Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1928
Peer-review started: April 28, 2021
First decision: June 16, 2021
Revised: June 23, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 15, 2021
Processing time: 200 Days and 16 Hours
Although much information is available regarding hepatitis C virus infection and diabetes, less is known about the relationship between hepatitis A virus (HAV) infection and diabetes.
To examine the roles of HAV in diabetes risk.
This cross-sectional study population included data from the National Health and Nutrition Examination Survey collected between 2005-2012. Adult subjects (≥ 20 years old) with available body mass index measurements, defined diabetes status, history of HAV vaccination, and HAV serology were included. HAV vaccination was based on self-reported history. Successful HAV immunization was defined as the presence of both vaccination and anti-HAV antibody. HAV infection was defined by the absence of vaccination but presence of anti-hepatitis A antibody. The odds ratio (OR) for diabetes with 95% confidence intervals (95%CI) was calculated for each HAV status and then adjusted for covariates. Sensitivity tests, based on different definitions of diabetes, were performed to verify the results.
Among 19942 subjects, 4229 subjects (21.21%) received HAV vaccination and HAV antibody was present in 9224 subjects (46.25%). Although HAV infection was associated with an increased risk of diabetes (OR: 1.13; 95%CI: 1.08-1.18), HAV vaccination was not associated with diabetes (OR: 1.06; 95%CI: 0.95-1.18), and successful HAV immunization had no impact on the risk of diabetes (OR: 1.11; 95%CI: 0.97-1.27). Thus, HAV infection was an unlikely cause of diabetes. Alternatively, in non-vaccinated subjects, diabetes increased the risk of HAV infection by 40% (OR: 1.40, 95%CI: 1.27-1.54).
An association between HAV infection and diabetes is observed which is best explained by an increased risk of HAV infection in diabetic patients. Diabetic subjects are more susceptible to HAV. Thus, HAV vaccination is highly recommended in diabetic patients.
Core Tip: The relationship of hepatitis A virus (HAV) infection and diabetes was examined. Although an association between HAV infection and diabetes was observed, neither HAV vaccination or successful HAV immunization had no impact on the risk of diabetes. Thus, HAV infection did not increase the risk of diabetes. In contrast, in non-vaccinated subjects, diabetes increased the risk of HAV infection by 40%. Thus, diabetic patients should receive HAV vaccination.
- Citation: Lin J, Ou HY, Karnchanasorn R, Samoa R, Chuang LM, Chiu KC. Role of hepatitis A virus in diabetes mellitus. World J Diabetes 2021; 12(11): 1928-1941
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1928.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1928
Diabetes is a multifactorial disorder with both genetic and environmental components. Infection is an environmental component that plays a role in the development of insulin resistance and diabetes[1]. Although infection per se cannot cause diabetes, it is a well-known cause of inflammation; thus, infection may increase the risk of insulin resistance and diabetes through increased inflammation[2]. Various infectious agents have been implied in diabetes, including helicobacter pylori[3], hepatitis C[4,5], hepatitis B[6], and cytomegalovirus[7]. Given the relationship between hepatitis C and B with diabetes, we reasoned that a similar relationship could exist between hepatitis A virus (HAV) status and diabetes.
HAV is an enveloped RNA virus[8] that is transmitted via the fecal-oral route, and produces both symptomatic and asymptomatic infections[9]. Jaundice, the cardinal manifestation, develops in less than 15% of HAV-infected patients from a community outbreak[10]. HAV infection is mostly self-limited, results in life-time immunity, and does not typically result in chronic infection or chronic liver disease[11]. In the United States, HAV infection rates have declined by 95% since the HAV vaccine first became available in 1995[12]. The Centers for Disease Control and Prevention (CDC) reported 1390 acute, symptomatic cases of HAV infection in the United States in 2015, with an overall incidence rate of 0.4 cases per 100000[13]. Further, incidence may be underestimated, as HAV infection is only estimated to cause identifiable illness in less than 5% of cases[14]. As it is a food-borne disease, epidemics can be widespread and lead to significant economic loss. Thus, HAV infection remains an important public health issue.
Case reports of diabetes developed after HAV infection have led to the suggestion that HAV infection may play a role in the development of diabetes[15,16]. Based on the presence or absence of immunoglobulin G antibody to HAV, the role of HAV in diabetes was excluded in one study[17]. However, the presence of immunoglobulin G antibody to HAV could be the result of either prior HAV infection or vaccination. To address this issue, we examined the role of HAV infection, vaccination history, and successful immunization in the risk of the development diabetes in a representative United States population.
The National Health and Nutrition Examination Survey (NHANES) is a major program of the National Center for Health Statistics (NCHS), which is part of the CDC. NHANES is designed to assess the health and nutritional status of adults and children in the United States through interviews, physical examinations, and laboratory tests. The main purpose of this survey is to provide vital and health statistics for the United States. The NCHS Research Ethics Review Board approved data collection for the NHANES 2005-2012. Informed consent was obtained from participants. The records/information were anonymized and de-identified prior to release in the NHANES website (http://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Analysis of de-identified data from the survey is exempt from the federal regulations for the protection of human research participants. This study only included de-identified data from the survey.
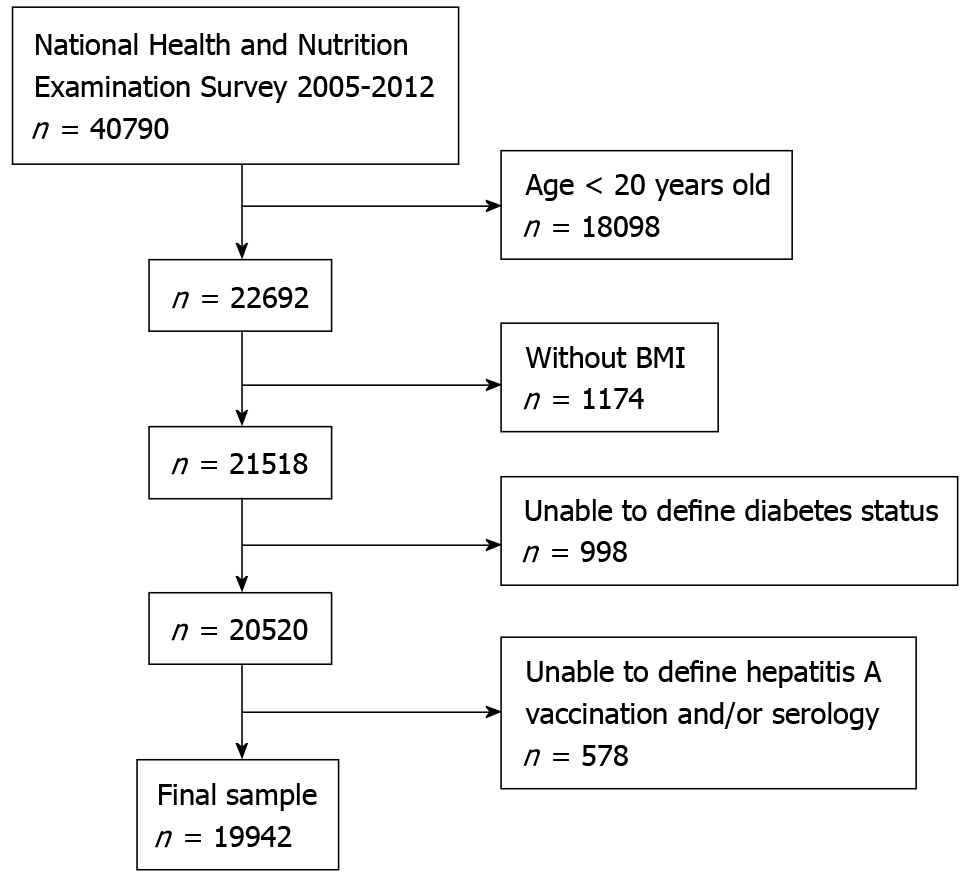
This study used data collected during the four cycles of the NHANES survey, 2005-2006, 2007-2008, 2009-2010, and 2011-2012, as the oral glucose tolerant tests was reintroduced since 2005-2006 cycle, yielded a total of 40790 potential subjects. As questionnaires were only collected for subjects 20 years or older, 18098 subjects younger than 20 years old were excluded from this study. Body mass index (BMI) is one of the major confounding factors for the development of diabetes; thus, the 1174 subjects lacking BMI data were excluded from this study. Undefined diabetes status (based on the criteria below) excluded an additional 998 subjects. Lack of HAV vaccination status and HAV serology data, including 6 subjects with intermediate HAV antibody titer, excluded an additional 578 subjects and yielded the final study population of 19942 subjects (Figure 1).
Plasma glucose concentration: NHANES collected blood samples for fasting plasma glucose concentration (FPG) after a 9-h fast and 2-h plasma glucose concentration (2hPG) in 2 h after oral administration of a standardized dose (75 g) of glucose loading in a subset of subjects after obtaining fasting samples. Plasma glucose concentration was determined by a hexokinase method. To realign plasma glucose concentrations from different assays between the cycles of 2005-2006 and 2007-2012, a regress equation as recommended by NHANES was used to line up plasma glucose concentrations from 2005-2006.
Glycosylated hemoglobin: NHANES measured glycosylated hemoglobin (HbA1c) using high performance liquid chromatography-based assays. The results of HbA1c were reviewed extensively by the National Glycohemoglobin Standardization Program (NGSP) for some minor drips between cycles which could be the results of different HbA1c laboratory instruments and laboratories were used between 2005 and 2012. Since both laboratories participated in the NGSP with the recommended standardization procedures to, the NGSP concluded that both laboratories met the NGSP criteria for bias and precision and no cross-over regression was required. In this study, the re-released HbA1c data in March 2012 for 2007-2008 (GHB_E) and 2009-2010 (GHB_F) were utilized.
Hepatitis A antibody: NHANES collected human serum or plasma and performed a qualitative determination of total anti-HAV antibody (both immunoglobulin G and immunoglobulin M) using competitive immunoassay. NHANES quality assurance and quality control protocols meet the 1988 Clinical Laboratory Improvement Act mandates. The assay used cannot differentiate between natural infection and vaccination; therefore, the presence of anti-HAV antibodies may reflect either acquired through infection or vaccine-induced immunity. Subjects with intermediate HAV antibody (n = 6) were excluded from the analysis.
To ensure inclusion of all diabetic subjects, we defined diabetes by any of the following: (1) Subjects used diabetic medications or insulin, or were informed to be diabetic by a health care provider; 92) HbA1c ≥ 47.5 mmol/mol or 6.5%; 93) FPG ≥ 7.0 mmol/L or 126 mg/dL; or 94) 2hPG ≥ 11.1 mmol/L or 200 mg/dL, according to the American Diabetes Association[18].
HAV vaccination status was self-reported to NHANES by participants. As the presence of anti-HAV antibody could be the result of prior infection or vaccination, we defined HAV infection as the presence of anti-HAV antibody in the absence of self-reported HAV vaccination. Since the successful HAV immunization rates between those who received at least 2 doses (n = 3730) or less than 2 doses (n = 499) were very similar (54.12% vs 52.10%, respectively, P = 0.39), they were pooled for further analysis. We defined successful HAV immunization as the presence of both anti-HAV antibody and HAV vaccination history.
Other covariate measures: NHANES-collected data on continuous covariates including age, BMI, and poverty index, and categorical covariates including gender, race/ethnicity, smoking status, alcohol consumption status, immediate family of diabetes, and education levels, were considered in this study. Age was calculated in years at the time of the screening interview form the reported date of birth. BMI (kg/m2) was computed from measured weight (kg) divided by the square of measured standing height (m). Based on the poverty guidelines of the United States Department of Health and Human Services, poverty index was computed as the ratio of family income to poverty, as described previously[19]. The reference poverty index of 1.0 denotes family income at the federal poverty level, and a poverty index of 2.0 signifies family income that is 200% of the federal poverty level. Gender was based on self-reported categories from the participants. Similarity, based on self-reported racial/ethnic groups, ethnicity/race were further categorized as Mexican Americans, other Hispanics, non-Hispanics whites, non-Hispanic blacks, and other ethnic/racial groups. Smoking status was defined as using tobacco/nicotine in the last 5 d. Status of alcohol consumption was defined as current alcohol consumption based on at least 12 alcohol drinks per year in the past year. Based on any blood relatives, including father, mother, sisters or brothers, ever being told by a health professional that they had diabetes, family history of diabetes was defined as positive. Based on the self-reported highest education, education level was categorized into 2 major categories: less or at least high school graduate.
SYSTAT 13.0 for windows package from SPSS, INC. (Chicago, IL, United States) was used for statistical analysis. Continuous data were presented as mean ± SD and examined using a two-tailed Student t-test or ANOVA test as if appropriate. Differences in categorical data were expressed in proportions and Chi-square test was used to examine categorical data. The odds ratio (OR) with 95% confidence intervals (CI) of risk of diabetes based on HAV vaccination history, HAV infection, and successful HAV immunization, or risk of HAV infection based on diabetes, were calculated based on logistic regression analyses with the contemplation of covariates (described above). No covariate was considered in Model 1; gender, age, and BMI were considered in Model 2; ethnic/racial group, gender, age, and BMI were considered in Model 3; all covariates including gender, age, BMI, race/ethnicity, current smoker, current alcohol consumption, family history of diabetes, poverty index, and education level were considered in Model 4. A nominal P value < 0.05 was considered to be significant.
To study the relationship between HAV and diabetes, we collected relevant data from NHANES. The sampling scheme was shown in Figure 1 and the clinical features of the study population (n = 19,942) were shown in Table 1. The prevalence of diabetes was 17.37%, with 12.19% established and 5.18% undiagnosed diabetic subjects. We used the distribution of anti-HAV antibody and vaccination history to calculate HAV immunization and infections rates (Table 2). Anti-HAV antibody was present in 9224 subjects (46.25% of total subjects), and 4229 subjects (21.21% of total subjects) received HAV vaccination (Table 2). We defined successful immunization as the intersection of the presence of anti-HAV antibody and HAV vaccination history; successful HAV immunization occurred in 2279 subjects (53.89% of vaccinated subjects). We defined HAV infection as the intersection of the presence of anti-HAV antibody with no history of HAV vaccination; infection occurred in 6945 subjects (44.20% of non-vaccinated subjects).
| n | 19942 | |
| Gender | Female | 10256 (51.43) |
| Age | Year | 49 ± 18 |
| Body mass index | Kg/m2 | 28.94 ± 6.75 |
| Fasting plasma glucose1 | mmol/L | 6.1 ± 2.1 |
| 2-h plasma glucose2 | mmol//L | 6.8 ± 2.9 |
| HbA1c3 | % | 5.7 ± 1.0 |
| HbA1c3 | mmol/mol | 38.90 ± 11.45 |
| Current smoker | Yes | 4651 (23.32) |
| Current alcohol consumption | Yes | 13069 (65.54) |
| Education: at least high school graduate | Yes | 14467 (72.55) |
| Poverty index | 2.72 ± 1.70 | |
| Family history of diabetes | Yes | 7936 (39.80) |
| States of glucose tolerance | ||
| Diabetes | Yes | 3463 (17.37) |
| Established diabetes | Yes | 2430 (12.19) |
| Undiagnosed diabetes | Yes | 1033 (5.18) |
| Hepatitis A vaccination | Yes | 4229 (21.21) |
| Presence of anti-HAV antibody | Yes | 9224 (46.25) |
| Ethnic/racial groups | ||
| Mexican American | 3307 (16.58) | |
| Other Hispanic | 1787 (8.96) | |
| Non-Hispanic white | 9205 (46.16) | |
| Non-Hispanic black | 4131 (20.72) | |
| Other | 1512 (7.58) |
To evaluate the role of HAV infection in the risk of development of diabetes, we compared the prevalence of diabetes between two groups: HAV infected subjects (the presence of anti-HAV antibody without a history of HAV vaccination) vs non-HAV infected subjects. As shown in Table 3, diabetes occurred in 1712 subjects (24.65%) with HAV infection and in 1751 subjects (13.47%) without HAV infection (P < 0.0001), with an OR of 1.45 (95%CI: 1.40-1.50; Table 4). Although no difference was noted in the gender distribution (P = 0.11) or BMI (P = 0.07), HAV-infected subjects were significantly older than non-infected subjects (56 ± 18 years and 46 ± 17 years, respectively; P < 0.0001). After adjustment for gender, age, and BMI covariates, HAV infection was significantly associated with an increased OR for diabetes (1.22; 95%CI: 1.17-1.27; Table 4). Although the frequency of HAV infection varied significantly across ethnic/racial groups (P < 0.0001), additional adjustment with ethnic/racial group had no significant effect on diabetes risk when considered as a covariate with gender, age, and BMI (OR: 1.22; 95%CI: 1.17-1.28; Table 4). In the fully adjusted Model 4 that includes all covariates, HAV infection remained a significant risk factor for the development of diabetes (OR: 1.13; 95%CI: 1.08-1.18; Table 4). Thus, there was an association between HAV infection diabetes.
| Non-infected | Infected | P | ||||||
| n | 12997 | 6945 | ||||||
| Gender | Female | 6738 (51.84) | 3518 (50.66) | NS | ||||
| Age | Yr | 46 ± 17 | 56 ± 18 | < 0.0001 | ||||
| Body mass index | Kg/m2 | 29.00 ± 7.01 | 28.82 ± 6.24 | NS | ||||
| Fasting plasma glucose1 | mmol/L | 5.8 ± 1.8 | 6.4 ± 2.4 | < 0.0001 | ||||
| 2-h postchallenged plasma glucose2 | mmol/L | 6.4 ± 2.7 | 7.4 ± 3.4 | < 0.0001 | ||||
| HbA1c3 | % | 5.6 ± 0.9 | 5.9 ± 1.2 | < 0.0001 | ||||
| HbA1c3 | mmol/mol | 37.66 ± 10.20 | 41.22 ± 13.18 | < 0.0001 | ||||
| Current smoker | Yes | 3418 (26.30) | 1233 (17.75) | < 0.0001 | ||||
| Current alcohol consumption | Yes | 9089 (69.93) | 3980 (57.31) | < 0.0001 | ||||
| Education: at least high school graduate | Yes | 10668 (82.08) | 3799 (54.70) | < 0.0001 | ||||
| Poverty index | 2.87 ± 1.70 | 2.44 ± 1.66 | < 0.0001 | |||||
| Family history of diabetes | Yes | 5074 (39.04) | 2862 (41.21) | 0.003 | ||||
| Established diabetes | Yes | 1217 (9.36) | 1213 (17.47) | < 0.0001 | ||||
| States of glucose tolerance | < 0.0001 | |||||||
| Normal glucose tolerance | 7512 (57.80) | 2799 (40.30) | ||||||
| Impaired glucose tolerance | 3734 (28.73) | 2434 (35.05) | ||||||
| Diabetes | 1751 (13.47) | 1712 (24.65) | ||||||
| Hepatitis A vaccination | Yes | 4229 (32.54) | 0 (0.00) | < 0.0001 | ||||
| Presence of hepatitis A antibody | Yes | 2279 (17.53) | 6945 (100.00) | < 0.0001 | ||||
| Ethnic/racial | < 0.0001 | |||||||
| Mexican American | 1184 (9.11) | 2123 (30.57) | ||||||
| Other Hispanic | 800 (6.16) | 987 (14.21) | ||||||
| Non-Hispanic white | 7346 (56.52) | 1859 (26.77) | ||||||
| Non-Hispanic black | 2807 (21.60) | 1,324 (19.06) | ||||||
| Other | 860 (6.62) | 652 (9.39) | ||||||
| Status | HAV infection | n | Diabetes | Model 1 | Model 2 | Model 3 | Model 4 |
| All diabetes | No | 12997 | 1751 (13.47) | 1.45 | 1.22 | 1.22 | 1.13 |
| Yes | 6945 | 1712 (24.65) | (1.40-1.50) | (1.17-1.27) | (1.17-1.28) | (1.08-1.18) | |
| Established diabetes | No | 12997 | 1217 (9.36) | 1.43 | 1.20 | 1.21 | 1.11 |
| Yes | 6945 | 1213 (17.47) | (1.37-1.49) | (1.14-1.26) | (1.15-1.27) | (1.05-1.16) | |
| Diabetes by fasting plasma glucose criterion | No | 4579 | 432 (9.43) | 1.44 | 1.22 | 1.21 | 1.12 |
| Yes | 2526 | 451 (17.85) | (1.35-1.55) | (1.13-1.32) | (1.12-1.31) | (1.03-1.21) | |
| Diabetes by 2-h postchallenged plasma glucose criterion | No | 4777 | 277 (5.80) | 1.46 | 1.21 | 1.17 | 1.11 |
| Yes | 2348 | 274 (11.67) | (1.34-1.60) | (1.10-1.33) | (1.07-1.29) | (1.01-1.23) | |
| Diabetes by HbA1c criterion | No | 12978 | 1023 (7.88) | 1.47 | 1.27 | 1.28 | 1.19 |
| Yes | 6932 | 1085 (15.65) | (1.41-1.54) | (1.21-1.34) | (1.22-1.35) | (1.12-1.25) |
To further evaluate the association of HAV with diabetes, we conducted sensitivity analyses with various definitions of diabetes (Table 4). As established diabetes accounted for 70.17% of diabetes in this sample set, we first examined the role of HAV infection in established diabetes. The results of all four models confirmed an association between HAV infection and established diabetes (Table 4). Using FPG and 2hPG concentrations as a proxy for diabetes confirmed the association between HAV infection and diabetes, demonstrating similar ORs to established diabetes for all four models (Table 4, respectively). As only a selected subset of subjects had either FPG or 2hPG measurements, the sample size was reduced significantly, but P-values, although increased, remained significant (P = 0.01 and P = 0.04, respectively). As HbA1c measurements were available for most subjects, the sample size, ORs, and P-values were very similar for the association of hepatitis A and HbA1c as for established diabetes (Table 4).
Since HAV infection was associated with an increased risk for diabetes, we next examined whether HAV vaccination was associated with a reduced risk of diabetes (Table 5). In this population, 12.84% of subjects who received HAV vaccination were diabetic, while 18.58% of non-vaccinated subjects were diabetic (P < 0.0001). The clinical characteristics of the two groups also differed significantly, except for the status of smoking and alcohol consumption. However, the association disappeared after adjustment for age, gender, and BMI in Model 2, and further analyses (Models 3 and 4) excluded the role of HAV vaccination in diabetes (Table 5). In the sensitivity analyses, the protective effect of diabetes by HAV vaccination was noted in Model 1 (unadjusted) by all diabetes definitions (Table 5), but adjustment of covariates (Models 2, 3, and 4) rendered no association between HAV vaccination and diabetes. Thus, HAV vaccination had no impact on the development of diabetes.
| Status | HAV vaccination | n | Diabetes | Model 1 | Model 2 | Model 3 | Model 4 |
| All diabetes | No | 15713 | 2920 (18.58) | 0.65 | 1.02 | 1.03 | 1.06 |
| Yes | 4229 | 543 (12.84) | (0.58-0.71) | (0.91-1.14) | (0.92-1.14) | (0.95-1.18) | |
| Established diabetes | No | 15713 | 2039 (12.98) | 0.68 | 1.08 | 1.08 | 1.12 |
| Yes | 4229 | 391 (9.25) | (0.61-0.77) | (0.96-1.22) | (0.96-1.22) | (0.99-1.28) | |
| Diabetes by fasting plasma glucose criterion | No | 5578 | 750 (13.45) | 0.61 | 0.95 | 0.95 | 0.97 |
| Yes | 1527 | 133 (8.71) | (0.51-0.75) | (0.77-1.16) | (0.77-1.17) | (0.78-1.19) | |
| Diabetes by 2-h postchallenged plasma glucose criterion | No | 5550 | 478 (8.61) | 0.52 | 0.85 | 0.86 | 0.88 |
| Yes | 1575 | 73 (4.63) | (0.40-0.66) | (0.65-1.10) | (0.66-1.12) | (0.67-1.15) | |
| Diabetes by HbA1c criterion | No | 15690 | 1772 (11.29) | 0.68 | 1.03 | 1.03 | 1.06 |
| Yes | 4220 | 336 (7.96) | (0.60-0.77) | (0.90-1.17) | (0.90-1.17) | (0.93-1.21) |
Since we noted the presence of anti-HAV antibody in only 53.89% of vaccinated subjects (Table 2), HAV vaccination per se might not be effective at all in the reduction of diabetes. We examined the role of successful HAV immunization on risk of diabetes, defined as the presence of both HAV vaccination and anti-HAV antibody. The prevalence of diabetes was significantly different between the subjects with and without successful immunization (14.79% vs 17.70%, respectively, P = 0.0006). Clinical characteristics also differed significantly between the two groups, except for education. As shown in Table 6, although an unadjusted analysis (Model 1) demonstrated a protective effect, additional analyses in Models 2, 3, and 4 failed to confirm the protective effect of successful HAV immunization on the risk of diabetes. Similar results were obtained in the sensitivity analyses (Table 6). Thus, successful HAV immunization played no role in the development of diabetes.
| Status | Successful HAV immunization | n | Diabetes | Model 1 | Model 2 | Model 3 | Model 4 |
| All diabetes | No | 17663 | 3126 (17.70) | 0.81 | 1.10 | 1.10 | 1.11 |
| Yes | 2279 | 337 (14.79) | (0.71-0.91) | (0.96-1.26) | (0.96-1.25) | (0.97-1.27) | |
| Established diabetes | No | 17512 | 2039 (11.64) | 0.83 | 1.13 | 1.13 | 1.14 |
| Yes | 2430 | 240 (9.88) | (0.72-0.96) | (0.97-1.31) | (0.97-1.31) | (0.98-1.33) | |
| Diabetes by fasting plasma glucose criterion | No | 6244 | 796 (12.75) | 0.77 | 1.06 | 1.05 | 1.05 |
| Yes | 861 | 87 (10.10) | (0.61-0.97) | (0.83-1.36) | (0.82-1.35) | (0.82-1.36) | |
| Diabetes by 2-h postchallenged plasma glucose criterion | No | 6243 | 498 (7.98) | 0.74 | 1.02 | 1.01 | 1.03 |
| Yes | 882 | 53 (6.01) | (0.55-0.99) | (0.75-1.39) | (0.74-1.37) | (0.76-1.40) | |
| Diabetes by HbA1c criterion | No | 17636 | 1900 (10.77) | 0.83 | 1.11 | 1.11 | 1.12 |
| Yes | 2274 | 208 (9.15) | (0.72-0.97) | (0.95-1.30) | 1.30) | (0.95-1.32) |
To examine the alternative hypothesis that diabetes was more susceptible for HAV infection, we restricted the sample to non-vaccinated subjects only and examined the association between diabetes status and HAV infection. In non-vaccinated subjects, 58.63% of diabetic subjects were seropositive for anti-HAV antibody, while only 40.91% of non-diabetic subjects were seropositive (P < 0.0001). The OR for HAV infection was 2.05 (95%CI: 1.89-2.22) for Model 1, which was unadjusted for covariates (Table 7). The association remained highly significant (P < 0.0001 for all models) after adjustment for age, gender, and BMI in Model 2 (OR: 1.59, 95%CI: 1.45-1.74), additional ethnic/racial groups in Model 3 (OR: 1.65, 95%CI: 1.50-1.81), and all covariates in Model 4 (OR: 1.40, 95%CI: 1.27-1.54). Sensitivity analyses with various definition of diabetes confirmed an increased OR for HAV infection in diabetic subjects (Table 7). Thus, in non-vaccinated subjects, subjects with diabetes had an increased risk of HAV infection than subjects without diabetes.
| Status | Diabetes | n | HAV infection | Model 1 | Model 2 | Model 3 | Model 4 |
| All diabetes | No | 12793 | 5233 (40.91) | 2.05 | 1.59 | 1.65 | 1.40 |
| Yes | 2920 | 1712 (58.63) | (1.89-2.22) | (1.45-1.74) | (1.50-1.81) | (1.27-1.54) | |
| Established diabetes | No | 13674 | 5732 (41.92) | 2.03 | 1.57 | 1.67 | 1.39 |
| Yes | 2039 | 1213 (59.49) | (1.85-2.24) | (1.42-1.73) | (1.50-1.85) | (1.24-1.56) | |
| Diabetes by fasting plasma glucose criterion | No | 4828 | 2075 (42.98) | 2 | 1.56 | 1.58 | 1.34 |
| Yes | 750 | 451 (60.13) | (1.71-2.34) | (1.32-1.85) | (1.32-1.87) | (1.11-1.61) | |
| Diabetes by 2-h postchallenged plasma glucose criterion | No | 5072 | 2074 (40.89) | 1.94 | 1.48 | 1.39 | 1.27 |
| Yes | 478 | 274 (57.32) | (1.61-2.35) | (1.21-1.81) | (1.13-1.71) | (1.02-1.58) | |
| Diabetes by HbA1c criterion | No | 13918 | 5847 (42.01) | 2.18 | 1.76 | 1.87 | 1.59 |
| Yes | 1772 | 1085 (61.23) | (1.97-2.41) | (1.58-1.96) | (1.67-2.09) | (1.42-1.80) |
In this study, we examined the relationship between HAV status and diabetes. The primary hypothesis was that HAV infection increases the risk of diabetes, while alternatively diabetes increased susceptibility to HAV infection, given another possibility would be no association between HAV infection and diabetes. We performed a stepwise hypothesis testing approach. First, the association of HAV infection with diabetes was established, followed by examination of the effect of HAV vaccination on diabetes. After demonstrating a lack of association with HAV vaccination, we further examined the effect of successful HAV immunization on diabetes, but again found no association. Instead, we confirmed an alternative hypothesis that diabetes increased susceptibility to HAV infection.
To examine the hypothesis that HAV was a risk factor for diabetes, we first examined the association between HAV infection and diabetes. In this representative U.S. population, the prevalence of HAV infection is 34.83% and HAV infection is associated with a 13% increased risk of diabetes (OR: 1.13, 95CI%: 1.08-1.18) after adjustment of covariates (Table 4). In contrast to viral hepatitis B and C, HAV infection is not associated with chronic liver disease[8,9,11]. The possibility of previous HAV infection affecting glucose metabolism could not be excluded. From a metabolic point of view, the HAV-infected subjects had significantly higher FPG, 2hPG, and HbA1c than non-HAV-infected subjects (Table 3). These results further suggest the possible interaction between a previously HAV-infected liver and glucose metabolism. Nevertheless, our results demonstrate a link between the serological evidence of HAV infection and diabetes initially.
If this observation was true, the HAV vaccination would reduce the risk of diabetes. Furthermore, we observed that the subjects with HAV infection history were less likely to be high school graduate or higher (54.70% vs 82.08%, P < 0.0001) than those without evidence of HAV infection. As it is well known that health-conscious persons are more likely to receive HAV vaccination, and less likely to develop diabetes, we next examined whether there was any association of HAV vaccination with diabetes, especially with reduced risk of diabetes. In this population, the HAV vaccination rate was relatively low, only 21.21%, and HAV vaccination had no impact of diabetes risk (OR: 1.06, 95%CI: 0.95-1.18) after adjustment for covariates (Table 5). Although an initial protective effect was noted in Model 1 (Table 5), no association between HAV vaccination and diabetes was found after adjustment for covariates. These results also suggested that the observed increased risk of diabetes with HAV infection was less likely through a selective process of a less health-conscious population (i.e., no HAV vaccination). However, this observation contradicted the initial observed association between HAV infection and diabetes. Next, we studied the effectiveness of HAV vaccination in the study population, which could affect the association between vaccination and diabetes. HAV vaccination has been reported to be very effective[20], but we found no evidence of reduced diabetes risk with HAV vaccination (Table 5). We examined the effectiveness of HAV vaccination in this population and found only 53.89% of vaccinated subjects were noted to have adequate anti-HAV antibody (Table 2). Among 4229 subjects who received vaccination, 543 subjects were diabetic. Anti-HAV antibody was present in 62.06% of diabetic subjects who received vaccination while in 52.69% of non-diabetic subjects who received vaccination (P < 0.0001). This suggested that either: 1) subjects with diabetes respond to HAV vaccination better than subjects without diabetes, or 2) subjects with diabetes were more likely to have received HAV vaccination than non-diabetic subjects. Due to relatively low seropositivity for HAV after vaccination, we further examined the effect of successful HAV immunization on diabetes risk. Again, no association was found between successful HAV immunization and diabetes (Table 6). If HAV infection was a risk factor of the development of diabetes as the observed association between diabetes and HAV infection, one would expect that successful immunization of HAV would reduce diabetes risk. However, the results demonstrated that successful HAV immunization had no impact on the risk of diabetes.
As this is a cross-sectional association study, the causal relationship cannot be obtained directly from this study. With the stepwise hypothesis testing approach, the original hypothesis was less likely to be true. To reconcile the results of diabetes with HAV infection, vaccination and successful immunization, alternative hypothesis that diabetic subjects was more prone for HAV infection was investigated in non-vaccinated subjects. One would expect to see more HAV infection in diabetic subjects than non-diabetic subjects without vaccination. To exclude the effect of HAV vaccination, the subset of non-vaccination subjects (n = 15713) was further examined for the association between HAV infection and diabetes (Table 7). Among 2920 diabetic subjects without HAV vaccination, the seropositive rate for anti-HAV antibody was 58.63%, while it was 40.91% in 12793 non-diabetic subjects without HAV vaccination (P < 0.0001). Thus, diabetes increased the risk of HAV with an OR of 2.05 (95%CI: 1.89-2.11). The association was confirmed after adjustment for covariates (Table 7) and also by sensitivity analyses based on the different definition of diabetes (Table 7). Thus, the results of this study are most consistent with the alternative hypothesis that diabetes increases the risk of HAV infection.
There are several features of this study that worthwhile to be mentioned. A representative United States population with fairly large sample size was used in the present study which makes the results more applicable to the United States population. As it is almost impossible to match the studied groups completely, the large sample size with fairly extensive data available allows adjustment of covariates properly. Indeed, adjustment of covariates did affect the results of HAV vaccination and successful HAV immunization, as protective effects were noted initially and they disappear after adjustment of covariates. In contrast, adjustment of covariates confirmed the results for the association between HAV infection and diabetes in the whole sample set as well as in the subset of subjects without prior HAV vaccination. Furthermore, we took extra steps to validate the result by sensitivity tests with different definition of diabetes to confirm the observations and the results of sensitivity tests were consistent with the original observations. Stepwise hypothesis testing approach allows this study to provide the insight of casual relationship between HAV infection and diabetes. Thus, there is a significant scientific merit of this study.
As this is a cross-sectional study, no causal relationship can be provided directly. However, with stepwise hypothesis testing approach, we are able to deduct the results and to infer diabetes as the cause of the increased susceptibility of HAV infection. Alternatively, one can examine the effect of aggressive prevention of diabetes on the incidence of HAV infection. Given the established benefit of diabetes prevention[21], it would not be ethical to conduct an interventional trial to examine the impact of diabetes prevention on the development of HAV infection. Thus, stepwise hypothesis testing approach is a useful alternative approach to infer the causal relationship when an interventional trial is not feasible to confirm the observation.
In the NHANES, there was no information collected regarding the type of diabetes and auto-antibodies for type 1 diabetes were not assessed. Thus, patients with type 1 diabetes cannot be separated out unambiguously. Based on the National Health Interview Survey in 2016, type 2 diabetes accounted for 90.9% of diabetes while type 1 diabetes accounted for 5.8% of diabetes[22] which was defined based on self-report type and also current use of insulin. In this Survey, the reported prevalence of type 1 diabetes was 0.55% of the adult population in the United Stated[22], which is much higher than the reported 0.18% from the Hispanic Community Health Study/Study of Latinos, which is a community-based epidemiologic study in Hispanic/Latino adults residing in four United States communities[23]. The overestimation in the 2016 Survey[22] could be from the assumption of type 1 diabetes based on the current use of insulin. Since type 2 diabetes develops when beta cell function to 55% left and beta cell declines at 5% per year regardless treatment modalities[24], it is expected that insulin treatment will be required sometime in the course of type 2 diabetes and it has been demonstrated that almost two-thirds of patient with type 2 diabetes received insulin treatment[25]. Thus, defining type 1 diabetes based on using insulin treatment is unreliable and could overestimate the prevalence of type 1 diabetes. Since type 1 and type 2 diabetes are the two most common form of diabetes and less than 10% of patients with diabetes have type 1 diabetes, the vast majority (≥ 90%) of diabetic patients in this cohort have type 2 diabetes. Thus, we are able to conclude the association of HAV infection with type 2 diabetes.
Hyperglycemia is associated with the increased risk of infection[26]. Infectious burden increases by 2.14 times in diabetes[27]. Furthermore, hospitalization rates from infection is almost 3.8-time higher in adults with diabetes than without diabetes, especially young adults with diabetes[28] who are more prevalent with type 1 diabetes. Thus, patients with type 1 diabetes could also have an increased risk of HAV infection. However, due to limited number of type 1 diabetes and unable to separate out type 1 diabetes unequivocally based the study design of the NHANES, we are not able to generalize the observed results in type 1 diabetes. Nevertheless, our observation of an increased risk of HAV infection in diabetes is in line with reported increased risk of infection in diabetes, regardless type of diabetes. Furthermore, the effectiveness of HAV vaccination is relatively low, 53.89% (Table 2), which is not in line with the published reports[20]. Further studies are required to address these issues.
In summary, we have demonstrated an association between diabetes and HAV infection. This association is not mediated through acute HAV infection, and HAV vaccination and successful HAV immunization do not reduce the risk of diabetes. In non-HAV-vaccinated subjects, diabetes is a risk factor for HAV infection with an OR of 1.40 (95%CI: 1.27-1.54). Thus, through the deduction process of a stepwise hypothesis testing approach, we suggest that the most rational explanation is that diabetes increases the risk of HAV infection by 40%. Furthermore, diabetes increases HAV mortality by 2.2 times[29]. Accordingly, HAV vaccination is highly recommended in diabetic subjects.
The liver plays an important role in glucose hemostasis. Hepatitis C infection increases the risk of diabetes. Thus, other liver infection could also play a role in the pathogenesis of diabetes.
This study is to dissect the relationship between hepatitis A and diabetes.
The objective of this study is to investigate the interaction between hepatitis A and diabetes through hypothesis testing in a representative adult cohort of the United States.
The information on hepatitis A vaccination history, hepatitis A antibody status, and diabetes status were obtained from the participants in the National Health and Nutrition Examination Survey 2005-2012. Hepatitis A infection was defined as the presence of hepatitis A antibody without hepatitis A vaccination. Hepatitis A vaccination was defined as the presence of hepatitis A vaccination, regardless hepatitis A antibody status. Successful hepatitis A immunization was defined as the presence of both hepatitis A vaccination and hepatitis A antibody. A stepwise hypothesis testing approach was used to dissect the interaction between hepatitis A and diabetes and the sensitivity tests based on the different definitions of diabetes were used to confirm the results.
Diabetes risk was increased in the participants with hepatitis A infection, suggesting a causal relationship between hepatitis A infection and diabetes. However, hepatitis A vaccination and also successful hepatitis A immunization did not reduce the risk of diabetes, excluding hepatitis A infection as a cause of diabetes. In non-hepatitis A vaccinated participants, diabetes increased the risk of hepatitis A infection by 40%.
Hepatitis A is not a cause of diabetes. Instead, diabetes increases the risk of hepatitis A infection.
Since hepatitis A mortality increases in patients with diabetes, hepatitis A vaccination is highly recommended in patients with diabetes.
A special acknowledgement is due to Chiu-Tien Chiu, MD, PhD for his unconditional support to KCC. We acknowledge Sarah T. Wilkinson, PhD for critical reading and editing of the manuscript, Henry Lin, PhD for excellent support of manuscript preparation, and Karen Ramos for excellent logistic and administrative support of this research endeavor.
Provenance and peer review: Invited article; Externally peer reviewed
Corresponding Author's Membership in Professional Societies: Endocrine Society; American Diabetes Association; American College of Endocrinology; American Association of Clinical Endocrinology; American College of Physicians.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorrell M S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 6372] [Article Influence: 354.0] [Reference Citation Analysis (1)] |
| 2. | Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1432] [Cited by in RCA: 1519] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 3. | Santos MLC, de Brito BB, da Silva FAF, Sampaio MM, Marques HS, Oliveira E Silva N, de Magalhães Queiroz DM, de Melo FF. Helicobacter pylori infection: Beyond gastric manifestations. World J Gastroenterol. 2020;26:4076-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 4. | Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Jeong D, Karim ME, Wong S, Wilton J, Butt ZA, Binka M, Adu PA, Bartlett S, Pearce M, Clementi E, Yu A, Alvarez M, Samji H, Velásquez García HA, Abdia Y, Krajden M, Janjua NZ. Impact of HCV infection and ethnicity on incident type 2 diabetes: findings from a large population-based cohort in British Columbia. BMJ Open Diabetes Res Care. 2021;9:e002145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Lao TT, Chan BC, Leung WC, Ho LF, Tse KY. Maternal hepatitis B infection and gestational diabetes mellitus. J Hepatol. 2007;47:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Chen S, de Craen AJ, Raz Y, Derhovanessian E, Vossen AC, Westendorp RG, Pawelec G, Maier AB. Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the Leiden 85-plus Study. Immun Ageing. 2012;9:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43:S164-S172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001;14:38-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Pavia AT, Nielsen L, Armington L, Thurman DJ, Tierney E, Nichols CR. A community-wide outbreak of hepatitis A in a religious community: impact of mass administration of immune globulin. Am J Epidemiol. 1990;131:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Matheny SC, Kingery JE. Hepatitis A. Am Fam Physician. 2012;86:1027-1034. [PubMed] |
| 12. | Centers for Disease Control and Prevention. Hepatitis A Questions and Answers for the Public, Viral Hepatitis. 2020. [cited 10 March 2021]. Available from: https://www.cdc.gov/hepatitis/hav/afaq.htm. |
| 13. | Centers for Disease Control and Prevention. Viral Hepatitis. [cited 10 March 2021]. Available from: http://www.cdc.gov/hepatitis/. |
| 14. | Levinthal G, Ray M. Hepatitis A: from epidemic jaundice to a vaccine-preventable disease. Gastroenterologist. 1996;4:107-117. [PubMed] |
| 15. | Makeen AM. The association of infective hepatitis type A (HAV) and diabetes mellitus. Trop Geogr Med. 1992;44:362-364. [PubMed] |
| 16. | Vesely DL, Dilley RW, Duckworth WC, Paustian FF. Hepatitis A-induced diabetes mellitus, acute renal failure, and liver failure. Am J Med Sci. 1999;317:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lutsey PL, Pankow JS, Bertoni AG, Szklo M, Folsom AR. Serological evidence of infections and Type 2 diabetes: the MultiEthnic Study of Atherosclerosis. Diabet Med. 2009;26:149-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | American Diabetes Association. . 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2247] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 19. | Malamug LR, Karnchanasorn R, Samoa R, Chiu KC. The Role of Helicobacter pylori Seropositivity in Insulin Sensitivity, Beta Cell Function, and Abnormal Glucose Tolerance. Scientifica (Cairo). 2014;2014:870165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Freeman E, Lawrence G, McAnulty J, Tobin S, MacIntyre CR, Torvaldsen S. Field effectiveness of hepatitis A vaccine and uptake of post exposure prophylaxis following a change to the Australian guidelines. Vaccine. 2014;32:5509-5513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 694] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 22. | Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, Orchard TJ, Rolka DB, Imperatore G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:359-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 312] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 23. | Kinney GL, Young KA, Lamb MM, Orchard TJ, Cai J, Hosgood D, Klein OL, Talavera G, Young L, Daviglus M. The Prevalence of Type 1 Diabetes in Hispanic/Latino Populations in the United States: Findings from the Hispanic Community Health Study/Study of Latinos. Epidemiology. 2020;31:e7-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249-1258. [PubMed] |
| 25. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4034] [Article Influence: 448.2] [Reference Citation Analysis (1)] |
| 26. | Zacay G, Hershkowitz Sikron F, Heymann AD. Glycemic Control and Risk of Cellulitis. Diabetes Care. 2021;44:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Hartog L, van Rooijen MS, Ujčič-Voortman J, Prins M, van Valkengoed IGM. Ethnic differences in infectious burden and the association with metabolic risk factors for cardiovascular disease: a cross-sectional analysis. BMC Public Health. 2018;18:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Harding JL, Benoit SR, Gregg EW, Pavkov ME, Perreault L. Trends in Rates of Infections Requiring Hospitalization Among Adults With Versus Without Diabetes in the United States, 2000-2015. Diabetes Care. 2020;43:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Hofmeister MG, Xing J, Foster MA, Augustine RJ, Burkholder C, Collins J, McBee S, Thomasson ED, Thoroughman D, Weng MK, Spradling PR. Factors Associated With Hepatitis A Mortality During Person-to-Person Outbreaks: A Matched Case-Control Study-United States, 2016-2019. Hepatology. 2021;74:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |