Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.2036
Peer-review started: September 3, 2021
First decision: October 3, 2021
Revised: October 16, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: December 15, 2021
Processing time: 104 Days and 3.6 Hours
Hypoglycemia is a common complication in patients with diabetes, mainly in those treated with insulin, sulfonylurea, or glinide. Impairments in counterregulatory responses and hypoglycemia unawareness constitute the main risk factors for severe hypoglycemia. Episodes of hypoglycemia are associated with physical and psychological morbidity. The fear of hypoglycemia constitutes a barrier that impairs the patient’s ability to reach good glycemic control. To prevent hypoglycemia, much effort must be invested in patient education regarding risk factors, warning signs, and treatment of hypoglycemia at an early stage, together with setting personalized goals for glycemic control. In this review, we present a comprehensive update on the treatment and prevention of hypoglycemia in type 1 and type 2 diabetic patients.
Core Tip: Hypoglycemia in diabetes is associated with increased morbidity and constitutes a barrier to glycemic control. Great effort must be invested in patient education on hypoglycemia prevention and management. Herein we present the recent data on the treatment and prevention of hypoglycemia in diabetes, with a focus on the benefits of treatment adjustment and the role of continuous glucose monitoring.
- Citation: Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J Diabetes 2021; 12(12): 2036-2049
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/2036.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.2036
Hypoglycemia is defined as a condition where plasma glucose concentration is low, which may expose patients to possible harm. This is common amongst persons who have type 1 diabetes, with an annual incidence of severe hypoglycemia ranging from 3.3% to 13.5%[1]. While patients treated with insulin or insulin secretagogues (sulfonylureas and meglitinides) are generally at higher risk[2], severe hypoglycemia is less common in patients with type 2 diabetes.
Glucose-lowering medications that do not cause unregulated insulin secretion, such as dipeptidyl peptidase-4 inhibitors, metformin, glucagon-like peptide-1 receptor agonists, thiazolidinediones, and sodium-glucose cotransporter-2 inhibitors are associated with lower risk of hypoglycemia, unless used in combination with insulin or insulin secretagogues[3].
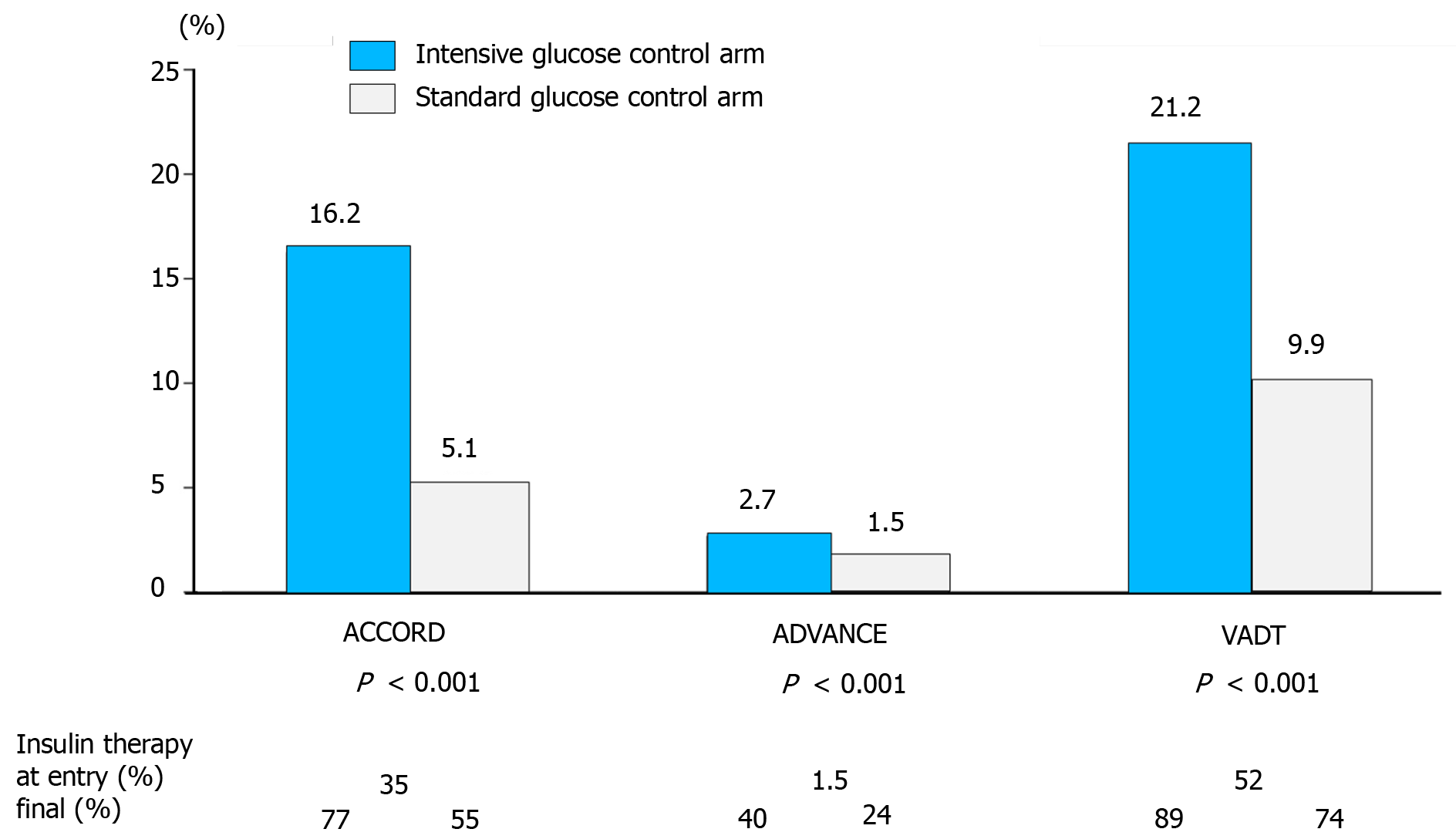
Historically, lowering the glycemic targets in diabetes in order to prevent microvascular and macrovascular complications has led to greater risk of hypoglycemia. In the United Kingdom Prospective Diabetes Study (UKPDS), when the HbA1c goal was 7% in the intensive treatment arm, the yearly incidence rate of severe hypoglycemia ranged from 0.7%-1.8% in type 2 diabetes patients receiving conventional treatment or treated with insulin, respectively[4]. In the ACCORD, ADVANCE, and VADT trials, significant increases in hypoglycemic episodes were observed in the intensive treatment as compared to the standard treatment groups (Figure 1)[5-8]. In the Diabetes Control and Complications Trial (DCCT), at least one episode of severe hypoglycemia during the follow-up period was experienced in ≤ 65% of type 1 diabetes patients in the intensive treatment arm[9]. Interestingly, observational studies point to a lack of significant reduction in the incidence of severe hypoglycemia over the last 20 years, albeit some recent studies have reported decreasing trends, especially among patients with type 2 diabetes[10-12].
In patients with diabetes, it is not easy to determine a specific plasma glucose concentration that is diagnostic of hypoglycemia, because the threshold for the appearance of hypoglycemia symptoms varies among patients. This threshold drops due to recurrent episodes of hypoglycemia and rises in individuals with uncontrolled diabetes.
The current classification of hypoglycemic episodes in diabetes includes three levels corresponding to the severity of hypoglycemia[13]:
Level 1 hypoglycemia: defined as plasma glucose concentration < 70 mg/dL but > 54 mg/dL. Plasma glucose of 70 mg/dL constitutes the threshold concentration below which neuroendocrine responses to hypoglycemia usually appear in individuals without diabetes. Many patients with diabetes suffer from impaired defense mechanisms against hypoglycemia and/or lack of hypoglycemia awareness; therefore, plasma glucose concentrations < 70 mg/dL are defined as clinically significant in diabetes and require intervention irrespective of symptom severity.
Level 2 hypoglycemia: defined as plasma glucose concentration below 54 mg/dL requiring immediate intervention to correct the hypoglycemia.
Level 3 hypoglycemia: defined as a serious event characterized by a change in the mental status or impairment in the patient’s physical ability to function that requires intervention by another person to correct the glucose concentration.
Symptoms of hypoglycemia include autonomic symptoms and neuroglycopenic symptoms. These vary among patients according to age and diabetes duration. For example, children may demonstrate emotional and behavioral changes secondary to hypoglycemia in addition to classic autonomic and neuroglycopenic symptoms.
Autonomic symptoms include anxiety, tremor, palpitations, diaphoresis, paresthesia, and sensation of hunger.
Neuroglycopenic symptoms include lack of concentration, headache, blurred vision, dizziness, confusion, convulsions, speech disturbance, restlessness, and loss of consciousness.
Neuroglycopenic symptoms result from brain neuronal glucose deprivation. The glycemic threshold for neuroglycopenic symptoms is typically around 54 mg/dL[13]. Unlike autonomic symptoms, the onset of neuroglycopenic symptoms is usually not affected by counter-regulatory hormonal failure or previous episodes of hypoglycemia[14].
Risk factors for hypoglycemia can be from therapeutic hyperinsulinemia or failure of “defense mechanisms” from a drop in plasma glucose concentration.
Conditions causing therapeutic hyperinsulinemia include: (1) Treatment with insulin, sulfonylureas, or glinides, if administered at high dose or with incorrect timing related to meal; (2) Lack of exogenous glucose, such as when eating a very low carbohydrate food portion, or during prolonged fasting; Lack of endogenic glucose production after drinking alcohol, (3) Increase in glucose consumption during or after physical exercise; (4) Increase in insulin sensitivity due to weight loss or physical exertion; and (5) Drop in insulin excretion under conditions such as renal failure, hepatic failure, and hypothyroidism[15] .
A decrease in plasma glucose concentration may lead to two main responses in the body under normal conditions: (1) Increase in endogenous glucose production by glycogenolysis and gluconeogenesis; and (2) Behavioral changes leading to a sensation of hunger and food seeking[8].
In non-diabetic patients, the initial response to a drop in glucose concentration is reduced insulin secretion. This occurs while the glucose concentration is still within the low physiological range. An additional drop in glucose will cause increased secretion of glucagon and epinephrine (also cortisol and growth hormone, whose roles are less significant) so that lower glucose concentrations activate an intensive sympathoadrenal reaction leading to the appearance of relevant symptoms, with additional lowering of glucose concentration liable to cause cognitive deterioration and severe neurological effects (e.g., convulsions, loss of consciousness)[16].
The above defense mechanisms are often impaired in patients with diabetes and significant beta-cell failure who lack an initial response to a drop in insulin. This leads to a delay in the secretion of glucose from the liver during hypoglycemia. The rate of hypoglycemic episodes increases with the duration of diabetes, perhaps due to the gradual lack of endogenous insulin, which occurs more rapidly in patients with type 1 diabetes and slower in those with type 2 diabetes[8].
In addition, although it is normal in the initial stages of diabetes, the glucagon reaction to hypoglycemia deteriorates over time in type 1 diabetes, and more slowly in persons with type 2 diabetes. In advanced stages, there is also a marked impairment in the sympathoadrenal reactions to hypoglycemia. The drop in the adrenal reaction is secondary to the reduction in the plasma glucose threshold required to activate this mechanism. In patients with type 1 diabetes, a combined reduction in glucagon and epinephrine reactions to hypoglycemia increases risk of hypoglycemia. These mechanisms also occur in the initial stages of type 2 diabetes but less as diabetes progresses[8].
It is believed that the impaired sympathoadrenal response is secondary to repeated episodes of hypoglycemia that reduce the autonomic response to other hypoglycemic events. This exposes patients to a vicious cycle of frequent hypoglycemia events and shifts glycemic thresholds for symptoms to lower plasma glucose concentrations close to levels that cause cognitive failure. After 25 years of treatment, the prevalence of this phenomenon in patients with type 1 diabetes reached 50%, as compared to a prevalence of approximately 10% in type 2 diabetics. It is unclear whether this phenomenon develops in diabetic patients taking oral medications alone[8]. This condition was defined by Cryer as hypoglycemia-associated autonomic failure (HAAF)[17].
The defense mechanisms may be impaired by repeated hypoglycemia events, physical exercise, and sleep thus contributing to the development of hypoglycemia.
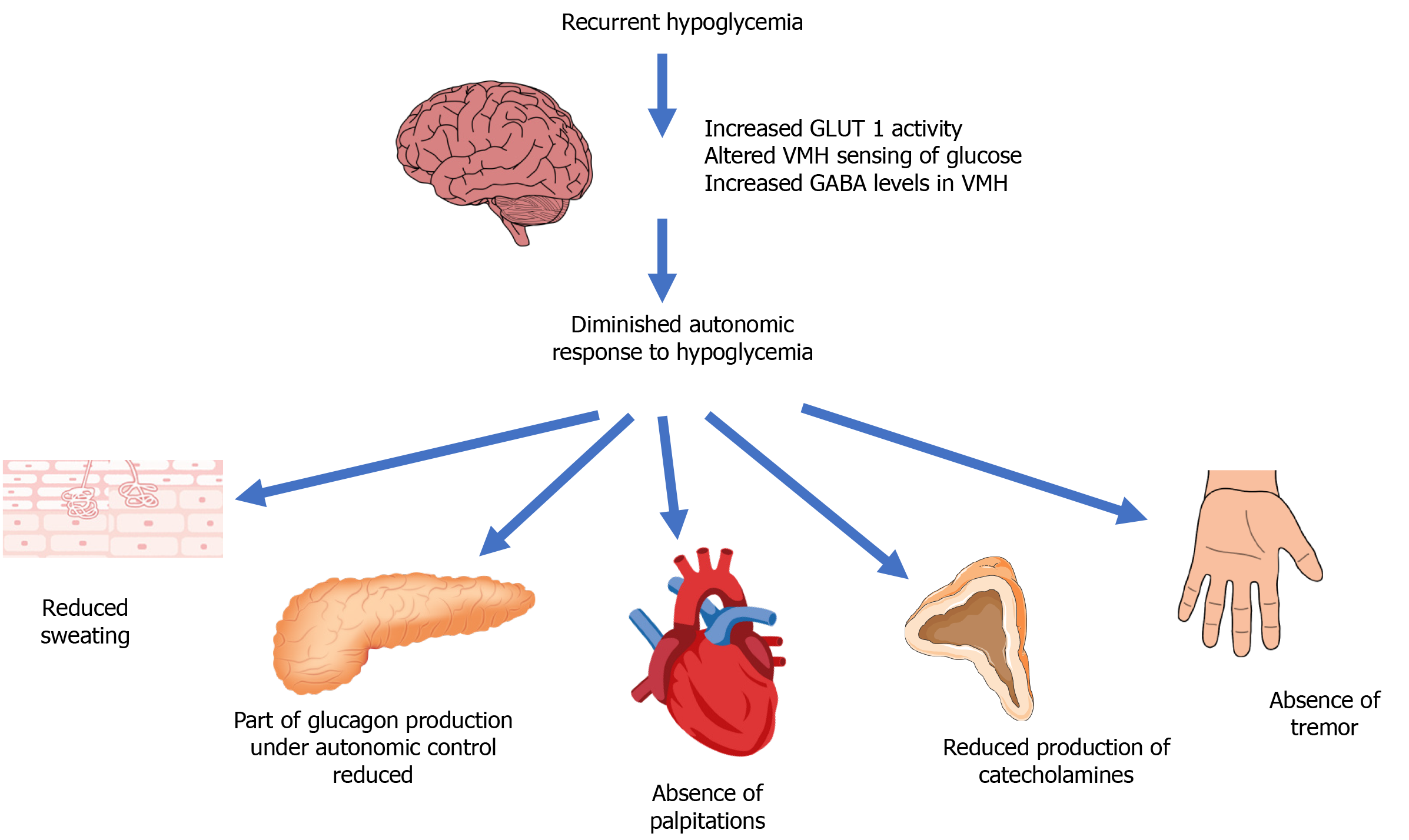
The presumed mechanisms of hypoglycemia unawareness are summarized in Figure 2[12].
Recurrent hypoglycemia can develop as a result of reduced autonomic response to hypoglycemia with attenuation of autonomic warning symptoms. The defective brain response is characterized by increased GLUT1 activity aimed at preserving brain function and altering glucose sensing in the ventromedial hypothalamus (VMH), mediated by elevated levels of Gamma-Aminobutyric Acid (GABA)[12]).
Hypoglycemia causes physical and psychological morbidity in diabetic patients. Symptomatic hypoglycemia constitutes a concern and a distraction. It can impair judgment, performance of simple daily activities such as driving, and behavior. In more severe cases, hypoglycemia may result in convulsions and loss of consciousness. Sometimes transient neurological deficits may appear, and rarely, there may be permanent neurological damage.
In systematic follow-up of patients over 18 years from the DCCT/EDIC, no significant reduction in long-term cognitive function was demonstrated in patients with type 1 diabetes[18]. However, the data did not include elderly patients or children with diabetes. Other studies show evidence of a relationship between hypoglycemia and cognitive decline in patients with type 1 or 2 diabetes. In one study, a relationship was found between hypoglycemia and reduction in cognitive function in children, including linguistic abilities, working memory, and speed of non-verbal processing[19]. Other studies suggest that among elderly diabetic patients hypoglycemia had twice the risk of developing dementia[20].
Concern about hypoglycemia is a barrier to diabetes treatment and control, while patients experiencing recurrent episodes of hypoglycemia are also at risk of depression and anxiety.
In a meta-analysis of more than 900000 patients, a 2-fold increase in the risk of cardiovascular morbidity was observed amongst patients with type 2 diabetes and severe hypoglycemia. This phenomenon may be explained by the sympathoadrenal response and marked increase in the level of catecholamines in the blood, causing a direct effect on the myocardium and vascular system, platelet activation, and aggregation[21].
In more severe cases, hypoglycemia is liable to cause mortality, responsible for 4%-10% of mortality in patients with type 1 diabetes[22,23]. In patients with type 2 diabetes, the mortality rate from hypoglycemia is unknown.
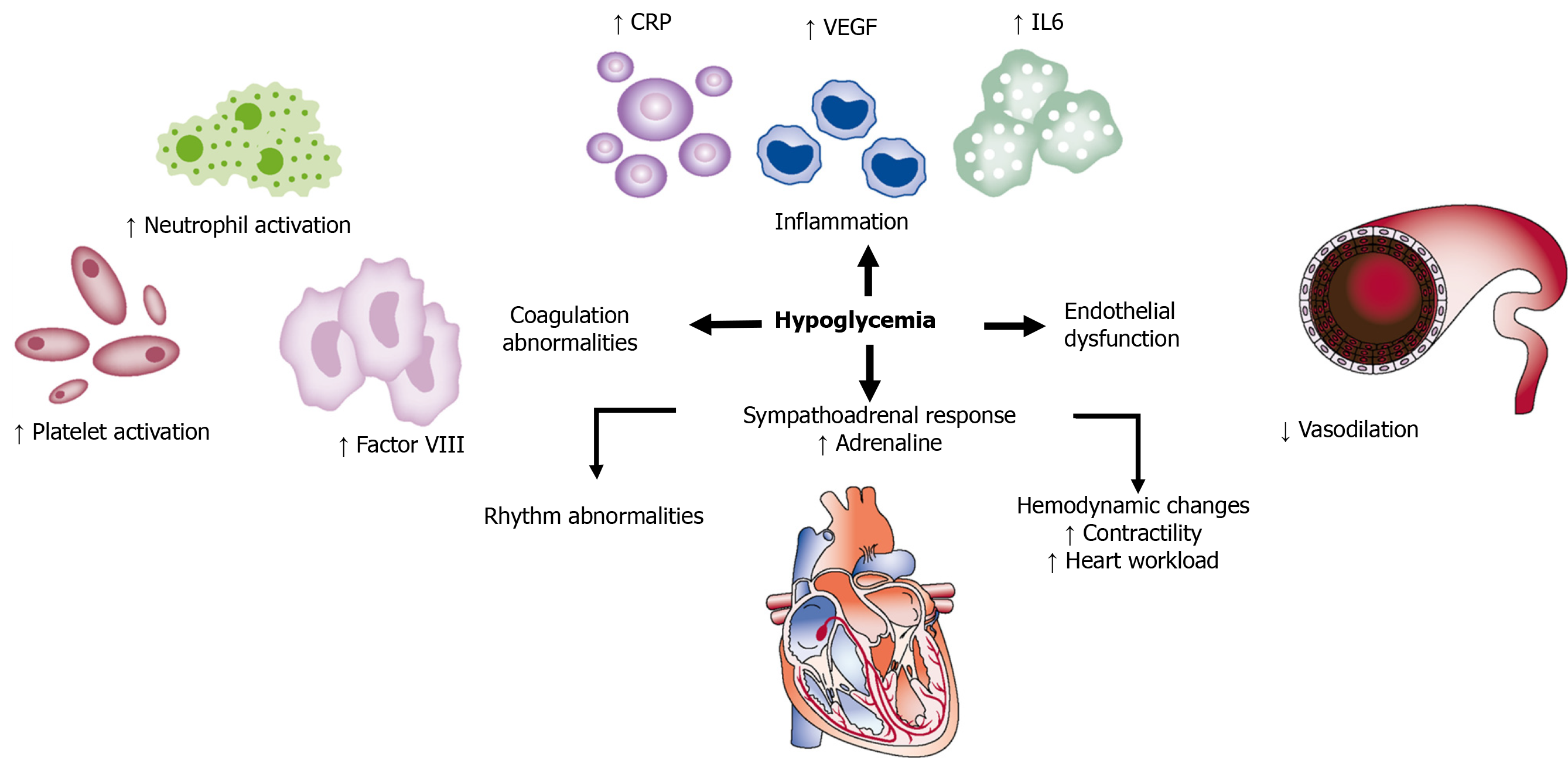
Although severe, sustained hypoglycemia may cause brain death, with most cases of sudden death related to cardiac arrhythmias due to enhanced sympathoadrenal reaction causing QT prolongation[24]. Hypoglycemia may affect cardiovascular events by several mechanisms, as detailed in Figure 3[25].
Clinical and epidemiological studies based on tens of thousands of patients with type 1 and type 2 diabetes from a variety of health services in different world regions showed a 1.5- to 6-fold increase in the risk of cardiovascular events and mortality among patients who experienced severe hypoglycemia[26].
It is very important to control diabetes in pregnant women to prevent maternal and fetal complications. The definition and diagnosis of hypoglycemia in pregnancy are challenging because glucose goals during pregnancy are 20% lower than prior to pregnancy[27]. A marked increase of up to 5-fold in the rate of severe hypoglycemia in the first trimester among women with type 1 diabetes has been found[28].
In general, the fetus is not in danger from maternal hypoglycemia if the mother is not injured during the episode. The risk of hypoglycemia in diabetic women treated with insulin also increases with breastfeeding[29].
At any given moment persons with diabetes constitute about 30 percent of hospitalized patients[30]. Attitudes towards glycemic control during hospitalization have altered considerably in the last decades, changing from a strict approach with tight control to a more lenient approach with less tight control. For the majority of critically and non-critically ill patients, a target glucose range of 140-180 mg/dL is recommended[31]. This change in recommendations is mostly based on observations that too much control often results in severe hypoglycemia and even endangers life, and thus, for diabetic patients, there is no improvement in morbidity or hospitalization parameters.
Most self-monitoring diagnoses of episodes of symptomatic or asymptomatic hypoglycemia can be treated effectively by rapid-acting carbohydrate (approximately 20 g of glucose constitutes a reasonable dose in most cases) with an expectation of clinical improvement within 20 min.
The importance of giving long-acting carbohydrates after correction of glucose level should be emphasized, because in prolonged hyperinsulinemia effects of oral glucose last fewer than 2 h.
Table 1 presents the protocol for treating hypoglycemia, established mostly in accordance with the Joint British Diabetes Societies guidelines[32].
| Steps | Procedure |
| Adults who are conscious, orientated, and able to swallow | |
| 1 | If the patient is receiving insulin (pump or IV infusion), stop it immediately |
| 2 | Give 15-20 g rapid-acting carbohydrate of the patient’s choice where possible. Examples include: 15-20 g chewable glucose tablets, 150-200 mL orange juice, or 3-4 heaped teaspoons of sugar dissolved in water |
| 3 | Repeat capillary blood glucose measurement 10-15 min later. If it is still less than 70 mg/dL, repeat the previous step up to 3 times |
| 4 | If the capillary blood glucose remains less than 70 mg/dL after 30-45 min or three cycles of treatment, consider IV 200 mL of 10% glucose over 15 min or administration of 1 mg of glucagon IM |
| 5 | Once blood glucose is above 70 mg/dL and the patient has recovered, it is recommended to give a long-acting carbohydrate. Examples: one slice of bread, a 200-300 mL glass of milk, or two biscuits |
| Adults who are conscious but confused, unable to cooperate but able to swallow | |
| 1 | If the patient is receiving insulin (pump or IV infusion), stop it immediately |
| 2 | If the patient is uncooperative but is able to swallow, give a 15g tube of glucose (e.g., Glucogel), squeezed into the mouth between the teeth and gums, or (if this is ineffective) glucagon 1mg IM |
| 3 | Repeat capillary blood glucose levels after 10-15 min. If it is still less than 70 mg/dL, repeat the previous step up to three times (glucagon injection should only be given once) |
| 4 | If the capillary blood glucose remains less than 70 mg/dL after 30-45 min (or three cycles of treatment), give IV 200 mL of 10% glucose over 15 min |
| 5 | Once blood glucose is above 70 mg/dL and the patient has recovered, giving a long-acting carbohydrate is recommended (as detailed above) |
| Adults who are unconscious and/or having seizures | |
| 1 | An urgent medical assessment is required. The following things should be checked and treated accordingly: Airway (administration of oxygen as appropriate), breathing, circulation (pulse), state of consciousness, blood glucose concentration, and body temperature |
| 2 | If the patient is receiving insulin (pump or IV infusion), stop it immediately |
| 3 | Request immediate assistance from medical staff |
| 4 | If IV access is available, give 100 mL of 20% glucose IV or 200 mL of 10% glucose over 15 min |
| 5 | If no immediate IV access is available, give 1mg glucagon IM. If no IV access is available initially, continue trying to obtain IV access as IM glucagon is less likely to be successful if required for a second time. If there is a need for prolonged treatment, IV administration of glucose is the treatment of choice |
| 6 | Capillary blood glucose test should be repeated after 10 min. If it is still less than 70 mg/dL repeat step 4 (or step 5 if IV access remains unavailable) |
| 7 | Once the blood glucose is greater than 70 mg/dL and the patient has recovered, give a long-acting carbohydrate (as previously detailed) |
The approach to hypoglycemia prevention includes patient education, appropriate dietary and exercise regimens, glucose monitoring, medication adjustment, and close clinical supervision[33].
The patients and those around them should be educated to identify symptoms of hypoglycemia and given appropriate treatment as soon as possible. It is important to routinely discuss the dangers of developing hypoglycemia and how it should be treated in patients treated with insulin, sulfonylurea, or glinide. In every hypoglycemia documented, the circumstances of the episode should be investigated together with the patient to try to detect the reason, for example, a skipped meal/prolonged fasting, physical exertion, alcohol consumption, and injection of a high insulin dose.
Patients with diabetes who are at increased risk of hypoglycemia are requested to carry glucagon with them at all times. Family members and people in the environment of patients with diabetes should be instructed regarding the administration of glucagon to the patient; they should also know where the glucagon is kept. Glucagon products include a solution for subcutaneous or intramuscular injection and intranasal glucagon (FDA approved in 2019).
Dietary intervention includes instruction regarding the amount of carbohydrates at meals and its effect on blood glucose concentration and building a personalized regular meal plan. In patients treated with insulin, there should be an emphasis on the importance of giving insulin with appropriate dosage and timing in relation to meals. Patients at risk of hypoglycemia should be instructed to equip themselves with glucose or foods containing carbohydrates and to always keep them at hand. In some patients, especially those with type 1 diabetes or at high risk of nocturnal hypoglycemia, a bedtime snack can be recommended with the purpose of preventing overnight hypoglycemia.
Physical exercise increases glucose consumption and the risk of hypoglycemia. Risk factors include strenuous, prolonged physical exertion, and lack of energy source relative to the insulin in the body. By monitoring blood glucose before and after physical exercise, early steps can be taken to prevent hypoglycemia. Small meals should be eaten prior to physical exercise if there are drops in glucose concentration. Patients are recommended to equip themselves with rapid-acting carbohydrates during physical exercise. When physical exercise is planned, it is important to adjust the insulin dose.
Self-monitoring of blood glucose (SMBG) and continuous glucose monitoring constitute essential tools to diagnose hypoglycemia in the early stages. SMBG constitutes an integral part of the efforts to prevent hypoglycemia. ADA recommendations for most patients on intensive insulin regimens (multiple daily injections (MDI) or pump) are to check glucose before meals and occasionally post-prandially, before sleep and physical exercise, when there is a suspicion of low blood glucose, after treatment of hypoglycemia, and before certain activities requiring high concentration like driving[34].
There is insufficient information in the literature regarding the frequency of glucose self-monitoring required in patients who do not use intensive insulin regimens, including those with type 2 diabetes using basal insulin and/or oral agents. According to most authorities, monitoring should be less intensive with fasting measurements in the morning and sometimes before supper.
Continuous glucose monitoring (CGM), which measures the interstitial glucose in real-time, constitutes a potential tool to improve diabetes control and reduce hypoglycemic episodes. There are two types of CGM devices: real-time CGM that provides information about current glucose concentrations and trends to a receiver; and intermittently scanned CGM which requires passing a scanner over the transmitter to determine the glucose concentration.
CGM has been investigated in many studies, with the efficacy of CGM in diabetes control tested in some studies and the integration of CGM intending to reduce hypoglycemic episodes in other studies.
In patients with uncontrolled diabetes (type 1 or type 2), the use of CGM contributed to improved control and reduction of 0.3%-0.6% in HbA1c[35].
Most studies that investigated the use of CGM to prevent hypoglycemia in type 1 diabetes showed a significant reduction in time spent in hypoglycemia within the range of 54-70 mg/dL[35]. To this day, only limited evidence is available on the effectiveness of CGM in reducing level 3 hypoglycemia episodes.
In a study of 120 patients, children and adults, with type 1 diabetes and HbA1c < 7.5% were divided for 26 wk into a group under CGM monitoring and a control group. Patients using CGM spent less time in hypoglycemia per day and was accompanied by better control than the control group[36].
A study of 322 patients with type 1 diabetes treated with an intensive insulin regimen showed that adults > 25 years who used CGM had a decrease of 0.5% in HbA1c vs those who performed SMBG, without a significant difference in hypoglycemia rate. No significant difference was observed in HbA1c or hypoglycemia episodes in people < 25 years[37].
In a recent trial, CGM was effective in reducing hypoglycemia as compared with standard blood glucose monitoring in adults > 60 years with type 1 diabetes[38].
An additional study in patients with type 1 diabetes and initial HbA1c < 7% showed advantages to using CGM with regard to diabetes control and reducing hypoglycemia[39].
The HypoDE trial showed that the use of CGM significantly reduced hypoglycemia rate in adult patients with type 1 diabetes with a history of hypoglycemia unawareness or severe hypoglycemia who were treated with an MDI regimen[40]. Nevertheless, a significant reduction in episodes of severe hypoglycemia requiring medical intervention (administration of glucose or glucagon) was not observed when compared to the control group[40].
In the DIAMOND trial that was conducted in patients with type 1 diabetes, use of CGM for 24 wk led to improvement in diabetes control (decrease of 0.6% in HbA1c) with a significant decrease in glycemic variability and reduction in time spent in hypoglycemia, but without change in number of severe hypoglycemia episodes[41].
For patients with type 1 diabetes who experience recurrent episodes of hypoglycemia and/or hypoglycemia unawareness, CGM technology may be useful, though its long-term efficacy has not yet been determined.
An additional technology that has come into use in recent years is flash technology to monitor blood glucose, which works by scanning without the need for finger prick or calibration, which has been proven to significantly reduce hypoglycemia rate in adult patients with well-controlled type 1 diabetes[42].
In the recent ALERTT1 trial, adults with type 1 diabetes who switched from intermittently scanned CGM (devoid of alarms) to real-time CGM (with alarms) had improved glycemic control and lower rates of grade 3 hypoglycemia[43]. However, the ALERTT1 trial results might become less applicable as intermittently scanned CGM devices are being updated to include alarms[44]. Further research comparing real-time CGM with updated intermittently scanned CGM technology is needed.
There is insufficient information in the literature regarding CGM efficacy in preventing hypoglycemia in type 2 diabetes. In a recent meta-analysis, no significant advantage was observed for CGM over SMBG in preventing hypoglycemia among patients with type 2 diabetes treated with insulin. Nevertheless, it should be noted that no increase in the risk of hypoglycemia was observed in patients who used CGM, despite the more significant reduction in HbA1c[45]. Recently, Karter et al[46] followed the outcomes of 3806 insulin-treated patients with diabetes (91% type 1, 9% type 2) who initiated real-time CGM. They demonstrated that patients with type 2 diabetes benefited from the use of CGM in terms of improved glycemic control and a significant decrease in the rate of hypoglycemia-related emergency department or hospital utilization. In a randomized clinical trial reported by Martens et al[47], CGM use in patients with type 2 diabetes treated with basal insulin resulted in better glycemic control with non-significant reduction of CGM-measured hypoglycemia.
However, further dedicated studies are needed to draw clear conclusions regarding CGM utility in hypoglycemia prevention among insulin-treated patients with type 2 diabetes.
Some hypoglycemia episodes in diabetes are associated with the treatment itself; therefore, it is important to use drugs with a low risk of hypoglycemia.
Metformin, DPP-4 inhibitors, GLP-1 analogs, SGLT-2 inhibitors, and pioglitazone are all associated with low risk of hypoglycemia in patients with type 2 diabetes. In contrast, sulfonylureas and glinides are associated with higher risk of hypoglycemia[48]; therefore, consideration of dose reduction or cessation of these drugs and switching to a different treatment is recommended in cases of recurrent hypoglycemia.
A decade ago, the transition to the use of long-acting basal insulin analogs (such as Detemir and Glargine U100) led to a significant reduction in episodes of nocturnal hypoglycemia compared to NPH insulin[49,50]. In patients with both type 1 and type 2 diabetes, the new ultra-long basal insulins Glargine U300 (300 units per mL) and Degludec have recently led to a significant additional reduction in the rate of nocturnal hypoglycemia[51-54].
The use of short-acting insulin analogs has also led to a significant reduction in rates of severe hypoglycemia as compared to human insulin[55].
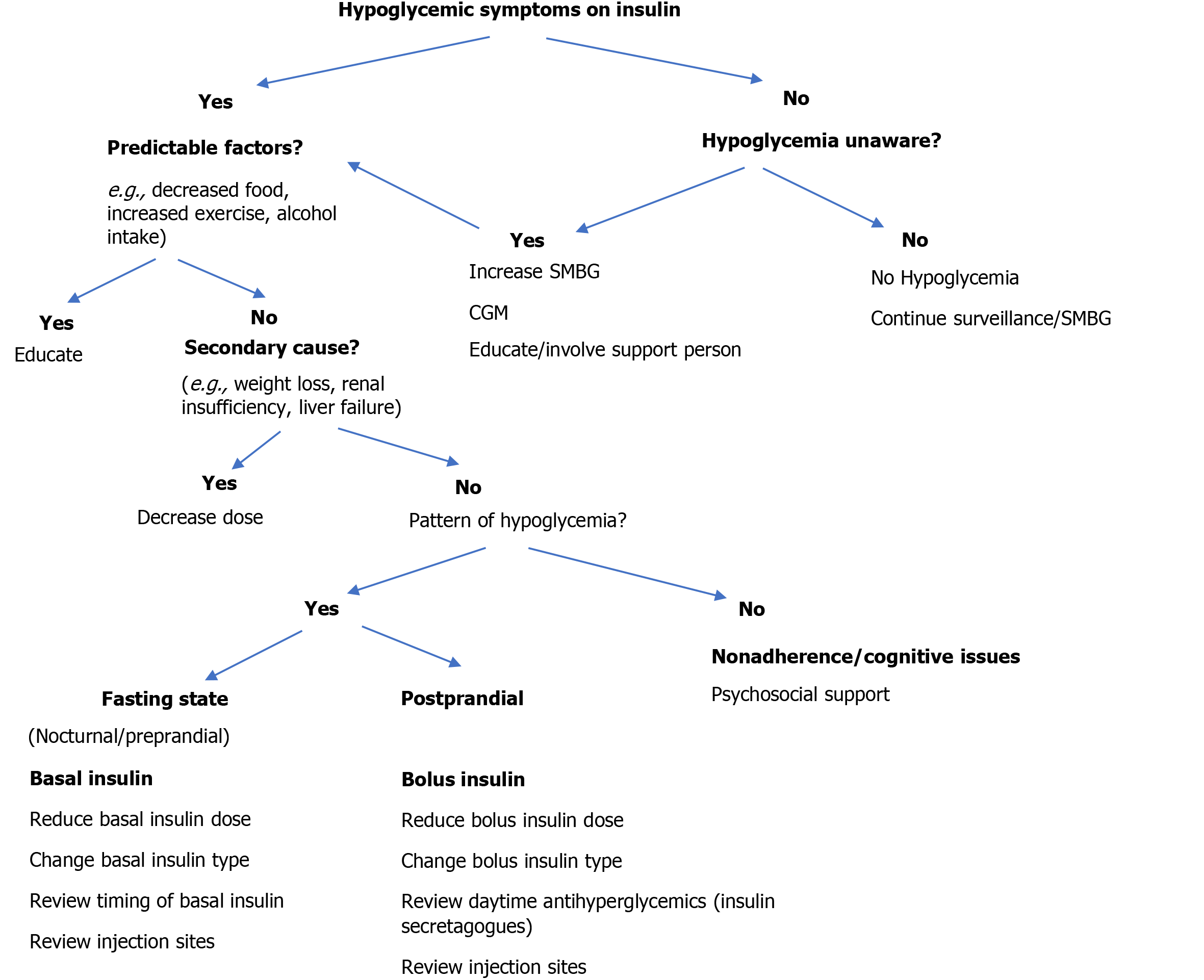
In patients with type 2 diabetes, the combination of basal insulin and GLP-1 analog in one syringe at a fixed ratio showed a significant improvement in diabetes control without increased risk of hypoglycemia[56]. The approach to preventing hypoglycemia in patients with diabetes treated with insulin is detailed in Figure 4[57].
A 2010 study showed that the use of continuous subcutaneous insulin infusion (CSII) prevented hypoglycemic episodes and improved the threshold of hypoglycemia awareness in patients with type 1 diabetes who suffered from recurrent episodes of non-severe or severe hypoglycemia[58]. An earlier meta-analysis showed that, compared to the MDI regimen, the use of CSII reduced the rate of severe hypoglycemia, but this conclusion was based on three randomized controlled studies that used NPH or Lente insulin[59]. Data has so far been inconsistent for patients with either type 1 or type 2 diabetes; two meta-analyses concluded there was no advantage to using a CSII over MDI regimen in terms of reducing the risk of severe hypoglycemic events. CSII, especially sensor-augmented insulin pump, showed an advantage in terms of glycemic control in adults with type 1 diabetes mellitus[60,61].
In the ASPIRE trial, using a sensor-augmented insulin pump programmed to suspend insulin infusion in response to low glucose concentrations led to a significant reduction in episodes of nocturnal hypoglycemia in patients with type 1 diabetes, without an increase in HbA1c[62].
The HypoCOMPaSS trial, which included patients with type 1 diabetes, compared a group treated with MDI and SMBG with a group treated with CSII and real-time CGM, showing a similar reduction in episodes of severe hypoglycemia and improvement in hypoglycemia awareness in both groups; patient satisfaction was higher in the pump group[63].
In recent years, much effort has been invested in building an “artificial pancreas”- a closed-loop system combining a real-time CGM and CSII using glucose control and safety algorithms that manage insulin delivery in a glucose-responsive manner. The use of an artificial pancreas can reduce the burden on patients by automatically adjusting the delivery of insulin based on sensor glucose levels. Single-hormone (insulin-only) and dual-hormone (insulin and glucagon) systems have been developed. In the dual-hormone system glucagon is also delivered in a similar glucose-responsive manner.
In a recent systematic review and meta-analysis, it was shown that the use of the “artificial pancreas” technology constitutes effective and safe treatment for patients with type 1 diabetes and leads to improved diabetes control, and reduced time in hypoglycemia[64]. However, current evidence for artificial pancreas systems is limited by inconsistent reporting of outcomes and short follow-up times[64-67].
Pancreatic islet transplantation might be of great promise for patients with type 1 diabetes. Significant progress has been made to improve islet function and clinical outcomes after transplantation. Pancreatic islet transplantation has provided glycemic control, reduced episodes of hypoglycemia, and improved hypoglycemia awareness in patients with type 1 diabetes[68-70].
Hypoglycemia in diabetes is associated with increased morbidity and constitutes a barrier to glycemic control. Much effort must be invested in hypoglycemia prevention, including patient education, appropriate dietary and exercise regimens, adjustment of the treatment regimen, and implementation of glucose monitoring systems as appropriate.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Israel
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fang ZH, Shuang WB S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Pettus JH, Zhou FL, Shepherd L, Preblick R, Hunt PR, Paranjape S, Miller KM, Edelman SV. Incidences of Severe Hypoglycemia and Diabetic Ketoacidosis and Prevalence of Microvascular Complications Stratified by Age and Glycemic Control in U.S. Adult Patients With Type 1 Diabetes: A Real-World Study. Diabetes Care. 2019;42:2220-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Thrasher J. Pharmacologic Management of Type 2 Diabetes Mellitus: Available Therapies. Am J Med. 2017;130:S4-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5771] [Cited by in RCA: 5268] [Article Influence: 195.1] [Reference Citation Analysis (0)] |
| 5. | ACCORD Study Group. , Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i-33i. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 6. | ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4759] [Cited by in RCA: 4888] [Article Influence: 287.5] [Reference Citation Analysis (0)] |
| 7. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3323] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 8. | Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34 Suppl 2:S132-S137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | . Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:271-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Lipska KJ, Yao X, Herrin J, McCoy RG, Ross JS, Steinman MA, Inzucchi SE, Gill TM, Krumholz HM, Shah ND. Trends in Drug Utilization, Glycemic Control, and Rates of Severe Hypoglycemia, 2006-2013. Diabetes Care. 2017;40:468-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 11. | Yang A, Wu H, Lau ESH, Ma RCW, Kong APS, So WY, Luk AOY, Chan JCN, Chow E. Trends in Glucose-Lowering Drug Use, Glycemic Control, and Severe Hypoglycemia in Adults With Diabetes in Hong Kong, 2002-2016. Diabetes Care. 2020;43:2967-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Iqbal A, Heller S. Managing hypoglycaemia. Best Pract Res Clin Endocrinol Metab. 2016;30:413-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, Kowalski AJ, Madden P, McAuliffe-Fogarty AH, McElwee-Malloy M, Peters A, Raman S, Reifschneider K, Rubin K, Weinzimer SA. Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 14. | McAulay V, Deary IJ, Frier BM. Symptoms of hypoglycaemia in people with diabetes. Diabet Med. 2001;18:690-705. [PubMed] [DOI] [Full Text] |
| 15. | International Hypoglycaemia Study Group. Minimizing Hypoglycemia in Diabetes. Diabetes Care. 2015;38:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 16. | Melmed S, Polonsky K, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology 13th edition. Philadelphia, PA: Elsevier, Inc., 2016: 1584. |
| 17. | Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 19. | Lin A, Northam EA, Rankins D, Werther GA, Cameron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes. 2010;11:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Yaffe K, Falvey CM, Hamilton N, Harris TB, Simonsick EM, Strotmeyer ES, Shorr RI, Metti A, Schwartz AV; Health ABC Study. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 21. | Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 22. | Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, Svensson J, Schober E, Gyürüs E, Castell C, Urbonaité B, Rosenbauer J, Iotova V, Thorsson AV, Soltész G. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia. 2007;50:2439-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 23. | Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 260] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Reno CM, Daphna-Iken D, Chen YS, VanderWeele J, Jethi K, Fisher SJ. Severe hypoglycemia-induced lethal cardiac arrhythmias are mediated by sympathoadrenal activation. Diabetes. 2013;62:3570-3581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 330] [Article Influence: 55.0] [Reference Citation Analysis (1)] |
| 27. | Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 28. | Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Damm P, Mathiesen ER. Hypoglycaemia during pregnancy in women with Type 1 diabetes. Diabet Med. 2012;29:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Riviello C, Mello G, Jovanovic LG. Breastfeeding and the basal insulin requirement in type 1 diabetic women. Endocr Pract. 2009;15:187-193. [PubMed] [DOI] [Full Text] |
| 30. | Gosmanov AR. A practical and evidence-based approach to management of inpatient diabetes in non-critically ill patients and special clinical populations. J Clin Transl Endocrinol. 2016;5:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | American Diabetes Association. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S211-S220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 32. | Joint British Diabetes Societies. The Hospital Management of Hypoglycaemia in Adults with Diabetes Mellitus. [cited 20 February 2021]. Available from: https://abcd.care/sites/abcd.care/files/site_uploads/JBDS_01_Hypo_Guideline_FINAL_23042021_0.pdf Accessed: 28 June 2021. |
| 33. | Diabetes Canada Clinical Practice Guidelines Expert Committee. Yale JF, Paty B, Senior PA. Hypoglycemia. Can J Diabetes. 2018;42 Suppl 1:S104-S108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S85-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 35. | American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S73-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 564] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 36. | Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 37. | Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1098] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 38. | Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, Bhargava A, Bode BW, Carlson A, Chaytor NS, Fox DS, Goland R, Hirsch IB, Kruger D, Kudva YC, Levy C, McGill JB, Peters A, Philipson L, Philis-Tsimikas A, Pop-Busui R, Shah VN, Thompson M, Vendrame F, Verdejo A, Weinstock RS, Young L, Miller KM; Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group. Effect of Continuous Glucose Monitoring on Hypoglycemia in Older Adults With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2020;323:2397-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 39. | Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 40. | Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, Hermanns N. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 380] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 41. | Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, Kollman C, Kruger D, McGill JB, Polonsky W, Toschi E, Wolpert H, Price D; DIAMOND Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 835] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 42. | Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 650] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 43. | Visser MM, Charleer S, Fieuws S, De Block C, Hilbrands R, Van Huffel L, Maes T, Vanhaverbeke G, Dirinck E, Myngheer N, Vercammen C, Nobels F, Keymeulen B, Mathieu C, Gillard P. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397:2275-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 44. | Olson DE. In type 1 diabetes, real-time vs. intermittently scanned continuous glucose monitoring improved glycemic control. Ann Intern Med. 2021;174:JC119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Dicembrini I, Mannucci E, Monami M, Pala L. Impact of technology on glycaemic control in type 2 diabetes: A meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes Metab. 2019;21:2619-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of Real-time Continuous Glucose Monitoring With Glycemic Control and Acute Metabolic Events Among Patients With Insulin-Treated Diabetes. JAMA. 2021;325:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 47. | Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, Pop-Busui R, Philis-Tsimikas A, Bao S, Umpierrez G, Davis G, Kruger D, Bhargava A, Young L, McGill JB, Aleppo G, Nguyen QT, Orozco I, Biggs W, Lucas KJ, Polonsky WH, Buse JB, Price D, Bergenstal RM; MOBILE Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. JAMA. 2021;325:2262-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 48. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S111-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 685] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 49. | Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2008;81:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 50. | Laranjeira FO, de Andrade KRC, Figueiredo ACMG, Silva EN, Pereira MG. Long-acting insulin analogues for type 1 diabetes: An overview of systematic reviews and meta-analysis of randomized controlled trials. PLoS One. 2018;13:e0194801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Matsuhisa M, Koyama M, Cheng X, Takahashi Y, Riddle MC, Bolli GB, Hirose T; EDITION JP 1 study group. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016;18:375-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Ritzel R, Harris SB, Baron H, Florez H, Roussel R, Espinasse M, Muehlen-Bartmer I, Zhang N, Bertolini M, Brulle-Wohlhueter C, Munshi M, Bolli GB. A Randomized Controlled Trial Comparing Efficacy and Safety of Insulin Glargine 300 Units/mL Versus 100 Units/mL in Older People With Type 2 Diabetes: Results From the SENIOR Study. Diabetes Care. 2018;41:1672-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, Renard E, Russell-Jones D, Philotheou A, Francisco AM, Pei H, Bode B; BEGIN Basal-Bolus Type 1 Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 54. | Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, Rosenstock J, Endahl LA, Francisco AM, Hollander P; NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 55. | Pedersen-Bjergaard U, Kristensen PL, Beck-Nielsen H, Nørgaard K, Perrild H, Christiansen JS, Jensen T, Hougaard P, Parving HH, Thorsteinsson B, Tarnow L. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol. 2014;2:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Perreault L, Rodbard H, Valentine V, Johnson E. Optimizing Fixed-Ratio Combination Therapy in Type 2 Diabetes. Adv Ther. 2019;36:265-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Blumer I, Clement M. Type 2 Diabetes, Hypoglycemia, and Basal Insulins: Ongoing Challenges. Clin Ther. 2017;39:S1-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Giménez M, Lara M, Conget I. Sustained efficacy of continuous subcutaneous insulin infusion in type 1 diabetes subjects with recurrent non-severe and severe hypoglycemia and hypoglycemia unawareness: a pilot study. Diabetes Technol Ther. 2010;12:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 60. | Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, Wilson LM, Haberl EB, Brick J, Bass EB, Golden SH. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 61. | Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2017;55:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 62. | Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Ahmann AJ, Welsh JB, Lee SW, Kaufman FR; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 63. | Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, Chadwick TJ, Barendse S, Stocken DD, Brennand C, Marshall SM, Wood R, Speight J, Kerr D, Flanagan D, Heller SR, Evans ML, Shaw JA. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37:2114-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 64. | Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich AB, Hovorka R, Tsapas A. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 65. | Bally L, Thabit H, Hartnell S, Andereggen E, Ruan Y, Wilinska ME, Evans ML, Wertli MM, Coll AP, Stettler C, Hovorka R. Closed-Loop Insulin Delivery for Glycemic Control in Noncritical Care. N Engl J Med. 2018;379:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 66. | Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 67. | Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, Schoelwer M, Ruedy KJ, Jost E, Carria L, Emory E, Hsu LJ, Oliveri M, Kollman CC, Dokken BB, Weinzimer SA, DeBoer MD, Buckingham BA, Cherñavvsky D, Wadwa RP; iDCL Trial Research Group. A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. N Engl J Med. 2020;383:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 68. | Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA; Clinical Islet Transplantation Consortium. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2016;39:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 69. | Foster ED, Bridges ND, Feurer ID, Eggerman TL, Hunsicker LG, Alejandro R; Clinical Islet Transplantation Consortium. Improved Health-Related Quality of Life in a Phase 3 Islet Transplantation Trial in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care. 2018;41:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 70. | Vantyghem MC, Chetboun M, Gmyr V, Jannin A, Espiard S, Le Mapihan K, Raverdy V, Delalleau N, Machuron F, Hubert T, Frimat M, Van Belle E, Hazzan M, Pigny P, Noel C, Caiazzo R, Kerr-Conte J, Pattou F; Members of the Spanish Back Pain Research Network Task Force for the Improvement of Inter-Disciplinary Management of Spinal Metastasis. Ten-Year Outcome of Islet Alone or Islet After Kidney Transplantation in Type 1 Diabetes: A Prospective Parallel-Arm Cohort Study. Diabetes Care. 2019;42:2042-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |