | 12.0 ± 10.1 | 15.5 ± 10.7 | 11.2 ± 5.6 | 12.9 ± 4.8 |
| BMI (kg/m2) | 24.1 ± 3.3 | 24.9 ± 1.6 | 23.5 ± 2.4 | 23.7 ± 3.4 |
| eGFR (mL/min/1.73 m2) | 63.6 ± 19.5 | 45.0 ± 22.2a | 76.1 ± 17.5 | 63.3 ± 15.7 |
| HbA1c (%) | 7.48 ± 1.28 | 6.33 ± 0.79 | 6.88 ± 0.37 | 7.14 ± 0.66 |
| Metformin use | 8 (44%) | 1 (17%) | 4 (40%) | 6 (40%) |
| Insulin secretagogues1 use | 5 (28%) | 0 (0%) | 4 (40%) | 8 (53%) |
| Insulin use | 2 (11%) | 0 (0%) | 1 (10%) | 3 (20%) |
To analyze the effect switching from daily DPP-4is to omarigliptin has on glucose variability, an additional ten outpatients with T2D treated with daily DPP-4is (sitagliptin, linagliptin, and vildagliptin) for a course greater than 3 mo were enrolled in the CGM study. These additional subjects consisted of 6 males and 4 females, with the mean age at examination, mean duration of diabetes, and mean hemoglobin A1c (HbA1c) being 66.1 ± 10.6 years, 13.2 ± 7.1 years, and 7.0% ± 0.8 %, respectively. Of these additional patients, 3 had been treated with sitagliptin 50 mg, 2 with linagliptin 5 mg, and 5 with vildagliptin 100 mg. All patients received a written or verbal explanation of the study before providing informed consent. This study’s protocol has been approved by the ethics committee of Shin-Koga Hospital.
In evaluating the efficacy of omarigliptin, clinical and laboratory data were collected through a review of electronic medical records at baseline and after 1, 2, and 3 mo of omarigliptin treatment. The study’s primary objective was to evaluate the efficacy of omarigliptin as add-on to or switched from daily DPP-4is (sitagliptin, linagliptin, or vildagliptin) over 3 mo by assessing the change in HbA1c from baseline. In one analysis, we divided the patients into three groups: improved (more than 0.3% decrease in HbA1c), worsened (more than 0.3% increase in HbA1c), and stable (-0.3% < change in HbA1c < 0.3%) and compared the clinical characteristics. Additionally, we examined the parameters affecting the therapeutic response to omarigliptin in the patients who were switched from daily DPP-4is.
The impact of switching from daily DPP-4is to omarigliptin on glycemic variability as measured by CGM, FreeStyle Libre Pro™ (Abbott Diabetes Care, Alameda, CA, United States) was also assessed in the additional 10 T2D patients treated with daily DPP-4is (sitagliptin, linagliptin, or vildagliptin). On day 1, using a self-adhesive pad, the FreeStyle Libre Pro™ was placed on the back of the patient’s upper arm and worn for 14 d. From days 1 to 7, all ongoing diabetes treatments using daily DPP-4is were maintained. On day 8, while still being assessed by CGM, daily DPP-4is were replaced with 25 mg of omarigliptin and administered once weekly.
Since sensor glucose values as determined by FreeStyle Libre Pro™ from days 2 to 14 have been reported to be comparable in accuracy to self-monitoring blood glucose devises in obtaining capillary blood glucose levels[3], the FreeStyle Libre Pro™ was used to collect and analyze the variability of daily DPP-4is data from days 2 to 7. Data were also collected to analyze the glucose variability of omarigliptin; however, to eliminate any residual effects of daily DPP-4is, the data was only taken from days 9 to 14. We then compared the mean sensor glucose levels, standard deviation (SD) of glycemic variability, mean amplitude of glycemic excursion (MAGE), and the mean of daily difference (MODD) for each period using the Glycemic Variability Analyzer Program in MATLAB (MathWorks, Natick, MA, United States).
Data are presented as a mean ± SD and as n (%) for frequencies unless otherwise specified. Where appropriate the prevalence was compared using the 2 test or Fisher’s exact test, and differences in nonparametric data were tested using the Mann-Whitney U test or Kruskal-Wallis test. A comparison of different time points within the same group was made using Friedman’s analysis of variance test for repeated measures, and multiple logistic regression analysis was used to determine the parameters affecting the therapeutic response to omarigliptin. Additionally, changes in glycemic variability parameters before and after administering omarigliptin were analyzed using the Wilcoxon signed-rank test within the groups, with P values of less than 0.05 being considered statistically significant. Statistical analysis for this study was performed using StatView statistical software (version 5.0, SAS Institute, Cary, NC, United States).
No significant difference was observed among the four groups (AO, L→Om, S→Om, and V→Om) relating to sex, age at examination, duration of diabetes, body mass index, or HbA1c (Table 1). Furthermore, there were no significant differences regarding the frequency of metformin, insulin secretagogues, or insulin use among the four groups. However, the mean estimated glomerular filtration rate at baseline in the L→Om group was significantly lower than the S→Om group due to the tendency for linagliptin to be used in patients with renal impairment since the elimination of this DPP-4i is primarily through non-renal routes.
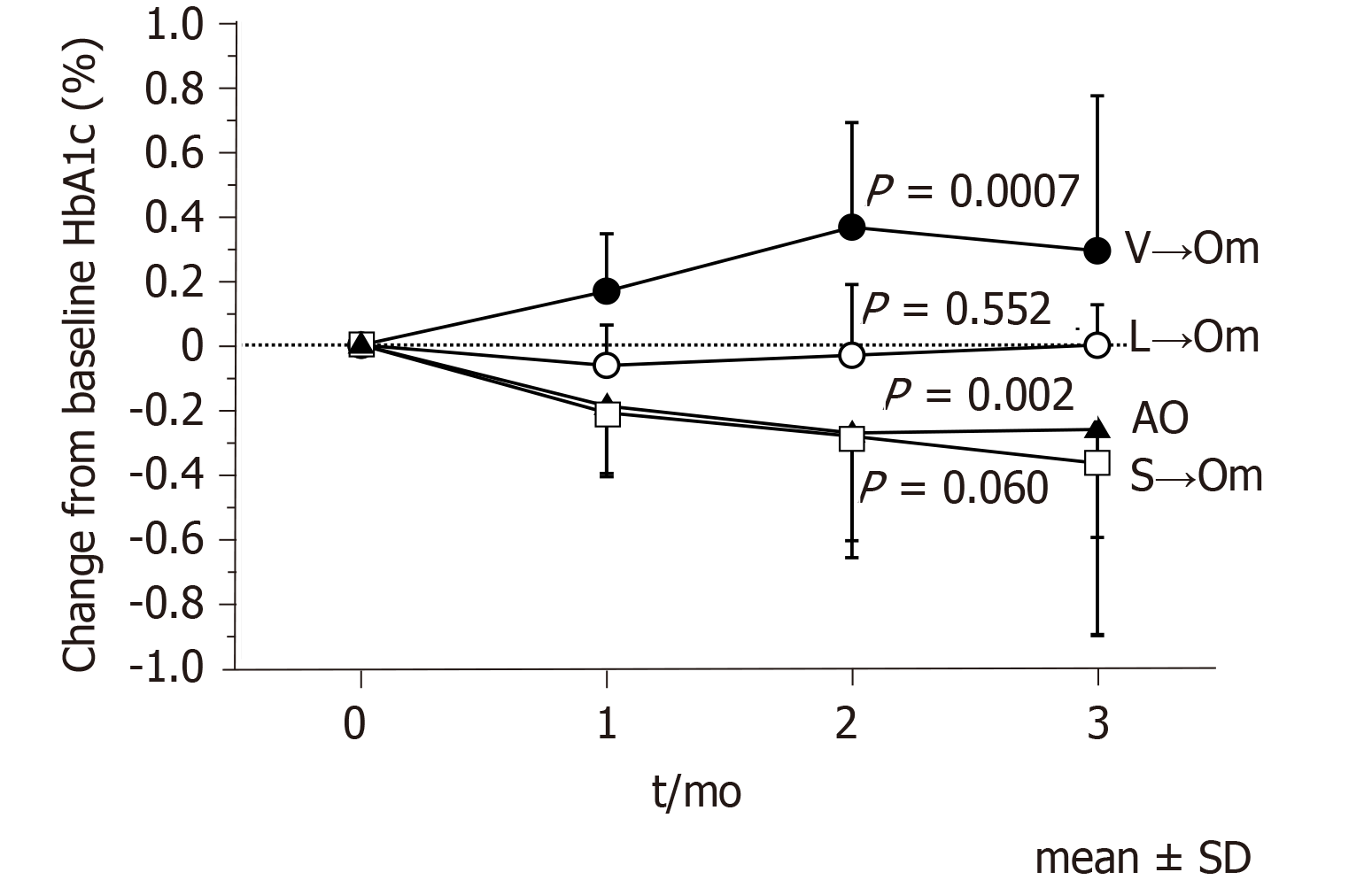
As shown in Figure 1, HbA1c levels improved significantly in the AO group (P = 0.002), with the maximum change from baseline after administering omarigliptin being -0.32% ± 0.41%. At the same time, however, there was some variability in the other three groups depending on the pre-switch daily DPP-4is; -0.05% ± 0.22% in the L→Om group, -0.17% ± 0.33% in the S→Om group, and 0.45% ± 0.42% in the V→Om group, which saw significant worsening (P = 0.0007). The change in HbA1c levels among the four groups revealed a statistically significant difference (Figure 2).
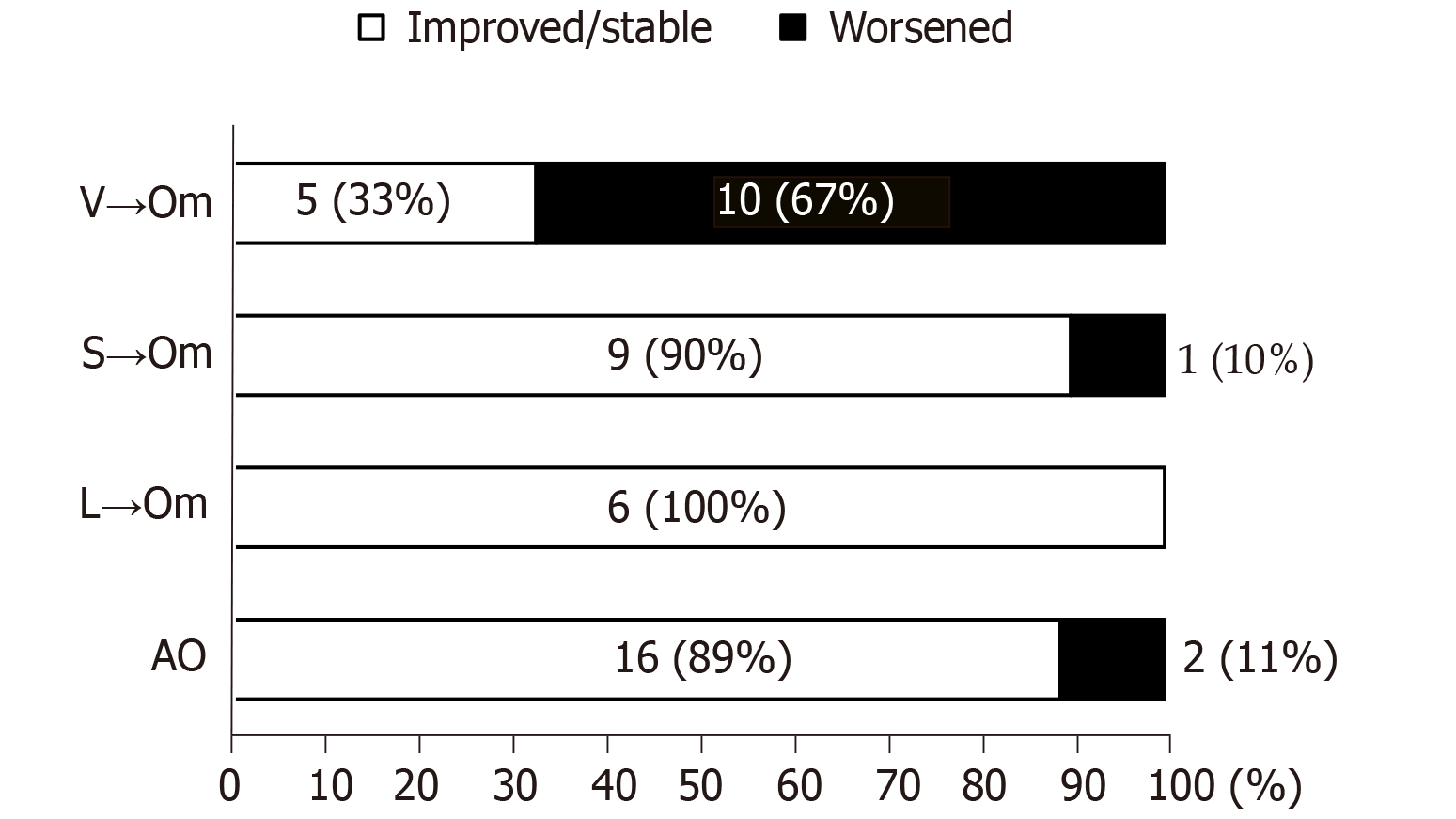
Three months after being administered omarigliptin, 13 patients (26.5%) showed an increase in HbA1c levels of more than 0.3% (worsened group). On the other hand, 36 patients (73.5%) revealed either no change or decreased HbA1c levels compared with the baseline value (improved/stable group). Of note, 10 of the 13 patients (76.9%) in the worsened group belonged to the V→Om group (Figure 3), indicating that vildagliptin may be more effective than omarigliptin concerning glycemic control.
The varying clinical characteristics of the improved/stable and worsened glycemic-control patients after switching from vildagliptin to omarigliptin are shown in Table 2. There was no significant difference in sex, age at examination, duration of diabetes, body mass index, HbA1c, estimated glomerular filtration rate, or the frequency of metformin, insulin secretagogues, and insulin use between the two groups (Table 2). Furthermore, multivariate logistic regression analysis revealed that switching from vildagliptin to omarigliptin was independently associated with worsening glycemic control (P = 0.0013, Table 3).
| Improved/stable (n = 5) | Worsened (n = 10) | P value | |
| Male : Female | 4 : 1 | 9 : 1 | NS |
| Age (yr) | 67.8 ± 10.2 | 71.2 ± 8.1 | NS |
| Duration (yr) | 9.8 ± 4.1 | 14.5 ± 4.5 | NS |
| BMI (kg/m2) | 25.1 ± 4.3 | 23.0 ± 2.8 | NS |
| eGFR (mL/min/1.73 m2) | 71.2 ± 19.2 | 59.4 ± 12.9 | NS |
| HbA1c (%) | 7.48 ± 0.97 | 6.97 ± 0.41 | NS |
| Metformin use | 2 (40%) | 4 (40%) | NS |
| Insulin secretagogues1 use | 3 (60%) | 5 (50%) | NS |
| Insulin use | 2 (40%) | 1 (10%) | NS |
| Odds ratio | 95%CI | P value | |
| Sex (male) | 0.837 | 0.03-24.30 | NS |
| Age | 1.004 | 0.82-1.23 | NS |
| Insulin secretagogues1 use | 0.575 | 0.04-7.72 | NS |
| Insulin use | 0.038 | 0.001-1.460 | NS |
| BMI at omarigliptin administration | 0.757 | 0.45-1.26 | NS |
| eGFR at omarigliptin administration | 0.941 | 0.84-1.06 | NS |
| HbA1c at omarigliptin administration | 5.862 | 0.86-39.80 | NS |
| Switching from vildagliptin | 146.62 | 7.00-3072.60 | 0.0013 |
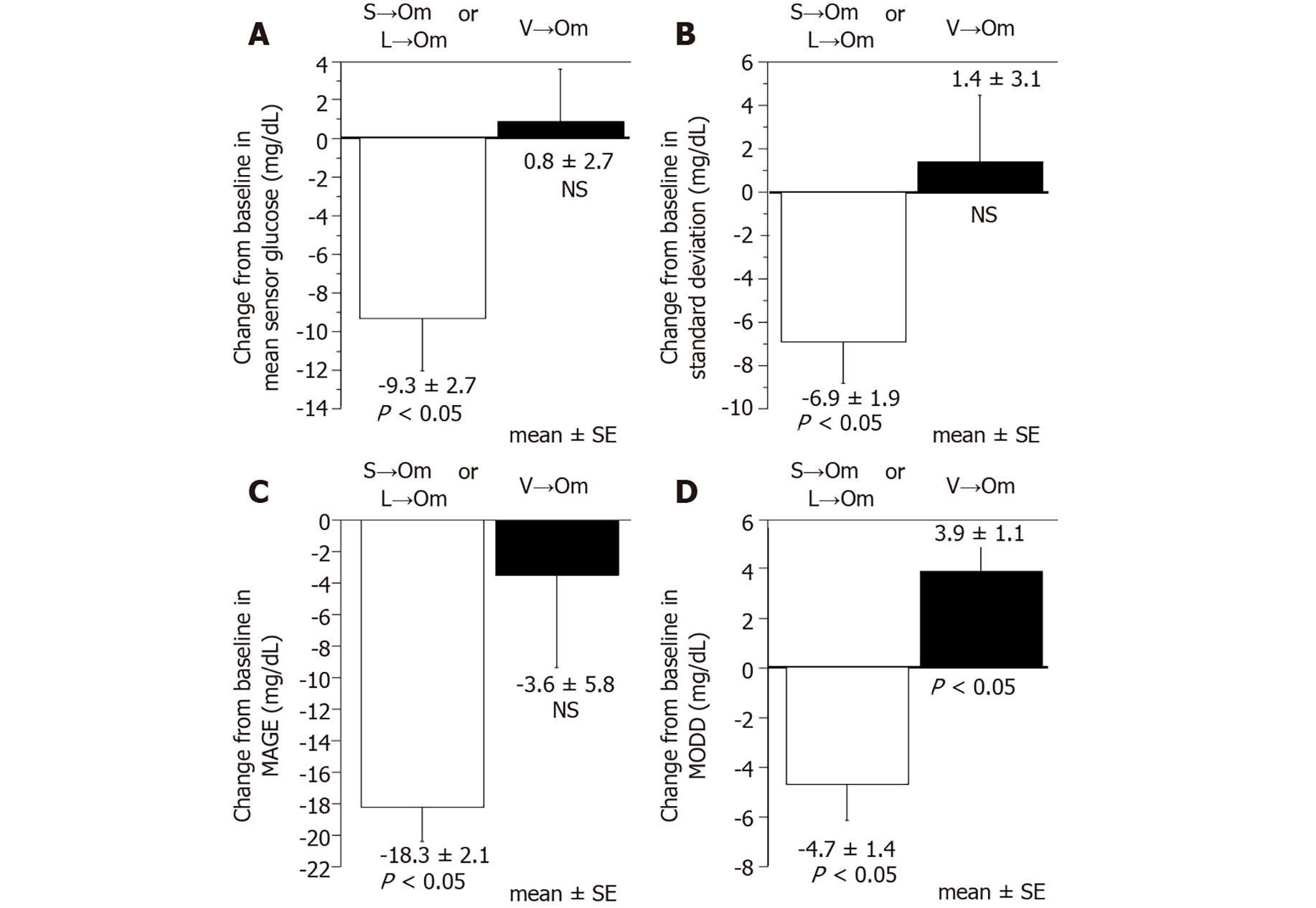
In determining the efficacy in glucose variability after switching from daily DPP-4is to omarigliptin, CGM analyses were performed in the 10 additional subjects who had been treated with either sitagliptin 50mg (n = 3), linagliptin 5mg (n = 2), or vildagliptin 100mg (n = 5). As shown in Figure 4, both the mean and SD of the sensor glucose value, MAGE, and MODD significantly improved when patients were switched from either linagliptin or sitagliptin to omarigliptin. However, except in the case of MODD, which worsened significantly after switching, no other significant changes were observed in any of the glucose variability indices when patients were switched from vildagliptin to omarigliptin.
In the present study, we demonstrate that: (1) Treatment using omarigliptin as add-on to other glucose-lowering agents decreased HbA1c levels; (2) There was a distinct drug effect regarding the efficacy of omarigliptin when switching from daily DPP-4is; and (3) Vildagliptin was more effective than omarigliptin for glycemic control.
DPP-4is that ameliorate β-cell dysfunction with low hypoglycemic risk are now widely used in the glycemic control of patients with T2D and are rapidly becoming a first-line antidiabetic drug in Japan. Moreover, recent technological advances have enhanced existing drugs and enabled prolonged actions such as in once-weekly DPP-4is. Due to the lower medication burden of once-weekly DPP-4is, there may also be improved patient adherence and satisfaction to these medication regimens[4]. In recent publications, omarigliptin, one such once-weekly DPP-4i medication, has been determined to be safe and effective as monotherapy or add-on therapy to other glucose-lowering agents[2,5-9]. Furthermore, reports have shown that T2D patients with non-alcoholic related fatty livers or non-alcoholic steatohepatitis are positively affected by omarigliptin through improved insulin resistance and reduced inflammation[10].
As current drug adherence rates among diabetic patients following a daily medication regimen are reported to be less than 70%[11], it is conceivable that switching from daily DPP-4is to once-weekly DPP-4is may lead to better glycemic control. However, data regarding the efficacy of omarigliptin after switching from various daily DPP4is in patients with T2D are still limited. While the present study is not definitive and did not reach statistical significance (P = 0.06), it demonstrates a decrease from baseline in mean HbA1c after switching patients from sitagliptin to omarigliptin exists and sets a precedent for future studies. Additionally, the mean and SD of the sensor glucose value, and the value of MAGE and MODD significantly improved when either linagliptin or sitagliptin was switched to omarigliptin (Figure 4). In contrast, switching from vildagliptin to omarigliptin resulted in significantly aggravated glycemic controls (P = 0.0007), no improvement in the glucose variability indices, and even significantly worsened MODD (Figure 4). These results indicate that vildagliptin may be more effective than omarigliptin concerning glycemic control.
DPP-4is possess distinct chemical structures categorized into three binding patterns (classes 1, 2, 3) based on the inhibitor binding subsites known as the S1, S2, S1’, S2’, and S2 extensive subsites[12-14]. The binding patterns of the DPP-4is used in this study are as follows: (1) Vildagliptin binds to S1 and S2 subsites (class 1); (2) Linagliptin binds to S1 and S2 as well as S1’ and/or S2’ subsites (class 2); while (3) Sitagliptin and omarigliptin bind to the S1, S2, and S2 extensive subsites (class 3)[15]. According to the previous study, the increased inhibitory activity of DPP-4is on DPP-4 tends to correlate with an increased number of binding subsites[13]. Furthermore, a recent meta-analysis revealed that the factor that explains most of the variance in HbA1 was baseline HbA1c levels: higher baseline HbA1c levels were associated with the greater fall in HbA1c seen after administering various DPP-4is[16]. However, contrary to this, the present study showed that the HbA1c levels increased after switching from class 1 DPP-4i (vildagliptin) with fewer binding subsites to class 3 DPP-4i (omarigliptin) with multiple binding subsites and that baseline HbA1c was similar among the V→Om, L→Om, and S→Om groups. Similar results published by other research groups have shown that switching from class 1 to class 3 DPP-4i worsened HbA1c levels by 0.33% in patients with T2D[17]. These results suggest the estimated reduction in the HbA1c levels does not correlate with the inhibitory activity of each DPP-4i and alludes to the number of binding subsites utilized by the various DPP-4is being a factor in determining selectivity between DPP-4 and other related enzymes.
This study has several limitations to report. To begin with, the number of patients in each study group was relatively small, and it was carried out retrospectively in the actual clinical setting and did not include a control group. Furthermore, we were unable to include some daily DPP-4is such as anagliptin, alogliptin, teneligliptin, and saxagliptin due to a lack of availability in our facility. Finally, no evaluation of medical nutrition therapy was carried out. Therefore, a further prospective study using a larger cohort that includes a control group and the effects of medical nutrition therapy is warranted to investigate the efficacy and safety of all types of daily DPP-4is.
In conclusion, the current study revealed that the change in HbA1c variables is dependent on the daily DPP-4i medication regimen followed before switching to a once-weekly DPP-4i. This study’s findings should help physicians in decision making regarding the selection and use of DPP-4is in patients with T2D by bringing awareness to the possibility of worsening glycemic control when switching from vildagliptin to omarigliptin.
Dipeptidyl peptidase-4 inhibitors (DPP-4is) have become standard medications for glycemic control in patients with type 2 diabetes (T2D). Despite the high frequency of switching from various daily DPP-4is to once-weekly DPP-4is in actual clinical practice, data regarding its efficacy in patients with T2D after switching are limited.
Compound-specific effects can be present and influence the efficacy of daily DPP-4is in patients with T2D.
The authors analyzed the efficacy of omarigliptin, one of several once-weekly DPP-4is, in Japanese patients with T2D who had previously received treatment with other glucose-lowering agents.
The 49 patients in this study were divided into four groups defined as either add-on or switched from daily DPP-4is (linagliptin, sitagliptin, and vildagliptin), and the clinical parameters among these groups were assessed and compared during a 3-mo follow-up. Additionally, glycemic variability measured by continuous glucose monitoring was also assessed in the switched groups.
The glycemic control saw significant improvement in the add-on group, while the switched from vildagliptin to omarigliptin group experienced significant worsening. Multivariate logistic regression analysis revealed that switching from vildagliptin to omarigliptin was independently associated with worsening glycemic control (P = 0.0013). However, the mean of daily difference significantly improved when the patient was switched from either linagliptin or sitagliptin to omarigliptin but significantly worsened when patients were switched from vildagliptin.
Administering omarigliptin as add-on therapy or switching from sitagliptin and linagliptin, but not vildagliptin, provides more effective glycemic control. These results should help in decision-making regarding the selection and use of DPP-4is in patients with T2D.
To investigate the efficacy and safety of all types of daily DPP-4is, a prospective study using a larger cohort and inclusive of a control group should be conducted in the future.
Provenance and peer review: Invited manuscript; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu QN S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: Focus on East Asian perspectives. J Diabetes Investig. 2016;7 Suppl 1:102-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Gantz I, Okamoto T, Ito Y, Okuyama K, O'Neill EA, Kaufman KD, Engel SS, Lai E; the Omarigliptin Study 020 Group. A randomized, placebo- and sitagliptin-controlled trial of the safety and efficacy of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther. 2015;17:787-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 499] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 4. | Suzuki K, Hasegawa K, Watanabe M. Efficacy and Patient Satisfaction of Dipeptidyl Peptidase-4 Inhibitor After Switching From Once-Daily DPP-4 Inhibitor to Once-Weekly Regimen. J Clin Med Res. 2018;10:641-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Gantz I, Okamoto T, Ito Y, Sato A, Okuyama K, O'Neill EA, Engel SS, Lai E; Omarigliptin Study 015 Group. A Randomized, Placebo-Controlled Trial Evaluating the Safety and Efficacy of Adding Omarigliptin to Antihyperglycemic Therapies in Japanese Patients with Type 2 Diabetes and Inadequate Glycemic Control. Diabetes Ther. 2017;8:793-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Shankar RR, Inzucchi SE, Scarabello V, Gantz I, Kaufman KD, Lai E, Ceesay P, Suryawanshi S, Engel SS. A randomized clinical trial evaluating the efficacy and safety of the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin. 2017;33:1853-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Lee SH, Gantz I, Round E, Latham M, O'Neill EA, Ceesay P, Suryawanshi S, Kaufman KD, Engel SS, Lai E. A randomized, placebo-controlled clinical trial evaluating the safety and efficacy of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus inadequately controlled by glimepiride and metformin. BMC Endocr Disord. 2017;17:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Home P, Shankar RR, Gantz I, Iredale C, O'Neill EA, Jain L, Pong A, Suryawanshi S, Engel SS, Kaufman KD, Lai E. A randomized, double-blind trial evaluating the efficacy and safety of monotherapy with the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in people with type 2 diabetes. Diabetes Res Clin Pract. 2018;138:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Kadowaki T, Seino Y, Kaku K, Okamoto T, Kameya M, Sato A, Hirano T, Oshima N, Gantz I, O'Neill EA, Engel SS; Omarigliptin Study 039 Group. A randomized, placebo-controlled study to evaluate the efficacy and safety of adding omarigliptin to insulin therapy in Japanese patients with type 2 diabetes and inadequate glycaemic control. Diabetes Obes Metab. 2021;23:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hattori S, Nomoto K, Suzuki T, Hayashi S. Beneficial effect of omarigliptin on diabetic patients with non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. Diabetol Metab Syndr. 2021;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Manteuffel M, Williams S, Chen W, Verbrugge RR, Pittman DG, Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt). 2014;23:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 12. | Fisman EZ, Tenenbaum A. Antidiabetic treatment with gliptins: focus on cardiovascular effects and outcomes. Cardiovasc Diabetol. 2015;14:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Nabeno M, Akahoshi F, Kishida H, Miyaguchi I, Tanaka Y, Ishii S, Kadowaki T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Watanabe YS, Yasuda Y, Kojima Y, Okada S, Motoyama T, Takahashi R, Oka M. Anagliptin, a potent dipeptidyl peptidase IV inhibitor: its single-crystal structure and enzyme interactions. J Enzyme Inhib Med Chem. 2015;30:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Nojima H, Kanou K, Terashi G, Takeda-Shitaka M, Inoue G, Atsuda K, Itoh C, Iguchi C, Matsubara H. Comprehensive analysis of the Co-structures of dipeptidyl peptidase IV and its inhibitor. BMC Struct Biol. 2016;16:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Esposito K, Chiodini P, Maiorino MI, Capuano A, Cozzolino D, Petrizzo M, Bellastella G, Giugliano D. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open. 2015;5:e005892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Suzuki Y, Tanaka A, Tanaka M, Hidaka N, Miyake T, Matsuura B, Hiasa Y, Araki H. Clinical effectiveness of switching between DPP-4 inhibitors in patients with type 2 diabetes mellitus . Int J Clin Pharmacol Ther. 2019;57:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |